Abstract
Background
The systemic immune-inflammation index (SII) showed an extensive link between immunological dysfunction and the activation of systemic inflammation. Several studies have confirmed the application of SII to orthopedic diseases. However, the significance of SII in critically ill elderly individuals with hip fracture who require intensive care unit (ICU) admission is not yet known. This study centered on exploring the relationship between SII and clinical outcomes among critically ill elderly hip fracture individuals.
Methods
The study centered around elderly patients experiencing severe illness following hip fractures and requiring admission to the ICU. These patients from the MIMIC-IV database formed the basis of this study’s cohort. We stratified them into quartiles according to their SII levels. The results involved the mortality at 30 days and 1 year post-admission. Then we employ Cox proportional hazards regression analysis as well as restricted cubic splines to explore the association between the SII and clinical results in critically ill elderly patients with hip fracture.
Results
The study encompassed 991 participants, among whom 63.98% identified as females. Notably, the mortality rates attributed to any cause within 30 days and 1 year after hospitalization stood at 19.68 and 33.40%, respectively. The multivariate Cox proportional hazards model disclosed a significant correlation between an elevated SII and all-cause mortality. Following adjustments for confounding variables, individuals with a high SII showed a notable correlation with 30-day mortality [adjusted hazard ratio (HR), 1.065; 95% confidence interval (CI), 1.044–1.087; p < 0.001] and 1-year mortality (adjusted HR, 1.051; 95% CI, 1.029–1.074; p < 0.001). Furthermore, the analysis of restricted cubic splines demonstrated a progressive increase in the risk of all-cause death as the SII value rose.
Conclusion
Among critically ill elderly patients with hip fracture, the SII exhibits a non-linear association that positively correlates with both 30-day and 1-year all-cause mortality rates. The revelation indicates that the SII may play a vital role in identifying patients with hip fractures who face an escalated risk of mortality due to any cause.
Keywords: systemic immune-inflammation index, critically ill elderly patients, hip fracture, prognostic value, mortality, MIMIC-IV database
1. Introduction
Hip fractures are common in individuals aged 70 years or older, and the incidence of hip fractures has risen by 93.0% from 1990 to 2019, with an estimated 14.2 million cases globally, which poses a significant threat to public health (1). Despite a considerable number of elderly adults receiving hospitalization post-injury, the risk of complications and mortality remains high. Approximately 9% of general patients succumb within a month, and this figure escalates to a staggering 36% for mortality within a year’s timeframe (2). Some researchers suggest that the elevated mortality rate of hip fractures is linked to the patient’s pathological and behavioral status, including nutritional deficiencies, immune system functioning, and the diminishment of skeletal muscle mass (3). Several studies have provided similar elucidation of the relationship between hip fracture mortality and immune health, particularly the inflammatory response (4). Published studies have demonstrated that the systemic immune-inflammation index (SII) functions as a distinct predictor of mortality subsequent to hip fracture incidents (5, 6). The SII is composed of the counts of neutrophils, lymphocytes, and platelets in peripheral blood, serving as a reliable indicator of inflammation. Furthermore, numerous studies have linked SII to predicting outcomes in various diseases among the general population and specific high-risk patient groups (6), for instance, malignancy (7), coronary artery disease (8), and acute ischemic stroke (9). Meanwhile, the SII has been progressively utilized for prognostication in orthopedics for preoperative evaluation (10), surgical trauma (11), and postoperative complications (12).
The SII provides a convenient and effective method for examining immunological dysfunction and the activation of systemic inflammation (13, 14). Prior research has mostly concentrated on evaluating SII levels in the general population to forecast adverse outcomes in hip fracture disease (3, 5). Several studies have found a significant link between high SII levels, inflammation advancement, and higher mortality rates for fractures (15). Moreover, multiple studies have demonstrated that SII is effective in forecasting the probability of post-surgical complications, including deep vein thrombosis (DVT), pneumonia, and even mortality in hip fracture patients (10, 16). Increasing evidence consistently connects high SII levels to elevated all-cause death rates. However, it is unclear whether this connection continues to exist in critically ill elderly individuals with hip problems who typically exhibit poorer pathophysiology. Therefore, evaluating the potential of SII in forecasting health complications among elderly individuals with severe hip conditions could aid in pinpointing those with an elevated risk of mortality from any cause, thereby facilitating timely medical intervention or emergency treatment.
The investigation sought to assess the prognostic value of the SII on overall mortality in aged critical patients with hip fracture, utilizing data sourced from the Medical Information Mart for Intensive Care IV (MIMIC-IV) database, considering the present state of scholarly research.
2. Methods
2.1. Data source
The research conducted a retrospective analysis of health-associated records sourced from the MIMIC-IV database, version 2.2, developed and managed by the MIT Computational Physiology Laboratory. The MIMIC-IV database encompasses numerical information related to the medical records of patients who were either admitted to the Intensive Care Unit (ICU) or received treatment in the emergency department at the Beth Israel Deaconess Medical Center, spanning the years between 2008 and 2019 (17). Authorization for Zhen-Jiang Liu, the first author (certification identifier: 12911307), was granted to access the MIMIC-IV database upon successful completion of the online educational program provided by the National Institutes of Health. The BIDMC Institutional Review Board evaluated the collection of patient data and the creation of the research resource. Then they gave approval to the data-sharing initiative and exempted the need for informed permission. All processes were executed in compliance with the regulations governing patient privacy and confidentiality.
2.2. Patient selection
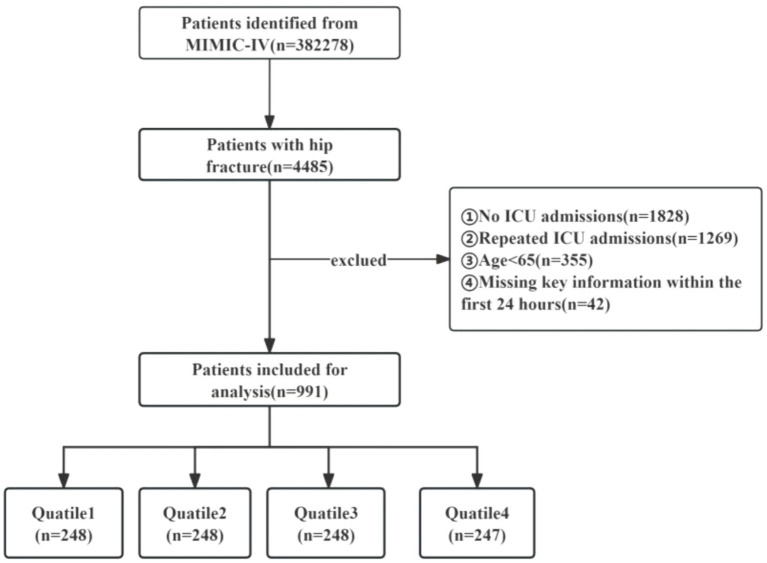
4,485 patients with hip fractures were identified in the MIMIC-IV database based on specific ICD codes: ICD-9:820, ICD-9:821, ICD-9:73314, ICD-9:73396, ICD-10:S72, ICD-10:S790, ICD-10:M9666, ICD-10:M8445, ICD-10:M8435, ICD-10:M8005, and ICD-10:M8085. The dataset encompasses a period ranging from January 2008 through December 2019. The eligibility criteria for inclusion were as follows: (I) participants aged 65 years or older; (II) those diagnosed with a hip fracture; and (III) patients necessitating ICU admission. Exclusion standards comprised the following: (I) instances where subjects had inadequate or untraceable documentation or pivotal medical records; (II) those who experienced multiple ICU admissions due to hip fracture, with only the initial admission data being considered; (III) individuals with missing survival outcome information; and (IV) patients devoid of critical data (neutrophil, lymphocyte, or platelet counts) on the day of admission. Ultimately, a collective of 991 participants were incorporated into the investigation and allocated into four categories in accordance with the quartiles of the SII (Figure 1).
Figure 1.
The flowchart of the patient selection process in the trial.
2.3. Data collectioin
Data extraction was carried out utilizing PostgreSQL software (version 13.7.2) alongside Navicate Premium (version 16), which facilitated the process through the execution of Sequential Query Language (SQL) commands. The procurement of feasible variables encompasses five primary aspects: (1) basic demographics, including age, gender, height, body mass, and body mass index (BMI). (2) vital signs, such as body temperature, heart rate, respiratory rate, mean blood pressure, systolic blood pressure, diastolic blood pressure, and pulse oximetry-derived oxygen saturation (SpO2). (3) comorbidities, including diabetes mellitus, rheumatic disease, chronic obstructive pulmonary disease (COPD), deep venous thrombosis (DVT), pulmonary embolism (PE), dementia, coronary heart disease, osteoporosis, sepsis, chronic kidney disease, pneumonia, cerebral infarction, and hypertension. (4) laboratory indicators, including anion gap、CK-MB, BUN, ALP, bicarbonate, total bilirubin, sodium, chloride, calcium, potassium, PT, PTT, PT-INR, creatinine, WBC counts, lymphocyte counts¸neutrophil counts, platelet counts, monocyte counts, hemoglobin, hematocrit. (5) treatment: heparin, mechanical ventilation. (6) Admission severity of disease scores, including the Sepsis-related Organ Failure Assessment Score (SOFA), the simplified Acute Physiology Score II (SAPS-II), the Logistic Organ Dysfunction System (LODS), and the Oxford Acute Severity of Illness Score (OASIS) (18–21). The follow-up period commenced on the admission date and ended on the day of death. The computation of the SII adheres to this equation: SII = platelets count×neutrophils count÷lymphocytes count (22). All variables were collected from records obtained within the first day of the individual’s admission to the ICU.
Variables with more than 20% of their values missing were removed from the analysis to avert any prejudice. For variables with missing data below 20%, an imputation process was executed utilizing a random forest method, which was educated by the available non-missing variables. This procedure was carried out via the “mice” package within the R programming environment (Supplementary Table S1) (23).
2.4. Clinical outcomes
The primary outcomes for this investigation centered on the 30-day all-cause mortality rate following hospital admission, with the secondary outcome being the all-cause mortality rate within a year of admission.
2.5. Statistical analysis
Continuous data were depicted as mean ± SD if normally distributed. If not, they were presented as the median together with the interquartile range. Categorical data were presented as percentages. The Kolmogorov–Smirnov test was utilized to assess the normal distribution of continuous variables. And if the variables followed a normal distribution, they were analyzed with either a t-test or an ANOVA. If the distribution was non-normal, statistical analysis was performed with the Mann–Whitney U-test or Kruskal-Wallis test. The Kaplan–Meier (K-M) survival analysis was utilized to evaluate the event rate in groupings categorized by varying SII levels, and any discrepancies were examined through log-rank analysis. Binary logistic regression was employed to assess variables that influence the likelihood of all-cause mortality. Employing Cox’s proportional hazards regression models, we ascertained the hazard ratio (HR), along with its 95% confidence interval (CI), relating to the SII and the endpoints, incorporating adjustments in select models. Variables having a p-value below 0.05 in univariate analysis were deemed confounders. Clinically pertinent factors along with those linked to prognosis were incorporated into the multivariate analysis for model: model 1: unadjusted; model 2: adjusted for age, sex, and BMI; model 3: adjusted for age, sex, BMI, sepsis, dementia, BUN, PT-INR, calcium, hemoglobin, and mechanical ventilation. For improved clarity in the representation of the hazard ratio (HR), the SII value was divided by 1,000.Further, employing restricted cubic splines (RCS) characterized by four knots, we investigated the non-linear correlation between the initial SII score and all-cause mortality rates within 30 days and 1 year after hospital admission. The assessment and identification of the SII’s cutoff point are carried out through an analysis of the receiver operating characteristic (ROC) curves. Models incorporated the SII either as a continuous metric or an ordinal variable, with the lowest quartile of the SII serving as the baseline category. Trend p-values were determined using quartile categories. Separate analyses were performed according to gender, age (above 80 and 80 years or less), BMI (over 30 and 30 kg/m2 or less), sepsis, dementia, and osteoporosis, in order to assess the uniformity of SII’s predictive strength for principal outcomes. To investigate the interaction effects between SII and the stratification factors, likelihood ratio tests were applied. A statistically noteworthy threshold was established with a two-tailed p-value below 0.05. In addition, we applied the Spearman correlation analysis to examine the relationship between different severity of illness scores and SII. Then we apply receiver operating characteristic (ROC) analysis to assess the predictive capacity of different severity of illness scores for 30-day and 1-year mortality in critically ill elderly patients with hip fracture. What’s more, the data analysis was carried out utilizing R software, specifically version 4.0.2, along with SPSS 25.0 (IBM SPSS Statistics, Armonk, NY, United States).
3. Results
This study encompassed a number of 991 geriatric patients with hip fractures, whose median age stood at 81.63 years (interquartile range: 75–89), among which 357 (36.02%) were males. The mean SII index across all participants was calculated to be 2115.26 (interquartile range: 655.11–2379.63). The recorded mortality rates were 19.68% within 30 days post-admission and 33.40% at the one-year mark (Table 1).
Table 1.
Features and results of participants grouped by SII.
| Variable | Total (n = 991) | Q1 (n = 248) | Q2 (n = 248) | Q3 (n = 248) | Q4 (n = 247) | X2/F | p-value |
|---|---|---|---|---|---|---|---|
| Age (year) | 81.63 (75–89) | 80.6 (73.5–88) | 82.4 (77.5–89) | 82.1 (74–90.5) | 81.4 (74–88) | 2.289 | 0.106 |
| Sex: male | 357 (36.02) | 87 (35.08) | 86 (34.68) | 95 (38.31) | 89 (36.03) | 0.283 | 0.837 |
| BMI | 25.89 (21.51–28.98) | 26.39 (22.54–29.99) | 25.21 (21.25–28.13) | 26.28 (21.90–29.27) | 25.66 (20.82–28.72) | 2.096 | 0.049 |
| Temperature (°C) | 36.79 (36.56–37.02) | 36.81 (36.55–37.02) | 36.77 (36.52–37.00) | 36.79 (36.58–37.03) | 36.79 (36.58–37.01) | 0.264 | 0.822 |
| Heart rate (beats/min) | 84.66 (73.92–83.69) | 83.45 (72.56–93.52) | 83.00 (72.54–93.45) | 85.50 (74.63–93.5) | 86.69 (75.58–96.52) | 3.393 | 0.019 |
| Respiratory rate (breaths/min) | 19.45 (16.72–21.71) | 19.18 (16.67–21.45) | 18.89 (16.39–20.98) | 19.45 (16.60–22.17) | 20.30 (17.35–22.55) | 6.701 | 0.001 |
| SBP (mmHg) | 118.28 (105.58–129.40) | 98.85 (95.54–98.41) | 96.89 (95.70–98.35) | 96.79 (95.52–98.30) | 96.45 (95.19–97.74) | 0.658 | 0.627 |
| DBP (mmHg) | 59.38 (51.69–66.08) | 60.40 (52.67–67.55) | 58.47 (51.42–64.69) | 59.31 (52.03–65.83) | 59.34 (50.61–66.16) | 1.346 | 0.239 |
| MBP (mmHg) | 75.12 (67.52–81.62) | 76.15 (68.54–83.51) | 74.63 (67.86–80.45) | 74.61 (66.87–81.37) | 75.09 (66.56–81.80) | 1.111 | 0.416 |
| SpO2 (%) | 96.74 (95.50–98.24) | 96.85 (95.54–98.41) | 96.89 (95.70–98.35) | 96.79 (95.51–98.30) | 96.45 (95.19–97.74) | 2.569 | 0.069 |
| Commorbidities | |||||||
| Diabetes mellitus | 229 (23.1) | 66 (26.61) | 54 (21.77) | 64 (25.81) | 45 (18.22) | 6.708 | 0.082 |
| Rheumatic Disease | 77 (7.77) | 19 (7.66) | 20 (8.06) | 22 (8.87) | 16 (6.48) | 0.106 | 0.776 |
| COPD | 131 (13.22) | 33 (13.31) | 21 (8.47) | 34 (13.71) | 43 (17.41) | 2.919 | 0.033 |
| PE | 59 (5.95) | 14 (5.65) | 13 (5.24) | 17 (6.85) | 15 (6.07) | 0.210 | 0.889 |
| DVT | 79 (7.97) | 18 (7.26) | 16 (6.45) | 28 (11.29) | 17 (6.88) | 1.694 | 0.166 |
| Dementia | 71(7.16) | 18 (7.26) | 15 (6.05) | 19 (7.66) | 19 (7.69) | 0.220 | 0.882 |
| Coronary heart disease | 324 (32.69) | 81 (32.66) | 83 (3.35) | 78 (31.45) | 82 (33.20) | 0.090 | 0.966 |
| Osteoporosis | 205 (20.69) | 52 (20.97) | 54 (21.77) | 51 (20.56) | 48 (19.43) | 0.143 | 0.934 |
| Sepsis | 61 (6.16) | 11 (4.44) | 9 (3.63) | 15 (6.05) | 26 (10.53) | 4.095 | 0.007 |
| CKD | 220 (22.20) | 56 (22.58) | 54 (21.77) | 55 (22.18) | 55 (22.27) | 0.016 | 0.997 |
| Pneumonia | 41 (4.14) | 6 (2.42) | 11 (4.44) | 10 (4.03) | 14 (5.67) | 1.122 | 0.338 |
| Cerebral infarction | 128 (12.92) | 35 (14.11) | 27 (10.89) | 32 (12.90) | 34 (13.77) | 0.459 | 0.710 |
| Hypertension | 185 (18.67) | 40 (16.13) | 57 (22.98) | 43 (17.34) | 45 (18.22) | 1.473 | 0.220 |
| Laboratory tests | |||||||
| Aniongap (mEq/L) | 14.8 (12–17) | 14.24 (12–16) | 14.54 (12–17) | 15.26 (13–17) | 15.25 (12.5–17) | 4.379 | 0.007 |
| CK-MB (ng/ml) | 9.9 (3.0–13.6) | 11.41 (3.5–15.68) | 9.79 (3–14.38) | 9.55 (3–12.06) | 8.90 (3–12.12) | 2.495 | 0.117 |
| BUN (mg/dL) | 30.0 (17–35.5) | 29.77 (18–34.5) | 27.84 (15.5–30.27) | 30.63 (16–38.5) | 31.94 (18–42) | 1.743 | 0.030 |
| ALP (U/L) | 100.6 (61.3–124.0) | 98.37 (58.74–121.17) | 101.16 (63.50–130.72) | 99.01 (60.18–117.35) | 104.02 (66–127) | 0.494 | 0.220 |
| Bicarbonate (mmol/L) | 24.9 (22–28) | 24.53 (22–28) | 25.15 (22.5–28) | 25.03 (23–27) | 24.86 (22–28) | 1.032 | 0.526 |
| Totalbilirubin (mg/dL) | 1.0 (0.4–1.2) | 1.09 (0.4–1.3) | 1.03 (0.45–1.29) | 1.01 (0.4–1.27) | 0.95 (0.35–1.1) | 0.946 | 0.025 |
| Sodium (mmol/L) | 137.8 (135–141) | 138.48 (136–141) | 137.42 (135.25–140) | 137.82 (134.75–141) | 137.68 (135–141) | 1.770 | 0.489 |
| Chloride (mmol/L) | 103.7 (100–107) | 104.73 (101–108) | 103.07 (100–107) | 103.52 (100–107) | 103.39 (100–107) | 3.274 | 0.036 |
| Calcium (mmol/L) | 5.5 (1.1–8.5) | 5.42 (1.12–8.5) | 5.81 (1.16–8.7) | 5.63 (1.13–8.6) | 4.95 (1.11–8.3) | 2.571 | 0.005 |
| Potassium(mmol/L) | 4.2 (3.6–4.6) | 4.29 (3.8–4.68) | 4.23 (3.73–4.6) | 4.32 (3.8–4.7) | 4.15 (3.6–4.5) | 2.377 | 0.175 |
| PT (s) | 16.3 (12.3–16.3) | 16.16 (12.3–16.3) | 15.69 (12.2–16.3) | 16.99 (12.5–16.65) | 16.38 (12.4–16.3) | 1.063 | 0.765 |
| PTT (s) | 37.0 (27.8–38.1) | 36.31 (27.9–38.43) | 36.24 (27.8–37.85) | 39.53 (27.7–39.35) | 36.05 (27.7–37.5) | 2.035 | 0.963 |
| PT-INR | 1.47 (1.1–1.5) | 1.48 (1.1–1.5) | 1.46 (1.1–1.5) | 1.54 (1.1–1.6) | 1.44 (1.1–1.45) | 0.762 | 0.224 |
| Creatinine(mg/dL) | 1.0 (0.6–1) | 1.06 (0.6–1.02) | 1.02 (0.6–1) | 1.01 (0.6–1.02) | 1.04 (0.6–1) | 0.140 | 0.622 |
| WBC counts (109/L) | 11.8 (7.4–14.1) | 9.24 (5.75–10.6) | 10.20 (6.85–12.2) | 11.84 (8.35–13.85) | 15.93 (10.6–18.7) | 53.606 | <0.001 |
| Platelet counts (109/L) | 209.8 (145–260) | 143.48 (92.5–187) | 201.64 (150.5–243.75) | 226.81 (162.25–274) | 267.53 (192–325) | 96.981 | <0.001 |
| Lymphocyte counts (109/L) | 1.4 (0.8–1.6) | 2.11 (1.12–2.11) | 1.43 (0.97–1.75) | 1.19 (0.83–1.42) | 0.81 (0.51–1.01) | 26.106 | <0.001 |
| Neutrophil counts (109/L) | 8.8 (5.0–10.9) | 4.67 (3.09–5.81) | 6.82 (4.89–8.55) | 9.46 (6.92–11.07) | 14.16 (9.27–16.91) | 196.272 | <0.001 |
| Monocyte count (109/L) | 0.6 (0.3–0.7) | 0.48 (0.32–0.58) | 0.55 (0.34–0.64) | 0.59 (0.37–0.72) | 0.69 (0.35–0.82) | 7.517 | <0.001 |
| Hemoglobin (g/dL) | 10.1 (8.6–11.4) | 10.03 (8.55–11.25) | 10.19 (8.75–11.6) | 10.02 (8.5–11.38) | 10.19 (8.6–11.5) | 0.623 | 0.733 |
| Hematocrit (%) | 31.2(26.9–35.2) | 31.00 (26.95–34.8) | 31.23 (26.85–35.53) | 31.02 (26.3–35.05) | 31.43 (27.1–35.4) | 0.308 | 0.873 |
| Heparin | 635 (64.08) | 144 (58.06) | 152 (61.29) | 168 (67.74) | 171 (68.95) | 3.025 | 0.029 |
| Mechanical ventilation | 173 (17.46) | 41 (16.53) | 36 (14.52) | 48 (19.35) | 48 (19.43) | 0.974 | 0.403 |
| SII | 2115.26 (655.11–2379.63) | 386.28 (247.90–529.49) | 931.46 (783.58–1084.92) | 1734.64 (1459.44–2019.21) | 5422.02 (3006.13–6,358) | 218 | <0.001 |
| Severity of illness scores | |||||||
| SOFA | 1.59 (1–2) | 1.40 (1–1.77) | 1.46 (1–2) | 1.58 (1–2) | 1.74 (1–2) | 1.537 | 0.039 |
| SAPS II | 41.46 (33–47) | 41.08 (32.5–48) | 40.71 (33–46) | 42.16 (34–48) | 41.89 (33–48) | 0.784 | 0.527 |
| LODS | 4.93 (3–6) | 4.76 (3–6) | 4.68 (3–6) | 5.29 (3–7) | 4.99 (3–6) | 2.055 | 0.072 |
| OASIS | 34.01 (28–39) | 33.33 (26–39) | 33.46 (28–38) | 34.38 (29–39) | 34.87 (29–40) | 1.992 | 0.122 |
| Events | |||||||
| 30-day mortality | 195 (19.68) | 25 (10.08) | 45 (18.15) | 52 (20.97) | 73 (29.55) | 10.386 | <0.001 |
| 1-year mortality | 331 (33.40) | 66 (26.61) | 75 (30.24) | 87 (30.08) | 103 (41.70) | 4.787 | 0.003 |
SII, systemic immune-inflammation index; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; SpO2, pulse oximetry-derived oxygen saturation; COPD, chronic obstructive pulmonary disease; PE, pulmonary embolism; DVT, deep venous thrombosis; CKD, chronic kidney disease; CK-MB, Creatine Kinase Isoenzyme-MB;BUN, blood urea nitrogen; ALP, alkaline phosphatase; PT, prothrombin time; PTT, partial thromboplastin time; WBC, white blood cell; SOFA, sequential organ failure assessment; SAPS II, simplifed acute physiological score II; LODS, the logistic organ dysfunction system; OASIS, oxford acute severity of illness score.
3.1. Baseline characteristics
Fundamental attributes of severely ill hip fracture patients segmented according to their SII quartiles are presented in Table 1.Participants were categorized into four distinct groups according to their SII levels at the time of hospital admission.[quartile Q1: 73.04–655.11; Q2: 655.11–1231.57; Q3: 1231.57–2379.63; Q4: 2379.63–12685.89]. The median SII value for each quartile was 386.28 (IQR: 247.90–529.49), 931.46 (IQR: 783.58–1084.92), 1734.64 (IQR: 1459.44–2019.21), and 5422.02 (IQR: 3006.13–6,358), respectively. Patients in the highest quartile of SII exhibited elevated heart rate and respiratory rate, along with a higher incidence of COPD and sepsis. Their white blood cell (WBC) counts, anion gap, and blood urea nitrogen (BUN) concentrations were heightened, while BMI, total bilirubin, chloride, and calcium levels exhibited a decline. Additionally, individuals in their group exhibited a greater severity of illness upon admission when compared with those in the lower quartile. The ranking in the upper quartile of the SII exhibited a notably increased mortality rate after 1 year when contrasted with individuals in the lower quartile (26.61% vs. 30.24% vs. 30.08% vs. 41.70%, p = 0.003). Similar results were observed in individuals with a 30-day mortality rate (10.08% vs. 18.15% vs. 20.97% vs. 29.55%, p < 0.001). Table 2 shows the differing initial characteristics of individuals who survived and those who did not survive 30 days after being admitted. Follow-up at 30 days after admission showed a mortality rate of 19.68%, with 195 patients dying and 796 patients surviving. No data loss occurred during the 30-day follow-up. Individuals in the non-survivor group exhibited a greater likelihood of being male, a greater incidence of dementia and sepsis, elevated levels of PT-INR, BUN, PT, and WBC, decreased levels of calcium and chloride, increased usage of mechanical ventilation, and higher severity of disease scores. The levels of SII were significantly higher in the group of patients who did not survive as compared to those who did (3389.64 vs. 1803.07, p < 0.001). Table 3 shows the differing initial characteristics of individuals who survived and those who did not survive 1 year after being admitted. Follow-up at 1 year after admission showed a mortality rate of 33.40%, with 331 patients dying and 660 patients surviving. No data loss occurred during the 1-year follow-up. The characteristics of patients in the non-survivor group 1 year after admission were similar to those at 1 month after admission, but we observed that this group tended to have a higher prevalence of chronic kidney disease, coronary artery disease, and osteoporosis, higher creatinine, and lower total bilirubin compared with survivors. In the group of patients who did not survive, the level of SII was significantly higher than in the group of patients who did survive (2646.79 vs. 1848.69, p < 0.001).
Table 2.
Baseline characteristics of the survivors and Non-survivors groups at 30 days following hospital admission.
| Variable | Total (n = 991) | Survivor (n = 796) | Non-survivor (n = 195) | p-value |
|---|---|---|---|---|
| Ages (year) | 81.63 (75–89) | 81.09 (74–88) | 83.85 (79–91) | <0.001 |
| Sex: male | 357 (36.02) | 275 (34.55) | 82 (42.05) | 0.050 |
| BMI | 25.89 (21.51–28.98) | 26.1 (21.7–29.1) | 25.0 (15.6–43.4) | 0.018 |
| Temperature (°C) | 36.79 (36.56–37.02) | 36.8 (36.6–37.0) | 36.7 (36.5–37.0) | 0.153 |
| Heart rate (beats/min) | 84.66 (73.92–83.69) | 84.4 (73.7–93.8) | 85.7 (75.6–95.4) | 0.119 |
| Respiratory rate (breaths/min) | 19.45 (16.72–21.71) | 19.3 (16.7–21.5) | 20.0 (17.1–22.3) | 0.065 |
| SBP (mmHg) | 118.28 (105.58–129.40) | 119.0 (106.3–130.2) | 115.4 (103.9–126.1) | 0.006 |
| DBP (mmHg) | 59.38 (51.69–66.08) | 59.6 (51.9–66.6) | 58.4 (50.8–64.9) | 0.081 |
| MBP (mmHg) | 75.12 (67.52–81.62) | 75.5 (68.0–82.2) | 73.7 (65.9–80.2) | 0.021 |
| SpO2 (%) | 96.74 (95.50–98.24) | 96.8 (95.6–98.2) | 96.6 (95.1–98.2) | 0.464 |
| Commorbidities | ||||
| Diabetes mellitus | 229 (23.1) | 187 (23.5) | 42 (21.54) | 0.317 |
| Rheumatic Disease | 77 (7.77) | 66 (8.29) | 11 (5.64) | 0.136 |
| COPD | 131 (13.22) | 109 (13.69) | 22 (11.28) | 0.373 |
| PE | 59 (5.95) | 45 (6.03) | 14 (7.18) | 0.420 |
| DVT | 79 (7.97) | 63 (7.91) | 16 (8.21) | 0.893 |
| Dementia | 71 (7.16) | 50 (6.28) | 21 (10.77) | 0.029 |
| Coronary heart disease | 324 (32.69) | 252 (31.66) | 72 (36.92) | 0.160 |
| Osteoporosis | 205 (20.69) | 172 (21.61) | 33 (16.92) | 0.148 |
| Sepsis | 61 (6.16) | 33 (4.15) | 28 (14.36) | <0.001 |
| CKD | 220 (22.20) | 169 (21.23) | 51 (26.15) | 0.138 |
| Pneumonia | 41 (4.14) | 31 (3.89) | 10 (5.13) | 0.438 |
| Cerebral infarction | 128 (12.92) | 96 (12.06) | 32 (16.41) | 0.105 |
| Hypertension | 185 (18.67) | 149 (18.72) | 36 (18.46) | 0.934 |
| Laboratory tests | ||||
| Anion gap (mEq/L) | 14.8 (12–17) | 14.73 (12–16.5) | 15.19 (12–17) | 0.261 |
| CK-MB(ng/ml) | 9.9 (3.0–13.6) | 9.90 (3–13.3) | 9.98 (3.10–14.55) | 0.490 |
| BUN (mg/dL) | 30.0 (17–35.5) | 28.95 (16–33.75) | 34.51 (20–43) | <0.001 |
| ALP(U/L) | 100.6 (61.3–124.0) | 98.76 (60–121.33) | 108.28 (67–134) | 0.060 |
| Bicarbonate(mmol/L) | 24.9 (22–28) | 24.95 (22.25–28) | 24.66 (22–28) | 0.470 |
| Total bilirubin (mg/dL) | 1.0 (0.4–1.2) | 1.01 (0.4–1.2) | 1.08 (0.4–1.28) | 0.661 |
| Sodium (mmol/L) | 137.8 (135–141) | 137.79 (135–141) | 138.10 (135–141) | 0.647 |
| Chloride (mmol/L) | 103.7 (100–107) | 103.50 (100–107) | 104.38 (101–108) | 0.110 |
| Calcium (mmol/L) | 5.5 (1.1–8.5) | 5.66 (1.14–8.55) | 4.61 (1.1–8.25) | 0.001 |
| Potassium (mmol/L) | 4.2 (3.6–4.6) | 4.24 (3.75–4.6) | 4.29 (3.7–4.7) | 0.106 |
| PT(s) | 16.3 (12.3–16.3) | 16.05 (12.3–16.3) | 17.35 (12.7–17.4) | 0.007 |
| PTT(s) | 37.0 (27.8–38.1) | 36.91 (27.8–37.9) | 37.54 (27.9–41.1) | 0.459 |
| PT-INR | 1.47 (1.1–1.5) | 1.45 (1.1–1.5) | 1.60 (1.1–1.7) | 0.043 |
| Creatinine (mg/dL) | 1.0 (0.6–1) | 1.01 (0.6–1) | 1.12 (0.6–1.2) | 0.371 |
| WBC counts (109/L) | 11.8 (7.4–14.1) | 11.40 (7.3–13.78) | 13.42 (8.1–16.8) | 0.001 |
| Platelet counts (109/L) | 209.8 (145–260) | 208.42 (145–259.75) | 215.47 (143–271) | 0.566 |
| Lymphocyte counts (109/L) | 1.4 (0.8–1.6) | 1.44 (0.84–1.66) | 1.16 (0.62–1.38) | <0.001 |
| Neutrophil counts (109/L) | 8.8 (5.0–10.9) | 8.31 (4.81–10.49) | 10.65 (6.16–13.50) | <0.001 |
| Monocyte counts (109/L) | 0.6 (0.3–0.7) | 0.57 (0.34–0.66) | 0.62 (0.37–0.76) | 0.014 |
| Hemoglobin (g/dL) | 10.1 (8.6–11.4) | 10.15 (8.6–11.5) | 9.53 (8.5–11.2) | 0.134 |
| Hematocrit (%) | 31.2 (26.9–35.2) | 31.2 (26.7–35.3) | 31.0 (27.3–34.7) | 0.612 |
| Heparin | 635 (64.08) | 508 (63.82) | 127 (65.13) | 0.773 |
| Mechanical ventilation | 173 (17.46) | 128 (16.08) | 45 (23.08) | 0.021 |
| SII | 2115.26 (655.11–2379.63) | 1803.07 (595.67–2178.48) | 3389.64 (927.3–3,652) | <0.001 |
| Severity of illness scores | ||||
| SOFA | 1.59 (1–2) | 1.51 (1–1.53) | 1.93 (1–3) | 0.009 |
| SAPS II | 41.46 (33–47) | 40.28 (33–46) | 46.27 (37–54) | <0.001 |
| LODS | 4.93 (3–6) | 4.67 (3–6) | 6.01 (4–8) | <0.001 |
| OASIS | 34.01 (28–39) | 33.34 (28–38) | 36.75 (31–43) | <0.001 |
SII, systemic immune-inflammation index; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; SpO2, pulse oximetry-derived oxygen saturation; COPD, chronic obstructive pulmonary disease; PE, pulmonary embolism; DVT, deep venous thrombosis; CKD, chronic kidney disease; CK-MB, Creatine Kinase Isoenzyme-MB;BUN, blood urea nitrogen; ALP, alkaline phosphatase; PT, prothrombin time; PTT, partial thromboplastin time; WBC, white blood cell; SOFA, sequential organ failure assessment; SAPS II, simplifed acute physiological score II; LODS, the logistic organ dysfunction system; OASIS, oxford acute severity of illness score.
Table 3.
Baseline characteristics of the survivors and Non-survivors groups at 1-year following hospital admission.
| Variable | Total (n = 991) | survivor (n = 660) | Non-survivor (n = 331) | p-value |
|---|---|---|---|---|
| Ages (year) | 81.63 (75–89) | 80.49 (74–87) | 83.92 (79–91) | <0.001 |
| Sex: male | 357 (36.02) | 221 (33.48) | 136 (41.09) | 0.019 |
| BMI | 25.89 (21.51–28.98) | 26.47 (22.20–29.32) | 24.72 (20.24–28.08) | <0.001 |
| Temperature (°C) | 36.79 (36.56–37.02) | 36.82 (36.58–37.05) | 36.72 (36.49–36.97) | 0.001 |
| Heart rate (beats/min) | 84.66 (73.92–83.69) | 84.51 (73.77–93.72) | 84.95 (74.21–94.75) | 0.658 |
| Respiratory rate (breaths/min) | 19.45 (16.72–21.71) | 19.27 (16.76–21.48) | 19.82 (16.65–22.48) | 0.154 |
| SBP (mmHg) | 118.28 (105.58–129.40) | 119.53 (107.21–130.72) | 115.79 (103.16–126.96) | 0.001 |
| DBP (mmHg) | 59.38 (51.69–66.08) | 60.10 (52.41–66.76) | 57.95 (50.56–64.88) | 0.004 |
| MBP (mmHg) | 75.12 (67.52–81.62) | 76.02 (68.48–82.44) | 73.34 (65.17–79.63) | 0.001 |
| SpO2 (%) | 96.74 (95.50–98.24) | 96.82 (95.61–98.24) | 96.60 (95.19–98.24) | 0.392 |
| Commorbidities | ||||
| Diabetes mellitus | 229 (23.1) | 148 (22.42) | 81 (24.47) | 0.260 |
| Rheumatic Disease | 77 (7.77) | 57 (8.64) | 20 (6.04) | 0.093 |
| COPD | 131 (13.22) | 87 (13.18) | 44 (13.29) | 0.961 |
| PE | 59 (5.95) | 31 (4.70) | 28 (8.46) | 0.018 |
| DVT | 79 (7.97) | 45 (6.82) | 34 (10.27) | 0.058 |
| Dementia | 71 (7.16) | 26 (3.94) | 45 (13.60) | <0.001 |
| Coronary heart disease | 324 (32.69) | 201 (30.45) | 123 (37.16) | 0.034 |
| Osteoporosis | 205 (20.69) | 152 (23.03) | 53 (16.01) | 0.010 |
| Sepsis | 61 (6.16) | 23 (3.48) | 38 (10.57) | <0.001 |
| CKD | 220 (22.20) | 124 (18.79) | 96 (29.00) | <0.001 |
| Pneumonia | 41 (4.14) | 22 (3.33) | 19 (5.74) | 0.073 |
| Cerebral infarction | 128 (12.92) | 81 (12.27) | 47 (14.20) | 0.394 |
| Hypertension | 185 (18.67) | 131 (19.85) | 54 (16.31) | 0.178 |
| Laboratory tests | ||||
| Anion gap (mEq/L) | 14.8 (12–17) | 14.63 (12–16.5) | 15.19 (12.5–17) | 0.081 |
| CK-MB (ng/ml) | 9.9 (3.0–13.6) | 10.29 (1.02–14.58) | 9.18 (3.52–12.25) | 0.953 |
| BUN (mg/dL) | 30.0 (17–35.5) | 27.59 (15.75–32) | 34.94 (21–43) | <0.001 |
| ALP (U/L) | 100.6 (61.3–124.0) | 98.78 (61.13–121.16) | 104.34 (62–130.5) | 0.133 |
| Bicarbonate (mmol/L) | 24.9 (22–28) | 25.01 (22.5–28) | 24.65 (22–28) | 0.459 |
| Total bilirubin (mg/dL) | 1.0 (0.4–1.2) | 1.03 (0.4–1.3) | 0.99 (0.4–1.1) | 0.029 |
| Sodium (mmol/L) | 137.8 (135–141) | 137.54 (135–141) | 138.46 (136–141) | 0.041 |
| Chloride (mmol/L) | 103.7 (100–107) | 103.44 (100–107) | 104.15(100–108) | 0.189 |
| Calcium (mmol/L) | 5.5 (1.1–8.5) | 5.73 (1.14–8.58) | 4.90 (1.12–8.4) | 0.010 |
| Potassium (mmol/L) | 4.2 (3.6–4.6) | 4.25 (3.78–4.6) | 4.25 (3.7–4.65) | 0.751 |
| PT(s) | 16.3 (12.3–16.3) | 15.82 (12.2–16.3) | 17.27 (12.7–17.4) | <0.001 |
| PTT(s) | 37.0 (27.8–38.1) | 36.62 (27.5–36.8) | 37.85 (28.1–40.9) | 0.063 |
| PT-INR | 1.47 (1.1–1.5) | 1.42 (1.1–1.5) | 1.59 (1.1–1.6) | 0.012 |
| Creatinine (mg/dL) | 1.0 (0.6–1) | 0.98 (0.6–1) | 1.15 (0.6–1.2) | 0.002 |
| WBC counts (109/L) | 11.8 (7.4–14.1) | 11.50 (7.4–13.7) | 12.39 (7.4–15.3) | 0.047 |
| Platelet counts (109/L) | 209.8 (145–260) | 210.98 (149.25–260) | 207.48 (138–262) | 0.180 |
| Lymphocyte counts (109/L) | 1.4 (0.8–1.6) | 1.42 (0.84–1.68) | 1.32 (0.69–1.40) | <0.001 |
| Neutrophil counts (109/L) | 8.8 (5.0–10.9) | 8.37 (4.88–10.27) | 9.58 (5.31–11.97) | 0.002 |
| Monocyte counts (109/L) | 0.6 (0.3–0.7) | 0.57 (0.34–0.66) | 0.58 (0.34–0.7) | 0.455 |
| Hemoglobin (g/dL) | 10.1 (8.6–11.4) | 10.19 (8.65–11.5) | 9.94 (8.5–11.25) | 0.059 |
| Hematocrit (%) | 31.2 (26.9–35.2) | 31.40 (26.95–35.6) | 30.71 (26.5–34.6) | 0.088 |
| Heparin | 635 (64.08) | 421 (63.79) | 214 (64.65) | 0.789 |
| Mechanical ventilation | 173 (17.46) | 99 (15.00) | 74 (22.36) | 0.004 |
| SII | 2115.26 (655.11–2379.63) | 1848.69 (605.52–2180.83) | 2646.79 (830.25–2867.27) | <0.001 |
| Severity of illness scores | ||||
| SOFA | 1.59 (1–2) | 1.57 (1–1.53) | 1.65 (1–2) | 0.880 |
| SAPS II | 41.46 (33–47) | 39.18 (32–44) | 46.01 (37–52) | <0.001 |
| LODS | 4.93 (3–6) | 4.41 (2–6) | 5.97 (4–8) | <0.001 |
| OASIS | 34.01 (28–39) | 32.82 (27–37.5) | 36.39 (31–42) | <0.001 |
SII, systemic immune-inflammation index; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; SpO2, pulse oximetry-derived oxygen saturation; COPD, chronic obstructive pulmonary disease; PE, pulmonary embolism; DVT, deep venous thrombosis; CKD, chronic kidney disease; CK-MB, Creatine Kinase Isoenzyme-MB;BUN, blood urea nitrogen; ALP, alkaline phosphatase; PT, prothrombin time; PTT, partial thromboplastin time; WBC, white blood cell; SOFA, sequential organ failure assessment; SAPS II, simplifed acute physiological score II; LODS, the logistic organ dysfunction system; OASIS, oxford acute severity of illness score.
3.2. Primary outcomes
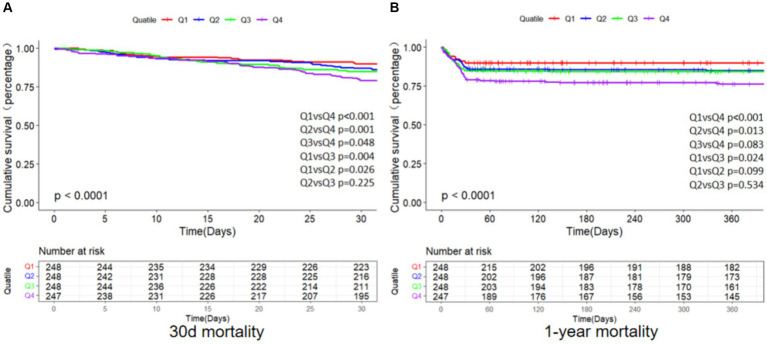
As depicted in Figure 2, the primary endpoints’ incidence across distinct clusters, demarcated by SII quartiles, was investigated through the employment of the K-M survival analysis curves. A notable disparity was observed in the mortality rates when comparing the brief (30-day) and extended (1-year) intervals, revealing a statistically noteworthy distinction (log-rank p-value less than 0.001).Furthermore, patients with an elevated SII faced an increased likelihood of mortality between 30 days and 1 year following admission. The SII’s clinical effectiveness was evaluated through ROC analysis. The AUC values for SII were not good enough: 30-day death AUC was 0.629 (p < 0.001) and 1-year death AUC was 0.576 (p < 0.001) (Supplementary Figure S1). Supplementary Table S2 displays the findings from binary logistic regression analysis regarding the likelihood of all-cause mortality among geriatric patients with hip fractures. Independent variables for logistic regression encompassed those factors that showed significance in univariate analysis with a threshold of p < 0.05, along with propositions from clinicians and insights drawn from clinical practice. The results found that sex, age, sepsis, calcium, hemoglobin, and SII were significant predictors.
Figure 2.
K-M survival analysis curves for all-cause mortality of 30 days (A) and 1 year (B). Footnote SII quartiles: Q1 (73.04–655.11), Q2 (655.11–1231.57), Q3 (1231.57–2379.63), Q4 (2379.63–12685.89).
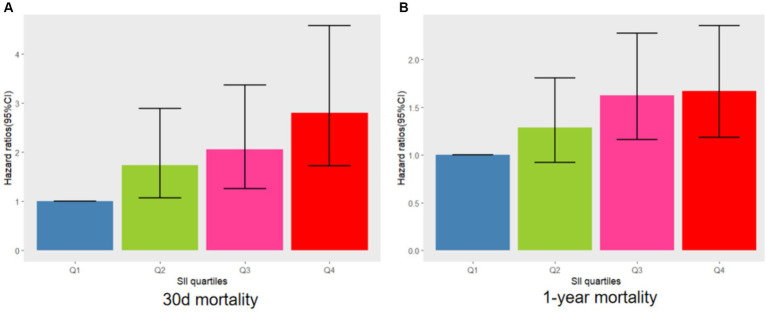
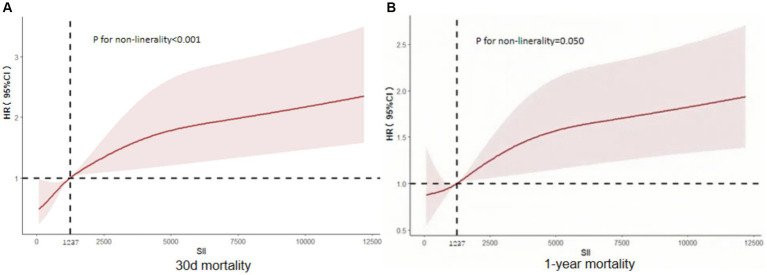
The relationship between SII and 30-day mortality was investigated using a Cox proportional hazards regression analysis. Analysis revealed that the SII consistently emerged as a significant risk factor across all models when examined as a continuous variable: model 1 [HR, 1.073 (95% CI 1.019–1.090) p = 0.002], model 2 [HR, 1.062 (1.043–1.08) p < 0.001], and model 3 [HR, 1.065 (1.044–1.087) p < 0.001]. In the context where SII was categorized, individuals falling into the highest quartile of SII demonstrated a notably heightened likelihood of 30-day mortality across three distinct Cox proportional hazards models: model 1 [HR, 2.649 (95% CI 1.30–5.399) p = 0.007], model 2 [HR, 2.635 (95% CI 1.658–4.188) p < 0.001], and model 3 [HR, 2.806 (95% CI 1.721–4.577) p < 0.001], compared to those in the lowest quartile. This risk tended to increase with higher SII values (Table 4; Figure 3A). The multivariate Cox proportional hazard analysis indicated similar outcomes for the SII and 1-year mortality (Table 4; Figure 3B). What’s more,the study utilized a confined cubic splines regression analysis to exhibit that both the 30-day and 1-year mortality rates escalated non-linearly with escalating SII (with p values for non-linearity equating to 0.050 and less than 0.001, respectively) (Figures 4A,B).
Table 4.
Cox proportional hazard ratios (HR) for all-cause mortality.
| Categories | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | p-value | P for trend | HR (95%CI) | p-value | P for trend | HR (95%CI) | p-value | P for trend | |
| 30-day mortality | |||||||||
| Continuous variable per unit | 1.053 (1.019–1.090) | 0.002 | 1.062 (1.043–1.081) | <0.001 | 1.065 (1.044–1.087) | <0.001 | |||
| Quartile | 0.037 | 0.019 | 0.01 | ||||||
| Q1 (N = 248) | Ref | ||||||||
| Q2 (N = 248) | 1.85 (1.072–3.192) | 0.027 | 1.630 (0.992–2.676) | 0.054 | 1.735 (1.062–2.895) | 0.028 | |||
| Q3 (N = 248) | 2.21 (1.22–4.003) | 0.009 | 2.006 (1.243–3.238) | 0.004 | 2.061 (1.261–3.368) | 0.004 | |||
| Q4 (N = 247) | 2.649 (1.3–5.399) | 0.007 | 2.635 (1.658–4.188) | <0.001 | 2.806 (1.721–4.577) | <0.001 | |||
| 1-year mortality | |||||||||
| Continuous variable per unit | 1.058 (1.028–1.089) | <0.001 | 1.049 (1.028–1.070) | <0.001 | 1.051 (1.029–1.074) | <0.001 | |||
| Quartile | 0.049 | 0.026 | 0.013 | ||||||
| Q1 (N = 248) | Ref | ||||||||
| Q2 (N = 248) | 1.176 (0.844–1.640) | 0.338 | 1.227 (0.877–1.718) | 0.232 | 1.290 (0.919–1.809) | 0.141 | |||
| Q3 (N = 248) | 1.405 (1.018–1.938) | 0.039 | 1.493 (1.072–2.079) | 0.018 | 1.627 (1.161–2.279) | 0.005 | |||
| Q4 (N = 247) | 1.582 (1.153–2.172) | 0.005 | 1.548 (1.099–2.181) | 0.012 | 1.670 (1.184–2.356) | 0.003 | |||
Model 1: unadjusted Model 2: adjusted for age, sex, BMI Model 3: adjusted for age, sex, BMI, sepsis, dementia, BUN, PT-INR, calcium, hemoglobin, and mechanical ventilation.
Figure 3.
Adjusted hazard ratios with 95% confidence intervals (95% CIs) for 30 days (A) and 1 year (B) mortality based on SII quartiles after controlling for age, BMI, sex, sepsis, dementia, BUN, PT-INR, calcium, hemoglobin, and mechanical ventilation. CI, confdence interval; SII, systemic immune-inflammation index.
Figure 4.
Restricted cubic spline curve depicting the hazard ratio of 30 days (A) and 1 year (B) for the SII. CI, confdence interval; SII, systemic immune-inflammation index.
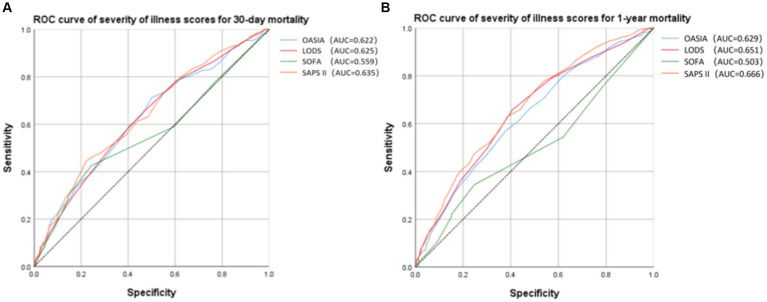
Receiver operating characteristic (ROC) analysis was conducted to assess the predictive capacity of different severity of illness scores for 30-day and 1-year mortality in critically ill elderly patients with hip fracture (Figure 5). Based on the AUC values (Figures 5A,B, Supplementary Tables S3, S4), the SAPS II score for 30-day mortality (AUC = 0.635, 95% CI 0.592–0.679) and for 1-year mortality (AUC = 0.666, 95% CI 0.631–0.701) exhibited the highest predictive value among the considered severity of illness scores. Furthermore, we applied the Spearman correlation analysis to examine the relationship between SOFA, OASIA, LODS, SAPS II score, and SII. According to Supplementary Tables S5–S8, we find that SOFA score and OASIS score show a positive correlation with SII (Spearman correlation = 0.085 and 0.070, respectively). LODS and SAPS II scores do not correlate with SII.
Figure 5.
Receiver operating characteristic (ROC) curve of severity of illness scores for 30-day (A) and 1-year (B) mortality.
3.3. Subgroup analysis
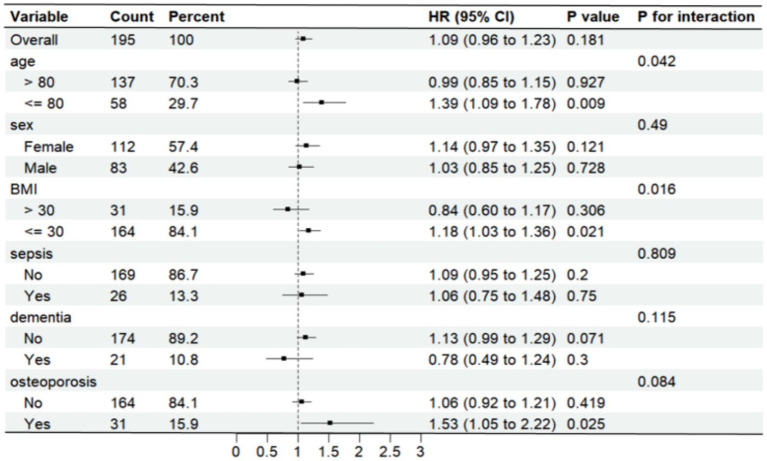
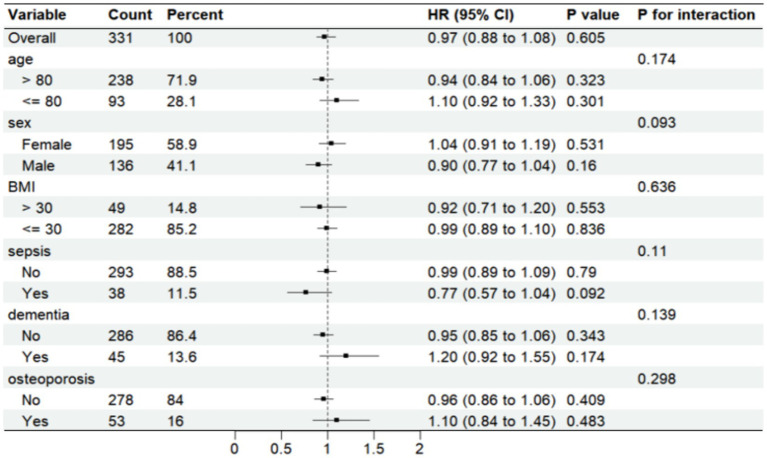
Further analysis was made of SII risk stratification values for primary endpoints, including age, sex, body weight index, sepsis, dementia, and osteoporosis, in multiple subgroups of the enrolled patients (Figures 6, 7). The results demonstrated that SII had no significant interaction with stratified variables such as sex, dementia, sepsis, and osteoporosis on 30-day mortality (all P for interactions were > 0.05). Similarly, SII also had no significant interaction with stratified variables such as age, sex, BMI, dementia, sepsis, and osteoporosis on 1-year mortality (all P for interactions were > 0.05). The SII was profoundly linked to an increased risk of 30-day mortality in critically ill elderly patients with hip fracture subgroups of those aged ≤80 years [HR (95% CI) 1.39 (1.09–1.78)], those with BMI ≤ 30 kg/m2 [HR (95% CI) 1.18 (1.03–1.36)], and those with osteoporisis [HR (95% CI) 1.53 (1.05–2.22)]. However, SII did not significantly affect subgroups of critically ill elderly patients with hip fracture in terms of 1-year mortality after hospital admission.
Figure 6.
Forest plots displaying hazard ratios for 30-day mortality across different subgroups. HR, hazard ratio; CI, confdence interval; BMI, body mass index.
Figure 7.
Forest plots displaying hazard ratios for 1-year mortality across different subgroups. HR, hazard ratio; CI, confdence interval; BMI, body mass index.
4. Discussion
The study investigated the relationship between the SII and clinical results in critically ill elderly patients with hip fractures using the MIMIC-IV database. Analysis found a strong connection between a raised SII and increased 30-day and 1-year mortality rates from all causes in critically ill elderly adults with hip fracture. The SII remained significantly correlated with all-cause 30-day and 1-year mortality after admission, even when accounting for confounding risk variables. Therefore, the SII could function as a crucial tool for clinical decision-making and potentially stand as an individual risk indicator in critically ill elderly patients with hip fractures.
Patients with elevated SII values exhibited increased heart rate, respiration rate, anion gap, BUN, SOFA scores, heparin utilization, and lower BMI, total bilirubin, chloride, and calcium, which suggests that these indicators are strongly linked to the negative outcomes of critically ill elderly patients, as shown in various prior research studies. For instance, prior research demonstrated that patients with a lower BMI often have concomitant complications such as malnutrition, hypoproteinemia, and osteoporosis, and they have a higher one-year postoperative mortality rate and poorer functional outcomes (24–26).Furthermore, patients with higher cardiac and respiratory rates had an increased likelihood of in-hospital mortality (27). Another research investigation demonstrated that those with a significantly higher plasma anion gap have a poorer clinical outcome and an increased likelihood of dying while in the hospital (28). Biochemical ionic disturbances, blood urea nitrogen, and total bilirubin levels on admission have also been associated with a poorer prognosis for hip fracture (29). The SOFA score, a metric for evaluating disease severity in ICU patients, has been linked to an unfavorable outcome in fracture patients (30). Heparin utilization reflects the coagulation status of the patient population, which tends to be at higher risk for VTE and DVT (31).
The SII, consisting of neutrophil counts, lymphocyte counts, and platelet counts, is proposed as a viable marker for preoperative status assessment, surgical trauma, and postoperative complications in the field of orthopedics (10–12). Numerous clinical studies have delved into the association between SII and the incidence as well as mortality rates of fractures and complications across the general populace and diverse patient categories. Zhang et al. (32) reported that there exists an association between increased SII and an increased vulnerability to systemic inflammation as well as osteoporosis in middle-aged and elderly populations. Fang et al. (33) found that monitoring changes in SII could play a role in predicting osteoporotic fractures. And for patients with hip fractures, Zeng et al. (34) reported that SII could potentially be a useful indication for predicting preoperative DVT development in individuals with intertrochanteric fractures of the femur. Moldovan et al. (11) found that SII was strongly associated with surgical trauma suffered by an elderly population with hip fractures. Bala (35) found that short-term functional projections in hip fracture patients who undergo hemiarthroplasty could potentially be foreseen through the utilization of SII. Further, a prospective study involving 290 participants showed that in elderly patients who underwent hip fracture surgery, a strong association was observed between SII and increased mortality due to any cause (5). In addition, several studies have demonstrated the role of SII in reflecting systemic inflammation and disease severity (36, 37), particularly the diagnostic significance of SAPSII in orthopedic diseases (38, 39). These studies suggested that SII showed potential for predicting clinical outcomes in critically ill elderly individuals with hip fractures.
The precise biological mechanisms that connect the SII with the occurrence of morbidity and mortality following hip fracture in older individuals remain unidentified. The possible pathways may be related to body immune dysfunction and systemic inflammatory activation after a hip fracture. The underlying mechanisms likely include a sequence of inflammatory cytokines and chemokines, which stem from the dysfunction of lymphocytes within the immune reaction, resulting in the accumulation of neutrophils and macrophages (40, 41). Also, injury, infection, or ischemia can trigger a defensive inflammatory response, leading to an increase in platelets (42). The nature of reduced physiological reserve in elderly patients makes them more vulnerable to post-injury release of aggressive cytokines (TNF-a, IL-6, and IL-1β, etc.), as well as the lack of sufficient anti-invasive mediators to counterbalance their negative effects (3). After a hip fracture, the hip fracture-induced plasma mitochondrial dried DNA (mtDNA) release leads to a systemic hyperinflammatory response and lung damage via activating the TLR9/NF-KB pathway, a process that has also been termed the “inflammatory storm” (43–46). Subsequent surgical trauma is rapidly exacerbated by sterile systemic invasive reactions and damage to vascular endothelial cells (47). In addition, pro-inflammatory cytokines can mediate oxidative stress injury, trigger osteoclasts, and enhance bone resorptive properties, which can gradually lead to bone remodeling and even osteoporosis (48). The inflammatory response increases the body’s pro-inflammatory mediators, aggravating excessive inflammation, and the excessive inflammatory factors pass through the bloodstream to the lungs, heart, and other distant organs, aggravating the damage to the heart, lungs, and other organs and making it easier to trigger coagulation dysfunction, respiratory infections, and other symptoms, which can lead to the failure of other organ functions. Inflammatory co-morbidities are accompanied by a progressive collapse of the immune system, which leads to negative clinical consequences (49).
Presently, there is little literature exploring the correlation between the SII and severely ill individuals. Alsabani et al. (50) discovered that in critically ill patients, SII was linked to a higher likelihood of undergoing prolonged hospitalization after orthopedic surgical procedures; IMA et al. noted that in critically ill patients undergoing extracorporeal coronary artery bypass grafting, the preoperative SII was found to be an indicator for postoperative complications, including cardiac arrest and acute myocardial infarction, as well as associated with an increased risk of mortality (51); research utilizing the MIMIC database revealed a substantial link between the SII and sepsis-related hospital mortality among critically ill individuals presenting with toxemia (52). However, in our ICU hip fracture patient group, the study found that SII emerged as a considerable predictor of heightened mortality among critically ill patients. Moreover, in the case of hip fracture, a common condition with significant morbidity and mortality, our research indicates that the SII might be beneficial for pinpointing high-risk patients before surgery and potentially help in minimizing severe future complications.
Furthermore, this study further conducted a detailed analysis of the risk stratification across various subpopulations. The stratified analysis revealed a uniform predictive strength of SII concerning 1-year mortality that was consistent in critically ill individuals with hip fracture. However, no association was discovered between the SII and 30-day or 1-year mortality in individuals with sepsis or dementia at baseline. The observed trend could stem from a reversed causal relationship, suggesting that individuals with specific co-existing conditions are more inclined to have received suitable treatment or embraced good lifestyle practices (53, 54). Moreover, the investigation revealed that the organism’s condition of 30-day after hospital admission, evaluated through the SII, exhibited a heightened predictive significance in individuals of those aged ≤80 years [HR (95% CI) 1.39 (1.09–1.78)], those with BMI ≤ 30 kg/m2 [HR (95% CI) 1.18 (1.03–1.36)], and those with osteoporisis [HR (95% CI) 1.53 (1.05–2.22)]. This suggests that treating osteoporosis could greatly affect the predictive accuracy of SII for all-cause mortality. Past research has shown a strong connection between the overall immunological and inflammatory condition of the body and osteoporosis, possibly due to the direct or indirect impact of immune cells on the functions of bone cells (55).What’s more, we confirm the association between SII and mortality is more pronounced in a population of critically ill hip fracture patients with a lower BMI. Another interesting finding of the present study was that patients with higher SII were younger, and the link between SII and all-cause death appeared to be more pronounced in younger patients. Our findings suggest that clinicians should offer equal attention to younger patients due to their potentially greater death rate rather than focusing solely on older patients with more comorbidities. The study revealed a positive association between SII and 30-day mortality and 1-year mortality among severely ill individuals who had suffered from hip fractures, but this correlation is a non-linear relationship, suggesting that SII could serve as an effective instrument for identifying high mortality risk in this patient population. Therefore, early detection of SII, screening of high-risk groups, and improved management of SII are crucial to decreasing significant negative clinical results in the future. In a word, the evaluation outcomes suggest that the SII should not be employed as a solitary diagnostic instrument. Its application should complement other medical and laboratory assessments to provide a thorough evaluation of a patient’s health condition and enhance the risk appraisal for occurrences like mortality subsequent to a hip fracture within clinical settings.
This study’s primary strength lies in the compelling evidence we present, establishing an elevated SII as a significant standalone predictor of higher mortality rates among critically ill elderly patients with hip fracture. Nevertheless, there are certain constraints to consider in our investigation. Firstly, this study was unable to prove causality because of its retrospective methodology. Despite utilizing multivariate adjustments and subgroup analysis, residual variables could still impact clinical outcomes. Important variables that could affect the results, like the exact type of hip fracture and whether it was treated with surgery, were not available in this database. Secondly, the research was executed exclusively within a single-center setting, concentrating on elderly participants with an elevated risk of hip fracture; therefore, the study may contain selection bias. Third, the study solely assessed the baseline SII index at the initial hospitalization. There was no significant change in the SII index before and after hospitalization. Hence, the prognostic value of alterations in the SII should be assessed in upcoming research as well. Fourthly, our data were extracted based on the MIMIC-IV database and the follow-up period commenced on the admission date and ended on the day of death, but the study fail to address potential changes in care over the years or clarify the number of patients treated each year of follow-up. Fifthly, our study lacked some blood indices, including cytokines such as interleukins and high-sensitivity C-reactive protein, which precluded us from exploring the association between the SII and conventional inflammatory markers. Consequently, the necessity arises for additional multicenter prospective studies aimed at validating the relevance of SII in predicting the long-term outcome among aged, critically ill patients with hip fractures.
5. Conclusion
Our findings expanded the applicability of the SII to older patients with hip fractures who are critically ill. The study showed that the SII could serve as a valuable tool for assessing the risk of all-cause mortality within 30 days and 1 year in this patient population. Observing the SII may be beneficial in decision-making and disease management in clinical settings. Additional research is required to further confirm the potential of SII to enhance clinical prognosis in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary materials, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the MIT Computational Physiology Laboratory. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
Z-JL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review & editing, Resources. G-HL: Writing – original draft, Data curation, Methodology, Validation. J-XW: Writing – original draft, Data curation, Investigation. Z-HM: Data curation, Investigation, Writing – original draft. K-YY: Software, Visualization, Writing – review & editing. C-LS: Project administration, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. Z-XS: Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The undertaking was funded by the Project of Guangdong Provincial Administration of Traditional Chinese Medicine (20220443) and Foshan Municipal Bureau of Science and Technology (20221381).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1408371/full#supplementary-material
References
- 1.Dong Y, Zhang Y, Song K, Kang H, Ye D, Li F. What was the epidemiology and global burden of disease of hip fractures from 1990 to 2019? Results from and additional analysis of the global burden of disease study 2019. Clin Orthop Relat Res. (2023) 481:1209–20. doi: 10.1097/CORR.0000000000002465, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu E, Killington M, Cameron ID, Li R, Kurrle S, Crotty M. Life expectancy of older people living in aged care facilities after a hip fracture. Sci Rep. (2021) 11:20266. doi: 10.1038/s41598-021-99685-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou L, Huang C, Zhu X, Ma Z. Combined systemic immune-inflammatory index (SII) and geriatric nutritional risk index (GNRI) predict survival in elderly patients with hip fractures: a retrospective study. J Orthop Surg Res. (2024) 19:125. doi: 10.1186/s13018-024-04585-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbour KE, Lui LY, Ensrud KE, Hillier TA, LeBlanc ES, Ing SW, et al. Inflammatory markers and risk of hip fracture in older white women: the study of osteoporotic fractures. J Bone Miner Res. (2014) 29:2057–64. doi: 10.1002/jbmr.2245, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang ZC, Jiang W, Chen X, Yang L, Wang H, Liu YH. Systemic immune-inflammation index independently predicts poor survival of older adults with hip fracture: a prospective cohort study. BMC Geriatr. (2021) 21:155. doi: 10.1186/s12877-021-02102-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Gu L, Chen Y, Chong Y, Wang X, Guo P, et al. Systemic immune-inflammation index is a promising non-invasive biomarker for predicting the survival of urinary system cancers: a systematic review and meta-analysis. Ann Med. (2021) 53:1827–38. doi: 10.1080/07853890.2021.1991591, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hua X, Long ZQ, Zhang YL, Wen W, Guo L, Xia W, et al. Prognostic value of preoperative systemic immune-inflammation index in breast Cancer: a propensity score-matching study. Front Oncol. (2020) 10:580. doi: 10.3389/fonc.2020.00580, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang YL, Wu CH, Hsu PF, Chen SC, Huang SS, Chan WL, et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Investig. (2020) 50:e13230. doi: 10.1111/eci.13230, PMID: [DOI] [PubMed] [Google Scholar]
- 9.Hou D, Wang C, Luo Y, Ye X, Han X, Feng Y, et al. Systemic immune-inflammation index (SII) but not platelet-albumin-bilirubin (PALBI) grade is associated with severity of acute ischemic stroke (AIS). Int J Neurosci. (2021) 131:1203–8. doi: 10.1080/00207454.2020.1784166, PMID: [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, He M, Jia W, Xie W, Song Y, Wang H, et al. Analysis of high-risk factors for preoperative DVT in elderly patients with simple hip fractures and construction of a nomogram prediction model. BMC Musculoskelet Disord. (2022) 23:441. doi: 10.1186/s12891-022-05377-8, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moldovan F, Ivanescu AD, Fodor P, Moldovan L, Bataga T. Correlation between inflammatory systemic biomarkers and surgical trauma in elderly patients with hip fractures. J Clin Med. (2023) 12:5147. doi: 10.3390/jcm12155147, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang X, Hu H, Zhang W, Shao Z. Prognostic value of prognostic nutritional index and systemic immune-inflammation index in patients with osteosarcoma. J Cell Physiol. (2019) 234:18408–14. doi: 10.1002/jcp.28476, PMID: [DOI] [PubMed] [Google Scholar]
- 13.Moldovan F. Sterile inflammatory response and surgery-related trauma in elderly patients with subtrochanteric fractures. Biomedicines. (2024) 12:354. doi: 10.3390/biomedicines12020354, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Targonska-Stepniak B, Grzechnik K. The usefulness of cellular immune inflammation markers and ultrasound evaluation in the assessment of disease activity in patients with Spondyloarthritis. J Clin Med. (2023) 12:5463. doi: 10.3390/jcm12175463, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo H, Song B, Zhou R, Yu J, Chen P, Yang B, et al. Risk factors and dynamic nomogram development for surgical site infection following open wedge high Tibial osteotomy for Varus knee osteoarthritis: a retrospective cohort study. Clin Interv Aging. (2023) 18:2141–53. doi: 10.2147/CIA.S436816, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao W, Wang W, Tang W, Lv Q, Ding W. Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic immune inflammation index (SII) to predict postoperative pneumonia in elderly hip fracture patients. J Orthop Surg Res. (2023) 18:673. doi: 10.1186/s13018-023-04157-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson A, Bulgarelli L, Shen L, Gayles A, Shammout A, Horng S, et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci Data. (2023) 10:1. doi: 10.1038/s41597-022-01899-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on Sepsis-related problems of the European Society of Intensive Care Medicine. Intens Care Med. (1996) 22:707–10. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 19.Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/north American multicenter study. J Am Med Assoc. (1993) 270:2957–63. doi: 10.1001/jama.1993.03510240069035, PMID: [DOI] [PubMed] [Google Scholar]
- 20.Le Gall JR, Klar J, Lemeshow S, Saulnier F, Alberti C, Artigas A, et al. The logistic organ dysfunction system. A new way to assess organ dysfunction in the intensive care unit. ICU scoring group. J Am Med Assoc. (1996) 276:802–10. doi: 10.1001/jama.1996.03540100046027 [DOI] [PubMed] [Google Scholar]
- 21.Johnson AE, Kramer AA, Clifford GD. A new severity of illness scale using a subset of acute physiology and chronic health evaluation data elements shows comparable predictive accuracy. Crit Care Med. (2013) 41:1711–8. doi: 10.1097/CCM.0b013e31828a24fe [DOI] [PubMed] [Google Scholar]
- 22.Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. (2014) 20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442, PMID: [DOI] [PubMed] [Google Scholar]
- 23.Cai W, Xu J, Wu X, Chen Z, Zeng L, Song X, et al. Association between triglyceride-glucose index and all-cause mortality in critically ill patients with ischemic stroke: analysis of the MIMIC-IV database. Cardiovasc Diabetol. (2023) 22:138. doi: 10.1186/s12933-023-01864-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu S, Bansal N, Denburg M, Ginsberg C, Hoofnagle AN, Isakova T, et al. Risk factors for hip and vertebral fractures in chronic kidney disease: the CRIC study. J Bone Miner Res. (2024) 39:433–42. doi: 10.1093/jbmr/zjae021, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiu CT, Lee JI, Lu CC, Huang SP, Chen SC, Geng JH. The association between body mass index and osteoporosis in a Taiwanese population: a cross-sectional and longitudinal study. Sci Rep. (2024) 14:8509. doi: 10.1038/s41598-024-59159-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun W, Wang P, Zhao Y. The characteristics of the body mass frequency index in dysmobility syndrome: a pilot study. Exp Gerontol. (2024) 191:112414. doi: 10.1016/j.exger.2024.112414, PMID: [DOI] [PubMed] [Google Scholar]
- 27.Bruijns SR, Guly HR, Bouamra O, Lecky F, Lee WA. The value of traditional vital signs, shock index, and age-based markers in predicting trauma mortality. J Trauma Acute Care Surg. (2013) 74:1432–1437. [DOI] [PubMed] [Google Scholar]
- 28.Sun X, Lu J, Weng W, Yan Q. Association between anion gap and all-cause mortality of critically ill surgical patients: a retrospective cohort study. BMC Surg. (2023) 23:226. doi: 10.1186/s12893-023-02137-w, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher A, Srikusalanukul W, Fisher L, Smith PN. Comparison of prognostic value of 10 biochemical indices at admission for prediction postoperative myocardial injury and hospital mortality in patients with osteoporotic hip fracture. J Clin Med. (2022) 11:6784. doi: 10.3390/jcm11226784, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heuer A, Muller J, Strahl A, Fensky F, Daniels R, Theile P, et al. Outcomes in very elderly ICU patients surgically treated for proximal femur fractures. Sci Rep. (2024) 14:1376. doi: 10.1038/s41598-024-51816-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu X, Lin J. Low molecular weight heparin versus other anti-thrombotic agents for prevention of venous thromboembolic events after total hip or total knee replacement surgery: a systematic review and meta-analysis. BMC Musculoskelet Disiord. (2018) 19:322. doi: 10.1186/s12891-018-2215-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang S, Ni W. High systemic immune-inflammation index is relevant to osteoporosis among middle-aged and older people: a cross-sectional study. Immun Inflamm Dis. (2023) 11:e992. doi: 10.1002/iid3.992, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang H, Zhang H, Wang Z, Zhou Z, Li Y, Lu L. Systemic immune-inflammation index acts as a novel diagnostic biomarker for postmenopausal osteoporosis and could predict the risk of osteoporotic fracture. J Clin Lab Anal. (2020) 34:e23016. doi: 10.1002/jcla.23016, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng G, Li X, Li W, Wen Z, Wang S, Zheng S, et al. A nomogram model based on the combination of the systemic immune-inflammation index, body mass index, and neutrophil/lymphocyte ratio to predict the risk of preoperative deep venous thrombosis in elderly patients with intertrochanteric femoral fracture: a retrospective cohort study. J Orthop Surg Res. (2023) 18:561. doi: 10.1186/s13018-023-03966-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bala MM. The benefit of dynamic neutrophil-lymphocyte ratio and systemic immune-inflammation index in predicting survival in patients undergoing hemiarthroplasty. Eur Rev Med Pharmacol Sci. (2022) 26:3878–85. doi: 10.26355/eurrev_202206_28955 [DOI] [PubMed] [Google Scholar]
- 36.Choe JY, Lee CU, Kim SK. Association between novel hematological indices and measures of disease activity in patients with rheumatoid arthritis. Medicina. (2023) 59:117. doi: 10.3390/medicina59010117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zinellu A, Mangoni AA. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio and disease activity in rheumatoid arthritis: a systematic review and meta-analysis. Eur J Clin Investig. (2023) 53:e13877. doi: 10.1111/eci.13877, PMID: [DOI] [PubMed] [Google Scholar]
- 38.Xing Z, Cai L, Wu Y, Shen P, Fu X, Xu Y, et al. Development and validation of a nomogram for predicting in-hospital mortality of patients with cervical spine fractures without spinal cord injury. Eur J Med Res. (2024) 29:80. doi: 10.1186/s40001-024-01655-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang K, Li X, Xu Y, Yang Q. Prognostic value of the systemic immuno-inflammatory index in critically ill patients with vertebral fractures. Medicine. (2024) 103:e36186. doi: 10.1097/MD.0000000000036186, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H, Liu J, Yao J, Zhong J, Guo L, Sun T. Fracture initiates systemic inflammatory response syndrome through recruiting polymorphonuclear leucocytes. Immunol Res. (2016) 64:1053–9. doi: 10.1007/s12026-016-8801-2, PMID: [DOI] [PubMed] [Google Scholar]
- 41.Husebye EE, Lyberg T, Opdahl H, Aspelin T, Stoen RO, Madsen JE, et al. Intramedullary nailing of femoral shaft fractures in polytraumatized patients. A longitudinal, prospective and observational study of the procedure-related impact on cardiopulmonary- and inflammatory responses. Scand J Trauma Resusc Emerg Med. (2012) 20:2. doi: 10.1186/1757-7241-20-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vulliamy P, Armstrong PC. Platelets in hemostasis, thrombosis, and inflammation after major trauma. Arterioscler Throm Vasc Biol. (2024) 44:545–57. doi: 10.1161/ATVBAHA.123.318801, PMID: [DOI] [PubMed] [Google Scholar]
- 43.Zhang JZ, Wang J, Qu WC, Wang XW, Liu Z, Ren JX, et al. Plasma mitochondrial DNA levels were independently associated with lung injury in elderly hip fracture patients. Injury. (2017) 48:454–9. doi: 10.1016/j.injury.2017.01.009, PMID: [DOI] [PubMed] [Google Scholar]
- 44.Gan L, Chen X, Sun T, Li Q, Zhang R, Zhang J, et al. Significance of serum mtDNA concentration in lung injury induced by hip fracture. Shock. (2015) 44:52–7. doi: 10.1097/SHK.0000000000000366, PMID: [DOI] [PubMed] [Google Scholar]
- 45.Gu X, Yao Y, Wu G, Lv T, Luo L, Song Y. The plasma mitochondrial DNA is an independent predictor for post-traumatic systemic inflammatory response syndrome. PLoS One. (2013) 8:e72834. doi: 10.1371/journal.pone.0072834, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou Y, Meng F, Kohler K, Bulow JM, Wagner A, Neunaber C, et al. Age-related exacerbation of lung damage after trauma is associated with increased expression of inflammasome components. Front Immunol. (2023) 14:1253637. doi: 10.3389/fimmu.2023.1253637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vallet H, Bayard C, Lepetitcorps H, O'Hana J, Fastenackels S, Fali T, et al. Hip fracture leads to transitory immune imprint in older patients. Front Immunol. (2020) 11:571759. doi: 10.3389/fimmu.2020.571759, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Azizieh F, Raghupathy R, Shehab D, Al-Jarallah K, Gupta R. Cytokine profiles in osteoporosis suggest a proresorptive bias. Menopause. (2017) 24:1057–64. doi: 10.1097/GME.0000000000000885, PMID: [DOI] [PubMed] [Google Scholar]
- 49.Tatum D, Taghavi S, Houghton A, Stover J, Toraih E, Duchesne J. Neutrophil-to-lymphocyte ratio and outcomes in Louisiana COVID-19 patients. Shock. (2020) 54:652–8. doi: 10.1097/SHK.0000000000001585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alsabani MH, Alotaibi BA, Olayan LH, Alghamdi AS, Alshammasi MA, Alqasir BA, et al. The value of preoperative systemic immune-inflammation index as a predictor of prolonged hospital stay in orthopedic surgery: a retrospective study. Int J Gen Med. (2023) 16:4773–82. doi: 10.2147/IJGM.S434630, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parmana I, Boom CE, Poernomo H, Gani C, Nugroho B, Cintyandy R, et al. High preoperative systemic immune-inflammation index values significantly predicted poor outcomes after on-pump coronary artery bypass surgery. J Inflamm Res. (2024) 17:755–64. doi: 10.2147/JIR.S449795, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu C, Wu X, Deng R, Xu X, Chen C, Wu L, et al. Systemic immune-inflammation index combined with quick sequential organ failure assessment score for predicting mortality in sepsis patients. Heliyon. (2023) 9:e19526. doi: 10.1016/j.heliyon.2023.e19526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang YR, Wang JJ, Chen SF, Wang HF, Li YZ, Ou YN, et al. Peripheral immunity is associated with the risk of incident dementia. Mol Psychiatry. (2022) 27:1956–62. doi: 10.1038/s41380-022-01446-5, PMID: [DOI] [PubMed] [Google Scholar]
- 54.Zhang H, Hao M, Hu Z, Li Y, Jiang X, Wang J, et al. Association of immunity markers with the risk of incident frailty: the Rugao longitudinal aging study. Immun Ageing. (2022) 19:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang Y, Peng B, Liu J, Liu Z, Xia Y, Geng B. Systemic immune-inflammation index and bone mineral density in postmenopausal women: a cross-sectional study of the national health and nutrition examination survey (NHANES) 2007-2018. Front Immunol. (2022) 13:975400. doi: 10.3389/fimmu.2022.975400, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary materials, further inquiries can be directed to the corresponding authors.