Abstract
Serological screening for human T-lymphotropic virus type 1 (HTLV-1) parallels the standard screening process for human immunodeficiency virus (HIV), in which samples found positive by enzyme-linked immunosorbent assay (ELISA) are confirmed with a modified Western blot procedure. There are a significant number of cases in which HTLV-1/2 ELISA-positive specimens demonstrate an incomplete banding pattern on this Western blot. Individuals providing these atypical antibody responses are categorized as seroindeterminate for HTLV-1/2. Although HTLV-1 genomic sequences are readily detectable in the peripheral blood lymphocytes (PBL) of seropositive individuals, previous studies have repeatedly demonstrated that PBL from the vast majority of HTLV-1/2 seroindeterminate individuals are PCR negative for HTLV-1. As a result, identification of the agent responsible for this indeterminate reactivity has been of interest. We have generated an HTLV-1-positive B-cell line (SI-1 B) from one of these seroindeterminate individuals. Previous screening for HTLV-1 in PBL from this patient had been routinely negative by primary PCR; however, HTLV-1 tax had been periodically detected by nested PCR. DNA sequence data generated with genomic DNA from the SI-1 B cell line and HTLV-1-specific primers demonstrated the presence of a full-length viral genome with >97% homology to the Cosmopolitan form of HTLV-1. A 12-bp deletion was identified in the 3′-gag/5′-prot region, which would predict translation of altered or nonfunctional proteins from these genes. We propose that this HTLV-1/2-seroindeterminate patient is infected with a prototypic form of HTLV-1 at an extremely low viral load and that this finding may explain HTLV-1/2 seroindeterminate reactivity in at least a subset of these individuals.
Human T-lymphotropic virus type 1 (HTLV-1) has been identified as the etiologic agent of two distinct human diseases: adult T-cell leukemia (ATL) and a chronic, progressive demyelinating disorder known as HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (3, 20). The virus has also been associated with a number of autoimmune diseases, including Sjögren's syndrome (18), uveitis (13), and an inflammatory arthropathy (14). Individuals infected with HTLV-1, or the closely related HTLV-2, have been discovered throughout the world. However, regions of HTLV-1 endemicity, with proportionately higher rates of infection, are clustered in southern Japan, the Caribbean, South America, the southern United States, equatorial Africa, and Iran (4).
Screening for HTLV-1/2 has become routine in blood banks in the United States and a small number of other developed nations. The screening process is initiated with an HTLV-1/2-specific enzyme-linked immunosorbent assay (ELISA). Samples which are found to be repeatedly positive by ELISA are confirmed through a radioimmunoprecipitation assay (RIPA) or, more commonly, a commercially available Western blot assay. The standardized Western blot assay incorporates viral proteins obtained from Hut102, an HTLV-1-infected cell line, and a number of recombinant HTLV-1/2 glycoproteins, which are included to increase the sensitivity and specificity of the assay. Established criteria for a seropositive HTLV-1/2 Western blot require reactivity to the viral p24 (Gag) protein as well as one of two viral Env proteins (rgp21 or rgp46). In a significant number of cases, which can be demonstrated throughout the world, the HTLV-1/2 ELISA is positive but an incomplete antibody response appears on the Western blot (7, 9, 12). Individuals providing these samples are categorized as HTLV-1/2 seroindeterminate. While HTLV-1 genomic sequences can be readily demonstrated in the peripheral blood lymphocytes (PBL) of virtually all HTLV-1-seropositive individuals by primary PCR or, in certain cases, by Southern blotting, previous studies have suggested that the vast majority of HTLV-1/2 seroindeterminate individuals are HTLV-1 genome negative (9, 12).
The causative agent(s) and medical significance of the HTLV-1/2 seroindeterminate status are unclear. A number of potential explanations have been provided for this serological finding, including (i) cross-reactivity with another infectious agent (e.g., Plasmodium falciparum) (10), (ii) infection with a truncated or deleted form of HTLV-1 (2, 5), (iii) infection with a novel retrovirus bearing significant homology to HTLV-1 (1), and (iv) infection with prototype HTLV-1 at viral loads which are below the range of current methods of detection. Previous studies have had limited success in addressing these possibilities.
We have previously described a cohort of patients with various neurological symptoms and seroindeterminate HTLV-1/2 Western blot profiles (17). Analysis of PBL from these individuals reflected data generated by other groups (9, 10, 12). HTLV-1 sequences were not detectable by primary PCR, while HTLV-1 tax could be periodically amplified by nested PCR. In this paper we describe an Epstein-Barr virus (EBV)-transformed B-cell line (SI-1 B), generated from one of these HTLV-1/2-seroindeterminate patients diagnosed with the relapsing, remitting form of multiple sclerosis, which was found to be positive for HTLV-1 by primary PCR. HLA typing of the cell line provided an exact match to the patient, ruling out contamination with another infected source. Southern blot analysis of SI-1 B genomic DNA demonstrated the expected HTLV-1 banding pattern for a number of restriction enzymes. The SI-1 B cell line was deficient in production of the viral p19 (Gag) protein, in contrast to other HTLV-1-infected cell lines, which were positive for expression of this protein. Overlapping PCR clones which spanned the entire HTLV-1 genome were generated using SI-1 B genomic DNA and primers specific for prototypic HTLV-1. These amplimers were subsequently subcloned and sequenced. The virus infecting the SI-1 B cell line was determined to fall in the Cosmopolitan subtype of HTLV-1 and was found to have >97% homology to the prototype sequence (16). In addition, a 12-bp deletion was discovered in the region coding for both the 3′ end of the viral Gag and the 5′ portion of the protease, resulting in predictable disruptions of the coding sequences for these proteins; potential effects of such alterations are discussed herein.
This is the first evidence demonstrating infection with full-length HTLV-1 in a primary-PCR negative, HTLV-1/2 seroindeterminate individual. We propose that this finding may provide an explanation for HTLV-1/2 seroindeterminate status in a subset of individuals, perhaps similarly infected with prototype HTLV-1 at an extremely low viral load.
MATERIALS AND METHODS
Patient history and generation of cell lines.
Patient SI-1 was referred to the Neuroimmunology Branch at the National Institute of Neurological Disease and Stroke, Bethesda, Md., with relapsing, remitting multiple sclerosis and HTLV-1/2 seroindeterminate status. She is an African American female from the southern United States with no other known risk factors for infection with HTLV-1. For generation of B-cell lines, 5 × 106 PBL were cultured for 2 weeks in complete RPMI (Life Technologies, Gaithersburg, Md.) (3 mM l-glutamine, 15 mM HEPES, 0.04 U of penicillin/streptomycin per ml) supplemented with 15% fetal calf serum, OKT3, and EBV stock. After 2 weeks of uninterrupted culture, the cells were passed into and maintained in complete RPMI–15% fetal calf serum alone.
HTLV-1/2 ELISA and Western blot analyses.
Patient serum was initially tested for the presence of HTLV-1/2 antibodies with an HTLV-1/2 enzyme immunoassay kit (Abbott Laboratories, North Chicago, Ill.) as specified in the kit instructions. A positive EIA was followed by testing by an HTLV-1/2 modified Western blot assay (HTLV Blot 2.4; Genelabs Diagnostics, Singapore City, Singapore); sera were diluted from 1:50 to 1:160,000 for the assay.
PCR screening of cell lines.
Genomic DNA was isolated from 5 × 106 cells by using the QiaAmp blood kit (Qiagen, Santa Clarita, Calif.), and PCR for HTLV-1 tax was performed with 1 μg of DNA as the template. Reactions were set up in a 50-μl volume containing target DNA, 10× buffer (100 mM Tris-HCl, 15 mM MgCl2, 500 mM KCl [pH 8.3]), 0.2 μM each SK43 primer and SK44 primer (see Table 1 for primer sequences), 200 μM total deoxynucleoside triphosphate, and 2.5 U of Taq polymerase (Boehringer Mannheim, Indianapolis, Ind.). Samples were cycled through 25 rounds of denaturation at 94°C for 1 min, annealing at 54°C for 1 min, and extension at 72°C for 1 min. Products were electrophoresed through a 1% agarose gel and were visualized by ethidium bromide staining.
TABLE 1.
Primer sequences and respective regions amplified for overlapping PCR clones spanning the entire prototypic HTLV-1 genome
| Amplimer | Region amplified | Primer and sequences |
|---|---|---|
| clone1 | 5′ LTR–3′ gag | BS1F, gaggcggccgcgcggccgcgtagttctgacaatgaccatgagcc |
| M3, gccagagttgctggtattctcgcct | ||
| clone2 | 3′ pol–5′ env | SK110, ccctacaatccaaccagctcag |
| SG453, gcgggatcctagggtgggaacag | ||
| clone3 | 3′ env–5′ pX | SG452, atcctcgagccctctataccatg |
| SK44, gagccgataacgcgtccatcg | ||
| clone4 | 3′ gag–3′ pol | M2, tccggcttgcggtgcagcagtttga |
| SK111, gtggtgaagctgccatcgggtttt | ||
| clone5 | 5′ pX–3′ LTR | SK43, cggatacccagtctacgtgt |
| BS2R, gaggaattcgaattctataggatgggctgtcgctggc |
Southern blotting.
Genomic DNAs were extracted with the Easy-DNA kit (Invitrogen, Carlsbad, Calif.) and purified on columns from the QiaAmp blood kit. Samples were digested to completion for 4 to 5 h at 37°C with EcoRI, PstI, or HindIII. The resulting digests were run on a 0.7% agarose gel, transferred to Nytran-supported nylon membranes (Schleicher & Schuell, Keene, N.H.), and UV cross-linked by Stratalinker (Strategene). The filters were hybridized for 1 h at 42°C with radiolabelled proviral HTLV-1 DNA lacking only long terminal repeat (LTR) sequences (Ready-to-Go random-primed labelling kit [Amersham Pharmacia, Piscataway, N.J.]) in Quick-Hyb solution (Stratagene). The filters were washed, and hybridization was visualized by overnight exposure to X-Omat film (Kodak) at −70°C.
p19 antigen capture assay.
Samples were prepared for use in the Retro-Tek HTLV p19 antigen ELISA kit (Cellular Products Inc., Buffalo, N.Y.). Supernatants were cleared by centrifugation at 800 × g in a microcentrifuge for 10 min, transferred to clean tubes, and used directly for ELISA. For cell lysates, 5 × 106 cells were centrifuged and the supernatant was discarded. The cell pellets were subjected to freezing-thawing and resuspended in 500 μl of phosphate-buffered saline with repeated up-and-down pipetting. The ELISA was performed as specified in the kit instructions, and concentrations of antigen were determined based on standards provided with the kit.
Western blot analysis for p19.
To analyze virus-specific protein synthesis, cell lysates and supernatants (prepared as above) obtained from an HTLV-1-uninfected B-cell line (UN-1), SI-1 B, a B-cell line derived from a HAM/TSP patient, and HUT102 were subjected to Western blot analysis. Proteins from lysates and supernatants (50 μg of protein per sample) were separated by electrophoresis on a 12% Tris–glycine gel and transferred to a 0.22-μm-pore-size nitrocellulose membrane (Schleicher & Schuell). Western blot analysis was performed with a monoclonal antibody to p19 (Chemicon, Inc., Temecula, Calif.).
RT-PCR for HTLV-1 tax expression.
RNA was purified from 5 × 106 cells with the RNeasy kit (Qiagen). After purification, RNA was treated with 10 U of DNase (Boehringer, Mannheim, Germany) and digested for 15 min at 37°C. DNase was then inactivated by heat treatment for 15 min at 70°C. First-strand cDNA synthesis was performed with the Ready-to-Go kit (Pharmacia) and 5 μg of total RNA primed with a combination of random hexamers and oligo(dT). The entire first-strand reaction was used as template for PCR amplification of HTLV-1 tax, env, pol and actin mRNAs; reactions were set up as described above in a total volume of 100 μl/reaction. Tax primers were RPX3 (5′-ATCCCGTGGAGACTCCTCAA-3′) and RTTAXB1 (5′-AGAGGTTCTCTGGGTGGGGAAG-3′), and Pol primers were SK110 (5′-CCCTACAATCCAACCCAGCTCAG-3′) and SK 1111 (5′-GTGGTGGATTTGCCATCGGGTTTT-3′). Nested reverse transcription-PCR (RT-PCR) for HTLV-1 env mRNA was performed with primers env-1 (5′-TGTGGTGCCTCCTGAACTGCG-3′) env-2 (5′-GTCTTAATAGCCGCCAGTGGA-3′), env-3 (5′AGGGGGCAGAACTGGAAGAAT-3′), and env-4 (5′-CAGCAGCTGGGGCTGTAATCA-3′). The actin primers were RTBACF1 (5′-GCATGGGTCAGAAGGATTCCTASI-1-3′) and RTBACB8 (5′-ACAGGGATAGCACAGCCTGGATAG-3′). Samples were cycled through 35 rounds of denaturation at 94°C for 30 min, annealing at 55°C for 30 s, and extension at 72°C for 1 min. Products were visualized as described above.
Generation of overlapping PCR clones.
Primers were chosen based on their location within the prototype HTLV-1 genomic sequence available in the GenBank database (16). Clones and respective primer sets are described in Table 1. PCR mixtures were prepared as above with 1 μg of genomic SI-1 B DNA as the template. The reactions consisted of 30 cycles of denaturation at 94°C for 30 s, annealing at either 55°C (clones 1, 2, and 3) or 60°C (clones 4 and 5) for 30 s, and extension at 72°C for 4 min (clones 1, 2, and 3) or 8 min (clones 4 and 5). Samples were visualized by gel electrophoresis as above. Expected product sizes were based on predictions from the prototypic HTLV-1 sequence. Each clone was amplified in triplicate to account for potential point mutations introduced during PCR by the Taq polymerase. The resulting PCR products were directly subcloned into the TA-cloning vector pCR2.1 (Invitrogen). Subclones (triplicates of each clone) were transformed into INVαF′, and positive colonies were selected by blue-white color screening. Positive colonies were miniprepped using the Qiagen miniprep kit, and predicted inserts were confirmed by restriction digestion.
Sequencing and data analysis.
Samples were sequenced on an ABI 373 automated sequencing system at the National Institute of Neurological Disorders and Stroke Core DNA facility on the National Institutes of Health campus. Dye-labelled vector-specific primers (M13F and M13R) were used to sequence the 3′ and 5′ regions of each clone, respectively. Nested deletions were generated for each clone using the Exo-Mung system (Stratagene), and sequence products of the resulting fragments were aligned to construct contigs for each clone. Sequence was obtained for both strands of each clone. Alignment and building of clone consensus sequence, as well as subsequent comparison to the prototype HTLV-1 sequence and translational analysis, were completed with the GeneWorks software package (IntelliGenetics), GCG-Lite (available at http://molbio.info.nih.gov/molbio/gcglite/), and analysis software available from the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/).
RESULTS
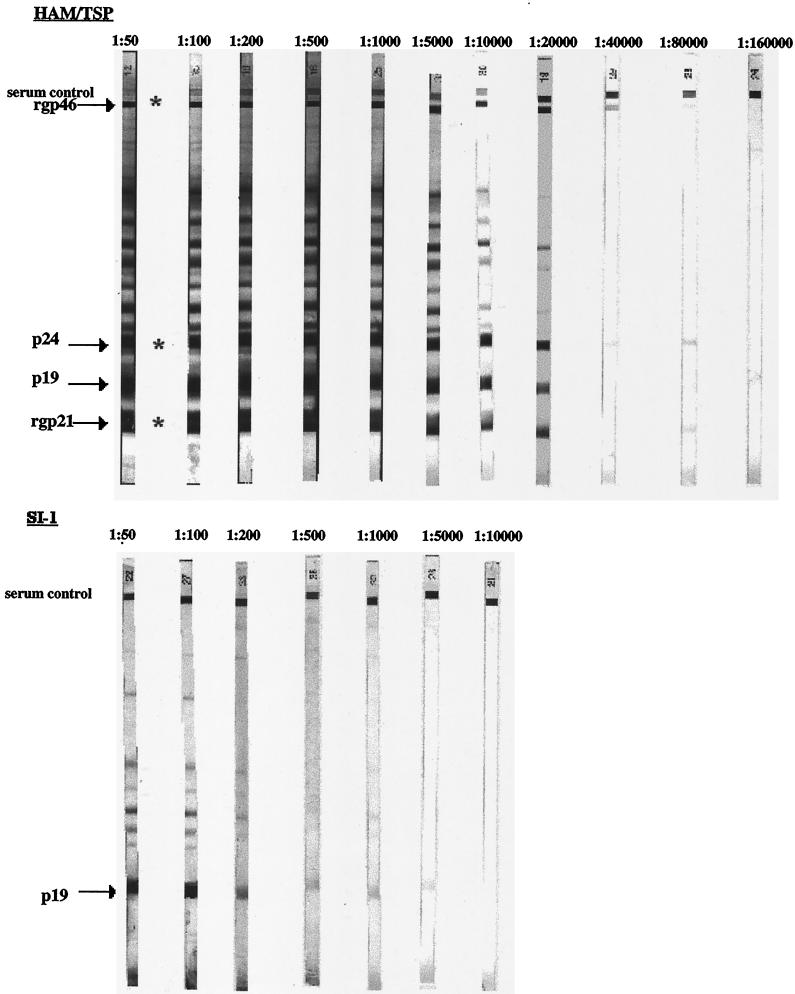
Patient SI-1 was positive in the HTLV-1/2 ELISA and was subsequently determined to be HTLV-1/2 seroindeterminate by Western blot analysis (Fig. 1). The blot was reminiscent of an HTLV-1/2-seroindeterminate Western blot pattern seen in samples throughout the world and known as the HTLV-1 Gag-indeterminate Profile (12). Reactivity to the p19 (Gag) element and a number of other antigens which are not considered diagnostic for infection with HTLV-1 was evident, but responses to the required viral p24, rgp21, and rgp46 antigens were absent. To exclude the possibility that this seroindeterminate pattern was due to low antibody titers, serum from a representative HAM/TSP patient was titrated to determine if a Western blot pattern comparable to that of patient SI-1 could be obtained. As can be seen in Fig. 1, the HAM/TSP patient serum, beginning at a 1:40,000 dilution, had a faint response to the p24, rgp21, and rgp46 bands. At a 1:80,000 dilution, only the p24 and rgp21 bands remained. Antibodies to HTLV-1 antigens were not detectable at the 1:160,000 dilution. At no dilution do any of these patterns resemble the Western blot pattern of patient SI-1 or the other seroindeterminate patterns previously described and typified by reactivity to p19 and absence of rgp46, p24, and rgp21 (17).
FIG. 1.
HTLV-1/2 Western blot. Serum from a representative HAM/TSP patient was blotted from 1:50 to 1:160,000 dilutions. Serum from seroindeterminate patient SI-1 was blotted from 1:50 to 1:10,000 dilutions. Reactivity to the p24 antigen and to either rgp21 or rgp46 (∗) is required for a classification of HTLV-1 seropositivity.
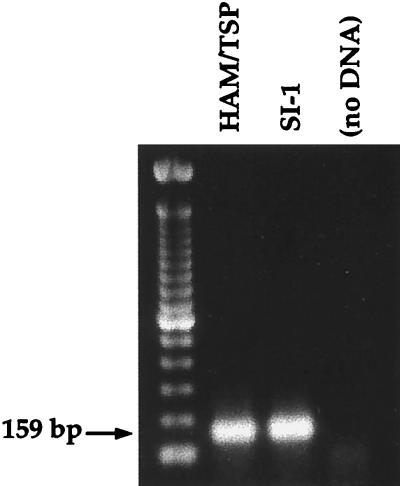
During routine screening for HTLV-1 tax in a number of B-cell lines from our cohort of HTLV-1/2-seroindeterminate patients (previously generated for use as autologous targets in cytotoxic T-lymphocyte assays), the SI-1 B cell line was found to be positive for this viral sequence by primary PCR (Fig. 2). To address issues of contamination or misidentification of the SI-1 B cell line, genomic DNA was isolated for PCR-based HLA subtyping. The results were identical to the previously determined HLA type of patient SI-1, confirming that the SI-1 B cell line originated from this seroindeterminate individual.
FIG. 2.
PCR for HTLV-1 tax using genomic DNA isolated from an HTLV-1-infected HAM/TSP B-cell line and the SI-1 B cell line.
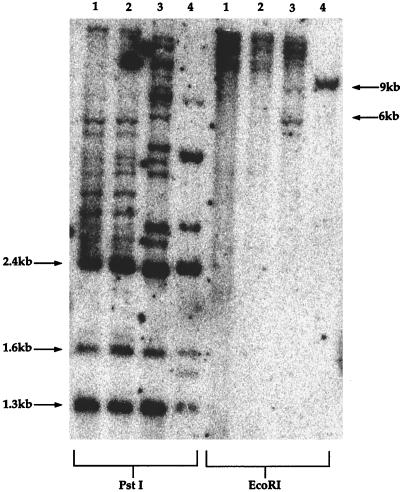
Southern blot analysis of DNA from the SI-1 B-cell line revealed that the virus present in this seroindeterminate patient was probably full-length and demonstrated the predicted banding pattern when digested with a number of restriction enzymes (Fig. 3). Since there are no internal EcoRI sites in prototypic HTLV-1, digestion with this enzyme predictably yields bands of 9 kb or larger (the size of the prototypic HTLV-1 genome). We observed a number of smaller bands from the SI-1 B cell line following EcoRI digestion. These nonprototypic bands were explained during subsequent sequence analysis, which identified a new EcoRI site present in the 3′ LTR of the HTLV-1 genome from the SI-1 B cell line. Digests of SI-1B DNA with PstI or HindIII (data not shown for HindIII) demonstrated the prototypic banding pattern for these enzymes and argued against any gross genetic changes in the internal portions of the viral genome (Fig. 3).
FIG. 3.
Southern blot for HTLV-1 integrated in B-cell genomic DNA generated from a patient with HAM/TSP (lanes 1 and 2), the SI-1 B cell line (lanes 3), and an HTLV-1-infected cell line from a patient with ATL (lanes 4). The line from the ATL patient contains a monoclonal integration of the HTLV-1 provirus. The other lines harbor multiple integration sites of the virus. Following digestion with PstI, expected virus-specific bands are 2.4, 1.6, and 1.3 kb; additional bands at higher molecular sizes are attributed to viral sequences contiguous with cellular genomic DNA external to adjacent PstI sites in the viral genome. Digestion of prototype virus with EcoRI predictably yields bands greater than 9 kb, due to a lack of internal EcoRI sites.
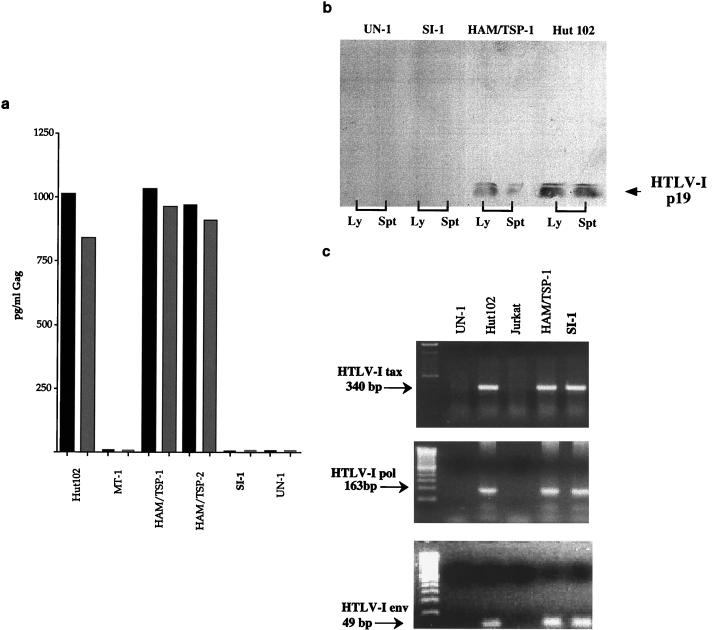
An antigen capture ELISA, specific for the p19 element of HTLV-1 Gag, was negative for both culture supernatant and cell lysate fractions of the SI-1 B cell line (Fig. 4a). Additionally, Western blot analysis demonstrated that the SI-1 B cell line was deficient in p19 production in both the lysate and supernatant fractions (Fig. 4b). In contrast, eight of eight B-cell lines established from HTLV-1-seropositive individuals were positive by the antigen capture assay (data shown for two of these cell lines [Fig. 4a]) and p19 was easily detectable by Western blot analysis in both HUT102 and a B-cell line derived from a HAM/TSP patient (Fig. 4b). To determine if the lack of Gag production was due to deletion or truncation of the HTLV-1 tax gene, a crucial factor for transactivation and transcription of other HTLV-1 genes, we performed RT-PCR for HTLV-1 tax. In this case, the SI-1 B cell line produced a full-length tax mRNA, as observed in other HTLV-1-infected cell lines (Fig. 4c). Additionally, the SI-1 B cell line was found to produce full-length mRNA for the env and pol regions of HTLV-1 (Fig. 4c). The potential for posttranscriptional abnormalities or alterations in expression of Tax in the SI-1 B cell line could not be addressed, since the protein was not detectable by Western blotting or immunofluorescence in any of the HTLV-1-infected B-cell lines tested (data not shown). Quantitative PCR for HTLV-1 genomic DNA indicated that there were no major differences in viral copy number between the SI-1 B cell line and other HTLV-1-infected B-cell lines, suggesting that the absence of detectable Gag production was not simply attributable to a low viral load in the cell line (data not shown).
FIG. 4.
(a) Antigen capture assay for HTLV-I Gag. Hut102 and MT-1 are HTLV-1-infected T-cell lines; MT-1 is known to have very low levels of viral transcriptional activity. UN-1 is a B-cell line generated from an HTLV-1-seronegative individual. Black and grey columns represent cell supernatant and cell lysate fractions, respectively. (b) Western blot assay for HTLV-1 p19. Western blot analysis was performed on supernatants (Spt) and cell lysates (Ly) from an uninfected B-cell line (UN-1), a B-cell line derived from seroindeterminate patient SI-1 (SI-1 B), a B-cell line from a HAM/TSP patient (HAM/TSP-1), and an HTLV-1 infected T-cell line (Hut102) with a monoclonal antibody to HTLV-1 p19 at a 1:50 dilution. SeeBlue (Novex, San Diego, Calif.) markers (not shown) were used for gel calibration. (c) RT-PCR assay for HTLV-1 tax, pol, and env. UN-1 is an HTLV-1-uninfected B-cell line; Hut102 is an HTLV-1-infected T-cell line; Jurkat is an HTLV-1-uninfected T-cell line; HAM/TSP is an HTLV-1-infected B-cell line from a HAM/TSP patient: SI-1 is a B-cell line derived from seroindeterminate patient SI-1.
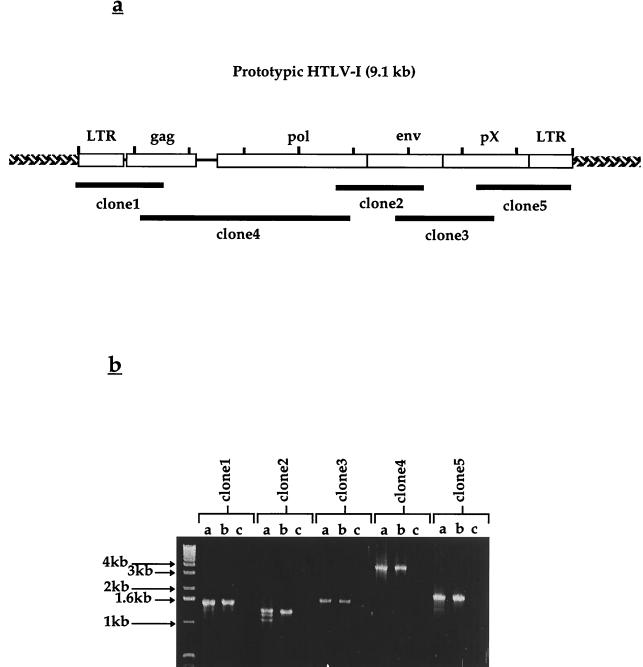
We thought that sequencing of the virus infecting the SI-1 B cell line would potentially shed light on viral genetic changes which could be associated with the HTLV-1/2-seroindeterminate antibody response. A strategy was devised which used overlapping PCR clones spanning the entire genome of prototypic HTLV-1 (Fig. 5a; Table 1). These regions were amplified by PCR (Fig. 5b) and were found to be of the expected sizes with respect to control samples and predictions from sequences in databases. Each individual region was amplified, subcloned, and sequenced in triplicate, so that subsequent comparison and alignment of individual clones from each region would allow for exclusion of mutations introduced by PCR amplification, cloning, or sequencing. The resulting contigs for each region, assembled from the combined sequence of nested deletions performed on each clone, were aligned and compiled to provide a full-length viral sequence for use in translational analysis and comparison to prototype HTLV-1. These comparisons are described in Table 2. The results indicate that the virus was globally >97% homologous to prototypic HTLV-1 on the nucleotide level. Fine analysis of the 5′ LTR indicated that the HTLV-1 strain infecting patient SI-1 was of the Cosmopolitan subtype (R. Mahieux, personal communication). A 12-bp deletion was identified, beginning at nucleotide 2109 of the viral genome, which would predict the following two alterations: (i) abrogation of the normal stop codon for the Gag polyprotein, resulting in a 20-amino-acid extension prior to termination at a new stop codon; and (ii) a deletion of amino acids 6 to 9 in the viral protease, with reemergence of prototype sequence at amino acid 10.
FIG. 5.
(a) Schematic representation of the HTLV-1 genome and relative locations of overlapping PCR clones generated from the SI-1 B cell line. (b) Overlapping PCR clones generated from the SI-1 B cell line using primers specific for prototypic HTLV-1. Primer sequences and expected amplimer sizes are shown in Table 1.
TABLE 2.
Comparison of the SI-1 B HTLV-1 sequence and various full-length HTLV-1 genomic sequences available in the GenBank database
| Region (SI-1 B viral genome)a | % Similarityb of region to that in:
|
|||
|---|---|---|---|---|
| AF033817 | AFO42071 | D13784 | I02029 | |
| Gag (nt 828–2177; 449 aa) | 98 (420/429) | 99 (426/429) | 99 (424/429) | 99 (423/429) |
| Protease (nt 2081–2798; 230 aa) | 95 (223/234) | NDc | 96 (225/234) | ND |
| Pol (nt 2515–5310; 895 aa) | 97 (857/878) | 99 (888/895) | 98 (884/895) | 98 (882/895) |
| Env (nt 5195–6658; 488 aa) | 98 (479/488) | 99 (485/488) | 98 (479/488) | 98 (478/488) |
| p40/Tax (nt 5195–5197, 7316–8374; 353 aa) | 98 (349/353) | ND | ND | ND |
| p27/Rex (nt 5139–5199, 7316–7822; 189 aa) | 100 (189/189) | ND | ND | ND |
| genome (9032 nt) | 98 | 99 | 97 | 98 |
nt, nucleotide sequence position in the SI-1 B viral genome; aa, predicted coding length of the respective gene product.
Percentages are based on comparisons of total number of aligned amino acids; numbers in parentheses represent actual alignments. Genomic comparisons are based on global genome-to-genome alignment. no. AF033817, unknown viral origin; AF042071, German isolate RKI-3; D13784, ATL isolate of Caribbean origin; J02029, ATL isolate of Japanese origin.
ND, not done.
DISCUSSION
The origins of seroindeterminate HTLV-1/2 Western blot patterns have been enigmatic since the initiation of screening for these viruses in the late 1980s. Interpretation of the significance of such patterns and counseling of these seroindeterminate individuals remain topics of concern for physicians in HTLV-1-endemic regions as well as for blood banks throughout the world. Although little progress has been made in the identification of an agent globally responsible for this phenomenon, several hypotheses have been proposed throughout the last decade.
A number of studies have demonstrated that antibodies specific for an antigen present in the blood stage of Plasmodium falciparum are cross-reactive with an epitope of the HTLV-1 Gag protein and that this cross-reactivity can result in binding to viral p19 elements in HTLV-1 screening assays (10). However, this finding was verified for seroindeterminate sera drawn from individuals residing in regions where malaria is endemic. In contrast, 15 HTLV-1/2-seroindeterminate serum samples taken from individuals residing in the United States were found to be negative for malarial cross-reactivity. HTLV-1/2-seroindeterminate prevalence rates increase with age in regions where malaria is endemic, while prevalence in other areas does not follow a similar pattern (12). In addition, the extraordinarily high rates of seroindeterminate findings (up to 25%) in some regions where malaria is endemic do not accurately reflect seroindeterminate rates in other regions, suggesting that the two situations are of different etiology. Of note, patient SI-1 had never lived in or traveled through a region where malaria is endemic. Alternative agents with HTLV-1-cross-reactive elements may account for seroindeterminate patterns in other areas, but no evidence has been provided for any such agent.
Seroindeterminate reactivities to the HTLV-1/2 Western blot have been found in sera from a small number of individuals infected with simian T-lymphotropic virus type 1 (STLV-1), which is homologous to HTLV-1 (21). Infection with such a virus could offer an explanation for an incomplete or faint banding pattern on the HTLV-1/2 Western blot, attributable to absence or minor changes of immunogenic epitopes recognized by the majority of HTLV-1-seropositive individuals. However, these STLV-1-infected individuals harbor viral loads which are easily detectable by PCR, a finding which contrasts sharply with data generated from the greater proportion of seroindeterminate samples worldwide.
The presence of truncated or deleted forms of HTLV-1 has been described in a number of studies, and these elements have been implicated as potential agents of disease in a number of cases (2, 5). Such deleted or truncated forms of HTLV-1 could theoretically account for an incomplete banding pattern on an HTLV-1/2 Western blot, again due to absence or alteration of crucial immunodominant viral epitopes. However, no evidence has been provided to support this theory, since individuals carrying truncated or altered forms of HTLV-1 have been primarily seronegative.
We report here the first evidence for infection with a full-length form of HTLV-1 in a primary-PCR-negative, HTLV-1/2 seroindeterminate individual. This patient exhibits a Western blot pattern demonstrating isolated reactivity to the viral p19 antigen, a pattern which has been previously described as the HTLV-1 Gag-indeterminate profile. Previous attempts to demonstrate infection with HTLV-1 in individuals with this pattern by using PCR have been largely unsuccessful (12). The fact that PBL from patient SI-1 were negative for viral sequences by primary PCR but occasionally positive by nested PCR suggested that this individual carries HTLV-1 at a viral load which is at or below the detectable limits of current PCR methods. Other groups have reported similarly positive results in nested PCR assays for HTLV-1 tax in some seroindeterminate individuals (A. Syrtsev, S. Van Dooren, and N. Senyuta, J. Acquired Immune Defic. Syndr. Hum. Retrovirol. 4:A51, 1999, abstract).
The presence of a 12-bp deletion in the HTLV-1 genome from patient SI-1, localized to a region which codes for portions of the viral Gag and protease, implies several potential functional defects of the virus that may infect this individual, although it is difficult to assess if this deletion is a major component in vivo. The absence of p19 from the SI-1 B cell line in the presence of a strong serological response to p19 from this patient suggests that defects in p19 may not be the major reason for the seroindeterminate reactivity. The N-terminal portion of the viral protease contains sites that are known to be involved in dimerization of this protein with the Gag-pro polyprotein, an interaction which is crucial for the activation of proteolytic function (6, 11). A deletion of four amino acids in this region could disrupt protease activity, precluding crucial processing steps required for liberation of individual viral proteins from their initial polyprotein forms and the subsequent assembly of a mature viral particle. It has been demonstrated that point mutations or deletion of glycosylation sites can result in a nonfunctional Env protein (15); failure in proteolytic processing of the polyprotein from its initial gp68 form to the gp46 and gp21 forms may result in a similar lack of Env expression. Therefore, the lack of reactivity from patient SI-1 to recombinant Env antigens on the HTLV-1 Western blot may simply be attributed to lack of antigen production. In addition, the inability to create functionally mature proteins, particularly those encoded by the viral pol gene, would result in potent attenuation of crucial replicative abilities of the virus.
The finding of an HTLV-1 PCR-positive B-cell line, initially generated from an otherwise primary PCR-negative individual, may have interesting ramifications for HTLV-1 on a broader scale. EBV-transformed B-cell lines are routinely generated in our laboratory for use as HLA-matched targets in cytotoxic T-lymphocyte assays. While B cells are not thought to be a natural reservoir for HTLV-1 in vivo, we found that all EBV-transformed B-cell lines generated from HTLV-1-seropositive individuals harbor the viral genome. As mentioned above, these HTLV-1-positive B-cell lines express detectable levels of the HTLV-1 Gag protein and tax mRNA. It is possible that these cells become infected during the in vitro EBV transformation of B cells from bulk PBL; the high levels of OKT3 used to activate and then exhaust the T-cell component of the PBL in this process may induce viral expression and promote syncytial contacts between T and B cells during the initial period of culture. In addition, there is some evidence that EBV itself may play a role in inducing a cellular environment conducive to HTLV-1 infection in cells which are normally not amenable to productive infection with the virus (19).
Although full-length mRNAs from the tax, env, and pol regions of HTLV-1 were found in the SI-1 B cell line, HTLV-1 p19 Gag production was markedly absent. The lack of detectable Gag expression from the SI-1 B cell line may have implications for the persistence of HTLV-1 in this individual in the absence of an active immune response. There is some evidence that a significant proportion of HTLV-1-infected cells from seropositive individuals may be transcriptionally silent, since tax mRNA can be detected in PBL from only a small proportion of patients with HAM/TSP through the use of a nested RT-PCR assay (unpublished results from our group). Since it has been reported that up to 10% of the peripheral CD4+ T cells in these individuals may carry the HTLV-1 genome (8), one would expect to detect tax mRNA in these cells if they were indeed transcriptionally active.
The demonstration of full-length HTLV-1 in a primary PCR-negative HTLV-1/2 seroindeterminate individual warrants further investigation. The potential for disease attributable to such a low-level infection is unclear but may be of significance for the subset of HTLV-1/2 seroindeterminate individuals who are similarly infected.
ACKNOWLEDGMENTS
We thank BBI Biotech (Rockville, Md.) for nested deletions (NIH contract NO1-NS-7-2372).
We thank Renaud Mahieux for helpful discussions. Additionally, we thank Colombe Chappey for assistance with our GenBank submission.
REFERENCES
- 1.Banki K, Maceda J, Hurley E, Ablonczy E, Mattson D H, Szegedy L, Hung C, Perl A. Human T-cell lymphotropic virus (HTLV)-related endogenous sequence, HRES-1, encodes a 28-kDa protein: a possible autoantigen for HTLV-1 gag-reactive autoantibodies. Proc Natl Acad Sci USA. 1992;89:1939–1943. doi: 10.1073/pnas.89.5.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daenke S, Parker C E, Niewiesk S, Newsom-Davis J, Nightingale S, Bangham C R. Spastic paraparesis in a patient carrying defective human T cell leukemia virus type I (HTLV-I) provirus sequences but lacking a humoral or cytotoxic T cell response to HTLV-I. J Infect Dis. 1994;169:941. doi: 10.1093/infdis/169.4.941. [DOI] [PubMed] [Google Scholar]
- 3.Gessain A, Barin F, Vernant J C, Gout O, Maurs L, Calender A, de The G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;ii:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 4.Gessain A. Epidemiology of HTLV-I and associated diseases. In: Höllsberg P, Hafler D A, editors. Human T-cell lymphotropic virus type I. London, United Kingdom: John Wiley & Sons Ltd.; 1996. pp. 33–64. [Google Scholar]
- 5.Hall W W, Liu C R, Schneewind O, Takahashi H, Kaplan M H, Roupe G, Vahlne A. Deleted HTLV-I provirus in blood and cutaneous lesions of patients with mycosis fungoides. Science. 1991;253:317–320. doi: 10.1126/science.1857968. [DOI] [PubMed] [Google Scholar]
- 6.Hayakawa T, Misumi Y, Kobayashi M, Yamamoto Y, Fujisawa Y. Requirement of N- and C-terminal regions for enzymatic activity of human T-cell leukemia virus type I protease. Eur J Biochem. 1992;206:919–925. doi: 10.1111/j.1432-1033.1992.tb17001.x. [DOI] [PubMed] [Google Scholar]
- 7.Khabbaz R F, Heneine W, Grindon A, Hartley T M, Shulman G, Kaplan J. Indeterminate HTLV serologic results in US blood donors: are they due to HTLV-I or HTLV-II? J Acquired Immune Defic Syndr. 1992;5:400–404. [PubMed] [Google Scholar]
- 8.Kubota R, Fujiyoshi T, Izumo S, Yashiki S, Maruyama I, Osame M, Sonoda S. Fluctuation of HTLV-I proviral DNA in peripheral blood mononuclear cells of HTLV-I associated myelopathy. J Neuroimmunol. 1993;42:147–154. doi: 10.1016/0165-5728(93)90004-i. [DOI] [PubMed] [Google Scholar]
- 9.Lal R B, Rudolph D L, Coligan J E, Brodine S K, Roberts C R. Failure to detect evidence of human T-lymphotropic virus (HTLV) type I and type II in blood donors with isolated gag antibodies to HTLV-I/II. Blood. 1992;80:544–550. [PubMed] [Google Scholar]
- 10.Lal R B, Rudolph D L, Alpers M P. Immunologic cross-reactivity between structural proteins of human T-cell lymphotropic virus (HTLV) type I and the blood stage of Plasmodium falciparum. Clin Diagn Lab Immunol. 1994;1:5–10. doi: 10.1128/cdli.1.1.5-10.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mamoun R Z, Dye D, Rebeyrotte N, Bouamr F, Cerutti M, Desgranges C. Mouse monoclonal antibodies directed against the HTLV-I protease recognize epitopes internal to the dimer. J Acquired Immune Defic Syndr Hum Retrovirol. 1997;14:184–188. doi: 10.1097/00042560-199702010-00014. [DOI] [PubMed] [Google Scholar]
- 12.Mauclère P, Le Hesran J Y, Mahieux R, Salla R, Mfoupouendoun J, Abada E T, Millan J, de The G, Gessain A. Demographic, ethnic, and geographic differences between human T cell lymphotropic virus (HTLV) type-I seropositive carriers and persons with HTLV-I gag-indeterminate western blots in Central Africa. J Infect Dis. 1997;176:505–509. doi: 10.1086/514071. [DOI] [PubMed] [Google Scholar]
- 13.Mochizuki M, Watanabe T, Yamaguchi K, Takatsuki K, Yoshimura K, Shirao M, Nakashima S, Mori S, Araki S, Miyata N. HTLV-I uveitis: a distinct clinical entity caused by HTLV-I. Jpn J Cancer Res. 1992;5:29–42. doi: 10.1111/j.1349-7006.1992.tb00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishioka K, Maruyama I, Sato K, Kitajima I, Nakajima Y, Osame M. Chronic inflammatory arthropathy associated with HTLV-I. Lancet. 1989;i:441. doi: 10.1016/s0140-6736(89)90038-x. [DOI] [PubMed] [Google Scholar]
- 15.Pique C, Tursz T, Dokhelar M C. Mutations introduced along the HTLV-I envelope gene result in a non-functional protein: a basis for envelope conservation? EMBO J. 1990;9:4243–4248. doi: 10.1002/j.1460-2075.1990.tb07872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seiki M, Hattori S, Hirayama Y, Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci USA. 1983;80:3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soldan S S, Graf M D, Waziri A, Flerlage A N, Robinson S M, Kawanishi T, Leist T P, Lehky T J, Levin M C, Jacobson S. HTLV-I/II seroindeterminate Western blot reactivity in a cohort of patients with neurologic disease. J Infect Dis. 1999;180:685–694. doi: 10.1086/314923. [DOI] [PubMed] [Google Scholar]
- 18.Terada K, Katamine S, Eguchi K, Moriuchi R, Kita M, Shimada H, Yamashita I, Iwata K, Tsuji Y, Nagataki S. Prevalence of serum and salivary antibodies to HTLV-1 in Sjogren's syndrome. Lancet. 1994;344:1116–1119. doi: 10.1016/s0140-6736(94)90630-0. [DOI] [PubMed] [Google Scholar]
- 19.Toth F D, Aboagye-Mathiesen G, Nemes J, Liu X, Andirko I, Hager H, Zdravkovic M, Szabo J, Kiss J, Aranyosi J, Ebbesen P. Epstein-Barr virus permissively infects human syncytiotrophoblasts in vitro and induces replication of human T cell leukemia-lymphoma virus type I in dually infected cells. Virology. 1997;229:400–414. doi: 10.1006/viro.1997.8449. [DOI] [PubMed] [Google Scholar]
- 20.Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481–492. [PubMed] [Google Scholar]
- 21.Vandamme A M, Van Laethem K, Liu H F, Van Brussel M, Delaporte E, de Castro Costa C M, Fleischer C, Taylor G, Bertazzoni U, Desmyter J, Goubau P. Use of a polymerase chain reaction assay detecting human T-lymphotropic virus (HTLV) types I, II and divergent simian strains in the evaluation of individuals with indeterminate HTLV serology. J Med Virol. 1997;52:1–7. [PubMed] [Google Scholar]