Abstract
Omphalitis, commonly caused by opportunistic bacteria has been significantly associated with morbidity and mortality in neonatal calves. Trueperella pyogenes is a commensal and opportunistic pathogen that can cause suppurative infection in farm animals. Our case involved a 10-day-old female Korean indigenous calf that presented with umbilical enlargement accompanied by a greenish-yellow purulent discharge and right forelimb lameness. The calf was diagnosed with failure of passive transfer at 24 h of age. Physical examination found hypothermia (38.1°C), tachycardia (110 beats/min), tachypnea (47 cycles/min), and open mouth breathing. Ultrasonography revealed hyperechoic pus in the 9th and 10th right intercostals, for which a liver abscess due to omphalophlebitis was suspected. After 3 days, the calf died. T. pyogenes was detected in the umbilical cord, lung, liver, kidney, intestine, mesenteric lymph node, urinary bladder, and bladder ligament. All genes related to the virulent factors (i.e., plo, cbpA, fimA, fimC, fimG, nanH, and nanP) were also identified, with plo and fimA being associated with pathogenicity. A final diagnosis of omphalitis was established based on the identification of virulent T. pyogenes and umbilical cord dilatation on ultrasonography. Antimicrobial susceptibility tests showed that the isolated T. pyogenes was susceptible to amoxicillin, ceftiofur, florfenicol, enrofloxacin, ofloxacin, and ciprofloxacin, suggesting the suitability of these antibiotics for treating T. pyogenes-induced omphalitis. Hence, accurate and rapid diagnosis of the involved bacteria and antimicrobial susceptibility patterns can help guide therapeutic decisions. Our case provides useful information that could aid large animal clinicians in the diagnosis and treatment of T. pyogenes-induced omphalitis.
Keywords: Trueperella pyogenes, omphalitis, failure of passive transfer, antimicrobial susceptibility patterns, Korean indigenous calf
Introduction
Omphalitis, often referred to as “navel ill” in farm animals, is an inflammation of the umbilicus and its connective tissue that mainly occurs on the extra-abdominal portion of the umbilicus approximately the first 30 days of life in calves. Although this condition is quite common among neonatal calves, no report has detailed its incidence aside from those that reported morbidity rates of 1.3 and 29.9%. Cleanliness of the calving and calf housing areas, transfer of passive immunity, and umbilical cord management of newborn calves are known to be associated with the occurrence of omphalitis. Various bacteria, such as members of family Enterobactericeae and Gram-positive cocci, have also been reported to cause omphalitis (1–3). Hence, the identification of the causative organism may be necessary for effective treatment.
Trueperella pyogenes is a Gram-positive, pleomorphic, non-spore-forming, non-motile, and facultatively anaerobic bacterium that has been identified as a commensal and opportunistic pathogen found in the mucous membranes of the upper respiratory, gastrointestinal, and urogenital tracts of farm animals (4–6). T. pyogenes is a major causative agent of mastitis, metritis, liver abscesses, lymphadenitis, otitis, peritonitis, pneumonia, pyodermitis, osteomyelitis, arthritis, endocarditis, umbilical thickening, and septicemia (4, 5, 7–9). T. pyogenes infections have been associated with decreased milk production and low quality of meat products, leading to severe economic losses in the livestock industry (10). The pathogenicity of T. pyogenes can be attributed to several virulence factors. Among them, pyolysin (plo), which is associated with tissue damage, has been considered the primary virulence factor of this pathogen, along with neuraminidases (nanH and nanP), fimbriae (fimA, fimC, fimE, and fimG), and collagen-binding protein (cbpA), which have been associated with virulence, mucosal adherence, and colonization (5). Sporadic cases of T. pyogenes infections that result in endocarditis and soft tissue infections have also been reported in humans (11, 12).
The umbilical cord is an organ that transmits nutrients and oxygen between the mother and her fetus (13). Following parturition, the umbilical cord of the calf that is cut and left behind dries and detaches, leaving a structure known as the navel (13). During this drying period, the umbilical cord is exposed to a contaminated environment that allows pathogens to easily enter the umbilical cord, causing infection of the urachus, umbilical arteries (omphaloarteritis), or umbilical vein (omphalophlebitis) (13). An infection in any or all umbilical structures (omphalitis) manifests as signs of inflammation, such as localized heat, swelling, purulent discharge, and pain, which may cause systemic infections, such as arthritis, peritonitis, pneumonia, uveitis, and even sepsis, due to the hematogenous spread of bacteria ascending along the structures of the umbilical cord (3).
Omphalitis is the third most common disease in newborn calves following neonatal diarrhea and bovine respiratory disease (BRD). Nevertheless, the available information regarding this condition in cattle is limited (14). We herein report a case of omphalitis caused by T. pyogenes, with subsequent pneumonia, arthritis, and liver abscess, in a Korean native calf, as well as present appropriate guidelines for its treatment. This case provides useful information that could help large animal clinicians diagnose and treat omphalitis, which is commonly observed on farms.
Case presentation
A 10-day-old female Hanwoo calf (Bos taurus coreanae) presented to our clinic with an enlarged umbilicus and right forelimb lameness as the main complaints. Failure of passive transfer (FPT) was diagnosed when the calf was 24 h of age, with a serum biochemical profile showing a total protein of 4.1 g/dL and globulin of 1.5 g/dL. The calf was also mildly anemic, as indicated by a hematocrit of 23.1% (Supplementary Figure S1). At the time of FPT diagnosis, the dam was not managing the calf, which did not exhibit any suckling reflex. A blood transfusion was performed at a dose of 15 mL/kg to treat the FPT, and the calf was subsequently reared on artificial milk. After the transfusion, the total protein increased slightly to 5.1 g/dL, but the calf was still weak and had no suckling reflex. Thus, it was force-fed 1,000–1,500 mL/day of replacement milk.
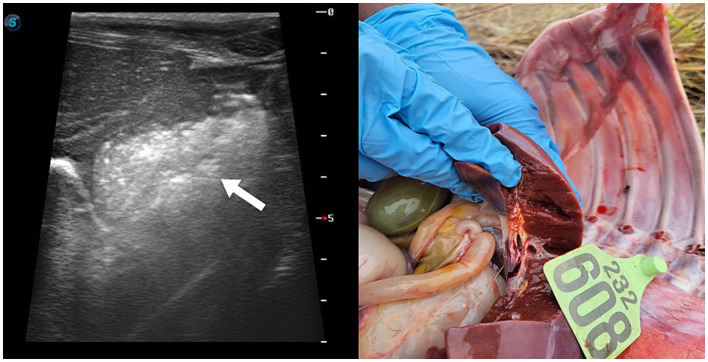
Upon arrival at the clinic, the calf had a temperature of 38.1°C, indicating mild hypothermia, a heart rate of 110 beats/min with tachycardia, and a respiratory rate of 47 cycles/min with tachypnea and open mouth breathing. Auscultation of the chest revealed crackles in the anterior lobe of the right lung. Swelling was observed at the knee of the right forelimb, which was warm to the touch. In addition, purulent material was noted on the umbilicus, which was warm but not painful. Ultrasonography revealed hyperechoic pus in the right 9th and 10th intercostals, adjacent to the liver and umbilical vein (Figure 1), as well as bladder distention. Based on the examination findings, a liver abscess due to omphalophlebitis was suspected, for which surgery was indicated. However, the calf’s condition failed to stabilize. After 3 days of treatment with penicillin at 22,000 IU/kg, surgical repair was planned. Unfortunately, the calf died within 2 h of diagnosis.
Figure 1.
Ultrasonography images showing a hyperechoic lesion at adjacent to the liver and umbilical vein in the 10-day-old female Hanwoo calf.
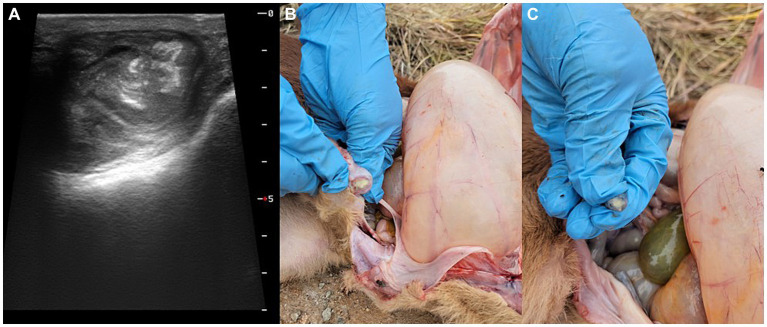
At necropsy, the umbilical cord and bladder ligament, where purulent substances were observed, were collected and transported to the laboratory (Figure 2). After the umbilical cord was incised, a greenish-yellow substance was found on the inner surface. The internal suppurative material was cultured on blood agar plates for 48 h at 37°C under facultative anaerobic condition. The colony was white, smooth, and glossy and surrounded by a narrow zone of hemolysis. Morphologically, distinct colonies were selected, and Gram-staining was performed, which confirmed the presence of Gram-positive bacteria (Figure 3). The single colony of Gram-positive bacteria was inoculated into brain heart infusion (BHI) broth and incubated overnight at 37°C. The bacterial DNA was extracted using the QIAamp UCP Pathogen Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. T. pyogenes isolate (provided by Yong-Il Cho, Sunchon National University, Sunchon, ROK) was cultivated at 37°C in BHI broth and used as positive control (15).
Figure 2.
Gross lesions of the umbilical cord and bladder ligaments vein in the 10-day-old female Hanwoo calf presenting (A) umbilical enlargement, (B) umbilical vein, and (C) umbilical artery with purulent discharge.
Figure 3.
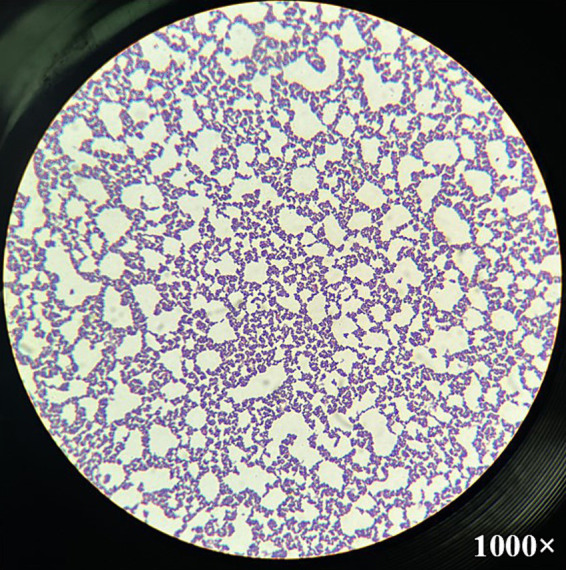
Microscopic image of Trueperella pyogenes after Gram-staining. Gram-positive coccobacilli were observed from the single colony cultured in greenish-yellow purulent discharge from the umbilical cord at magnification 1,000×.
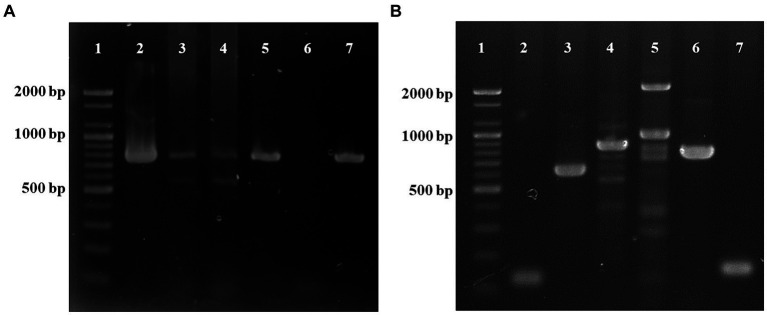
DNA was extracted from the umbilical cord, umbilical vein, umbilical artery, lungs, kidneys, intestines, mesenteric lymph nodes, and bladder using the DNeasy Blood & Tissue Kit (Qiagen) according to the manufacturer’s instructions. Polymerase chain reaction (PCR) analysis was conducted to detect the genes of T. pyogenes infection. For detection of the plo gene, a specific primer set (F: 5′-CAGTCAAGGTGAGTGAGTGGAAA-3′ and R: 5′-CTTGAACTGGGAAA-3′) was used (16). The predicted sized of the amplicon was 773 bp using the following cycling conditions: 94°C for 5 min, followed by 40 cycles at 94°C for 30 s, 47°C for 30 s, and 72°C for 50 s. Distilled water was included in each PCR run as a negative control. T. pyogenes was detected in all the samples examined (Figure 4A). Escherichia coli was also tested from all the tissue samples (17), and the hemolysin gene was found only in the kidney and intestine.
Figure 4.
PCR analysis for the detection of Trueperella pyogenes using the plo gene (773 bp) (A). Lane 1: ladder (1 kb), lane 2: umbilical cord, lane 3: lung, lane 4: mesenteric lymph nodes, lane 5: bladder, lane 6: negative control, lane 7: positive control. PCR analysis of virulent genes (B). Lane 1: ladder (1 kb), lane 2: collagen-binding protein (cbpA, 124 bp), lane 3: type A fimbriae (fimA, 605 bp), lane 4: type C fimbriae (fimC, 843 bp), lane 5: type G fimbriae (fimG, 929 bp), lane 6: neuraminidase H (nanH, 781 bp), lane 7: neuraminidase P (nanP, 150 bp).
Additionally, as gross lesions were markedly found in the lungs, PCR analysis was also performed to detect the main pathogens causing BRD, such as Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, E. coli, and T. pyogenes. However, no other bacteria besides T. pyogenes were detected in the lungs. All PCR products were separated by electrophoresis on 1.2% agarose gels and visualized after staining with ethidium bromide. The T. pyogenes-positive PCR products were purified using the Accupower PCR Purification Kit (Bioneer, Daejeon, ROK) according to the manufacturer’s instructions and directly sequenced (Bioneer). The nucleotide sequence obtained in this study was aligned using the BioEdit software and then compared with reference sequences from the National Center for Biotechnology Information database1 to determine its similarity using Geneious Prime 2022.2 software. 2 Phylogenetic analysis of T. pyogenes was performed using the maximum-likelihood method implemented in MEGA11 using the best substitution model. Bootstrap values were calculated by analyzing 1,000 replicates to evaluate the reliability of clusters, and the sequence obtained in this calf was grouped with the reference strains of T. pyogenes (Supplementary Figure S2). Our sequence showed 97.3–99.1% identity to those detected in other countries. The nucleotide sequence obtained in this study was assigned accession number PP731570.
Subsequently, the presence of virulence genes, including cbpA, fimC, fimA, fimG, nanP, nanH, and plo, was investigated in T. pyogenes-positive isolates using specific primers (Supplementary Table S1), and all the virulence genes were identified (Figure 4B).
Antimicrobial susceptibility tests of the isolated T. pyogenes were conducted using the disk diffusion method on Mueller-Hilton agar with the following 12 antibacterial disks: amoxicillin (10 μg), penicillin G (10 IU), ceftiofur (30 μg), oxytetracycline (30 μg), florfenicol (30 μg), enrofloxacin (5 μg), ofloxacin (5 μg), ciprofloxacin (5 μg), erythromycin (15 μg), streptomycin (10 μg), lincomycin (2 μg), and clindamycin (2 μg). The results of antimicrobial susceptibility tests were recorded after 48 h of incubation. For amoxicillin, penicillin G, ceftiofur, florfenicol, enrofloxacin, ofloxacin, ciprofloxacin, erythromycin, lincomycin, and clindamycin, criteria for T. pyogenes were used to interpret the results and catalog the strains as susceptible or non-susceptible according to CLSI (2023). For the rest of antimicrobial agents, no standards are available. Accordingly, the T. pyogenes isolates obtained from omphalitis were found to be susceptible to amoxicillin, ceftiofur, florfenicol, enrofloxacin, ofloxacin, and ciprofloxacin but showed high resistance to the remaining antibiotics (Supplementary Table S2).
Discussion
In newborn calves, the umbilicus, along with the gastrointestinal and respiratory tracts can serve as entry points for infection. Several pathogens cause infectious neonatal diseases by exploiting the umbilicus as the main route of entry. Omphalitis contributes significantly to neonatal morbidity in cattle (18, 19). Previous studies have shown that umbilical infections occur in 1.3 to 14% of dairy calves (20, 21). According to a recent study, omphalitis is the most commonly detected disorder in pre-weaned calves, with the largest proportion being 15.9% at herd level (22). Additionally, omphalitis has been found to cause 23% of preslaughter mortality and 54% of post-slaughter in veal calves (23). Although omphalitis can cause growth retardation, increased susceptibility to other diseases, and higher mortality rates, this has not garnered much attention from farmers and veterinarians.
Good sanitation at parturition, maternity pen hygiene, cleanliness of the calf environment, proper colostrum intake, and umbilical disinfection have been reported as management practices that reduce the incidence of omphalitis in newborn calves (24). In the current case, the calf was diagnosed with FPT, with a total serum protein of 4.1 g/dL. Despite receiving blood transfusion, this calf still exhibited signs of FPT and no suckling reflex. The calf was not born in dystocia, had a well-maintained and hygienic maternity pen, and did not spend much time in the cow-calf barn due to artificial lactation. Although FPT was considered to cause omphalitis, recent studies have reported that FPT does not cause omphalitis (14). However, the occurrence of omphalitis in a calf with FPT, as in the current case, is believed to increase septicemia through hematogenous transmission.
The distinction between local and systemic omphalitis infection in newborn calves is clinically important because these differences can affect both prognosis and treatment decisions. The diagnosis of omphalitis in neonatal calves is primarily based on palpation, ultrasonography, and aspiration (25–27). Palpation is probably the most frequently used approach in clinical practice given its simplicity and availability. In cases of omphalophlebitis or omphaloarteritis, which are usually not localized infections, deep palpation may reveal dilated veins or signs of pain in the calf. In this case, however, while the calf exhibited both omphalophlebitis and omphaloarteritis, it did not show any signs of pain on palpation. In this case, dilatation of the umbilical vein or arteries was not palpable on deep palpation and was not observed on ultrasonography. At necropsy, pus was found in the umbilical vein and arteries, but no dilatation could be seen in both. This may be explained by the result of chronic infection with omphalitis. The calf was primarily in sternal or lateral recumbency and was very depressed. As demonstrated in the current case, ultrasonography should be considered as an adjunct to visualization and palpation in septic calves. Indeed, studies have shown that ultrasonography can be used to determine the site and extent of the infection, as well as detect the dilatation of the umbilical veins and arteries with hypo/hyper echogenic fluid (26–29).
In the current case, the bacteria detected in the umbilical cords were identified as T. pyogenes by PCR method, and this was confirmed by sequencing analysis. However, a limitation of our study is that biochemical tests, including urease, oxidase, catalase, nitrate reduction, gelatin hydrolysis, and fermentation of sucrose, mannitol, maltose, lactose, glucose, and xylose, for identification of T. pyogenes were not performed. Nevertheless, the possibility of T. pyogenes as the causative organism can be considered by colony morphology and Gram-staining results. In general, the initial identification of bacteria is based on cell morphology and colony characteristics (6). The colonies cultured in the umbilical cords typically exhibited beta-hemolysis zones with hemolytic rings on blood agar and the glistering colonies were morphologically identified as T. pyogenes. In addition to morphological characteristics (30), the clinical presentation of the calf, particularly the presence of hyperechoic pus, pointed to a potential T. pyogenes infection. T. pyogenes is a common pathogen implicated in various clinical manifestations among farm animals (8, 31), which it is an important opportunistic pathogen, particularly associated with suppurative infections (4, 6, 8). Its prevalence has recently been increasing worldwide (32). However, reports of omphalitis caused by T. pyogenes in cattle have been very limited (8), with only one related case described in foals (3). The present study demonstrated that T. pyogenes can cause omphalitis. Currently, the transmission route of this bacteria remains unclear. However, we assumed that this calf became infected by contact between the umbilical cord and contaminated utensils or environment. Another possible route of entry for this pathogen could be the gastrointestinal tract, indicating that infection may be caused by dissemination of septicemia in the vessels.
Moreover, all genes for virulence factors were identified in this calf. Studies have been shown differences in the expressions of genes encoding virulence factors among various clinical manifestations (5). In particular, plo and fimA virulence factors have been identified as highly crucial to the pathogenesis of T. pyogenes infection and useful genetic markers in the detection of this pathogen in clinical samples (5, 33). Considering that other virulence genes aside from these two were expressed in the current case, the T. pyogenes detected in this study can be considered virulent. Although few reports have been available on T. pyogenes infections in humans, farm animals have been considered a major source of human infection. Hence, the risk to public health due to pathogenic T. pyogenes through animal-to-human transmission should not be overlooked.
The clinical course of these suppurative infections caused by T. pyogenes may be severe, with misdiagnosis or inappropriate treatment only leading to increased mortality rates (6). As such, bacterial identification and antimicrobial susceptibility tests should be performed to decide the appropriate treatment plan. Generally, cephalosporins, penicillin and other beta-lactams, tetracyclines, and macrolides are the first line of antimicrobial therapies of choice for T. pyogenes infections in livestock (2, 27, 34). However, the T. pyogenes isolates identified in the current case were found to be mainly resistant to penicillin G, oxytetracycline, erythromycin, streptomycin, lincomycin, and clindamycin but susceptible to amoxicillin, ceftiofur, florfenicol, enrofloxacin, ofloxacin, and ciprofloxacin. Moreover, previous studies have reported that T. pyogenes are resistant to macrolides, tetracyclines, and beta-lactam antibiotics (33, 35–37), which is somewhat consistent with our results. Although macrolides and penicillin had been frequently used to treat T. pyogenes infections in the past, the inappropriate use of antimicrobials increases the selection rate of multidrug-resistant bacteria. Especially in the current case, the resistance of T. pyogenes to penicillin G may be attributed to its inefficacy against slow-growing bacteria and inability to infiltrate biological membranes. Quinolones (e.g., enrofloxacin, ofloxacin, and ciprofloxacin) and amphenicols (e.g., florfenicol) primarily targeted Gram-negative bacteria and have been approved for the treatment of BRD in cattle. Interestingly, these antibiotics were found to be effective against T. pyogenes in this case. Therefore, the appropriate and responsible use of antimicrobials for farm animals can prevent the emergence of multidrug-resistant bacterial pathogens and mitigate one health concerns.
Conclusion
This case describes the occurrence of omphalitis caused by T. pyogenes in a 10-day-old Hanwoo calf. All the virulence genes were detected in this case. Moreover, T. pyogenes isolated in this study are susceptible to amoxicillin, ceftiofur, florfenicol, enrofloxacin, ofloxacin, and ciprofloxacin, suggesting the use of these antibiotics as the first line of treatment for omphalitis caused by T. pyogenes to prevent multidrug resistance.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by this study was approved by the Institutional Animal Care and Use Committee (IACUC) at Kyungpook National University (No. KNU-2023-0280). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YK: Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. MJJ: Data curation, Formal analysis, Investigation, Validation, Visualization, Writing – original draft. JP: Validation, Resources, Writing – review & editing. KSC: Conceptualization, Formal analysis, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the National Research Foundation of Korea (NRF), which is funded by the Mid-Career Research Program (grant No. NRF-2021R1A2C1011579).
Footnotes
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1362352/full#supplementary-material
References
- 1.Ortega J, Daft B, Assis RA, Kinde H, Anthenill L, Odani J, et al. Infection of internal umbilical remnant in foals by Clostridium sordellii. Vet Pathol. (2007) 44:269–75. doi: 10.1354/vp.44-3-269, PMID: [DOI] [PubMed] [Google Scholar]
- 2.Radostitis OM, Gay CC, Hinchcliff KW, Constable P. (2007). Veterinary medicine: a textbook of the disease of cattle, sheep, goats, pigs, and horses. Philadelphia, PA: Saunders Elsevier. 722–724. [Google Scholar]
- 3.Rampacci E, Passamonti F, Bottinelli M, Stefanetti V, Cercone M, Nannarone S, et al. Umbilical infections in foals: microbiological investigation and management. Vet Rec. (2017) 180:543. doi: 10.1136/vr.103999, PMID: [DOI] [PubMed] [Google Scholar]
- 4.Ashrafi Tamai I, Mohammadzadeh A, Zahraei Salehi T, Mahmoodi P, Pakbin B. Investigation of antimicrobial susceptibility and virulence factor genes in Trueperella pyogenes isolated from clinical mastitis cases of dairy cows. Food Sci Nutr. (2021) 9:4529–38. doi: 10.1002/fsn3.2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Risseti RM, Zastempowska E, Twaruzek M, Lassa H, Pantoja JCF, de Vargas APC, et al. Virulence markers associated with Trueperella pyogenes infections in livestock and companion animals. Lett Appl Microbiol. (2017) 65:125–32. doi: 10.1111/lam.12757, PMID: [DOI] [PubMed] [Google Scholar]
- 6.Rzewuska M, Kwiecień E, Chrobak-Chmiel D, Kizerwetter-Świda M, Stefańska I, Gieryńska M. Pathogenicity and virulence of Trueperella pyogenes: a review. Int J Mol Sci. (2019) 20:2737. doi: 10.3390/ijms20112737, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujimoto H, Nakamura T, Sato A, Chuma T. Antimicrobial susceptibility of Trueperella pyogenes isolated from cattle and pigs with septicemia in southern Kyushu, Japan. J Vet Med Sci. (2023) 85:379–82. doi: 10.1292/jvms.22-0460, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ribeiro MG, Risseti RM, Bolanos CA, Caffaro KA, de Morais AC, Lara GH, et al. Trueperella pyogenes multispecies infections in domestic animals: a retrospective study of 144 cases (2002 to 2012). Vet Q. (2015) 35:82–7. doi: 10.1080/01652176.2015.1022667, PMID: [DOI] [PubMed] [Google Scholar]
- 9.Zhang D, Zhao J, Wang Q, Liu Y, Tian C, Zhao Y, et al. Trueperella pyogenes isolated from dairy cows with endometritis in Inner Mongolia, China: tetracycline susceptibility and tetracycline-resistance gene distribution. Microb Pathog. (2017) 105:51–6. doi: 10.1016/j.micpath.2017.02.010, PMID: [DOI] [PubMed] [Google Scholar]
- 10.Maan MK, Sattar A, Mi K, Bakr Shabbir MA, Xie S, Xin L, et al. Integration of PK/PD for dose optimization of aditoprim against Trueperella pyogenes causing endometritis in bovines. Microb Pathog. (2020) 142:104097. doi: 10.1016/j.micpath.2020.104097, PMID: [DOI] [PubMed] [Google Scholar]
- 11.Kavitha K, Latha R, Udayashankar C, Jayanthi K, Oudeacoumar P. Three cases of Arcanobacterium pyogenes-associated soft tissue infection. J Med Microbiol. (2010) 59:736–9. doi: 10.1099/jmm.0.016485-0, PMID: [DOI] [PubMed] [Google Scholar]
- 12.Plamondon M, Martinez G, Raynal L, Touchette M, Valiquette L. A fatal case of Arcanobacterium pyogenes endocarditis in a man with no identified animal contact: case report and review of the literature. Eur J Clin Microbiol Infect Dis. (2007) 26:663–6. doi: 10.1007/s10096-007-0354-9 [DOI] [PubMed] [Google Scholar]
- 13.Van Camp MB, Renaud DL, Duffield TF, Gomez DE, McFarlane WJ, Marshall J, et al. Describing and characterizing the literature regarding umbilical health in intensively raised cattle: a scoping review. Vet Sci. (2022) 9:288. doi: 10.3390/vetsci9060288, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perrot F, Joulié A, Herry V, Masset N, Lemaire G, Barral A, et al. Failure of passive immunity transfer is not a risk factor for omphalitis in beef calves. Vet Sci. (2023) 10:544. doi: 10.3390/vetsci10090544, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aftabuzzaman M, Espiritu H, Kim SH, Mamuad L, Jin SJ, Lee SS, et al. Characterization of Trueperella pyogenes isolated from caseous lymphadenitis lesions in Korean native goats. Korean J Vet Serv. (2021) 44:321–6. doi: 10.7853/kjvs.2021.44.4.321 [DOI] [Google Scholar]
- 16.Zhang W, Liu X, Liu M, Ma B, Xu L, Wang J. Development of a multiplex PCR for simultaneous detection of Pasteurella multocida, Mannheimia haemolytica and Trueperella pyogenes. Acta Vet Hung. (2017) 65:327–39. doi: 10.1556/004.2017.032, PMID: [DOI] [PubMed] [Google Scholar]
- 17.Ryu JH, Kim S, Park J, Choi KS. Characterization of virulence genes in Escherichia coli strains isolated from pre-weaned calves in the Republic of Korea. Acta Vet Scand. (2020) 62:45. doi: 10.1186/s13028-020-00543-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miessa L, Silva A, Botteon R, Botteon P. Morbidity and mortality by umbilical cord inflammation in dairy calves. Hora Vet. (2003) 23:16–8. [Google Scholar]
- 19.Steerforth DD, Van Winden S. Development of clinical sign-based scoring system for assessment of omphalitis in neonatal calves. Vet Rec. (2018) 182:549. doi: 10.1136/vr.104213 [DOI] [PubMed] [Google Scholar]
- 20.Hathaway SC, Bullians JA, Johnstone AC, Biss ME, Thompson A. A pathological and microbiological evaluation of omphalophlebitis in very young calves slaughtered in New Zealand. N Z Vet J. (1993) 41:166–70. doi: 10.1080/00480169.1993.35763, PMID: [DOI] [PubMed] [Google Scholar]
- 21.Svensson C, Lundborg K, Emanuelson U, Olsson SO. Morbidity in Swedish dairy calves from birth to 90 days of age and individual calf-level risk factors for infectious diseases. Prev Vet Med. (2003) 58:179–97. doi: 10.1016/s0167-5877(03)00046-1, PMID: [DOI] [PubMed] [Google Scholar]
- 22.Dachrodt L, Bartel A, Arndt H, Kellermann LM, Stock A, Volkmann M, et al. Benchmarking calf health: assessment tools for dairy herd health consultancy based on reference values from 730 German dairies with respect to seasonal, farm type, and herd size effects. Front Vet Sci. (2022) 9:990798. doi: 10.3389/fvets.2022.990798, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas GW, Jordaan P. Pre-slaughter mortality and post-slaughter wastage in bobby veal calves at a slaughter premises in New Zealand. N Z Vet J. (2013) 61:127–32. doi: 10.1080/00480169.2012.734374, PMID: [DOI] [PubMed] [Google Scholar]
- 24.Mee JF, Berry DP, Cromie AR. Prevalence of, and risk factors associated with, perinatal calf mortality in pasture-based Holstein-Friesian cows. Animal. (2008) 2:613–20. doi: 10.1017/S1751731108001699, PMID: [DOI] [PubMed] [Google Scholar]
- 25.Anderson DE, Rings DM. Current veterinary therapy: food animal practice. St. Louis, MO, USA: Saunders Elsevier; (2009). [Google Scholar]
- 26.House JK, Smith GW, Gunn AA, McGuirk SM, Izzo M. Manifestations and management of disease in neonatal ruminants In: Smith BP, Pusterla N, editors. Large animal internal medicine. St. Louis, MO, USA: Mosby; (2020) 335–81. [Google Scholar]
- 27.Peek SF, Divers TJ. Rebhun’s diseases of dairy cattle. Amsterdam, Netherlands: Elsevier; (2018). [Google Scholar]
- 28.O'Brien RT, Forrest LJ. A retrospective study of umbilical sonography in calves. Vet Radiol Ultrasound. (1996) 37:63–47. doi: 10.1111/j.1740-8261.1996.tb00815.x [DOI] [Google Scholar]
- 29.Steiner A, Lejeune B. Ultrasonographic assessment of umbilical disorders. Vet Clin North Am Food Anim Pract. (2009) 25:781–94. doi: 10.1016/j.cvfa.2009.07.012, PMID: [DOI] [PubMed] [Google Scholar]
- 30.Qin L, Meng F, He H, Yang YB, Wang G, Tang YD, et al. A virulent Trueperella pyogenes isolate, which causes severe bronchoconstriction in porcine precision-cut lung slices. Front Vet Sci. (2022) 8:824349. doi: 10.3389/fvets.2021.824349, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rzewuska M, Stefanska I, Osinska B, Kizerwetter-Swida M, Chrobak D, Kaba J, et al. Phenotypic characteristics and virulence genotypes of Trueperella (Arcanobacterium) pyogenes strains isolated from European bison (Bison bonasus). Vet Microbiol. (2012) 160:69–76. doi: 10.1016/j.vetmic.2012.05.004, PMID: [DOI] [PubMed] [Google Scholar]
- 32.Morley PS. 313 new insights on liver abscess syndrome in cattle using metagenomic investigations. J Anim Sci. (2020) 98:53. doi: 10.1093/jas/skaa278.095 [DOI] [Google Scholar]
- 33.Rezanejad M, Karimi S, Momtaz H. Phenotypic and molecular characterization of antimicrobial resistance in Trueperella pyogenes strains isolated from bovine mastitis and metritis. BMC Microbiol. (2019) 19:305. doi: 10.1186/s12866-019-1630-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quinn PJ, Markey BK, Leonard FC, Hartigan PJ, Fanning S, Fitzpatrick ES. Veterinary microbiology and microbial disease. Hoboken: Wiley-Blackwell; (2011) 245–257. [Google Scholar]
- 35.Ashrafi Tamai I, Mohammadzadeh A, Zahraei Salehi T, Mahmoodi P. Genomic characterisation, detection of genes encoding virulence factors and evaluation of antibiotic resistance of Trueperella pyogenes isolated from cattle with clinical metritis. Antonie Van Leeuwenhoek. (2018) 111:2441–53. doi: 10.1007/s10482-018-1133-6, PMID: [DOI] [PubMed] [Google Scholar]
- 36.Santos TM, Caixeta LS, Machado VS, Rauf AK, Gilbert RO, Bicalho RC. Antimicrobial resistance and presence of virulence factor genes in Arcanobacterium pyogenes isolated from the uterus of postpartum dairy cows. Vet Microbiol. (2010) 145:84–9. doi: 10.1016/j.vetmic.2010.03.001, PMID: [DOI] [PubMed] [Google Scholar]
- 37.Zastempowska E, Lassa H. Genotypic characterization and evaluation of an antibiotic resistance of Trueperella pyogenes (Arcanobacterium pyogenes) isolated from milk of dairy cows with clinical mastitis. Vet Microbiol. (2012) 161:153–8. doi: 10.1016/j.vetmic.2012.07.018, PMID: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.