Author's summary
Vaccine-related myocarditis (VRM) is a rare but significant adverse event associated primarily with mRNA vaccines against coronavirus disease 2019 (COVID-19). This review explores the incidence, risk factors, clinical presentation, pathogenesis, management strategies, and outcomes associated with COVID-19 VRM. The incidence of VRM is notably higher in male adolescents and young adults, especially after the second dose of mRNA vaccines. The pathogenesis appears to involve an immune-mediated process, but the precise mechanism remains mostly unknown so far. Most studies have suggested that VRM is mild and self-limiting, and responds well to conventional treatment. However, a recent nationwide study in Korea warns that severe cases, including fulminant myocarditis or death, are not uncommon in patients with COVID-19 VRM. This review also briefly addresses the critical balance between the substantial benefits of COVID-19 vaccination and the rare risks of VRM in the coming endemic era. It emphasizes the need for continued surveillance, research to understand the underlying mechanisms, and strategies to mitigate risk. Filling these knowledge gaps would be vital to refining vaccination recommendations and improving patient care in the evolving COVID-19 pandemic landscape.
Keywords: COVID-19, Vaccines, Myocarditis
Abstract
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 has led to a global health crisis with substantial mortality and morbidity. To combat the COVID-19 pandemic, various vaccines have been developed, but unexpected serious adverse events including vaccine-induced thrombotic thrombocytopenia, carditis, and thromboembolic events have been reported and became a huddle for COVID-19 vaccination. Vaccine-related myocarditis (VRM) is a rare but significant adverse event associated primarily with mRNA vaccines. This review explores the incidence, risk factors, clinical presentation, pathogenesis, management strategies, and outcomes associated with VRM. The incidence of VRM is notably higher in male adolescents and young adults, especially after the second dose of mRNA vaccines. The pathogenesis appears to involve an immune-mediated process, but the precise mechanism remains mostly unknown so far. Most studies have suggested that VRM is mild and self-limiting, and responds well to conventional treatment. However, a recent nationwide study in Korea warns that severe cases, including fulminant myocarditis or death, are not uncommon in patients with COVID-19 VRM. The long-term cardiovascular consequences of VRM have not been well understood and warrant further investigation. This review also briefly addresses the critical balance between the substantial benefits of COVID-19 vaccination and the rare risks of VRM in the coming endemic era. It emphasizes the need for continued surveillance, research to understand the underlying mechanisms, and strategies to mitigate risk. Filling these knowledge gaps would be vital to refining vaccination recommendations and improving patient care in the evolving COVID-19 pandemic landscape.
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is a highly contagious disease caused by the virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1) It quickly spread worldwide after the first case emerged in late 2019, posing a significant public health challenge due to its high mortality and morbidity rates, as well as its substantial socio-economic cost.2)
To prevent COVID-19, new vaccines against SARS-CoV-2 have been developed and are being rapidly used worldwide.3) The European Medicines Agency has authorized the use of the first 4 vaccine preparations, including Oxford-AstraZeneca’s ChAdOx1, Johnson & Johnson’s AD26.COV2·S, Pfizer’s BNT162b2, and Moderna’s mRNA-1273.4) ChAdOx1 and AD26.COV2·S are adenoviral vaccines composed of recombinant adenovirus vectors that contains a codon-optimized DNA encoding the spike protein of SARS-CoV-2. BNT162b2 and mRNA-1273 vaccines are mRNA vaccines that consist of lipid nanoparticle envelope containing a strand of nucleoside-modified mRNA encoding the viral spike protein of SARS-CoV-2. The recombinant spike proteins stimulate the production of antibodies against SARS-CoV-2, which in turn helps prevent COVID-19 by these increased antibodies.
While clinical trials have proven the safety of various COVID-19 vaccines with a large number of subjects, challenges have emerged in the field. These include doubts about vaccine effectiveness, vaccine hesitancy, and the emergence of immune-evading mutant viruses following the introduction of novel vaccines.3)
The primary cause of vaccine hesitancy is thought to be concerns about vaccine side effects. Several COVID-19 vaccines have been developed using innovative platforms, which may result in newly reported adverse reactions. Thrombosis with thrombocytopenia syndrome has been a common side effect of adenovirus-vectored vaccines, while myocarditis and pericarditis have been associated with mRNA vaccines.5),6),7),8)
This review explores the incidence, pathogenesis, clinical presentation, diagnosis, management strategies, and outcomes of COVID-19 vaccine-related myocarditis (VRM) based on the published studies. This review also briefly discusses the critical balance between the substantial benefits of COVID-19 vaccination and the rare risks of VRM in the upcoming endemic era, as well as the unmet needs concerning COVID-19 VRM.
CORONAVIRUS DISEASE 2019 MYOCARDITIS VERSUS CORONAVIRUS DISEASE 2019 VACCINE-RELATED MYOCARDITIS
Myocarditis is an inflammation of the myocardium that can cause structural abnormalities in the myocardium, leading to a range of clinical presentations from asymptomatic to heart failure and death.9),10) It can be caused by various infectious agents, most commonly viral infections, autoimmune diseases, environmental toxins, and, in rare cases, as an adverse reaction to drugs or vaccines.
Myocarditis may develop during a COVID-19 infection due to direct viral invasion and endothelial dysfunction. The Centers for Disease Control and Prevention (CDC) reports that the risk of developing myocarditis during the course of a SARS-CoV-2 infection is approximately 150 per 100,000 individuals.11) The reported incidence of COVID-19 myocarditis has been reported to range from 1,000 to 4,000 cases per 100,000 patients, according to the Vaccine Adverse Event Reporting System (VAERS), a national early warning system for detecting potential safety problems with US-licensed vaccines.12) Therefore, COVID-19 has become a potentially important cause of myocarditis.
Following the introduction of COVID-19 vaccines, cases of acute myocarditis with non-viral causes have been reported. These vaccines have also been identified as potential causes of myocarditis.13) The reported incidence of COVID-19 VRM varies depending on the type of vaccines, age, and sex. According to VAERS data, the incidence ranges from approximately 0.3 to 5.0 cases per 100,000 vaccinated individuals.13)
To diagnosis VRM, it is important to exclude COVID-19 infection as the risk of myocarditis is significantly higher in individuals with COVID-19 infection than in those who have received the COVID-19 infection than in those who have received the COVID-19 vaccination. Table 1 summarized the differences between COVID-19 infection and VRM.
Table 1. Differentiation between COVID-19 myocarditis and COVID-19 VRM.
| Timing | Incidence | Severity and prognosis | Possible mechanism | |
|---|---|---|---|---|
| COVID-19 myocarditis | During the course of a COVID-19 infection, often during the acute phase of illness | • 150/100,000 by US CDC11) | • Survival: 30–80% | • Direct viral invasion |
| • 1,000 to 4,000/100,000 by US VAERS | • Can vary in severity and may be associated with more severe COVID-19 cases | • Immune reaction | ||
| • Endothelial dysfunction secondary to infection of adjacent cells or increased inflammation | ||||
| • Genetic susceptibility | ||||
| COVID-19 VRM | A few days to weeks after receiving a COVID-19 vaccine | • 0.3–5.0/100,000 vaccinated people | • Survival: >99% | • Immune response |
| • Depending on the vaccine platform, age, and sex | • Generally mild and self-limiting, with most individuals recovering fully with appropriate medical care | • Sex-related factor | ||
| • Predominantly in males aged 12 to 40 years | • Rarely, fulminant cases needing ICU care, or transplantation | • Genetic susceptibility |
CDC = Center for Disease Control and Prevention; COVID-19 = coronavirus disease 2019; ICU = intensive care unit; VAERS = Vaccine Adverse Event Reporting System; VRM = vaccine-related myocarditis.
PATHOGENESIS OF CORONAVIRUS DISEASE 2019 VACCINE-RELATED MYOCARDITIS
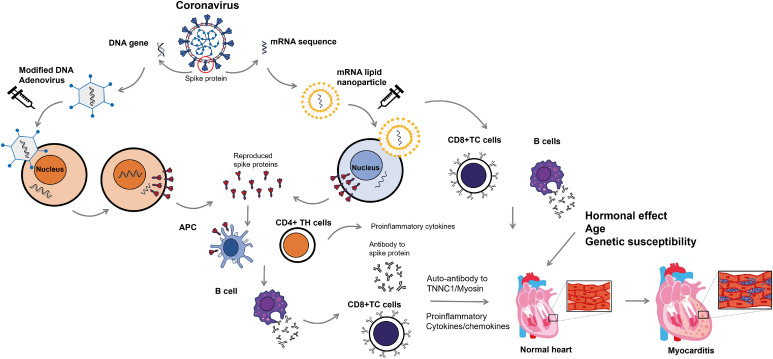
The pathophysiologic mechanisms of VRM are not fully understood. Possible mechanisms include a vaccine-induced immune response, molecular mimicry between self-antigens and viral antigens, and factors such as host age, sex, and genetic predisposition (Figure 1).
Figure 1. Proposed pathophysiologic mechanism of coronavirus disease 2019 vaccine-related myocarditis. Vaccines for severe acute respiratory syndrome coronavirus-2 use the viral spike protein as their target. Adenoviral vaccines use a modified form of DNA adenovirus to elicit an immune response to the viral spike proteins. Upon injection, the DNA is released into the cytoplasm and later migrates into the cell nucleus without being incorporated into the cellular DNA. DNA produces mRNA that codes for viral spike proteins. In contrast, mRNA vaccines use mRNA for viral spike protein that is included in lipid nanoparticles. The mRNA then migrates back into the cytoplasm. After injection of these vaccines, the mRNA inserts itself into the cytoplasm, where it synthesizes the viral spike proteins via interaction with ribosomes. Viral spike proteins can elicit an immune response from the host’s helper T cells (CD4+ TH) and APC, resulting in the production of antibodies against viral spike proteins by B cells. Infected cells are attacked by cytotoxic T cells (CD8+ TC) coated with antibodies against viral spike proteins. It is important to note that antibodies against spike proteins may also have cross-reactivity with TNNC1 and myosin, potentially leading to an attack on the myocardium. Increased immune responses, including elevated serum concentrations of proinflammatory cytokines and chemokines, can enhance the attack. In females, sex hormones such as estrogen inhibit T cell activity and reduce the immune response. Additionally, genetic factors may contribute to the pathogenesis of myocarditis.
APC = antigen-presenting cells; TC = cytotoxic T cells; TH = helper T cells; TNNC1 = cardiac troponin C1.
Vaccines against COVID-19 typically encode similar forms of the SARS-CoV-2 viral spike glycoproteins, which can activate both innate and adaptive immune responses.14) Antibodies can be produced against these spike proteins after the introduction of mRNA or DNA encoding them. It is worth noting that there is molecular mimicry between the viral spike glycoprotein and myosin heavy chain or cardiac troponin C1. Myocardial inflammation can be induced by the interaction between antibodies against the spike protein and cardiac antigens. Additionally, mRNA molecules in mRNA vaccines can activate the immune response.15),16) Some of these immune responses can activate cardiotropic clones of T and B cells, which can subsequently damage the myocardium.
Vaccines may enhance self-reactive T-cell responses and exacerbate pre-existing autoimmune cardiac diseases, increasing the risk of severe myocarditis in affected patients. It is believed that sex hormones, particularly in genetically susceptible men, may contribute to the higher incidence of VRM in young men.14) Normally, estrogen inhibits proinflammatory T-cell responses, while testosterone activates specific helper T-cell responses.
EPIDEMIOLOGIC STUDIES FOR CORONAVIRUS DISEASE 2019 VACCINE-RELATED MYOCARDITIS
Following the introduction of the live attenuated smallpox vaccine, several cases of VRM have been reported.17) Interest in vaccine-related adverse events has increased, particularly after the introduction of COVID-19 vaccines using novel technology. Countries have established organizations to monitor these adverse events, including VAERS in the US. According to VAERS data, the peak incidence of VRM was observed in young males aged 15 to 17 years, particularly after the second dose. The incidence rate is approximately 0.3 to 5.0 per 100,000 COVID-19 vaccinated individuals.12),14)
Table 2 summarizes various epidemiological studies on COVID-19 VRM. The incidence of VRM can be influenced by factors such as vaccine type, age, and sex. Additionally, the reporting system (passive or active surveillance) and the definition of myocarditis used can affect the incidence. Therefore, readers should take these limitations into account when interpreting the data.
Table 2. Epidemiologic studies of COVID-19 vaccine-related myocarditis/myopericarditis.
| Authors | Type of study | Location | Number | Vaccine platform | Number of cases | Incidence | Case of FM | Method of case definition | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Straus et al.18) | Pharmacovigilance study | Worldwide | 252,000,000 | mRNA-1273: | 3,017 | Myocarditis/myopericarditis: | 44 (1.8%) | • Mostly voluntarily submitted event reports | |||||
| n=252,000,000 | 9.23/100,000 person-years | • Case verified by BC and CDC | |||||||||||
| 568,668,391 doses | 53.76/100,000 person-years in males aged <40 years, particularly those 18–24 years (RR, 3.10, 95% CI, 2.68–3.58) | ||||||||||||
| Usually after second dose | |||||||||||||
| Oster et al.19) | Population based study | US | 192,405,448 | BNT162b2: | 1,626 | BNT162b2: | 0 (12 needs ICU treatment, 2 with mechanical ventilation) | • VAERS | |||||
| n=114,246,837 | BNT162b2: 1,136 | 4.94/1,000,000 doses | |||||||||||
| 229,779,233 doses | mRNA-1273: 490 | 1.29/1,000,000 doses after first dose | |||||||||||
| mRNA-1273: | 9.71/1,000,000 doses after second dose | ||||||||||||
| n=78,158,611 | Male: 84.4% | ||||||||||||
| 144,321,612 doses | Males 12–15 years: 70.7/1,000,000 doses | ||||||||||||
| Males 16–17 years: 105.9/1,000,000 doses | |||||||||||||
| Males 18–24 years: 52.4/1,000,000 doses | |||||||||||||
| mRNA-1273: | |||||||||||||
| 3.40/1,000,000 doses | |||||||||||||
| 1.61/1,000,000 doses after first dose | |||||||||||||
| 5.09/1,000,000 doses after second dose | |||||||||||||
| Male: 76.7% | |||||||||||||
| Males 18–24 years: 56.3 per 1,000,000 doses | |||||||||||||
| Goddard et al.20) | Population based study | US | 2,403,307 (aged 18–39 years) | BNT162b2: | 79 | BNT162b2: | 0 | • Medical record review | |||||
| n=1,479,596 | BNT162b2: 41 | 14.2/1,000,000 doses | • CDC criteria | ||||||||||
| 2,891,498 doses | mRNA-1273: 38 | 4.7/1,000,000 doses after first dose | |||||||||||
| mRNA-1273: | 24.1/1,000,000 doses after second dose | ||||||||||||
| n=923,711 | Male: 88.0% | ||||||||||||
| 1,803,267 doses | mRNA-1273: | ||||||||||||
| 21.1/1,000,000 doses | |||||||||||||
| 9.7/1,000,000 doses after first dose | |||||||||||||
| 33.0/1,000,000 doses after second dose | |||||||||||||
| Male: 84.0% | |||||||||||||
| Simone et al.21) | Population based study | US | 2,392,924 | BNT162b2 (50%) | 15 | 0.8/1,000,000 after first dose | 0 | • Self-reporting from clinicians | |||||
| mRNA-1273 (50%) | BNT162b2: 8 | 5.8/1,000,000 after second dose | |||||||||||
| mRNA-1273: 7 | Male: 100% | ||||||||||||
| All under 40 years of age-most under 25 years | |||||||||||||
| Diaz et al.22) | Population based study | US | 2,000,287 | BNT162b2 (52.6%) | 20 | 1.0/100,000 | N/A | • Medical record review | |||||
| mRNA-1273 (44.1%) | BNT162b2: 9 | Male: 75% | |||||||||||
| Ad26.COV2.S (3.1%) | mRNA-1273: 11 | Usually under 40 years | |||||||||||
| Montgomery et al.23) | Population based study | US | 2,810,000 doses | 23 | 0.8/100,000 doses | 0 | • VAERS | ||||||
| BNT162b2: 7 | |||||||||||||
| mRNA-1273: 16 | |||||||||||||
| Canada government24) | Population based study | Canada | 28,769,834 | 99,034,764 doses | 1,207 | 1.22/100,000 doses | N/A | • Mostly voluntarily submitted event reports | |||||
| BNT1626b2: 713 | 97.8% associated with mRNA vaccines | • BC | |||||||||||
| mRNA-1273: 458 | BNT162b2: | ||||||||||||
| ChAdOx1: 17 | 54.3% of those associated with second dose | ||||||||||||
| BNT162b2-Omi.BA.4/BA.5: 5 | 66.2% male, median age 26 years | ||||||||||||
| mRNA-1273.214: 3 | mRNA-1273: | ||||||||||||
| mRNA-1273.222: 1 | 71.8% of those associated with second dose | ||||||||||||
| Ad26.COV2.S: 1 | 74.7% male, median age 29 years | ||||||||||||
| NVX-CoV2373: 1 | |||||||||||||
| Unspecified: 8 | |||||||||||||
| Karlstad et al.25) | Population based study | Denmark, Finland, Norway, and Sweden | 23,122,522 | 18,814,068 | 295 | BNT162b2: | BNT162b2: | • Hospital diagnosis code (ICD codes) | |||||
| ChAdOx1: n=1,356,457 | BNT162b2: 220 | In males: | 2.3% with first dose | ||||||||||
| BNT162b2: n=15,064,585 | mRNA-1273: 75 | 0.27/100,000 (95% CI, 0.09–0.46) for the first dose | 0.2% with second dose | ||||||||||
| mRNA-1273: n=2,390,870 | 0.67/100,000 (95% CI, 0.46–0.88) for the second dose: | mRNA-1273: | |||||||||||
| Other: n=2,156 | In females: | 4.5% with second dose | |||||||||||
| 0.15/100,000 (95% CI, 0.02–0.28) for the first dose | |||||||||||||
| 0.09/100,000 (95% CI, −0.09–0.26) for the second dose | |||||||||||||
| mRNA-1273: | |||||||||||||
| In males: | |||||||||||||
| 0.33/100,000 (95% CI, −0.11–0.78) for the first dose | |||||||||||||
| 4.97/100,000 (95% CI, 3.62–6.32) for the second dose | |||||||||||||
| In females: | |||||||||||||
| 0.05/100,000 (95% CI, −0.13–0.23) for the first dose | |||||||||||||
| 0.48/100,000 (95% CI, 0.07–0.89) for the second dose | |||||||||||||
| Patone et al.26) | Population based study | UK | 38,615,491 | 38,615,491 | 397 | Only in younger than 40 years old | N/A | • English National Immunisation | |||||
| ChAdOx1: n=20,615,911 | ChAdOx1: 226 | aRR for the first dose: | • Database of COVID-19 vaccination | ||||||||||
| BNT162b2: n=16,993,389 | BNT162b2: 158 | ChAdOx1: 1.29 (95% CI, 1.05–1.58) | |||||||||||
| mRNA-1273: n=1,006,191 | mRNA-1273: 9 | BNT1626b2: 1.31 (95% CI, 1.03–1.66) | |||||||||||
| mRNA-1273: 2.97 (95% CI, 1.34–6.58) | |||||||||||||
| aRR for the second dose: | |||||||||||||
| BNT1626b2: 1.30 (95% CI, 0.98–1.72) | |||||||||||||
| mRNA-1273: 9.84 (95% CI, 2.69–36.03) | |||||||||||||
| Le Vu et al.27) | Population based study | France | 32,000,000 | BNT162b2: | 534 | BNT162b2: | 0 (15 needs ICU treatment, 17 with mechanical ventilation) | • Hospital discharge data | |||||
| n=21,200,000 | BNT162b2: 405 | OR, 1.8 (95% CI, 1.3–2.5) for the first dose | |||||||||||
| 40,500,000 doses | mRNA-1273: 129 | OR, 8.1 (95% CI, 6.7–9.9) for the second dose | |||||||||||
| mRNA-1273: | mRNA-273: | ||||||||||||
| n=2,860,000 | OR, 3.0 (95% CI, 1.4–6.2) for the first dose | ||||||||||||
| 5,440,000 doses | OR, 30 (95% CI, 21–43) for the second dose | ||||||||||||
| Highest association with mRNA-1273 in persons aged 18 to 24 years | |||||||||||||
| Husby et al.28) | Population based study | Denmark | 4,931,775 | BNT162b2: | 269 with myocarditis or pericarditis | BNT162b2: | N/A | • Hospital diagnosis code of myocarditis or pericarditis (ICD-10 codes) | |||||
| n=3,482,295 | BNT162b2: 248 | 1.4/100,000 (95% CI, 1.0–1.8) | |||||||||||
| mRNA-1273: | mRNA-1273: 21 | 1.3/100,000 (95% CI, 0.8–1.9) for females | |||||||||||
| n=498,814 | 1.5/100,000 (95% CI, 1.0–2.2) for males | ||||||||||||
| aRR, 1.34 (95% CI, 0.90–2.00) | |||||||||||||
| 3.73 (95% CI, 1.82–7.65) for females | |||||||||||||
| 0.82 (95% CI, 0.50–1.34) for males | |||||||||||||
| mRNA-1273: | |||||||||||||
| 4.2/100,000 (95% CI, 2.6–6.4) | |||||||||||||
| 2.0/100,000 (95% CI, 0.7–4.8) for females | |||||||||||||
| 6.3/100,000 (95% CI,3.6–10.2) for males | |||||||||||||
| aRR, 3.92 (95% CI, 2.30–6.68) | |||||||||||||
| 6.33 (95% CI, 2.11–18.96) for females | |||||||||||||
| 3.22 (95% CI, 1.75–5.93) for males | |||||||||||||
| Massari et al.29) | Population based study | Italy | 2,816,809 | Total 5,109,231 doses | 441 with myocarditis/pericarditis | 68.5% were males | N/A | • Emergency care/hospital discharge databases (ICD-9 codes) | |||||
| BNT162b2: n=2,405,759 | BNT162b2: 346 | BNT162b2: | |||||||||||
| mRNA-1273: n=456,050 | mRNA-1273: 95 | aRR, 1.08/100,000 (95% CI, 0.70–1.67) for the first dose | |||||||||||
| 1.99/100,000 (95% CI, 1.30–3.05) for the second dose | |||||||||||||
| mRNA-1273: | |||||||||||||
| aRR, 2.22/100,000 (95% CI, 1.00–4.91) for the first dose | |||||||||||||
| 2.63/100,000 (95% CI, 1.21–5.71) for the second dose | |||||||||||||
| Highest risks in males of 12 to 39 years and in males and females 18 to 29 years with mRNA-1273 | |||||||||||||
| Cho et al.30) | Population based study | Korea | 44,276,704 | BNT162b2: n=24,828,152 | 480 | 62.3% were males | 36 (9 deaths, and 1 heart transplantation) | • Mandatory reporting system | |||||
| mRNA-1273: n=6,781,796 | BNT162b2: 306 | Overall: 1.08/100,000 (95% CI, 0.99–1.19) | • Case verified by the Expert Adjudication Committee on COVID-19 Vaccination Pericarditis/Myocarditis | ||||||||||
| ChAdOx1: n=11,156,646 | mRNA-1273: 156 | BNT162b2: 1.23/100,000 (95% CI, 1.10–1.38) | |||||||||||
| Ad26.COV2.S: n=1,510,110 | ChAdOx1: 15 | mRNA-1273: 2.30/100,000 (95% CI, 1.95–2.69) | |||||||||||
| Ad26.COV2.S: 3 | ChAdOx1: 0.14/100,000 (95% CI, 0.08–0.22) | ||||||||||||
| Ad26.COV2.S: 0.20/100,000 (95% CI, 0.04–0.58) | |||||||||||||
| Highest in males between the ages of 12–17 years | |||||||||||||
| Mevorach et al.31) | Population based study | Israel | 5,442,696 | BNT162b2: n=5,442,696 | 142 | Males: 3.19/100,000 (95% CI, 2.37–4.02) | N/A | • Active and passive surveillance with ICD-9 | |||||
| Females: 0.39/100,000 (95% CI, 0.10–0.68) | • Case verified by BC | ||||||||||||
| Highest in males between the ages of 16 and 19 years, after second dose | |||||||||||||
| Witberg et al.32) | Population based study | Israel | 2,558,421 | BNT162b2: n=2,558,421 | 54 | Overall: 2.13/100,000 (95% CI, 1.56–2.70) after first dose | 1 | • Database of Clalit Health Services with ICD-9 | |||||
| Males: 4.12/100,000 (95% CI, 2.99–5.26) | • Case verified by CDC | ||||||||||||
| Females: 0.23/100,000 (95% CI, 0–0.49) | |||||||||||||
| Highest in males between the ages of 16–29 years | |||||||||||||
| Wong et al.33) | Population based study | Hong Kong | 2,333,379 | BNT162b2: n=1,308,820 | Myocarditis and pericarditis | N/A | • Hospital record of Hong Kong Hospital Authority (ICD-9-CM) | ||||||
| CoronaVac: n=955,859 | BNT162b2: 10/100,000 (95% CI, 0.76–13.66) | ||||||||||||
| CoronaVac: 3/100,000 (95% CI, 0.31–5.46) | |||||||||||||
aRR = adjusted relative risk; BC = Brighton Collaboration; CDC = Center for Disease Control and Prevention; CI = confidence interval; COVID-19 = coronavirus disease 2019; FM = fulminant myocarditis; ICD = International Classification of Diseases; ICD-9-CM = International Classification of Diseases, 9th Revision, Clinical Modification; ICU = intensive care unit; N/A = not available; RR = relative risk; VAERS = Vaccine Adverse Events Reporting System.
A worldwide pharmacovigilance study on mRNA-1273, involving more than 252 million individuals, showed that the incidence of VRM associated with mRNA-1273 is 9.23 per 100,000 person-years. It was highest in males aged 18 to 24 years old who had received the second dose of the vaccine.18) The VAERS data including more than 192 million revealed that young men were the highest risk group, especially after the second dose of an mRNA vaccines. The incidence of adverse effects after the first dose of BNT162b2 vaccine was 1.29 per 1,000,000 doses, while after the second dose it was 9.71 per 1,000,000 doses. For mRNA-1273 vaccine, the incidences were 1.61 and 5.09 per 1,000,000 doses after the first and the second doses, respectively.19) The English National Immunisation database reported data on myocarditis or pericarditis data following vaccination with ChAdOx1, BNT162b2 and mRNA-1273 among a population of approximately 38.6 million population in the UK.26) As the ChAdOx1 vaccine was developed and made available in the UK, this database includes data on more than 20 million individuals who received the ChAdOx1 vaccine. The database includes 397 VRM cases, with 226 occurring after ChAdOx1 vaccination, 158 after BNT162b2 vaccination, and 9 after mRNA-1273 vaccination. They showed increased risk of VRM in a week after the first dose of both mRNA and adenoviral vaccines and after the second dose of mRNA vaccines only in younger than 40 years old. Another nationwide population-based study by Le Vu et al.27) reported that 534 cases of VRM of total 32 million persons after mRNA vaccines in France, and the highest risk of VRM was in individuals aged 18 to 24 years old vaccinated with mRNA-1273 vaccine, especially after the second dose. In other population-based studies, VRM was more commonly associated with mRNA vaccines in young males and after the second doses.20),21),22),23),25),28),29),31),32),33)
Cho et al.30) recently reported nationwide surveillance data on COVID-19 VRM in Korea. The Korean government established a nationwide reporting system for adverse events following COVID-19 vaccination before the vaccination campaign began, as they initiated the vaccination later than Western countries. Additionally, the Expert Adjudication Committee on COVID-19 Vaccine-related Myocarditis/Pericarditis was organized within the Korea CDC. The Korean government requires the reporting of all adverse events related to the COVID-19 vaccination, mandatory. The committee reviewed all reported cases and confirmed myocarditis or pericarditis after the vaccination, based on diagnostic appropriateness. According to the study, 44,276,704 individuals aged 12 and older received at least one dose of COVID-19 vaccine. Of these, 480 patients were confirmed to have VRM by an expert committee, resulting in an overall incidence rate of 1.08 per 100,000 vaccinated persons in Korea. This incidence was particularly high in males after receiving the second dose of mRNA vaccines, consistent with other studies. However, unlike other studies, this research provided more precise clinical outcomes for patients with VRM. Out of 480 VRM patients, 95 cases (19.8%) were identified as severe, including 85 intensive care unit admissions (17.7%), 36 cases of fulminant myocarditis (7.5%), 21 patients (4.4%) treated with extracorporeal membrane oxygenator (ECMO), 21 deaths (4.4%), and 1 heart transplantation (0.2%). In addition, 8 cases of sudden cardiac death (SCD) were reported following COVID-19 vaccination. SCD occurred in individuals under 45 years of age and within one week of the vaccination.
DIAGNOSIS OF CORONAVIRUS DISEASE 2019 VACCINE-RELATED MYOCARDITIS
The diagnostic criteria for COVID-19 VRM in published large cohort studies generally align with standard myocarditis diagnostic criteria, with additional consideration given to the temporal relationship between symptom onset and COVID-19 vaccination.21),23),25),26),27),30),31) Therefore, the diagnosis of COVID-19 VRM typically follows a combination of clinical criteria, laboratory findings, and imaging results, mirroring the diagnostic approach used for myocarditis in general but within the specific context of recent COVID-19 vaccination.
Symptoms of myocarditis of VRM are similar to common myocarditis, including chest pain or pressure sense, shortness of breath, palpitations, or syncope.10),14) Chest pain or discomfort is the most frequent symptom and is generally mild and can be worsened by inspiration (respiratory-dependency). Also, patients may also experience malaise, general weakness, and febrile senses. These symptoms typically start within several days after the vaccination against COVID-19. Although there are several cases of VRM without symptoms and a possibility of selection bias, almost all patients with VRM complain of chest pain.13),14),34)
The diagnosis of VRM requires signs of laboratory data supporting myocarditis, including measures of myocardial injury and evidence of systemic inflammation.10),35) Specific serum markers of myocardial injury include cardiac troponin I or T, and creatine kinase-MB. In patients with myocarditis, cardiac troponin levels are typically elevated and reach their peak between 48 and 72 hours after symptoms onset.14),23) Serum level of cardiac troponins shows a weak correlation with the severity of ventricular dysfunction.36) Leukocytosis, elevated C-reactive protein (CRP) and erythrocyte sedimentation rate can be present with systemic inflammation, and CRP is elevated in 80% to 95% of the patients.10) Other biomarkers associated with myocardial inflammation may aid the diagnosis.
Electrocardiography is a crucial diagnostic tool for identifying myocarditis and eliminating other potential causes of chest pain. Abnormal electrocardiographic findings can be found in about 85% of patients with myocarditis, and ST-segment elevation is the most frequent abnormal finding.10),37) Also, suspicion of acute myocarditis should be raised and high-risk forms suggested by atrioventricular block, QRS width greater than 120 ms, symptomatic bradycardia or tachycardia, or ventricular arrhythmias.10),37),38)
Echocardiography is an imaging test of choice that should be used in patients with suspected myocarditis to help detect structural and functional abnormalities of the heart.10) Several echocardiographic findings suggesting acute myocarditis include increased ventricular wall thickness, decreased segmental contractility (hypokinesia), particularly in the inferior and inferolateral wall, and increased myocardial echogenicity.10) Also, abnormal tissue Doppler finding, mild right ventricular dysfunction, pericardial effusion, and abnormal strain echocardiographic finding can be found in myocarditis patients.10),14),39) Echocardiographic measurement of global longitudinal strain was significantly lower in patients with myocarditis than control subjects among patients presenting chest pain and elevated troponins.39) The assessment of left ventricular ejection fraction (LVEF) can give prognostic information, and patients with lower LVEF on admission had worse survival.38)
Cardiac magnetic resonance imaging (CMR) is a powerful imaging study in myocarditis patients and it can measure LVEF after quantifying ventricular volumes, along with cardiac mass.10),14) Additionally, it can detect and quantify the extent of myocardial inflammation in patients with myocarditis. The presence of myocardial edema and/or late-gadolinium enhancement on CMR is a part of diagnostic criteria for myocarditis along with another 3 clinical criteria including electrocardiographic findings, elevated marker of serum myocardiolysis, and functional and structural abnormalities on cardiac imaging tests.10),14),40) Original Lake Louise Criteria has been proposed to detect myocardial inflammation by CMR in 2009.41) The 2018 update of the Lake Louise Criteria suggests that a positive diagnosis of myocarditis requires the presence of at least one criterion from each of the following 2 categories: T2-based marker of myocardial edema and T1-based marker for associated myocardial injury (myocardial edema, hyperemia, necrosis or fibrosis).42)
Endomyocardial biopsy (EMB) is the gold standard diagnostic method for detecting myocardial inflammation. Recent expert consensus recommend EMB is indicated in cases with acute myocarditis presenting cardiogenic shock or acute heart failure, ventricular arrhythmias or high-degree atrioventricular block, peripheral eosinophilia, suspicion of chronic inflammatory cardiomyopathy, and accuracy of CMR for diagnosis is uncertain.10),14) While EMB is rarely indicated in mild cases of myocarditis, it remains the most reliable diagnostic tool for severe cases. Patients with COVID-19 VRM exhibit numerous macrophages in the myocardium with low levels of T-cell infiltrates.14)
CHALLENGES OR ISSUES IN THE DIAGNOSIS OF CORONAVIRUS DISEASE 2019 VACCINE-RELATED MYOCARDITIS
The diagnosis of COVID-19 VRM presents several challenges and issues, primarily due to its rarity, the variability in clinical presentation, and the overlap with other conditions. Some of the key problems and issues are as follows:
1) Non-specific symptoms: The symptoms of VRM, including chest pain, shortness of breath, and palpitations, are non-specific and can be associated with a wide range of cardiovascular and non-cardiovascular conditions. Therefore, this non-specificity can lead to delays in diagnosis or misdiagnosis.
2) Overlap with other conditions: VRM can mimic other acute cardiac conditions. Thus, it can be challenging to differentiate between VRM and other acute cardiac conditions, such as acute coronary syndrome, based solely on clinical presentation.
3) Similar laboratory and imaging findings: Although elevated cardiac biomarkers and imaging findings are important for diagnosing myocarditis, they are not specific to this condition. Additionally, not all healthcare settings have access to advanced imaging techniques like CMR, which can provide more definitive evidence of myocarditis.
4) Temporal association between symptom onset and vaccination: Establishing a causal link between the COVID-19 vaccination and myocarditis is challenging. Temporal association does not necessarily imply causation, and other potential causes or coincidental occurrences must be carefully excluded.
5) Lack of standardized diagnostic criteria of VRM: Although the Brighton Collaboration (BC) and other organizations provide case definitions for vaccine-related adverse events, there may be variability in how these criteria are applied in clinical practice. This can lead to inconsistencies in diagnosis and reporting. For instance, Cho et al.30) conducted a nationwide study in Korea using the modified criteria of BC case definition for VRM, while Oster et al.19) conducted a US cohort study using CDC criteria for the diagnosis of VRM.
6) Underreporting and misclassification: Some cases of VRM may not be reported or may be misclassified due to the mild nature of symptoms in some individuals or the attribution of symptoms to other causes.
7) Differential diagnosis: The differentiation of VRM from myocarditis caused by other factors, such as viral infections including SARS-CoV-2, adds complexity to the diagnostic process.
8) Evolving understanding: As the COVID-19 pandemic progresses and new vaccine platforms are introduced, the clinical understanding of VRM continues to develop. It is important to note that this evolving knowledge base can impact diagnostic approaches and criteria over time.
Addressing these challenges requires a high degree of clinical suspicion, access to comprehensive diagnostic tools, and a multidisciplinary approach involving cardiologists, infectious disease specialists, and other healthcare professionals. Ongoing research and updated guidelines are essential for improving the diagnosis and management of COVID-19 VRM.
MANAGEMENT OF CORONAVIRUS DISEASE 2019 VACCINE-RELATED MYOCARDITIS
Currently, there is no agreement on how to manage VRM patients who experience acute symptoms. Typically, most VRM cases have a normal or near-normal LVEF, and patients experience a quick resolution of their symptoms. It is important to distinguish chest pain caused by VRM from chest pain caused by coronary artery disease. If coronary artery disease is suspected, it is recommended to perform coronary angiography to confirm its presence. However, in cases of reduced left ventricular systolic function (LVSF) and clinical heart failure, management should be based on heart failure guidelines.43),44) If there is a decline in LVSF, heart failure medications such as angiotensin receptor-neprilysin inhibitors or angiotensin-converting enzyme inhibitors, beta-blockers, mineralocorticoid receptor antagonists, and sodium-glucose cotransporter-2 inhibitors should be used. If an arrhythmia is present, management should follow the guidelines for arrhythmia. In cases of severe form of VRM, EMB is indicated as described above.
The pathophysiology of VRM has not been established. However, one mechanism is thought to be the activation of cellular immunity. Anti-inflammatory therapy or the use of immunosuppressive drugs may be considered as a therapeutic option. Nevertheless, the selective use of short-term corticosteroids in patients with severe LV dysfunction or in patients presenting with cardiogenic shock is recommended to balance the benefits and risks of immunosuppression in VRM.14)
In cases of shock due to severe heart failure, mechanical ventricular support and/or ECMO may be considered to reduce LV load and provide a bridge to recovery.14),45) However, it is important to note that fulminant myocarditis has a high mortality rate. In the Korean study,30) 36 patients (7.5%) were diagnosed with fulminant myocarditis, 21 of whom received ECMO. Of those 21 patients, 11 recovered, 9 died, and 1 underwent heart transplantation.
SPECIAL CONSIDERATION: VACCINATION IN THE UPCOMING ENDEMIC ERA
With the widespread use of COVID-19 vaccines and an increasing number of individuals recovering from the virus, COVID-19 has now transitioned into an endemic phase. In this era, the importance of COVID-19 vaccination may seem less significant, and some people may be hesitant to get vaccinated due to concerns about adverse events. However, COVID-19 vaccination still plays a crucial role for several reasons.46)
1) COVID-19 vaccines have proven highly effective in reducing rates of severe disease, hospitalization, and death. Ongoing vaccination efforts will continue to be crucial in protecting populations, particularly vulnerable groups such as the elderly and those with underlying comorbidities, including cardiovascular diseases, chronic kidney disease, diabetes, or lung diseases.
2) Although COVID-19 has entered the endemic phase, localized outbreaks or spikes in cases could still occur, potentially resulting in a pandemic. High vaccination rates can help prevent these outbreaks and minimize their impact.
3) Vaccinations help reduce the burden on healthcare systems by preventing severe cases of COVID-19. This enables hospitals and healthcare providers to better manage other medical conditions and emergencies, thereby maintaining overall healthcare capacity and efficiency.
4) The pandemic has presented a challenge with the emergence of new variants. To increase their effectiveness against prevalent strains, vaccines have been adapted. It will be essential to continue updating and administering vaccines during the endemic phase to counteract the virus’s evolution.
5) A retrospective large US cohort study found that COVID-19 survivors had a significantly higher risk of incidental cardiovascular diseases compared to non-COVID-19 controls.47) Therefore, individuals with a history of COVID-19 and clinicians should pay attention to their cardiovascular health in the long term. COVID-19 vaccination can be an effective tool in reducing the risk of infection and potential long-term health consequences.48)
6) Booster doses or updated vaccines may be necessary during an endemic phase due to the emergence of new COVID-19 variants or waning immunity.
For these reasons, COVID-19 vaccination will remain a crucial aspect of public health strategy in the upcoming endemic era. It will allow societies to live with COVID-19 more safely and with less disruption, highlighting the significance of vaccine development, distribution, and ongoing public health measures to effectively manage the disease.
FUTURE PERSPECTIVES OR UNMET NEEDS FOR CORONAVIRUS DISEASE 2019 VACCINE-RELATED MYOCARDITIS
COVID-19 VRM, although rare, has revealed several unmet needs in understanding and managing this adverse event. It is crucial to address these needs to enhance vaccine safety, improve patient outcomes, and maintain public trust in vaccination programs in the future.
1) Future strategies may prioritize the need for strong surveillance systems to promptly detect and report cases of VRM. Improved data collection efforts can clarify incidence rates, identify at-risk populations, and track long-term outcomes.
2) The pathophysiological mechanism of COVID-19 VRM is still unknown. Research into the immunological and genetic mechanisms underlying VRM is critical. Identifying specific risk factors, including genetic predispositions and interactions with other environmental or biological factors, can inform targeted prevention strategies.
3) Developing next-generation vaccines with high efficacy and reduced risk of adverse events, including VRM, is a priority. Ongoing research may focus on modifying existing vaccine platforms or exploring new technologies to minimize potential cardiac effects.
4) Future research could investigate personalized vaccination strategies, such as adjusting vaccine dosing, scheduling, or administration based on individual risk factors for myocarditis. Tailoring vaccination approaches could help reduce risks for susceptible individuals.
5) Standardized treatment guidelines for the management of VRM are essential. This includes protocols for diagnosis, monitoring, and therapy, ranging from conservative management for mild cases to advanced interventions for severe cases.
6) Long-term longitudinal studies that monitor individuals who have experienced VRM will provide valuable insights into long-term clinical outcomes, recurrences, and the effectiveness of various treatment strategies.
7) During the COVID-19 pandemic, countries worldwide have addressed health issues related to COVID-19, including VRM, in their own ways. Therefore, international collaboration among researchers, healthcare organizations, and regulatory agencies will be vital for sharing knowledge, standardizing surveillance and treatment approaches, and coordinating responses to VRM.
CONCLUSIONS
In conclusion, the experiences obtained from COVID-19 VRM have significantly contributed to our understanding of this rare but important adverse event. Our journey through COVID-19 vaccine risk management has been illuminating, underscoring the delicate balance between vaccine efficacy and safety. This experience has taught us the critical need for robust surveillance, rapid response to adverse events, and the importance of public trust. Moving forward, it is crucial to enhance our comprehension of the immunological mechanisms underlying myocarditis, improve vaccine technologies, and develop precise risk mitigation strategies. Transparent communication and global collaboration will be essential in adapting our approaches. These insights will not only enhance future vaccine safety but also improve our ability to address emerging health threats, ensuring that we protect public health while minimizing risks.
ACKNOWLEDGMENTS
We thank to Sung-Won Park for her excellent illustration.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest: Jae-Hyeong Park has nothing to declare. Kye Hun Kim serves on the editorial boards as a deputy editor in the Korean Circulation Journal.
Data Sharing Statement: The data generated in this study is available from the corresponding author upon reasonable request.
- Conceptualization: Park JH, Kim KH.
- Data curation: Park JH, Kim KH.
- Formal analysis: Park JH.
- Supervision: Kim KH.
- Writing - original draft: Park JH.
- Writing - review & editing: Park JH, Kim KH.
References
- 1.Berlin DA, Gulick RM, Martinez FJ. Severe COVID-19. N Engl J Med. 2020;383:2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 2.Jung J. Preparations for the assessment of COVID-19 infection and long-term cardiovascular risk. Korean Circ J. 2022;52:808–813. doi: 10.4070/kcj.2022.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barouch DH. COVID-19 vaccines - immunity, variants, boosters. N Engl J Med. 2022;387:1011–1020. doi: 10.1056/NEJMra2206573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu R, Pan J, Zhang C, Sun X. Cardiovascular complications of COVID-19 vaccines. Front Cardiovasc Med. 2022;9:840929. doi: 10.3389/fcvm.2022.840929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh EE, Frenck RW, Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falsey AR, Sobieszczyk ME, Hirsch I, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 vaccine. N Engl J Med. 2021;385:2348–2360. doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollack A, Kontorovich AR, Fuster V, Dec GW. Viral myocarditis--diagnosis, treatment options, and current controversies. Nat Rev Cardiol. 2015;12:670–680. doi: 10.1038/nrcardio.2015.108. [DOI] [PubMed] [Google Scholar]
- 10.Ammirati E, Frigerio M, Adler ED, et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ Heart Fail. 2020;13:e007405. doi: 10.1161/CIRCHEARTFAILURE.120.007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data - United States, March 2020-January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1228–1232. doi: 10.15585/mmwr.mm7035e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rafaniello C, Gaio M, Zinzi A, et al. Disentangling a thorny issue: myocarditis and pericarditis post COVID-19 and following mRNA COVID-19 vaccines. Pharmaceuticals (Basel) 2022;15:525. doi: 10.3390/ph15050525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verma AK, Lavine KJ, Lin CY. Myocarditis after COVID-19 mRNA vaccination. N Engl J Med. 2021;385:1332–1334. doi: 10.1056/NEJMc2109975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heidecker B, Dagan N, Balicer R, et al. Myocarditis following COVID-19 vaccine: incidence, presentation, diagnosis, pathophysiology, therapy, and outcomes put into perspective. A clinical consensus document supported by the Heart Failure Association of the European Society of Cardiology (ESC) and the ESC Working Group on Myocardial and Pericardial Diseases. Eur J Heart Fail. 2022;24:2000–2018. doi: 10.1002/ejhf.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19:75–77. doi: 10.1038/s41569-021-00662-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rout A, Suri S, Vorla M, Kalra DK. Myocarditis associated with COVID-19 and its vaccines - a systematic review. Prog Cardiovasc Dis. 2022;74:111–121. doi: 10.1016/j.pcad.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckart RE, Love SS, Atwood JE, et al. Incidence and follow-up of inflammatory cardiac complications after smallpox vaccination. J Am Coll Cardiol. 2004;44:201–205. doi: 10.1016/j.jacc.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Straus W, Urdaneta V, Esposito DB, et al. Analysis of Myocarditis among 252 million mRNA-1273 recipients worldwide. Clin Infect Dis. 2023;76:e544–e552. doi: 10.1093/cid/ciac446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327:331–340. doi: 10.1001/jama.2021.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goddard K, Lewis N, Fireman B, et al. Risk of myocarditis and pericarditis following BNT162b2 and mRNA-1273 COVID-19 vaccination. Vaccine. 2022;40:5153–5159. doi: 10.1016/j.vaccine.2022.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simone A, Herald J, Chen A, et al. Acute myocarditis following COVID-19 mRNA vaccination in adults aged 18 years or older. JAMA Intern Med. 2021;181:1668–1670. doi: 10.1001/jamainternmed.2021.5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson IV, Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 2021;326:1210–1212. doi: 10.1001/jama.2021.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6:1202–1206. doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canada government. Reported side effects following COVID-19 vaccination in Canada [Internet] Ottawa: Canada government; 2023. [cited 2024 January 1]. Available from: https://health-infobase.canada.ca/covid-19/vaccine-safety/#detailedSafetySignals. [Google Scholar]
- 25.Karlstad Ø, Hovi P, Husby A, et al. SARS-CoV-2 vaccination and myocarditis in a Nordic cohort study of 23 million residents. JAMA Cardiol. 2022;7:600–612. doi: 10.1001/jamacardio.2022.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28:410–422. doi: 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Vu S, Bertrand M, Jabagi MJ, et al. Age and sex-specific risks of myocarditis and pericarditis following COVID-19 messenger RNA vaccines. Nat Commun. 2022;13:3633. doi: 10.1038/s41467-022-31401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Husby A, Hansen JV, Fosbøl E, et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ. 2021;375:e068665. doi: 10.1136/bmj-2021-068665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massari M, Spila Alegiani S, Morciano C, et al. Postmarketing active surveillance of myocarditis and pericarditis following vaccination with COVID-19 mRNA vaccines in persons aged 12 to 39 years in Italy: a multi-database, self-controlled case series study. PLoS Med. 2022;19:e1004056. doi: 10.1371/journal.pmed.1004056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho JY, Kim KH, Lee N, et al. COVID-19 vaccination-related myocarditis: a Korean nationwide study. Eur Heart J. 2023;44:2234–2243. doi: 10.1093/eurheartj/ehad339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA vaccine against COVID-19 in Israel. N Engl J Med. 2021;385:2140–2149. doi: 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Witberg G, Barda N, Hoss S, et al. Myocarditis after COVID-19 vaccination in a large health care organization. N Engl J Med. 2021;385:2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong CK, Lau KT, Xiong X, et al. Adverse events of special interest and mortality following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines in Hong Kong: a retrospective study. PLoS Med. 2022;19:e1004018. doi: 10.1371/journal.pmed.1004018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heymans S, Eriksson U, Lehtonen J, Cooper LT., Jr The quest for new approaches in myocarditis and inflammatory cardiomyopathy. J Am Coll Cardiol. 2016;68:2348–2364. doi: 10.1016/j.jacc.2016.09.937. [DOI] [PubMed] [Google Scholar]
- 35.Sexson Tejtel SK, Munoz FM, Al-Ammouri I, et al. Myocarditis and pericarditis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2022;40:1499–1511. doi: 10.1016/j.vaccine.2021.11.074. [DOI] [PubMed] [Google Scholar]
- 36.Gilotra NA, Minkove N, Bennett MK, et al. Lack of relationship between serum cardiac troponin I level and giant cell myocarditis diagnosis and outcomes. J Card Fail. 2016;22:583–585. doi: 10.1016/j.cardfail.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 37.Younis A, Matetzky S, Mulla W, et al. Epidemiology characteristics and outcome of patients with clinically diagnosed acute myocarditis. Am J Med. 2020;133:492–499. doi: 10.1016/j.amjmed.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 38.Ammirati E, Veronese G, Brambatti M, et al. Fulminant versus acute nonfulminant myocarditis in patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2019;74:299–311. doi: 10.1016/j.jacc.2019.04.063. [DOI] [PubMed] [Google Scholar]
- 39.Schauer J, Caris E, Soriano B, et al. The diagnostic role of echocardiographic strain analysis in patients presenting with chest pain and elevated troponin: a multicenter study. J Am Soc Echocardiogr. 2022;35:857–867. doi: 10.1016/j.echo.2022.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. 2648a–2648d. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 41.Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 43.McDonagh TA, Metra M, Adamo M, et al. 2023 Focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023;44:3627–3639. doi: 10.1093/eurheartj/ehad195. [DOI] [PubMed] [Google Scholar]
- 44.Park SM, Lee SY, Jung MH, et al. Korean Society of Heart Failure guidelines for the management of heart failure: management of the underlying etiologies and comorbidities of heart failure. Korean Circ J. 2023;53:425–451. doi: 10.4070/kcj.2023.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kociol RD, Cooper LT, Fang JC, et al. Recognition and initial management of fulminant myocarditis: a scientific statement from the American Heart Association. Circulation. 2020;141:e69–e92. doi: 10.1161/CIR.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 46.Kim KH. The role of COVID-19 vaccination for patients with atherosclerotic cardiovascular disease in the upcoming endemic era. J Lipid Atheroscler. 2024;13:21–28. doi: 10.12997/jla.2024.13.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang WJ, Wang CY, Wang SI, Wei JC. Long-term cardiovascular outcomes in COVID-19 survivors among non-vaccinated population: a retrospective cohort study from the TriNetX US collaborative networks. EClinicalMedicine. 2022;53:101619. doi: 10.1016/j.eclinm.2022.101619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Notarte KI, Catahay JA, Velasco JV, et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: a systematic review. EClinicalMedicine. 2022;53:101624. doi: 10.1016/j.eclinm.2022.101624. [DOI] [PMC free article] [PubMed] [Google Scholar]