Abstract
Epidemiological evidence has revealed a potential relationship between periodontal disease and cardiovascular disease (CVD). Consensus regarding a link between these pathologies remains elusive, however, largely secondary to the considerable overlap between risk factors and comorbidities common to both disease processes. This review article aims to update the evidence for an association by summarizing the evidence for causality between periodontitis and comorbidities linked to CVD, including endocarditis, hypertension (HTN), atrial fibrillation (AF), coronary artery disease (CAD), diabetes mellitus (DM) and hyperlipidemia (HLD). This article additionally discusses the role for periodontal therapy to improved management of the comorbidities, with the larger goal of examining the value of periodontal therapy on reduction of CVD risk. In doing so, we endeavor to further the understanding of the commonality between periodontitis, and CVD.
Keywords: Teeth, Oral health, CVD
Graphical abstract
1. Introduction
Periodontitis is a bacterially induced chronic inflammatory disease that destroys the connective tissue and bone that support the teeth [1]. It is estimated that roughly half of all adults in the United States have mild to moderate forms of the disease, with severe periodontitis affecting up to 15 % of all adults in the United States [2]. Globally, severe periodontitis is estimated to affect 19 % of the global adult population, representing over 1 billion cases [3]. Mounting evidence suggests that chronic inflammation increases the risk of cardiovascular disease (CVD) [4]. This has led to speculation that periodontitis may be a modifiable risk factor contributing to the development of CVD, with epidemiological studies supporting an association between CVD and periodontitis [5,6]. CVD and its sequalae – coronary artery disease (CAD), acute myocardial infarction, stroke and peripheral arterial disease – is a leading cause of morbidity and mortality worldwide [7]. Endocarditis, diabetes mellitus (DM), hypertension (HTN), hyperlipidemia (HLD), CAD and atrial fibrillation (AF) have all been shown to be risk factors for or sequalae of CVD. In this review, we will discuss the evidence regarding association and pathophysiology between these pathologies and periodontitis in the broader context of CVD. Specifically, we summarize the significant evidence for causality between periodontitis and the aforementioned comorbidities of CVD, and the findings for periodontal therapy in the setting of improved management of said comorbidities in order to reduce CVD risk.
2. Pathophysiology
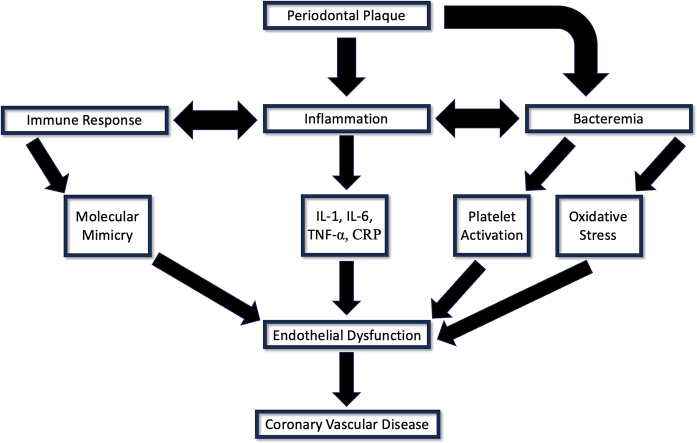
Several mechanisms have been proposed to explain the association between periodontal and cardiovascular disease. Bacteria introduced to the bloodstream from periodontal plaques have been shown to induce platelet activation, aggregation, and thrombosis. Platelet activation by oral bacteria, specifically Streptococcus mutans and S. sanguinis, can lead to localized thrombus formation, and secretion of pro-inflammatory cytokines from the platelets themselves, which contribute to inflammation, atherogenesis, and thrombogenesis [8,9]. Porphyromonas gingivalis has also been shown to induce CVD by activating factor X, prothrombin and protein C, promoting thrombotic tendency and intravascular clot formation [10].
Systemic inflammation has additionally been theorized to underscore the observed association between CVD and periodontitis. Strong evidence exists that increased CVD risk is associated with elevated levels of pro-inflammatory cytokines, such as IL-1, IL-6 and TNF-alpha (TNF-α), and acute phase proteins such as C-reactive protein (CRP) [11,12]. Periodontitis has also been associated with chronic increased systemic inflammatory cytokines and acute phase proteins [13,14]. Systemic inflammation secondary to these inflammatory mediators stimulates immune cell migration to the walls of the endothelium and increased low-density lipid (LDL) uptake by macrophages, leading to increased foam cell formation and subsequently plaque formation [15]. Additionally, increased reactive oxygen species (ROS) due to elevated CRP in periodontitis leads to stress on cellular structures and causes changes in molecular pathways that underpin the pathogenesis of CVD [16].
The systemic inflammation induced by periodontitis can promote molecular mimicry and generation of self-reactive antibodies targeting heat shock proteins (HSP). Bacterial invasion and systemic inflammation have been theorized to increase HSP production in endothelial cells [17]. Due to the homologous nature of HSPs across species, oral bacterial HSP generate antibodies that in turn attack the HSPs produced by the stressed endothelium [18]. A study by Perschinka et al. found that CVD promotes expression of HSP60 and increased adhesion molecules by endothelial cells, causing progression of fatty streaks to irreversible atherosclerotic changes [19].
Establishing causality between periodontitis and CVD remains challenging despite evidence supporting the aforementioned theories. To date, there have been few high-quality studies examining periodontitis with CVD endpoints. Rather, the majority of studies examining causality between these pathologies use surrogate biomarkers to reflect CVD outcomes [20]. Perhaps most challenging to elucidating the relationship between periodontitis and CVD is the fact that there exists considerable overlap in regard to risk factors and comorbidities in the development of both disease processes [21]. Cardiovascular and periodontal diseases share several risk factors, including age, smoking, diabetes, and poor socioeconomic conditions [22].The comorbidities frequently associated with CVD – endocarditis, HTN, AF, CAD, HLD, DM – are also commonly associated with periodontitis, raising the question if any association between periodontitis and CVD is direct, or in fact mediated through a shared comorbidity. This is especially of concern when evaluating any causality in the context of DM, as literature supports a “two-way relationship” between periodontitis and DM, suggesting that any periodontitis and CVD linkage may in fact lie in a hyperglycemic driven periodontal disease state [23]. To further elucidate the potential confounding of comorbidities, we discuss below the relationship between periodontitis and several diseases known to be risk factors for CVD.
3. Endocarditis
A relationship between oral health and endocarditis dates as far back as the early 1900s [24]. Thomas Horder was among the first to acknowledge the role “oral sepsis” played in the development of infective endocarditis in high-risk patients [25]. With the advent of penicillin in the 1940s, evidence emerged that antibiotics lowered the incident of bacteremia following dental extraction [26]. In 1955, this evidence led to the American Heart Association recommendations regarding the use of antibiotics to reduce the risk of endocarditis in patients with underlying heart disease [27]. These recommendations, coupled with good oral hygiene became the standard of care for endocarditis prevention in high-risk patients for over 50 years [23].
In the interim, several species of microorganisms have been identified as the main culprits of endocarditis. Roughly 90 % of causative bacteria are transient or stable components of the oral microbiome, [28] including Staphylococcus aureus (S. aureus), Streptococcus viridians and Streptococcus bovis, and Enterococcus faecalis. Furthermore, a landmark study by Lockhart et. al revealed an association between poor oral hygiene and endocarditis-related bacteremia, revealing an almost eightfold increase in bacteremia risk with bleeding induced by routine tooth brushing [29]. This and other evidence has led the American Heart Association to continue to recommend antimicrobial prophylaxis in high risk cardiac patients undergoing dental procedures, including patients with congenital heart defects, prosthetic valves, previous endocarditis, and cardiac transplant recipients. [30]
4. Hypertension
Epidemiological studies have suggested the existence of a positive relationship between oral health disorders and HTN. [31] Periodontitis specifically has been theorized to contribute to the low-grade inflammatory state underlying CVD and HTN. [32] The identification of periodontitis as a possible risk factor for HTN could be explained by multiple mechanisms [4,5]. Periodontitis has been linked to systemic inflammatory mediators such as CRP and IL-6, both of which are known to affect endothelial function [4]. It has further been posited that immune cells are primed in the chronically inflamed periodontium, thus making them pre-disposed to chemotactic recruitment to perivascular tissues, a step that has been shown precede development of outright HTN and atherosclerotic disease [33,34].
Recent investigations have aimed to establish an association between oral status, specifically periodontitis, and HTN risk. The prevalence of HTN in adults with concurrent periodontitis was summarized in a meta-analysis by Aguilera et al. using 30 prospective and retrospective studies between 2003 and 2018. [35] In 25 of the 30 analyzed studies, the prevalence of HTN was higher in adults with a diagnosis of periodontitis (range 7–77 %) compared with those not suffering from the disease (range 4–70 %) [6]. Periodontitis was also found to be greater in individuals with HTN in the reviewed studies (29–61 %) compared to those without HTN (17–39 %) [7].
Risk factors such as old age, smoking, obesity, and diabetes have been considered common denominators underlying the observed prevalence of periodontitis in HTN patients [ 36]. However, recent literature suggests an association between periodontitis and HTN that is causal in nature and independent of the aforementioned risk factors [2,37,38]. A cross-sectional study among participants in Sweden demonstrated a linear trend between severity of periodontal disease and HTN after adjusting for confounding factors such as age, gender, and smoking status [1]. Another study with 12,000 participants from the 3rd National Health and Nutrition Examination Survey (NHANESIII) found participants with moderate and severe periodontitis had higher systolic blood pressure than those with mild periodontal disease [39]. The evidence of a causal relationship between periodontal disease and HTN is further supported by prospect cohort studies demonstrating improvement in blood pressure following periodontal therapy [[40], [41], [42]].
5. Atrial fibrillation
Systemic inflammation has been shown to be a significant factor in the development of atrial remodeling in animal and human studies [43]. Atrial remodeling has been established as an initial substrate for the development of atrial fibrillation (AF) [44]. This has prompted investigations into potential associations between the inflammatory state induced by periodontitis and AF. Chen et al. found that patients with periodontitis had a 31 % higher risk of developing AF than patients without periodontal disease, an increased risk which remained statistically significant after adjusting for common comorbidities [45]. Studies have also suggested a linear relationship between severity of periodontal disease and AF risk. A recent univariate analysis revealed a dose-response relationship between an increased number of missing teeth and risk of AF, although the relationship was not seen in multivariate analysis [46]. Further research has demonstrated an association between prevalent AF and increased dental plaque levels, bleeding on probing, and periodontal inflamed surface area [47,48].
The atria of AF patients are infiltrated by inflammatory cells, underlying the theory that systemic inflammation can drive the development of arrythmia [31]. Other studies have demonstrated that patients with AF have increased levels of inflammatory markers, including CRP, TNF-α, and plasma IL-6 [49]. CRP downregulates nitric oxide, promoting endothelial cell apoptosis [50]. TNF has also been shown to contribute to the pathogenesis of AF via augmenting pulmonary vein arrhythmogenicity and inducing abnormal calcium homeostasis [51]. Several studies have confirmed increases of these systemic biomarkers in patients with periodontitis [52,53].
A reduction in systemic inflammatory biomarkers have been observed in periodontitis patients following increased oral health maintenance [54]. Evidence exists linking improved periodontitis control and lowered risk of AF. Chen et al. found that dental scaling at least once a year for three consecutive years was associated with reduced AF risk [44]. A dose response relationship between increased frequency of daily tooth brushing and AF has also been shown, with subjects brushing their teeth at least three times daily having significantly less risk of AF than those brushing only once or twice [45].
6. Coronary artery disease
Coronary artery disease (CAD) and periodontitis have been associated since a landmark study in 1989 by Mattila et al. revealed significantly worse dental health in patients with acute myocardial infarction after adjusting for other risk factors [55]. In the following decades, several studies have analyzed the linkage between these disease processes. Mattila provided further evidence of this linkage in 1993, with a study demonstrating coronary atherosclerosis on diagnostic coronary angiography was associated with dental infections in males after adjusting for risk factors [56]. These findings were echoed in a publication by Buhlen et al., which found metrics of periodontitis to be associated with angiographically verified coronary artery narrowing in patients with stable CAD or ACS [57]. A similar study by Costa et al. quantified the association by demonstrating a 2.79 times higher risk of developing CAD in patients with pre-existing periodontitis [58]. Yakob et. al revealed that Porphyromonas gingivalis and Porphyromonas nigrescens increased the odds of carotid artery atherosclerosis as confirmed by ultrasound, a condition associated with CAD [59,60]. Angina, a common symptom manifestation of CAD, was shown by Söder et. al to be more common in patients with a high dental calculus score [61]. In a separate study, Söder et. al higher dental calculus scores were associated with cardiac death due to myocardial infarction [62]. A systematic review by Dietrich et al. analyzing six studies on CAD demonstrated an increased risk of a first coronary event in patients with clinically diagnosed periodontitis or more severe periodontitis compared to patients without periodontitis or less severe periodontitis [63].
There is increasing evidence that periodontitis contributes to atherosclerosis and subsequently CAD. One mechanism proposed is invasion of the endothelium by periodontal pathogens. An immunohistologic study by Ford et al. found that periodontopathic bacteria species were found more frequently in atherosclerotic lesions than other commonly associated pathogens [64]. A follow up study by Ford and coauthors found oral bacteria in 75 % of atherosclerotic plaque specimens analyzed [65]. Analysis of cardiovascular specimens containing thrombus tissues demonstrated that S. mutans, a common pathogen in periodontitis, was the most prevalent bacteria (78 %) [66]. Other periodontitis organisms, including P. gingivalis, Prevotella intermedia, and Tannerella forsythia, have been isolated in atherosclerotic lesions of coronary arteries [67]. A second mechanism through which periodontal disease may contribute to CAD lies in elevation of systemic inflammatory cytokines secondary to periodontitis. Cytokines such as IL-1β, IL-6, IL-8, TNF-α, which are elevated in patients with periodontitis, have been implicated in endothelial dysfunction and initiation of atherosclerosis [68]. A cross sectional analysis by Beukers et al. found periodontitis to be independently associated with CVD after adjusting for confounders such as hypertension, diabetes, and smoking status [69].
There is a dearth of literature regarding periodontitis treatment and impact on CAD. Promising findings were reported by Bokhari et al. in a study revealing significantly decreased CRP and white blood cells in CAD patients with periodontitis following non-surgical periodontal therapy [70]. Tonetti et al. found improved endothelial function in the form of increased flow mediated dilation six months following periodontal treatment in patients with CAD [71]. These studies in conjunction provide evidence of an endothelial response to reduced systemic inflammation following periodontal treatment.
7. Diabetes mellitus
The causal relationship between oral disease, specifically periodontitis, and type 2 diabetes mellitus (T2DM) is well established and dates back to the 1960s [72]. Several studies suggest the association between T2DM and periodontitis is bidirectional [73]. Individuals with T2DM are more likely to develop periodontitis, and diabetic patients with periodontitis have worse glycemic control [74,75]. Several randomized controlled trials have found that treatment of chronic periodontitis improves glycemic control in patients with T2DM, chiefly through reduction of hemoglobin A1c, and furthermore reduces the risk of cardiovascular disease [76,77].
Several mechanisms have been proposed to underly the association between T2DM and periodontitis. Oxidate stress appears to be a major link, as it can activate proinflammatory pathways common to both disease processes. [78] Allen et al. observed that T2DM patients with periodontitis had compromised glycemic control and increased oxidative stress markers such as small molecule antioxidant capacity (pSMAC) and protein carbonyl levels [79]. CRP has alternatively been suggested to be the linchpin between T2DM and periodontitis. Lei et al. presented data suggesting that increased CRP in the setting of periodontitis was associated with increased levels of HbA1c. [80] Animal studies have further suggested that periodontitis aggravates pancreatic β-cell failure and insulin resistance in diabetic mice [81].
A prospective cohort study of 126,805 patients with diabetes and periodontal disease by Merchant et al. revealed an association between periodontal therapy and reduced HbA1c while controlling for confounding lifestyle factors such as smoking and BMI, demonstrating a potential rationale for periodontal treatment as a means for enhanced glycemic control [82]. Scaling and root planning (SRP) is the mainstay treatment for periodontal disease [83]. Recent studies have found that SRP is associated with reduced metabolism and systemic inflammation in T2DM patients [84]. The role antibiotic therapy as additional periodontal treatment in diabetic patients is controversial. A systemic review of Grellmann et al. suggested that adjunctive antibiotic therapy may improve the efficacy of SRP in reducing periodontitis in T2DM patients [85]. However, Lira Junior et al. found that the use of systemic antibiotics in the periodontal treatment of diabetic patients had no significant decrease in HbA1c [86].
8. Hyperlipidemia
Several studies show a bi-directional relationship between hyperlipidemia (HLD) and periodontal disease [87]. Patients with mild or moderate HLD manifested higher values of periodontal parameters compared to normolipidemic individuals [88,89]. In parallel, Cutler et al. and Moeintaghavi et al. concluded that periodontitis is associated with statistically significantly increased levels of serum lipids, demonstrating periodontitis patients as having 12 % and 52 % higher mean total cholesterol and triglycerides, respectively, as compared to patients without periodontal disease [90,91]. However, controversy exists regarding whether the association with periodontal disease lies in serum cholesterol or triglyceride levels. Buhlin et al. demonstrated an association between high cholesterol and periodontitis, while Morita et al. found that serum triglyceride level might be a potential indicator for the presence of periodontal disease [92,93].
Evidence suggests that a cyclic relationship exists between serum lipids, periodontitis and systemic health. HLD has a deregulatory effect on the immune system and wound healing, resulting in increased susceptibility to periodontitis and other infections [94]. This mechanism may be partly explained by HLD induced white blood cell hyperactivity, subsequently leading to increased oxygen radicals which in turn are associated with progression of periodontitis in adults [95,96]. Concomitantly, increased cytokines in the setting of periodontitis alter the amino acid utilization of various tissues involved in lipid metabolism, and stimulate the hypothalamic-pituitary-adrenal axis, leading to increased serum cortisol and glucagon [97,98]. The resultant elevated serum lipids are cleared less effectively due to cytokine interference in lipoprotein lipase activity [99].
The bi-directional nature of HLD and periodontitis is underscored by the evidence of treatment response seen in both pathologies via therapeutic intervention. D'aiuto et al. investigated the impact intensive periodontal therapy on HLD, revealing a decrease in total and LDL cholesterol following two months of periodontal treatment [100]. Significant increase in the ratio of HDL cholesterol to LDL cholesterol after periodontal therapy was demonstrated in a study by Pussinen et al., further supporting the linkage between HLD and periodontitis [101]. In parallel, statin usage in the setting of HLD has been shown to be protective against periodontal attachment loss [102].
9. Management
To date, no well-powered studies of the effects of periodontal treatment on hard CVD endpoints (myocardial infarction, stroke, cardiovascular death) have been conducted [103]. However, the aforementioned studies regarding an association between periodontal therapy and HTN, DM, CAD, HLD, and AF suggest that treatment of periodontitis may improve the CVD risk profile. This hypothesis is underscored by a population-based study conducted in 2015 in Taiwan by Chou et al. suggested that a dose-response relationship exists between periodontitis severity and CVD risk [104]. A similar study by Park et al. found that ≥1 tooth brushing per day or ≥ 1 regular dental visit for professional cleaning per year reduced cardiovascular risk by 9 % and 14 %, respectively [105]. This may be secondary to the reduction in systemic markers of inflammation following periodontal treatment, as demonstrated in a study of CVD patients undergoing periodontitis therapy by Higashi et al. [40] These findings suggest that adherence to daily oral hygiene may be an effective way to control periodontal disease and ultimately reduce the risk of CVD.
10. Current challenges
Randomized controlled trials to evaluate the effect of periodontitis and periodontal therapy on CVD are challenging to perform for the following reasons. First, because periodontal therapy ranges from non-surgical debridement to surgical intervention potentially with antibiotic coverage, it is difficult to define intervention and control groups. This is compounded by the wide-ranging spectrum that is periodontitis, as any defined treatment modality would either be too aggressive or insufficient to meet each patient at their individual level of periodontal disease. Third, a trial would require a very large sample size to detect any potential effect of periodontitis on CVD risk [106,107]. In the interim, using causal inference methods on large clinical databases can continue to further understanding of the relationship between periodontitis and CVD [89,108]. Finally, a true case-control treatment study may not be possible for ethical reasons.
11. Future research
Studies support a link between oral health and CVD, but causality remains elusive. A longitudinal assessment of periodontal health and assessment of CVD surrogate such as inflammatory markers and changes in periodontal parameters would further support a causal relationship between periodontitis and CVD. Prospective interventional studies are necessary to further evaluate the theory that periodontal treatment may reduce the risk of CVD. Specifically, literature quantifying the impact of periodontal therapy on CVD hard endpoints such as myocardial infarction or stroke are warranted. Given the significant overlapping lifestyle risk factors shared in both periodontitis and CVD, any future research must control for confounding variables.
12. Conclusion
Available data strongly suggest that periodontitis may have overall health consequences, specifically pertaining to CVD and associated diagnoses. Significant evidence exists that periodontal therapy may contribute to improved outcomes in these pathologies, largely due to decreased systemic inflammation. Prospective studies are warranted to confirm these findings and further guide clinical practice for the management of periodontitis in regard to CVD risk and outcomes. The high prevalence of periodontitis in populations, the disease that is fully preventable with proper oral hygiene practices, emphasizes the need for further evidence on these associations (Table 1).
Table 1.
CVD Comorbidities and periodontal therapy summary of sources.
| First author | Year | Type of study | Key findings | Limitations |
|---|---|---|---|---|
| Chang | 2020 | Population-based cohort study |
|
|
| Baeza | 2020 | Meta-analysis of Randomized clinical trials |
|
|
| Lockhart | 2017 | Placebo – controlled study |
|
|
| Söder | 2016 | Prospective cohort study |
|
|
| Chen | 2016 | Retrospective cohort study |
|
|
| Merchant | 2015 | Prospective cohort study |
|
|
| Söder | 2014 | Prospective cohort study |
|
|
| Vidal | 2013 | Interventional prospective cohort study |
|
|
| Bokhari | 2012 | Single blind, parallel arm randomized controlled clinical trial |
|
|
| Yakob | 2012 | Prospective cohort study |
|
|
| Fajardo | 2010 | Controlled, double blind randomized clinical trial |
|
|
| Tonetti | 2007 | Parallel-group, single-blind, randomized, controlled trial |
|
|
| D'Aiuto | 2005 | Three-arm preliminary randomized trial |
|
|
CRediT authorship contribution statement
Steven Hopkins: Writing – original draft, Writing – review & editing. Saivaroon Gajagowni: Writing – original draft, Writing – review & editing. Yusuf Qadeer: Validation, Visualization, Writing – review & editing. Zhen Wang: Validation, Visualization, Writing – review & editing. Salim S. Virani: Supervision, Validation, Visualization, Writing – review & editing. Jukka H. Meurman: Supervision, Validation, Visualization, Writing – review & editing. Roman Leischik: Supervision, Validation, Writing – review & editing. Carl J. Lavie: Supervision, Validation, Writing – review & editing. Markus Strauss: Supervision, Validation, Visualization, Writing – review & editing. Chayakrit Krittanawong: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Markus Strauss, Email: markus.strauss@ukmuenster.de.
Chayakrit Krittanawong, Email: Chayakrit.Krittanawong@va.gov.
References
- 1.Friedewald V.E., Kornman K.S., Beck J.D., et al. The American Journal of Cardiology and Journal of Periodontology editors’ consensus: periodontitis and atherosclerotic cardiovascular disease. J. Periodontol. 2009;80(7):1021–1032. doi: 10.1902/jop.2009.097001. [DOI] [PubMed] [Google Scholar]
- 2.Burt B., Research, Science and Therapy Committee of the American Academy of Periodontology Position paper: epidemiology of periodontal diseases. J. Periodontol. 2005;76(8):1406–1419. doi: 10.1902/jop.2005.76.8.1406. [DOI] [PubMed] [Google Scholar]
- 3.Oral health. World Health Organization Factsheets. Published March 14, 2023. Accessed February 15, 2024. https://www.who.int/news-room/fact-sheets/detail/oral-health#:~:text=Periodontal%20(gum)%20disease&text=Severe%20periodontal%20diseases%20are%20estimated,than%201%20billion%20cases%20worldwide.
- 4.Naderi S., Merchant A.T. The association between periodontitis and cardiovascular disease: an update. Curr. Atheroscler. Rep. 2020;22(10):52. doi: 10.1007/s11883-020-00878-0. Published 2020 Aug 9. [DOI] [PubMed] [Google Scholar]
- 5.Bahekar A.A., Singh S., Saha S., Molnar J., Arora R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: a meta-analysis. Am. Heart J. 2007;154(5):830–837. doi: 10.1016/j.ahj.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 6.Cairo F., Castellani S., Gori A.M., et al. Severe periodontitis in young adults is associated with sub-clinical atherosclerosis. J. Clin. Periodontol. 2008;35(6):465–472. doi: 10.1111/j.1600-051X.2008.01228.x. [DOI] [PubMed] [Google Scholar]
- 7.Libby P. The changing landscape of atherosclerosis. Nature. 2021;592(7855):524–533. doi: 10.1038/s41586-021-03392-8. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald J.R., Foster T.J., Cox D. The interaction of bacterial pathogens with platelets. Nat. Rev. Microbiol. 2006;4(6):445–457. doi: 10.1038/nrmicro1425. [DOI] [PubMed] [Google Scholar]
- 9.Kerrigan S.W., Cox D. Platelet-bacterial interactions. Cell. Mol. Life Sci. 2010;67(4):513–523. doi: 10.1007/s00018-009-0207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imamura T., Potempa J., Tanase S., Travis J. Activation of blood coagulation factor X by arginine-specific cysteine proteinases (gingipain-Rs) from Porphyromonas gingivalis. J. Biol. Chem. 1997;272(25):16062–16067. doi: 10.1074/jbc.272.25.16062. [DOI] [PubMed] [Google Scholar]
- 11.Kaptoge S., Seshasai S.R., Gao P., et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur. Heart J. 2014;35(9):578–589. doi: 10.1093/eurheartj/eht367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lagrand W.K., Visser C.A., Hermens W.T., et al. C-reactive protein as a cardiovascular risk factor: more than an epiphenomenon? Circulation. 1999;100(1):96–102. doi: 10.1161/01.cir.100.1.96. [DOI] [PubMed] [Google Scholar]
- 13.Cardoso E.M., Reis C., Manzanares-Céspedes M.C. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad. Med. 2018;130(1):98–104. doi: 10.1080/00325481.2018.1396876. [DOI] [PubMed] [Google Scholar]
- 14.Polepalle T., Moogala S., Boggarapu S., Pesala D.S., Palagi F.B. Acute phase proteins and their role in periodontitis: a review. J. Clin. Diagn. Res. 2015;9(11):ZE01–ZE5. doi: 10.7860/JCDR/2015/15692.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danesh J., Muir J., Wong Y.K., Ward M., Gallimore J.R., Pepys M.B. Risk factors for coronary heart disease and acute-phase proteins. A population-based study. Eur. Heart J. 1999;20(13):954–959. doi: 10.1053/euhj.1998.1309. [DOI] [PubMed] [Google Scholar]
- 16.Ray P.D., Huang P.W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seymour G.J., Ford P.J., Cullinan M.P., Leishman S., Yamazaki K. Relationship between periodontal infections and systemic disease. Clin. Microbiol. Infect. 2007;13(Suppl. 4):3–10. doi: 10.1111/j.1469-0691.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- 18.Mollenhauer J., Schulmeister A. The humoral immune response to heat shock proteins. Experientia. 1992;48(7):644–649. doi: 10.1007/BF02118310. [DOI] [PubMed] [Google Scholar]
- 19.Perschinka H., Mayr M., Millonig G., et al. Cross-reactive B-cell epitopes of microbial and human heat shock protein 60/65 in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2003;23(6):1060–1065. doi: 10.1161/01.ATV.0000071701.62486.49. [DOI] [PubMed] [Google Scholar]
- 20.Febbraio M., Roy C.B., Levin L. Is there a causal link between periodontitis and cardiovascular disease? A concise review of recent findings. Int. Dent. J. 2022;72(1):37–51. doi: 10.1016/j.identj.2021.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Heart Federation What is Cardiovascular Disease? https://world-heart-federation.org/what-is-cvd/#:~:text=Cardiovascular%20disease%20(CVD)%20is%20a,vessels%20(veins%20and%20arteries Available from: (Accessed 14 October 2023)
- 22.Zhou M, Dong J, Zha L, Liao Y. Causal association between periodontal diseases and cardiovascular diseases. Genes 2022; 13(1):13. https://doi.org/ 10.3390/genes13010013. [DOI] [PMC free article] [PubMed]
- 23.Gurav A.N. Periodontitis and insulin resistance: casual or causal relationship? Diabetes Metab. J. 2012;36(6):404–411. doi: 10.4093/dmj.2012.36.6.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cahill T.J., Baddour L.M., Habib G., et al. Challenges in infective endocarditis. J. Am. Coll. Cardiol. 2017;69(3):325–344. doi: 10.1016/j.jacc.2016.10.066. [DOI] [PubMed] [Google Scholar]
- 25.Horder T. Oral sepsis,. Lancet 1921;197(5084):300. doi:https://doi.org/ 10.1016/s0140-6736(00)55895-4. [DOI]
- 26.Okell C.C., Elliott S.D. Bacteraemia and oral sepsis: with special reference to the aetiology of subacute endocarditis. Lancet. 1935;2:869–872. doi: 10.1016/S0140-6736(00)47788-3. [DOI] [Google Scholar]
- 27.Del Giudice C., Vaia E., Liccardo D., et al. Infective endocarditis: a focus on oral microbiota. Microorganisms. 2021;9(6):1218. doi: 10.3390/microorganisms9061218. Published 2021 Jun 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isoshima D., Yamashiro K., Matsunaga K., et al. Assessment of pathogenesis of infective endocarditis by plasma IgG antibody titer test against periodontal bacteria. Clin. Case Reports. 2017;5(10):1580–1586. doi: 10.1002/ccr3.1066. Published 2017 Aug 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lockhart P.B., Brennan M.T., Thornhill M., et al. Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J. Am. Dent. Assoc. 2009;140(10):1238–1244. doi: 10.14219/jada.archive.2009.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prophylaxis against infective endocarditis: antimicrobial prophylaxis against infective endocarditis in adults and children undergoing interventional procedures. London: National Institute for Health and Care Excellence (NICE); July 2016. [PubMed]
- 31.Holmlund A., Holm G., Lind L. Severity of periodontal disease and number of remaining teeth are related to the prevalence of myocardial infarction and hypertension in a study based on 4,254 subjects. J. Periodontol. 2006;77(7):1173–1178. doi: 10.1902/jop.2006.050233. [DOI] [PubMed] [Google Scholar]
- 32.Darnaud C., Thomas F., Pannier B., Danchin N., Bouchard P. Oral health and blood pressure: the IPC cohort. Am. J. Hypertens. 2015;28(10):1257–1261. doi: 10.1093/ajh/hpv025. [DOI] [PubMed] [Google Scholar]
- 33.Guzik T.J., Skiba D.S., Touyz R.M., Harrison D.G. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc. Res. 2017;113(9):1009–1023. doi: 10.1093/cvr/cvx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mikolajczyk T.P., Nosalski R., Szczepaniak P., et al. Role of chemokine RANTES in the regulation of perivascular inflammation, T-cell accumulation, and vascular dysfunction in hypertension. FASEB J. 2016;30(5):1987–1999. doi: 10.1096/fj.201500088R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muñoz Aguilera E., Suvan J., Buti J., et al. Periodontitis is associated with hypertension: a systematic review and meta-analysis. Cardiovasc. Res. 2020;116(1):28–39. doi: 10.1093/cvr/cvz201. [DOI] [PubMed] [Google Scholar]
- 36.Del Pinto R., Landi L., Grassi G., et al. Hypertension and periodontitis: a joint report by the Italian Society of Hypertension (SIIA) and the Italian Society of Periodontology and Implantology (SIdP) High Blood Press. Cardiovasc. Prev. 2021;28(5):427–438. doi: 10.1007/s40292-021-00466-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woo H.G., Chang Y., Lee J.S., Song T.J. Tooth loss is associated with an increased risk of hypertension: a nationwide population-based cohort study. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0253257. (Published 2021 Jun 15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Czesnikiewicz-Guzik M., Osmenda G., Siedlinski M., et al. Causal association between periodontitis and hypertension: evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur. Heart J. 2019;40(42):3459–3470. doi: 10.1093/eurheartj/ehz646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsakos G., Sabbah W., Hingorani A.D., et al. Is periodontal inflammation associated with raised blood pressure? Evidence from a National US survey. J. Hypertens. 2010;28(12):2386–2393. doi: 10.1097/HJH.0b013e32833e0fe1. [DOI] [PubMed] [Google Scholar]
- 40.D’Aiuto F., Parkar M., Andreou G., Brett P.M., Ready D., Tonetti M.S. Periodontitis and atherogenesis: causal association or simple coincidence? J. Clin. Periodontol. 2004;31(5):402–411. doi: 10.1111/j.1600-051X.2004.00580.x. [DOI] [PubMed] [Google Scholar]
- 41.Higashi Y., Goto C., Jitsuiki D., et al. Periodontal infection is associated with endothelial dysfunction in healthy subjects and hypertensive patients. Hypertension. 2008;51(2):446–453. doi: 10.1161/HYPERTENSIONAHA.107.101535. [DOI] [PubMed] [Google Scholar]
- 42.Vidal F., Cordovil I., Figueredo C.M., Fischer R.G. Non-surgical periodontal treatment reduces cardiovascular risk in refractory hypertensive patients: a pilot study. J. Clin. Periodontol. 2013;40(7):681–687. doi: 10.1111/jcpe.12110. [DOI] [PubMed] [Google Scholar]
- 43.Leelapatana P., Limpuangthip N. Association between oral health and atrial fibrillation: a systematic review. Heliyon. 2022;8(3) doi: 10.1016/j.heliyon.2022.e09161. Published 2022 Mar 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korantzopoulos P., Letsas K.P., Tse G., Fragakis N., Goudis C.A., Liu T. Inflammation and atrial fibrillation: a comprehensive review. J. Arrhythm. 2018;34(4):394–401. doi: 10.1002/joa3.12077. Published 2018 Jun 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen D.Y., Lin C.H., Chen Y.M., Chen H.H. Risk of atrial fibrillation or flutter associated with periodontitis: a nationwide, population-based, cohort study. PLoS One. 2016;11(10) doi: 10.1371/journal.pone.0165601. (Published 2016 Oct 31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang Y., Woo H.G., Park J., Lee J.S., Song T.J. Improved oral hygiene care is associated with decreased risk of occurrence for atrial fibrillation and heart failure: a nationwide population-based cohort study. Eur. J. Prev. Cardiol. 2020;27(17):1835–1845. doi: 10.1177/2047487319886018. [DOI] [PubMed] [Google Scholar]
- 47.Struppek J., Schnabel R.B., Walther C., et al. Periodontitis, dental plaque, and atrial fibrillation in the Hamburg City Health Study. PLoS One. 2021;16(11) doi: 10.1371/journal.pone.0259652. (Published 2021 Nov 22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyauchi S., Nishi H., Ouhara K., et al. Relationship between periodontitis and atrial fibrosis in atrial fibrillation. J. Am. Coll. Cardiol. EP. 2023 Jan;9(1):43–53. doi: 10.1016/j.jacep.2022.08.018. [DOI] [PubMed] [Google Scholar]
- 49.Conway D.S., Buggins P., Hughes E., Lip G.Y. Relationship of interleukin-6 and C-reactive protein to the prothrombotic state in chronic atrial fibrillation. J. Am. Coll. Cardiol. 2004;43(11):2075–2082. doi: 10.1016/j.jacc.2003.11.062. [DOI] [PubMed] [Google Scholar]
- 50.Verma S., Wang C.H., Li S.H., et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106(8):913–919. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 51.Lee S.H., Chen Y.C., Chen Y.J., et al. Tumor necrosis factor-alpha alters calcium handling and increases arrhythmogenesis of pulmonary vein cardiomyocytes. Life Sci. 2007;80(19):1806–1815. doi: 10.1016/j.lfs.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 52.Loos B.G. Systemic markers of inflammation in periodontitis. J. Periodontol. 2005;76(11 Suppl):2106–2115. doi: 10.1902/jop.2005.76.11-S.2106. [DOI] [PubMed] [Google Scholar]
- 53.Bretz W.A., Weyant R.J., Corby P.M., et al. Systemic inflammatory markers, periodontal diseases, and periodontal infections in an elderly population. J. Am. Geriatr. Soc. 2005;53(9):1532–1537. doi: 10.1111/j.1532-5415.2005.53468.x. [DOI] [PubMed] [Google Scholar]
- 54.D’Aiuto F., Parkar M., Andreou G., et al. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J. Dent. Res. 2004;83(2):156–160. doi: 10.1177/154405910408300214. [DOI] [PubMed] [Google Scholar]
- 55.Mattila K.J., Nieminen M.S., Valtonen V.V., et al. Association between dental health and acute myocardial infarction. BMJ. 1989;298(6676):779–781. doi: 10.1136/bmj.298.6676.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mattila K.J., Valle M.S., Nieminen M.S., Valtonen V.V., Hietaniemi K.L. Dental infections and coronary atherosclerosis. Atherosclerosis. 1993;103(2):205–211. doi: 10.1016/0021-9150(93)90263-t. [DOI] [PubMed] [Google Scholar]
- 57.Buhlin K., Mäntylä P., Paju S., et al. Periodontitis is associated with angiographically verified coronary artery disease. J. Clin. Periodontol. 2011;38(11):1007–1014. doi: 10.1111/j.1600-051X.2011.01775.x. [DOI] [PubMed] [Google Scholar]
- 58.Costa T.H., de Figueiredo Neto J.A., de Oliveira A.E., Lopes e Maia Mde F., de Almeida A.L. Association between chronic apical periodontitis and coronary artery disease. J. Endod. 2014;40(2):164–167. doi: 10.1016/j.joen.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 59.Yakob M., Söder B., Meurman J.H., Jogestrand T., Nowak J., Söder P.Ö. Prevotella nigrescens and Porphyromonas gingivalis are associated with signs of carotid atherosclerosis in subjects with and without periodontitis. J. Periodontal Res. 2011;46(6):749–755. doi: 10.1111/j.1600-0765.2011.01398.x. [DOI] [PubMed] [Google Scholar]
- 60.Yakob M., Meurman J.H., Jogestrand T., Nowak J., Söder P.Ö., Söder B. C-reactive protein in relation to early atherosclerosis and periodontitis. Clin. Oral Investig. 2012;16(1):259–265. doi: 10.1007/s00784-010-0487-6. [DOI] [PubMed] [Google Scholar]
- 61.Söder B., Meurman J.H., Söder P.Ö. Dental calculus links statistically to angina pectoris: 26-year observational study. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0157797. Published 2016 Jun 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Söder B., Meurman J.H., Söder P.Ö. Dental calculus is associated with death from heart infarction. Biomed. Res. Int. 2014;2014 doi: 10.1155/2014/569675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dietrich T., Sharma P., Walter C., Weston P., Beck J. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease [published correction appears in J Clin Periodontol. 2013 Apr;40 Suppl 14:S210–5] J. Clin. Periodontol. 2013;40(Suppl. 14):S70–S84. doi: 10.1111/jcpe.12062. [DOI] [PubMed] [Google Scholar]
- 64.Ford P.J., Gemmell E., Hamlet S.M., et al. Cross-reactivity of GroEL antibodies with human heat shock protein 60 and quantification of pathogens in atherosclerosis. Oral Microbiol. Immunol. 2005;20(5):296–302. doi: 10.1111/j.1399-302X.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 65.Ford P.J., Gemmell E., Chan A., et al. Inflammation, heat shock proteins and periodontal pathogens in atherosclerosis: an immunohistologic study. Oral Microbiol. Immunol. 2006;21(4):206–211. doi: 10.1111/j.1399-302X.2006.00276.x. [DOI] [PubMed] [Google Scholar]
- 66.Nakano K., Nemoto H., Nomura R., Inaba H., Yoshioka H., Taniguchi K., et al. Detection of oral bacteria in cardiovascular specimens. Oral Microbiol. Immunol. 2009;24:64–68. doi: 10.1111/j.1399-302X.2008.00479.x. [DOI] [PubMed] [Google Scholar]
- 67.Pucar A., Milasin J., Lekovic V., Vukadinovic M., Ristic M., Putnik S., et al. Correlation between atherosclerosis and periodontal putative pathogenic bacterial infections in coronary and internal mammary arteries. J. Periodontol. 2007;78:677–682. doi: 10.1902/jop.2007.060062. [DOI] [PubMed] [Google Scholar]
- 68.Zardawi F., Gul S., Abdulkareem A., Sha A., Yates J. Association between periodontal disease and atherosclerotic cardiovascular diseases: revisited. Front. Cardiovasc. Med. 2021;7 doi: 10.3389/fcvm.2020.625579. . Published 2021 Jan 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beukers N.G., van der Heijden G.J., van Wijk A.J., Loos B.G. Periodontitis is an independent risk indicator for atherosclerotic cardiovascular diseases among 60 174 participants in a large dental school in the Netherlands. J. Epidemiol. Community Health. 2017;71(1):37–42. doi: 10.1136/jech-2015-206745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bokhari S.A., Khan A.A., Butt A.K., et al. Non-surgical periodontal therapy reduces coronary heart disease risk markers: a randomized controlled trial. J. Clin. Periodontol. 2012;39(11):1065–1074. doi: 10.1111/j.1600-051X.2012.01942.x. [DOI] [PubMed] [Google Scholar]
- 71.Tonetti M.S., D’Aiuto F., Nibali L., et al. Treatment of periodontitis and endothelial function [published correction appears in N Engl J Med. 2018 Jun 13;:null] N. Engl. J. Med. 2007;356(9):911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 72.Liccardo D, Cannavo A, Spagnuolo G, Ferrara N, Cittadini A, Rengo C, Rengo G. Periodontal disease: a risk factor for diabetes and cardiovascular disease. Int. J. Mol. Sci. 2019; 20(6):1414. https://doi.org/ 10.3390/ijms20061414. [DOI] [PMC free article] [PubMed]
- 73.Santos C.M., Lira-Junior R., Fischer R.G., Santos A.P., Oliveira B.H. Systemic antibiotics in periodontal treatment of diabetic patients: a systematic review. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0145262. (Published 2015 Dec 22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guzman S., Karima M., Wang H.Y., Van Dyke T.E. Association between interleukin-1 genotype and periodontal disease in a diabetic population. J. Periodontol. 2003;74(8):1183–1190. doi: 10.1902/jop.2003.74.8.1183. [DOI] [PubMed] [Google Scholar]
- 75.Tsai C., Hayes C., Taylor G.W. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent. Oral Epidemiol. 2002;30(3):182–192. doi: 10.1034/j.1600-0528.2002.300304.x. [DOI] [PubMed] [Google Scholar]
- 76.Altamash M., Klinge B., Engström P.E. Periodontal treatment and HbA1c levels in subjects with diabetes mellitus. J. Oral Rehabil. 2016;43(1):31–38. doi: 10.1111/joor.12339. [DOI] [PubMed] [Google Scholar]
- 77.Wang X., Han X., Guo X., Luo X., Wang D. The effect of periodontal treatment on hemoglobin a1c levels of diabetic patients: a systematic review and meta-analysis. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0108412. (Published 2014 Sep 25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patil V.S., Patil V.P., Gokhale N., Acharya A., Kangokar P. Chronic periodontitis in type 2 diabetes mellitus: oxidative stress as a common factor in periodontal tissue injury. J. Clin. Diagn. Res. 2016;10(4):BC12–BC16. doi: 10.7860/JCDR/2016/17350.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Allen E.M., Matthews J.B., O’Halloran D.J., Griffiths H.R., Chapple I.L. Oxidative and inflammatory status in type 2 diabetes patients with periodontitis. J. Clin. Periodontol. 2011;38(10):894–901. doi: 10.1111/j.1600-051X.2011.01764.x. [DOI] [PubMed] [Google Scholar]
- 80.Chen L., Wei B., Li J., et al. Association of periodontal parameters with metabolic level and systemic inflammatory markers in patients with type 2 diabetes. J. Periodontol. 2010;81(3):364–371. doi: 10.1902/jop.2009.090544. [DOI] [PubMed] [Google Scholar]
- 81.Liu Y., Zhang Q. Periodontitis aggravated pancreatic β-cell dysfunction in diabetic mice through interleukin-12 regulation on Klotho. J. Diabetes Investig. 2016;7(3):303–311. doi: 10.1111/jdi.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Merchant A.T., Georgantopoulos P., Howe C.J., Virani S.S., Morales D.A., Haddock K.S. Effect of long-term periodontal care on hemoglobin A1c in type 2 diabetes. J. Dent. Res. 2016;95(4):408–415. doi: 10.1177/0022034515622197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tang Z., Fan Q., Jiang Q., et al. The effect of antibiotics on the periodontal treatment of diabetic patients with periodontitis: a systematic review and meta-analysis. Front. Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1013958. Published 2023 Jan 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baeza M., Morales A., Cisterna C., et al. Effect of periodontal treatment in patients with periodontitis and diabetes: systematic review and meta-analysis. J. Appl. Oral Sci. 2020;28 doi: 10.1590/1678-7757-2019-0248. (Published 2020 Jan 10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grellmann A.P., Sfreddo C.S., Maier J., Lenzi T.L., Zanatta F.B. Systemic antimicrobials adjuvant to periodontal therapy in diabetic subjects: a meta-analysis. J. Clin. Periodontol. 2016;43(3):250–260. doi: 10.1111/jcpe.12514. [DOI] [PubMed] [Google Scholar]
- 86.Lira Junior R., Santos C.M.M., Oliveira B.H., Fischer R.G., Santos A.P.P. Effects on HbA1c in diabetic patients of adjunctive use of systemic antibiotics in nonsurgical periodontal treatment: a systematic review. J. Dent. 2017;66:1–7. doi: 10.1016/j.jdent.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 87.Fentoglu O., Bozkurt F.Y. The bi-directional relationship between periodontal disease and hyperlipidemia. Eur. J. Dent. 2008;2(2):142–146. [PMC free article] [PubMed] [Google Scholar]
- 88.Fentoğlu O., Oz G., Taşdelen P., Uskun E., Aykaç Y., Bozkurt F.Y. Periodontal status in subjects with hyperlipidemia. J. Periodontol. 2009;80(2):267–273. doi: 10.1902/jop.2009.080104. [DOI] [PubMed] [Google Scholar]
- 89.Shivakumar T., Patil V.A., Desai M.H. Periodontal status in subjects with hyperlipidemia and determination of association between hyperlipidemia and periodontal health: a clinicobiochemical study. J. Contemp. Dent. Pract. 2013;14(5):785–789. doi: 10.5005/jp-journals-10024-1403. (Published 2013 Sep 1) [DOI] [PubMed] [Google Scholar]
- 90.Cutler C.W., Shinedling E.A., Nunn M., Jotwani R., Kim B.O., Nares S., et al. Association between periodontitis and hyperlipidemia: cause or effect? J. Periodontol. 1999;70(12):1429–1434. doi: 10.1902/jop.1999.70.12.1429. [DOI] [PubMed] [Google Scholar]
- 91.Moeintaghavi A., Haerian-Ardakani A., Talebi-Ardakani M., Tabatabaie I. Hyperlipidemia in patients with periodontitis. J. Contemp. Dent. Pract. 2005;6(3):78–85. (Published 2005 Aug 15) [PubMed] [Google Scholar]
- 92.Buhlin K., Gustafsson A., Pockley A.G., Frostegård J., Klinge B. Risk factors for cardiovascular disease in patients with periodontitis. Eur. Heart J. 2003;24(23):2099–2107. doi: 10.1016/j.ehj.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 93.Morita M., Horiuchi M., Kinoshita Y., Yamamoto T., Watanabe T. Relationship between blood triglyceride levels and periodontal status. Community Dent. Health. 2004;21(1):32–36. [PubMed] [Google Scholar]
- 94.Iacopino A.M., Cutler C.W. Pathophysiological relationships between periodontitis and systemic disease: recent concepts involving serum lipids. J. Periodontol. 2000;71(8):1375–1384. doi: 10.1902/jop.2000.71.8.1375. [DOI] [PubMed] [Google Scholar]
- 95.Croft K.D., Beilin L.J., Vandongen R., Rouse I., Masarei J. Leukocyte and platelet function and eicosanoid production in subjects with hypercholesterolaemia. Atherosclerosis. 1990;83(2–3):101–109. doi: 10.1016/0021-9150(90)90155-c. [DOI] [PubMed] [Google Scholar]
- 96.Krause S., Brachmann P., Brandes C., Lösche W., Hoffmann T., Gängler P. Aggregation behaviour of blood granulocytes in patients with periodontal disease. Arch. Oral Biol. 1990;35(1):75–77. doi: 10.1016/0003-9969(90)90119-u. [DOI] [PubMed] [Google Scholar]
- 97.Fukushima R., Saito H., Taniwaka K., et al. Different roles of IL-1 and TNF on hemodynamics and interorgan amino acid metabolism in awake dogs. Am. J. Phys. 1992;262(3 Pt 1):E275–E281. doi: 10.1152/ajpendo.1992.262.3.E275. [DOI] [PubMed] [Google Scholar]
- 98.Gwosdow A.R., Kumar M.S., Bode H.H. Interleukin 1 stimulation of the hypothalamic-pituitary-adrenal axis. Am. J. Phys. 1990;258(1 Pt 1):E65–E70. doi: 10.1152/ajpendo.1990.258.1.E65. [DOI] [PubMed] [Google Scholar]
- 99.Fried S.K., Zechner R. Cachectin/tumor necrosis factor decreases human adipose tissue lipoprotein lipase mRNA levels, synthesis, and activity. J. Lipid Res. 1989;30(12):1917–1923. [PubMed] [Google Scholar]
- 100.D’Aiuto F., Nibali L., Parkar M., Suvan J., Tonetti M.S. Short-term effects of intensive periodontal therapy on serum inflammatory markers and cholesterol. J. Dent. Res. 2005;84(3):269–273. doi: 10.1177/154405910508400312. [DOI] [PubMed] [Google Scholar]
- 101.Pussinen P.J., Vilkuna-Rautiainen T., Alfthan G., et al. Severe periodontitis enhances macrophage activation via increased serum lipopolysaccharide. Arterioscler. Thromb. Vasc. Biol. 2004;24(11):2174–2180. doi: 10.1161/01.ATV.0000145979.82184.9f. [DOI] [PubMed] [Google Scholar]
- 102.Fajardo M.E., Rocha M.L., Sánchez-Marin F.J., Espinosa-Chávez E.J. Effect of atorvastatin on chronic periodontitis: a randomized pilot study. J. Clin. Periodontol. 2010;37(11):1016–1022. doi: 10.1111/j.1600-051X.2010.01619.x. [DOI] [PubMed] [Google Scholar]
- 103.Hansen, P.R., Holmstrup, P. (2022). Cardiovascular diseases and periodontitis. In: Santi-Rocca, J. (Eds) Periodontitis. Advances in Experimental Medicine and Biology, vol 1373. Springer, Cham. doi: 10.1007/978-3-030-96881-6_14. [DOI] [PubMed]
- 104.Chou S.H., Tung Y.C., Lin Y.S., et al. Major adverse cardiovascular events in treated periodontitis: a population-based follow-up study from Taiwan. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0130807. Published 2015 Jun 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park S.Y., Kim S.H., Kang S.H., et al. Improved oral hygiene care attenuates the cardiovascular risk of oral health disease: a population-based study from Korea. Eur. Heart J. 2019;40(14):1138–1145. doi: 10.1093/eurheartj/ehy836. [DOI] [PubMed] [Google Scholar]
- 106.Merchant, A.T., Virani, S.S. Evaluating periodontal treatment to prevent cardiovascular disease: challenges and possible solutions. Curr. Atheroscler. Rep. 19, 4 (2017). https://doi.org/ 10.1007/s11883-017-0640-7. [DOI] [PubMed]
- 107.Merchant A.T., Virani S.S. Childhood oral infections and subclinical atherosclerosis in adulthood: should we wait for causality or just treat? JAMA Netw. Open. 2019;2(4) doi: 10.1001/jamanetworkopen.2019.2489. (Published 2019 Apr 5) [DOI] [PubMed] [Google Scholar]
- 108.Robins J.M. Association, causation, and marginal structural models. Synthese. 1999;121:151–179. [Google Scholar]