Abstract
Background and Objectives
The effect of different coffee and tea consumption on postprandial glucose and lipid metabolism has never been reported previously. The aim of the present study was to investigate the effect of different coffee or tea consumption at breakfast on postprandial cardiometabolic risk factors in healthy individuals.
Methods and Study Design
Eighteen healthy young subjects completed the trial. After 8-hour overnight fast, volunteers either ingested water, freeze-dried coffee, spray-dried coffee, green tea, black tea or oolong tea together with a breakfast consisting of an egg and 180g deep-fried dough sticks. Blood was drawn at 0h, 0.5h, 1h, 2h, and 3h.
Results
The differences in triglyceride (TG) values relative to the baseline levels at 2h and 3h of green tea was significantly decreased compared with black tea and oolong tea (p<0.05). Compared with black tea, green tea and oolong tea significantly reduced postprandial total cholesterol (TC) levels (p<0.05, p<0.01), respectively. Furthermore, the serum concentrations of high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were substantially decreased after oolong tea consumption compared with black tea (p<0.05, p<0.01).
Conclusions
Green tea ingestion can lower the elevation of serum TG and TC levels after high-fat or high-cholesterol diets. Our findings have far-reaching implications given the widespread use of coffee and tea and the current concern over cardiometabolic risk factors.
Key Words: beverage, coffee, tea, postprandial cardiometabolic risk, triglyceride
Introduction
Coffee is one of the most frequently consumed beverages worldwide. Drinking three to five cups of coffee a day (a cup of regular coffee contains about 70 to 150mg of caffeine) is associated with a reduced risk of cardiovascular disease (CVD) and type 2 diabetes (T2D). Coffee, brewed and drunk in myriad ways, has attracted special attention regarding its effects on certain diseases. Previous epidemiological studies have shown that coffee consumption largely reduces the risk of T2D.1 Cohort studies from Dutch,2 US,3 Sweden,4 and Finland5, 6 have reported that the risk of T2D is lowered with increased coffee consumption. Habitual coffee consumption was also reported to delay the manifestations of metabolic syndrome.7 Coffee contains substantial amounts of caffeine, chlorogenic acid, magnesium, and other micronutrients that may promote glucose metabolism.8 Coffee consumption reduces waist circumference, blood pressure, triglycerides, and fasting plasma glucose levels.1 The latest research indicated that higher caffeine concentrations in plasma may reduce the risk of obesity and T2D, and 43% of the effects of caffeine on susceptibility to T2D were achieved by reduced body mass index (BMI).9
In 2020, the global tea consumption is about 6.3 billion kg, and about 3 billion people like drinking tea. Tea from Camellia sinensis is the second most commonly con-
sumed beverage worldwide after water, and different processing methods produce different types of tea. Tea is produced worldwide. Approximately 78% is black tea, which is favored by Europeans and North Americans; 20% is green tea, which is commonly consumed in Asian countries; and < 2% is oolong tea, popular in South China.9 Various degrees of processing and fermentation lead to differences among the three tea types. Specifically, black tea is fully fermented, oolong tea is partially fermented, and green tea is processed without fermentation. Tea contains more than 4000 phytochemicals, some of which have health benefits.10 Unlike green tea, black tea and oolong tea contain a mixture of catechins, which are good sources of oxidized polymeric substances such as thearubigins and theaflavins.11
The polyphenolic compounds catechins are thought to contribute to the health-promoting effects of green tea.12 These catechins have the potential to prevent CVD and cancer owing to their antioxidant properties.13 Green tea may also have beneficial effects on fasting glucose, insulin levels, glucose metabolism, or insulin sensitivity. However, Josic et al. reported no benefit of green tea on insulin sensitivity or glucose levels.14 Flavonoids found in black tea are associated with a reduced risk of CVD mortality. In older women, greater habitual dietary flavonoids intake was associated with reduced abdominal aortic calcification (AAC). AAC is a predictor of cardiovascular risk such as heart attack and stroke, while it was also found to be a reliable predictor of senile dementia. Those who drank two, four, and six cups of black tea were 16%, 30%, 42% less likely to develop extensive AAC than those who drank zero cups of black tea per day.15 Some randomized trials have reported significant hypocholesterolemic effects of black tea consumption,15, 16 whereas others have reported no effect on lipid profiles.17, 18 Research on the biological activity of oolong tea is limited, but the hypolipidemic effects of oolong tea have been demonstrated both in humans19 and animal models.20 Oolong tea has also been found to improve lipid metabolism21 and decrease body weight.13, 22
However, in vitro and in vivo studies on the mechanisms underlying the effects of coffee consumption on the development of T2D and insulin resistance have yielded inconsistent results. Moreover, well-controlled human studies are lacking. A traditional Chinese breakfast includes high-fat items such as egg and deep-fried dough sticks. The acute effects of coffee or tea on glucose, insulin, or lipid profile when ingested along with such high-fat foods have not been studied before. We hypothesized that coffee or tea consumption may reduce postprandial cardiometabolic risk factors.
Therefore, the aim of this trial was to assess the effects of freeze-dried coffee, spray-dried coffee, green tea, black tea, and oolong tea on serum glucose, insulin, and lipid levels after consumption of Chinese deep-fried dough sticks and an egg in healthy individuals.
Methods
Study design and participants
This trial employed a randomized, placebo-controlled, crossover design to evaluate the effects of five different beverages. The protocol was approved by the Ethics Committee of Beijing Luhe Hospital affiliated with Capital Medical University and registered in the Chinese Clinical Trial Registry (ChiCTR1900025675).
Eligible participants included healthy adults, aged 18-45 years, restricted to a BMI of 18-28kg/m2. Criteria for inclusion required participants to be non-smokers and non-drinkers and not be diagnosed with diabetes mellitus, hyperglycemia, hyperlipemia, and hyperuricemia. Pregnant and lactating women and individuals with intolerance toward eggs, wheat, tea, coffee were excluded from the study. Individuals with heart and liver disease or other acute digestive tract diseases, respiratory diseases, and systemic disorders such as blood, endocrine, neurological and mental disorders were also excluded.
All participants were selected from among students at Capital Medical University or workers at Beijing Luhe Hospital. A total of 26 healthy individuals met the inclusion criteria. Two women were excluded on the first occasion due to the difficulties in drawing blood, two women were excluded due to an inability to adhere to the diet, and one man was excluded due to fainting during blood withdrawal. The rest were excluded mainly due to loss to follow-up, work, or personal reasons. Thus, 18 individuals (9 women and 9 men) were included in this study. Data were thus collected from nine men and nine women [(mean ± SE): age 25.8 ± 1.9 years (range 18-44 years); BMI 23.1 ± 0.5kg/m2 (range 19.2-26.4kg/m2)].
The trial was conducted at Beijing Luhe Hospital, Capital Medical University. A questionnaire was completed by each participant, which included health history, diet, and physical activity. All eligible participants signed an informed consent form before any study procedures began and were informed about the study protocol. In addition, all participants were asked to maintain their physical activity and normal dietary intake during the washout period of one week.
Randomization
After screening for age and BMI of the enrolled subjects, 18 participants who met the inclusion criteria were randomized by a computer-generated random number sequence into one of the test beverage groups. CMZ was responsible for preparing and providing the beverages for the participants.
Treatments
Participants were required to abstain from caffeine consumption, and medication in the morning prior to and during the test. After an 8-hour overnight fast, venous blood was collected from each participant. Subsequently, the participants were randomly assigned to either the water group (200mL), freeze-dried coffee group (150mL, containing 2g coffee solids), spray-dried coffee group (150mL, containing 2g coffee solids), green tea group (250mL, contain 2g tea solids), black tea group (250mL, containing 2g tea solids), or oolong tea group (250mL, containing 2g tea solids). Participants were asked to wait in a quiet and comfortable room during the morning (8:00 am-11:00 am). The assigned beverage was consumed together with breakfast consisting of an egg and 180g deep-fried dough sticks. The meal was to be finished within 25min. Then, blood samples were taken at 0.5h, 1h, 2h, and 3h from each participant after the meal. At 4°C, all blood samples were centrifuged at 3000×g for 10 min. Then, the serum was instantly stored at −80°C until subsequent analysis. After a washout period, participants were crossed over to another group to receive the test protocol repeated until every participant had finished each beverage.
Anthropometric measurements
Anthropometric measurements of all participants were performed by an interviewer at entry, including height, body weight, blood pressure, waist circumference, and hip circumference.
Laboratory measurements
Serum uric acid (UA), glucose, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were analyzed using enzyme-based colorimetric test kits on a Beckman AU5800 fully automatic biochemical analyzer (Beckman Coulter Commercial Enterprise Co., Ltd, Shanghai, China). Insulin was analyzed by chemiluminescence immunoassay (Cobas E601 automatic analyzer, Roche Diagnostics Ltd, Shanghai, China).
Statistical analysis
Sample size was determined based on previous randomized controlled trials with green tea.14 The trial was designed to detect a difference of 1.5mg/dL in serum glucose and 0.5 mmol/L in TG. We assumed a dropout rate of 25% and a standard error (SE) of 0.7mg/dL for serum glucose and 0.4mmol/L for TG. Thus, according to these assumptions, 18 participants were enrolled into the study to have a power of 80% for a two-sided test with a significance level of 0.05.
All data were examined for normal distribution using the Shapiro-Wilk test before analysis. One-way analysis of variance (ANOVA) was used to evaluate baseline characteristics to ensure that important covariates were equally distributed among random groups. The effects of the different treatments and at different timepoints on all parameters were analyzed using one-way repeated measures ANOVA. Within-group differences were examined using ANOVA and post hoc multiple comparisons. The results for baseline characteristics and measured values are shown as the mean ± SE. Area under the curve (AUC) was evaluated using the trapezoidal rule (GraphPad Prism 5, San Diego, CA, USA) for changes in different parameters. Differences were considered significant if p<0.05. All statistical calculations were performed using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA).
Results
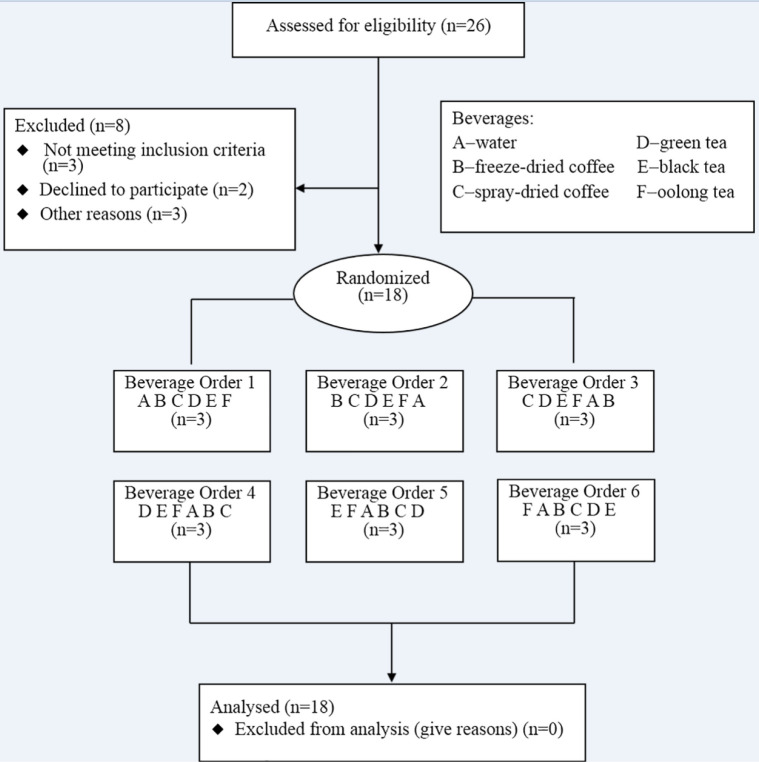
Figure 1 shows participant flow through the study. 18 participants were randomly assigned to one of six beverage orders, with three subjects per beverage order. Table 1 shows the baseline characteristics of the participants. There was no statistically significant difference between the groups. We observed no significant change in parameters related to liver and kidney functions (data not shown). Changes within 3h in all measured parameters after consuming the different beverages are shown in Figure 2 and Figure 3.
Figure 1.
Flow chart of study participants
Table 1.
Baseline characteristics of study participants
| Characteristic | Value |
|---|---|
| Age (year) | 25.8±1.90 |
| BMI (kg/m2) | 23.1±0.50 |
| Body weight (kg) | 65.7±2.20 |
| TG (mmol/L) | 1.12±0.14 |
| TC (mmol/L) | 3.99±0.15 |
| HDL-C (mmol/L) | 1.22±0.05 |
| LDL-C (mmol/L) | 2.41±0.13 |
| Systolic blood pressure (mmHg) | 121±2.50 |
| Diastolic blood pressure (mmHg) | 71.2±1.60 |
| Waist circumference (cm) | 82.7±1.80 |
| Hip circumference (cm) | 97.6±1.30 |
| Glucose (mmol/L) | 5.27±0.12 |
| UA (µmol/L) | 347±24.7 |
| Insulin (mmol/L) | 11.4±2.25 |
BMI: body mass index; TG: triglyceride; TC: total cholesterol; HDL-C: high-density lipoprotein cholestero; LDL-C: low-density lipoprotein cholesterol; UA: uric acid
All values are presented as the mean ± SE
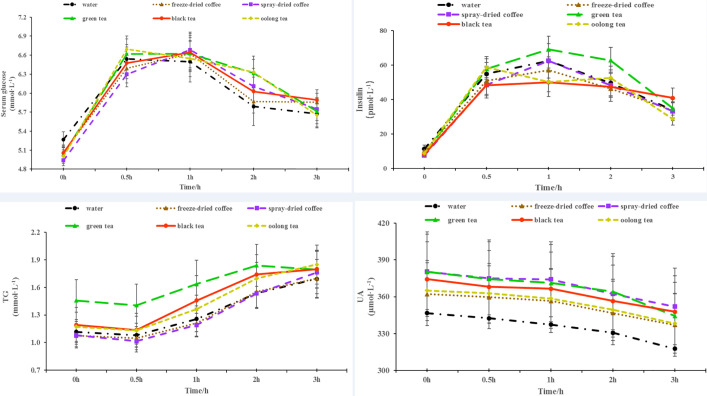
Figure 2.
Changes in serum glucose, insulin, triglycerides (TG) and uric acid (UA) within 3 h after consuming different beverages. All values are shown as the mean ± SE.
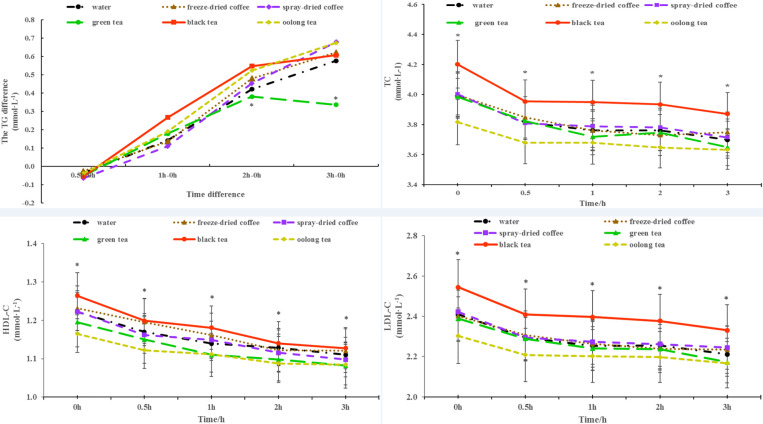
Figure 3.
The differences in TG values (0.5h-0h,1h-0h, 2h-0h, 3h-0h) of different beverages and changes in serum total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) within 3 h after consuming different beverages. The differences in TG values: * Between-group significance, the TG difference (2h-0h, 3h-0h) of green tea compared with black tea and oolong tea p < 0.05. TC: * Between-group significance, green tea and oolong tea compared with black tea (p < 0.05, p < 0.01). HDL-C: * Between-group significance, oolong tea compared with black tea p < 0.05. LDL-C: * Between-group significance, oolong tea compared with black tea p < 0.01. All values are shown as the mean ± SE
There were no significant differences in glucose, insulin, TG, and UA levels among the five beverages (Figure 2). However, considering the varying baseline levels of these indices, we analyzed the differences in TG values relative to the baseline levels at different time points. The results indicated a significant reduction in TG levels for green tea compared to black tea and oolong tea (p<0.05) (Figure 3). This finding suggests that green tea consumption led to an improvement in serum TG levels. Meanwhile, ingestion of green tea and oolong tea resulted in significant lower TC level than black tea consumption (p<0.05, p<0.01), respectively. Moreover, compared with black tea, the concentrations of serum HDL-C and LDL-C were substantially decreased after oolong tea ingestion (p<0.05, p<0.01), respectively (Figure 3). No significant differences were observed in the 3-hour AUC of all parameters after ingestion of any one of these six beverages with the traditional high-fat breakfast.
Discussion
The primary outcome in this study was the effects of six beverages on postprandial glucose, insulin levels, and lipid profile after consumption of a high-fat diet. Our results showed that no significant improvement with ingestion of coffee or tea on serum glucose, insulin and UA. One interpretation may be that the beneficial effect of tea or coffee occurs only with long-term intervention or in individuals with glucose or lipid metabolism problems. Unlike black tea and oolong tea, intake of green tea was inversely associated with TG differences (2h-0h, 3h-0h), which revealed that green tea are beneficial for healthy individuals in improving the postprandial cardiometabolic outcomes. Furthermore, consumption of green tea or oolong tea were associated with significant reduced serum TC, LDL-C and HDL-C levels compared with black tea.
Tea (Camellia sinensis L.) is the second most widely consumed therapeutic and flavored non-alcoholic beverage in the world next to water.23 Considering the extensive intake of tea worldwide, the important health implications couldn't be ignored even its small metabolic effects. In vitro and in vivo studies have shown that tea and its components may improve the pathogenesis of diabetes and glucose metabolism through several mechanisms, such as enhancing insulin sensitivity.24 Tea and its components have been found to have beneficial effects on both glucoregulatory control and endothelial function in the clinical setting.25 Striegel et al. identified that both black tea pomace and black tea water extract reduce postprandial hyperglycemia linked to T2D.26 However, Gavrieli et al. reported no hypoglycemic effect of green and black tea extracts in adults with T2D.27 Moreover, no significant effect of green and black tea extracts was found on glucose control in adults with T2D in a double-blind randomized study.28 Consumption of green tea and oolong tea was associated with a reduced risk of mortality from CVD.29 In brief, the results obtained from different animal or human studies of glucose regulation are inconsistent.
Green tea extract (GTE) and the major component epigallocatechin-3-gallate has been reported to improve hypertriglyceridemia and related lipid dysregulation30 through the inhibition of pancreatic lipase,31 regulation of AMP-activated protein kinase,32 modulation of gut microbiota and bile acid metabolism,33 or inflammatory control in hypothalamus and liver.34 In a randomized, single-blind, parallel-group clinical trial including 40 healthy young women, after the ingestion of 200mL of green tea beverage, postprandial blood TG level at 4h was significantly lower compared to the control group, which suggest that green tea ingestion can lower the elevation of blood TG level after a high-fat meal in young women.35 Similarly, in the present study, we observed decreased TG concentration after green tea intake, which indicated that green tea improved the postprandial serum TG level in healthy individuals.
Green tea consumption was associated with a lower risk of total cardiovascular mortality. For women, the total CVD mortality risk was decreased by 23%, 19%, 38% respectively from drinking 1-2 cups, 3-5 cups, and >6 cups of green tea per day compared with those who did not drink green tea (<1 cup/week) . Drinking ≥1 cup of oolong tea per day was associated with a reduced risk of total CVD mortality. This association was more pronounced in men and was associated with a 61% decrease in the overall risk of CVD mortality. However, the amount of black tea consumed was not associated with coronary heart disease, stroke, or all-cause CVD in either men or women.36 In conclusion, there was a negative correlation between the consumption of green tea, oolong tea and the risk of cardiovascular death, but not with black tea. We speculated that the antioxidant properties were reduced in fully fermented black tea compared with medium fermented oolong tea and unfermented green tea.
In an animal study, dietary epigallocatechin gallate-the main component of green tea polyphenols decreased the plasma TC and non-HDL-C levels in a dose-dependent manner by inhibiting cholesterol absorption associated with mild fat malabsorption.37 In another animal study, metabolic changes induced by green tea intake activated AMP-activated protein kinase and modulated the expression of genes involved in metabolism, particularly in the adipose tissue, thus showing its therapeutic potential against obesity, insulin resistance, and dyslipidemia in rats.38 Green tea is an easily accessible and popular drink and it may indirectly lead to lower morbidity and mortality rates due to CVD by ameliorating hyperlipidemia. In a human trial, administration of GTE for 12 months significantly reduced the TC, LDL-C, and non-HDL-C levels in postmenopausal women. The same trial suggested that GTE may be recommended for reducing cholesterol levels, particularly in individuals with high cholesterol concentrations.39 Current meta-analyses of clinical trials of the effect of short-term (3-24 weeks) green tea consumption on lipid profile showed that green tea or GTE significantly decreased both TC (by 5mg/dL) and LDL-C (by 2mg/dL) across different populations.40, 41 Similar conclusions were also obtained in this study that green tea intake reduced the TC concentrations in healthy individuals, which showed the beneficial improvement effects from green tea on postprandial cardiometabolic risk factors.
Habitual coffee consumption is associated with a lower incidence of glucose intolerance,2-6 and it influences postprandial glucose metabolism rather than fasting glucose metabolism. However, caffeine is reported to negatively affect postprandial glucose homeostasis and lipid profile.42 High caffeine intake might lower the risk of diseases related to adiposity, such as CVD through reducing weight, BMI, and fat mass.37 There were similar reports that consumption of coffee and total caffeine intake was associated with a reduced risk of mortality from CVD, coronary heart disease and metabolic syndrome.36, 43, 44 There were no strong associations were reported between genetically predicted plasma caffeine concentrations and cardiometabolic risk factors.45 In the present study, freeze-dried coffee and spray-dried coffee were not associated with improvements on glucose and lipid profile, which may be owing to the acute and short-term intervention or the small amount of caffeine.
A limitation of our study was that the diets of the participants differed before the test. Some participants failed to maintain a light diet. For example, some participants consumed barbecue, hot pot, and other heavy meals on the day before the test. In addition, some participants had dinner too late resulting in the absence of an effect on the lipid profile or UA levels.
Conclusion
Concerns about the correct choice of beverage seem to exist. The present results suggest that it may be better to drink green tea when consuming high-fat or high-cholesterol diets. These findings emphasized the potential role of healthy choices of beverages in managing the cardiometabolic risk factors. Well-controlled studies with a larger sample size may further enhance our understanding of the acute effects and mechanisms of coffee or tea consumption on the levels of serum glucose and blood lipids in healthy individuals.
Acknowledgements
We thank all the participants of the study and the staff at Beijing Luhe Hospital, Capital Medical University.
Conflict of Interest and Funding Disclosure
The authors declare no conflict of interest. This research was supported by Qingdao Postdoctoral Application Research Project (RZ1900010484)
Funding Statement
This research was supported by Qingdao Postdoctoral Application Research Project (RZ1900010484)
References
- 1.Tunnicliffe JM, Shearer J. Coffee, glucose homeostasis, and insulin resistance: physiological mechanisms and mediators. Appl Physiol Nutr Metab. 2008;33:1290–1300. doi: 10.1139/H08-123. [DOI] [PubMed] [Google Scholar]
- 2.van Dam RM, Feskens EJ. Coffee consumption and risk of type 2 diabetes mellitus. Lancet. 2002;360:1477–1478. doi: 10.1016/S0140-6736(02)11436-X. [DOI] [PubMed] [Google Scholar]
- 3.Salazar-Martinez E, Willett WC, Ascherio A, Manson JE, Leitzmann MF, Stampfer MJ, Hu FB. Coffee consumption and risk for type 2 diabetes mellitus. Ann Intern Med. 2004;140:1–8. doi: 10.7326/0003-4819-140-1-200401060-00005. [DOI] [PubMed] [Google Scholar]
- 4.Rosengren A, Dotevall A, Wilhelmsen L, Thelle D, Johansson S. Coffee and incidence of diabetes in Swedish women: a prospective 18-year follow-up study. J Intern Med. 2004;255:89–95. doi: 10.1046/j.1365-2796.2003.01260.x. [DOI] [PubMed] [Google Scholar]
- 5.Tuomilehto J, Hu G, Bidel S, Lindström J, Jousilahti P. Coffee consumption and risk of type 2 diabetes mellitus among middle-aged Finnish men and women. JAMA. 2004;291:1213–1219. doi: 10.1001/jama.291.10.1213. [DOI] [PubMed] [Google Scholar]
- 6.Carlsson S, Hammar N, Grill V, Kaprio J. Coffee consumption and risk of type 2 diabetes in Finnish twins. Int J Epidemiol. 2004;33:616–617. doi: 10.1093/ije/dyh185. [DOI] [PubMed] [Google Scholar]
- 7.Hino A, Adachi H, Enomoto M, Furuki K, Shigetoh Y, Ohtsuka M, et al. Habitual coffee but not green tea consumption is inversely associated with metabolic syndrome: an epidemiological study in a general Japanese population. Diabetes Res Clin Pract. 2007;76:383–389. doi: 10.1016/j.diabres.2006.09.033. 10.1016/j.diabres. 2006.09.033 . [DOI] [PubMed] [Google Scholar]
- 8.van Dam RM, Dekker JM, Nijpels G, Stehouwer CDA, Bouter LM, Heine RJ. Coffee consumption and incidence of impaired fasting glucose, impaired glucose tolerance, and type 2 diabetes: the Hoorn Study. Diabetologia. 2004;47:2152–2159. doi: 10.1007/s00125-004-1573-6. [DOI] [PubMed] [Google Scholar]
- 9.Singh BN, Rawat AK, Bhagat RM, Singh BR. Black tea: phytochemicals, cancer chemoprevention and clinical studies. Crit Rev Food Sci Nutr. 2015;57:1394–1410. doi: 10.1080/10408398.2014.994700. [DOI] [PubMed] [Google Scholar]
- 10.Yung LM, Leung FP, Wong WT, Tian XY, Yung LH, Chen ZY, Yao XQ, Huang Y. Tea polyphenols benefit vascular function. Inflammopharmacology. 2008;16:230–234. doi: 10.1007/s10787-008-8022-y. [DOI] [PubMed] [Google Scholar]
- 11.Miyata Y, Tamaru S, Tanaka T, Tamaya K, Matsui T, Nagata Y, Tanaka K. Theflavins and theasinensin A derived from fermented tea have antihyperglycemic and hypotriacylglycerolemic effects in KK-A(y) mice and Sprague-Dawley rats. J Agric Food Chem. 2013;61:9366–9372. doi: 10.1021/jf400123y. 10.1021/jf 400123y . [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Ho CT. Polyphenolic chemistry of tea and coffee: a century of progress. J Agric Food Chem. 2009;57:8109–8114. doi: 10.1021/jf804025c. [DOI] [PubMed] [Google Scholar]
- 13.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 14.Josic J, Olsson AT, Wickeberg J, Lindstedt S, Hlebowicz J. Does green tea affect postprandial glucose, insulin and satiety in healthy subjects: a randomized controlled trial. Nutr J. 2010;9:63. doi: 10.1186/1475-2891-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahorun T, Luximon-Ramma A, Neergheen-Bhujun VS, Gunness TK, Googoolye K, Auger C, Crozier A, Aruoma OI. The effect of black tea on risk factors of cardiovascular disease in a normal population. Prev Med. 2012;54:S98–S102. doi: 10.1016/j.ypmed.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Fujita H, Yamagami T. Antihypercholesterolemic effect of Chinese black tea extract in human subjects with borderline hypercholesterolemia. Nutr Res. 2008;28:450–456. doi: 10.1016/j.nutres.2008.04.005. 10.1016/j.nutres. 2008.04.005 . [DOI] [PubMed] [Google Scholar]
- 17.Kubota K, Sumi S, Tojo H, Sumi-Inoue Y, I-chin H, Oi Y, Fujita H, Urata H. Improvements of mean body mass index and body weight in preobese and overweight Japanese adults with black Chinese tea (Pu-Erh) water extract. Nutr Res. 2011;31:421–428. doi: 10.1016/j.nutres.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Trautwein EA, Du Y, Meynen E, Yan X, Wen Y, Wang H, Molhuizen HOF. Purified black tea theaflavins and theaflavins/catechin supplements did not affect serum lipids in healthy individuals with mildly to moderately elevated cholesterol concentrations. Eur J Nutr. 2010;49:27–35. doi: 10.1007/s00394-009-0045-7. [DOI] [PubMed] [Google Scholar]
- 19.He RR, Chen L, Lin BH, Matsui Y, Yao XS, Kurihara H. Beneficial effects of oolong tea consumption on diet-induced overweight and obese subjects. Chin J Integr Med. 2009;15:34–41. doi: 10.1007/s11655-009-0034-8. [DOI] [PubMed] [Google Scholar]
- 20.Kuo KL, Weng MS, Chiang CT, Tsai YJ, Lin-Shiau SY, Lin JK. Comparative studies on the hypolipidemic and growth suppressive effects of oolong, black, pu-erh, and green tea leaves in rats. J Agric Food Chem. 2005;53:480–489. doi: 10.1021/jf049375k. [DOI] [PubMed] [Google Scholar]
- 21.Yang TT, Koo MW. Hypocholesterolemic effects of Chinese tea. Pharmacol Res. 1997;35:505–512. doi: 10.1006/phrs.1997.0176. [DOI] [PubMed] [Google Scholar]
- 22.Han LK, Takaku T, Li J, Kimura Y, Okuda H. Antiobesity action of oolong tea. Int J Obes Relat Metab Disord. 1999;23:98–105. doi: 10.1038/sj.ijo.0800766. [DOI] [PubMed] [Google Scholar]
- 23.Hayat K, Iqbal H, Malik U, Bilal U, Mushtaq S. Tea and its consumption: benefits and risks. Crit Rev Food Sci Nutr. 2015;55:939–954. doi: 10.1080/10408398.2012.678949. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen CT, Lee AH, Pham NM, Do VV, Ngu ND, Tran BQ, Binns C. Habitual tea drinking associated with a lower risk of type 2 diabetes in Vietnamese adults. Asia Pac J Clin Nutr. 2018;27:701–706. doi: 10.6133/apjcn.072017.08. [DOI] [PubMed] [Google Scholar]
- 25.Stote KS, Baer DJ. Tea consumption may improve biomarkers of insulin sensitivity and risk factors for diabetes. J Nutr. 2008;138:1584S–1588S. doi: 10.1093/jn/138.8.1584S. [DOI] [PubMed] [Google Scholar]
- 26.Striegel L, Kang B, Pilkenton SJ, Rychlik M, Apostolidis E. Effect of black tea and black tea pomace polyphenols on α-glucosidase and α-amylase inhibition, relevant to type 2 diabetes prevention. Front Nutr. 2015;2:3. doi: 10.3389/fnut.2015.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gavrieli A, Fragopoulou E, Mantzoros CS, Yannakoulia M. Gender and body mass index modify the effect of increasing amounts of caffeinated coffee on postprandial glucose and insulin concentrations: a randomized, controlled, clinical trial. Metab Clin Exp. 2013;62:1099–1106. doi: 10.1016/j.metabol.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Mackenzie T, Leary L, Brooks WB. The effect of an extract of green and black tea on glucose control in adults with type 2 diabetes mellitus: double-blind randomized study. Metab Clin Exp. 2007;56:1340–1344. doi: 10.1016/j.metabol.2007.05.0018. [DOI] [PubMed] [Google Scholar]
- 29.Wu LY, Juan CC, Ho LT, Hsu YP, Hwang LS. Effect of green tea supplementation on insulin sensitivity in Sprague-Dawley rats. J Agric Food Chem. 2004;52:643–648. doi: 10.1021/jf030365d. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Wu S. Epigallocatechin gallate suppresses hepatic cholesterol synthesis by targeting SREBP-2 through SIRT1/FOXO1 signaling pathway. Mol Cell Biochem. 2018;448:175–185. doi: 10.1007/s11010-018-3324-x. [DOI] [PubMed] [Google Scholar]
- 31.Lee ES, Lee MK. Effect of extraction condition on the content of EGCG and caffeine of green tea: comparison with the inhibitory activity on pancreatic lipase. Natur Prod Sci. 2013;19:166–172. Available from: https://kiss.kstudy.com/Detail/Ar?key=3177204. [Google Scholar]
- 32.Li F, Gao C, Yan P, Zhang M, Wang Y, Hu Y, Wu X, Wang X, Sheng J. EGCG reduces obesity and white adipose tissue gain partly through AMPK activation in mice. Front Pharmacol. 2018;22:1366. doi: 10.3389/fphar.2018.01366. 10.3389/f phar.2018.01366 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naito Y, Ushiroda C, Mizushima K, Inoue R, Yasukawa Z, Abe A, Takagi T. Epigallocatechin-3-gallate (EGCG) attenuates nonalcoholic fatty liver disease via modulating the interaction between gut microbiota and bile acids. J Clin Biochem Nutr. 2020;67:2–9. doi: 10.3164/jcbn.20-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou H, Yang W, Bao S, Cao Y. Epigallocatechin gallate suppresses inflammatory responses by inhibiting toll-like receptor 4 signaling and alleviates insulin resistance in the livers of high-fat-diet rats. J Oleo Sci. 2020;69:479–486. doi: 10.5650/jos.ess19303. [DOI] [PubMed] [Google Scholar]
- 35.Trismiyanti T, Gunawan I, Tarigan THE. Effect of green tea ingestion on postprandial triglyceride levels in young women. World Nutr J. 2017;1:23–27. doi: 10.25220/WNJ.V01i1.0006. [DOI] [Google Scholar]
- 36.Mineharu Y, Koizumi A, Wada Y, Iso H, Watanabe Y, Date C, et al. Coffee, green tea, black tea and oolong tea consumption and risk of mortality from cardiovascular disease in Japanese men and women. J Epidemiol Community Health. 2011;65:230–240. doi: 10.1136/jech.2009.097311. [DOI] [PubMed] [Google Scholar]
- 37.Raederstorff DG, Schlachter MF, Elste V, Weber P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J Nutr Biochem. 2003;14:326–332. doi: 10.1016/S0955-2863(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 38.Rocha A, Bolin AP, Cardoso CAL, Otton R. Green tea extract activates AMPK and ameliorates white adipose tissue metabolic dysfunction induced by obesity. Eur J Nutr. 2016;55:2231–2244. doi: 10.1007/s00394-015-1033-8. [DOI] [PubMed] [Google Scholar]
- 39.Samavat H, Newman AR, Wang R, Yuan JM, Wu AH, Kurzer MS. Effects of green tea catechin extract on serum lipids in postmenopausal women: a randomized, placebo-controlled clinical trial. Am J Clin Nutr. 2016;104:1671–1682. doi: 10.3945/ajcn.116.137075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Onakpoya I, Spencer E, Heneghan C, Thompson M. The effect of green tea on blood pressure and lipid profile: a systematic review and meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis. 2014;24:823–836. doi: 10.1016/j.numecd.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 41.Khalesi S, Sun J, Buys N, Jamshidi A, Nikbakht-Nasrabadi E, Khosravi-Boroujeni H. Green tea catechins and blood pressure: a systematic review and meta-analysis of randomised controlled trials. Eur J Nutr. 2014;53:1299–1311. doi: 10.1007/s00394-014-0720-1. [DOI] [PubMed] [Google Scholar]
- 42.Beaudoin MS, Robinson LE, Graham TE. An oral lipid challenge and acute intake of caffeinated coffee additively decrease glucose tolerance in healthy men. J Nutr. 2011;141:574–581. doi: 10.3945/jn.110.132761. [DOI] [PubMed] [Google Scholar]
- 43.Noh HM, Park YS, Kim JH. Coffee consumption and coronary heart disease risk using the Framingham risk score. Asia Pac J Clin Nutr. 2017;26:931–938. doi: 10.6133/apjcn.082016.05. [DOI] [PubMed] [Google Scholar]
- 44.Kim JH, Park YS, Kim H. Association between metabolic syndrome and coffee consumption in the Korean population by gender: A cross-sectional study in Korea. Asia Pac J Clin Nutr. 2018;27:1131–1140. doi: 10.6133/apjcn.022018.04. [DOI] [PubMed] [Google Scholar]
- 45.Larsson SC, Woolf B, Gill D. Appraisal of the causal effect of plasma caffeine on adiposity, type 2 diabetes, and cardiovascular disease: two sample mendelian randomisation study. BMJ Med. 2023;2:1–8. doi: 10.1136/bmjmed-2022-000335. [DOI] [PMC free article] [PubMed] [Google Scholar]