Abstract
We have investigated the mechanism of duck hepatitis B virus (DHBV) entry into susceptible primary duck hepatocytes (PDHs), using mutants of carboxypeptidase D (gp180), a transmembrane protein shown to act as the primary cellular receptor for avian hepatitis B virus uptake. The variant proteins were abundantly produced from recombinant adenoviruses and tested for the potential to functionally outcompete the endogenous wild-type receptor. Overexpression of wild-type gp180 significantly enhanced the efficiency of DHBV infection in PDHs but did not affect ongoing DHBV replication, an observation further supporting gp180 receptor function. A gp180 mutant deficient for endocytosis abolished DHBV infection, indicating endocytosis to be the route of hepadnaviral entry. With further gp180 variants, carrying mutations in the cytoplasmic domain and characterized by an accelerated turnover, the ability of gp180 to function as a DHBV receptor was found to depend on a wild-type-like sorting phenotype which largely avoids transport toward the endolysosomal compartment. Based on these data, we propose a model in which a distinct intracellular DHBV traffic to the endosome, but not beyond, is a prerequisite for completion of viral entry, i.e., for fusion and capsid release. Furthermore, the deletion of the two enzymatically active carboxypeptidase domains of gp180 did not lead to a loss of receptor function.
The first step in viral entry is the attachment of the infectious particle to a cell surface molecule on the host cell. For enveloped viruses, fusion with a cellular membrane must follow, liberating the viral capsid into the cytoplasm. Two modes of fusion have been described: pH-independent fusion at the cell surface, as in the case of human immunodeficiency virus triggered by association with a second receptor, and fusion following endocytosis, triggered by acidification in the endosome (14).
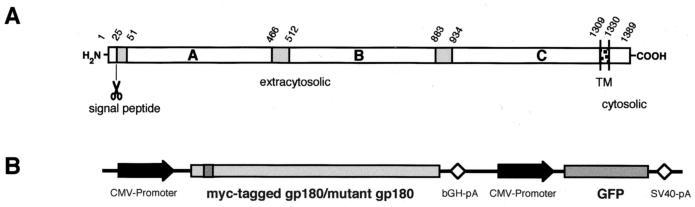
Hepatitis B viruses (hepadnaviruses, HBVs) are small, enveloped DNA viruses causing acute and chronic infection in mammals and birds (7). For the prototypic human HBV, none of the various receptor candidates proposed have been shown to actually function in viral entry, due to the lack of an in vitro infection system (3). In the duck HBV (DHBV) animal model, primary hepatocytes can be readily prepared and infected with DHBV in vitro (21). This system has therefore served as a model to study hepadnaviral entry. Previous work has demonstrated that DHBV uses duck carboxypeptidase D (gp180) as a cellular attachment molecule as a first step for entering the host hepatocyte (2, 12, 20, 23). gp180 is a ubiquitously expressed 180-kDa glycoprotein. Cloning and characterization revealed it to be a type I transmembrane protein with a large extracytoplasmic portion and a short cytoplasmic tail (13, 18) (Fig. 1A). The extracytoplasmic part consists of three homologous, carboxypeptidase E-like domains, of which only the first two (A and B) are enzymatically active; the third, membrane-proximal domain (C) has been shown to be sufficient for DHBV binding (5). However, it has not been assessed whether domains A and B themselves or their enzymatic activities, are dispensable for DHBV receptor function. It is noteworthy that gp180, being a virus receptor, is concentrated in the Golgi complex and only marginally present at the plasma membrane, from where it is rapidly endocytosed (2). As for other proteins with gp180-like sorting properties, such as furin (17), intracellular gp180 traffic has been shown to depend on signals contained in the short cytoplasmic domain (2, 6). In a heterologous cell line, gp180 expression was shown to mediate uptake of fluorescently labeled DHBV particles (2). However, gp180 expression did not render production competent heterologous cells susceptible for DHBV infection (2, 13). Therefore, it has been proposed that a further host-specific component, i.e., a coreceptor, is necessary for fusion to occur, to complete viral entry.
FIG. 1.
(A) Schematic representation of duck carboxypeptidase D (gp180). Three homologous extracytosolic domains (A to C) are preceded by a signal peptide and followed by a transmembrane (TM) region and a short cytosolic domain. Numbers at the top indicate amino acid positions (13, 18). (B) Schematic representation of the expression cassettes, inserted in reverse direction into the deleted E1 region of the recombinant adenovirus vector (for details, see Materials and Methods). bGH-pA and SV40-pA, bovine growth hormone and simian virus 40 poly(A) sites.
Earlier studies have shown that raising the pH in endosomal vesicles cannot inhibit DHBV infection (11, 16). This finding indicates that an acidic pH is not required to activate the fusion reaction of these viruses and implicates a fusion mechanism at the cell surface. On the other hand, energy dependence of DHBV entry (11) and cellular gp180 and fluorescent DHBV particle traffic (2) suggest endocytosis as the route of infection. We have investigated the mechanism of DHBV entry in more detail, using a novel assay in which primary duck hepatocytes (PDHs) were transduced with recombinant adenoviruses encoding various gp180 mutants prior to infection with DHBV. Our findings support the notion that the fusion reaction occurs postendocytosis and that it requires proper intracellular gp180 traffic but not the gp180 carboxypeptidase activity.
MATERIALS AND METHODS
Mutant gp180 expression plasmids.
Plasmids pC-myc-gp180, encoding a Myc epitope-tagged gp180 under cytomegalovirus (CMV) promoter control and serving as the parental plasmid for all constructs, and pC-gp180tl (previously termed pC-gp180Tm) were described previously (2). pC-gp180stl was generated by digesting pC-myc-gp180 with SacI (partially) and EcoRI, cleaving just after the gp180 coding region and in the tail-encoding region, respectively (1). The plasmid was then ligated in the presence of an oligo-DNA duplex introducing a stop codon after gp180 amino acid Phe1373 (…SHEF). For pC-gp180ADA and -DDD (Table 1), EcoRI/XhoI-digested pC-myc-gp180 was ligated with PCR fragments containing the relevant mutations. To obtain gp180-furin chimeric mutants, pC-gp180tl, which contains an artificial PstI site (Ser1333-codon TCA→AGG), was digested with PstI (partially) or XhoI and with XbaI (encoding the stop codon). PCR fragments derived from bovine furin cDNA (kindly provided by Wolfgang Garten) were inserted to yield plasmids encoding the proteins gp180-furint (…CVCS1333-R742SGFS…) and gp180-furintm+t (…LPRE1308-V719VAGL…). pC-gp180ΔAB was constructed by digestion of pCB-myc-gp180 with BspEI (located in front of the introduced Myc tag [1]) and SalI and ligation in the presence of an oligo-DNA duplex encoding another Myc epitope tag (…ELRY47-SGEQKLISEEDL-V867DSK…). All mutations were confirmed by restriction analysis or sequencing. Proper protein expression was verified by Western blotting and immunofluorescence using anti-gp180 and antifurin antisera.
TABLE 1.
Distribution and postendocytotic sorting of gp180 cytoplasmic domain mutants
| gp180 constructa | Cytosolic sequenceb | Distributionc
|
|
|---|---|---|---|
| Steady state | Internalized antibodies | ||
| wt | ..[tm]-SIKSNRHKDGFHRLRQHHDDYEDEIRMMSTGSKKSLLSHEFQDETDTEEETLYSSKH | G | G |
| tl | ..[tm]-SI | S/I | S |
| stl | ..[tm]-SIKSNRHKDGFHRLRQHHDDYEDEIRMMSTGSKKSLLSHEF | V/G | V |
| ADA | ..[tm]-SIKSNRHKDGFHRLRQHHDDYEDEIRMMSTGSKKSLLSHEFQDEADAEEETLYSSKH | G | G/V |
| DDD | ..[tm]-SIKSNRHKDGFHRLRQHHDDYEDEIRMMSTGSKKSLLSHEFQDEDDDEEETLYSSKH | G | G |
| furint | ..[tm]-SRSGFSFRGVKVYTMDRGLISYKGLPPEAWQEECPSDSEEDEGRGERTAFIKDQSAL | G | V |
| furint+tm | ..[tmf]-SRSGFSFRGVKVYTMDRGLISYKGLPPEAWQEECPSDSEEDEGRGERTAFIKDQSAL | G | V |
wt, Myc-tagged gp180; construction of mutants stl, ADA, DDD, gp180-furint and furint+tm chimeras is described in Materials and Methods.
Amino acid sequence starting at position S-1333; tm and tmf, gp180 and furin transmembrane regions, respectively; the casein kinase II recognition motifs are underlined, and point mutations introduced at the predicted phosphorylation sites are indicated in bold type.
G, Golgi; S, cell surface; S/I, mostly cell surface, some intracellular; V, vesicular; V/G predominantly vesicular, some Golgi-like; G/V vice versa. For the experimental setup, see Fig. 5 and Materials and Methods.
Recombinant adenoviruses.
Recombinant adenoviruses were generated using the AdenoEasy system, kindly provided by Tong-Chuan He and Bert Vogelstein and described in detail in reference 8 and online (http://www.coloncancer.org/). gp180 wild-type (gp180wt) or mutant expression cassettes [containing promoter and poly(A) signal sequences from the pC vector] were cloned into the multiple cloning site of pAdTrack, unidirectionally with the CMV-green fluorescent protein (GFP) cassette contained in this vector (8). All subsequent steps up to virus production in 293 cells were carried out as described previously (8). In the infection experiments, crude, high-titer lysates from 293 cells (about 109 GFP expression-forming units [EFU]/ml) were used as source of recombinant adenovirus.
Localization of gp180 and gp180 variants.
For localizing variant gp180 in the cell line HuH7, cells were cultivated on coverglass chamber slides (two wells; Nunc) and transfected with gp180wt- or mutant gp180-expressing plasmids using a standard calcium phosphate protocol or Superfect (Qiagen), 0.1 μg of gp180 DNA, and 1 μg of unspecific plasmid DNA per well. One to two days after transfection, cells were washed with phosphate-buffered saline and fixed with 3% paraformaldehyde for 20 min. The fixed cells were permeabilized with 0.25% Triton X-100 in phosphate-buffered saline and immunostained with monoclonal antibody (MAB) 9E10 (recognizing the Myc epitope tag; a kind gift from Martin Eilers), anti-TGN46 antiserum (2), and fluorescent secondary antibodies (goat anti-rabbit and anti-mouse; Dianova). PDHs were transduced with amounts of the appropriate recombinant adenovirus to yield about 5 to 10% GFP fluorescent cells. After 4 days of further culture, cells were fixed and immunostained with MAB 9E10 and a tetramethylrhodamine isothiocyanate-conjugated secondary antibody (goat anti-mouse; Dianova). For fluorescence analysis, we used a Leica TCS NT confocal laser scanning microscope (63×/1.2 NA w PLAPO objective).
Internalization assay in HuH7 cells.
One to two days after transfection, HuH7 cells were incubated with a 1:1,000 dilution in culture medium of anti-gp180 antiserum (raised against the recombinant ectodomain of gp180 [24]) for 1 to 3 h at 37°C, washed twice, fixed, permeabilized, and immunostained with anti-Myc MAB 9E10 and fluorescent secondary antibodies (Dianova). Generation and uptake reaction of fluorescently labeled viral substrates were performed as described elsewhere (2). Fluorescence analysis was carried out by confocal laser scanning microscopy.
Biochemical pre-S binding assay.
HuH7 cells were transfected with pC-myc-gp180, or the appropriate mutant, and lysed 2 days later by addition of 150 mM NaCl–10 mM Tris-HCl (pH 7.4)–2 mg of aprotinin and leupeptin per ml–1 mM phenylmethylsulfonyl fluoride–1% Triton X-100 (1 ml per 10-cm-diameter dish). After clearing by centrifugation, lysates were subjected to an affinity precipitation with pre-S–Sepharose as described previously (2).
PDHs and DHBV infection interference assay.
PDHs were prepared and cultured in 12-well culture dishes (about 8 × 105 cells/well) as described elsewhere (9). For the infection interference assay, the culture medium was changed 16 h postplating and supplemented with 5% fetal calf serum (Gibco BRL). At 24 h postplating, adenovirus lysates were added to infect >90% of the hepatocytes after overnight incubation (about 100 EFU/cell). Cells were further cultured in serum-free medium for 3 to 4 days prior to DHBV infection. The hepatocytes were incubated for 3 to 5 h with 8 × 107 DNA-containing DHBV particles/well and then washed with glycine buffer (50 mM glycine, 150 mM NaCl [pH 2.2]) for 1.5 min. After 4 days of further culture, cells were harvested, digested with proteinase K, and extracted with phenol and chloroform. This preparation of intracellular viral DNA was subjected to DNA dot blot analysis as previously described (16). The radioactive signals were quantified with a Molecular Dynamics PhosphorImager.
RESULTS
gp180 and gp180 mutants are strongly expressed in PDHs after transduction with recombinant adenoviruses.
Expression of gp180 in heterologous hepatocytes or hepatoma cell lines mediates particle uptake but does not render these susceptible for DHBV infection (2, 13), precluding a mutational analysis of gp180 receptor function beyond physical DHBV binding. We have therefore devised a novel strategy to overcome this limitation, by outcompeting the endogenous gp180 phenotype in DHBV-susceptible duck hepatocytes, through strong expression of mutant gp180 prior to DHBV infection. Since PDHs can be only poorly transfected, with efficiency usually below 10% (our unpublished observations), we used recombinant adenovirus transduction, a powerful tool for efficient gene transfer into various animal cells, including primary hepatocytes.
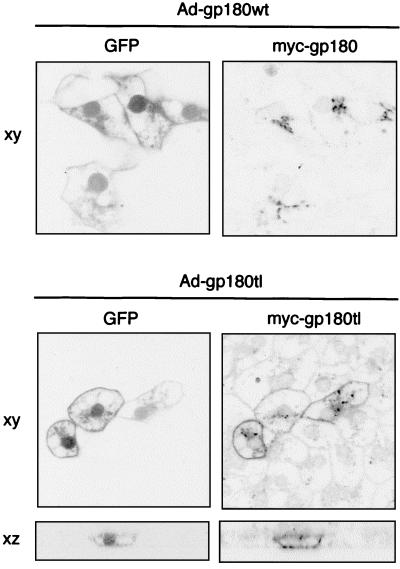
Recombinant adenoviruses were generated using the AdenoEasy system (8). The recombinant E1- and E3-deleted adenoviruses contained a CMV promoter-driven GFP expression cassette, alone or with a gp180 expression cassette under the same promoter, inserted into the ΔE1 region (Fig. 1B; Materials and Methods). In PDHs, GFP autofluorescence (22) became detectable about 24 h after adenovirus transduction and served as a marker for productively infected cells. To confirm expression of variant gp180, PDHs infected with recombinant adenoviruses were fixed and immunostained 4 days posttransduction (Fig. 2 shows data for Myc-tagged gp180 and gp180tl, a mutant lacking the gp180 cytoplasmic domain). In transduced PDHs, Myc-gp180 was properly localized in a Golgi-like compartment, while gp180tl showed in addition to internal staining a pronounced surface distribution. Anti-Myc antibody allowed us to differentiate Myc-tagged gp180 from the endogenous receptor. From Western blot analysis (not shown), we estimated the levels of transduced receptor to be 10- to 100-fold above that of the endogenous protein.
FIG. 2.
Selective visualization in duck hepatocytes of Myc-tagged mutant gp180 expressed from recombinant adenoviruses. Primary hepatocytes were infected with recombinant adenoviruses encoding GFP plus Myc-tagged gp180 (Ad-gp180wt) or GFP plus a Myc-tagged gp180 mutant lacking the gp180 cytoplasmic domain (Ad-gp180tl). On day 4 postinfection, cells were fixed and stained with an anti-Myc antibody (tetramethylrhodamine), and GFP- and anti-Myc-related fluorescence was analyzed by confocal laser scanning microscopy (xy, in the plane of the culture; xz, perpendicular to the culture).
For an infection inhibition assay based on competition, high transduction efficiency is a prerequisite for a reliable macroscopic analysis. Using high amounts of recombinant adenovirus (about 102 EFU/cell) allowed us to transduce more than 90% of the PDHs in culture (not shown).
Arresting gp180 at the cell surface blocks DHBV infection.
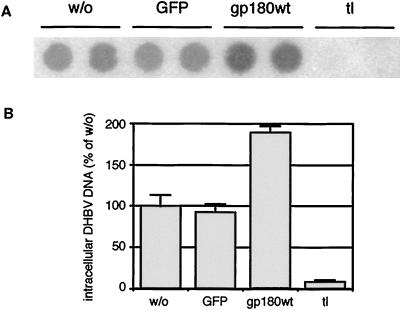
Deletion of the gp180 cytoplasmic tail leads to a surface accumulation of mutant gp180 (gp180tl), and anti-gp180 antibodies, or the viral ligand, are bound to gp180tl at the cell surface but not endocytosed (2). To determine whether DHBV is able to infect PDHs while being arrested receptor bound at the cell surface, we transduced PDHs with recombinant adenoviruses encoding GFP, GFP and gp180wt, or GFP and gp180tl. Four days after adenovirus infection, PDHs were superinfected with DHBV and cultured for 4 further days prior to analysis of intracellular DHBV DNA (Fig. 3). Preinfection with an adenovirus encoding GFP did not influence the susceptibility of PDHs to DHBV. In the case of transduction with gp180tl, and with the transduction efficiency estimated to be about 90%, intracellular DHBV DNA levels were reduced to about 10% of the control level. Thus, the receptor, when arrested at the cell surface, not only is itself unable to initiate a productive infection of PDHs but also outcompetes endogenous gp180 function.
FIG. 3.
Expression of gp180 lacking the cytoplasmic domain blocks DHBV infection. PDHs were transduced with recombinant adenoviruses encoding GFP (GFP), GFP plus gp180 (gp180wt), or GFP plus gp180tl (tl) and after further 4 days infected with DHBV. w/o, without adenovirus transduction. Four days later, the cells were harvested and assayed by DNA dot blotting for intracellular DHBV DNA (A, duplicate determinations). (B) Relative quantification of radioactive signals. Error bars indicate the deviation between duplicate experiments.
Supplementing the endogenous gp180 levels with additional Myc-tagged gp180wt increased the infection level to about twofold, supporting the role of gp180 as the DHBV receptor. However, as intracellular receptor levels were estimated to be raised at least 10-fold, this increase does not correspond to a proportional gain in DHBV susceptibility.
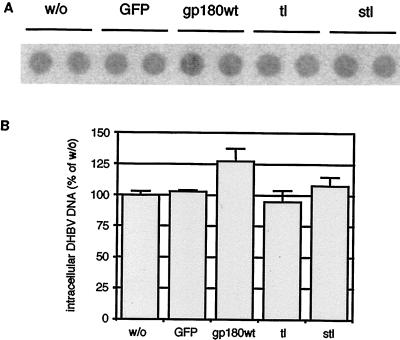
To rule out effects of mutant gp180 expression at steps of viral replication unrelated to uptake, we transduced PDHs prepared from DHBV-positive ducklings and compared the levels of intracellular DHBV DNA after 6 days (Fig. 4). The latter were not significantly influenced by transduction with any of the recombinant adenoviruses used, as were levels of intracellular DHBV envelope and capsid proteins and virus secretion (not shown).
FIG. 4.
Expression of mutant gp180 has no significant influence on ongoing DHBV replication. PDHs, prepared from a DHBV-positive duckling, were infected with recombinant adenoviruses encoding GFP only (GFP) or GFP plus gp180 (gp180wt) or deletion mutants tl and stl. w/o, control without adenovirus infection. At day 6 after adenovirus transduction, the cells were harvested and assayed by DNA dot blot for intracellular DHBV DNA (A, two parallel experiments). (B) Relative quantification of radioactive signals. Error bars indicate the deviation between duplicate measurements.
Accelerated transport to the endolysosomal compartment hinders successful DHBV entry.
Similar to the well-studied cytoplasmic domain of furin, the gp180 tail contains C terminally a stretch of acidic amino acids, encompassing a putative casein kinase II recognition site (Table 1, underlined residues) that has been implicated in regulating delivery of the protein to the lysosomal compartment (4, 6).
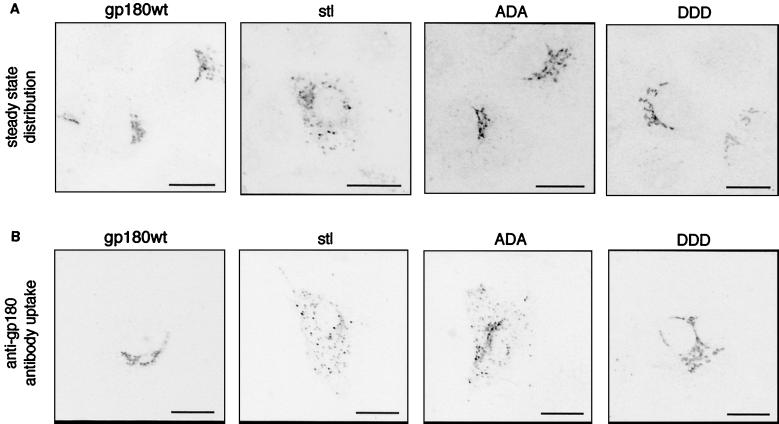
We investigated the role of this signal for gp180 sorting by mutating the putative phosphorylation residues from threonine to alanine (ADA) or aspartate (DDD); alternatively, we deleted the acidic stretch together with the 16 C-terminal amino acids (Table 1, stl). To analyze the steady-state distribution of these gp180 variants, HuH7 cells were transfected with one of the expression constructs, fixed 2 days posttransfection, and immunostained with an anti-gp180 antiserum (Fig. 5A). While gp180wt and the ADA and DDD mutants were all contained in a Golgi-like compartment, most of gp180stl was randomly distributed in vesicular structures throughout the cytoplasm. Next we monitored the sorting events of mutant gp180 following endocytosis. For this purpose, gp180-transfected HuH7 cells were incubated with anti-gp180 antibody and then were fixed and stained with a fluorescent secondary antibody (Fig. 5B). In cells expressing gp180 or the DDD mutant, internalized anti-gp180 antibodies were transported to the Golgi compartment, while in cells expressing gp180stl these were retained in vesicular structures. A similar pattern was observed, although to a lesser extent, in case of the ADA mutant. These observations are consistent with a recent report showing that gp180 mutants identical to stl and ADA have a high tendency of lysosomal sorting, resulting also in a much-reduced half-life (6). In these latter cases, internalized antibodies might thus have been diverted more frequently to the lysosomal pathway.
FIG. 5.
Changes in localization and endocytotic sorting of gp180 mutants carrying mutations in the cytoplasmic domain. HuH7 cells were transfected with plasmids encoding gp180wt or the indicated mutants of gp180 (Table 1). One day later, cells were immunostained with anti-gp180 antiserum and analyzed for gp180 localization by confocal laser scanning microscopy (A). Another set of cells was incubated with anti-gp180 antibodies for 2 h prior to fixation, staining with a fluorescent secondary antibody, and confocal microscopy (B). For a summary of mutant gp180 distribution, see Table 1. The scale bars represent 20 μm.
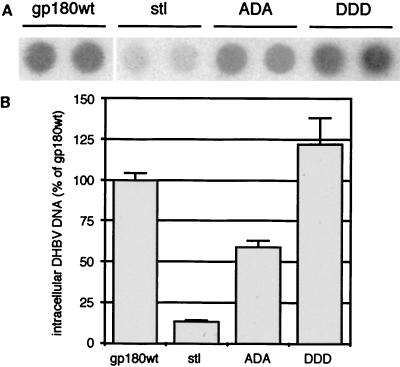
To determine whether the differential sorting events described above influence the receptor function of gp180, we raised recombinant adenoviruses transducing one of the gp180 mutants into PDHs. In the same experimental setup as described for Fig. 3, the DDD mutant functioned like gp180wt, while deletion of the acidic signal (gp180stl) led to a strong reduction of DHBV infection (Fig. 6). Knocking out the putative casein kinase II phosphorylation site (ADA) caused a twofold reduction compared to the wild type. These observations suggest that a defined intracellular targeting of incoming DHBV, mediated by gp180, is important for productive infection.
FIG. 6.
Disruption of intracellular sorting of gp180 influences DHBV infection. PDHs, preinfected with recombinant adenoviruses encoding gp180wt or the indicated mutants of gp180 were superinfected with DHBV. After further 4 days, the cells were harvested and assayed by DNA dot blotting for intracellular DHBV DNA (A, duplicate measurements). (B) Quantification of relative radioactive signals. Error bars indicate the deviation between two parallel experiments.
This conclusion was sustained with gp180-furin chimera, in which either the cytoplasmic domain or both the transmembrane region and the cytoplasmic domain of gp180 were replaced by the respective domains of furin (Table 1, gp180-furint and gp180-furintm+t [Table 1]). Both mutants exhibited a sorting phenotype similar to that of gp180stl and gp180ADA (Table 1) and influenced DHBV infection negatively when expressed in PDH (25 and 20% of intracellular DHBV DNA, relative to gp180wt, for gp180-furint and gp180-furintm+t, respectively).
The enzymatically active domains of gp180 are not required for DHBV entry.
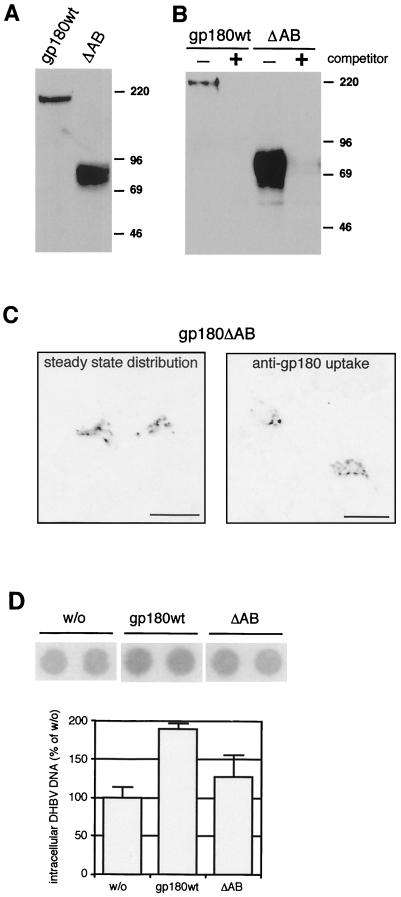
gp180 contains two enzymatically active carboxypeptidase domains, A and B, while the third, inactive domain C contains the virus binding site (Fig. 1A and reference 5). To investigate a possible involvement of A and B in the DHBV entry process, we constructed a deletion mutant lacking these two domains (gp180ΔAB). The corresponding protein, when expressed in transfected HuH7 cells (Fig. 7A) or in transduced hepatocytes, had an apparent size of about 80 kDa in a sodium dodecyl sulfate-gel, due to glycosylation; digestion with endoglycosidase F shifted the protein to a sharper-migrating band of approximately the calculated molecular mass (61 kDa; not shown). gp180ΔAB behaved like full-length gp180 with regard to its localization and traffic in transfected cells (Fig. 7C) and ligand binding (Fig. 7B and data from surface plasmon resonance spectroscopy [S. Urban, G. Multhaup, and H. Schaller, unpublished data]).
FIG. 7.
The enzymatically active domains A and B of gp180 are not essential for DHBV binding and receptor function. (A) Anti-gp180 immunoblot of lysates from HuH7 cells, transfected with plasmids encoding gp180wt or gp180ΔAB (ΔAB; see Fig. 1A and Materials and Methods). Numbers on the right indicate molecular masses of marker proteins (in kilodaltons). (B) Anti-gp180 immunoblot detecting gp180 or gp180ΔAB bound to a pre-S matrix in the absence (−) or presence (+) of free pre-S polypeptide as a competitor (2). (C) Localization and sorting of gp180ΔAB in transfected HuH7 cells. Cells were treated and analyzed by confocal microscopy as described for Fig. 5. The scale bars represent 20 μm. (D) Influence on DHBV infection. PDHs were infected with DHBV after transduction with recombinant adenoviruses encoding gp180wt or gp180ΔAB (ΔAB). w/o, without adenovirus transduction. Cells were assayed by DNA dot blotting for intracellular DHBV DNA as described for Fig. 3 and 6.
For functional analysis, a recombinant adenovirus encoding gp180ΔAB was used in an infection interference assay as described for the gp180 sorting mutants (Fig. 3 and 6). Compared to the control cells, no dominant-negative effect was observed (Fig. 7D), arguing against a requirement of the two N-terminal domains in the receptor for DHBV entry. However, expression of gp180ΔAB increased productive DHBV infection of transduced PDHs not as efficiently as full-length gp180, possibly as a result of subtle trafficking differences between the two receptor species in their virus-bound state. While gp180-bound virus particles were retained exclusively in endosomal structures of transfected HuH7 cells (2), particles internalized by gp180ΔAB were found in addition in tubular structures, resembling the Golgi apparatus (not shown).
DISCUSSION
Our previous studies have demonstrated that the duck carboxypeptidase D, or gp180, serves as cellular receptor for avian HBVs (2, 23). Here, we have complemented these studies by the characterization of gp180 traffic needed for productive DHBV entry. When gp180 mutants with defined sorting properties were overexpressed in DHBV-susceptible PDHs from recombinant adenovirus, the mutant protein outcompeted the endogenous receptor, and their receptor functionality could be assessed through a quantification of DHBV infection. As all mutants bound DHBV and had an at least transitory surface exposure, we interpret the negative influence on DHBV infection displayed by some mutants as a gradual incapability of the respective mutant receptor-virus complexes to undergo fusion, due to improper intracellular sorting.
While DHBV has been shown to be highly infectious in test animals (10), experimental infection is rather inefficient in cell culture (about 10% of cells infected after incubation with 100 DNA-containing virus particles per hepatocyte for 5 h at 37°C (B. Glass and H. Schaller, unpublished data). One explanation for the latter might be the extremely low gp180 levels at the cell surface, as revealed by confocal microscopic analysis (2). In addition, antibody uptake experiments in PDHs showed no detectable uptake after 5 h at 37°C, which implies a slow cycling rate of the receptor in cultured, primary hepatocytes (1). Finally, an adenovirus-mediated increase of intracellular receptor levels (about 10- to 100-fold) did not lead to a proportional increase in PDH susceptibility. Collectively, these observations suggest that the regulated gp180 transport to the plasma membrane is the rate-limiting step of DHBV entry in cultured hepatocytes. However, they also indicate that steps involving additional factors (e.g., the fusion receptor) may become rate limiting for virus entry once gp180 concentrations are raised much above the steady-state levels.
In control experiments, high expression of exogenous gp180 or gp180 mutants from adenovirus showed no effect on an established DHBV infection (Fig. 4) or on superinfection of PDHs with herpes simplex virus type 1 or Semliki Forest virus (our unpublished observations). We thus conclude that all of the observed effects on DHBV infection reflect a direct interaction between incoming DHBV and the respective receptor variants, rather than nonspecific membrane or traffic disturbances due to protein overexpression.
Previous studies have suggested DHBV entry to be independent of lysosomal acidification (11, 16). Generally, pH-independent fusion occurs at the plasma membrane (as is the case for herpesviruses or lentiviruses), while endocytotic viruses use low pH as a trigger for the fusion reaction (14). However, the lack of requiring low endosomal pH does not necessarily imply cell surface fusion, as Epstein-Barr virus, for example, enters B cells via endocytosis, while it fuses with epithelial cells at the cell surface (15). Expression of cell surface-arrested gp180 (gp180tl) abolished DHBV infection. gp180tl expression had no significant effect on ongoing replication (Fig. 4), which implies that the cell surface arrest of the virus-receptor complex blocks the delivery of infectious capsids to the nucleus. There are at least three different ways to account for this effect: (i) the gp180-virus complex requires endocytosis to encounter a second receptor, triggering fusion; (ii) fusion occurs, but the capsid is unable to penetrate the cytosceletal filament which underlies the plasma membrane; or (iii) the fusion reaction or the attraction of the secondary receptor requires the gp180 cytoplasmic domain. Since hepadnaviral capsids are rather small (with a diameter of about 28 nm [7]), and since changing the gp180 tail to the furin cytoplasmic domain resulted in a less drastic effect on DHBV infection (compared to gp180tl), the latter two possibilities appear less likely. Together with previous data suggesting an energy-dependent step in entry (11), our results support a model in which endocytosis into hepatocytes is required to initiate productive DHBV infection.
Most endocytotic viruses are adapted to escape the early or late endosome, avoiding their delivery to lysosomes, rapid inactivation, and degradation (14); an exception are (nonenveloped) reoviruses, where specific proteolytic cleavage is needed for infectivity (19). Together with previous data (6), the accelerated turnover of the mutants gp180stl, gp180ADA, and the gp180-furin chimeras, as well as antibody uptake experiments with these mutants in HuH7 cells, suggest an increased tendency for these mutants, relative to the wild type, to be transported from the endosomal to the lysosomal compartment. Upon expression of these mutants in susceptible duck hepatocytes, we found an inverse correlation of infection levels with the efficiency by which DHBV was targeted to the lysosomal pathway. This suggests that DHBV entry is abrogated through delivery of internalized virus to the lysosomes, where in the absence of the fusion receptor the virus particles are proteolytically degraded. Additionally, DHBV may be inactivated by the low lysosomal pH, in accordance with the observation that lowering the pH rapidly inactivates DHBV (16; E. V. L. Grgacic, D. Anderson, and H. Schaller, unpublished data).
In view of its long half-life (in the range of days [Urban et al., unpublished data]), gp180 has the potential to cycle several times between the trans-Golgi network and the plasma membrane, without being subjected to lysosomal degradation. Confocal analysis (2), together with the high stability of the receptor-ligand complex within the physiological pH range (24), suggests that DHBV remains receptor bound postendocytosis. Thus, this type of receptor may provide a so far unrecognized means for an incoming virus particle to escape the normal lysosomal delivery, which is the fate of many other endocytosed substrates. In keeping with this concept, viral substrates did not reach the Golgi complex of gp180-transfected HuH7 cells but were retained in endosomal structures, together with gp180 (2). If this were also the case in susceptible hepatocytes, it would explain how the fusion reaction, which would require the engagement of a second receptor in the endosome, becomes kinetically favorable over transport events abrogating infection.
gp180 has been shown to be an enzymatically active carboxypeptidase (5). However, carboxypeptidase D inhibitors did not block DHBV infection (Urban et al., unpublished data), suggesting that DHBV entry does not involve the enzymatic function of gp180. In support of this notion, deletion of the two active carboxypeptidase domains (gp180ΔAB) had no effect in our infection interference assay. As the isolated C domain binds the DHBV pre-S ligand as well as the full-length receptor does (Fig. 7B; Urban et al., unpublished data), this observation implies that gp180 can also function as a DHBV receptor in the absence of domains A and B.
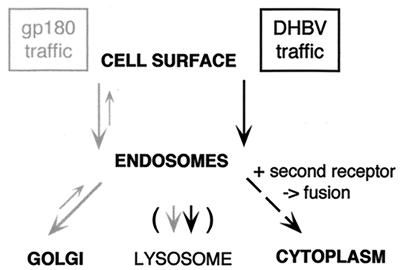
From the data accumulated so far, we propose a model for the entry of DHBV which in principle may extend also to the mammalian hepadnaviruses (albeit these might use different cellular molecules). In this model (schematically depicted in Fig. 8), viral entry begins at the plasma membrane with the attachment of the infectious virus particle to the receptor molecule, gp180, which is normally efficiently retrived to the trans-Golgi network. After the binding step, DHBV is coendocytosed along with its receptor and transported to an endosomal compartment but not to the trans-Golgi network (as was the case with receptor-bound antibodies) or to the lysosome. Instead, the particle-receptor complex appears to be arrested, by an as yet unknown mechanism, in the endosomes until a second receptor triggers the fusion between the cellular and the virus membrane, liberating the nucleocapsid into the cytoplasm. Accordingly, DHBV appears to be a first example of an enveloped virus whose uptake depends on endocytosis but not on low pH for membrane fusion.
FIG. 8.
Model of cellular gp180 traffic and DHBV entry. DHBV-complexed gp180 is arrested in the endosome to allow interaction with the second receptor and membrane fusion.
ACKNOWLEDGMENTS
We thank Bärbel Glass and Uta Klöcker for preparation of PDHs, Andreas Vonderheit for his contribution in obtaining and characterizing pC-gp180ΔAB, and Martin Sprinzl and Ulla Protzer for initial help in generating recombinant adenoviruses. We are also grateful to Stephan Urban for helpful discussions, Tong-Chuan He and Bert Vogelstein for providing the AdenoEasy system, Kazuyuki Kuroki for gp180 cDNA, Wolfgang Garten for bovine furin cDNA, Vas Ponnambalam, Martin Eilers, Stephan Urban, and Christa Kuhn for antisera, Bernhard Dobberstein and Elizabeth Grgacic for critically reading the manuscript, and Karin Coutinho for expert editorial assistance.
This work was supported by a Boehringer Ingelheim Fonds predoctoral fellowship to K.M.B. and by the Fonds der Chemischen Industrie.
ADDENDUM IN PROOF
In an experiment concerning the nature of the vesicular structures characteristic for the mutant gp180 proteins (Fig. 5B), we found that most of the gp180-stl-containing vesicles (and, to lesser extent, gp180-ADA) also contained cathepsin D, a lysosomal marker protein. These results further support the notion that a large fraction of these mutant proteins is transported toward the lysosomal compartment after endocytosis from the cell surface, sustaining the model shown in Fig. 8 that lysosomal transport of incoming virus may lead to abrogation of infection.
REFERENCES
- 1.Breiner K M. Carboxypeptidase D (gp180): Rezeptor, Transrezeptor und Signalmolekül für Vogelhepatitis B Viren. Ph.D. thesis. Heidelberg, Germany: University of Heidelberg; 1998. [Google Scholar]
- 2.Breiner K M, Urban S, Schaller H. Carboxypeptidase D (gp180), a Golgi-resident protein, functions in the attachment and entry of avian hepatitis B viruses. J Virol. 1998;72:8098–8104. doi: 10.1128/jvi.72.10.8098-8104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeMeyer S, Gong J Z, Suwandhi W, van Pelt J, Soumillon A, Yap S H. Organ and species specificity of hepatitis B virus (HBV) infection: a review of literature with a special reference to preferential attachment of HBV to human hepatocytes. J Viral Hepat. 1997;4:145–153. doi: 10.1046/j.1365-2893.1997.00126.x. [DOI] [PubMed] [Google Scholar]
- 4.Dittie A S, Thomas L, Thomas G, Tooze S A. Interaction of furin in immature secretory granules from neuroendocrine cells with the AP-1 adaptor complex is modulated by casein kinase II phosphorylation. EMBO J. 1997;16:4859–4870. doi: 10.1093/emboj/16.16.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eng F J, Novikova E G, Kuroki K, Ganem D, Fricker L D. gp180, a protein that binds duck hepatitis B virus particles, has metallocarboxypeptidase D-like enzymatic activity. J Biol Chem. 1998;273:8382–8388. doi: 10.1074/jbc.273.14.8382. [DOI] [PubMed] [Google Scholar]
- 6.Eng F J, Varlamov O, Fricker L D. Sequences within the cytoplasmic domain of gp180/carboxypeptidase D mediate localization to the trans-Golgi network. Mol Biol Cell. 1999;10:35–46. doi: 10.1091/mbc.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganem D. Hepadnaviridae. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2703–2737. [Google Scholar]
- 8.He T C, Zhou S, da Costa L T, Yu J, Kinzler K W, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hild M, Weber O, Schaller H. Glucagon treatment interferes with an early step of duck hepatitis B virus infection. J Virol. 1998;72:2600–2606. doi: 10.1128/jvi.72.4.2600-2606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jilbert A R, Miller D S, Scougall C A, Turnbull H, Burrell C J. Kinetics of duck hepatitis B virus infection following low dose virus inoculation: one virus DNA genome is infectious in neonatal ducks. Virology. 1996;226:338–345. doi: 10.1006/viro.1996.0661. [DOI] [PubMed] [Google Scholar]
- 11.Köck J, Borst E M, Schlicht H J. Uptake of duck hepatitis B virus into hepatocytes occurs by endocytosis but does not require passage of the virus through an acidic intracellular compartment. J Virol. 1996;70:5827–5831. doi: 10.1128/jvi.70.9.5827-5831.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuroki K, Cheung R, Marion P L, Ganem D. A cell surface protein that binds avian hepatitis B virus particles. J Virol. 1994;68:2091–2096. doi: 10.1128/jvi.68.4.2091-2096.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuroki K, Eng F, Ishikawa T, Turck C, Harada F, Ganem D. gp180, a host cell glycoprotein that binds duck hepatitis B virus particles, is encoded by a member of the carboxypeptidase gene family. J Biol Chem. 1995;270:15022–15028. doi: 10.1074/jbc.270.25.15022. [DOI] [PubMed] [Google Scholar]
- 14.Marsh M, Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller N, Hutt-Fletcher L M. Epstein-Barr virus enters B cells and epithelial cells by different routes. J Virol. 1992;66:3409–3414. doi: 10.1128/jvi.66.6.3409-3414.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rigg R J, Schaller H. Duck hepatitis B virus infection of hepatocytes is not dependent on low pH. J Virol. 1992;66:2829–2836. doi: 10.1128/jvi.66.5.2829-2836.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schäfer W, Stroh A, Berghofer S, Seiler J, Vey M, Kruse M L, Kern H F, Klenk H D, Garten W. Two independent targeting signals in the cytoplasmic domain determine trans-Golgi network localization and endosomal trafficking of the proprotein convertase furin. EMBO J. 1995;14:2424–2435. doi: 10.1002/j.1460-2075.1995.tb07240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song L, Fricker L D. Purification and characterization of carboxypeptidase D, a novel carboxypeptidase E-like enzyme, from bovine pituitary. J Biol Chem. 1995;270:25007–25013. doi: 10.1074/jbc.270.42.25007. [DOI] [PubMed] [Google Scholar]
- 19.Sturzenbecker L J, Nibert M, Furlong D, Fields B N. Intracellular digestion of reovirus particles requires a low pH and is an essential step in the viral infectious cycle. J Virol. 1987;61:2351–2361. doi: 10.1128/jvi.61.8.2351-2361.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tong S, Li J, Wands J R. Interaction between duck hepatitis B virus and a 170-kilodalton cellular protein is mediated through a neutralizing epitope of the pre-S region and occurs during viral infection. J Virol. 1995;69:7106–7112. doi: 10.1128/jvi.69.11.7106-7112.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuttleman J S, Pugh J C, Summers J W. In vitro experimental infection of primary duck hepatocyte cultures with duck hepatitis B virus. J Virol. 1986;58:17–25. doi: 10.1128/jvi.58.1.17-25.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsien R Y. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- 23.Urban S, Breiner K M, Fehler F, Klingmüller U, Schaller H. Avian hepatitis B virus infection is initiated by the interaction of a distinct pre-S subdomain with its cellular receptor gp180. J Virol. 1998;72:8089–8097. doi: 10.1128/jvi.72.10.8089-8097.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urban S, Kruse C, Multhaup G. A soluble form of the avian hepatitis B virus receptor: biochemical characterisation and functional analysis of the receptor ligand complex. J Biol Chem. 1999;274:5707–5715. doi: 10.1074/jbc.274.9.5707. [DOI] [PubMed] [Google Scholar]