Abstract
Myostatin (MSTN) is a negative regulator of skeletal muscle growth and a popular target for enhancing the productivity of farmed fish. We previously developed an mstn-knockout breed of the aquaculture fish red sea bream (Pagrus major) using genome editing technology. However, little is known about the effects of mstn disruption on the fillet quality of red sea bream and other fish species. In this study, we used fillets of mstn-deficient red sea bream to evaluate their compositional and textural changes during refrigeration. Compared to the wild type, the mutant fillets exhibited an increase in moisture content and a decrease in drippings, indicating an enhanced water-holding capacity. Furthermore, the mutant fillets showed increased water retention and marginally lower collagen content, resulting in lower breaking force, an index of texture. In conclusion, we demonstrated that mstn disruption alters the compositional and textural properties of red sea bream fillets.
Keywords: Myostatin, Genome editing, Red sea bream, Fillet quality, Water-holding capacity, Texture
Highlights
-
•
Fillet quality of adult homozygous mstn mutants (HMs) red sea bream was investigated.
-
•
Compared to the wild type, HM fillets had a higher water-holding capacity.
-
•
Decrease in lactic acid content slowed the decrease in pH during refrigerated storage.
-
•
Breaking force of HM fillets was reduced, suggesting a softening of their texture.
1. Introduction
Fish are valuable foods that contain vitamins, minerals, highly unsaturated fatty acids, and other nutrients necessary for human health. The worldwide consumption of fish has almost doubled in the past half-century owing to economic development and increased health consciousness [1]. Fish are extremely important in world food production; however, they have been bred for only a few decades or remain in a near-wild state because of the difficulty in establishing aquaculture techniques and the dependence on catching wild juveniles. This fact is in sharp contrast with agricultural and livestock products that have undergone thousands of years of selective breeding [2,3]. To meet the increasing demand for fish as foods, it is necessary to develop new economically valuable breeds using various biotechnologies.
Genome editing technology such as the CRISPR-Cas9 system has been a powerful tool for accelerating the molecular breeding of various organisms over the past decade. This system has been applied to more than ten fish species and has developed desirable traits, such as growth promotion and disease resistance, through the functional modification of specific genes [4,5]. In particular, myostatin (mstn) is a popular target gene used to increase the edible portions. MSTN, also known as growth and differentiation factor 8, is a negative regulator of skeletal muscle growth [6]. Mutations of mstn cause the hypertrophy of skeletal muscle in mice and promote the development of a “double muscling phenotype” in various livestocks [7,8]. This phenomenon has attracted the attention of researchers not only in the livestock sector but also in the aquaculture sector because of the importance of increasing productivity [9]. In fish, mstn mutants have been generated in medaka (Oryzias latipes) and tiger pufferfish (Takifugu rubripes) by random chemical mutagenesis, which is a time-consuming and labor-intensive process [10,11]. Since the availability of genome editing technology has reduced the workload for the establishment of genetically modified strains, research on the production of mstn mutants in farmed fish has been widespread [4,5]. It has been demonstrated that mstn mutations induced an increase in the number of muscle fibers in some farmed fish, such as channel catfish (Ictalurus punctatus), common carp (Cyprinus carpio), and Nile tilapia (Oreochromis niloticus) [[12], [13], [14]]. The rapid progress made in these studies highlights the versatility and simplicity of the application of the CRISPR-Cas9 system to cultured fish.
Red sea bream (Pagrus major) is an important aquaculture fish in Japan, known for its bright red body color, ease of breeding, and excellent taste [15]. The aquaculture production of this species in Japan was 62,300 tons in 2020, ranking second in the total aquaculture production of fish in the country. In our previous study, we established an mstn-deficient breed of red sea bream using the CRISPR-Cas9 system [16]. We previously reported that the homozygous mstn mutants (HMs) of red sea bream exhibited a 17 % increase in skeletal muscle mass and had a higher ability to accumulate ingested protein compared to the wild type (WT) [[16], [17], [18]]. The red sea bream breed was notified to the Ministry of Agriculture, Forestry and Fisheries and the Ministry of Health, Labor and Welfare, which has been marketed in Japan since 2021 [19]. To date, phenotypic analyses of mstn mutated fish, including red sea bream and other fish species, have mainly focused on morphological changes in muscle fibers, body shape, and growth performance. However, few studies have specifically validated fillet qualities such as texture and nutritional components in mstn mutated fish. Identification of food characteristics may provide hints for establishing production and processing methods suitable for mstn mutated fish. Thus, we used skeletal muscle from adult red sea bream HMs to evaluate fillet quality-related factors, such as composition, pH, drip volume, and physical properties until four days postmortem. Refrigeration for four days after harvest is a critical period that has a great impact on consumers because the quality of fresh fish can change rapidly. Furthermore, many markets and retailers recommend that fresh fish be sold within four days of harvest to ensure optimal freshness. We therefore examined the fillet quality up to four days after harvest to reflect the actual market situation.
2. Materials and methods
2.1. Experimental fish
The genome-edited red sea bream used in this study were cultured at Regional Fish Institute, Ltd, Kyoto, Miyazu-shi, Japan. We obtained HM fish by injecting CRISPR-Cas9 reagents into fertilized eggs and mating parent fish with mutations in their germline [16]. HM fish harbor a 14-base deletion that causes a frame-shift in the coding sequence of mstn and exhibits a double muscling phenotype [16]. Adult WT fish were purchased from an aquaculture company in Wakayama, Japan and used as a control group. HM and WT fish were transferred to the land-based aquaculture facility of the Regional Fish Institute, Ltd, in Kyoto, Miyazu-shi, Japan, and kept in the same tank in a semi-circulating system for eight months. Each fish was fasted for one day prior to analysis to eliminate gastrointestinal contents. A total of 10 fish, including five each HM or WT individuals at approximately three years of age (1,019–1,124 days post fertilization), were randomly collected from the tank for analysis. The WT group comprised one male and four females, while the HM group had three males and two females. The gonads of each individual were relatively degenerated because the breeding season was a couple of months past. All fish were killed by the ikijime method (quickly inserting a spike into the brain), which is regarded as one of the fastest and most humane [20]. The caudal fork length (FL) and body weight (BW) of the fish were measured, and the condition factor was calculated as 1000 × BW/FL3 (BW in grams, FL in centimeters). To evaluate the fillet quality, two fillets were cut from each individual, stored in a polystyrene box on ice, and transported from the farm to the laboratory 6 h after death. One whole fillet was stored at 4 °C until the proximate composition analysis was performed. The other fillet was used to cut the dorsal portion into approximately 40-g pieces, which were assayed for drip volume and pH. The remaining dorsal muscle was used to validate the physical properties, collagen content, and lactic acid content. Each muscle sample was wrapped in cooking paper, placed in a zippered plastic bag, and stored in a polystyrene box at 4 °C until use. The cooking papers were replaced each time sampling was conducted.
2.2. Proximate composition analysis
Proximate composition analysis was outsourced to the commercial laboratory, Bureau Veritas FEAC Co., Ltd. The fillets were sent on the second day postmortem, and each component was quantified on the third day postmortem. Moisture content was quantified using the weight loss on drying method by drying the samples at 105 °C for 5 h. The crude protein content was determined using the nitrogen combustion method. The crude lipid content was analyzed using the Soxhlet diethyl ether solvent extraction method. The crude ash content was quantified after combustion at 600 °C for 16 h in an electric muffle furnace. The carbohydrate content was calculated as the difference between 100 and the sum of moisture, crude protein, crude lipids, and crude ash contents. The results were expressed in grams per 100 g wet weight of raw fillets.
2.3. Measurement of drip loss and pH
The drip loss (DL) was determined for each portion of dorsal muscle (40 g) cut from the fillets. Each muscle sample was dabbed with cooking paper to remove excess water, and its initial weight (Wi) was recorded at 9 h postmortem. Thereafter, weighing was conducted at 24 h intervals (Wf) until the fourth day postmortem. The DL at each time point was calculated as follows: DL (%) = [(Wi − Wf)/Wi] × 100 %.
Concurrently with the weight measurements, pH was also determined using the same samples. The intracellular muscle pH was measured by inserting a glass electrode attached to a hand-held pH meter Testo 206-pH2 (Testo, Lenzkirch, Germany), into the samples. The electrode was inserted at two different sites per sample for duplicate measurements.
2.4. Quantification of lactic acid
A portion of the fillet (1 g) was collected from each dorsal muscle at 9 h postmortem, and stored in a 2 mL microtube at −80 °C until time of analysis. The same treatments were followed at 24 h intervals until the fourth day postmortem. The frozen fish samples were thawed at approximately 20 °C and homogenized in 5 mL of 10 % perchloric acid. The homogenate was centrifuged at 15,000×g for 10 min at 4 °C. The supernatant was neutralized with 1 N KOH on ice and centrifuged at 15,000×g for 10 min at 4 °C. The concentration of lactic acid in the supernatant was determined using a commercially available kit ENZYTEC Liquid L-lactic acid (J. K. International, Tokyo, Japan). The Enzytec™ Multi-acid standard low (J. K. International, Tokyo, Japan) was used to calibrate the apparatus. All the samples were diluted until the optimal concentration was reached by direct comparison with the calibration curve.
2.5. Analysis of textural properties
Five-millimeter thick slices, simulating sashimi, were cut from the dorsal muscle vertically to the orientation of muscle fibers at 18, 42, and 90 h postmortem. The breaking force and strain of the slices were assessed using a RHEONER II creep meter RE2-33005B equipped with a 20 N load cell (Yamaden, Tokyo, Japan). A cylindrical plunger (8 mm in diameter) simulating a molar tooth was inserted into the slices parallel to the orientation of the muscle fibers at a speed of 1 mm/s. The breaking force (N) of the slices was represented as the maximum load value (N) at the point at which the slices were broken by the plunger. The breaking strain (mm) of each slice was defined as the penetration distance (mm) from the slice contact point to the breaking point. Three slices per fish were used to calculate the average values.
2.6. Measurement of collagen content
A portion (1 g) was obtained from each dorsal muscle at 18, 42, and 90 h postmortem, and stocked in a 2 mL microtube at −80 °C until analysis was performed. Muscle samples were freeze-dried and suspended in 2 mL of 6 M HCl in a 40 mL Pierce vial with a resealable valve (Thermo Fisher Scientific, MA, USA). The suspension was hydrolyzed by heating under vacuum at 150 °C for 1 h and the hydrolysate was diluted to 50 mL with water. The hydroxyproline (Hyp) content in the hydrolysate was measured using an LC-20 liquid chromatographic system (Shimadzu, Kyoto, Japan) according to the method of Bidlingmeyer et al. [21]. The Hyp contents were converted to collagen content by multiplying with the coefficient (collagen weight/Hyp weight) obtained from the amino acid composition of collagen in red sea bream [22].
2.7. Measurement of microstructure
Histological analysis was performed according to the method of Inoué and Osatake [23], with modifications. A portion (1 g) was cut from the dorsal muscle at 18 h postmortem, fixed in 4 % formaldehyde at approximately 20 °C for 24 h, and then stored at 4 °C until analysis was performed. The tissues were dehydrated in ascending concentrations of ethanol and fitted into gelatine capsules, which were then immersed in liquid nitrogen and fractured. The fractured specimens were freeze-dried in tert-butyl alcohol and mounted on aluminum stubs under a microscope. Specimens were coated with gold using a JFC-1100E sputtering-ion instrument (Nihon Denshi, Tokyo, Japan). The muscle microstructures were visualized using an MT-3030 scanning electron microscope (SEM) at an accelerating voltage of 15 kV (Hitachi, Tokyo, Japan). The number and area of muscle fibers were quantified using ImageJ digital image analysis software.
2.8. Statistical analysis
Before proceeding with the analysis, the assumption of normality of the data was confirmed using the Shapiro–Wilk test. The FL, BW, nutritional compositions including lipid and carbohydrate contents, and muscle fiber area were analyzed using Student's t-test. Condition factors and nutritional compositions, including moisture, protein, and ash contents, were analyzed using Welch's t-test. The drip volume, pH, lactic acid content, breaking force, breaking strain, and collagen content were analyzed using the two-way ANOVA, followed by Sidak's multiple comparison test. The distribution of muscle fiber area was analyzed using the χ2 Pearson test. P values less than 0.05 were considered to indicate statistical significance. P values less than 0.1 were assumed to indicate a trend. Results are presented as means ± standard deviation (SD). Statistical analyses were performed using the GraphPad Prism 10 (GraphPad Software, CA, USA) and R software (http://www.r-project.org).
3. Results
3.1. Body size evaluation
The results of the body size evaluation are shown in Table 1. The FL of the HM group (35.8 ± 1.5 cm) was significantly shorter than that of the WT group (44.9 ± 0.9 cm). In contrast, there was no difference in the BW between the two groups (HM: 1.5 ± 0.3 kg and WT: 1.6 ± 0.1 kg). The condition factor, which is an index of the BW–FL relationship in fish, of the HM fish (40.1 ± 3.6) was significantly higher than that of the WT fish (21.4 ± 0.5).
Table 1.
Body size evaluation of the wild type (WT) and the homozygous mstn mutants (HM).
| WT | HM | |||||
|---|---|---|---|---|---|---|
| Fork length (cm) | 42.5 | ± | 0.9 | 33.8 | ± | 1.5a |
| Body weight (kg) | 1.6 | ± | 0.1 | 1.5 | ± | 0.3 |
| Condition factor | 21.4 | ± | 0.5 | 40.1 | ± | 3.6a |
Means ± SD (n = 5).
Condition factor = Body weight (g)/Fork length3 (cm) × 1000.
Significant difference between WT and HM (P < 0.05).
3.2. Proximate composition analysis of the fillets
Proximate composition analysis of the fillets was performed to assess the basic nutritional properties of the fish. As shown in Table 2, the moisture and protein contents of HM fillets (73.3 ± 0.2 and 22.6 ± 0.2 g/100 g, respectively) were higher than those of WT fillets (72.0 ± 1.1 and 21.3 ± 0.5 g/100 g). The lipid content was significantly lower in HM fillets (2.1 ± 0.3 g/100 g) than in WT fillets (5.0 ± 0.7 g/100 g). The ash and carbohydrate contents of the HM group (1.7 ± 0.1 and 0.2 ± 0.1 g/100 g, respectively) were comparable to those of the WT group (1.6 ± 0.1 and 0.2 ± 0.2 g/100 g).
Table 2.
Proximate composition of the fillets of the wild type (WT) and the homozygous mstn mutants (HM).
| WT | HM | |||||
|---|---|---|---|---|---|---|
| Moisture | 72.0 | ± | 1.1 | 73.3 | ± | 0.2b |
| Protein | 21.3 | ± | 0.5 | 22.6 | ± | 0.2a |
| Lipid | 5.0 | ± | 0.7 | 2.1 | ± | 0.3a |
| Ash | 1.6 | ± | 0.1 | 1.7 | ± | 0.1 |
| Carbohydrate | 0.2 | ± | 0.2 | 0.2 | ± | 0.1 |
Means ± SD (n = 5).
Significant difference between WT and HM (P < 0.05).
Significant tendency between WT and HM (P < 0.1).
3.3. Changes in drippings, pH, and lactic acid content of the fillets during refrigeration
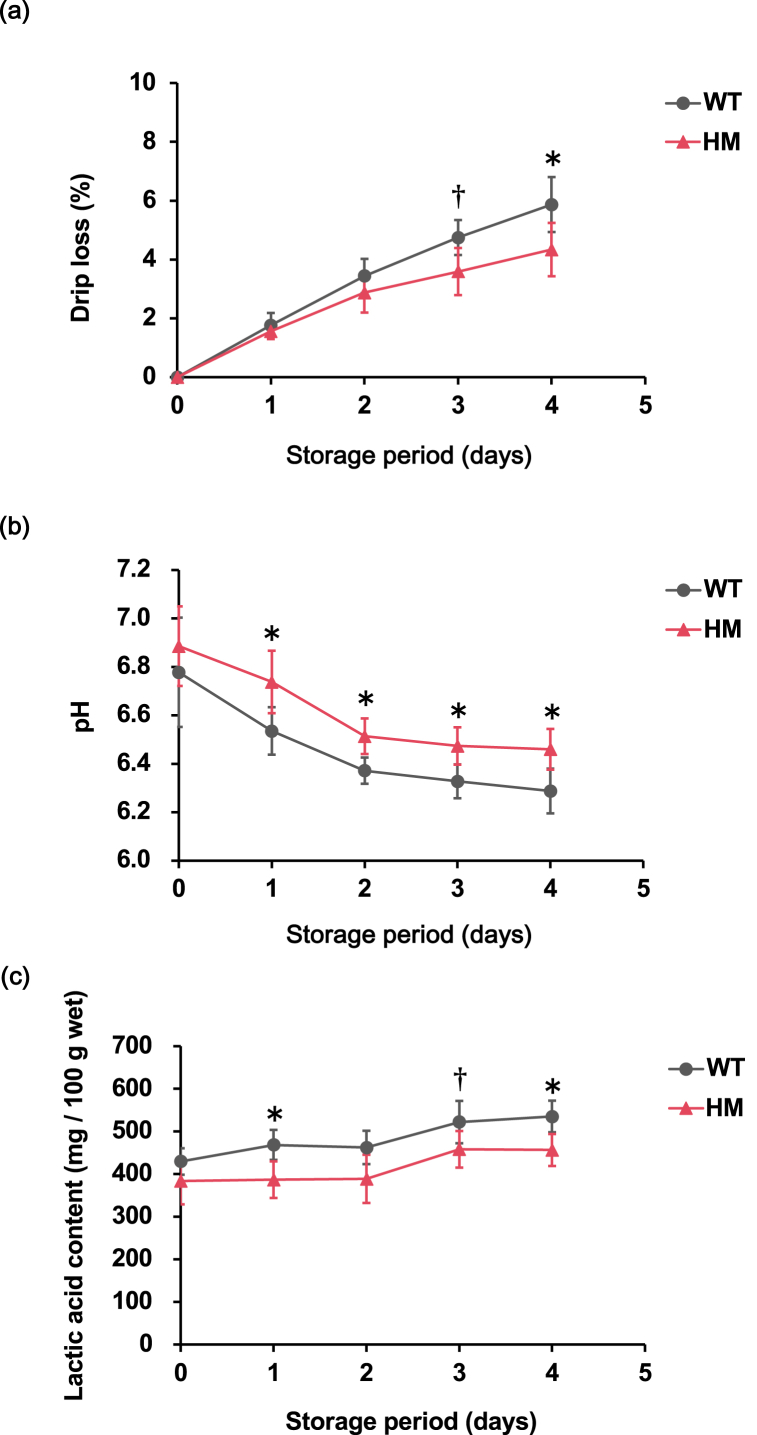
To investigate the properties of HM fillets, we evaluated temporal changes in the amount of drippings during storage (Fig. 1a). The drip volume of the HM and WT groups gradually increased in a time-dependent manner. The difference in the drip volume between the HM and WT groups was small at the beginning of storage and then increased with time. The drip volume of the HM group (4.3 ± 0.9 %) was significantly lower than that of the WT group (5.9 ± 0.9 %) at the fourth day postmortem. The drip volume was significantly affected by genotype in the two-way ANOVA (P = 0.000837). As shown in Fig. 1b, the pH of both the HM and WT groups gradually decreased over time. The pH values of the HM group were significantly higher than those of the WT group during the first four days postmortem. The pH value was significantly affected by genotype in the two-way ANOVA (P = 0.000292). To investigate the factors responsible for the inhibition of the pH decrease in HM fillets, we measured the lactic acid content of the fillets (Fig. 1c). The amount of lactic acid in the HM group was significantly lower than that in the WT group at the first and fourth day postmortem. The lactic acid content was significantly affected by genotype in the two-way ANOVA (P = 0.00000644).
Fig. 1.
Biochemical analysis of skeletal muscle in mstn-deficient red sea bream. (a) Drip loss (DL), (b) pH, and (c) lactic acid content in fish fillets stored in refrigeration. The first measurement was conducted at 9 h postmortem, followed by periodic evaluations every 24 h until the fourth day postmortem. The group of wild type and homozygous mstn mutants are designated as “WT” and “HM”, respectively. The genotype has a significant impact on the DL, pH, and lactic acid content according to the two-way ANOVA test (P = 0.000837, 0.000292, and 0.00000644, respectively). The asterisks indicate that the values are significantly different by Sidak's test, *: P < 0 0.05. The daggers indicate that the values tend to be different by Sidak's test, †: P < 0.1. Data are presented as the mean ± SD (n = 5). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.4. Changes in textural properties of the fillets during refrigeration
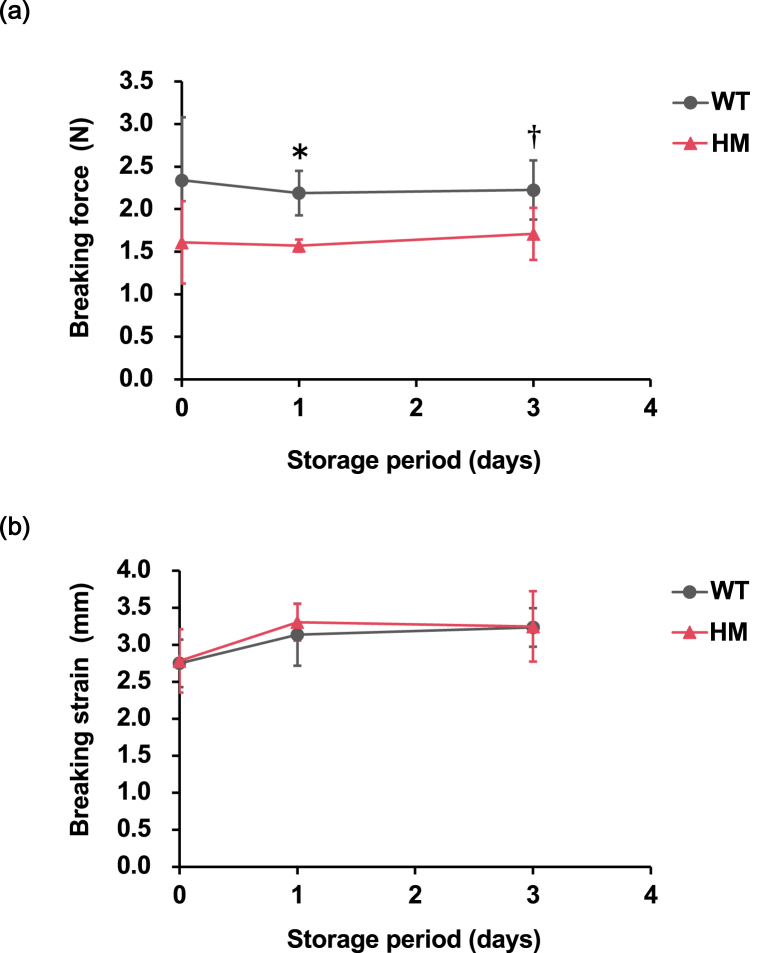
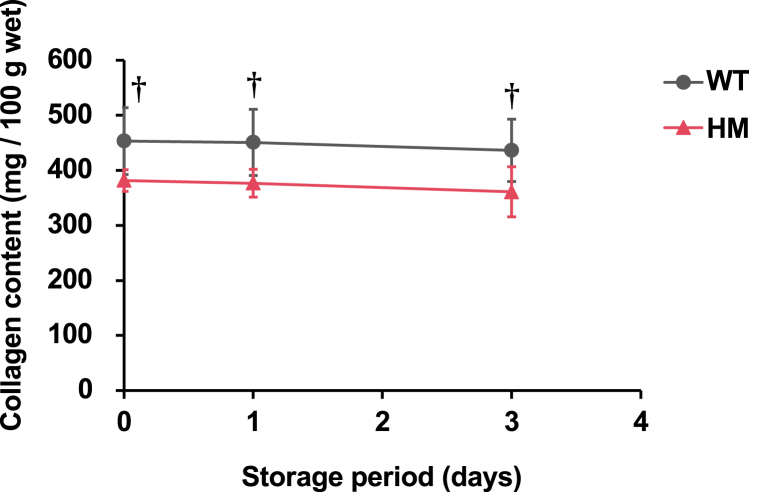
The breaking force and strain of the fillet were measured to evaluate the physical properties of the fillets. The breaking force of the HM group tended to be lower than that of the WT group throughout the measurement period (Fig. 2a). HM fillets exhibited a significantly lower breaking force (1.6 ± 0.1 N) than WT fillets (2.2 ± 0.3 N) at the first day postmortem. The breaking force was significantly affected by genotype in the two-way ANOVA (P = 0.000949). In contrast, the breaking strain of the HM group was comparable to that of the WT group at every sampling point, and no significant differences or trends were observed between the two groups (Fig. 2b). To explore the factors that caused the difference in the breaking force between the two groups, the amount of collagen was calculated by quantifying Hyp in the muscle hydrolysate (Fig. 3). The collagen content in the HM group tended to be lower than that in the WT group. The collagen content was significantly affected by genotype in the two-way ANOVA (P = 0.000539).
Fig. 2.
Evaluation of physical properties in mstn-deficient red sea bream. (a, b) Breaking force and strain of red sea bream muscle. A total of three measurements were performed at 18, 42, and 90 h postmortem, which are labeled “Day 0”, “Day 1”, and “Day 3”, respectively. The group of wild type and homozygous mstn mutants are designated as “WT” and “HM”, respectively. The genotype has a significant impact on the breaking force, according to the two-way ANOVA test (P = 0.000949). The asterisks indicate that the values are significantly different by Sidak's test, *: P < 0.05. Daggers indicate that the values tend to be different compared to those of WT by Sidak's test, †: P < 0.1. Data are presented as the mean ± SD (n = 5). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Quantification of collagen content in mstn-deficient red sea bream. A total of three measurements were performed at 18, 42, and 90 h postmortem, which are labeled “Day 0”, “Day 1”, and “Day 3”, respectively. The group of wild type and homozygous mstn mutants are designated as “WT” and “HM”, respectively. The genotype has a significant impact on the collagen content, according to the two-way ANOVA test (P = 0.000539). The daggers indicate that the values tend to be different by Sidak's test, †: P < 0.1. Data are presented as the mean ± SD (n = 5). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.5. Changes in the microstructure of the fillets
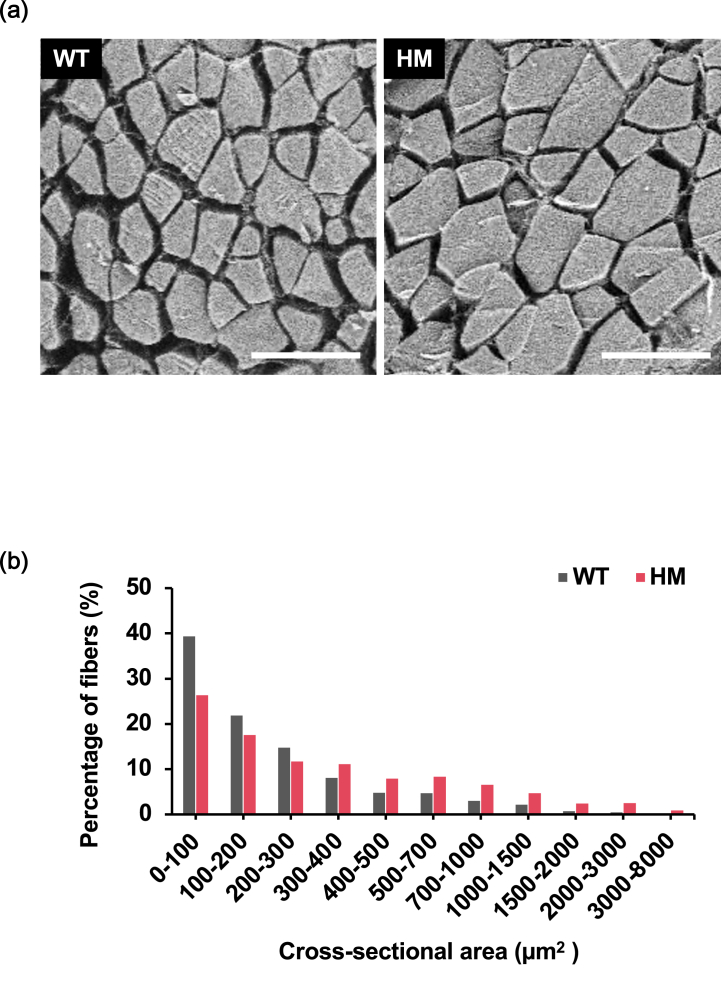
To examine microstructural alterations in the fillets, we observed the muscle fibers of the HM and WT groups using SEM (Fig. 4a). The average cross-sectional areas of myofibers in HM fillets (440.8 ± 59.8 μm2) was significantly higher than in WT fillets (248.5 ± 33.8 μm2). The myofiber distribution in HM fillets was significantly right-shifted compared to that in WT fillets, confirming that the myofibers of HM fillets were larger than those of WT fillets (Fig. 4b).
Fig. 4.
Histological evaluation of muscle fibers in mstn-deficient red sea bream. (a) Representative images of cross-sectional areas of dorsal muscle at 18 h postmortem. The group of wild type and homozygous mstn mutants are designated as “WT” and “HM”, respectively. Scale bar: 100 μm. (b) Myofiber distribution of skeletal muscle. A minimum of 1,000 myofibers were measured per fish (n = 5). The myofiber distribution of the HM fillets was significantly right-shifted compared to that of the WT fillets by χ2 Pearson test (P < 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Compared to the WT group, the HM group had a significantly shorter FL, comparable BW, and significantly higher conditioning factor. These results are consistent with those of our previous study on adult fish at 559 days of age, showing an increase in skeletal muscle mass in the HM group [16]. Thus, the individuals randomly selected from the breeding population were used as typical examples of HMs or WTs for the fillet quality evaluation.
Our proximate composition analysis revealed the contents of various components in HM and WT fillets. The moisture content of HM fillets was higher than that of WT fillets, and the protein content of HM fillets was significantly higher than that of WT fillets. This is similar to our previous proximate composition analysis results for juveniles (ca. 10 cm) [17]. We speculate that these results are due to the disruption of the mstn gene, which promotes the growth and hypertrophy of muscle tissue. Our research group previously confirmed a 17 % increase in skeletal muscle volume in HM fish using computed tomography [16]. In the current study, we histologically demonstrated the hypertrophy of HM myofibers through SEM observations. We assume that the increase in skeletal muscle volume and hypertrophy led to an increase in the moisture and protein contents because muscle is rich in both components [24].
A comparison of the lipid content between the genotypes showed a significant decrease in HM fillets compared to WT fillets, which is similar to a previous study on juveniles [17]. Furthermore, it has been demonstrated that the mstn mutations caused a decreased lipid mass in mammals such as mice, cattle, and sheep [25,26]. In contrast, gene disruption or knockdown of mstn induced an increase in lipid content of skeletal muscle in some fish species, such as zebrafish (Danio rerio), loach (Misgurnus anguillicaudatus), and Nile tilapia [[27], [28], [29]]. MSTN plays two distinct roles: promoting adipogenesis [30] and inhibiting adipogenesis [31], however, its specific function in adipogenesis is currently controversial. Considering the fact that the disruption of mstn in red sea bream reduces the amount of lipids, this gene may play a role in promoting lipid synthesis, but the exact mechanism is unclear. Further studies are required to understand the detailed roles of mstn in lipid metabolism in red sea bream and other species.
The lipid content of adult fish was considerably lower than that of juveniles, regardless of the genotype. Indeed, the lipid content of adult fish used in this study (HM: 2.1 ± 0.3 g/100 g and WT: 5.0 ± 0.7 g/100 g) was markedly lower than that of juveniles used in our previous study (HM: 11.6 ± 0.4 g/100 g and WT: 12.1 ± 0.3 g/100 g) [17]. The amount of lipids in fish generally varies depending on various factors such as tissue type, age, feed, and water temperature [32]. The difference in lipid content between the present study and our previous study [17] is presumably due to the difference in whether the viscera was used in the measurements. It has been reported that the whole viscera or hepatopancreas of red sea bream contain several to dozens of times more lipid than skeletal muscle [33]. In our previous study using juveniles [17], the entire body, including the viscera, was used for the proximate composition analysis. However, in the current study, only the fillets were subjected to the analysis as the aim was to assess their quality-related properties. Thus, we presume that the use of fillets without the viscera resulted in a low lipid content.
The ash content of the HM group did not differ from that of the WT group. In contrast, we previously showed that the ash content of HM juveniles was lower than that of WT juveniles [17]. A possible explanation for this discrepancy is whether an ash-rich bone was used in the proximate composition analysis [34]. Although the exact reason remains unknown, the ratio of the vertebral body to the whole body is smaller in the HM fish than in the WT fish [16]. The smaller vertebrae of HM fish may have resulted in their lower ash content than in WT fish because the whole body, including the vertebral body, was used for the proximate composition analysis in our previous study [17]. In contrast, in the current study, we used only fillets that did not contain the vertebral body. Thus, the lack of a difference in the ash content between the HM and WT groups may be because the smaller vertebrae of HM was not reflected in the quantitative results.
The carbohydrate content was comparable between the HM and WT fillets. Carbohydrates are originally a minor component of fish fillet, compared to water and protein [32]. Our results confirmed that the mstn mutation had no significant effect on the carbohydrate content.
The HM group also exhibited other interesting fillet quality-related properties. The drip volumes of HM fillets were reduced compared to those of WT fillets. Combined with the moisture content results, HM fillets had a higher water retention capacity than WT fillets. We speculate that the reason for the high water-holding capacity of HM fillets is the suppression of the postmortem pH decrease. Fish maintain a muscular pH of approximately 7.0 when alive, but the pH steadily declines over time after death [35]. As the pH level approaches the isoelectric point of the myofibrillar protein (approximately 5.0), the protein structure shrinks or is partially denatured, resulting in the loss of water from the fillet [36]. In this study, we found that the pH of HM fillets was higher than that of WT fillets at each sampling point. Thus, the suppression of a decrease in pH may alleviate the postmortem shrinkage of HM fillet, thus reducing the amount of dripping during storage. The suppression of the postmortem pH decrease in HM may be associated with reduced lactate levels. The postmortem decrease in pH in muscles is usually caused by the formation and accumulation of lactic acid derived from the anaerobic glycolytic system [36]. The lactic acid content in skeletal muscle in HM fillets was lower than that in WT fillets. Thus, the reduced amount of lactic acid in HM fillets may have been a factor in the suppressed postmortem pH decrease. The exact reason for the decrease in lactic acid content is unknown, but mstn mutations may alter the expression levels of lactic acid metabolism-related genes. We plan to investigate the mechanism of lactic acid reduction in HM fillets by quantifying the metabolites and enzyme activities involved in anaerobic glycolysis in future studies.
The properties of HM fillets with high water retention are worth noting because of their potential economic value. Meat producers and processors consider meat lacking water retention undesirable for three reasons [36]. First, because the price of meat is determined by its weight, loss of water during storage and cooking results in economic losses. Second, the dripping that leaks around raw meat is unpleasant for retail and grocery store consumers. Third, the loss of water content is associated with the loss of desirable sensory properties, such as tenderness and juiciness. Thus, HM fillet with high water retention capacity may contribute to solving these issues.
We evaluated the physical properties of HM fillet by measuring its breaking force and strain, which are indicators of food hardness and elasticity. The breaking strain of HM fillets was comparable to that of WT fillets, but the breaking force of HM fillets was lower than that of WT fillets, indicating that HM fillets have a softer texture than WT fillets. Because meat with high water retention is generally considered to be soft in texture, the high water retention of HM fillet may reduce its breaking force [37]. Another possible reason for the decrease in the breaking force of HM fillet is its reduced collagen content. Collagen is abundant in the connective tissue of fish muscle and is positively correlated with fillet texture indicators such as hardness [38]. Farmed sea bass (Dicentrarchus labrax L.) have a lower collagen content than natural individuals, resulting in lower hardness and other textural properties [39]. Thus, we speculate that the decreasing trend in collagen content in HM fillets may be a factor contributing to its reduced breaking force. The exact reason for the decreasing trend in collagen content in HM fillets is not clear; however, structural alterations in the myofibers may be involved. SEM observations confirmed that the myofibers of HM fillets were markedly more enlarged than those of WT fillets. This result suggests that myofiber hypertrophy leads to a decrease in the proportion of connective tissue per unit meat amount, resulting in collagen loss per unit.
The texture that consumers desire in fish fillet generally varies according to their personal preferences and food culture. This study showed that the breaking force of HM fillets, an index of hardness, may be reduced due to a decreasing trend in collagen content. In contrast, it has been reported that collagen synthesis in the skeletal muscle of red sea bream tends to be promoted by feeding diets containing catechin, a type of polyphenol [40]. Faba bean feeding also increases collagen content and enhances textural parameters such as hardness and springiness in grass carp (Ctenopharyngodon idellus) muscles [41]. Thus, the application of these feeding methods to HM fish may induce an increase in collagen content and tune the fillet texture according to individual consumer needs. Whether existing texture control techniques are effective for HM fish will be of scientific interest in the future.
The collagen content of HM fillets tended to be lower than that of WT fillets, but the difference was not conclusive. This suggests that the reduction in breaking force of HM fillets may be due to a combination of factors. In general, the reduction in breaking force of fish fillets during storage is not only caused by collagen collapse, but also by structural changes in their myofibrils [42]. Considering that the collagen content of HM fillets was not notable reduced compared to that of WT fillets, the disruption of mstn may also affect the myofibrillar structure. It has been reported that the collapse of the Z-line of myofibrils is responsible for fillet softening [43,44]. Thus, a detailed examination of the constituent proteins of the Z-line, or the activity of the relevant proteases, may further clarify the factors responsible for the reduction in breaking force of HM fillets.
In summary, we demonstrated that the disruption of mstn in red sea bream alters the compositional and textural aspects of fillet quality. To further characterize the food properties of HM fillets, we will focus on the heating-induced changes in weight, nutritional and flavor components, and sensory properties in future studies. An interesting prospect for research on mstn-deficient fish is comparisons between fish species. The disruption of mstn function has already been demonstrated in several fish species, and given the rapid progress and versatility of genome editing technology, will be extended to many other species [4,5]. Therefore, comprehensive studies of the fillet quality of mstn mutants across fish species are required. Because the fillet quality of mstn mutant fish has not yet been extensively studied, our findings may serve as a basis for such comparisons.
5. Conclusions
In the present study, we used the homozygous mstn mutants (HM) of red sea bream to assess the fillet quality of fillets over time during refrigeration. Proximate component analysis showed that HM fillets had a high moisture content. Furthermore, the amount of drippings leaking from HM fillets was reduced. These results suggest that the postmortem skeletal muscles of HM fish display a high water-holding capacity. The enhanced water retention of HM fillets was attributed to the slow decrease in pH during storage caused by a decrease in lactic acid content. In addition, the breaking force of HM fillets was reduced, suggesting a softening of their texture. The variation in textural properties was explained by the increased water retention and decreasing trend in collagen content in HM fillets. Electron microscopy indicated that the myofiber hypertrophy decreased the ratio of collagen in the total muscle tissue. In conclusion, we demonstrated the potential of HM fillets as a viable new fishery product owing to their high water retention and altered texture. Our insights into the alteration of the food properties of HM fillets may contribute to a comprehensive understanding of the fillet quality of mstn mutants, including other fish species.
Declaration of ethics
All animal experiments were approved by the Animal Experimentation Committee of Kyoto University (R6-102). Our research was performed in accordance with the ARRIVE guidelines.
Availability of data and materials
Data associated with the study has not been deposited into a publicly available repository. Data are available from the corresponding author on reasonable request.
CRediT authorship contribution statement
Yu Murakami: Writing – review & editing, Writing – original draft, Visualization, Validation, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Masashi Ando: Validation, Supervision, Methodology, Investigation. Kenta Kishimoto: Writing – review & editing, Resources, Funding acquisition. Mitsuki Ohama: Resources. Yuto Uemura: Resources. Reoto Tani: Resources, Investigation. Atsushi Akazawa: Resources. Kentaro Matsumiya: Writing – review & editing, Supervision. Kenji Sato: Writing – review & editing, Supervision. Masato Kinoshita: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Yu Murakami reports financial support was provided by Japan Society for the Promotion of Science (JSPS) Japan Science and Technology Agency (JST). Kenta Kishimoto and Masato Kinoshita has patent #JP 2024–038254 pending to Licensee. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by Grants-in-Aid from JSPS KAKENHI Early-Career Scientists 22K14940 and JST COI-NEXT JPMJPF2114. The funders had no role in the design of the study; collection, analysis, and interpretation of data; or in the writing of the manuscript.
Contributor Information
Yu Murakami, Email: murakami.yu.7z@kyoto-u.ac.jp.
Masashi Ando, Email: ando@nara.kindai.ac.jp.
Kenta Kishimoto, Email: kishimoto@regional.fish.
Mitsuki Ohama, Email: ohama@regional.fish.
Yuto Uemura, Email: uemura@regional.fish.
Reoto Tani, Email: tani@regional.fish.
Atsushi Akazawa, Email: akazawa@regional.fish.
Kentaro Matsumiya, Email: matsumiya.kentaro.6w@kyoto-u.ac.jp.
Kenji Sato, Email: sato.kenji.7x@kyoto-u.ac.jp.
Masato Kinoshita, Email: kinoshita.masato.4e@kyoto-u.ac.jp.
References
- 1.FAO The state of world fisheries and aquaculture: sustainability in action. 2020. [DOI]
- 2.Groeneveld L.F., Lenstra J.A., Eding H., Toro M.A., Scherf B., Pilling D., Negrini R., Finlay E.K., Jianlin H., Groeneveld E., Weigend S., GLOBALDIV Consortium Genetic diversity in farm animals—a review. Anim. Genet. 2010;41(suppl. 1):6–31. doi: 10.1111/j.1365-2052.2010.02038.x. [DOI] [PubMed] [Google Scholar]
- 3.Natalini A., Acciarri N., Cardi T. Breeding for nutritional and organoleptic quality in vegetable crops: the case of tomato and cauliflower. Agriculture. 2021;11 doi: 10.3390/agriculture11070606. [DOI] [Google Scholar]
- 4.Okoli A.S., Blix T., Myhr A.I., Xu W., Xu X. Sustainable use of CRISPR/Cas in fish aquaculture: the biosafety perspective. Transgenic Res. 2022;31:1–21. doi: 10.1007/s11248-021-00274-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mokrani A., Liu S. Harnessing CRISPR/Cas9 system to improve economic traits in aquaculture species. Aquaculture. 2024;579 doi: 10.1016/j.aquaculture.2023.740279. [DOI] [Google Scholar]
- 6.Egerman M.A., Glass D.J. Signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 2014;49:59–68. doi: 10.3109/10409238.2013.857291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kambadur R., Sharma M., Smith T.P., Bass J.J. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res. 1997;7:910–916. doi: 10.1101/gr.7.9.910. [DOI] [PubMed] [Google Scholar]
- 8.Mcpherron A.C., Lawler A.M., Lee S.J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 9.Shoyombo A.J., Abdulmojeed Y., Alabi O.O., Popoola M.A., Okon E.M., Arije D.O. Applications of myostatin in poultry and aquaculture—a review. Open Agric. J. 2022;16 doi: 10.2174/18743315-v16-e2208010. [DOI] [Google Scholar]
- 10.Chisada S.I., Okamoto H., Taniguchi Y., Kimori Y., Toyoda A., Sakaki Y., Takeda S., Yoshiura Y. Myostatin-deficient medaka exhibit a double-muscling phenotype with hyperplasia and hypertrophy, which occur sequentially during post-hatch development. Dev. Biol. 2011;359:82–94. doi: 10.1016/j.ydbio.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Kuroyanagi M., Katayama T., Imai T., Yamamoto Y., Chisada S., Yoshiura Y., Ushijima T., Matsushita T., Fujita M., Nozawa A., Suzuki Y., Kikuchi K., Okamoto H. New approach for fish breeding by chemical mutagenesis: establishment of TILLING method in fugu (Takifugu rubripes) with ENU mutagenesis. BMC Genom. 2013;14:786. doi: 10.1186/1471-2164-14-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khalil K., Elayat M., Khalifa E., Daghash S., Elaswad A., Miller M., Abdelrahman H., Ye Z., Odin R., Drescher D., Vo K., Gosh K., Bugg W., Robinson D., Dunham R. Generation of myostatin gene-edited channel catfish (Ictalurus punctatus) via zygote injection of CRISPR/Cas9 system. Sci. Rep. 2017;7:7301. doi: 10.1038/s41598-017-07223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahi N., Mallik S.K., Sarma D. Muscle growth in targeted knockout common carp (Cyprinus carpio) mstn gene with low off-target effects. Aquaculture. 2022;547 doi: 10.1016/j.aquaculture.2021.737423. [DOI] [Google Scholar]
- 14.Wu Y., Wu T., Yang L., Su Y., Zhao C., Li L., Cai J., Dai X., Wang D., Zhou L. Generation of fast growth Nile tilapia (Oreochromis niloticus) by myostatin gene mutation. Aquaculture. 2023;562 doi: 10.1016/j.aquaculture.2022.738762. [DOI] [Google Scholar]
- 15.Murata O., Harada T., Miyashita S., Izumi K., Maeda S., Kato K., Kumai H. Selective breeding for growth in red sea bream. Fish. Sci. 1996;62:845–849. doi: 10.2331/fishsci.62.845. [DOI] [Google Scholar]
- 16.Kishimoto K., Washio Y., Yoshiura Y., Toyoda A., Ueno T., Fukuyama H., Kato K., Kinoshita M. Production of a breed of red sea bream Pagrus major with an increase of skeletal muscle mass and reduced body length by genome editing with CRISPR/Cas9. Aquaculture. 2018;495:415–427. doi: 10.1016/j.aquaculture.2018.05.055. [DOI] [Google Scholar]
- 17.Ohama M., Washio Y., Kishimoto K., Kinoshita M., Kato K. Growth performance of myostatin knockout red sea bream Pagrus major juveniles produced by genome editing with CRISPR/Cas9. Aquaculture. 2020;529 doi: 10.1016/j.aquaculture.2020.735672. [DOI] [Google Scholar]
- 18.Washio Y., Ohama M., Kishimoto K., Kinoshita M., Kato K. Growth performance and edible ratio of myostatin-knockout young red sea bream Pagrus major produced by genome editing with CRISPR/Cas9. Aquaculture Science. 2021;69:101–112. doi: 10.11233/aquaculturesci.69.101. [DOI] [Google Scholar]
- 19.Author anonymous. Japan embraces CRISPR-edited fish. Nat. Biotechnol. 2022;40:10. doi: 10.1038/s41587-021-01197-8. [DOI] [PubMed] [Google Scholar]
- 20.Diggles B.K. Educational resources to promote best practice in the humane dispatch of fish caught by recreational Fishers. Fish. Manag. Ecol. 2016;23:200–207. https://doi-org.kyoto-u.idm.oclc.org/10.1111/fme.12127 [Google Scholar]
- 21.Bidlingmeyer B.A., Cohen S.A., Tarvin T.L. Rapid analysis of amino acids using pre-column derivatization. J. Chromatogr. 1984;336:93–104. doi: 10.1016/S0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- 22.Nagai T., Izumi M., Ishii M. Fish scale collagen. Preparation and partial characterization. Int. J. Food Sci. Technol. 2004;39:239–244. doi: 10.1111/j.1365-2621.2004.00777.x. [DOI] [Google Scholar]
- 23.Inoué T., Osatake H. A new drying method of biological specimens for scanning electron microscopy: the t-butyl alcohol freeze-drying method. Arch. Histol. Cytol. 1988;51:53–59. doi: 10.1679/aohc.51.53. [DOI] [PubMed] [Google Scholar]
- 24.Zhan X., Sun D.W., Zhu Z., Wang Q.J. Improving the quality and safety of frozen muscle foods by emerging freezing technologies: a review. Crit. Rev. Food Sci. Nutr. 2018;58:2925–2938. doi: 10.1080/10408398.2017.1345854. [DOI] [PubMed] [Google Scholar]
- 25.Guo T., Jou W., Chanturiya T., Portas J., Gavrilova O., Mcpherron A.C. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS One. 2009;4 doi: 10.1371/journal.pone.0004937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aiello D., Patel K., Lasagna E. The myostatin gene: an overview of mechanisms of action and its relevance to livestock animals. Anim. Genet. 2018;49:505–519. doi: 10.1111/age.12696. [DOI] [PubMed] [Google Scholar]
- 27.Gao Y., Dai Z., Shi C., Zhai G., Jin X., He J., Lou Q., Yin Z. Depletion of myostatin b promotes somatic growth and lipid metabolism in zebrafish. Front. Endocrinol. 2016;7:88. doi: 10.3389/fendo.2016.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao B., Tan J., Chen L., Xu Y., Liao X., Li Y., Chen J., Song Y., Hu W. CRISPR/Cas9 system-based myostatin-targeted disruption promotes somatic growth and adipogenesis in loach, Misgurnus anguillicaudatus. Aquaculture. 2021;544 doi: 10.1016/j.aquaculture.2021.737097. [DOI] [Google Scholar]
- 29.Wang Q., Yan Y., Tao Y., Lu S., Xu P., Qiang J. Transcriptional knock-down of mstn encoding myostatin improves muscle quality of Nile tilapia (Oreochromis niloticus) Mar. Biotechnol. 2023;25:951–965. doi: 10.1007/s10126-023-10252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Artaza J.N., Bhasin S., Magee T.R., Reisz-Porszasz S., Shen R., Groome N.P., Meerasahib M.F., Gonzalez-Cadavid N.F. Myostatin inhibits myogenesis and promotes adipogenesis in C3H 10T(1/2) mesenchymal multipotent cells. Endocrinology. 2005;146:3547–3557. doi: 10.1210/en.2005-0362. [DOI] [PubMed] [Google Scholar]
- 31.Guo W., Flanagan J., Jasuja R., Kirkland J., Jiang L., Bhasin S. The effects of myostatin on adipogenic differentiation of human bone marrow-derived mesenchymal stem cells are mediated through cross-communication between Smad3 and Wnt/beta-catenin signaling pathways. J. Biol. Chem. 2008;283:9136–9145. doi: 10.1074/jbc.M708968200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmed I., Jan K., Fatma S., Dawood M.A.O. Muscle proximate composition of various food fish species and their nutritional significance: a review. J. Anim. Physiol. Anim. Nutr. 2022;106:690–719. doi: 10.1111/jpn.13711. [DOI] [PubMed] [Google Scholar]
- 33.Osato S., Miyata K., Matsuo S., Itou T., Kora H., Misima T., Tachibana K., Tsuchimoto M. Change of fat in various parts of fish body accompanying growth in cultured red sea bream. Nippon Suisan Gakkaishi. 1991;57:905–913. (in Japanese) [Google Scholar]
- 34.Toppe J., Albrektsen S., Hope B., Aksnes A. Chemical composition, mineral content and amino acid and lipid profiles in bones from various fish species. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007;146:395–401. doi: 10.1016/j.cbpb.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 35.Simeonidou S., Govaris A., Vareltzis K. Quality assessment of seven Mediterranean fish species during storage on ice. Food Res. Int. 1997;30:479–484. doi: 10.1016/S0963-9969(98)00008-8. [DOI] [Google Scholar]
- 36.Toldrá F. Muscle foods: water, structure and functionality. Food Sci. Technol. Int. 2003;9:173–177. doi: 10.1177/1082013203035048. [DOI] [Google Scholar]
- 37.Gault N.F. The relationship between water-holding capacity and cooked meat tenderness in some beef muscles as influenced by acidic conditions below the ultimate pH. Meat Sci. 1985;15:15–30. doi: 10.1016/0309-1740(85)90071-3. [DOI] [PubMed] [Google Scholar]
- 38.Sato K., Yoshinaka R., Sato M., Shimizu Y. Collagen content in the muscle of fishes in association with their swimming movement and meat texture. Nippon Suisan Gakkaishi. 1986;52:1595–1600. doi: 10.2331/suisan.52.1595. [DOI] [Google Scholar]
- 39.Periago M.J., Ayala M.D., López-Albors O., Abdel I., Martínez C., García-Alcázar A., Ros G., Gil F. Muscle cellularity and flesh quality of wild and farmed sea bass. Dicentrarchus labrax L. Aquaculture. 2005;249:175–188. doi: 10.1016/j.aquaculture.2005.02.047. [DOI] [Google Scholar]
- 40.Nakagawa H.E., Mustafa M.D.G.H., Takii K.E., Umino T.E., Kumai H.I. Effect of dietary catechin and Spirulina on vitamin C metabolism in red sea bream. Fish. Sci. 2000;66:321–326. doi: 10.1046/j.1444-2906.2000.00050.x. [DOI] [Google Scholar]
- 41.Ma L.L., Kaneko G., Wang X.J., Xie J., Tian J.J., Zhang K., Wang G.J., Yu D.G., Li Z.F., Gong W.B., Yu E.M., Li H.H. Effects of four faba bean extracts on growth parameters, textural quality, oxidative responses, and gut characteristics in grass carp. Aquaculture. 2020;516 doi: 10.1016/j.aquaculture.2019.734620. [DOI] [Google Scholar]
- 42.Cheng J.H., Sun D.W., Han Z., Zeng X.A. Texture and structure measurements and analyses for evaluation of fish and fillet freshness quality: a review. Compr. Rev. Food Sci. Food Saf. 2014;13:52–61. doi: 10.1111/1541-4337.12043. [DOI] [PubMed] [Google Scholar]
- 43.Tachibana K., Suzuki H., Yagi M., Mishima T., Hara K., Tsuchimoto M., Limited degradation of α-actinin in the ordinary muscle of cultured red sea bream during storage in ice, Nippon Suisan Gakkaishi 67 (2001) 723–727 (in Japanese).
- 44.Liang J., Miyazaki R., Zhao X., Hirasaka K., Taniyama S., Tachibana K. Changes in the pericellular connective tissue and breaking strength of the three types of muscles of the cultured carp Cyprinus carpio during storage in ice. Fish. Sci. 2014;80:1083–1088. doi: 10.1007/s12562-014-0769-z. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with the study has not been deposited into a publicly available repository. Data are available from the corresponding author on reasonable request.