Abstract
Treatment with a 2-week course of anti-CD154 antibody and a single transfusion of donor leukocytes (a donor-specific transfusion or DST) permits skin allografts to survive for >100 days in thymectomized mice. As clinical trials of this methodology in humans are contemplated, concern has been expressed that viral infection of graft recipients may disrupt tolerance to the allograft. We report that acute infection with lymphocytic choriomeningitis virus (LCMV) induced allograft rejection in mice treated with DST and anti-CD154 antibody if inoculated shortly after transplantation. Isografts resisted LCMV-induced rejection, and the interferon-inducing agent polyinosinic:polycytidylic acid did not induce allograft rejection, suggesting that the effect of LCMV is not simply a consequence of nonspecific inflammation. Administration of anti-CD8 antibody to engrafted mice delayed LCMV-induced allograft rejection. Pichinde virus also induced acute allograft rejection, but murine cytomegalovirus and vaccinia virus (VV) did not. Injection of LCMV ∼50 days after tolerance induction and transplantation had minimal effect on subsequent allograft survival. Treatment with DST and anti-CD154 antibody did not interfere with clearance of LCMV, but a normally nonlethal high dose of VV during tolerance induction and transplantation killed graft recipients. We conclude that DST and anti-CD154 antibody induce a tolerant state that can be broken shortly after transplantation by certain viral infections. Clinical application of transplantation tolerance protocols may require patient isolation to facilitate the procedure and to protect recipients.
Among the most important risks faced by allograft recipients are viral infections. These may arise from infected transplanted organs, from the reactivation of latent host viruses as a consequence of an allogeneic stimulus and immunosuppressive treatment, or from exposure of the immunosuppressed host to exogenous environmental pathogens (3, 5, 12, 17, 23).
Recent developments in our understanding of T-cell activation, anergy, and tolerance have led to treatment protocols that permit durable graft survival without the need for prolonged immunosuppressive therapy. These protocols are based on interference with costimulatory signal pathways. When naïve T cells encounter antigen, they require ligation of both the T-cell receptor (“signal 1”) and certain costimulatory molecules (“signal 2”) in order to proliferate and differentiate. Signal 1 in the absence of signal 2 leads to anergy or possibly apoptosis (21). One important costimulatory molecule is CD154 (CD40 ligand), which binds to CD40 on antigen-presenting cells (6, 11, 14).
We have shown in mice that a very brief course of anti-CD154 antibody together with a single transfusion of allogeneic splenocytes prolongs the survival of fully allogeneic skin grafts (16). About 20% of grafts survive for >275 days in euthymic recipients (16), and the majority survive for >100 days in thymectomized recipients (15). Although we initially interpreted graft survival to be the result of anergy of effector T-cell populations (16), the mechanism appears to be more complex. For example, tolerance to the allograft can be abrogated by treatment of recipients with antibody to CD4 (15). The data suggest that allograft rejection, which is normally mediated by CD8+ T cells, may be regulated by a CD4+ T-cell population that arises as a consequence of the tolerization procedure. Whatever the mechanism, donor-specific transfusion (DST) and anti-CD154 antibody treatment are being studied intensively for possible use in human transplantation (20).
This two-element tolerance induction protocol is simple and appears benign, but its adaptation in the clinic will require documentation of the safety and durability of transplanted allografts. The requirement for CD4+ T cells to maintain allotolerance in this system suggests that allograft survival could be unstable in the presence of infection, which may significantly disrupt immune regulation and CD4+-to-CD8+ T-cell ratios. Many viral infections not only induce transient shifts in the CD4/CD8 ratio from 2:1 to 1:2 or 1:3 but also induce cytotoxic T lymphocytes (CTLs) lytic to uninfected allogeneic targets (4, 19, 25, 26, 28). The degeneracy of the T-cell response to viral infection is such that many virus-specific T-cell clones cross-react with specific allogeneic major histocompatibility complex (MHC) antigens expressed on cells not infected with the virus (1, 2, 19, 22). Such cross-reactivity between viral antigens and alloantigens has been observed in T cells isolated from mice infected with lymphocytic choriomeningitis virus (LCMV), vaccinia virus (VV), Pichinde virus (PV), and murine cytomegalovirus (MCMV) (19). It has also been observed in T cells from humans infected with Epstein-Barr virus (4, 25, 26, 28). Recent studies have documented at the molecular level how the T-cell receptor (TcR) of virus-specific T cells may interact with allogeneic MHC molecules expressing endogenous peptides (1, 4, 7, 24). Virus infections also have the potential to overcome T-cell unresponsiveness by inducing high levels of interleukin-2 and other cytokines (9, 10, 10). The present studies were designed to determine whether viral infection of C57BL/6 (H2b) mice treated with BALB/c (H2d) DST and anti-CD154 monoclonal antibody (MAb) and transplanted with allogeneic BALB/c skin grafts would influence graft survival.
MATERIALS AND METHODS
Animals.
C57BL/6 (H2b) and MHC-incompatible BALB/c (H2d) mice were obtained from the National Cancer Institute (Frederick, Md.). In all experiments, male C57BL/6 recipients and female BALB/c skin and DST donors were used. All animals were certified to be free of Sendai virus, pneumonia virus of mice, murine hepatitis virus, minute virus of mice, ectromelia virus, lactate dehydrogenase-elevating virus, mouse poliovirus, reovirus type 3 virus, murine adenovirus, LCMV, polyomavirus, Mycoplasma pulmonis, and Encephalitozoon cuniculi. All animals were housed in microisolator cages and given ad libitum access to autoclaved food. They were maintained in accordance with recommendations in the Guide for the care and use of laboratory animals (11a) and the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the University of Massachusetts Medical School.
Transplantation procedures.
Thymectomized male C57BL/6 recipients 6 weeks of age were tolerized and transplanted, using previously published techniques (15, 16). Briefly, 107 BALB/c splenocytes from adult female donors were injected intravenously in a volume of 0.5 ml into recipients 7 days before grafting. Four doses of anti-CD154 MAb (0.25 mg) were administered intraperitoneally (i.p.) twice weekly beginning on the day of spleen cell injection. BALB/c skin grafts 1 to 2 cm in diameter were transplanted onto the dorsal flanks of C57BL/6 mice 7 days after the spleen cell injection and the initiation of anti-CD154 MAb treatment. Graft rejection was defined as the first day on which the entire graft was rejected (15, 16). In one experiment, thymectomized graft recipients treated with DST and anti-CD154 MAb were given the interferon-inducing agent polyinosinic:polycytidylic acid (poly I:C; Sigma, St. Louis, Mo.) at a dose (each) of 0.5 mg intravenously 1 day after graft placement.
Transplantation recipients were thymectomized for two reasons. First, after tolerization they maintain skin allografts for prolonged periods, often indefinitely, thereby permitting long-term studies (15). Second, thymectomy permitted us to define the effects of viral infection on tolerized lymphocyte populations in the absence of new thymic emigrants in the periphery.
Virus infection procedures.
In most experiments, mice were inoculated i.p. with 5 × 104 PFU of LCMV strain Armstrong propagated in baby hamster kidney cells. In the C57BL/6 (H2b) mouse, the LCMV Armstrong strain induces a particularly high virus-specific CTL response and, in addition, significant levels of cytotoxicity against uninfected target cells expressing H2d MHC alloantigens (19, 28). In other experiments, mice were inoculated with varying concentrations of VV, strain WR, MCMV strain Smith, PV, or PV strain AN3739 (28). Routes of administration are indicated in the descriptions of the individual experiments. LCMV, VV, MCMV, and PV have all been reported to induce anti-H2d allospecific CTLs in C57BL/6 mice (28). Certain mice were inoculated with recombinant VV expressing the gene for the LCMV glycoprotein (VV-GP) or the gene for the LCMV nucleoprotein (VV-NP); both viruses were the kind gift of J. Lindsay Whitton, Scripps Research Institute, La Jolla, Calif. (27, 28).
CTL assay.
CTL analysis for virus-specific killing was performed by chromium-51-release microcytotoxicity assays on LCMV-infected syngeneic MC57G (H2b) target cells and control uninfected syngeneic target cells as previously described (19). Allospecific CTL activity induced by virus infection was analyzed on uninfected P-815 (H2d) target cells.
Statistics.
Average duration of graft survival is presented as the median. Graft survival among groups was compared by the method of Kaplan and Meier (13). The equality of allograft survival distributions for animals in different treatment groups was tested by using the log rank statistic (13). P values of <0.05 were considered statistically significant.
RESULTS
Infection with LCMV abrogates transplantation tolerance to skin allografts.
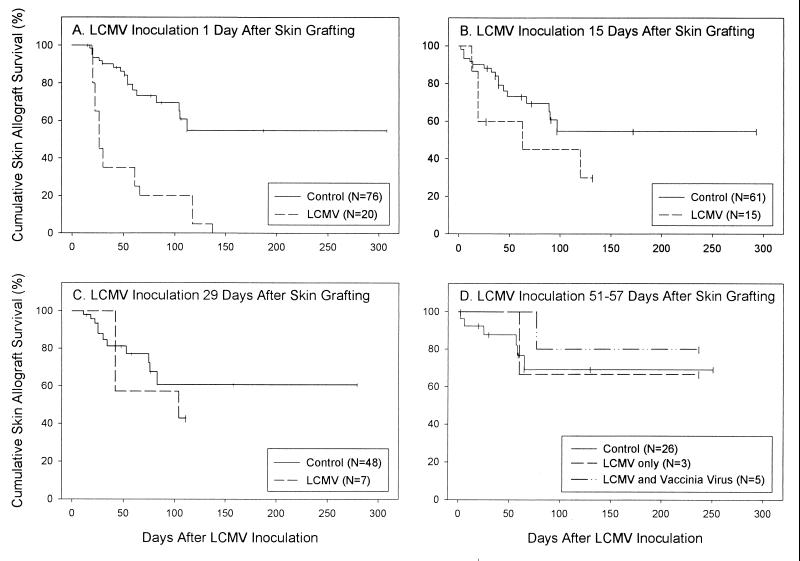
Skin allograft recipients treated with DST and anti-CD154 MAb and then infected with LCMV 1 day after transplantation uniformly rejected their grafts (Fig. 1A). In the majority of cases, the earliest signs of rejection appeared 10 to 11 days after infection, but in some cases, signs of rejection did not appear for nearly a month. In contrast, more than half of skin allografts in uninfected mice treated with DST and anti-CD154 MAb survived permanently. Median graft survival time (MST) was >308 days in controls versus 26 days in LCMV-infected recipients (P < 0.001).
FIG. 1.
Skin allograft survival in uninfected and LCMV-infected mice. C57BL/6 mice were treated with a single donor-specific BALB/c spleen cell transfusion and a brief course of anti-CD154 MAb and given a BALB/c skin graft as described in Materials and Methods. Transplantation was performed on small groups of animals over the course of several weeks, as animals became available. A cumulative total of 96 tolerized, transplanted animals was used in this experiment. (A) On the day after transplantation, a cohort of 20 mice was injected with LCMV; the remaining animals were untreated. Skin allograft survival in this group was statistically significantly shorter than that in the pool of all other uninfected transplanted mice (P < 0.001). (B) On day 15 after transplantation, 15 recipients from among the uninfected controls with successful grafts (A) were injected with LCMV. There was no statistically significant difference in overall graft survival between this group and the pool of all remaining uninfected animals (P = 0.06). (C) On day 29 after transplantation, seven of the recipients from among the uninfected controls that had not spontaneously rejected their grafts (B) were selected and injected with LCMV. Graft survival in this group was statistically similar to that observed in the pool of all remaining uninfected control animals (P = 0.54). (D) On days 51 to 57 after transplantation, eight recipients from among the uninfected controls that had not spontaneously rejected their grafts (C) were selected and injected with LCMV. Three of the tolerized recipients infected with LCMV received no other intervention; one recipient rejected its graft 60 days after LCMV infection. Five of the tolerized recipients infected with LCMV, whose grafts then survived 47 days after infection, were further challenged with 106 PFU of VV strain WR injected directly into their grafts. One of these animals rejected its graft 30 days later; the remaining grafts survived through the end of the experiment. Graft survival in the two virus-treated groups was statistically similar to that in the pool of all remaining uninfected controls. Each panel shows graft survival relative to the day of LCMV injection. Cumulative graft survival was calculated by the method of Kaplan and Meier, which takes into account the successive removal of mice from the pool of control animals. Vertical bars indicate censored data (i.e., animals with intact grafts at the conclusion of the study and animals removed at successive intervals for use in the timed infection experiments shown in panels B and C).
In contrast, LCMV infection at progressively later time points after tolerance induction had progressively less effect on graft survival. Skin allograft recipients treated with DST and anti-CD154 MAb and then infected with LCMV 15 days after transplantation experienced a rate of survival that was only somewhat compromised (Fig. 1B). MST after infection was 78 days in LCMV-infected recipients versus >293 days in controls (P = 0.06).
Survival of skin allografts in recipients treated with DST and anti-CD154 MAb and then infected with LCMV 29 days after transplantation was not statistically different from that observed in controls (Fig. 1C). MST of grafts after infection was 104 days in LCMV-infected recipients versus >279 days in controls (P = 0.54).
Survival of skin allografts in tolerized recipients infected with LCMV 51 to 57 days after transplantation was indistinguishable from that in controls (Fig. 1D). In this arm of the experiment, eight mice received LCMV 51 to 57 days after transplantation. Three of the eight mice received no other intervention. One mouse rejected its graft 60 days after LCMV inoculation; the remaining two survived through the end of the experiment. As an additional test of the durability of tolerance, the other five tolerized recipients that had been infected with LCMV on days 51 to 57 after grafting were challenged with a second virus on day 47 after LCMV infection. They were inoculated with 106 PFU of VV directly into their grafts. This procedure was designed to determine whether profound viral infection within the dermis of the allograft would induce rejection. Twenty-four days after VV infection, one of the five mice showed signs of graft rejection, and the graft was completely rejected 6 days later. The other four mice retained their grafts with no sign of rejection. MST after initial LCMV infection was >237 days in the total group of 8 infected recipients versus >251 days in controls (P = 0.26).
Finally, three of three successful skin allografts on tolerized recipients that were then infected with LCMV 87 days after transplantation survived through the end of the experiment (MST, >221 days relative to infection and >308 days overall). These experiments collectively indicate that allografts that remain stable for ∼50 days become remarkably resistant to virus-induced rejection.
Histology.
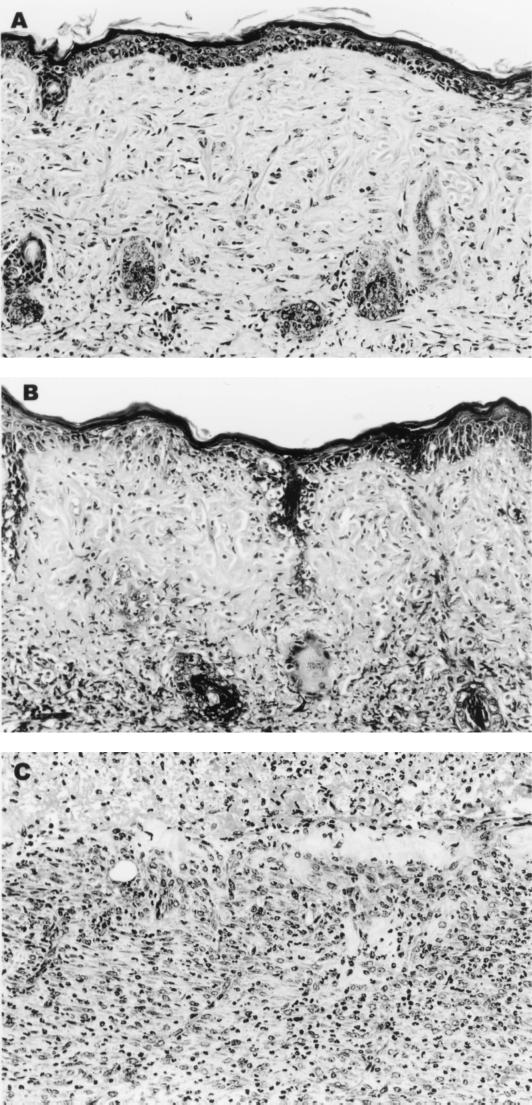
Histological analysis of control skin grafts that had survived for 30 days (Fig. 2A) revealed scattered lymphocytes in the basal layer of the epidermis and surrounding hair follicles. Grafts in the process of rejection taken from LCMV-infected mice 15 days after inoculation (day 16 after transplantation) were characterized by lymphocytic infiltration of the basal layer of the epidermis and degeneration of the basal epithelium (Fig. 2B). Apoptotic bodies and a destructive lymphocytic infiltrate of the hair follicles were present (Fig. 2B). The initial phase of rejection was followed by progressive denudation of the graft site. Histologic study of a fully rejected graft site from an LCMV-infected mouse at day 15 revealed a subjacent acute and chronic inflammatory reaction (Fig. 2C). Once an area of necrosis became visible on a graft, it invariably expanded, and the graft was rejected.
FIG. 2.
LCMV-induced infiltration of leukocytes into the rejecting allograft. C57BL/6 mice were pretreated with a single donor-specific BALB/c spleen cell transfusion and a brief course of anti-CD154 MAb and then given a BALB/c skin graft as described in Materials and Methods. Mice were either left untreated (A) or infected with LCMV 1 day after transplantation (B and C). (B) The early phase of graft rejection characterized by mononuclear cell infiltration of the epidermal basal layer and hair follicles. This is followed by denudation of the graft (C). (Hematoxylin and eosin stain; magnification, ×160).
Antigen specificity.
To determine whether graft rejection in response to early LCMV infection (Fig. 1A) was an antigen-specific phenomenon or simply the consequence of a nonspecific inflammatory response to infection, control isografts were performed. C57BL/6 mice were treated with a BALB/c DST and anti-CD154 and then given a C57BL/6 skin isograft in accordance with the standard protocol. Each of seven recipients was then infected with LCMV on the day after transplantation. In two independent trials, all seven isografts survived indefinitely (MST, >259 days) and showed no evidence of rejection.
Depletion of CD8+ cells delays LCMV-induced rejection of allografts.
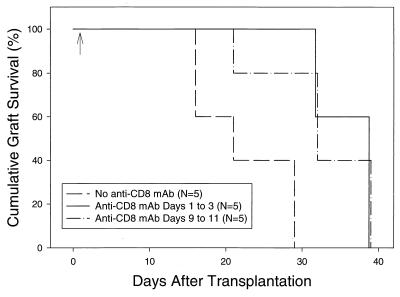
CD8+ T-cell responses to LCMV in C57BL/6 mice are known to include H2d alloreactive T cells (19), and in other systems, CD8+ T cells have been found to be important in mediating graft rejection (20). We therefore tested the hypothesis that CD8+ T cells mediated graft rejection in LCMV-infected mice harboring BALB/c allografts. C57BL/6 mice were tolerized, transplanted with BALB/c skin allografts, infected with LCMV on the day after transplantation, and then randomized into three groups of 5 animals each. Group 1 received no further treatment. Group 2 received anti-CD8 MAb on days 0, 1, and 2 after infection. Group 3 received anti-CD8 MAb on days 8, 9, and 10 after infection. Mice depleted of CD8+ cells immediately after infection (group 2) became long-term LCMV carriers. The blood concentration of LCMV was uniformly >105 PFU per ml (range, 1.5 × 105 to 5.3 × 105) 126 days after infection. This finding is consistent with reports that LCMV persists in the presence of a weak CTL response that would result from treatment with anti-CD8 MAb (18). Mice depleted of CD8+ cells on days 8 to 10 (group 3) completely cleared LCMV (<10 PFU/ml of blood), again consistent with reports that this virus is cleared by CD8+ T cells prior to day 8 after infection (18). In both group 2 (MST = 39 days) and group 3 (MST = 32 days), allograft rejection was significantly delayed compared with group 1 controls that did not receive anti-CD8 MAb (MST = 21 days; P < 0.0025) (Fig. 3).
FIG. 3.
Skin allograft survival in LCMV-infected mice treated with anti-CD8 MAb. C57BL/6 mice were pretreated with a single donor-specific BALB/c spleen cell transfusion and a brief course of anti-CD154 MAb and then given a BALB/c skin graft as described in Materials and Methods. All mice were infected with LCMV 1 day after transplantation (arrow) and randomized into three groups. Group 1 received no further treatment. Group 2 was treated with anti-CD8 MAb on days 0, 1, and 2 relative to infection (days 1 to 3 relative to transplantation). Group 3 was treated with anti-CD8 MAb on days 8, 9, and 10 relative to infection (days 9, 10, and 11 relative to transplantation), by which time the induced T-cell response has usually cleared LCMV infection. Graft survival in groups 2 and 3 differs from that in group 1, where P values are ≤0.025.
The delay in LCMV-induced allograft rejection in anti-CD8 MAb-treated mice suggests that CD8+ T cells participate in the abrogation of transplantation tolerance in this model system. However, the fact that rejection ultimately did occur in mice depleted of CD8+ cells suggests either that the CD8 depletion was incomplete or that an additional rejection mechanism, perhaps mediated by CD4+ cells, was active. To test the former possibility, we measured the percentage of CD8+ cells present after graft rejection and observed that depletion was, in fact, incomplete. After graft rejection, the percentage of peripheral blood CD8+ T cells was found to be 6.1% ± 1.5% in group 1 (controls, n = 5), 0.6% ± 0.06% in group 2 (anti-CD8 treatment on days 0 to 2; n = 5), and 3.4% ± 0.9% in group 3 (anti-CD8 treatment on days 8 to 10; n = 3). The long-term depletion of CD8+ cells appeared to be more complete in group 2 than in group 3, possibly because the number of CD8+ cells was very high on day 8 after infection, when mice in group 3 were given anti-CD8 MAb. Of interest are the mice in group 2, whose CD8+ T cells were depleted at the time of infection. They had very low levels of CD8+ cells, but nonetheless ultimately rejected their grafts, albeit with delayed kinetics. Depletion of CD4+ T cells was not studied, as we have previously shown that anti-CD4 MAb treatment induces allograft rejection in uninfected tolerized mice (5).
Levels of LCMV-induced allospecific CTL activity are depressed in tolerized mice.
Because allograft rejection was delayed by depletion of CD8+ T cells and because LCMV induces anti-H2d-specific CTLs in C57BL/6 mice (19, 22, 28), we measured splenic anti-H2d-specific CTL activity in transplanted, LCMV-infected animals. Groups of thymectomized and tolerized C57BL/6 recipient mice with intact BALB/c (H2d) skin allografts were infected with LCMV 1, 15, 50, or 70 days after transplantation. A separate group of thymectomized, LCMV-infected mice that received no graft or other treatment was used as a positive control. CTL assays were performed 8 days after infection.
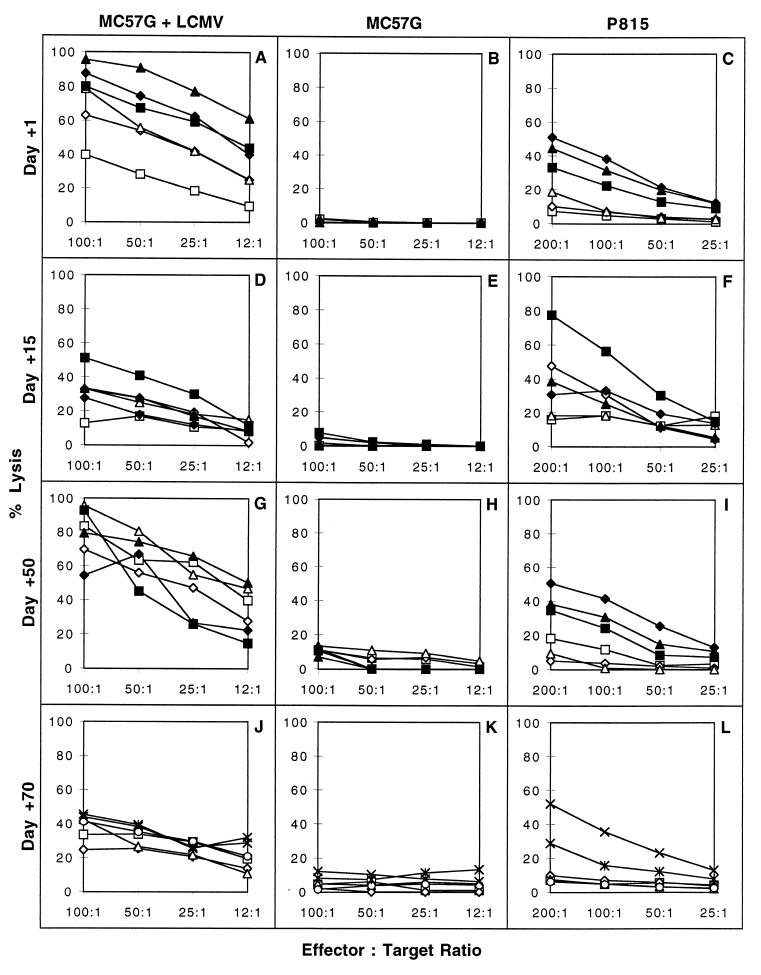
Control levels of CTL activity were high on LCMV-infected syngeneic targets, very low on uninfected syngeneic targets, and intermediate on allogeneic uninfected P-815 (H2d) targets (Fig. 4, filled symbols). Killing of uninfected syngeneic targets was very low in all experiments (Fig. 4B, E, H, and K). Mice infected with LCMV 1 day after transplantation evidenced somewhat reduced but still substantial levels of LCMV-specific CTL activity on day 8 after infection (Fig. 4A) and cleared the virus. This finding is noteworthy because it documents that, although reduced, a protective LCMV-specific CTL response was not prevented by the anti-CD154 MAb and the trauma of surgery. There was no response to uninfected syngeneic cells (Fig. 4B), and the allografted, infected mice displayed only low levels of cytotoxicity against P-815 targets (Fig. 4C). LCMV-infected mice given allogeneic BALB/c splenocytes, anti-CD154 MAb, and skin isografts also displayed very low levels of allospecific CTL activity (data not shown). This demonstrates that the continued presence of an allogeneic skin graft was not required to maintain relatively depressed levels of virus-induced allospecific CTL activity in mice previously tolerized with BALB/c splenocytes.
FIG. 4.
Effect of DST and anti-CD154 MAb on LCMV-induced CTL activity. C57BL/6 mice were pretreated with a single donor-specific BALB/c spleen cell transfusion and a brief course of anti-CD154 MAb and then given a BALB/c skin graft as described in Materials and Methods. Groups of mice bearing intact grafts were infected with LCMV on day 1, 15, 50, or 70 after skin transplantation. T cells from these mice were tested in 7- to 8-h chromium-51-release assays for cytotoxicity against LCMV-infected syngeneic MC57G cells (A, D, G, and J), uninfected MC57G cells (B, E, H, and K), or allogeneic P-815 cells (C, F, I, and L). Open symbols represent the percent lysis mediated by splenocytes from mice treated with DST and anti-CD154 MAb and skin grafted. Solid symbols represent cytotoxicity mediated by control thymectomized mice infected with LCMV. In the day 70 experiment, the ×-× symbols refer to lysis by cells from LCMV-infected normal nonthymectomized mice that were used as the controls.
Allografted mice infected with LCMV 15 days after transplantation generated virus-specific CTL responses nearly as high as those generated by the thymectomized nontolerized control mice (Fig. 4D). In contrast, although some killing of allogeneic P-815 targets was detectable, the tolerized mice exhibited substantially lower allospecific CTL activity than did the control thymectomized mice (Fig. 4F). Similar results were obtained from mice infected with LCMV 50 (Fig. 4G, H, and I) or 70 (Fig. 4J, K, and L) days after transplantation. With the sole exception of one transplanted, infected mouse with high allospecific CTL activity (Fig. 4F, open diamond), generally low but still detectable levels of allospecific CTL activity were observed. In comparison with the thymectomized, nontolerized controls, the LCMV-induced allospecific CTL activity in all groups of tolerized, grafted, virus-infected mice was significantly, but not completely, eliminated. This finding was also true for mice that were infected on the day after transplantation and were destined to reject their allografts.
In contrast to the high levels of virus-specific and allospecific cytotoxicity observed on day 8 postinfection in thymectomized control mice (Fig. 4C), CTL activity was undetectable on day 15 postinfection (data not shown). This was true both in control, nontransplanted, thymectomized mice and in tolerized mice given LCMV on the day after transplantation (data not shown). The results confirm the transient nature of the LCMV-induced CTL response in the spleen. They also show that no delayed allospecific CTL response was generated in the spleens of mice harboring allografts. The data do not, however, exclude the possibility that allospecific CTL may have infiltrated the allografts.
poly I:C does not abrogate graft survival in mice treated with DST and anti-CD154 MAb.
poly I:C is a synthetic double-stranded polyribonucleotide. Presumably due to structural resemblance to double-stranded viral RNA, poly I:C elicits cytokine responses that mimic the early stages of viral infection. These responses include the stimulation of type I interferon interleukin-1, tumor necrosis factor alpha, and the activation of NK cells, macrophages, and endothelial cells (8). To determine if the deleterious effect of peritransplant LCMV infection on graft survival was in part due to nonspecific inflammation, thymectomized C57BL/6 recipients were treated with DST and anti-CD154 MAb and given BALB/c skin allografts. These mice were then given either no further treatment (n = 5) or poly I:C (n = 5) 1 day after transplantation. MST was >113 days in both controls and in poly I:C-treated recipients.
Infection with other viruses at the time of tolerance induction.
These data document that tolerized mice inoculated with LCMV 1 day after transplantation control the infection but rapidly reject their grafts. We next examined the response of tolerized, thymectomized C57BL/6 mice to various other viruses that were inoculated 1 day after transplantation of BALB/c skin grafts. The results are summarized in Table 1.
TABLE 1.
Survival of skin allografts on tolerized mice infected with various virusesa
| Virus | Dose (PFU) | n | Graft survival (days) | MST |
|---|---|---|---|---|
| VV Wt | 106 | 8b | >75b | |
| VV Wt | 105 | 6 | 10, >75, >75, >75, >75, >75 | >75 |
| VV Wt | 104 | 6 | >75, >75, >75, >75, >75, >75 | >75 |
| MCMV | 105 | 5 | 10, >75, >75, >75, >75 | >75 |
| MCMV | 3 × 104 | 5 | 36, 41, >75, >75, >75 | >75 |
| MCMV | 104 | 11 | One graft, 10; ten grafts, >75 each | >75 |
| PV | 5 × 106 | 5 | 21, 21, 22, 61, >64 | 22c |
| VV-NP | 8 × 106 | 5 | 59, >64, >64, >64, >64 | >64 |
| VV-GP | 8 × 106 | 5 | >64, >64, >64, >64, >64 | >64 |
| Uninfected | NA | 19 | 7, 8, 20, >64, >64, >64, >64, and 12 grafts, >75 each | >75 |
C57BL/6 mice were treated with DST and anti-CD154 MAb and grafted with BALB/c skin as described in Materials and Methods. One day after skin grafting, mice were injected i.p. with virus as indicated. The day of graft rejection is indicated. Wt, wild type.
A total of 7 of the 8 mice were found dead before day 14, but had intact grafts to that point.
Significantly different from values obtained with uninfected controls (P = 0.013).
Only 3 of 19 tolerized, thymectomized, but uninfected control mice rejected their grafts by day 75 after transplantation. This rate of skin allograft survival is consistent with previous reports (15).
Seven of eight tolerized, transplanted mice infected i.p. with 106 PFU of VV in two experiments died within 1 week of infection. In contrast, none of six mice treated with DST and anti-CD154 MAb but no skin graft died when given 106 PFU of VV, suggesting that the surgical trauma of transplantation may have enhanced the susceptibility of these mice to infection. Tolerized, grafted mice given either 105 PFU (n = 6) or 104 PFU (n = 6) of VV uniformly survived the infection (Table 1). The effect of VV infection on graft survival in mice that cleared the infection was relatively minimal. By day 75 after infection, only 1 of 13 surviving VV-infected mice rejected its graft.
Tolerized, grafted mice given either 104 (n = 11), 3 × 104 (n = 5), or 105 (n = 5) PFU of MCMV i.p. uniformly survived; normal mice given 106 PFU of the same stock died. This result demonstrates that the tolerized mice were able to control the potentially lethal virus. By day 75 after transplantation, 4 of 21 tolerized recipients rejected their grafts after MCMV infection, but this allograft survival distribution was not statistically different from that observed in tolerized, uninfected mice (Table 1).
Tolerized mice survived infection with PV at a dose of 5 × 106 PFU. PV, like LCMV, was a potent inducer of graft rejection; three of five grafts on PV-infected mice were rejected by day 22, and a fourth by day 61. PV and LCMV are both arenaviruses.
These results suggest that tolerization and transplantation procedures may make a host more susceptible to some but not all viral infections. In general, however, tolerized mice controlled virus infections. The data demonstrate that viruses differ greatly in their ability to induce acute graft rejection in recently tolerized recipients. Because LCMV very rapidly induced the rejection of BALB/c allografts whereas VV did not, we hypothesized that an LCMV gene product expressed in a VV recombinant might induce rejection by eliciting T cells cross-reactive with the allograft. To test this hypothesis, tolerized, skin-grafted mice were inoculated i.p. with 8 × 106 PFU of either VV-NP or VV-GP. These high doses were used because VV recombinants grow more poorly in vivo than does wild-type VV. Grafts on four of five of the VV-NP-infected mice showed signs of rejection during the first 10 days after infection. The grafts shrank to 20 to 60% of the normal size but then stabilized. Only one graft was eventually rejected on day 59. Grafts on five VV-GP-infected mice showed no signs of rejection at any time and survived throughout the duration of this experiment. Studies into the nature of this response are continuing.
DISCUSSION
These results demonstrate that acute viral infection can interfere with tolerance induction and reduce skin allograft survival in mice tolerized with DST and anti-CD154 MAb. In the majority of cases, however, the tolerized, transplanted recipients cleared the infection and survived. Infection at later time points after tolerization had progressively less effect on graft survival and no deleterious effect on recipient survival.
Statistically significant effects of viral infection on graft survival were observed only when infection occurred soon after transplantation. This observation suggests that cells responsible for rejection may initially be only partially suppressed or tolerized and that this early tolerant state may be overcome by factors induced during the viral infection. After grafts had survived for several weeks, however, LCMV infection no longer interfered with the allotolerant state.
Our data suggest that the mechanism by which infection in the peritransplant period interferes with tolerization and graft survival involves CD8+ T cells. LCMV-induced CD8+ T-cell-mediated allograft rejection could be the consequence of T-cell cross-reactivity between self-presented LCMV peptides and alloantigens. Alternatively, rejection could be the consequence of bystander stimulation of non-cross-reactive but anergized or “ignorant” allospecific T cells whose TcR can be stimulated by the allograft (10). However, if acute graft rejection were a consequence of bystander stimulation, it is surprising that poly I:C, VV, and MCMV were so inefficient in this process. These results, coupled with the results obtained with anti-CD8 MAb, suggest that the abrogation of graft survival by LCMV infection immediately after transplantation is more likely to be the result of allospecific CTL generation than the result of nonspecific inflammation. Surprisingly, however, only very low levels of LCMV-induced allospecific CTL could be detected in the spleen during LCMV-induced graft rejection. It is possible that some of the CTLs migrated into the graft and were therefore underrepresented in the spleen. Consistent with this concept, rejecting grafts had significant lymphocyte infiltrates, and rejection was impaired by the depletion of CD8+ T cells.
The resistance of grafts to LCMV-induced allospecific CTLs at later time points could involve several factors. We have shown in both the present study and previous reports (15) that after 7 weeks of graft survival, a more stable form of tolerance or suppression is present. This appears not to be due to complete absence of graft-rejecting T cells because alloreactive cells can be detected in uninfected animals bearing grafts for >100 days (15). Although the present data show that a long-term tolerant state cannot be overcome by infection, it is known that it can be broken by depletion of CD4+ cells (15). It is plausible to suggest that the difference in the ability of low levels of alloreactive CD8+ CTLs to abrogate allograft tolerance over time is due to the development of a population of CD4+ suppressor T cells.
LCMV, VV, PV, and MCMV all induce anti-H2d allospecific CTL in C57BL/6 mice, but LCMV is a more potent stimulator of CTL than are the other viruses (19, 22, 28). LCMV appears to be more efficient than VV or MCMV in inducing graft rejection, but to date only LCMV has been investigated in detail. It is noteworthy that in one experiment, PV, which is distantly related to LCMV by virtue of being an arenavirus, induced graft rejection with a time course similar to that of LCMV.
Clonal exhaustion of high-affinity LCMV-specific CTLs occurs under conditions of high antigen load and persistent infection (18). Treatment with anti-CD8 MAb led to LCMV persistence but still allowed for graft rejection. These findings indicate that high-affinity LCMV-specific CD8+ CTLs are not required for rejection, but evidence has suggested that some LCMV-induced allospecific CTLs have higher affinity to allogeneic targets than to LCMV-infected syngeneic targets with which they cross-react (19).
It is noteworthy that, unlike LCMV and PV, VV had no detectable effect on graft survival. In an attempt to determine the mechanism underlying this difference, we investigated the ability of immunogenic LCMV-encoded proteins in VV vectors to enhance the ability of VV to stimulate graft rejections. Our initial investigation has shown that a VV-NP recombinant substantially reduced graft size after transplantation but did not ultimately lead to complete graft rejection. Additional analysis of VV recombinants is in progress.
For the most part, tolerized and transplanted animals survived infection, and as shown with LCMV, mounted a sterilizing CTL response. Only high-dose VV infection was associated with recipient mortality. It should be noted that the fatal outcome required both the tolerization procedure and surgical trauma; tolerized mice that had not been operated on survived the infection.
In conclusion, we demonstrate that DST and anti-CD154 MAb induce a state of allotolerance that can be broken shortly after transplantation by certain viral infections. In addition, infection with at least one virus during treatment may endanger host survival. We recognize that data from this limited survey of infection in murine graft recipients cannot be extrapolated with any assurance to other species. However, clinical trials of tolerance-based transplantation are being planned, and the data do suggest that it may be prudent to initially isolate patients during tolerance induction in order to facilitate the procedure and to reduce the risk of infection.
ACKNOWLEDGMENTS
We thank Linda Paquin, Linda Leehy, and Carey L. O'Donnell for technical assistance.
This work was supported in part by grants AR35506 and 3PO3-DK32520 and by program projects 1PO1-DK53006 and 1PO1-AI42669 from the National Institutes of Health.
REFERENCES
- 1.Alexander-Miller M A, Burke K, Koszinowski U H, Hansen T H, Connolly J M. Alloreactive cytotoxic T lymphocytes generated in the presence of viral-derived peptides show exquisite peptide and MHC specificity. J Immunol. 1993;151:1–10. [PubMed] [Google Scholar]
- 2.Braciale T J, Andrew M E, Braciale V L. Simultaneous expression of H-2-restricted and alloreactive recognition by a cloned line of influenza virus-specific cytotoxic T lymphocytes. J Exp Med. 1981;153:1371–1376. doi: 10.1084/jem.153.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briggs J D, Timbury M C, Paton A M, Bell P R. Viral infection and renal transplant rejection. Br Med J. 1972;4:520–522. doi: 10.1136/bmj.4.5839.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burrows S R, Khanna R, Burrows J M, Moss D J. An alloresponse in humans is dominated by cytotoxic T lymphocytes (CTL) cross-reactive with a single Epstein-Barr virus CTL epitope: implications for graft-versus-host disease. J Exp Med. 1994;179:1155–1161. doi: 10.1084/jem.179.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caldas C, Ambinder R. Epstein-Barr virus and bone marrow transplantation. Curr Opin Oncol. 1995;7:102–106. doi: 10.1097/00001622-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Clark L B, Foy T M, Noelle R J. CD40 and its ligand. Adv Immunol. 1996;63:43–78. doi: 10.1016/s0065-2776(08)60854-8. [DOI] [PubMed] [Google Scholar]
- 7.Daniel C, Horvath S, Allen P M. A basis for alloreactivity: MHC helical residues broaden peptide recognition by the TCR. Immunity. 1998;8:543–552. doi: 10.1016/s1074-7613(00)80559-2. [DOI] [PubMed] [Google Scholar]
- 8.Doukas J, Cutler A H, Mordes J P. Polyinosinic:polycytidylic acid is a potent activator of endothelial cells. Am J Pathol. 1994;145:137–147. [PMC free article] [PubMed] [Google Scholar]
- 9.Ehl S, Hombach J, Aichele P, Hengartner H, Zinkernagel R M. Bystander activation of cytotoxic T cells: studies on the mechanism and evaluation of in vivo significance in a transgenic mouse model. J Exp Med. 1997;185:1241–1251. doi: 10.1084/jem.185.7.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehl S, Hombach J, Aichele P, Rulicke T, Odermatt B, Hengartner H, Zinkernagel R, Pircher H. Viral and bacterial infections interfere with peripheral tolerance induction and activate CD8+ T cells to cause immunopathology. J Exp Med. 1998;187:763–774. doi: 10.1084/jem.187.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foy T M, Aruffo A, Bajorath J, Buhlmann J E, Noelle R J. Immune regulation by CD40 and its ligand gp39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 11a.Institute of Laboratory Animal Resources. Guide for the care and use of laboratory animals. Washington, D.C.: National Research Council, National Academy of Sciences; 1996. [Google Scholar]
- 12.Kadakia M P, Rybka W B, Stewart J A, Patton J L, Stamey F R, Elsawy M, Pellett P E, Armstrong J A. Human herpesvirus 6: infection and disease following autologous and allogeneic bone marrow transplantation. Blood. 1996;87:5341–5354. [PubMed] [Google Scholar]
- 13.Kaplan E L, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 14.Larsen C P, Pearson T C. The CD40 pathway in allograft rejection, acceptance, and tolerance. Curr Opin Immunol. 1997;9:641–647. doi: 10.1016/s0952-7915(97)80043-x. [DOI] [PubMed] [Google Scholar]
- 15.Markees T G, Phillips N E, Gordon E J, Noelle R J, Shultz L D, Mordes J P, Greiner D L, Rossini A A. Long-term survival of skin allografts induced by donor splenocytes and anti-CD154 antibody in thymectomized mice requires CD4+ T cells, interferon-gamma, and CTLA4. J Clin Investig. 1998;101:2446–2455. doi: 10.1172/JCI2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markees T G, Phillips N E, Noelle R J, Shultz L D, Mordes J P, Greiner D L, Rossini A A. Prolonged survival of mouse skin allografts in recipients treated with donor splenocytes and antibody to CD40 ligand. Transplantation. 1997;64:329–335. doi: 10.1097/00007890-199707270-00026. [DOI] [PubMed] [Google Scholar]
- 17.May A G, Betts R F, Freeman R B, Andrus C H. An analysis of cytomegalovirus infection and HLA antigen matching on the outcome of renal transplantation. Ann Surg. 1978;187:110–117. doi: 10.1097/00000658-197802000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moskophidis D, Lechner F, Pircher H, Zinkernagel R M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 19.Nahill S R, Welsh R M. High frequency of cross-reactive cytotoxic T lymphocytes elicited during the virus-induced polyclonal cytotoxic T lymphocyte response. J Exp Med. 1993;177:317–327. doi: 10.1084/jem.177.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossini A A, Greiner D L, Mordes J P. Induction of immunological tolerance for transplantation. Physiol Rev. 1999;79:99–141. doi: 10.1152/physrev.1999.79.1.99. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz R H. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 22.Sheil J M, Bevan M J, Lefrancois L. Characterization of dual-reactive H-2Kb-restricted anti-vesicular stomatitus virus and alloreactive cytotoxic T cells. J Immunol. 1987;138:3654–3660. [PubMed] [Google Scholar]
- 23.Simmons R L, Lopez C, Balfour H J, Kalis J, Rattazzi L C, Najarian J S. Cytomegalovirus: clinical virological correlations in renal transplant recipients. Ann Surg. 1974;180:623–634. doi: 10.1097/00000658-197410000-00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Speir J A, Garcia K C, Brunmark A, Degano M, Peterson P A, Teyton L, Wilson I A. Structural basis of 2C TCR allorecognition of H-2Ld peptide complexes. Immunity. 1998;8:553–562. doi: 10.1016/s1074-7613(00)80560-9. [DOI] [PubMed] [Google Scholar]
- 25.Strang G, Rickinson A B. Multiple HLA class I-dependent cytotoxicities constitute the “non-HLA-restricted” response in infectious mononucleosis. Eur J Immunol. 1987;17:1007–1013. doi: 10.1002/eji.1830170717. [DOI] [PubMed] [Google Scholar]
- 26.Tomkinson B E, Maziarz R, Sullivan J L. Characterization of the T cell-mediated cellular cytotoxicity during acute infectious mononucleosis. J Immunol. 1989;143:660–670. [PubMed] [Google Scholar]
- 27.Whitton J L, Southorn P A, Oldstone M B. Analyses of the cytotoxic T lymphocyte responses to glycoprotein and nucleoprotein components of lymphocytic choriomeningitis virus. Virology. 1999;162:321–327. doi: 10.1016/0042-6822(88)90471-0. [DOI] [PubMed] [Google Scholar]
- 28.Yang H Y, Dundon P L, Nahill S R, Welsh R M. Virus-induced polyclonal cytotoxic T lymphocyte stimulation. J Immunol. 1989;142:1710–1718. [PubMed] [Google Scholar]