Key Points
Question
Is there a difference in mortality between continuous and intermittent infusions of β-lactam antibiotics in critically ill patients with sepsis?
Findings
In this randomized clinical trial that included 7031 adult patients with sepsis, there was not a statistically significant difference in the proportion of patients who died within 90 days who received continuous (24.9%) compared with intermittent (26.8%) β-lactam antibiotic infusions (odds ratio, 0.91).
Meaning
In critically ill patients with sepsis, continuous vs intermittent β-lactam antibiotic infusions did not significantly reduce 90-day mortality in the primary analysis.
Abstract
Importance
Whether β-lactam antibiotics administered by continuous compared with intermittent infusion reduces the risk of death in patients with sepsis is uncertain.
Objective
To evaluate whether continuous vs intermittent infusion of a β-lactam antibiotic (piperacillin-tazobactam or meropenem) results in decreased all-cause mortality at 90 days in critically ill patients with sepsis.
Design, Setting, and Participants
An international, open-label, randomized clinical trial conducted in 104 intensive care units (ICUs) in Australia, Belgium, France, Malaysia, New Zealand, Sweden, and the United Kingdom. Recruitment occurred from March 26, 2018, to January 11, 2023, with follow-up completed on April 12, 2023. Participants were critically ill adults (≥18 years) treated with piperacillin-tazobactam or meropenem for sepsis.
Intervention
Eligible patients were randomized to receive an equivalent 24-hour dose of a β-lactam antibiotic by either continuous (n = 3498) or intermittent (n = 3533) infusion for a clinician-determined duration of treatment or until ICU discharge, whichever occurred first.
Main Outcomes and Measures
The primary outcome was all-cause mortality within 90 days after randomization. Secondary outcomes were clinical cure up to 14 days after randomization; new acquisition, colonization, or infection with a multiresistant organism or Clostridioides difficile infection up to 14 days after randomization; ICU mortality; and in-hospital mortality.
Results
Among 7202 randomized participants, 7031 (mean [SD] age, 59 [16] years; 2423 women [35%]) met consent requirements for inclusion in the primary analysis (97.6%). Within 90 days, 864 of 3474 patients (24.9%) assigned to receive continuous infusion had died compared with 939 of 3507 (26.8%) assigned intermittent infusion (absolute difference, −1.9% [95% CI, −4.9% to 1.1%]; odds ratio, 0.91 [95% CI, 0.81 to 1.01]; P = .08). Clinical cure was higher in the continuous vs intermittent infusion group (1930/3467 [55.7%] and 1744/3491 [50.0%], respectively; absolute difference, 5.7% [95% CI, 2.4% to 9.1%]). Other secondary outcomes were not statistically different.
Conclusions and Relevance
The observed difference in 90-day mortality between continuous vs intermittent infusions of β-lactam antibiotics did not meet statistical significance in the primary analysis. However, the confidence interval around the effect estimate includes the possibility of both no important effect and a clinically important benefit in the use of continuous infusions in this group of patients.
Trial Registration
ClinicalTrials.gov Identifier: NCT03213990
This clinical trial compares the efficacy of continuous vs intermittent infusion of a β-lactam antibiotic (piperacillin-tazobactam or meropenem) in decreasing all-cause mortality at 90 days in critically ill patients with sepsis.
Introduction
Defined as life-threatening organ dysfunction due to a dysregulated response to infection,1 sepsis is a major cause of mortality worldwide. Early antibiotic therapy directed at the likely infective microorganism is a cornerstone of treatment,2,3,4 of which β-lactam antibiotics are an important class of antibiotics.4 For patients treated in the intensive care unit (ICU), meropenem and piperacillin-tazobactam are commonly used drugs in this antibiotic class.5,6 β-Lactam antibiotics have been primarily administered as multiple, short (eg, 30-minute) intermittent infusions. Due to time-dependent kill characteristics, there is a biological rationale that continuous infusion may be more effective than intermittent administration.7,8,9
Recent clinical trials have reported that the administration of β-lactam antibiotics by continuous infusion resulted in concentrations above the minimum inhibitory concentration of typical pathogens.10,11 However, clinical trials and meta-analyses have not provided conclusive evidence of microbiological cure and improved patient-centered outcomes.12,13,14,15,16 To address uncertainty about the optimal method of β-lactam antibiotic administration in the ICU setting,17,18 a randomized clinical trial was conducted to determine whether continuous infusion of piperacillin-tazobactam or meropenem resulted in decreased all-cause mortality at 90 days in critically ill patients with sepsis compared with intermittent infusion.
Methods
Trial Design and Oversight
The Beta-Lactam Infusion Group (BLING) III trial was an international, open-label, phase 3, randomized clinical trial comparing continuous vs intermittent infusions of β-lactam antibiotics on all-cause 90-day mortality in critically ill patients with sepsis. The study protocol (Supplement 1) and statistical analysis plan (Supplement 2) have been published previously.19,20 The trial was approved by the relevant human research ethics committee or equivalent in each region; institutional approval was obtained as per site requirements. In some jurisdictions, approval was obtained for enrollment prior to consent in certain circumstances (eMethods in Supplement 3); in all countries, written informed consent was obtained from patients or their legal surrogate with documented verbal consent obtained in some regions during the SARS-CoV-2 (COVID-19) pandemic as per ethics committee approval (eMethods in Supplement 3).
The trial management committee was responsible for the design of the study. The George Institute for Global Health generated the allocation sequence and conducted the statistical analysis. An independent data and safety monitoring committee conducted a prespecified midpoint safety analysis (Supplement 2). Study reporting adhered to the CONSORT 2010 Statement for randomized clinical trials.
The trial was conducted in 104 adult ICUs in Australia, Belgium, France, Malaysia, New Zealand, Sweden, and the United Kingdom. Participants were enrolled between March 26, 2018, and January 11, 2023; 90-day follow-up was completed on April 12, 2023.
Participants
Eligible participants (Table 1)21,22 were adult (≥18 years) ICU patients with (1) a documented site or strong suspicion of infection, (2) treatment with piperacillin-tazobactam or meropenem commenced within the previous 24 hours, and (3) 1 or more organ dysfunction criteria met in the previous 24 hours as defined in the study inclusion criteria (eMethods in Supplement 3). Eligible participants were expected to remain in the ICU for at least the next calendar day and in whom the administration of either piperacillin-tazobactam or meropenem by intermittent or continuous infusion was considered equally appropriate for the patient by the attending clinicians. Full details of inclusion and exclusion criteria are provided in the eMethods in Supplement 3.
Table 1. Baseline Characteristics of Participants in the BLING III Trial.
| Characteristic | Continuous infusion (n = 3498)a | Intermittent infusion (n = 3533)a |
|---|---|---|
| Age, mean (SD), y | 59.3 (16.4) | 59.6 (16.1) |
| Median (IQR) | 62 (49-72) | 63 (50-72) |
| Sex, No. (%) | ||
| Female | 1190 (34.0) | 1233 (34.9) |
| Male | 2308 (66.0) | 2300 (65.1) |
| Weight, mean (SD) [No.], kg | 82.7 (22.6) [3493] | 82.7 (22.9) [3530] |
| Median (IQR) | 80 (68-94) | 80 (67-94) |
| Height, mean (SD) [No.], cm | 170.5 (9.9) [3468] | 170.3 (10.2) [3509] |
| Median (IQR) | 170 (164-178) | 170 (164-178) |
| Source of ICU admission, No./total (%) | ||
| Accident and emergency department | 1203/3495 (34.4) | 1178/3532 (33.4) |
| Hospital floor (ie, wards) | 982/3495 (28.1) | 1047/3532 (29.6) |
| Operating theater following emergency surgery | 732/3495 (20.9) | 720/3532 (20.4) |
| Operating theater following elective surgery | 254/3495 (7.3) | 265/3532 (7.5) |
| Transfer from another hospital (except from another ICU) | 179/3495 (5.1) | 173/3532 (4.9) |
| Transfer from another ICUb | 145/3495 (4.1) | 149/3532 (4.2) |
| Time from ICU admission to randomization, mean (SD) [No.], h | 80.2 (135.2) [3496] | 78.9 (148.3) [3532] |
| Median (IQR) | 25.2 (12.6-102.9) | 24.8 (12.1-104.1) |
| APACHE II score, mean (SD) [No.]c | 19.6 (7.6) [3495] | 19.5 (7.4) [3529] |
| Median (IQR) | 19 (14-25) | 19 (14-24) |
| Lowest Pao2/Fio2 ratio in the 24 h prior to randomization, mean (SD) [No.] | 190.5 (102.5) [3322] | 192.4 (103.6) [3371] |
| Median (IQR) | 169 (111-252) | 172 (113-252) |
| Highest creatinine, mean (SD) [No.], μmol/L | 122.6 (95.2) [3481] | 123.6 (106.5) [3519] |
| Median (IQR) | 92 (64-150) | 91 (63-148) |
| Highest bilirubin, mean (SD) [No.], μmol/L | 25.9 (45.8) [3308] | 24.7 (43.9) [3346] |
| Median (IQR) | 14 (8-24) | 13 (8-24) |
| Lowest platelet count, mean (SD) [No.], ×109/L | 232.3 (141.0) [3460] | 228.6 (137.6) [3492] |
| Median (IQR) | 208 (138-298) | 204 (136-295) |
| Lowest MAP in the 24 h prior to randomization, mean (SD) [No.], mm Hg | 63.8 (12.4) [3485] | 64.0 (12.1) [3512] |
| Median (IQR) | 63 (57-70) | 63 (57-70) |
| Worst Glasgow Coma Scale score (nonsedated), mean (SD) [No.]d | 12.2 (4.1) [2776] | 12.3 (4.0) [2814] |
| Median (IQR) | 14 (10-15) | 14 (11-15) |
| Received inotropes/vasopressors in the 24 h prior to randomization, No./total (%) | 2481/3496 (71.0) | 2482/3532 (70.3) |
| Received antibiotics in the 24 h prior to randomization, No./total (%)e | 2340/3496 (66.9) | 2460/3532 (69.6) |
| Primary site of infection, No./total (%) | ||
| Pulmonary | 2062/3494 (59.0) | 2119/3532 (60.0) |
| Intra-abdominal | 469/3494 (13.4) | 447/3532 (12.7) |
| Blood | 268/3494 (7.7) | 294/3532 (8.3) |
| Urinary | 214/3494 (6.1) | 166/3532 (4.7) |
| Skin | 184/3494 (5.3) | 186/3532 (5.3) |
| Gut | 98/3494 (2.8) | 120/3532 (3.4) |
| Central nervous system | 65/3494 (1.9) | 74/3532 (2.1) |
| Intravenous catheter | 18/3494 (0.5) | 20/3532 (0.6) |
| Endocarditis | 13/3494 (0.4) | 4/3532 (0.1) |
| Otherf | 103/3494 (2.9) | 102/3532 (2.9) |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; BLING, Beta-Lactam Infusion Group; Fio2, fraction of inspired oxygen; ICU, intensive care unit; MAP, mean arterial pressure; Pao2, arterial partial pressure of oxygen.
SI conversion factors: To convert bilirubin to mg/dL, divide by 17.104; creatinine to mg/dL, divide by 88.4.
Descriptive statistics are reported for all participants with available data by group. The group denominator for each characteristic is reported.
From the same or a different hospital.
The APACHE II score is a severity of illness scoring system that ranges from 0 to 71; higher scores correspond to more severe disease and a higher risk of death.21 A median APACHE II score of 19 equates to a 90-day mortality of approximately 22% in critically ill patients.22
The Glasgow Coma Scale score ranges from 3 to 15, with lower scores indicating a greater degree of neurologic dysfunction. A score of 14 most commonly indicates a patient who opens their eyes spontaneously and follows verbal commands but is confused.
Other than piperacillin-tazobactam or meropenem.
Other includes, for continuous vs intermittent infusion, respectively, musculoskeletal, 18 (0.5%) vs 20 (0.6%); oronasopharyngeal, 20 (0.6%) vs 15 (0.4%); intrathoracic, 15 (0.4%) vs 13 (0.4%); soft tissue, 13 (0.4%) vs 13 (0.4%); gynecological, 3 (0.1%) vs 2 (0.1%); ear/eye infection, 2 (0.1%) vs 2 (0.1%), infected graft/hardware, 2 (0.1%) vs 2 (0.1%); and unknown, 30 (0.9%) vs 35 (1.0%).
Randomization
Participants were randomized in a 1:1 ratio to receive the prescribed antibiotic by either continuous (intervention group) or intermittent (control group) infusion.19 Randomization was generated using a minimization algorithm via a password-protected, encrypted, web-based interface with stratification by study site.
Interventions
In both groups, the total 24-hour β-lactam antibiotic dose was determined by the attending clinicians. A defined daily dose of 14 g for piperacillin-tazobactam and 3 g for meropenem was used as a reference measure.23 All participants received at least 1 β-lactam antibiotic infusion dose prior to open-label randomized treatment. Randomized drug administration by continuous infusion (over 24 hours) or intermittent infusion (over 30 minutes) was continued for the duration of the treatment course or until ICU discharge, whichever occurred first. A switch between piperacillin-tazobactam and meropenem (or vice versa) was permitted in the same treatment group after receipt of a loading dose by intermittent infusion for participants in the continuous infusion group. Additional details on study drug administration appear in the eMethods in Supplement 3.
Outcome Measures
The primary outcome was all-cause mortality at 90 days from the date of randomization. Secondary outcomes were clinical cure, defined as the completion of the β-lactam antibiotic treatment course by day 14 without recommencement of antibiotic therapy within 48 hours of cessation for the same infectious episode (eMethods in Supplement 3); new acquisition, colonization, or infection with a multiresistant organism or Clostridioides difficile infection up to 14 days after randomization (eMethods in Supplement 3); all-cause ICU mortality; and all-cause hospital mortality. Tertiary outcomes were the number of days free of ICU, hospital, mechanical ventilation, and kidney replacement therapy up to 90 days after randomization.19 Study days are defined in the eMethods in Supplement 3. Drug reactions thought to have a causal relationship (ie, possible, probable, or definitely related) to the study-assigned administration method were reported as adverse events. Protocol deviations related to administration of the β-lactam antibiotic included predefined events (eMethods in Supplement 3).
Trial Population
A sample size of 7000 patients provided 90% power to detect an absolute difference of 3.5% in all-cause mortality at 90 days from an estimated baseline mortality of 27.5%, at an α of .05.20 This calculation allowed for a rate of withdrawal and loss to follow-up of up to 5% of participants. Recruitment proceeded for 6 months at 13 sites after 7000 participants to facilitate recruitment in a prespecified pharmacokinetic-pharmacodynamic substudy.
Statistical Analysis
The effectiveness of the intervention was evaluated by a modified intention-to-treat analysis of all participants randomized to the trial where consent or approval to use their data was obtained, regardless of protocol adherence. This primary analysis consisted of logistic regression with treatment allocation as a fixed effect and trial site as a random effect. Results are presented as odds ratios (ORs) and 95% CIs and converted to absolute differences (ADs) in proportions.
The primary outcome was also examined in a prespecified adjusted analysis with the following covariates added to the main logistic regression model: sex, Acute Physiology and Chronic Health Evaluation Score (APACHE) II score (a severity of illness score ranging from 0 to 71, with higher scores indicating an increased risk of death) at randomization,21 source of admission (admitted following emergency or elective surgery vs other), and type of β-lactam antibiotic used before randomization (piperacillin-tazobactam or meropenem). Five prespecified subgroup analyses were also carried out according to the following prerandomization variables: presence vs absence of presumed pulmonary infection at baseline, type of β-lactam antibiotic first administered (piperacillin-tazobactam or meropenem), age (<65 years vs ≥65 years), sex (male vs female), and low vs high severity of illness (defined by an APACHE II score at randomization <25 or ≥25).15,19,24
Secondary binary outcomes were analyzed using a similar logistic regression to the primary analysis. Tertiary duration outcomes were analyzed using linear regression. Time to discharge or cessation were summarized using a cumulative incidence function treating death as a competing risk, censored at 90 days. Intervention effects were estimated as hazard ratios and 95% CIs obtained from a cause-specific Cox model with a fixed effect of treatment and a random site effect. Proportions of patients with adverse events were compared using a Fisher exact test among patients who received 1 or more doses of the β-lactam antibiotic in the assigned treatment group.
There was no imputation of missing data across all analyses. All tests were 2-sided with a nominal significance level at 5%. Analyses of the primary outcome are unadjusted for multiplicity; however, the family-wise error rate was controlled across secondary outcomes (1 family) and tertiary outcomes (1 family) using a Holm-Bonferroni correction.25 Analyses were performed using SAS Enterprise Guide 8.3, SAS version 9.4 (SAS Institute Inc).
Results
Trial Population
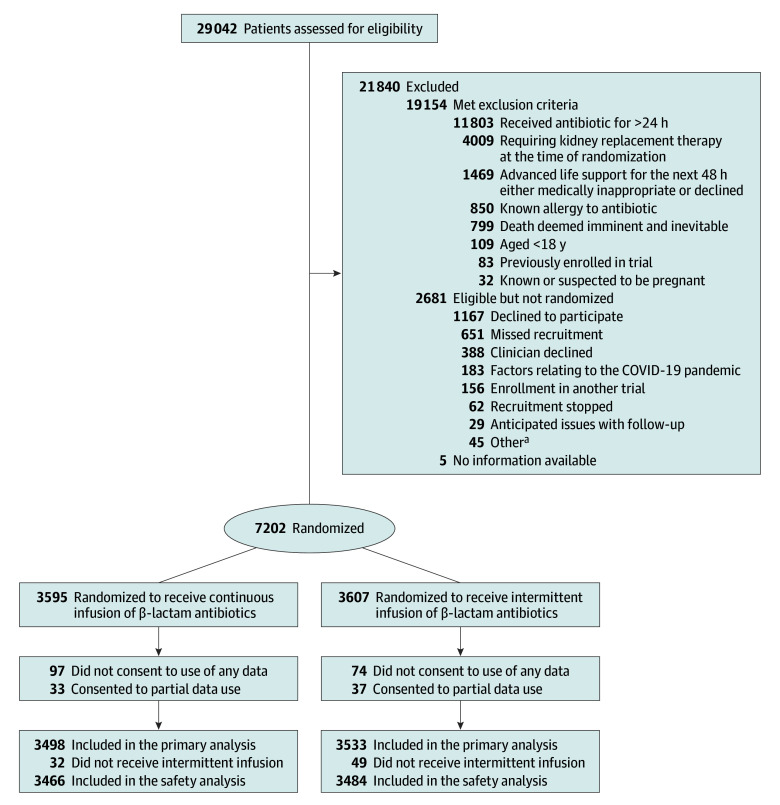
A total of 7202 participants were randomized, of whom 7031 with consent or approval to use data were included in the primary analysis (Figure 1). Of these, 6950 received at least 1 dose of the β-lactam antibiotic by allocation group and were included in the safety analysis. Enrollment by country and site is shown in eTable 1 in Supplement 3. The intervention and control groups had similar baseline characteristics (Table 1 and eTable 2 in Supplement 3).
Figure 1. Screening, Randomization, and Analysis of Study Participants in the BLING III Trial.
aOther reasons included not a public patient (n = 35), pharmacy issues (n = 3), infection control risk (n = 2), website issues (n = 1), inclusion criteria not confirmed (n = 1), and unknown (n = 3).
Of 7026 participants, the most common primary sites of infection were pulmonary in 4181 participants (59.5%), intra-abdominal in 916 (13.0%), blood in 562 (8.0%), and urinary in 380 (5.4%) (Table 1). Infective organisms for the primary site of infection are listed in eTable 3 in Supplement 3. Secondary and tertiary sites of infection and associated infective organisms are shown in eTables 4 through 6 in Supplement 3. The median duration of randomized treatment was 5.8 days (IQR, 3.1-10.2) and 5.7 days (IQR, 3.1-10.3) in the continuous and intermittent infusion groups, respectively (eTable 7 in Supplement 3). The median defined daily dose was 1.0 in both groups on the first whole day (day 2) post randomization with an IQR of 0.8 to 1.2 defined daily doses (eTable 7 in Supplement 3). The daily dose of study drugs was similar in both groups over the postrandomization treatment period up to day 16 (eFigures 1 and 2 in Supplement 3). The reasons for cessation of a β-lactam antibiotic are listed in eTable 8 in Supplement 3. Prior to randomization, 2658 of 3415 patients (77.8%) in the continuous infusion group and 2809 of 3445 (81.5%) in the intermittent group were prescribed the study drug by intermittent infusion (eTable 7 in Supplement 3). Other antibiotics administered in the 24 hours before randomization up to day 16 are listed in eTable 9 in Supplement 3; 16.2% (568/3498) and 14.0% (496/3533) in the continuous and intermittent infusion groups, respectively, did not receive any other antibiotics during this period.
Primary Outcome
At 90 days after randomization, 864 of 3474 patients (24.9%) randomized to the continuous infusion group and 939 of 3507 (26.8%) randomized to the intermittent infusion group had died (AD, −1.9% [95% CI, −4.9% to 1.1%]; OR, 0.91 [95% CI, 0.81 to 1.01]; P = .08); day 90 status was missing for 0.7% of participants (50/7031) (Table 2). After adjusting for prespecified covariates, the AD was −2.2% (95% CI, −5.5% to 1.1%; OR, 0.89 [95% CI, 0.79 to 0.99]; P = .04). The cumulative incidence function of time to death is shown in eFigure 3 in Supplement 3. There was no significant heterogeneity in the effect of the intervention assignment on mortality at 90 days in any of the 5 predefined subgroup pairs (Figure 2). The place and cause of death are listed in eTable 10 in Supplement 3.
Table 2. Reporting of Primary, Secondary, and Tertiary Outcomes.
| Outcome | Continuous infusion (n = 3498)a | Intermittent infusion (n = 3533)a | Absolute difference, % (95% CI) | Odds ratio or mean difference (95% CI) | P valueb |
|---|---|---|---|---|---|
| Primary outcome | |||||
| All-cause mortality at day 90, No./total (%) | 864/3474 (24.9) | 939/3507 (26.8) | −1.9 (−4.9 to 1.1) | 0.91 (0.81 to 1.01) | .08 |
| Adjusted analysis | −2.2 (−5.5 to 1.1) | 0.89 (0.79 to 0.99) | .04 | ||
| Secondary outcomes | |||||
| Clinical cure at day 14, No./total (%) | 1930/3467 (55.7) | 1744/3491 (50.0) | 5.7 (2.4 to 9.1) | 1.26 (1.15 to 1.38) | <.001 |
| New acquisition, colonization, or infection with an MRO or C difficile, No./total (%)c | 253/3498 (7.2) | 266/3533 (7.5) | −0.3 (−1.9 to 1.4) | 0.96 (0.80 to 1.15) | .65 |
| All-cause ICU mortality, No./total (%) | 595/3474 (17.1) | 645/3507 (18.4) | −1.3 (−4.0 to 1.4) | 0.92 (0.81 to 1.04) | .35 |
| All-cause hospital mortality, No./total (%) | 808/3474 (23.3) | 878/3507 (25.0) | −1.8 (−4.8 to 1.2) | 0.91 (0.81 to 1.02) | .27 |
| Tertiary outcomes | |||||
| Days alive and free of ICU up to day 90, mean (SD) [No.] | 59.4 (33.9) [3481] | 57.8 (34.2) [3516] | NA | 1.5 (−0.1 to 3.0) | .18 |
| Median (IQR) | 78 (31 to 84) | 76 (22 to 84) | |||
| Days alive and free of hospital up to day 90, mean (SD) [No.] | 42.4 (32.2) [3476] | 40.6 (32.5) [3515] | NA | 1.8 (0.3 to 3.3) | .08 |
| Median (IQR) | 52 (0 to 73) | 48 (0 to 72) | |||
| Days alive and free of mechanical ventilation up to day 90, mean (SD) [No.] | 64.2 (34.5) [3486] | 62.9 (34.8) [3520] | NA | 1.2 (−0.4 to 2.8) | .28 |
| Median (IQR) | 84 (41 to 89) | 83 (32 to 89) | |||
| Days alive and free of KRT up to day 90, mean (SD) [No.] | 71.2 (32.8) [3489] | 70.2 (33.1) [3521] | NA | 0.9 (−0.6 to 2.4) | .25 |
| Median (IQR) | 90 (70 to 90) | 90 (54 to 90) | |||
Abbreviations: ICU, intensive care unit; KRT, kidney replacement therapy; MRO, multiresistant organism; NA, not applicable.
Descriptive statistics and odds ratio or mean difference are reported for all participants with available data by group. The group denominator for each outcome is reported.
P values for secondary and tertiary outcomes corrected with a Holm-Bonferroni adjustment.
The number and percentage of new acquisition, colonization, or infection with an MRO, excluding Clostridioides difficile, was 6.1% (213/3498) in the continuous infusion group and 6.6% (232/3533) in the intermittent infusion group.
Figure 2. Subgroup Analysis of Mortality at Day 90.
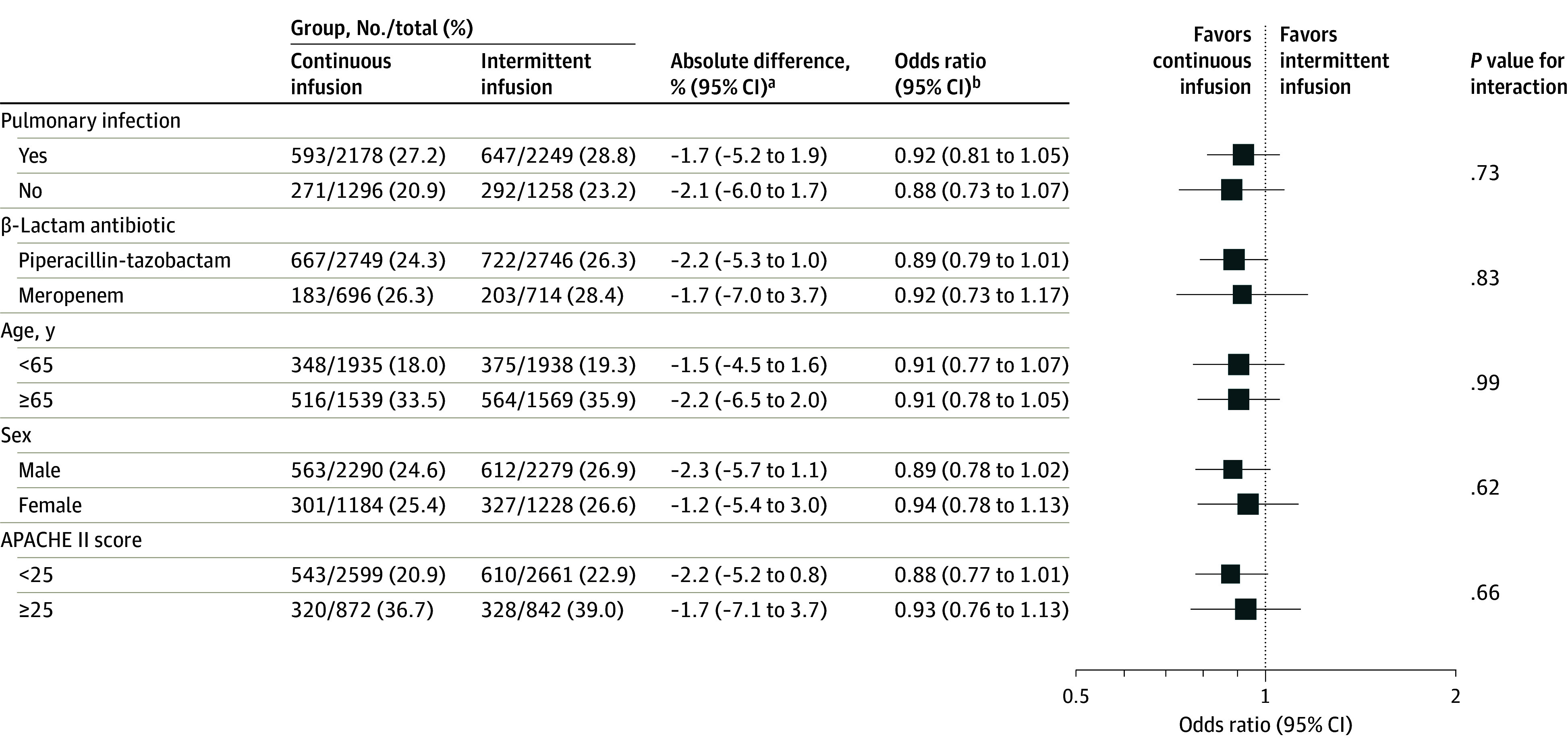
APACHE indicates Acute Physiology and Chronic Health Evaluation Score.
aAbsolute differences (95% CIs) were derived from the logistic regression by applying an inverse link transformation.
bOdds ratios (95% CIs) were obtained from logistic regression with treatment group and the subgroup variable and its interaction with the treatment group as a fixed effect and site as a random effect.
Secondary Outcomes
There was a statistically significant difference in the rates of clinical cure at 14 days post randomization in favor of the continuous infusion group (AD, 5.7% [95% CI, 2.4% to 9.1%]; OR, 1.26 [95% CI, 1.15 to 1.38]). There were no statistically significant differences between groups in the rates of new acquisition, colonization, or infection with a multiresistant organism or C difficile infection (AD, −0.3% [95% CI, −1.9% to 1.4%]; OR, 0.96 [95% CI, 0.80 to 1.15]); ICU mortality (AD, −1.3% [95% CI, −4.0% to 1.4%]); and hospital mortality (AD, −1.8% [95% CI, −4.8% to 1.2%]).
Tertiary Outcomes
There were no statistical differences in days alive and free of ICU stay, hospital stay, mechanical ventilation, and kidney replacement therapy (Table 2). Daily participant disposition is shown in Figure 3 and cumulative incidence functions of time to alive discharge from the index ICU and hospital admission are shown in eFigures 4 and 5 in Supplement 3.
Figure 3. Daily Participant Disposition.
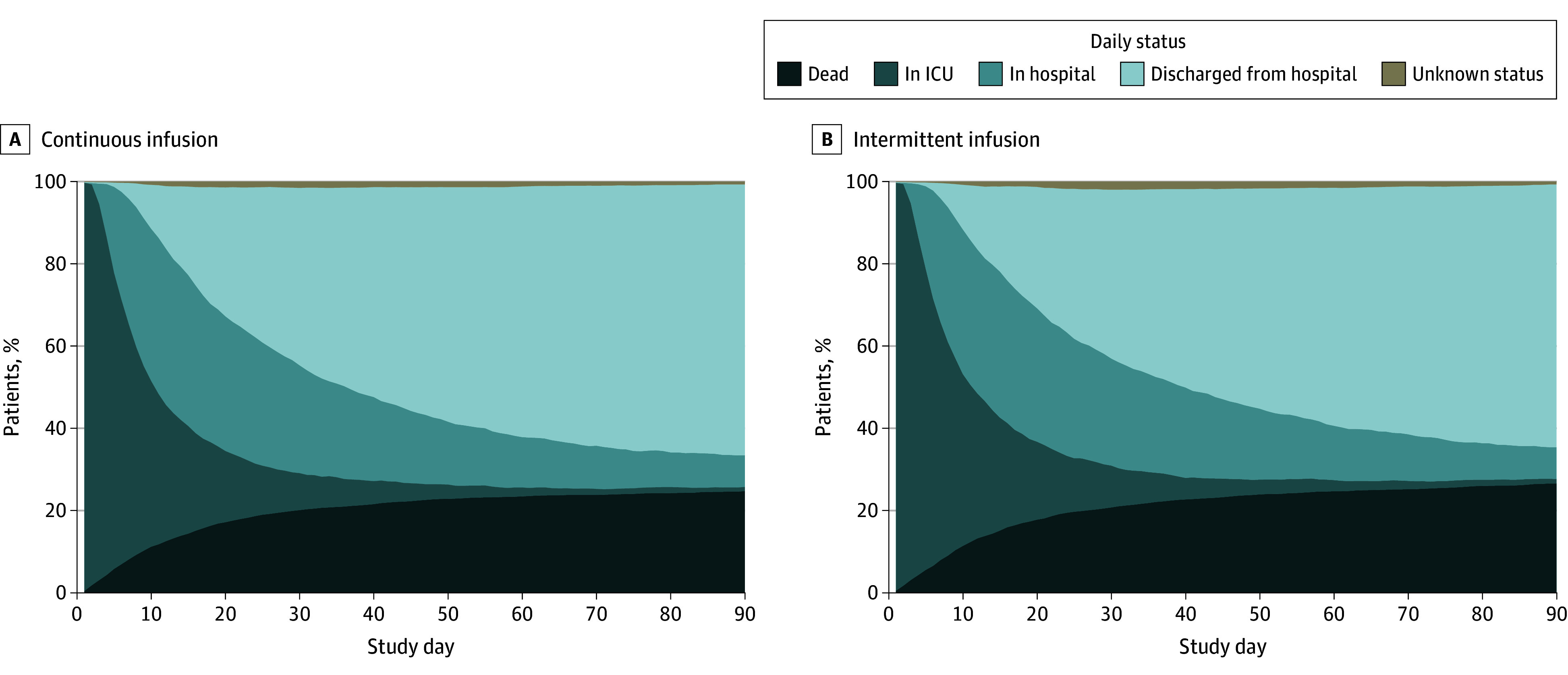
ICU indicates intensive care unit.
Adverse Events
There were 10 adverse events in the continuous infusion group (0.3%) and 6 adverse events in the intermittent infusion group (0.2%), including 1 serious adverse event in the continuous infusion group (eTable 11 in Supplement 3). The serious adverse event was severe encephalopathy resulting in aspiration pneumonia, cardiac arrest, and death in the setting of septic shock. The event was assessed by site clinicians as possibly related to meropenem treatment. Administration by continuous infusion was hypothesized as potentially contributing to a higher minimum meropenem blood concentration leading to a higher cerebrospinal fluid concentration.
Protocol Deviations
There were no statistical differences in the proportions of protocol deviations in both groups (eTable 12 in Supplement 3).
Discussion
In this randomized clinical trial, the use of continuous compared with intermittent infusions of β-lactam antibiotics in critically ill patients with sepsis did not meet statistical significance in reducing mortality at 90 days. However, the confidence interval around the effect estimate includes a clinically important benefit. The observed absolute reduction in mortality of around 2 percentage points with the use of continuous infusion represents a number needed to treat of 50 patients to prevent 1 death. The clinical significance of this finding is further supported by increased rates of clinical cure in the continuous infusion group and consistent directional changes in point estimates across other secondary and tertiary outcomes.
This pragmatic trial has several strengths. An extensive body of work informed the design of the trial that included feasibility and phase 2 trials previously conducted by the BLING investigators.10,18 This work facilitated the establishment of an international collaboration to conduct the trial in multiple ICUs in 7 countries under routine clinical conditions over a relatively short trial inception period. The effect of continuous infusion of β-lactam antibiotics was assessed using a robust, patient-centered primary outcome that was not susceptible to adjudication bias. The definition of clinical cure used in this trial was also not susceptible to adjudication bias. The size of this trial greatly exceeded all previous trials. The recent Continuous Infusion vs Intermittent Administration of Meropenem in Critically Ill Patients (MERCY) trial (N = 607) comparing continuous and intermittent infusions of meropenem with double-dummy administration reported a difference of 2.5 percentage points in mortality at 28 days in favor of continuous infusions that is consistent with the current trial despite differences in mortality, rates of drug resistance, and trial end points.26
Effective delivery of β-lactam antibiotics by continuous infusion present some practical considerations for patients treated in the ICU.27,28 Pharmacokinetic and pharmacodynamic considerations mandate that the same daily doses are used for both continuous and intermittent infusions.27 While overall drug costs may remain consistent, the time taken to prepare and administer drugs with continuous infusions may be shorter than intermittent infusions,8,16,29 although this may be negated by a higher incidence of interruptions of continuous infusions for clinical reasons. Continuous infusions of carbapenems that require multiple infusions in a 24-hour period for drug stability reasons and use of dedicated infusion pumps and intravenous portals pose additional pragmatic considerations, particularly in resource-limited environments.8,28,29,30 Consistent with previous trials, there were no observed differences in adverse events between continuous and intermittent infusions of β-lactam antibiotics.16,18,26,27
Limitations
This study had some limitations. First, blinding was not feasible in a pragmatic trial of this size. This was mitigated by using a robust primary outcome and blinded outcome adjudication. Second, some participants may have been randomized despite having a noninfectious cause of organ dysfunction. Third, while this international study has high indices of external validity and generalizability, extrapolation of these results to regions with high levels of antibiotic resistance requires caution. Fourth, there was no adjustment for susceptibility to the β-lactam antibiotic or the impact of additional antibiotics and dosing strategies, although the groups were observed to have a high degree of baseline balance. Fifth, the difference in outcomes may have been reduced by the use of intermittent dosing prior to randomization in the continuous group and after ICU discharge. Sixth, these results relate to the use of continuous infusions primarily in a high-income ICU setting and extrapolation to low- and middle-income settings in and outside the ICU cannot be inferred.
Conclusions
The observed difference in 90-day mortality between continuous vs intermittent infusions of β-lactam antibiotics did not meet statistical significance in the primary analysis. However, the confidence interval around the effect estimate includes the possibility of both no important effect and a clinically important benefit in the use of continuous infusions in this group of patients.
Educational Objective: To identify the key insights or developments described in this article.
-
Why might continuous infusion of β-lactam antibiotics be more effective than intermittent dosing?
Continuous infusion allows for a lower but continuous serum concentration, reducing adverse effects.
Higher total daily doses can be administered safely with continuous infusion.
Time-dependent kill characteristics of β-lactam antibiotics are better achieved through continuous infusion.
-
Which of the following was a trial outcome?
Adverse events occurred more frequently with intermittent dosing.
All-cause mortality was significantly higher in the continuous infusion group 90 days after randomization (the primary outcome).
Clinical cure 14 days after randomization was significantly better in the continuous infusion group (a secondary outcome).
-
Why might the results of this trial challenge straightforward interpretation?
Continuous dosing proved challenging to implement with the result that many participants received a combination of continuous and intermittent infusions.
Secondary and tertiary outcomes demonstrated inconsistent effects, some of which supported continuous infusion and some of which supported intermittent dosing.
While not statistically significant, the confidence interval around the primary outcome effect estimate included a clinically important benefit.
Section Editor: Christopher Seymour, MD, Associate Editor, JAMA (christopher.seymour@jamanetwork.org).
Trial Protocol
Statistical Analysis Plan
eMethods
eTable 1. Enrolment by Country and Site
eTable 2. APACHE III Diagnoses
eTable 3. Infective Organisms Identified from the Primary Site of Infection
eTable 4. Secondary and Tertiary Sites of Infection
eTable 5. Infective Organisms Identified from Second Site of Infection
eTable 6. Infective Organisms Identified from Third Site of Infection
eTable 7. β-Lactam Antibiotic Administration Details
eTable 8. Reasons for Cessation of β-Lactam Antibiotic
eTable 9. Other Antibiotics Administered
eTable 10. Place and Cause of Death
eTable 11. Summary of Adverse Events
eTable 12. Summary of Protocol Deviations
eFigure 1. Longitudinal Mean Plot of the Daily Dose of Piperacillin-Tazobactam
eFigure 2. Longitudinal Mean Plot of the Daily Dose of Meropenem
eFigure 3. Cumulative Incidence Function of Time to Death
eFigure 4. Cumulative Incidence Function of Time to Alive Discharge from Index ICU Admission
eFigure 5. Cumulative Incidence Function of Time to Alive Discharge from Index Hospital Admission
eReferences
Nonauthor Collaborators. BLING III Study Investigators
Data Sharing Statement
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200-211. doi: 10.1016/S0140-6736(19)32989-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleischmann-Struzek C, Mellhammar L, Rose N, et al. Incidence and mortality of hospital- and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 2020;46(8):1552-1562. doi: 10.1007/s00134-020-06151-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans L, Rhodes A, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181-1247. doi: 10.1007/s00134-021-06506-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dulhunty JM, Paterson D, Webb SAR, Lipman J. Antimicrobial utilisation in 37 Australian and New Zealand intensive care units. Anaesth Intensive Care. 2011;39(2):231-237. doi: 10.1177/0310057X1103900212 [DOI] [PubMed] [Google Scholar]
- 6.Roberts JA, Paul SK, Akova M, et al. ; DALI Study . DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58(8):1072-1083. doi: 10.1093/cid/ciu027 [DOI] [PubMed] [Google Scholar]
- 7.Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26(1):1-10. doi: 10.1086/516284 [DOI] [PubMed] [Google Scholar]
- 8.Mouton JW, Vinks AA. Continuous infusion of beta-lactams. Curr Opin Crit Care. 2007;13(5):598-606. doi: 10.1097/MCC.0b013e3282e2a98f [DOI] [PubMed] [Google Scholar]
- 9.Roberts JA, Webb S, Paterson D, Ho KM, Lipman J. A systematic review on clinical benefits of continuous administration of beta-lactam antibiotics. Crit Care Med. 2009;37(6):2071-2078. doi: 10.1097/CCM.0b013e3181a0054d [DOI] [PubMed] [Google Scholar]
- 10.Dulhunty JM, Roberts JA, Davis JS, et al. Continuous infusion of beta-lactam antibiotics in severe sepsis: a multicenter double-blind, randomized controlled trial. Clin Infect Dis. 2013;56(2):236-244. doi: 10.1093/cid/cis856 [DOI] [PubMed] [Google Scholar]
- 11.Roberts JA, Kirkpatrick CM, Roberts MS, Robertson TA, Dalley AJ, Lipman J. Meropenem dosing in critically ill patients with sepsis and without renal dysfunction: intermittent bolus versus continuous administration? Monte Carlo dosing simulations and subcutaneous tissue distribution. J Antimicrob Chemother. 2009;64(1):142-150. doi: 10.1093/jac/dkp139 [DOI] [PubMed] [Google Scholar]
- 12.Chytra I, Stepan M, Benes J, et al. Clinical and microbiological efficacy of continuous versus intermittent application of meropenem in critically ill patients: a randomized open-label controlled trial. Crit Care. 2012;16(3):R113. doi: 10.1186/cc11405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhodes NJ, Liu J, O’Donnell JN, et al. Prolonged infusion piperacillin-tazobactam decreases mortality and improves outcomes in severely ill patients: results of a systematic review and meta-analysis. Crit Care Med. 2018;46(2):236-243. doi: 10.1097/CCM.0000000000002836 [DOI] [PubMed] [Google Scholar]
- 14.Vardakas KZ, Voulgaris GL, Maliaros A, Samonis G, Falagas ME. Prolonged versus short-term intravenous infusion of antipseudomonal β-lactams for patients with sepsis: a systematic review and meta-analysis of randomised trials. Lancet Infect Dis. 2018;18(1):108-120. doi: 10.1016/S1473-3099(17)30615-1 [DOI] [PubMed] [Google Scholar]
- 15.Roberts JA, Abdul-Aziz MH, Davis JS, et al. Continuous versus intermittent β-lactam infusion in severe sepsis: a meta-analysis of individual patient data from randomized trials. Am J Respir Crit Care Med. 2016;194(6):681-691. doi: 10.1164/rccm.201601-0024OC [DOI] [PubMed] [Google Scholar]
- 16.Thabet P, Joshi A, MacDonald E, et al. Clinical and pharmacokinetic/dynamic outcomes of prolonged infusions of beta-lactam antimicrobials: an overview of systematic reviews. PLoS One. 2021;16(1):e0244966. doi: 10.1371/journal.pone.0244966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cotta MO, Dulhunty JM, Roberts JA, Myburgh J, Lipman J. Should β-lactam antibiotics be administered by continuous infusion in critically ill patients? a survey of Australia and New Zealand intensive care unit doctors and pharmacists. Int J Antimicrob Agents. 2016;47(6):436-438. doi: 10.1016/j.ijantimicag.2016.02.017 [DOI] [PubMed] [Google Scholar]
- 18.Dulhunty JM, Roberts JA, Davis JS, et al. ; BLING II Investigators for the ANZICS Clinical Trials Group . A multicenter randomized trial of continuous versus intermittent β-lactam infusion in severe sepsis. Am J Respir Crit Care Med. 2015;192(11):1298-1305. doi: 10.1164/rccm.201505-0857OC [DOI] [PubMed] [Google Scholar]
- 19.Lipman J, Brett SJ, De Waele JJ, et al. A protocol for a phase 3 multicentre randomised controlled trial of continuous versus intermittent β-lactam antibiotic infusion in critically ill patients with sepsis: BLING III. Crit Care Resusc. 2019;21(1):63-68. doi: 10.1016/S1441-2772(23)00582-3 [DOI] [PubMed] [Google Scholar]
- 20.Billot L, Lipman J, Brett SJ, et al. ; BLING III Investigators . Statistical analysis plan for the BLING III study: a phase 3 multicentre randomised controlled trial of continuous versus intermittent β-lactam antibiotic infusion in critically ill patients with sepsis. Crit Care Resusc. 2023;23(3):273-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818-829. doi: 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- 22.Finfer S, Micallef S, Hammond N, et al. ; PLUS Study Investigators and the Australian New Zealand Intensive Care Society Clinical Trials Group . Balanced multielectrolyte solution versus saline in critically ill adults. N Engl J Med. 2022;386(9):815-826. doi: 10.1056/NEJMoa2114464 [DOI] [PubMed] [Google Scholar]
- 23.WHO Collaborating Centre for Drug Statistics Methodology . ATC/DDD Index 2024. January 26, 2024. Accessed April 14, 2024. https://atcddd.fhi.no/atc_ddd_index/
- 24.Taccone FS, Laupland KB, Montravers P. Continuous infusion of β-lactam antibiotics for all critically ill patients? Intensive Care Med. 2016;42(10):1604-1606. doi: 10.1007/s00134-016-4241-7 [DOI] [PubMed] [Google Scholar]
- 25.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat Theory Appl. 1979;6(2):65-70. [Google Scholar]
- 26.Monti G, Bradic N, Marzaroli M, et al. ; MERCY Investigators . Continuous vs intermittent meropenem administration in critically ill patients with sepsis: the MERCY randomized clinical trial. JAMA. 2023;330(2):141-151. doi: 10.1001/jama.2023.10598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts JA, Paratz J, Paratz E, Krueger WA, Lipman J. Continuous infusion of beta-lactam antibiotics in severe infections: a review of its role. Int J Antimicrob Agents. 2007;30(1):11-18. doi: 10.1016/j.ijantimicag.2007.02.002 [DOI] [PubMed] [Google Scholar]
- 28.De Waele JJ, Lipman J, Carlier M, Roberts JA. Subtleties in practical application of prolonged infusion of β-lactam antibiotics. Int J Antimicrob Agents. 2015;45(5):461-463. doi: 10.1016/j.ijantimicag.2015.01.007 [DOI] [PubMed] [Google Scholar]
- 29.Fawaz S, Barton S, Whitney L, Nabhani-Gebara S. Differential antibiotic dosing in critical care: survey on nurses’ knowledge, perceptions and experience. JAC Antimicrob Resist. 2020;2(4):dlaa083. doi: 10.1093/jacamr/dlaa083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berthoin K, Le Duff CS, Marchand-Brynaert J, Carryn S, Tulkens PM. Stability of meropenem and doripenem solutions for administration by continuous infusion. J Antimicrob Chemother. 2010;65(5):1073-1075. doi: 10.1093/jac/dkq044 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eMethods
eTable 1. Enrolment by Country and Site
eTable 2. APACHE III Diagnoses
eTable 3. Infective Organisms Identified from the Primary Site of Infection
eTable 4. Secondary and Tertiary Sites of Infection
eTable 5. Infective Organisms Identified from Second Site of Infection
eTable 6. Infective Organisms Identified from Third Site of Infection
eTable 7. β-Lactam Antibiotic Administration Details
eTable 8. Reasons for Cessation of β-Lactam Antibiotic
eTable 9. Other Antibiotics Administered
eTable 10. Place and Cause of Death
eTable 11. Summary of Adverse Events
eTable 12. Summary of Protocol Deviations
eFigure 1. Longitudinal Mean Plot of the Daily Dose of Piperacillin-Tazobactam
eFigure 2. Longitudinal Mean Plot of the Daily Dose of Meropenem
eFigure 3. Cumulative Incidence Function of Time to Death
eFigure 4. Cumulative Incidence Function of Time to Alive Discharge from Index ICU Admission
eFigure 5. Cumulative Incidence Function of Time to Alive Discharge from Index Hospital Admission
eReferences
Nonauthor Collaborators. BLING III Study Investigators
Data Sharing Statement