Abstract
Background
No standardized treatment strategy exists for managing oligoprogression during maintenance therapy in driver-negative advanced non-small cell lung cancer (NSCLC). Similarly, a uniform response to oligoprogression during maintenance therapy using immune checkpoint inhibitors (ICIs) has not been established. Consequently, our investigation focused on assessing the efficacy and safety of employing stereotactic total body radiotherapy in conjunction with ICIs to address oligoprogression in advanced NSCLC.
Methods
We conducted a retrospective analysis of patients diagnosed with driver-negative advanced NSCLC who received stereotactic body radiotherapy (SBRT) in combination with ICIs to manage oligoprogressive lesions within the period from October 2018 to October 2023 at our institution. Oligoprogression, defined as progression occurring in three or fewer disease sites, was the focus of our investigation. Our assessment encompassed various parameters including the local control rate (LCR), progression-free survival post-oligoprogression (PFS-P), overall survival post-oligoprogression (OS-P), progression-free survival (PFS), overall survival (OS), and the safety profile associated with SBRT followed by sequential ICIs after oligoprogression.
Results
A total of 15 patients were enrolled in this study, all at stage IV, with 12 (80%) receiving a diagnosis of adenocarcinoma. Before oligoprogression, 11 (73.3%) patients had undergone immunotherapy. Following the treatment of oligoprogressed lung cancer with SBRT sequential ICIs, the median PFS-P and OS-P were 8 months (95% CI: 2.7–13.3) and 12 months (95% CI: 7.3–16.7), respectively. Additionally, the median PFS and OS were 26 months (95% CI: 8.0–44.0) and 30 months (not reached), respectively. The median local control (LC) of 15 oligoprogressed lesions was 13 months (95% CI: 5.3–20.2), with a 1-year LCR of 77.9%. Notably, patients with a performance status (PS) score of less than 2 demonstrated a more favorable survival benefit.
Conclusions
Stereotactic systemic radiation therapy, combined with sequential ICIs, enhances both LC and survival in advanced NSCLC characterized by oligoprogression and negative driver gene mutations. This approach also exhibits the potential to postpone the transition between systemic chemotherapy regimens. Manageable adverse reactions were observed, with the absence of grade 4 reactions.
Keywords: Non-small cell lung cancer (NSCLC), immune checkpoint inhibitors (ICIs), oligoprogression, stereotactic body radiotherapy (SBRT)
Highlight box.
Key findings
• The combination of stereotactic body radiotherapy (SBRT) alongside immune checkpoint inhibitors (ICIs) demonstrates effectiveness in treating patients who have developed immunotherapy resistance, specifically those presenting with oligometastases stemming from driver-negative non-small cell lung cancer (NSCLC).
What is known and what is new?
• Immunotherapy markedly enhances the prognosis for patients diagnosed with NSCLC. Nevertheless, the effectiveness and safety associated with incorporating SBRT into the treatment regimen remain uncertain, primarily owing to the infrequency of patients displaying oligometastases.
• In addition to assessing the effectiveness and safety of SBRT in conjunction with ICIs for managing oligometastases subsequent to immunotherapy resistance, our investigation encompassed an analysis of the local control rate and discerned variations in efficacy among distinct subgroups.
What is the implication, and what should change now?
• SBRT combined with ICIs exhibits promise as a prospective therapeutic avenue for addressing ICI resistance in advanced NSCLC lacking driver mutations. Nonetheless, the substantiation of its efficacy necessitates more extensive cohort studies, with a particular emphasis on prospective investigations.
Introduction
Lung cancer remains the foremost contributor to global cancer-related mortality (1). The introduction of programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) inhibitors has revolutionized the therapeutic landscape for advanced non-small cell lung cancer (NSCLC). Multiple studies in immunocombination therapy have yielded promising outcomes, leading to their integration into established treatment protocols outlined in clinical guidelines (2-4). Despite the survival advantages associated with this shift in the treatment paradigm for advanced NSCLC patients, instances of drug-resistant responses persist, with oligoprogression observed in 13–55.3% of cases (5,6).
The concept of oligometastases was initially proposed by Hellman and Weichselbaum, who defined oligometastases as an intermediate state in the progression from limited to extensive disease (7). Oligoprogressive disease (OPD) is characterized by the localized progression of only a small number of metastatic sites in patients with extensive disease, occurring within the context of controlling the remaining systemic lesions (8). Recent studies have demonstrated the effectiveness of localized therapy in managing pulmonary oligoprogressive metastases (9,10). Furthermore, radiation therapy now enables the interruption of systemic therapy at multiple disease sites, allowing for the delivery of high radiation doses to the tumor while minimizing damage to adjacent normal tissue, rapidly and safely (11-13).
In the realm of driver-negative advanced NSCLC, a standardized treatment strategy for oligoprogression during maintenance therapy is conspicuously absent. Likewise, there exists no universally accepted response protocol for cases of oligoprogression emerging during maintenance therapy involving immune checkpoint inhibitors (ICIs). Consequently, our investigation delved into assessing the efficacy and safety of employing stereotactic total body radiotherapy in sequence with ICIs for managing instances of oligoprogression in advanced NSCLC. We present this article in accordance with the STROBE reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-2232/rc).
Methods
Patients characteristics
This retrospective study involved the screening of patient data spanning from October 2018 to October 2023 at Taizhou Cancer Hospital. The inclusion criteria encompassed patients meeting the following conditions: (I) diagnosis of stage IV NSCLC; (II) absence of driver genes; (III) receipt of at least one line of antitumor therapy; (IV) oligoprogression, defined as progression at up to three metastatic sites, development of new metastatic lesions, or progression of existing metastatic lesions; and (V) oligoprogression followed by stereotactic body radiotherapy (SBRT) in conjunction with sequential ICI therapy. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Committee at Taizhou Cancer Hospital (SL2023052) and individual consent for this retrospective analysis was waived.
Stereotactic radiotherapy
Stereotactic treatment was administered using an electron linear accelerator radiation therapy system (Trilogy 5916, Varian, USA). Simulated computed tomography (CT) scans were conducted under free-breathing conditions. The gross tumor volume (GTV) was delineated as the visible tumor volume on the simulated CT image, incorporating image fusion with magnetic resonance imaging (MRI) or positron emission tomography-computed tomography (PET-CT) when available. Motion management strategies were implemented based on the treatment site, involving patient immobilization with a vacuum mattress, daily cone-beam CT scans, and defining a planned target volume (PTV) by expanding the GTV isotropically by 3–5 mm. The dose was quantified as the biologically effective dose, assuming an α/β ratio of 10 (BED10). The total dose for stereotactic radiation therapy ranged from 25 to 50 Gy administered over 3 to 10 days. Dosage and fractionation protocols were individualized based on the size and location of the patient’s tumor.
Endpoints and assessment
The concept of local control (LC) in this study pertained to the duration from the initiation of SBRT to either the occurrence of radiographic progression or the date of the last follow-up assessment. Progression-free survival post-oligoprogression (PFS-P) was operationally defined as the time elapsed from the commencement of SBRT to the point of radiologic progression. Meanwhile, overall survival post-oligoprogression (OS-P) was characterized as the interval between the initiation of SBRT and either the date of death or the last follow-up. In instances where patients had received prior treatment with ICIs, progression-free survival (PFS) was specifically delineated as the span between the initial administration of ICI treatment and the onset of radiologic progression post-SBRT. Furthermore, overall survival (OS) was construed as the timeframe between the commencement of ICI treatment and the occurrence of death or the last follow-up. The assessment of drug toxicity was conducted following the Common Terminology Criteria for Adverse Events (CTCAE 5.0), whereas adverse reactions related to SBRT were evaluated utilizing the criteria outlined by the Radiation Therapy Oncology Group (RTOG).
Statistical analysis
The statistical analyses were conducted employing IBM SPSS v.27 software. Kaplan-Meier analyses were utilized for assessing LC, PFS, PFS-P, OS, and OS-P. Meanwhile, Cox regression models were applied in the univariate analyses to examine prognostic factors linked to both SBRT and ICIs. To compare lesion subgroups, the log-rank test was employed. In the statistical analysis, a P value less than 0.05 was deemed statistically significant.
Results
Patients characteristics
Patients diagnosed with advanced NSCLC lacking driver mutations were subjected to a treatment regimen involving SBRT followed by ICIs for oligoprogressive lesions within our medical facility. Oligoprogression, denoting progression in three or fewer disease sites, served as our specific criterion. Our assessment focused on determining the local control rate (LCR), PFS-P, OS-P, and the safety profile associated with the sequential administration of SBRT and ICIs after oligoprogression.
We conducted a retrospective analysis of 15 patients diagnosed with stage IV NSCLC lacking driver gene mutations. These individuals underwent stereotactic radiation therapy followed by sequential ICIs from October 2018 to October 2023 at the Radiation Therapy Center of Taizhou Cancer Hospital. Among them, 11 patients underwent SBRT following either monoimmunization or combination therapy, while 4 initially received combination chemotherapy. Table 1 comprehensively outlines patient characteristics; notably, 80% were diagnosed with adenocarcinoma, and 86.7% exhibited an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0–1. Specific details regarding ICIs and oligoprogression are presented in Table 2. Before oligoprogression, 53.3% of patients utilized immunotherapy as their first-line treatment, with 26.7% receiving monotherapy, 46.6% combining immunotherapy with chemotherapy, and 4 patients undergoing a combination of chemotherapy and antiangiogenesis. The 20 sites of oligoprogression comprised the lung (n=17, 85%), brain (n=1, 5%), bone (n=1, 5%) and lymph nodes (n=1, 5%). A total of 16 organs with metastatic lesions underwent treatment by SBRT. Among the 15 individuals included in our study, 14 received treatment for lung conditions, encompassing both primary pulmonary cases and intrapulmonary metastases. Additionally, one individual underwent treatment for brain-related issues. Furthermore, another participant received targeted palliative radiotherapy to alleviate pain from bone metastases, in addition to undergoing lung treatment. Following oligoprogression, after the sequential administration of SBRT to ICIs, 40% of patients maintained the same single ICI for maintenance therapy, while 60% altered their ICI strategy, remaining on a single drug for maintenance.
Table 1. Clinicopathologic features among 15 patients.
| Characteristics | Values |
|---|---|
| Gender, n (%) | |
| Female | 1 (6.7) |
| Male | 14 (93.3) |
| Age (years) | |
| Mean | 61 |
| <65, n (%) | 3 (20.0) |
| ≥65, n (%) | 12 (80.0) |
| Smoking status, n (%) | |
| No | 5 (33.3) |
| Yes | 10 (66.7) |
| ECOG PS, n (%) | |
| 0–1 | 13 (86.7) |
| 2 | 2 (13.3) |
| Pathology, n (%) | |
| Adenocarcinoma | 12 (80.0) |
| Squamous cell carcinoma | 3 (20.0) |
| LIPI, n (%) | |
| 0 | 2 (13.3) |
| 1 | 8 (53.3) |
| 2 | 5 (33.3) |
| PD-L1, n (%) | |
| Positive (≥1%) | 4 (26.7) |
| Negative (<1%) | 1 (6.7) |
| Unknown | 10 (66.7) |
| Lung metastasis, n (%) | |
| No | 3 (20.0) |
| Yes | 12 (80.0) |
| Bone metastasis, n (%) | |
| No | 9 (60.0) |
| Yes | 6 (40.0) |
| Brain metastasis, n (%) | |
| No | 9 (60.0) |
| Yes | 6 (40.0) |
LIPI, lung immune prognostic index; ECOG PS, Eastern Cooperative Oncology Group Performance Status; PD-L1, programmed cell death ligand 1.
Table 2. Disease characteristics at the time of oligoprogression.
| Variable | Values, n (%) |
|---|---|
| Type of immunotherapy (ICIs strategy) | |
| Monotherapy | 4 (26.7) |
| Combination | 7 (46.6) |
| None | 4 (26.7) |
| Lines of immunotherapy before oligoprogression | |
| First line | 8 (53.3) |
| Second line | 3 (26.7) |
| None | 4 (26.7) |
| Response to immunotherapy before oligoprogression | |
| CR | 0 |
| PR | 3 (20.0) |
| SD | 12 (80.0) |
| Number of oligoprogression | |
| One | 12 (80.0) |
| Two | 2 (13.3) |
| Three | 1 (6.7) |
| Organs of oligoprogression | |
| Lung | 17 (85.0) |
| Brain | 1 (5.0) |
| Lymph node | 1 (5.0) |
| Bone | 1 (5.0) |
| Pattern of oligoprogression | |
| New metastasis | 3 (20.0) |
| Existing metastasis | 12 (80.0) |
| Type of immunotherapy after oligoprogression | |
| Monotherapy | 15 (100.0) |
| Combination | 0 |
| Change of ICIs strategy | |
| Continue previous treatment or maintenance strategy | 6 (40.0) |
| Adjusted maintenance strategy | 9 (60.0) |
ICIs, immune checkpoint inhibitors; SD, stable disease; PR, partial response; CR, complete response.
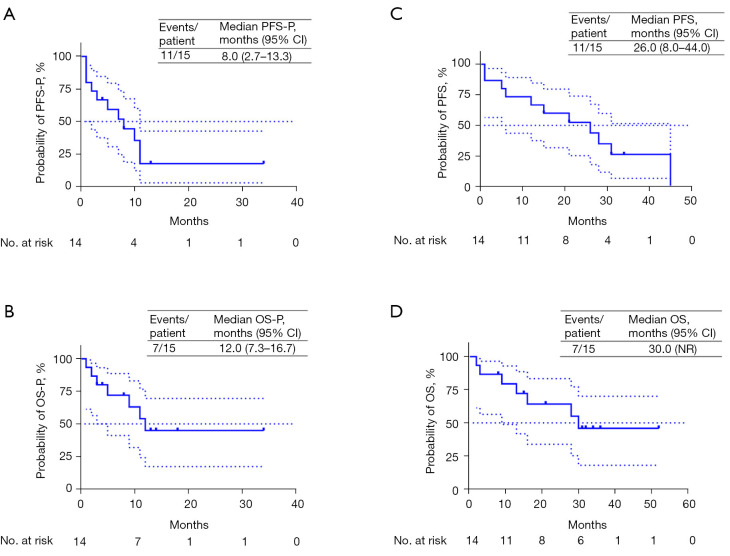
Clinical outcomes
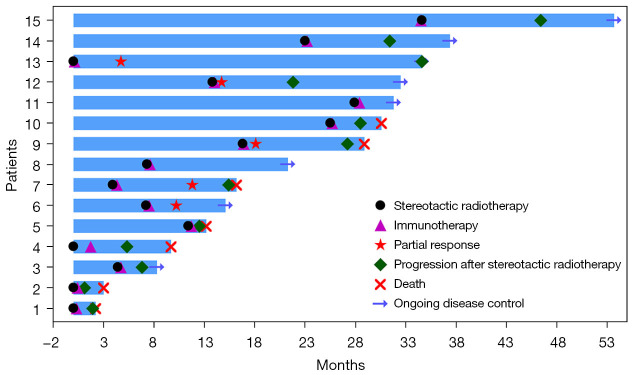
The median progression-free survival post-oligoprogression (mPFS-P) and median overall survival post-oligoprogression (mOS-P) following oligoprogression were 8.0 months [95% confidence interval (CI): 2.7–13.3] and 12 months (95% CI: 7.3–16.7), respectively (see Figure 1A,1B). Additionally, the median progression-free survival (PFS) after the initial immunotherapy was 26 months (95% CI: 8.0–44.0) as depicted in Figure 1C. The median overall survival (mOS) was 30 months (not available) (see Figure 1D). The treatment status of each patient was visually represented in the swim lane diagram (refer to Figure 2). Univariate analysis (refer to Tables 3,4) indicated that patients with ECOG PS scores of 0–1 predicted longer PFS-P, PFS, OS, and OS-P.
Figure 1.
Kaplan-Meier curves of PFS-P (A) and PFS (C), OS-P (B) and OS (D). NR, not reached; PFS-P, progression-free survival post-oligoprogression; PFS, progression-free survival; OS-P, overall survival post-oligoprogression; OS, overall survival; CI, confidence interval.
Figure 2.
Swimming plot visualizing details of the response to SBRT sequential ICIs therapy after oligoprogression in 15 patients. SBRT, stereotactic body radiotherapy; ICIs, immune checkpoint inhibitors.
Table 3. Univariable analysis of PFS and PFS-P.
| Variable | PFS-P | PFS | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Gender (female vs. male) | 0.647 (0.080–5.213) | 0.68 | 0.038 (0.000–161.997) | 0.44 | |
| Age (<65 vs. ≥65 years) | 3.334 (0.740–15.013) | 0.12 | 4.979 (1.084–22.877) | 0.04 | |
| Smoking status (no vs. yes) | 0.806 (0.234–2.782) | 0.73 | 0.483 (0.119–1.958) | 0.31 | |
| ECOG PS (0–1 vs. 2) | 0.078 (0.074–15.013) | 0.04 | 0.170 (0.028–1.031) | 0.054 | |
| Brain metastasis (no vs. yes) | 0.635 (0.183–2.200) | 0.47 | 0.284 (0.063–1.284) | 0.10 | |
| Liver metastasis (no vs. yes) | 0.143 (0.013–1.575) | 0.11 | 0.071 (0.004–1.142) | 0.06 | |
| Bone metastasis (no vs. yes) | 0.574 (0.173–1.910) | 0.37 | 0.613 (0.176–2.132) | 0.44 | |
| Lung metastasis (no vs. yes) | 0.857 (0.184–3.985) | 0.11 | 1.348 (0.260–6.977) | 0.72 | |
| Previous immunotherapy (no vs. yes) | 1.397 (0.366–5.328) | 0.63 | 2.156 (0.538–8.634) | 0.28 | |
| LIPI (0 vs. ≥1) | 0.555 (0.114–2.692) | 0.46 | 0.460 (0.092–2.299) | 0.34 | |
PFS, progression-free survival; PFS-P, progression-free survival post-oligoprogression; CI, confidence interval; ECOG PS, performance score of Eastern Cooperative Oncology Group; LIPI, lung immune prognostic index.
Table 4. Univariable analysis of OS and OS-P.
| Variable | OS-P | OS | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Gender (female vs. male) | 0.041 (0.000–1,562.7) | 0.55 | 0.041 (0.000–1,749.4) | 0.56 | |
| Age (<65 vs. ≥65 years) | 1.717 (0.330–8.929) | 0.52 | 2.367 (0.453–12.359) | 0.31 | |
| Smoking status (no vs. yes) | 0.015 (0.000–7.699) | 0.19 | 0.016 (0.000–8.506) | 0.20 | |
| ECOG PS (0–1 vs. 2) | 0.049 (0.004–0.052) | 0.02 | 0.124 (0.017–0.892) | 0.04 | |
| Brain metastasis (no vs. yes) | 0.618 (0.138–2.772) | 0.53 | 0.457 (0.099–2.114) | 0.32 | |
| Liver metastasis (no vs. yes) | 0.154 (0.014–1.703) | 0.13 | 0.074 (0.005–1.186) | 0.07 | |
| Bone metastasis (no vs. yes) | 0.896 (0.199–4.047) | 0.89 | 0.902 (0.200–4.068) | 0.89 | |
| Lung metastasis (no vs. yes) | 0.572 (0.068–6.838) | 0.61 | 0.625 (0.075–5.234) | 0.67 | |
| Previous immunotherapy (no vs. yes) | 2.7 (0.595–12.258) | 0.20 | 3.946 (0.857–18.164) | 0.08 | |
| LIPI (0 vs. ≥1) | 0.766 (0.089–6.581) | 0.81 | 0.663 (0.077–5.724) | 0.71 | |
OS, overall survival; OS-P, overall survival post-oligoprogression; CI, confidence interval; ECOG PS, performance score of Eastern Cooperative Oncology Group; LIPI, lung immune prognostic index.
Following SBRT and ICIs, the optimal efficacy was observed with partial response (PR) in 5 loci (25%) and stable disease (SD) in 15 loci (75%), without any instances of complete response (CR) or progressive disease (PD). The median LC was 13.0 months (95% CI: 5.8–20.2) (refer to Figure 3). The 1-year LC rate was 77.9%. Specifically, for the most prevalent lung lesions, PR was achieved at 5 sites (25%) and SD at 9 sites (45%). No discernible differences in PFS and OS were identified between pulmonary and nonpulmonary lesions.
Figure 3.
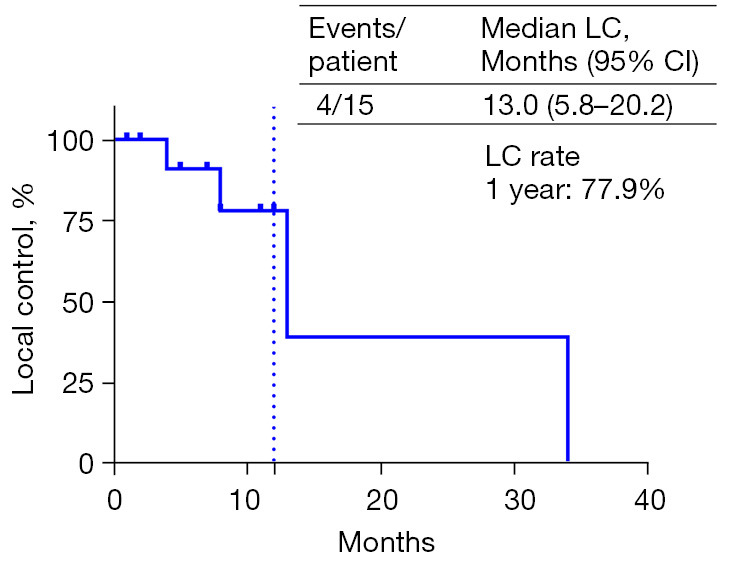
Kaplan-Meier curve of LC in 15 oligoprogressed lesions. LC, local control; CI, confidence interval.
Toxicity
No Grade 4 toxic reactions were detected in our study. Grade 1–2 fatigue was reported in five cases, while grade 1–2 pneumonitis and grade 1–2 elevation of thyroid-stimulating hormone (TSH) were observed in three cases each. Notably, grade 3 adverse reactions included thrombocytopenia (n=1), myocarditis (n=1), and rash (n=1). It is worth mentioning that no adverse events (AEs) related to radiation were identified (Table 5).
Table 5. Main toxicities during treatment since oligoprogression.
| Adverse event category | ICIs related, n (%) | SBRT related, n | ||
|---|---|---|---|---|
| Grades 1–2 | Grade 3 | Grades 1–4 | ||
| Fatigue | 5 (33.3) | 0 | 0 | |
| Liver dysfunction | 2 (13.3) | 0 | 0 | |
| Thrombocytopenia | 0 | 1 (6.7) | 0 | |
| TSH elevation | 3 (20.0) | 0 | 0 | |
| Proteinuria | 1 (6.7) | 0 | 0 | |
| Skin rashes | 1 (6.7) | 1 (6.7) | 0 | |
| Hypopituitarism | 2 (13.3) | 0 | 0 | |
| Pneumonitis | 3 (20.0) | 0 | 0 | |
| Myocarditis | 0 | 1 (6.7) | 0 | |
| High BNP | 1 (6.7) | 0 | 0 | |
ICIs, immune checkpoint inhibitors; SBRT, stereotactic body radiotherapy; TSH, thyroid-stimulating hormone; BNP, B-type natriuretic peptide.
Discussion
The advent of ICIs has markedly altered the prognosis for lung cancer patients, particularly offering renewed optimism to those grappling with advanced stages of the disease. However, this newfound promise is inevitably tempered by the emergence of resistance, leading to oligoprogression in a considerable percentage of patients, ranging from 13% to 55.3% (5,6,14). Notably, Rheinheimer et al.’s investigation yielded comparable probabilities of OPD following both chemoimmunotherapy and monoimmunotherapy (5). Notably, there is a lack of a standardized treatment protocol for addressing oligoprogression during maintenance therapy for driver-negative advanced NSCLC. Likewise, a consistent response to oligoprogression during maintenance therapy with ICIs is absent. This underscores the imperative for additional research to delineate potentially efficacious approaches.
The development of resistance to ICIs involves several mechanisms, including ICI escape resulting from disruption of the human leukocyte antigen (HLA) class I antigen processing machinery (APM) (15), deletion and alteration of the interferon signaling pathway (16,17), and innate anti-PD-1 resistance (18). Notably, radiotherapy exhibits a synergistic effect on the immune system by modifying cell surface molecules and activating the innate immune response, leading to a systemic impact that contributes to tumor shrinkage and enhances the efficacy of ICIs (19-21). Furthermore, local radiotherapy targeted at a single unirradiated metastasis can induce an anti-CTLA-4 response, initiating a systemic anti-tumor immune response (22,23). Emerging data indicate a correlation between the resistance of residual clones to cytotoxic chemotherapy and the size of tumor deposits; larger deposits exhibit greater resistance and more favorable LC of the lesion (24). Theoretically, proactive management of oligoprogressive lesions through local interventions before extensive metastasis occurs, post-drug resistance, holds promise for achieving disease control. This approach not only addresses local symptoms resulting from tumor growth but also aligns with the intention to control disease progression.
The mOS for patients diagnosed with advanced NSCLC, who experienced progression following a minimum of one line of treatment without further advancement, was 3.7 months. In contrast, individuals treated with natalizumab exhibited a significantly prolonged mOS of 17.8 months (25). The manifestation of complete or PR after initial treatment with ICIs, followed by disease progression or mortality, was categorized as acquired resistance (AR). Among AR patients, the mOS after treatment was notably extended to 18.9 months (26). Previous studies have indicated that the phenomenon of oligoprogression, when managed alongside localized therapy, contributes to an overall prolongation of PFS and OS, thereby delaying the transition to a systemic treatment approach (6,27,28). However, a comprehensive summary of survival rates related to the combination of SBRT and ICIs for patients experiencing AR and oligoprogression was lacking. To address this gap, relevant studies were systematically reviewed and summarized in Table 6. Xu et al. (6) reported noteworthy outcomes in their investigation, revealing that the integration of radiotherapy with ICIs following oligoprogression resulted in a second PFS (PFS2: time from the initiation of first immunotherapy to the occurrence of second progression or death) of 15 months, accompanied by an OS of 26.4 months. Wang et al. (28) documented that the integration of SBRT with checkpoint inhibitors (CPIs) yielded a median PFS following oligoprogressive therapy and oligoprogressive therapy-associated OS of 11 and 34 months, respectively. The OS from the initiation of CPI therapy to death or last follow-up was reported as 51 months, accompanied by a noteworthy 1-year LCR of 100%. Notably, their study encompassed patients with stage III and EGFR mutations, different from our investigation. In our study, patients manifesting oligoprogression exhibited a PFS-P of 8 months, an OS-P of 12 months, an overall OS of 30 months, and a 1-year LCR of 77.9%. Our findings suggest that the sequencing of SBRT with ICIs can effectively address OPD stemming from AR, offering a survival advantage.
Table 6. Oligoprogressive disease comparison summary.
| Author | Disease site | PFS (months) | OS (months) | LC (12 months) | Number of oligoprogressive cases |
|---|---|---|---|---|---|
| Current study | NSCLC | 8 | 30 | 77.9% | 15 |
| Weickhardt et al., 2012 (29) | NSCLC | 6.2 | NA | NA | 25 |
| Gan et al., 2014 (30) | NSCLC | 5.5 | 39 | 86% | 14 |
| Chan et al., 2017 (31) | NSCLC | 7 | 28.2 | NA | 24 |
| Merino Lara et al., 2018 (32) | NSCLC | 7.6 | 39.3 | 84.4% | 20 |
| Jiang et al., 2019 (33) | NSCLC | 13.9 | 28.3 | NA | 24 |
| Borghetti et al., 2019 (34) | NSCLC | NA | 23 | NA | 49 |
| Santarpia et al., 2020 (35) | NSCLC | 6.3 | 38.7 | NA | 36 |
| Kagawa et al., 2020 (36) | NSCLC | 7.37 | NA | NA | 10 |
| Buglione et al., 2020 (37) | NSCLC | 10.6 | 29.6 | 90% | 198 |
| Wang et al., 2021 (28) | NSCLC | 11 | 51 | 100% | 24 |
| Mahmood et al., 2022 (38) | NSCLC | 6.41 | 29.8 | NA | 59 |
| Xu et al., 2021 (6) | NSCLC | 15 | 26.4 | NA | 38 |
PFS, progression-free survival; OS, overall survival; LC, local control; NSCLC, non-small cell lung cancer; NA, not available.
In our investigation, patients exhibiting PS scores of 0–1 demonstrated a higher likelihood of deriving benefits from combination therapy, aligning with the findings of the study by Hosoya et al. (39) (Tables 3,4). Nevertheless, the limited sample size of only 2 patients with ECOG PS 0–1 underscores the necessity for a more extensive dataset to validate our findings. Other studies have indicated that elderly patients, those with a minimum of three metastatic organs or bone metastases, tend to exhibit shorter durable responses to ICIs (28,39). Conversely, patients with favorable lung immune prognostic index (LIPI), positive PD-L1 expression, or adenocarcinoma are more predisposed to benefit from ICIs (28,39). Eighty-five percent of the 15 patients presented with oligoprogressive lung lesions and underwent radiotherapy. Only one patient underwent extrapulmonary treatment—specifically, brain radiotherapy—which might have influenced the decision to extend radiotherapy to all oligoprogressive sites. Owing to the constraints posed by our sample size, discernible variations in PFS, PFS-P, OS, and OS-P were not evident among groups stratified by different pathologic types, LIPI, and metastatic organ involvement. Consequently, a more expansive analysis involving a larger sample size is imperative to elucidate pertinent predictive biomarkers for identifying patients poised to derive maximal benefits from such therapeutic approaches.
This investigation entailed a retrospective analysis characterized by a modest sample size and inherent selection bias. To surmount these constraints, there is a pressing imperative for extensive prospective controlled cohort studies.
Conclusions
The utilization of stereotactic systemic radiation therapy, coupled with sequential ICIs, exhibits enhanced LC and confers a survival advantage in advanced NSCLC characterized by oligoprogression and the absence of driver gene mutations. Moreover, this treatment approach holds the potential to prolong the intervals before transitioning between systemic chemotherapy regimens. The management of adverse effects remains feasible.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study received support from the Key Laboratory of Minimally Invasive Intervention and Big Data Artificial Intelligence, located in Taizhou, China.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Committee at Taizhou Cancer Hospital (SL2023052) and individual consent for this retrospective analysis was waived.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-2232/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-2232/dss
Peer Review File: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-2232/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-23-2232/coif). The authors have no conflicts of interest to declare.
References
- 1.Bade BC, Dela Cruz CS. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin Chest Med 2020;41:1-24. 10.1016/j.ccm.2019.10.001 [DOI] [PubMed] [Google Scholar]
- 2.Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N Engl J Med 2020;383:1328-39. 10.1056/NEJMoa1917346 [DOI] [PubMed] [Google Scholar]
- 3.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 4.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 5.Rheinheimer S, Heussel CP, Mayer P, et al. Oligoprogressive Non-Small-Cell Lung Cancer under Treatment with PD-(L)1 Inhibitors. Cancers (Basel) 2020;12:1046. 10.3390/cancers12041046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Y, Li H, Fan Y. Progression Patterns, Treatment, and Prognosis Beyond Resistance of Responders to Immunotherapy in Advanced Non-Small Cell Lung Cancer. Front Oncol 2021;11:642883. 10.3389/fonc.2021.642883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. 10.1200/JCO.1995.13.1.8 [DOI] [PubMed] [Google Scholar]
- 8.Patel PH, Palma D, McDonald F, et al. The Dandelion Dilemma Revisited for Oligoprogression: Treat the Whole Lawn or Weed Selectively? Clin Oncol (R Coll Radiol) 2019;31:824-33. 10.1016/j.clon.2019.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kagawa Y, Furuta H, Uemura T, et al. Efficacy of local therapy for oligoprogressive disease after programmed cell death 1 blockade in advanced non-small cell lung cancer. Cancer Sci 2020;111:4442-52. 10.1111/cas.14605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klement RJ, Hoerner-Rieber J, Adebahr S, et al. Stereotactic body radiotherapy (SBRT) for multiple pulmonary oligometastases: Analysis of number and timing of repeat SBRT as impact factors on treatment safety and efficacy. Radiother Oncol 2018;127:246-52. 10.1016/j.radonc.2018.02.016 [DOI] [PubMed] [Google Scholar]
- 11.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019;393:2051-8. 10.1016/S0140-6736(18)32487-5 [DOI] [PubMed] [Google Scholar]
- 12.Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol 2009;27:1579-84. 10.1200/JCO.2008.19.6386 [DOI] [PubMed] [Google Scholar]
- 13.Timmerman RD, Kavanagh BD, Cho LC, et al. Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol 2007;25:947-52. 10.1200/JCO.2006.09.7469 [DOI] [PubMed] [Google Scholar]
- 14.Harada D, Takigawa N. Oligoprogression in Non-Small Cell Lung Cancer. Cancers (Basel) 2021;13:5823. 10.3390/cancers13225823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gettinger S, Choi J, Hastings K, et al. Impaired HLA Class I Antigen Processing and Presentation as a Mechanism of Acquired Resistance to Immune Checkpoint Inhibitors in Lung Cancer. Cancer Discov 2017;7:1420-35. 10.1158/2159-8290.CD-17-0593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunn GP, Sheehan KC, Old LJ, et al. IFN unresponsiveness in LNCaP cells due to the lack of JAK1 gene expression. Cancer Res 2005;65:3447-53. 10.1158/0008-5472.CAN-04-4316 [DOI] [PubMed] [Google Scholar]
- 17.Sharma P, Hu-Lieskovan S, Wargo JA, et al. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017;168:707-23. 10.1016/j.cell.2017.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hugo W, Zaretsky JM, Sun L, et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 2016;165:35-44. 10.1016/j.cell.2016.02.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin AJ, Roach M, Bradley J, et al. Combining stereotactic body radiation therapy with immunotherapy: current data and future directions. Transl Lung Cancer Res 2019;8:107-15. 10.21037/tlcr.2018.08.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang AC, Postow MA, Orlowski RJ, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017;545:60-5. 10.1038/nature22079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharabi AB, Lim M, DeWeese TL, et al. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol 2015;16:e498-509. 10.1016/S1470-2045(15)00007-8 [DOI] [PubMed] [Google Scholar]
- 22.Tang C, Wang X, Soh H, et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res 2014;2:831-8. 10.1158/2326-6066.CIR-14-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 2013;105:256-65. 10.1093/jnci/djs629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strøm HH, Bremnes RM, Sundstrøm SH, et al. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer 2013;109:1467-75. 10.1038/bjc.2013.466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ricciuti B, Genova C, Bassanelli M, et al. Safety and Efficacy of Nivolumab in Patients With Advanced Non-small-cell Lung Cancer Treated Beyond Progression. Clin Lung Cancer 2019;20:178-185.e2. 10.1016/j.cllc.2019.02.001 [DOI] [PubMed] [Google Scholar]
- 26.Schoenfeld AJ, Rizvi H, Memon D, et al. Acquired resistance to PD-1 blockade in NSCLC. J Clin Oncol 2020;38:9621. 10.1200/JCO.2020.38.15_suppl.9621 [DOI] [Google Scholar]
- 27.Ramadan S, Quan K, Schnarr K, et al. Impact of stereotactic body radiotherapy (SBRT) in oligoprogressive metastatic disease. Acta Oncol 2022;61:705-13. 10.1080/0284186X.2022.2063067 [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, Wei L, Li J, et al. Combing stereotactic body radiotherapy with checkpoint inhibitors after oligoprogression in advanced non-small cell lung cancer. Transl Lung Cancer Res 2021;10:4368-79. 10.21037/tlcr-21-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol 2012;7:1807-14. 10.1097/JTO.0b013e3182745948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gan GN, Weickhardt AJ, Scheier B, et al. Stereotactic radiation therapy can safely and durably control sites of extra-central nervous system oligoprogressive disease in anaplastic lymphoma kinase-positive lung cancer patients receiving crizotinib. Int J Radiat Oncol Biol Phys 2014;88:892-8. 10.1016/j.ijrobp.2013.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan OSH, Lee VHF, Mok TSK, et al. The Role of Radiotherapy in Epidermal Growth Factor Receptor Mutation-positive Patients with Oligoprogression: A Matched-cohort Analysis. Clin Oncol (R Coll Radiol) 2017;29:568-75. 10.1016/j.clon.2017.04.035 [DOI] [PubMed] [Google Scholar]
- 32.Merino Lara T, Helou J, et al. Multisite stereotactic body radiotherapy for metastatic non-small-cell lung cancer: Delaying the need to start or change systemic therapy? Lung Cancer 2018;124:219-26. 10.1016/j.lungcan.2018.08.005 [DOI] [PubMed] [Google Scholar]
- 33.Jiang T, Chu Q, Wang H, et al. EGFR-TKIs plus local therapy demonstrated survival benefit than EGFR-TKIs alone in EGFR-mutant NSCLC patients with oligometastatic or oligoprogressive liver metastases. Int J Cancer 2019;144:2605-12. 10.1002/ijc.31962 [DOI] [PubMed] [Google Scholar]
- 34.Borghetti P, Bonù ML, Giubbolini R, et al. Concomitant radiotherapy and TKI in metastatic EGFR- or ALK-mutated non-small cell lung cancer: a multicentric analysis on behalf of AIRO lung cancer study group. Radiol Med 2019;124:662-70. 10.1007/s11547-019-00999-w [DOI] [PubMed] [Google Scholar]
- 35.Santarpia M, Altavilla G, Borsellino N, et al. High-dose Radiotherapy for Oligo-progressive NSCLC Receiving EGFR Tyrosine Kinase Inhibitors: Real World Data. In Vivo 2020;34:2009-14. 10.21873/invivo.11999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kagawa Y, Furuta H, Uemura T, et al. Efficacy of local therapy for oligoprogressive disease after programmed cell death 1 blockade in advanced non-small cell lung cancer. Cancer Sci 2020;111:4442-52. 10.1111/cas.14605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buglione M, Jereczek-Fossa BA, Bonù ML, et al. Radiosurgery and fractionated stereotactic radiotherapy in oligometastatic/oligoprogressive non-small cell lung cancer patients: Results of a multi-institutional series of 198 patients treated with "curative" intent. Lung Cancer 2020;141:1-8. 10.1016/j.lungcan.2019.12.019 [DOI] [PubMed] [Google Scholar]
- 38.Mahmood U, Huynh MA, Killoran JH, et al. Retrospective Review of Outcomes After Radiation Therapy for Oligoprogressive Disease on Immune Checkpoint Blockade. Int J Radiat Oncol Biol Phys 2022;114:666-75. 10.1016/j.ijrobp.2022.05.008 [DOI] [PubMed] [Google Scholar]
- 39.Hosoya K, Fujimoto D, Morimoto T, et al. Clinical factors associated with shorter durable response, and patterns of acquired resistance to first-line pembrolizumab monotherapy in PD-L1-positive non-small-cell lung cancer patients: a retrospective multicenter study. BMC Cancer 2021;21:346. 10.1186/s12885-021-08048-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as