Abstract
Objective: To explore the risk factors for ventricular arrhythmia after percutaneous coronary intervention (PCI) in elderly patients with acute myocardial infarction (AMI). Methods: A retrospective cohort of 201 elderly AMI patients who underwent PCI in the emergency department of No. 215 Hospital of Shaanxi Nuclear Industry from April 2020 to January 2023 was analyzed. The patients were randomly divided into a training set (n=134) for model development and a test set (n=67) for model validation. The training set was divided into a ventricular arrhythmia group (n=51) and a non-ventricular arrhythmia group (n=83), based on the occurrence of ventricular arrhythmia post-PCI. The factors affecting ventricular arrhythmias were analyzed by logistic regression and Lasso regression models.Results: Lasso regression screened 12 characteristic factors at λ=0.1 se. In the training set, the area under the ROC curve (AUC) of the Lasso model for predicting ventricular arrhythmia was 0.954, which was significantly higher than 0.826 for the Logistic model (P < 0.001). In the test set, the AUC of the Lasso model was 0.962, which was also significantly higher than 0.825 for the Logistic model (P=0.003). Conclusion: Compared to the logistic regression model, the Lasso regression model can more accurately predict the occurrence of ventricular arrhythmia after PCI in elderly AMI patients. The Lasso regression model constructed in this study can provide a reference for the clinical identification of high-risk elderly AMI patients and the development of targeted monitoring and treatment.
Keywords: Acute myocardial infarction, percutaneous coronary intervention, ventricular arrhythmia, risk factors
Introduction
Acute ST-segment elevation myocardial infarction (STEMI) is characterized by rapid onset, high lethality, and poor prognosis [1]. For STEMI patients, percutaneous coronary intervention (PCI) is crucial for salvaging ischemic myocardium and preventing ischemia-reperfusion injury [2]. PCI can effectively alleviate coronary stenosis, restore blood supply to the myocardium, improve perfusion to the ischemic myocardium, and enhance long-term prognosis for patients [3]. However, even after PCI, reperfusion-induced malignant ventricular arrhythmias (including ventricular tachycardia and ventricular fibrillation) remain a leading cause of death in STEMI patients [4]. Following PCI, accumulation of inflammatory mediators in the damaged vascular endothelium contributes to arrhythmogenesis by lowering the arrhythmia threshold [5].
Ventricular arrhythmia is a severe complication after STEMI, leading to hemodynamic changes, syncope, and cardiac arrest [5]. Most ventricular arrhythmias occur within 48 hours after the onset of STEMI symptoms and are a common cause of cardiac arrest in hospitalized patients [6]. Despite improvements in STEMI reperfusion therapies, ventricular arrhythmias continue to contribute to high in-hospital mortality rates [7]. Patients who develop ventricular arrhythmias have worse clinical outcome compared to those who do not, with a risk of in-hospital cardiac death as high as 31% and a significantly lower 30-day survival rate [8]. However, the impact of ventricular arrhythmia may be underestimated, as it often leads to sudden and unexpected cardiac arrest and is frequently recognized as the primary result of myocardial infarction. Therefore, it is crucial to increase awareness of ventricular arrhythmia and implement effective preventive measures in the early stages of STEMI to improve patient survival [9]. Early identification and intervention for STEMI patients at high risk of ventricular arrhythmia is currently a pressing challenge. Reported risk factors for ventricular arrhythmia in STEMI patients include early hospital admission, smoking, male sex, and younger age. However, there is a lack of simple and effective individualized risk assessment tools [10]. Scoring models such as Thrombolysis in Myocardial Infarction (TIMI) and Global Registry of Acute Coronary Events (GRACE) have been developed to assess long-term mortality risk rather than early ventricular arrhythmias. As a result, practical and straightforward risk prediction models for early-stage ventricular arrhythmias in STEMI patients are scarce, both in China and abroad.
In this study, we reviewed the medical records of STEMI patients at No. 215 Hospital of Shaanxi Nuclear Industry. We synthesized the risk factors reported in the literature that affect ventricular arrhythmia and investigated the risk factors associated with the occurrence of ventricular arrhythmia during hospitalization in STEMI patients. Additionally, we compared the predictive performances for ventricular arrhythmias between the two risk models that were constructed based on Logistic regression and Lasso regression. The findings of this study can guide clinicians in adopting appropriate strategies to reduce the occurrence of ventricular arrhythmia.
Materials and methods
Sample size calculation
Based on previous literature [4], the prevalence of patients with ventricular arrhythmias is about 25% to 40%, with a mean prevalence of 32.5%. Assuming an optimal effective threshold of 10%, a significance level (α) of 0.05, and a power (1-β) of 0.95, the sample size formula suggests that a total of 83 cases are needed. Considering a potential 10% loss to follow-up, the required sample size was increased to approximately 92.13 cases. The actual number of cases will depend on the availability of clinical collections.
Clinical information
The clinical data of elderly STEMI patients who underwent PCI in the Emergency Department of No. 215 Hospital of Shaanxi Nuclear Industry from April 2020 to January 2023 were retrospectively collected. The study was approved by the Medical Ethics Committee of No. 215 Hospital of Shaanxi Nuclear Industry.
Inclusion and exclusion criteria
Inclusion criteria: ① Patients meeting the diagnostic criteria for a myocardial infarction (AMI) [11]; ② Patients with an age ≥ 60 years; ③ Patients who received emergency PCI treatment; and ④ Patients with complete clinical data.
Exclusion criteria: ① Patients with concurrent atrial fibrillation and atrioventricular block; ② Patients with presence of acute cerebrovascular disease, including acute cerebral ischemia; ③ Patients with recent use of oral antiarrhythmic drugs; ④ Patients with evidence of impaired liver or kidney function as indicated by abnormal liver and kidney function tests.
Training set and test set definition and treatment
A total of 264 patients were initially considered, and after applying the inclusion and exclusion criteria, 201 eligible patients were included. The patients were randomly divided into a training set and a test set using a table of randomized numbers, ensuring a balanced distribution of characteristics in each group. This approach was critical for developing and validating our predictive models for postoperative ventricular arrhythmias.
The training set (n=134) comprised approximately two-thirds of the total sample, and its primary function was to develop the predictive model. Within this group, patients were further categorized into either a ventricular arrhythmia group (n=51) or a non-ventricular arrhythmia group (n=83) based on the development of ventricular arrhythmia postoperatively. This categorization facilitated a detailed analysis of the risk factors for ventricular arrhythmia and their characteristics. We employed various statistical methods, including logistic regression and Lasso regression, to identify significant predictors and construct a predictive model. The test set (n=67) was consisted of the remaining one-third of the patients, and its function was to validate the predictive model developed from the training set. This group included 26 patients with ventricular arrhythmias and 41 patients without. The efficacy of risk models constructed based on Logistic regression and Lasso regression was assessed using an ROC curve and compared using Delong test.
Clinical data collection
Patients’ clinical data and laboratory values were collected through electronic medical records. The clinical data included age, gender, BMI (body mass index), history of smoking, history of hypertension, history of diabetes mellitus, history of hyperlipidemia, history of myocardial infarction, electrocardiogram, TIMI classification [12], Killip Class IV [13], and time from onset to PCI. Laboratory tests were performed at the time of admission, including SBP (systolic blood pressure), DBP (diastolic blood pressure), TC (total cholesterol), TG (triglycerides), HDL-C (high-density lipoprotein cholesterol), LDL-C (low-density lipoprotein cholesterol), FPG (fasting plasma glucose), SCr (serum creatinine), UA (uric acid), cTnI (cardiac troponin I), K (kalium), NT-proBNP (N-terminal pro-b-type natriuretic peptide), WBC (white blood cell count), and LYM (lymphocyte count).
Measurement of results
1. Comparison of baseline information between patients in the training and test sets was conducted. 2. Risk factors affecting patients’ ventricular arrhythmia in the training set were analyzed using univariate and multivariate logistic analysis. 3. Factors influencing patients’ ventricular arrhythmia were identified through Lasso regression screening. 4. Two prediction models were developed based on the results of logistic regression and Lasso regression, respectively. 5. The predictive efficacy of each of the models was validated separately on the training set and test set (Figure 1).
Figure 1.

Patient screening flowchart.
Statistical analysis
SPSS 26.0 was used for data analysis. Counted data were expressed as a rate (%) and compared using chi-square test. For measured data, the distribution of data was assessed by the Kolmogorov-Smirnov test. Data that conformed to normal distribution were expressed as Mean ± SD and analyzed by independent samples t-test. Data not conforming to a normal distribution were described as quartiles P50 (P25, P75). The critical variables for predicting postoperative ventricular arrhythmia were identified by both Logistic regression and Lasso regression, and the corresponding risk model was established. The clinical value of the two models for predicting ventricular arrhythmias in patients was analyzed using ROC (receiver operating characteristics) curve. A DeLong test was used to compare the effectiveness of the two risk models. A difference was considered significant when P < 0.05.
Results
Comparison of baseline information of patients between the training and test sets
The baseline data of the two groups of patients were compared, and the results showed that there were no significant differences in terms of age, gender, BMI, SBP, DBP, history of smoking, history of hypertension, history of diabetes mellitus, history of hyperlipidemia, history of myocardial infarction, electrocardiogram, TIMI classification, Killip class IV, onset to PCI treatment time, TC, TG, HDL-C, LDL-C, FPG, SCr, UA, cTnI, K, NT-proBNP, WBC, or LYM (all P > 0.05, Table 1).
Table 1.
Comparison of baseline data between training and test sets
| Variable | Training set (n=134) | Test set (n=67) | t/x2/Z value | P-value |
|---|---|---|---|---|
| Age | 72.33±9.29 | 70.37±9.19 | 1.415 | 0.159 |
| Gender | ||||
| Male | 71 | 43 | 0.039 | 0.844 |
| Female | 53 | 34 | ||
| BMI (kg/m2) | 25.76±3.35 | 24.73±3.62 | 1.944 | 0.054 |
| SBP (mm Hg) | 129.00 [117.33, 149.18] | 125.00 [115.40, 149.70] | 4644.000 | 0.691 |
| DBP (mm Hg) | 68.69±10.68 | 66.09±11.31 | 1.560 | 0.121 |
| Smoking history | ||||
| Yes | 65 | 36 | 0.610 | 0.435 |
| No | 59 | 41 | ||
| History of hypertension | ||||
| Yes | 62 | 35 | 0.393 | 0.531 |
| No | 62 | 42 | ||
| History of diabetes | ||||
| Yes | 18 | 13 | 0.204 | 0.651 |
| No | 106 | 64 | ||
| History of hyperlipidemia | ||||
| Yes | 71 | 47 | 0.280 | 0.597 |
| No | 53 | 30 | ||
| History of myocardial infarction | ||||
| Yes | 9 | 8 | 0.602 | 0.438 |
| No | 115 | 69 | ||
| Electrocardiography | ||||
| J-wave | 69 | 52 | 2.802 | 0.094 |
| (sth. or sb) else | 55 | 25 | ||
| TIMI classification | ||||
| 0 level | 18 | 18 | 2.537 | 0.111 |
| Class I-III | 106 | 59 | ||
| Killip Level IV | ||||
| Be | 9 | 12 | 3.520 | 0.061 |
| Clogged | 115 | 65 | ||
| Time from onset to PCI | ||||
| ≥ 6 h | 31 | 28 | 3.427 | 0.064 |
| < 6 h | 93 | 49 | ||
| TC (mmol/L) | 4.60 [4.10, 5.00] | 4.50 [3.95, 5.00] | 4664.500 | 0.652 |
| TG (mmol/L) | 1.92±0.55 | 1.95±0.52 | -0.321 | 0.749 |
| HDL-C (mmol/L) | 1.10 [0.90, 1.20] | 1.20 [0.95, 1.30] | 3903.000 | 0.129 |
| LDL-C (mmol/L) | 2.68±0.75 | 2.49±0.84 | 1.558 | 0.122 |
| FPG (mmol/L) | 6.39±1.73 | 6.66±1.53 | -1.102 | 0.272 |
| SCr (µmol/L) | 77.93±8.08 | 78.86±7.71 | -0.796 | 0.427 |
| UA (µmol/L) | 382.65±114.67 | 380.09±101.82 | 0.161 | 0.872 |
| cTnI (µg/L) | 4.61±1.69 | 4.51±1.64 | 0.385 | 0.701 |
| K (mmol/L) | 6.55±1.90 | 6.36±1.61 | 0.744 | 0.458 |
| NT-proBNP (pg/mL) | 606.38±266.61 | 707.52±306.68 | -2.300 | 0.023 |
| WBC (×10^9/L) | 10.65±4.01 | 10.61±3.38 | 0.077 | 0.938 |
| LYM (×10^9/L) | 2.22±0.76 | 2.32±0.70 | -0.984 | 0.327 |
Note: BMI, Body Mass Index; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; TC, Total Cholesterol; TG, Triglycerides; HDL-C, High-Density Lipoprotein Cholesterol; LDL-C, Low-Density Lipoprotein Cholesterol; FPG, Fasting Plasma Glucose; SCr, Serum Creatinine; UA, Uric Acid; cTnI, cardiac Troponin I; K, Kalium; NT-proBNP, N-Terminal pro-b-type Natriuretic Peptide; WBC, White Blood Cell count; LYM, Lymphocyte count; PCI, Percutaneous Coronary Intervention; TIMI, Thrombolysis In Myocardial Infarction.
Screening of risk factors for ventricular arrhythmia by Logistic regression in the training set
We compared the baseline data of patients with ventricular arrhythmias to those without in the training set. The results revealed some interesting findings. First, we observed that the numbers of patients with electrocardiographic J waves, TIMI classification grade 0, Killip grade IV, and time from onset to PCI treatment ≥ 6 h in the ventricular arrhythmia group were significantly higher compared to the non-ventricular arrhythmia group (all P < 0.05, Table 2). Furthermore, we found that the level of K was significantly lower in patients with ventricular arrhythmia compared to those with non-ventricular arrhythmia (P < 0.001, Table 2).
Table 2.
Comparison of baseline data of patients with and without ventricular arrhythmia in the training set
| Variable | Ventricular arrhythmia group (n=51) | Non-ventricular arrhythmia group (n=83) | t/x2/Z value | P-value |
|---|---|---|---|---|
| Age | 71.08±10.18 | 70.69±7.68 | 0.237 | 0.813 |
| Gender | ||||
| Male | 29 | 46 | 0.027 | 0.870 |
| Female | 22 | 37 | ||
| BMI (kg/m2) | 25.59±3.24 | 25.57±3.61 | 0.035 | 0.972 |
| SBP (mm Hg) | 133.50 [120.70, 150.70] | 125.70 [116.35, 144.70] | 2468.000 | 0.108 |
| DBP (mm Hg) | 68.61±10.43 | 67.58±10.54 | 0.554 | 0.581 |
| Smoking history | ||||
| Yes | 24 | 43 | 0.285 | 0.594 |
| No | 27 | 40 | ||
| History of hypertension | ||||
| Yes | 26 | 39 | 0.202 | 0.653 |
| No | 25 | 44 | ||
| History of diabetes | ||||
| Yes | 11 | 11 | 1.592 | 0.207 |
| No | 40 | 72 | ||
| History of hyperlipidemia | ||||
| Yes | 35 | 43 | 3.674 | 0.055 |
| No | 16 | 40 | ||
| History of myocardial infarction | ||||
| Yes | 6 | 8 | 0.153 | 0.696 |
| No | 45 | 75 | ||
| Electrocardiography | ||||
| J-wave | 43 | 36 | 21.881 | < 0.001 |
| else | 8 | 47 | ||
| TIMI classification | ||||
| 0 level | 16 | 11 | 6.446 | 0.011 |
| I-III level | 35 | 72 | ||
| Killip Level IV | ||||
| Yes | 11 | 3 | 10.884 | < 0.001 |
| No | 40 | 80 | ||
| Time from onset to PCI | ||||
| ≥ 6 h | 24 | 16 | 11.643 | < 0.001 |
| < 6 h | 27 | 67 | ||
| TC (mmol/L) | 4.50 [4.00, 5.00] | 4.60 [4.20, 5.00] | 2042.000 | 0.734 |
| TG (mmol/L) | 1.97±0.57 | 1.94±0.54 | 0.323 | 0.747 |
| HDL-C (mmol/L) | 1.00 [0.90, 1.30] | 1.10 [0.90, 1.30] | 1922.500 | 0.371 |
| LDL-C (mmol/L) | 2.66±0.78 | 2.69±0.78 | -0.167 | 0.868 |
| FPG (mmol/L) | 6.64±1.78 | 6.58±1.68 | 0.188 | 0.851 |
| SCr (µmol/L) | 78.30 [75.35, 84.00] | 77.20 [71.65, 81.80] | 2460.000 | 0.116 |
| UA (µmol/L) | 377.19±111.26 | 383.75±116.00 | -0.326 | 0.745 |
| cTnI (µg/L) | 4.85±1.63 | 4.55±1.76 | 1.015 | 0.312 |
| K (mmol/L) | 5.60±1.63 | 6.99±1.69 | -4.696 | < 0.001 |
| NT-proBNP (pg/mL) | 590.45±248.23 | 660.22±289.96 | -1.480 | 0.141 |
| WBC (×10^9/L) | 10.90±3.65 | 10.37±3.74 | 0.813 | 0.418 |
| LYM (×10^9/L) | 2.26±0.81 | 2.19±0.69 | 0.529 | 0.598 |
Note: BMI, Body Mass Index; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; TC, Total Cholesterol; TG, Triglycerides; HDL-C, High-Density Lipoprotein Cholesterol; LDL-C, Low-Density Lipoprotein Cholesterol; FPG, Fasting Plasma Glucose; SCr, Serum Creatinine; UA, Uric Acid; cTnI, cardiac Troponin I; K, Kalium; NT-proBNP, N-Terminal pro-b-type Natriuretic Peptide; WBC, White Blood Cell count; LYM, Lymphocyte count; PCI, Percutaneous Coronary Intervention; TIMI, Thrombolysis In Myocardial Infarction.
In an effort to identify independent risk factors for ventricular arrhythmia, variables showing differences in univariate analysis were further examined using logistic regression after dichotomization based on predefined cutoffs (Table 3). This multifactorial analysis determined that electrocardiogram abnormalities, Killip class IV, and potassium levels independently contributed to the risk of developing ventricular arrhythmia (all P < 0.05, Table 4).
Table 3.
Assignment table
| Variable | Assignment of values |
|---|---|
| Electrocardiography | J-wave =1, other =0 |
| TIMI classification | Class 0=1, Class I-III=0 |
| Killip Level IV | Yes =1, No =0 |
| Time from onset to PCI treatment | ≥ 6 h =1, < 6=0 |
| K (mmol/L) | ≥ 6.35=0, < 6.35=1 |
Note: K, Kalium; PCI, Percutaneous Coronary Intervention; TIMI, Thrombolysis in Myocardial Infarction.
Table 4.
Risk factors for ventricular arrhythmia in training set
| Variable | β | Standard error | Chi-square value | P-value | OR | 95% CI | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lower limit | Limit | ||||||
| Electrocardiography | 1.868 | 0.556 | 11.305 | 0.001 | 6.475 | 2.179 | 19.235 |
| TIMI classification | -0.952 | 0.840 | 1.286 | 0.257 | 0.386 | 0.074 | 2.001 |
| Killip Level IV | 1.866 | 0.908 | 4.224 | 0.040 | 6.461 | 1.090 | 38.287 |
| Time from onset to PCI | 0.367 | 0.697 | 0.276 | 0.599 | 1.443 | 0.368 | 5.659 |
| K | -0.527 | 0.139 | 14.279 | < 0.001 | 0.59 | 0.449 | 0.776 |
Note: K, Kalium; PCI, Percutaneous Coronary Intervention; TIMI, Thrombolysis in Myocardial Infarction.
Lasso regression analysis for identifying ventricular arrhythmia risk factors
In our investigation, Lasso regression was used to screen the factors affecting the occurrence of ventricular arrhythmia, and found that 21 distinct factors existed at λ=.min, while 12 typical elements existed at λ=.1 se (Figure 2A). Considering the model generalization performance, we chose the 12 distinctive factors at λ=.1 se for model construction, including DBP, smoking history, history of diabetes, history of myocardial infarction, electrocardiogram, Killip class IV, time from onset to PCI treatment, HDL-C, SCr, K and NT-proBNP (Figure 2B).
Figure 2.
Number of features and coefficient paths in Lasso regression. A. Number of features selected for different regularization strengths (λ values). B. Coefficient paths of the final 12 feature factors selected.
Construction of a risk models for ventricular arrhythmia in training set
Two risk models were constructed, using logistic and Lasso regression. The risk score based on logistic regression (herein referred as Logistic regression risk score) was calculated as follows: ECG * 1.868 + Killip class IV * 1.866 + K * -0.527. The risk score based on Lasso regression (herein referred as Lasso regression risk score) was calculated as follows: 0.438166736 + DBP * 0.002706921 + Smoking history * -3.124405182 + History of diabetes * -0.502621028 + History of myocardial infarction * -0.812023095 + Electrocardiogram * 2.949793237 + Killip class IV * 1.785153882 + Duration of onset of disease to PCI treatment * 1.699460129 + TC * -0.04767599 + HDL-C * -0.063388104 + SCr * 0.01033912 + K * -0.3454136 + NT-proBNP * -0.000590218. Comparing patients in the training set, we found that those in the ventricular arrhythmia group had significantly higher risk scores than those in the non-ventricular arrhythmia group (Figure 3A). ROC curve analysis showed that the areas under the curve (AUC) of the risk score of both models for ventricular arrhythmia were 0.826 and 0.954 in the training set, respectively (Figure 3B).
Figure 3.

Risk scores and ROC curves for the two risk models in training set. A. Comparison of risk scores between the two models. B. ROC curve of the risk scores based on the two models. Note: a denotes the ventricular arrhythmia group, b represents the non-ventricular arrhythmia group, ***P < 0.001, ROC, Receiver Operating Characteristics.
Validation of risk models in test set
The generalizability of the two models was validated using the test set. By comparison, it was found that the patients in the ventricular arrhythmia group in the test set had significantly higher ventricular arrhythmia risk scores inboth models than those in the non-ventricular arrhythmia group (Figure 4A). The AUC of risk score of both models for ventricular arrhythmia was 0.825 and 0.962, respectively (Figure 4B).
Figure 4.
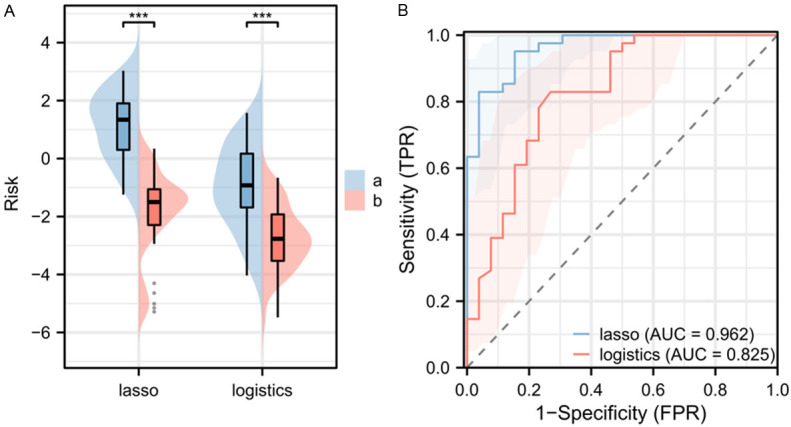
Risk scores and ROC curves of the two risk models in the test set. A. Comparison of risk scores between the two models. B. ROC curve of the risk scores based on the two models. Note: a indicates ventricular arrhythmia group, b indicates non-ventricular arrhythmia group, ***P < 0.001, ROC, Receiver Operating Characteristic.
Evaluation of the effectiveness of logistics and Lasso models
To further compare the predictive efficiency of the two models, we compared the AUCs of both models in the test set and training set by the Delong test. The findings indicated that the AUC for the Lasso model was significantly superior to that of the logistic model in both the training and test sets (both P < 0.05, Table 5).
Table 5.
Delong test comparing the effectiveness of logistic and Lasso model
| Z-value | P-value | AUC difference | Standard error margin | 95% CI | |
|---|---|---|---|---|---|
| Lasso-Logistic (training set) | 4.972 | < 0.001 | 0.129 | 0.224 | 0.078-0.179 |
| Lasso-Logistic (test set) | 2.984 | 0.003 | 0.136 | 0.271 | 0.047-0.226 |
Note: AUC, area under the curve.
Discussion
ST segment elevation myocardial infarction STEMI is marked by acute myocardial ischemic necrosis, typically triggered by a lesion in the coronary artery, which leads to a significant reduction or cessation of blood supply to the heart muscle and results in severe and prolonged acute ischemia of the affected myocardium [14,15]. STEMI is characterized by sudden onset, rapid progression, poor prognosis, and high mortality rate [16]. Early restoration of myocardial perfusion is crucial to protect the dying myocardium, prevent the expansion of infarct size, reduce the extent of myocardial ischemia, and preserve cardiac function, thereby playing a significant role in improving the prognosis of patients [17,18].
A meta-analysis of previous large clinical trials has indicated that the occurrence of ventricular arrhythmias in patients with AMI increases the 30-day mortality rate, significantly affects patient outcome and prognosis during hospitalization, and diminishes the benefit of clinical care [19]. Therefore, it is crucial to develop an accurate risk prediction model for ventricular arrhythmia. Such a model would enable more precise treatment and prognostic evaluation of STEMI patients. In our study, the training set consisted of 134 cases, out of which 51 elderly STEMI patients developed ventricular arrhythmia after PCI, resulting in a prevalence rate of 38.06%. This aligns with previous studies. Through multivariate logistic regression analysis, we identified ECG, Killip class IV, and K as independent risk factors for ventricular arrhythmia in STEMI patients. The presence of J waves on the ECG indicates cardiac electrophysiologic instability and increases the risk of sudden death and ventricular arrhythmia [20]. Furthermore, it was discovered that frequent ventricular arrhythmia is a significant health concern even in middle-aged and elderly populations without significant heart disease. Patients classified as Killip class IV exhibit severe heart failure and impaired cardiac function, which significantly raises the risk of ventricular arrhythmia after PCI. Studies have demonstrated that a high Killip class independently predicts mortality and major adverse cardiovascular events in AMI patients upon admission [21]. Additionally, elevated blood potassium levels interfere with the electrical signaling function of the cardiac myocardium, thereby increasing the risk of arrhythmia. Hyperkalemia has also been associated with various health problems, including cardiac excitability effects, peripheral neuropathy, and renal tubular acidosis [22]. Therefore, recognizing these risk factors is critical in the management of elderly STEMI patients to prevent fatal complications that can follow ventricular arrhythmia.
In this study, we constructed a risk prediction model based on logistic regression with an AUC of 0.826 in predicting ventricular arrhythmias in STEMI patients. Similarly, the AUC in our validation set was 0.825, indicating that the risk score based on logistic regression is highly effective in predicting post-emergency PCI ventricular arrhythmia in elderly patients. Sun et al. [23] reported an AUC of 0.815 in their training set model and an AUC of 0.755 for the validation set model. Another study by Sun [24] also constructed a logistic regression predictive model for ventricular arrhythmia in 2649 AMI patients, identifying Killip classification ≥ grade 3, randomized blood glucose > 11.1 mmol/L, LVEF < 50%, and creatinine > 100 µmol/L were associated with ventricular arrhythmias, and the model had an AUC of 0.779 for predicting ventricular arrhythmias. These studies collectively demonstrate the high efficiency and robustness of the logistic regression models in predicting ventricular arrhythmia in elderly patients with AMI undergoing emergency PCI.
Although logistic regression models have shown considerable effectiveness and robustness in predicting ventricular arrhythmia in elderly STEMI patients undergoing emergency PCI, they may not fully capture the complex and nonlinear relationships associated with ventricular arrhythmia. Recently, Lasso regression models have shown to be significantly more effective than logistic models in predicting various clinical outcomes. For instance, Shao et al. [25] demonstrated that the Lasso model outperformed the logistic model in predicting the progression to diabetic foot in elderly diabetic patients. The main advantage of the Lasso model lies in its ability to perform variable selection and regularization, making it particularly suitable for handling high-dimensional data and addressing multicollinearity issues. Due to these characteristics, the Lasso model offers greater flexibility and accuracy when analyzing complex clinical datasets [26,27]. In our study, we compared the prediction models constructed by Lasso regression and logistic regression and found that the Lasso model had a larger AUC than the logistic regression model in both the training and test sets. This suggests that the Lasso model is more valuable for predicting the occurrence of ventricular arrhythmias in patients with STEMI undergoing emergency PCI. Based on our findings, we believe that the superiority of Lasso regression over logistic regression may be partly attributed to its ability to screen relevant variables during the modeling process. The Lasso model identifies and retains the variables that have the most influence on the predictive target while excluding those that contribute less to the model. This approach not only simplifies the model but also enhances the accuracy and interpretability of the predictions.
This study also has limitations, such as small sample size and being a single center study. Future studies involving joint multicenter survey with expanded sample size should be planned to include more STEMI patients from different hospitals of various regions to enhance the robustness and representativeness of the study results. Furthermore, only variables at admission were considered in this study, and postoperative and follow-up indicators were not included. To construct a more comprehensive model, it is necessary to collect additional variables such as surgery-related indicators, discharge indicators, and follow-up data. Moreover, only two models, logistic regression and Lasso regression, were compared. Exploring additional machine learning models like random forest and neural networks can be planned to improve prediction outcome. By implementing these improvements, we aim to develop a ventricular arrhythmia risk prediction model for STEMI patients with a larger sample size, comprehensive consideration of variables, and an optimized statistical model to enhance the predictive accuracy and provide better guidance for clinical practice.
In conclusion, compared to the logistic regression model, the Lasso regression model can more accurately predict the occurrence of ventricular arrhythmia after PCI in elderly STEMI patients. The Lasso regression model constructed in this study can provide a reference for the clinical identification of high-risk elderly STEMI patients and the development of targeted monitoring and treatment strategies.
Disclosure of conflict of interest
None.
References
- 1.Buiten RA, Ploumen EH. Drug-eluting stents for ST-segment elevation myocardial infarction: extending the biodegradable versus durable polymer debate. Lancet. 2023;402:1942–1943. doi: 10.1016/S0140-6736(23)02297-3. [DOI] [PubMed] [Google Scholar]
- 2.Jollis JG, Granger CB, Zegre-Hemsey JK, Henry TD, Goyal A, Tamis-Holland JE, Roettig ML, Ali MJ, French WJ, Poudel R, Zhao J, Stone RH, Jacobs AK. Treatment time and in-hospital mortality among patients with ST-segment elevation myocardial infarction, 2018-2021. JAMA. 2022;328:2033–2040. doi: 10.1001/jama.2022.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Liang Z, Qin L, Wang M, Wang X, Zhang H, Liu Y, Li Y, Jia Z, Liu L, Zhang H, Luo J, Dong S, Guo J, Zhu H, Li S, Zheng H, Liu L, Wu Y, Zhong Y, Qiu M, Han Y, Stone GW. Bivalirudin plus a high-dose infusion versus heparin monotherapy in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: a randomised trial. Lancet. 2022;400:1847–1857. doi: 10.1016/S0140-6736(22)01999-7. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Wei L, Wu Y, Yan J, Zhao L, Yue X, Gao C. ST-segment elevation predicts the occurrence of malignant ventricular arrhythmia events in patients with acute ST-segment elevation myocardial infarction. BMC Cardiovasc Disord. 2023;23:61. doi: 10.1186/s12872-023-03099-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pu J, Ding S, Ge H, Han Y, Guo J, Lin R, Su X, Zhang H, Chen L, He B EARLY-MYO Investigators. Efficacy and safety of a pharmaco-invasive strategy with half-dose alteplase versus primary angioplasty in ST-segment-elevation myocardial infarction: EARLY-MYO trial (early routine catheterization after alteplase fibrinolysis versus primary PCI in acute ST-segment-elevation myocardial infarction) Circulation. 2017;136:1462–1473. doi: 10.1161/CIRCULATIONAHA.117.030582. [DOI] [PubMed] [Google Scholar]
- 6.Perera D, Morgan HP, Ryan M, Dodd M, Clayton T, O’Kane PD, Greenwood JP, Walsh SJ, Weerackody R, McDiarmid A, Amin-Youssef G, Strange J, Modi B, Lockie T, Hogrefe K, Ahmed FZ, Behan M, Jenkins N, Abdelaal E, Anderson M, Watkins S, Evans R, Rinaldi CA, Petrie MC REVIVED-BCIS2 Investigators. Arrhythmia and death following percutaneous revascularization in ischemic left ventricular dysfunction: prespecified analyses from the REVIVED-BCIS2 trial. Circulation. 2023;148:862–871. doi: 10.1161/CIRCULATIONAHA.123.065300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander C, Bishop MJ, Gilchrist RJ, Burton FL, Smith GL, Myles RC. Initiation of ventricular arrhythmia in the acquired long QT syndrome. Cardiovasc Res. 2023;119:465–476. doi: 10.1093/cvr/cvac103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takada T, Shishido K, Hayashi T, Yokota S, Miyashita H, Yokoyama H, Nishimoto T, Ochiai T, Moriyama N, Tobita K, Mizuno S, Yamanaka F, Murakami M, Tanaka Y, Takahashi S, Saito S. Impact of late ventricular arrhythmias on cardiac mortality in patients with acute myocardial infarction. J Interv Cardiol. 2019;2019:5345178. doi: 10.1155/2019/5345178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cadrin-Tourigny J, Bosman LP, Nozza A, Wang W, Tadros R, Bhonsale A, Bourfiss M, Fortier A, Lie OH, Saguner AM, Svensson A, Andorin A, Tichnell C, Murray B, Zeppenfeld K, van den Berg MP, Asselbergs FW, Wilde AAM, Krahn AD, Talajic M, Rivard L, Chelko S, Zimmerman SL, Kamel IR, Crosson JE, Judge DP, Yap SC, van der Heijden JF, Tandri H, Jongbloed JDH, Guertin MC, van Tintelen JP, Platonov PG, Duru F, Haugaa KH, Khairy P, Hauer RNW, Calkins H, Te Riele ASJM, James CA. A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. 2022;43:e1–e9. doi: 10.1093/eurheartj/ehac180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin WC, Hsiung MC, Yin WH, Tsao TP, Lai WT, Huang KC. Electrocardiography score for left ventricular systolic dysfunction in non-ST segment elevation acute coronary syndrome. Front Cardiovasc Med. 2022;8:764575. doi: 10.3389/fcvm.2021.764575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan S, Pol D, Splatt L, Abrahams T, Mydin M, Nelson AJ, Nicholls SJ, Brown AJ. Diagnostic accuracy of reperfusion criteria following fibrinolysis for ST-elevation myocardial infarction. AsiaIntervention. 2023;9:49–51. doi: 10.4244/AIJ-D-22-00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Şeker T, Türkoğlu C, Akkuş O, Gür M. The relationship between visible thrombus aspiration material with no-reflow and in-hospital mortality ratio in patients with anterior ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Turk Kardiyol Dern Ars. 2019;47:95–102. doi: 10.5543/tkda.2019.49940. [DOI] [PubMed] [Google Scholar]
- 13.Milwidsky A, Greidinger D, Frydman S, Hochstadt A, Ifrach-Kashtan N, Mizrachi M, Topilsky Y. Echocardiographic Killip classification. J Am Soc Echocardiogr. 2022;35:287–294. doi: 10.1016/j.echo.2021.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Jimenez JV, Banna S, Desai N. A peculiar ST elevation mimicking STEMI. JAMA Intern Med. 2023;183:158–159. doi: 10.1001/jamainternmed.2022.5070. [DOI] [PubMed] [Google Scholar]
- 15.Bouisset F, Gerbaud E, Bataille V, Coste P, Puymirat E, Belle L, Delmas C, Cayla G, Motreff P, Lemesle G, Aissaoui N, Blanchard D, Schiele F, Simon T, Danchin N, Ferrières J FAST-MI Investigators. Percutaneous myocardial revascularization in late-presenting patients with STEMI. J Am Coll Cardiol. 2021;78:1291–1305. doi: 10.1016/j.jacc.2021.07.039. [DOI] [PubMed] [Google Scholar]
- 16.Bates ER, Jacobs AK. Time to treatment in patients with STEMI. N Engl J Med. 2013;369:889–892. doi: 10.1056/NEJMp1308772. [DOI] [PubMed] [Google Scholar]
- 17.Lim GB. Sonothrombolysis improves PCI after STEMI. Nat Rev Cardiol. 2019;16:320. doi: 10.1038/s41569-019-0188-z. [DOI] [PubMed] [Google Scholar]
- 18.Rathod KS, Comer K, Casey-Gillman O, Moore L, Mills G, Ferguson G, Antoniou S, Patel R, Fhadil S, Damani T, Wright P, Ozkor M, Das D, Guttmann OP, Baumbach A, Archbold RA, Wragg A, Jain AK, Choudry FA, Mathur A, Jones DA. Early hospital discharge following PCI for patients with STEMI. J Am Coll Cardiol. 2021;78:2550–2560. doi: 10.1016/j.jacc.2021.09.1379. [DOI] [PubMed] [Google Scholar]
- 19.Dalager-Pedersen M, Sogaard M, Carl Schonheyder H, Nielsen H, Thomsen RW. Response to letter regarding article, “risk for myocardial infarction and stroke after community-acquired bacteremia: a 20-year population-based cohort study”. Circulation. 2015;131:e9. doi: 10.1161/CIRCULATIONAHA.114.012813. [DOI] [PubMed] [Google Scholar]
- 20.Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Frederiksen BS, Davanlou M, Hansen JF. Ventricular arrhythmias and risk of death and acute myocardial infarction in apparently healthy subjects of age >or=55 years. Am J Cardiol. 2006;97:1351–1357. doi: 10.1016/j.amjcard.2005.11.067. [DOI] [PubMed] [Google Scholar]
- 21.Del Buono MG, Montone RA, Rinaldi R, Gurgoglione FL, Meucci MC, Camilli M, Iannaccone G, Sanna T, Pedicino D, Trani C, Niccoli G, Crea F. Clinical predictors and prognostic role of high Killip class in patients with a first episode of anterior ST-segment elevation acute myocardial infarction. J Cardiovasc Med (Hagerstown) 2021;22:530–538. doi: 10.2459/JCM.0000000000001168. [DOI] [PubMed] [Google Scholar]
- 22.Hunter RW, Bailey MA. Hyperkalemia: pathophysiology, risk factors and consequences. Nephrol Dial Transplant. 2019;34(Suppl 3):iii2–iii11. doi: 10.1093/ndt/gfz206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun L, Han B, Wang Y, Zhu W, Jiang J, Zou A, Chi B, Mao L, Ji Y, Wang Q, Tang L. A new scoring system for predicting ventricular arrhythmia risk in patients with acute myocardial infarction. Clin Interv Aging. 2023;18:283–292. doi: 10.2147/CIA.S395121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun L, Mao L, Zou A, Chi B, Chen X, Ji Y, Jiang J, Zhou X, Wang Q. Development and validation of a clinical predictive model for the risk of malignant ventricular arrhythmia during hospitalization in patients with acute myocardial infarction. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021;33:438–442. doi: 10.3760/cma.j.cn121430-20201217-00760. [DOI] [PubMed] [Google Scholar]
- 25.Shao Z, Wang Z, Bi S, Zhang J. Establishment and validation of a nomogram for progression to diabetic foot ulcers in elderly diabetic patients. Front Endocrinol (Lausanne) 2023;14:1107830. doi: 10.3389/fendo.2023.1107830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan Y, Gong H, Hu J, Wu D, Zheng Z, Wang L, Lei C. Perioperative parameters-based prediction model for acute kidney injury in Chinese population following valvular surgery. Front Cardiovasc Med. 2023;10:1094997. doi: 10.3389/fcvm.2023.1094997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, He X, Bai Y, Du G, Cai M. Identification and validation of novel biomarkers affecting bladder cancer immunotherapy via machine learning and its association with M2 macrophages. Front Immunol. 2022;13:1051063. doi: 10.3389/fimmu.2022.1051063. [DOI] [PMC free article] [PubMed] [Google Scholar]



