Abstract
Objective: Formulate a gel and test its scientific efficacy for treating musculoskeletal ailments with or without phonophoresis. Methods: Gel was made from Jasminum sambac leaf extract (30:70 aqueous-methanolic). A pragmatic, community-based, double-blinded randomized clinical study (IRCT20230202057310N1) was undertaken on 380 pre-diagnosed individuals with 1st and 2nd-grade musculoskeletal injuries, divided into four parallel groups (n = 95 per group): Group I got phonophoresis-applied J. sambac 10% gel. Group II got phonophoresis-applied diclofenac diethylammonium 2% gel. J. sambac 10% gel was superficially massaged onto Group III. Group IV received a superficial massage with diclofenac diethylammonium 2% gel. Color, stability, pH, spreadability, beginning of pain relief, discomfort, stiffness, and activities of daily living were recorded using the Numeric Pain Rating Scale (NPRS) and Western Ontario and McMaster Universities Arthritis Index (WOMAC) Scale. Methods included phytochemical analysis, molecular docking, and antioxidant quantification using 2,2-diphenylpicrylhydrazyl (DPPH), nitric oxide (NO), and superoxide dismutase (SOD) tests. Results: J. sambac gel worked better than diclofenac gel in phonophoresis and massage, with regard to NPRS P<0.001, WOMAC pain P<0.001, WOMAC stiffness P<0.003, and WOMAC activities of daily living (ADLs) P<0.001. There were also significant differences in pain, stiffness, and ADLs. J. sambac showed significant (P<0.005-0.001) results. Conclusion: J. sambac gel relieved pain and inflammation in musculoskeletal injury patients. J. sambac gel is natural, cheap, and easy to make. Better drug absorption may explain the effectiveness of phonophoresis.
Keywords: Jasminum sambac gel, pain, inflammation, musculoskeletal injuries, phonophoresis
Introduction
Musculoskeletal health concerns the presentation of the locomotor system. Musculoskeletal disorders encompass more than 150 conditions characterized by damage to the muscles, joints, bones, and adjacent connective tissues, resulting in temporary or lifelong restrictions on working. Musculoskeletal conditions are typically characterized by pain (often persistent) and limitations in mobility and dexterity, decreasing the ability to work and participate in society [1]. Musculoskeletal conditions occur at all ages, from childhood to old age [2]. The number of people with musculoskeletal disorders related to functional restrictions is quickly increasing. They range from short-term, including strains, sprains, and fractures related to pain and restrictions in working, to long-term conditions, including osteoarthritis and low back pain [3].
Musculoskeletal injuries are a real public health problem globally, as they are the most common cause of long-term disability as well as poor performance in activities of daily living (ADLs), resulting in early retirement from work and decreased levels of comfort and ability to partake in society, mostly in low- and middle-income countries like Pakistan [1]. Low back pain is the single chief reason for disability in 160 countries. A recent Global Burden of Disease (GBD) study from 2019 shows that almost 1.71 billion people have musculoskeletal disorders. The World Health Organization (WHO) is responding to the increased burden of musculoskeletal issues by an increased number of programmatic areas [4]. Pain and inflammation are an increasing global public health concern. There are various types of musculoskeletal injuries (muscular and soft tissue injuries), like overuse injuries, compression injuries, sports injuries, and traumatic injuries, which are the most common causes of pain and long-term disabilities, as well as poor performance in activities of daily living (ADLs) [5].
Nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, and opioids are commonly used to treat symptoms of pain and inflammation [5]. These drugs also have adverse reactions like cardiovascular, gastrointestinal, renal, and hepatic toxicities [6-8]. When NSAIDs are given orally, they have various adverse effects, just as aspirin causes GI bleeding, paracetamol causes an increased risk of hepatotoxicity, and nimsulide causes an increased risk of cardiotoxicity [9]. Also, corticosteroids are the most commonly used drugs, orally or intravenously, for musculoskeletal issues, and these have adverse effects like osteoporosis, skeletal deformities, and muscle wasting. These drugs also burden the kidney in terms of excretion and cause nephrotoxicity [10]. If not used properly, these drugs cause more adverse effects than benefits, putting a substantial economic burden on public health. Putting drugs on the skin lowers the risks of intravenous treatment, keeps the liver from having to deal with removal, lowers the risk of an overdose or underdose, makes finishing easy, and allows for both local and systemic treatment [11]. Topical drugs’ pharmacologic effects will depend on several things, such as how much, how fast, and how deeply they penetrate the skin, as well as the drugs’ toxic effects on tissues that are still hidden [12]. So, making drugs that can be put on the skin is still very important for getting deeper skin tissues and having localised effects.
Therapeutic ultrasound is the most commonly used modality in physiotherapy clinics for its anti-inflammatory effect. Using the piezoelectric crystals in the transducer to do phonophoresis also makes it easier for topical drugs to enter the skin. The aqueous gel is commonly used as a coupling medium to reduce friction between the transducer and skin and enhance the penetration of sound waves into the skin [4,13].
The climatic conditions of Pakistan are very favorable for the growth of different types of medicinal plants, and some local manufacturers are also producing different herbal medicines on a commercial scale for export to foreign countries. The yearly income from these products is comparable to that of any international pharmaceutical company [14]. Moreover, due to the lack of proper techniques and multiple other insignificant approaches, scientific investigations into traditional practices have yet to be done in detail. However, further research on different medicinal herbs would significantly impact the cost of medicine in many countries, where the primary cost is spent on import taxes [15,16].
Jasminum sambac, commonly known as jasmine, belongs to the family Oleaceae. The entire plant is of great interest in traditional herbal use for treatment purposes. It has fragrant flowers and is one of the oldest cultivated plants by humans. Its native land is in subtropical regions, from where it has spread to other global regions [17]. Pharmacologic studies showed that it had effects on the gastrointestinal system [18], the central nervous system (CNS), the cardiovascular system (CVS), the peripheral nervous system (PNS), and the immune system. It also killed insects, lowered cholesterol, fought obesity, and was an antiseptic, antifungal, sedative, and tonic for the uterus. In brewed form, it helps prevent breast cancer and stops uterine bleeding. The roots of J. sambac are very beneficial for treating wounds and snake bites. Flowers and leaves possess decongestant and antipyretic properties. Moreover, due to their lovely fragrance, the essential oils of J. sambac are used to produce perfumes [19].
Various studies investigated the anti-inflammatory and analgesic activities of various parts of J. sambac, such as oils and different extracts of roots, flowers, and leaves. Most of these studies gave oral doses to animal models (references). Studies also revealed the analgesic and anti-inflammatory effects of the topical gel of the leaves of J. sambac, on the animal model [19,20]. However, no data exist on anti-inflammatory and analgesic activity in humans with gel or phonophoresis. Topical salicylate is commonly used to decrease pain and inflammation. Salicylate in combination with therapeutic ultrasound, in various studies revealed that salicylate moved into the subdermal tissues [12]. The presence of salicylate in the leaves of J. sambac was also reported in various studies [21].
As the climatic conditions of Pakistan are very favourable for the growth of different types of medicinal plants, J. sambac is widespread in Punjab, Pakistan. This formulation is more cost-effective, easy to formulate, and less toxic than other costly medicines. It was decided to test how well the gel of J. sambac reduced pain and inflammation when combined with superficial massage and phonophoresis. Implementing this affordable and justifiable strategy might decrease pain and inflammation, decrease long-term disabilities, and enhance performance in activities of daily living (ADLs). Decreasing musculoskeletal issues’ burden may strengthen orthopedic trauma care, especially in low- and middle-income countries.
Materials and methods
Drugs, chemicals, and instruments
Diclofenac diethyl ammonium 2% w/w was purchased from GlaxoSmithKline Pharmaceuticals (Pvt) Ltd., Pakistan. Doxorubicin was purchased from Indus Pharma Lahore. Methanol was purchased from Merck, Germany. Carbpol-940 was purchased from Duksan Pure Chemicals Co., Ltd., South Korea. Sodium benzoate, 2,2-diphenylpicrylhydrazyl (DPPH), nitric oxide (NO) assay, and superoxide dismutase Assay (SOD) triethanolamine, and glycerol were purchased from Sigma-Aldrich, Pakistan. Distilled water purchased from SARCO Chemicals, Multan, PU, Pakistan. Therapeutic ultrasound purchased from Enraf Nonius Sonoplus 590. All other chemicals and reagents used in this study were of pharmaceutical grade.
Collection of plant materials
J. sambac was obtained from the Muhammad Institute of Medical and Allied Sciences garden, Multan. An experienced taxonomist from Muhammad Nawaz Shareef University of Agriculture, Multan (R.R. Steward, F.W. Pak. 625-3) helped identify the plant. The plant’s newly grown leaves were left to undergo the process of shadow drying. Before the grinding process, the special herb grinder removed dirt and debris from the dried leaves, resulting in a coarsely powdered state. The hermetically sealed container was utilised for the conservation of pulverised botanical matter. The manufacture of the extract from the powdered material was carried out using a well-documented approach, which involved the maceration process in a mixture of water and methanol (70:30). The crude extract pool was evaporated on a rotatory evaporator at 37°C under low pressure until it reached a thick paste consistency. The resulting stock solutions were then stored in airtight jars in a lab refrigerator at -2°C [21-25]. The estimated 10% yield of extract was taken using the formula (Formula (1)):
Preliminary phytochemical evaluation
Phytochemical evaluation of major classes in aqueous methanolic leaf extract of J. sambac was evaluated using standard protocol [15,16].
High-performance liquid chromatography (HPLC) analysis
A binary solvent gradient system was used in HPLC with the C-18 column (250 mm internal diameter), which is suitable for isolating different constituents in 36 minutes. The flow rate was 0.0008 L/min, and 5 m was the film depth at a temperature of 30°C in the oven. Linalool, iso-quercetin, and β-sitosterol were prepared as a reference and purchased from Aldrich (St. Louis, USA). To achieve g/mL, the dilutions were prepared with methanol. The retention times of samples were compared to standards. The efficacy of HPLC-separated components was assessed using the separation factor and resolution [15,16].
Fourier transform infrared spectroscopy (FTIR)
J. sambac gel was used for FTIR analysis to document the functional groups present in leaves of J. sambac, based on peak portions with functional groups and their fingerprint characters. In this study, various functional groups were characterized as C-O, N = O, C = O, C-N, C-H, and C-O and identified at their respective absorption peaks. These functional groups have responsibility for the alkyl, or either anhydrite deoxyribose, esters, or alcoholic group formation [26].
Antioxidant activity
Antioxidant activity was performed using 2,2-diphenylpicrylhydrazyl (DPPH), Nitric oxide (NO) assay, and Superoxide Dismutase Assay (SOD).
DPPH assay
J. sambac aqueous methanolic leaf extract was diluted to make a final volume of 5 mL with different concentrations. Then, for 40 minutes, this mixture was stored in the dark. The stated solution’s 517 nm absorbance was measured using a spectrophotometer. Each study was done three times, and the percentage of inhibition in ascorbic acid equivalency was measured [27-29]. The equation below was used to compute the percentage of DPPH scavenging potential (Formula (2)):
Measurement of NO scavenging capacity
J. sambac was extracted using an aqueous-ethanolic solution at 10 mg/mL. Ascorbic acid and extract were diluted with distilled water to achieve 1000 and 2000 µg/mL concentrations, respectively. Experiment solutions were stored at a constant temperature of 4 degrees C. A freshly made Griess reagent was utilised in the process. To find the best concentration of extract (1000 and 2000 µg/mL), 0.5 mL of 10 mM sodium nitroprusside in phosphate-buffered saline was mixed with 1 mL of each concentration and left to sit at 25 degrees C for three hours. The extract was spiked with a freshly made Griess reagent of the same volume. The extracts were left out of the control samples, but the volume of buffer used was kept constant. There were adequate quantities of extracts in the various coloured tubes, but no sodium nitroprusside. A 96-well plate was loaded with 150 L of the reaction mixture. Based on our prior correspondence, a UV-Vis microplate reader (Alibaba, Hangzhou, China) was used to quantify the absorbance at 546 nm [27-29]. Using the following formula, this study determined the percentage of NO scavenging activity of ascorbic acid and extracts and the percentage of inhibition by the standard (Formula (3)):
Superoxide dismutase assay
Using a slightly altered version of the method reported, the herb’s capacity to scavenge superoxide anion radicals was determined. The PMS, NADH, and NBT systems were applied to generate superoxide radicals. Then we put the tested materials (50 µl) at different dilutions, along with 125 µl of NBT (300 M) and 125 µl of NADH (468 M) in a test tube containing Tris-HCl buffer (625 µl, 16 mM, pH 8.0). The reaction commenced after 125 µl (60 mM) of PMS was added to the mixture - a vigorous vortexing of the mixture followed by five minutes of room temperature incubation [29]. Then, absorbance was analyzed using a spectrophotometer (Hitachi, U-1900 UV/VIS, Hitachi High-Technologies Corporation).
Gel formulation and quality tests
Aqueous-methanolic leaf extract of J. sambac 10% was mixed in distilled water and carbpol-940 (gelling agent), sodium benzoate (preservative), triethanolamine, and glycerol (emulsifiers) added and shaken vigorously to mix them properly and then transferred in plastic bottles with spray caps for application. A stability test of J. sambac gel was performed at 40°C±2 temperature and 75%±5 relative humidity as accelerated, 30°C±2 temperature and 65%±5 relative humidity as long term. Color, appearance, and spreadability were checked through visual inspection, density using a pycnometer, and pH using a Hanna HI-98127 pH tester. The gel formulation was done with MEDIVET Pharmaceutical, Lahore (Batch No. MV-QC/23-04/03).
Study design and ethical consideration
Participants were blinded. The institutional ethical committee stored the product-related information and informed consent forms duly signed by the participants. This study was approved by the WHO primary clinical trial registry with registration reference IRCT20230202057310N1 for authentication before research on humans. The research was planned according to the WMA Declaration of Helsinki guidelines, “ethical principles for medical research involving human subjects”. The clinical study was conducted after written consent from the participants at Muhammad Physiotherapy and Rehabilitation Center, Multan, Pakistan (Approval. No. MIMAS/01/26/Qurat).
Inclusion and exclusion criteria
Participants (either sex), aged 18-70 years, who were already diagnosed with 1st and 2nd-grade musculoskeletal injuries were enrolled. Participants who had allergies to any of the listed ingredients used in the formulation of the J. sambac gel or other skin problems, cancer, diabetes, neurological disorders, fractures, open wounds, or were immobilized were excluded from the study.
Grouping and procedure of application
Participants with musculoskeletal injuries (n = 380 calculated through Rao-soft sample size calculator) [2] were equally randomized into four parallel groups (n = 95) through a lottery method and kept blinded. The initial baseline history was taken to measure equality of distribution and effectiveness. The first and second groups received topical application of J. sambac 10% and diclofenac diethyl-ammonium 2% w/w gels, respectively, through phonophoresis with therapeutic ultrasound (continuous mode, 1 MH, 0.8 W/cm2), on the affected areas of participants, in comparison, the third and fourth groups received topical application of J. sambac 10% and diclofenac diethyl-ammonium 2% w/w gels, respectively, through superficial massage on the affected areas of participants, as shown in Figure 1. If symptoms persisted, participants were followed up with and advised to apply both gels topically to the affected area 2-3 times daily for 2-14 days (depending upon the grade of injury) with special instructions (do not bandage tightly and avoid eye contact). The quantity of gels depended on the injury site and the affected parts’ surface area during the study.
Figure 1.
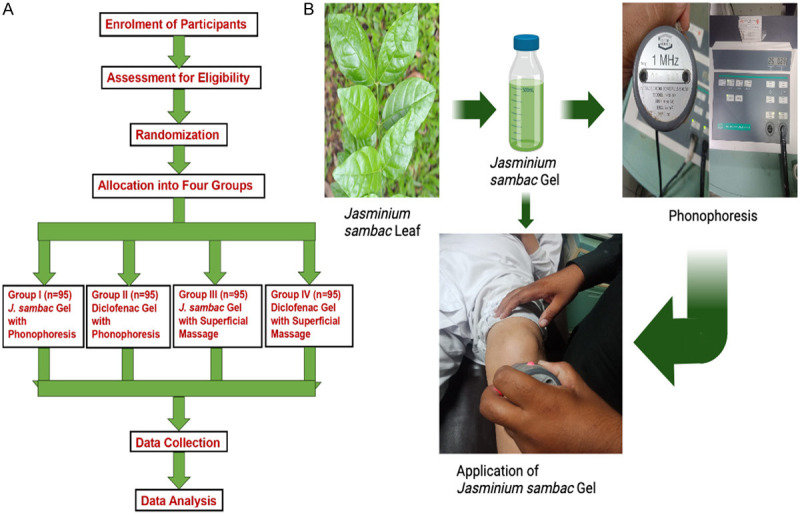
A. CONSORT diagram of methodology used for anti-inflammatory activity. B. Photographic representation of methodology used for anti-inflammatory activity.
Assessment of outcome
The intensity of the pain was assessed using the NPRS, global pain relief, and WOMAC scales both before and after applying both gels [5,28]. Participants did not report any allergies or side effects throughout the study. Following the application of the J. sambac gel, the cooling sensation was assessed, and the duration was recorded to compare the variations between the two groups and the initial conditions. The time was recorded following the application of both gels when there was a reduction of 2 points out of 11 points on the NPRS scale, which measures the commencement of pain relief. The duration of pain relief was measured until the end point, indicating the overall duration of the pain relief impact.
Data collection
The data were computed following authorization from the institutional ethical committee. The participants signed a written consent form. At the beginning and end of the session, each criterion of the NPRS, global pain relief, and WOMAC scales was fulfilled to check that all the parameters had been addressed. During the data-gathering process, researchers were accessible to address any inquiries from the subjects. The trial was carried out under the stringent supervision of a physician and a senior physiotherapist.
Molecular docking analysis
All prepared ligands and enzymes were docked using ‘AutoDock 4.2’. The remaining dynamisms of the ligands were decreased to a minimum by using the force field of MMFF-94. Similarly, ligand atoms were supplemented with Gasteiger fractional charges, whereas rotatable bonds were determined by combining the non-polar hydrogen atoms. The AutoDock techniques incorporated relevant parameters and necessary hydrogen atoms into the protein molecule, which were subsequently utilized for docking calculations. Visualizer 3.1 software was used to observe interactions between all proteins and ligands [29].
Statistical analysis
Results were communicated using GraphPad Prism version 8.4.2 software. Mean ± SD was calculated for the quantitative data. Paired t-test was used to compare the means of each group before and after applying gels, and one-way analysis of variance (ANOVA) was used to compare the mean of four independent groups using SPSS-23. The confidence interval was 95%, with P<0.05 considered significant.
Results
Preliminary phytochemical evaluation
Phytochemical evaluation of aqueous methanolic leaf extract of J. sambac showed presence of major phytochemical classes saponins, coumarins, phenols, and flavonoids while alkaloids, tannins and anthraquinones were found absent.
HPLC analysis
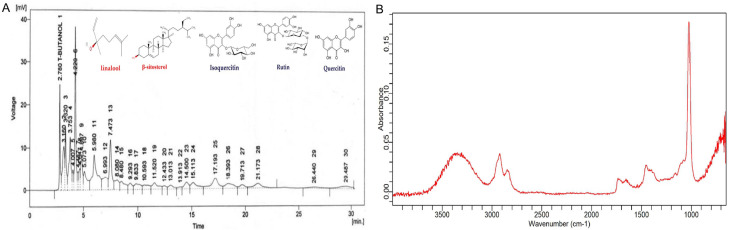
HPLC of aqueous-methanolic leaf extract of J. sambac is displayed in Figure 2A.
Figure 2.
Section of HPLC chromatogram of aqueous-methanolic leaf extract of J. sambac indicating the presence of different constituents including linalool, iso-quercetin, β-sitosterol, quercetin, and rtin (A). FTIR spectra of J. sambac gel (B).
FTIR peak values and functional groups
Different compounds were confirmed with the help of many functional groups at different numbers of wave such as alkanes at 3275/cm, the terpenes at 2919/cm, nitrites at 2850/cm peak value, saponins at 2351/cm, esters at 1012/cm, alkenes at 1730/cm, alkaloids at 1602/cm and amines at 1514/cm respectively through the analysis of FTIR in J. sambac leaf extract Figure 2B.
Antioxidant assay
DPPH assay
J. sambac gel showed significant antioxidant potential in comparison to standard (ascorbic acid) as shown in Figure 3A.
Figure 3.
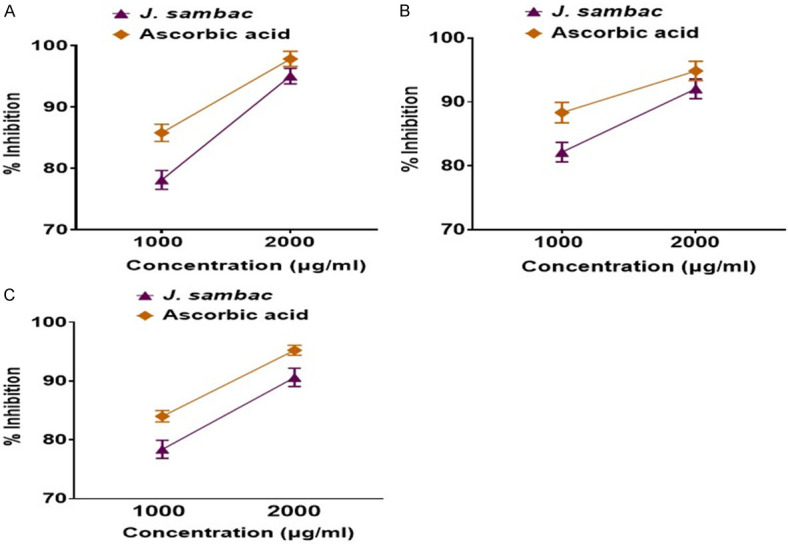
Antioxidant potential of J. sambac with respect to ascorbic acid. A. DPPH assay; B. NO scavenger assay; C. Superoxide dismutase assay.
NO scavenging assay
J. sambac gel showed significant NO scavenging potential in comparison to standard (ascorbic acid) as shown in Figure 3B.
Superoxide dismutase assay
J. sambac gel showed significant SOD inhibition in comparison to standard (ascorbic acid) as shown in Figure 3C.
Stability test of J. sambac gel
Visual examination revealed a product with good spreadability properties, as shown in Figure 4. It has a mustard green color, a distinctive herbal smell, and a pH of 6.3±0.2. The density, pH, and appearance of the J. sambac gel remained intact for 18 months.
Figure 4.
Visual representation of spreadability of J. sambac gel.
Incidence of musculoskeletal injuries
The incidence of musculoskeletal injuries is shown in Figure 5.
Figure 5.
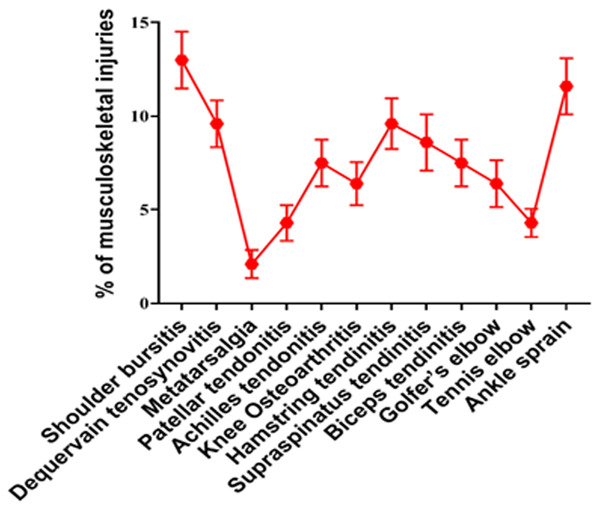
Incidence of musculoskeletal injuries included in this study.
Cooling effect
Results showed that J. sambac gel had a higher cooling effect on applied areas than diclofenac diethyl ammonium 2%, as shown in Figure 6. This may be due to the ingredients’ presence.
Figure 6.
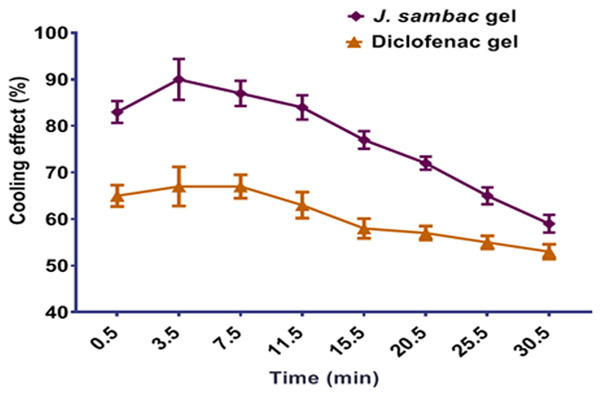
% cooling effect of the J. sambac and diclofenac gels.
Time difference in onset of pain relief for anti-nociceptive activity
The J. sambac gel revealed a highly significant quick response in pain and inflammation relief in the group receiving J. sambac gel with phonophoresis (P<0.001) and significant for the group receiving J. sambac gel with massage (P<0.02) when compared to diclofenac with phonophoresis (P<0.01) and massage (P<0.05). Results are shown in Figure 7.
Figure 7.
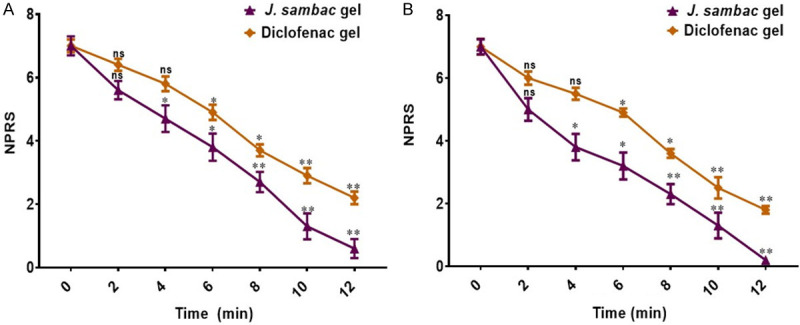
Time difference to onset pain relief for the anti-nociceptive activity of applied gels with (A) massage, and (B) phonophoresis. nsnon significant, *significant, **highly significant.
Efficacy of gels on different outcomes
J. sambac gel with massage (P<0.002 for WOMAC pain, P<0.003 for WOMAC stiffness, P<0.002 for WOMAC ADLs) and with phonophoresis (P<0.0002 for WOMAC pain, P<0.0003 for WOMAC stiffness, P<0.0004 for WOMAC ADLs) was superior compared to diclofenac diethyl-ammonium gel with massage (P<0.04 for WOMAC pain, P<0.05 for WOMAC stiffness, P<0.04 for WOMAC ADLs) and with phonophoresis (P<0.001 for WOMAC pain, P<0.003 for WOMAC stiffness, P<0.002 for WOMAC ADLs). Results are shown in Figure 8. The J. sambac gel showed significant anti-nociceptive activity evaluated through NPRS and global pain relief in groups receiving J. sambac gel with phonophoresis (P<0.000 of NPRS), with massage (P<0.002 of NPRS) when compared to diclofenac diethyl-ammonium gel with massage (P<0.05 of NPRS) and phonophoresis (P<0.001 of NPRS). Results of NPRS and global pain relief are shown in Figure 9.
Figure 8.
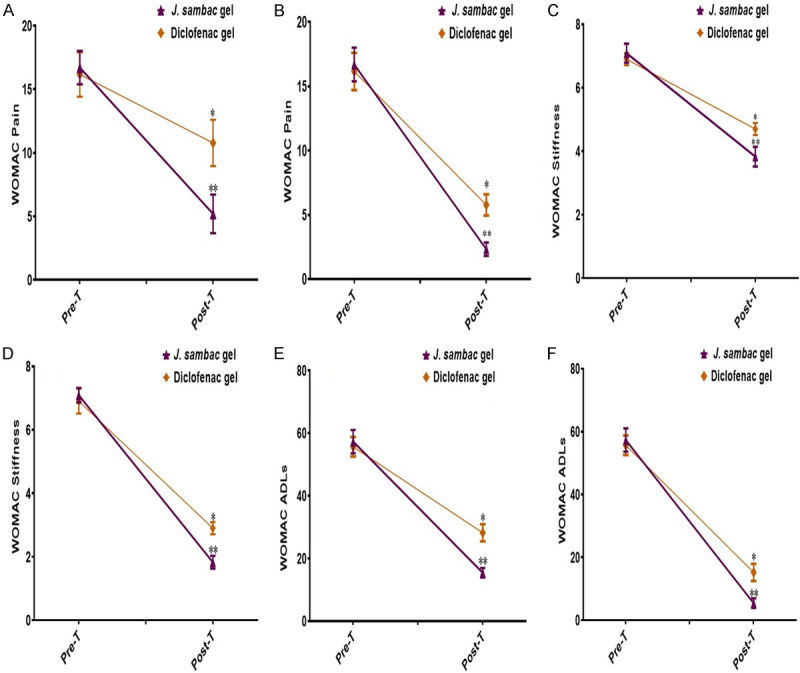
Results of efficacy difference of anti-inflammatory and anti-nociceptive activities in group (A) WOMAC pain (massage); (B) WOMAC pain (phonophoresis); (C) WOMAC stiffness (massage); (D) WOMAC stiffness (phonophoresis); (E) WOMAC ADLs (massage); (F) WOMAC ADLs (phonophoresis). nsnon-significant, *significant, **highly significant.
Figure 9.
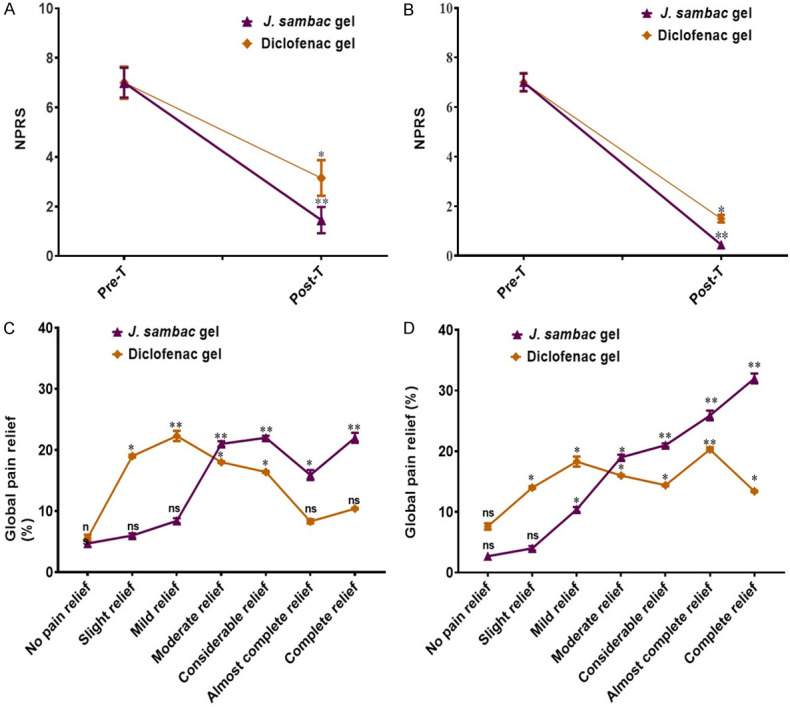
The anti-nociceptive activity of J. sambac gel and diclofenac gels with (A) massage and (B) phonophoresis. Global pain relief difference between polyherbal and diclofenac gels with (C) massage and (D) phonophoresis. nsnon-significant, *significant, **highly significant.
Molecular docking study
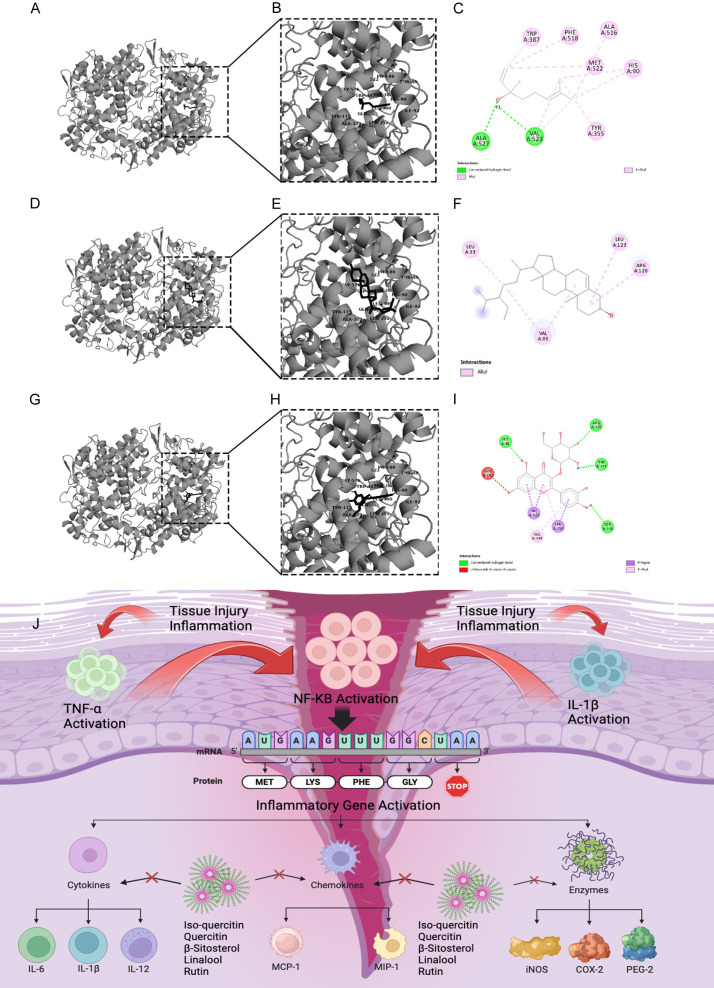
The automated docking technique showed the orientation of quercetin, iso-quercetin, β-sitosterol and linalool bounded with the active pockets of the COX-2 enzyme. The interaction of linalool with the active pockets of COX-2 was found to have a significant fitness score and hydrogen bonding against COX-2. Isoquercetin and β-sitosterol demonstrated minimum binding energy with enzyme via non-covalent interaction, as shown in Figure 10A-I.
Figure 10.
Schematic representation of interaction between COX-2 receptor and three distinct ligands (Linalool in the first row, quercetin in the second row and beta-sitosterol in the third row). Each column depicts a specific ligand-protein complex. (A, D and G) represent the 3D structures showing the overall docked configuration, while (B, E and H) offer magnified views of the ligand binding pockets within the protein structure. Complementary to the 3D structures, (C, F and I) provide 2D diagrams illustrating the key molecular interactions between the ligands and the protein residues. (J) The figure represents molecular mechanism of anti-inflammatory and antinociceptive effect of J. sambac gel in musculoskeletal injury.
Discussion
We treated different musculoskeletal injuries such as shoulder bursitis, dequervain tenosynovitis, metatarsalgia, patellar tendonitis, achilles tendonitis, knee osteoarthritis, hamstring tendonitis, supraspinatus tendonitis, biceps tendonitis, golfer elbow, tennis elbow, and ankle sprain (Figure 5), which are mainly due to overuse or overstressing of workload. The results of this study revealed that the J. sambac gel was faster in action than diclofenac for antinociceptive and anti-inflammatory activities when evaluated through NPRS, WOMAC, and the global pain relief scales (Figures 7, 8 and 9). This is because the occurrence of active constituents in J. sambac (Figure 2) which have the anti-inflammatory and antinociceptive tendency, results in rapid action through different pathways in response (Figure 10J).
Inflammation involves the transcriptional regulation of numerous genes implicated in inflammatory processes, such as iNOS, COX-2, TNF-, IL-1, and IL-6. NO is an essential inflammatory mediator macrophage cells generate during an inflammatory reaction. Thus, quercetin and its isoquercetin can decrease NO generation [30]. It was found that quercetin inhibited NF-κB activation while actively inhibiting NO production, iNOS, and COX-2 expression, significantly impacting its anti-inflammatory effects. β-sitosterol additionally inhibited the secretion of inflammatory factors from keratinocytes and macrophages that were stimulated by PGN, TNF-α, or LPS, including TNF-α, IL-1β, IL-6, IL-8, and ROS, individually. In macrophages, there was a partial suppression of NF-κB [31]. Based on experimental and preclinical data, rutin has the potential to successfully mitigate inflammation by lowering levels of pro-inflammatory markers like TNF-α, IL-6, COX-2, and IL-1β. Additionally, it may improve inflammation by inhibiting nuclear factor kappa B (NF-κB)/mitogen-activated protein kinase (MAPK) activation [32]. The HPLC analysis of J. sambac has shown the presence of rutin, quercetin, isoquercetin, β-sitosterol, and linolool (Figure 2). Although β-sitosterol is a well-known phytosterol found in plants, understanding its cellular processes has proven to be challenging due to its limited solubility in standard media. One reason could be that the effect of J. sambac gel through phonophoresis shows better drug penetration and absorption than massage.
In previous studies, it was reported that J. sambac has been used for many years for its anti-inflammatory and antinociceptive activities in oral drugs, creams, and, most commonly, oil [18,19]. This study showed the topical anti-inflammatory and analgesic effect of J. sambac gel for topical application to manage pain and inflammation with superficial massage and phonophoresis. Results revealed that the J. sambac 10% gel and diclofenac diethyl ammonium 2% gel presented more significant antinociceptive and anti-inflammatory activities with phonophoresis than by superficial massage (Figures 7, 8 and 9).
Previous studies in animals revealed that J. sambac could inhibit prostaglandins synthesis by down-regulating cyclooxygenase 2 (COX-2), interleukin-6 (IL-6), nuclear factor-κB (NF-κB), and tumor necrosis factor-α (TNF-α) [31]. Previous studies also demonstrate bioactivity assays that show that J. sambac has anti-arthritic and immunomodulatory effects by acting on RAW267 macrophages by activating NF-κB translocation, TNF-α, reactive oxygen species (ROS), and IL-6. Also, inhibiting Toll-like receptor-4 (TLR-4) can suppress the activities of NO and cytokines [32]. Previous studies also revealed that J. sambac has anti-inflammatory activity by inhibiting NO release and macrophages stimulated by LPS. This may be due to triggering pathways of NRF-2/ARE. NRF-2 enhances HO-1 expression, which creates a reducing environment and inhibits NF-κB [33]. All these inflammatory pathways are involved in different musculoskeletal injuries, so the results of this study also revealed that J. sambac gel has a significant anti-inflammatory effect, which may be due to active constituents that may act on different inflammatory pathways. Decreasing inflammation and the prostaglandin level may be helpful in antinociceptive activity (Figure 10). J. sambac gel has shown significant antioxidant activity (Figure 3). It is logical to believe that the anti-inflammatory and antinociceptive effect of J. sambac gel may be due to downregulating oxidative stress in inflammation and pain.
Finally, this study revealed that the most significant results were shown in groups treated with phonophoresis through therapeutic ultrasound (Figures 7, 8 and 9). This can increase tissue permeability and improve circulation. It is widely used for its anti-inflammatory activity and can enhance drug penetration in the skin and tissues. Previous studies revealed that it was beneficial in different musculoskeletal injuries by upregulating TGF-β1 and decreasing inflammatory response by downregulating COX-2 and PGE-2 [34]. However, a few studies revealed that it can upregulate COX-2 and is helpful in the regeneration phase [35]. This may be due to different effects of COX-2 at different stages of inflammation. In the early stage of inflammation, ultrasound suppresses COX-2 expression, and in later stages, it promotes the regeneration of muscle fibers mediated by COX-2. Previous studies also proved that muscles treated with low-intensity ultrasound therapy have increased levels of myogenic markers such as myogenin, actin, desmin, and arranged multinucleated myotubes [36]. The most commonly used gel as coupling media between the transducer and skin is aqueous-base; therefore, we used aqueous-base in a J. sambac gel formulation to enhance the transmission of sound waves in the skin and soft tissues.
The proposed mechanisms of action for the anti-inflammatory activity of the active constituents present in different ingredients of J. sambac gel acting through different pathways, are shown in Figure 10D.
Results of this study revealed that the gel made from J. sambac leaf extract can reduce pain and swelling, leading to fewer long-term disabilities and increasing the ability to do activities of daily living (ADLs). Decreasing the burden of musculoskeletal issues can strengthen orthopaedic trauma care, especially in low and middle-income countries. It might prove less expensive because the traditional method is used to formulate it, and J. sambac is widely available and can be used safely to manage musculoskeletal injury more effectively by involvement of multiple pathways.
Conclusions
The J. sambac gel, when applied topically with superficial massage or phonophoresis, exhibits anti-inflammatory and antinociceptive properties. These effects may be attributed to the diverse phytochemical constituents found in the gel. These constituents are believed to act on different pharmacological and biochemical pathways. Therefore, it possesses the capability to manage various grades of pain effectively.
Acknowledgements
This research was funded by Taif University, Saudi Arabia, project number (TU-DSPP-2024-138).
Disclosure of conflict of interest
None.
Abbreviations
- GBD
global burden of disease
- ADLs
activities of daily living
- WOMAC
Western Ontario and McMaster Universities Arthritis Index
- NPRS
numeric pain rating scale
- NSAIDs
nonsteroidal anti-inflammatory drugs
- COX-2
cyclooxygenase 2
- IL
interleukin
- NF-κB
nuclear factor-κB
- TNF-α
tumor necrosis factor-α
- DPPH
2,2-diphenylpicrylhydrazyl
- NO
nitric oxide
- SOD
superoxide dismutase
- WHO
World Health Organization
- WMA
World Medical Association
- ROS
reactive oxygen species
- TLR-4
Toll-like receptor-4
- TGF-β1
Transforming growth factor-β1
- PGE-2
prostaglandin E-2
- MAPK
mitogen-activated protein kinase
- FTIR
fourier transform infrared spectroscopy
- HPLC
high performance liquid chromatography
- iNOS
inducible nitric oxide synthase
- PGN
peptidoglycan
- LPS
lipopolysaccharides
References
- 1.Rathore FA, Attique R, Asmaa Y. Prevalence and perceptions of musculoskeletal disorders among hospital nurses in Pakistan: a cross-sectional survey. Cureus. 2017;9:e1001. doi: 10.7759/cureus.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irfan M, Qurat-Ul-Ain, Iqbal MO, Chen J, Khan MK, Sattar M, Khan IA. Association between anthropometric measurements of chairs and biomechanical variables leading to musculoskeletal problems in students at different government universities of Multan. Am J Transl Res. 2023;15:6136–6147. [PMC free article] [PubMed] [Google Scholar]
- 3.Williams A, Kamper SJ, Wiggers JH, O’Brien KM, Lee H, Wolfenden L, Yoong SL, Robson E, McAuley JH, Hartvigsen J, Williams CM. Musculoskeletal conditions may increase the risk of chronic disease: a systematic review and meta-analysis of cohort studies. BMC Med. 2018;16:167. doi: 10.1186/s12916-018-1151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cieza A, Causey K, Kamenov K, Hanson SW, Chatterji S, Vos T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2021;396:2006–2017. doi: 10.1016/S0140-6736(20)32340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ain QU, Khan IA, Raza MA, Anjum A, Khan MK, Perwasha P, Ishaq S. Evaluation of polyherbal gel for musculoskeletal injuries in industrial workers. Work. 2024;18:1–4. doi: 10.3233/WOR-230178. [DOI] [PubMed] [Google Scholar]
- 6.Iqbal MO, Naeem M, Mumtaz A, Ahmed MM, Ahmad A, Riaz R, Mesbah Z, Munawar N. Biochemical evaluation and medicinal ability of Jatropha mollissima in hepatic disorders. Am J Transl Res. 2022;14:7178–7188. [PMC free article] [PubMed] [Google Scholar]
- 7.Iqbal MO, Manzoor M, Mumtaz A, Riaz R, Arshad S, Khan IA, Javaid U, Manzoor Z, Munawar SH, Andleeb S, Ahmed MM, Aslam A. Evaluation of the hepatoprotective activity of hydroalcoholic extract of Alhagi camelorum against valproic acid-induced hepatotoxicity in rats. Biomed Pharmacother. 2022;150:112953. doi: 10.1016/j.biopha.2022.112953. [DOI] [PubMed] [Google Scholar]
- 8.Iqbal MO, Yahya EB. In vivo assessment of reversing aminoglycoside antibiotics nephrotoxicity using Jatropha mollissima crude extract. Tissue Cell. 2021;72:101525. doi: 10.1016/j.tice.2021.101525. [DOI] [PubMed] [Google Scholar]
- 9.Iqbal MO, Yahya EB, Andleeb S, Ahmed MM, Javaid MU, Shakeel W, Iqbal I. In vivo assessment of reversing cisplatin-induced nephrotoxicity using Jatropha mollissima crude extract and its potential cytotoxicity. Saudi J Biol Sci. 2021;28:7373–7378. doi: 10.1016/j.sjbs.2021.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iqbal MO, Ahmed MM, Arshad S, Javaid U, Khan IA, Manzoor M, Andleeb S, Riaz R, Munawar SH, Manzoor Z, Mumtaz A. Nephroprotective effects of Alhagi camelorum against cisplatin-induced nephrotoxicity in albino wistar rats. Molecules. 2022;27:941. doi: 10.3390/molecules27030941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wade AG, Crawford GM, Young D, Corson S, Brown C. Comparison of diclofenac gel, ibuprofen gel, and ibuprofen gel with levomenthol for the topical treatment of pain associated with musculoskeletal injuries. J Int Med Res. 2019;47:4454–4468. doi: 10.1177/0300060519859146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veloso C, Cardoso C, Vitorino C. Topical fixed-dose combinations: a way of progress for pain management? J Pharm Sci. 2021;110:3345–3361. doi: 10.1016/j.xphs.2021.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Byl NN. The use of ultrasound as an enhancer for transcutaneous drug delivery: phonophoresis. Phys Ther. 1995;75:539–553. doi: 10.1093/ptj/75.6.539. [DOI] [PubMed] [Google Scholar]
- 14.Khan IA, Janbaz KH, Saqib F. Antidiarrheal activity of methanolic leaf extract of Rumex vesicarius . Bangladesh J Pharmacol. 2016;11:175–180. [Google Scholar]
- 15.Ain QU, Abid MUH, Hashim M, Ishaq S, Manzoor A, Perwasha P, Mizgan GE, Iqbal MO, Munawar SH, Manzoor Z, Khan IA. Anticoagulant and thrombolytic activities of leaf extract of mangifera indica in smokers. Tob Regul Sci. 2022;8:1189–1201. [Google Scholar]
- 16.Ain QU, Iqbal MO, Khan IA, Bano N, Naeem M, Jamaludin MI, Devaraj S. Phytochemical, antioxidant, antipyretic and anti-inflammatory activities of aqueous-methanolic leaf extract of Mangifera indica . Am J Transl Res. 2023;15:4533–4543. [PMC free article] [PubMed] [Google Scholar]
- 17.Mizgan GE, Kousar S, Khan IA, Perwasha P, Gul H, Aslam I, Abbas A, Ul-Ain Q. Evaluation of anti-clotting and thrombolytic potential of the aqueous-methanolic extract of Jasminum sambac . Pak J Pharm Sci. 2022;35:1747–1754. [PubMed] [Google Scholar]
- 18.Kunhachan P, Banchonglikitkul C, Kajsongkram T, Khayungarnnawee A, Leelamanit W. Chemical composition, toxicity and vasodilatation effect of the flowers extract of Jasminum sambac (L.) Ait. “G. Duke of Tuscany”. Evid Based Complement Alternat Med. 2012;2012:471312. doi: 10.1155/2012/471312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Snafi AE. Pharmacological and therapeutic effects of Jasminum sambac-a review. Indo Am J Pharm Sci. 2018;5:1766–1778. [Google Scholar]
- 20.Belango YMC, Cruz AF, Miguel RB, Rotairo CRL, Oli RAT. Anti-inflammatory property of the formulated topical gel from the crude leaf extracts of Sampaguita (Jasminum sambac L. family: Oleaceae) Int J Chem Eng Appl. 2016;7:199. [Google Scholar]
- 21.Khan IA, Hussain M, Munawar SH, Iqbal MO, Arshad S, Manzoor A, Shah MA, Abbas K, Shakeel W, Syed SK. Jasminum sambac: a potential candidate for drug development to cure cardiovascular ailments. Molecules. 2021;26:5664. doi: 10.3390/molecules26185664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan IA, Lodhi AH, Munawar SH, Manzoor A, Manzoor Z, Raza MA. Formulation and evaluation of rutin-allicin gel against diabetic foot ulcer. Lat Am J Pharm. 2020;39:725–729. [Google Scholar]
- 23.Khan IA, Aziz A, Sattar M, Munawar SH, Manzoor Z, Raza MA, Fatima G, Hannan A. Evaluation of wound healing potential of Rumex vesicarius L. leaf extract and fractions in rabbit. Afr J Tradit Complement Altern Med. 2015;12:60–64. [Google Scholar]
- 24.Khan IA, Aziz A, Manzoor Z, Munawar SH, Sarwar HS, Afzal A, Raza MA. Study on antipyretic activity of Rumex vesicarius leaves extract in albino rabbits. Vet World. 2014;7:44–48. [Google Scholar]
- 25.Aziz A, Saqib F, Khan IA, Ashraf MM, Ashraf MN, Raza MA. Dermatological evaluation of anti-irritant and anti-inflammatory effect of plumerin-R isolated from the latex of plumeria rubra Linn. Lat Am J Pharm. 2018;37:317–20. [Google Scholar]
- 26.Rafique R, Khan ZU, Akram MAN, Javid K, Iltaf S, Shaukat M, Shaukat H, Ali S. Phytochemical analysis of stem and leaf extracts of Jasminum sambac (L.) aiton by FTIR method: analysis of stem and leaf of Jasminum sambac (L.) Biol Sci-PJSIR. 2023;66:199–204. [Google Scholar]
- 27.Khan IA, Hussain M, Syed SK, Saadullah M, Alqahtani AM, Alqahtani T, Aldahish AA, Asiri S, Zeng LH. Pharmacological justification for the medicinal use of Plumeria rubra Linn. in cardiovascular disorders. Molecules. 2021;27:251. doi: 10.3390/molecules27010251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iqbal MO, Khan IA, Manzoor A, Arshad S, Sial AS, Dar E, Shaikh AR. Cardioprotective effect of hydroalcoholic leaf extract of Jatropha mollissima on isoproterenol-induced myocardial infarction in rats. Pharmacogn Mag. 2021;17:251–256. [Google Scholar]
- 29.Khan IA, Hussain M, Hussain N, Alqahtani AM, Alqahtani T. Cardioprotective effect of Rumex vesicarius Linn. Leaf extract against catecholamine-induced cardiotoxicity. Molecules. 2022;27:3383. doi: 10.3390/molecules27113383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Liang X, Wang B, Lin Z, Ye M, Ma R, Zheng M, Xiang H, Xu P. Six herbs essential oils suppressing inflammatory responses via inhibiting COX-2/TNF-α/IL-6/NF-κB activation. Microchem J. 2020;156:104769. [Google Scholar]
- 31.Huang H, Yang X, Li W, Han Q, Xu Z, Xia W, Wu M, Zhang W. Structural characterization and immunomodulatory activity of an arabinogalactan from Jasminum sambac (L.) aiton tea processing waste. Int J Biol Macromol. 2023;235:123816. doi: 10.1016/j.ijbiomac.2023.123816. [DOI] [PubMed] [Google Scholar]
- 32.Ceccacci S, Lucia A, Tortora A, Colantuono A, Carotenuto G, Tito A, Monti MC. Jasminum sambac cell extract as antioxidant booster against skin aging. Antioxidants (Basel) 2022;11:2409. doi: 10.3390/antiox11122409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alvarez AM, DeOcesano-Pereira C, Teixeira C, Moreira V. IL-1β and TNF-α modulation of proliferated and committed myoblasts: IL-6 and COX-2-derived prostaglandins as key actors in the mechanisms involved. Cells. 2020;9:2005. doi: 10.3390/cells9092005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin H, Du L, Luo Z, He Z, Wang Q, Chen S, Zhu YL. The therapeutic effects of low-intensity pulsed ultrasound in musculoskeletal soft tissue injuries: focusing on the molecular mechanism. Front Bioeng Biotechnol. 2022;10:1080430. doi: 10.3389/fbioe.2022.1080430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sang F, Xu J, Chen Z, Liu Q, Jiang W. Low-intensity pulsed ultrasound alleviates osteoarthritis condition through focal adhesion kinase-mediated chondrocyte proliferation and differentiation. Cartilage. 2021;13:196S–203S. doi: 10.1177/1947603520912322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klaiman MD, Shrader JA, Danoff JV, Hicks JE, Pesce WJ, Ferland J. Phonophoresis versus ultrasound in the treatment of common musculoskeletal conditions. Med Sci Sports Exerc. 1998;30:1349–1355. doi: 10.1097/00005768-199809000-00002. [DOI] [PubMed] [Google Scholar]