Abstract
Hyperglycemic status is associated with the development and prognosis of colorectal cancer (CRC), although the exact mechanisms are not fully understood. Hyperglycemia can promote the development of CRC by influencing cell proliferation and apoptosis, inflammatory responses, oxidative stress, immunomodulation, angiogenesis, and other pathways. In terms of prognosis, hyperglycemia may affect the survival and recurrence of CRC patients as well as chemotherapy resistance, but the results of related studies are not consistent. Hypoglycemic treatment may have a positive impact on the prognosis of CRC patients, but its specific effects need further research. Therefore, this article systematically explores the relationship between hyperglycemia and CRC, analyzes the impact of hyperglycemia on the occurrence and prognosis of CRC, and discusses the role of managing hyperglycemia in CRC.
Keywords: Hyperglycemia, colorectal cancer, cell proliferation and apoptosis, hypoglycemic therapy, prognosis, survival rate
Introduction
Colorectal cancer (CRC), an epithelial malignancy, holds a high prevalence in China. Due to the similar early symptoms with hemorrhoids, which are often neglected, coupled with the fact that the intestinal tract has virtually no pain-sensing nerves, more than 80% of the patients are diagnosed at mid-to-late stages [1]. A study across 23 domestic hospitals involving nearly 8,000 diagnosed CRC patients revealed that over half of the diagnoses were at an advanced stage (III, IV), with 33% at stage II [1]. Data indicate that by 2030, there may be over 2.2 million new CRC cases and 1.1 million associated deaths globally [2]. Tincidence of CRC is increasing every year in both young and older adult populations, posing a serious threat and a significant economic burden on society [3,4].
Hyperglycemia refers to a state where blood glucose levels surpass the normal range, indicating elevated sugar concentrations in the bloodstream. Recent studies have explored the correlation between metabolic abnormalities and tumors, focusing particularly on the relationship between hyperglycemia and tumor development. Hyperglycemia can indirectly affect cancer cells by raising the levels of insulin/IGF-1 and inflammatory cytokines within the circulatory system [5]. Evidence also suggests that hyperglycemia itself can directly impact the growth and apoptosis of cells [5-10], as well as activate multiple signaling pathways to coordinate the behavior of cancer cells, such as proliferation, migration, and invasion [5,7-11]. These findings highlight a relationship between hyperglycemia and CRC. However, the exact association and mechanism of action of hyperglycemia in CRC have not been fully explored. Further studies are essential to elucidate the mechanisms. Therefore, this paper aims to comprehensively analyze the current research progress on the relevance of hyperglycemia to CRC, providing insight for further CRC treatment and research.
Correlation between hyperglycemia and CRC
Over time, a hyperglycemic state leads to changes in the intracellular environment, predisposing cells to abnormal proliferation and mutation, thus increasing the risk of CRC. In addition, hyperglycemia, a hallmark of diabetes, is often accompanied by a state of metabolic disorder and chronic inflammation, which further exacerbate the development of CRC. A study followed 300,039 patients with type 2 diabetes mellitus (T2DM) and compared them to an equal number of age and sex-matched non-T2DM individuals, revealing that the CRC risk in the T2DM patients was approximately 1.3 times that of the non-T2DM population [12]. This is consistent with previous reports that revealed a 1.2-1.5 times greater CRC risk in T2DM patients [13]. A meta-analysis study pooling data from 151 studies involving 32 million people confirmed a causal relationship between T2DM and CRC [14]. However, another meta-analysis found no association between T1DM and CRC in comparison to T2DM [15].
The results obtained from these clinical observations or Meta-analyses are not entirely consistent due to the presence of heterogeneity, selection bias and other confounding factors. We identified several Mendelian randomization studies that have explored the relationship between diabetes and colorectal cancer (CRC). For example, a prospective Mendelian randomization study conducted by Goto et al. [16] showed that each doubling of diabetes risk was associated with a hazard ratio (HR) for CRC of 0.90, with a 95% confidence interval (CI) ranging from 0.74 to 1.10, indicating no significant association between diabetes and CRC risk. In contrast, a study by Murphy et al. [17] using Mendelian randomization methods found that individuals genetically predisposed to T2DM had a 1.04 times higher risk of CRC for each one-unit increase in log odds, with a 95% CI of 1.01-1.07. Higher levels of glycated hemoglobin (HbA1c) were also associated with a slight increase in colorectal cancer risk, with a risk ratio of 1.09 per one standard deviation (1-SD) increase and a 95% CI ranging from 1.00 to 1.19. However, these findings may be subject to pleiotropy, meaning that the genetic variants studied may affect multiple traits simultaneously, not just diabetes and CRC risk. Furthermore, a study by Xiao et al. [18] concluded that the presence of T2DM does not increase the risk of CRC. These findings underscore the complex and sometimes contradictory results emerging from different studies, reflecting variations in study design, sample selection, and choice of genetic instrumental variables.
Conversely, CRC patients are a high-risk group for diabetes. Within 5 years of CRC diagnosis, the annual incidence of diabetes increases in CRC patients compared to non-CRC patients [19]. The pooled analysis, based on 13 studies, revealed that the combined risk ratio of diabetes in survivors of blood, gynecological, breast, colorectal, and urological cancers was 1.39 (95% CI: 1.29-1.50) [20]. Additionally, glucocorticoids and targeted drugs are commonly chosen to prevent recurrence and treat postoperative and advanced CRC patients, but these treatments may impair pancreatic β-cell function and reduce insulin sensitivity. Similarly, cancer-targeted drugs may also weaken insulin sensitivity and increase insulin resistance [11]. There are complex interactions between hyperglycemia, diabetes mellitus, and CRC.
Mechanism of hyperglycemia promoting and influencing the occurrence, development, and prognosis of CRC
Effects of hyperglycemia on cell proliferation, metastasis, and apoptosis
Even in the presence of oxygen, cancer cells preferentially utilize glycolysis rather than oxidative phosphorylation to obtain adenosine triphosphate (ATP) for proliferation. This phenomenon, known as the Warburg effect [21], has been observed in the early stages of CRC [22]. At the same time, it was also discovered that the APC gene frequently mutates in CRC patients (in over 80% of patients), significantly contributing to the development of CRC by enhancing the activity of the glycolysis pathway. APC gene mutations cause the accumulation of the β-catenin/T-cell factor-4 complex, which activates the transcription of β-catenin target genes, such as myeloblastosis viral oncogene homolog (MYC), pyruvate kinase M2 (PKM2), pyruvate dehydrogenase kinase 1 (PDK1), and monocarboxylate transporter 1 (MCT1) [23-26]. This indirectly promotes glycolysis, generating more ATP to meet the growth demands of tumor cells [21] (Figure 1). Furthermore, the APC/β-catenin pathway activation also modulates the expression of two transcription factors, HIF1α and MYC [26]. Overall, the development of CRC is closely related to the enhancement of the glycolytic metabolic pathway, and in particular, the APC gene mutation plays a key role in promoting this metabolic transformation. These findings highlight the importance of a better understanding of the metabolic characteristics of CRC for the development of new treatment strategies.
Figure 1.
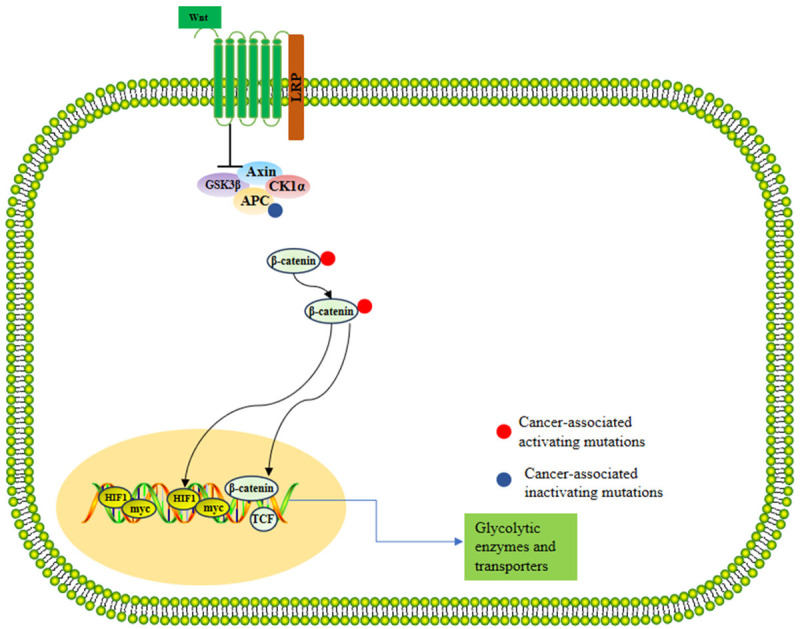
Glycolytic pathway in colorectal cancer. Note: Axin: Axis inhibition protein; GSK3β: Glycogen synthase kinase-3 beta; CK1α: Casein kinase 1 alpha; HIF1: Hypoxia-inducible factor 1; TCF: T-cell factor.
Normal cells grow slowly under hyperglycemic conditions and show a tendency to apoptosis [27,28]. However, the opposite is true in cancer. In a hyperglycemic environment. Both in vitro and in vivo studies have shown that cancer cells demonstrate increased proliferation and reduced rates of apoptosis under hyperglycemia [29]. The transwell experiments have shown that high levels of glucose (11 mM) promote the proliferation of the CRC cells. Additionally, it can also promote the proliferation and migration of CRC cells through the activation of phosphoinositide 3-kinase (PI3K), protein kinase C alpha (PKCα), and myosin light chain kinase (MLCK) [30]. Additionally, high glucose levels may promote cancer cell proliferation and migration through the PI3K/AKT serine (Akt) pathway [29].
Acting as a tumor suppressor, miRNA-9 (miR-9) is capable of targeting cyclin B1 and N-cadherin by the insulin-like growth factor 1 receptor (IGF1R) pathway, while simultaneously upregulating E-cadherin in CRC cells exposed to high glucose levels [31]. In these conditions, high glucose levels promote cell proliferation, migration, and invasion by negatively regulating the IGF1R/sarcoma viral oncogene homolog (Src)/extracellular regulatory protein kinase (ERK) axis through the mediation of miR-9 [31] (Figure 2). Furthermore, a high glucose level has been linked to a higher likelihood of liver metastasis in CRC, where overexpression of integrin αvβ6 plays a role [32]. High glucose leads to increased high mobility group AT-hook 2 (HMGA2) expression, which in turn fuels cancer cell proliferation, migration, and invasion while inhibiting apoptosis, all through the activation of the epithelial-mesenchymal transition (EMT) pathway [33]. In CRC cells, literature has documented at least 11 EMT-associated molecular pathways, including those involving β-linker proteins, transforming growth factor-β, Wnt pathways, and aberrant notch homolog 1 (NOTCH-1) signaling [34]. Although hyperglycemia promotes the development of CRC, it generally fails to proliferate cancer cells in the absence of insulin [35], which may be an important reason for the lack of correlation between T1DM and CRC.
Figure 2.
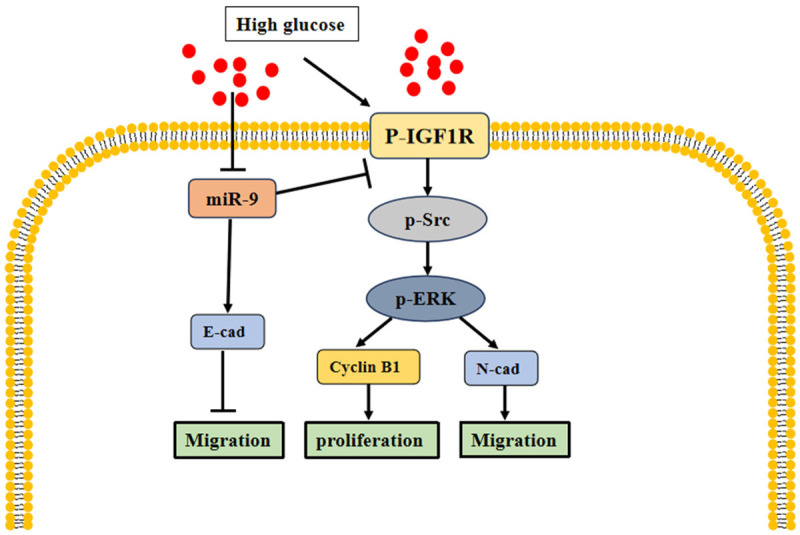
Hyperglycemia promotes colorectal cancer cell proliferation and migration through the IGF1R/Src/ERK pathway. Note: IGF1R: insulin-like growth factor 1 receptor; p-Src: sarcoma viral oncogene homolog phosphorylation; p-ERK: Extracellular regulatory protein kinase phosphorylation; E-cad: E-cadherin; N-cad: N-cadherin.
Overall, these discoveries offer valuable insight into the mechanisms of hyperglycemia-induced CRC cell invasion and metastasis and provide new ideas for the development of future therapies (Table 1). For example, treatments targeting PI3K, PKCα, MLCK and other pathways may have efficacy in hyperglycemic settings.
Table 1.
Hyperglycemia affects the occurrence and development of colorectal cancer through different pathways
| Author | Materials | Outcome | Mechanism |
|---|---|---|---|
| Tomas et al. [29] | SW480 | Promote cancer cell proliferation and migration | PI3K/Akt pathway |
| Masur et al. [30] | HT29, SW480 | Promote cancer cell proliferation and migration | PKCα transcription, PI3K transcription, MLCK protein |
| Wang et al. [32] | CRC tissue, CRC liver metastasis mouse model | Promote the invasion and metastasis of cancer cells | ERK pathway up-regulates αvβ6 and induces MMP-9 up-regulation |
| Chang et al. [31] | HCT15, CRC tissue, colitis-associated CRC mouse models | Promote cancer cell proliferation and migration | Collpasin response mediator protein-2 |
| Wu et al. [33] | HCT-116, HT-29, CRC tissue | Promote cancer cell proliferation, migration and invasion, inhibit apoptosis | EMT |
| Yang et al. [36] | Caco2, SW480, SW620 | Promote cell proliferation, reduce apoptosis and increase cell migration | Inhibition of miR-16, and VEGFR2 gene |
| Lee et al. [37] | HCT116 | Promote cell proliferation and inhibit apoptosis | Endoplasmic reticulum stress response |
Note: CRC: colorectal cancer; PI3K/Akt: phosphoinositide 3-kinase/AKT serine; PKCα: protein kinase C alpha; MLCK: myosin light chain kinase; MMP-9: matrix metalloproteinase 9; EMT: epithelial-mesenchymal transition; VEGFR2: a vascular endothelial growth factor receptor 2.
Effects of hyperglycemia on inflammatory response
Inflammatory responses intensify in the body during hyperglycemic states. Inflammation is a self-protective response of the body to cellular damage, infection, or other stimuli, but a long-term chronic inflammatory state may lead to tissue damage and tumorigenesis. In hyperglycemic state, the accumulation of advanced glycation end products (AGEs) may lead to increased release of inflammatory factors, such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). The inflammatory factors activate the Akt and Wnt signaling pathways, which may promote the development of colorectal carcinogenesis [38,39]. The presence of these cytokines also contributes to the development of insulin resistance, as well as to the activation of signaling pathways downstream of oncogenes, for example, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), jun proto-oncogene (c-Jun), and c-Jun n-terminal kinases (JNK)/mitogen-activated protein kinases (MAPK) [40]. Indeed, activation of NF-κB is also a well-known mechanism implicated in CRC development and progression. The pathologic characteristics linked to these cytokines encompass oxidative stress, reduced β-oxidation, intracellular lipid accumulation in the liver or skeletal muscle, and mitochondrial dysfunction, all of which are associated with cancer progression. That is to say, the presence of these cytokines and the associated pathologic features may play a role in cancer progression. In addition, increased expression of AGEs promotes the binding of AGEs to the receptor and thus mediates the ERK/specificity protein 1 (SP1)/MMP2 pathway. This activation results in the migration of CRC cells [41].
Effects of hyperglycemia on oxidative stress
Elevated blood glucose levels can result in increased production of reactive oxygen species, and triggers a strong oxidative stress response through processes such as non-enzymatic protein glycosylation and glucose auto-oxidation [42]. Briefly, hyperglycemia directly stimulates the activation of several molecular pathways, including glycolysis, AGE production, protein kinase C activation, and amino caproate and polyol pathways, all of which contribute to oxidative stress [43]. During oxidative stress, proteins, lipids, and DNA are susceptible to oxidative damage. Protein oxidation alters their structure and function, affecting normal cellular metabolism and signaling. Lipid peroxidation can disrupt the integrity of cell membranes, leading to abnormal membrane function. DNA oxidation may result in gene mutations and erroneous transmission of genetic information, increasing the cancer risk. Growing evidence supports the association of oxidative stress, particularly oxidatively modified DNA and lipid peroxidation products, that may contribute to a heightened risk of CRC in individuals with diabetes [44,45].
Interestingly, the involvement of the NOD-like receptor pyrin domain-3 (NLRP3) inflammasome appears to be involved in CRC development. High blood glucose levels have been shown to activate the NLRP3 inflammasome, further supporting the association between NLRP3 and CRC. In colon adenocarcinoma tissue, the expression of NLRP3 is considerably higher than that of normal tissue. Moreover, elevated levels of NLRP3 are correlated with lower survival rates in CRC patients [46]. The main function of the inflammasome is to regulate caspase-1 enzyme activity, thereby promoting the secretion of IL-1β and IL-18. IL-1β may facilitate colon tumor growth and invasion by activating colon cancer stem cells, increasing the expression of EMT activator Zeb1, and promoting EMT [47].
Effects of hyperglycemia on immunomodulation
Hyperglycemia also impacts immune regulation, which is crucial in combating the onset and progression of tumors [48]. Elevated sugar levels may lead to abnormal immune cell function, such as reduced activity of macrophages and T lymphocytes, and impaired function of natural killer cells. Such alterations may compromise the body’s immune surveillance and clearance capabilities against tumors, increasing the risk of tumor development. Hyperglycemia can influence the infiltration of CD4+ T cells, CD8+ T cells, and mucosa-associated invariant T cells in CRC tissues [49]. This suggests that hyperglycemia may cause immune system dysfunction, affecting the ability of immune cells to recognize and attack CRC. These changes in immune response may accelerate tumor growth and spread, thereby influencing the progression of CRC.
Effects of hyperglycemia on angiogenesis
Elevated insulin and IGF levels in hyperglycemic states may promote tumor cell growth and survival by activating the IGF-1-PI3K-AKT-mechanistic target of rapamycin (mTOR) signaling pathway [50]. This process may be associated with the widespread expression of insulin receptors on tumor cells, allowing for enhanced responses to these hormones and thus meeting their bioenergetic needs. Additionally, a high level of IGF also promotes the transcription of vascular endothelial growth factor (VEGF) genes, which further promotes angiogenesis and tumor invasion [5]. Hyperglycemia also overproduces late oxidized protein products, which interact with late glycosylation end product receptors to trigger a series of signal transduction cascades, including activation of MAPK, p38 mitogen-activated protein kinase, protein kinase C (PKC), and NF-κB [51]. These events may trigger inflammatory responses, inhibit apoptosis, and promote neovascularization generation, providing favorable conditions for the progression of CRC [52,53].
Effects of hyperglycemia on epigenetic change
Epigenetic modifications, which affect gene expression by altering chromatin structure and DNA methylation without changing the DNA sequence, can be disrupted by high blood glucose levels. This disruption can lead to the permanent activation of cancer pathways in tumor cells, a phenomenon known as “glucose memory”. Studies have found that CD34+ stem cells exposed to a high glucose environment undergo changes in oxidative status and an increase in DNA methylation associated with migration ability and expression of C-X-C chemokine receptor type 4 (CXCR4) [54]. These changes persist even when returning to normal glucose conditions, suggesting that epigenetic alterations in hyperglycemia can induce lasting functional change in CD34+ stem cells. These CD34+ stem cells “memorize” this condition through epigenetic modifications, resulting in altered CXCR4 receptor expression and migration ability [54]. Inhibiting CXCR4 affects the chemotaxis of various immune cell types, leading to immune suppression in CRC by impairing the chemokine receptor function that mediates the aggregation of immune cells within the tumor [55]. Furthermore, it has been confirmed that the C-X-C motif chemokine ligand 12 (CXCL12)-CXCR4 axis plays a role in tumor growth, invasion, angiogenesis, and metastasis in CRC [56].
Another example is NFκB-p65 subunit (NFκB-p65), which is a transcription factor that may lead to changes in gene expression under hyperglycemic conditions [57]. NF-κB-p65 can activate multiple proliferation-related genes, such as cyclin D1 and c-Myc, promoting the proliferation and growth of tumor cells [58]. In a hyperglycemic environment, lactoferrin combined with the NT5DC3 protein can inhibit the growth of colorectal tumors by altering abnormal epigenetic marks [59]. Furthermore, the expression of nucleoside triphosphate diphosphatase 3 (NT5DC3) protein can be used to distinguish between T2D or T2D-induced CRC and healthy individuals [59]. It is evident that hyperglycemia levels may lead to an increase in the methylation levels of some tumor suppressor genes, thereby inhibiting their expression and promoting the occurrence of CRC. Conversely, it may also lead to a decrease in the methylation of some tumor-related genes, thereby increasing their expression.
Effects of hyperglycemia on prognosis
Hyperglycemia may affect the prognosis of CRC patients by influencing the biologic behavior of tumor cells and response to therapy. Previous research has indicated that under high glucose concentrations (15 mM), the growth inhibitory effect of 5-Fluorouracil (5-FU) on cancer cells may be diminished, and apoptosis may be reduced due to stimulated DNA replication [60]. Hyperglycemia can also influence OS and disease-free survival (DFS) in CRC patients by modulating the phosphorylation of SMAD family member 3 (SMAD3) and Myc, as well as upregulating the expression of euchromatic histone lysine methyltransferase 2 (EHMT2) [36]. This is consistent with previous findings [61]. In a hyperglycemic environment, the expression of HIF-1α may also be promoted through the glucose transporter 1/O-GlcNAc transferase/hypoxia-inducible factor 1 alpha (HIF-1α) pathway. This enhancement could subsequently reduce the tolerance of cancer cells to 5-FU and radiotherapy. However, the combined use of HIF-1α inhibitors has been shown to reverse radiotherapy resistance in hyperglycemia environments, improving treatment outcomes and offering new insight for the clinical treatment of CRC patients with hyperglycemia [62]. These findings highlight the need for further research to investigate the mechanisms by which hyperglycemia impacts the prognosis of CRC.
Effects of hyperglycemia on survival and recurrence in CRC patients
Hyperglycemia affects the prognosis of CRC patients in several ways. Research indicates that hyperglycemia may impact the survival time in CRC patients. For instance, among CRC patients receiving bevacizumab and/or cetuximab treatment, those with diabetes experienced a modestly reduced overall survival time (OS) and progression-free survival (PFS). Specifically, the diabetic group had an OS of 22.7 months and PFS of 9.7 months, in contrast to the OS of 27.1 months and PFS of 10.8 months in nondiabetic group [63]. This finding aligns with another study where, in patients with metastatic CRC, the median PFS and median OS were 8 months and 15 months in the T2DM group and 16 months and 29 months in the non-T2DM group, respectively [64].
Furthermore, high blood glucose levels may increase the risk and recurrence in CRC patients. One study indicated that the postoperative recurrence rate in CRC patients with high blood glucose was 52.34%, compared to 30.94% in those with normal blood glucose levels [36]. Additionally, high blood glucose can reduce the tolerance of CRC patients to chemotherapy, enhancing resistance to treatments like 5-FU, fluorouracil, oxaliplatin, and irinotecan [60,63], and increasing treatment-related toxicity. Diabetic cancer patients are more likely to experience adverse events during chemotherapy, including infection, hematologic disease, endocrine system disease, and deterioration of overall condition, highlighting the necessity of considering the impact of diabetes on personalized care plans for cancer patients [65].
In conclusion, hyperglycemia has multifaceted impacts on the efficacy and prognosis of CRC patients, including lowered survival rate, increased risk of recurrence, reduced chemotherapy resistance and tolerance, and heightened treatment-related toxicity. These findings underscore the importance of meticulous blood sugar control in CRC patients and the necessity for tailored treatments, taking into account the unique challenges of diabetes.
Effect of hypoglycemic drugs on CRC
Metformin
Lowering blood glucose with metformin was associated with a lower risk of CC mortality (HR: 0.74, 95% CI: 0.50-1.09) [66]. In a meta-analysis of 58 studies, metformin treatment not only reduced the incidence and recurrence of CRC but also improved OS and CRC-specific survival. Particularly in patients with mCRC, a more significant improvement in OS was observed (HR: 0.77, 95% CI: 0.68-0.87) [67]. Beyond its glucose-lowering effects, metformin has shown potential in reducing cancer risk. It reduces blood glucose and insulin levels by inhibiting hepatic glucose output, increasing glucose uptake and utilization, and improving insulin sensitivity. Metformin can activate the AMPK signaling pathway, which subsequently leads to reduced protein synthesis and inhibition of tumor cell proliferation, thereby lowering the risk of malignant tumor development [68].
Research has found that metformin exerts its anticancer effects on CRC cells by activating the AMPK pathway and concurrently inhibiting the mTOR pathway, thereby suppressing cell proliferation, migration, and invasion. It also reduces tumor proliferation and EMT process while promoting apoptosis. Furthermore, the sensitivity of different CRC cell lines to metformin is related to the response of the AMPK-mTOR pathway, which has been validated [69,70]. Metformin significantly reduces the risk of CRC in patients with type 2 diabetes in a dose-dependent manner. However, results vary across studies. For instance, in patients with diabetes treated with metformin after surgery, there was no significant difference in DFS, OS, or recurrence time compared to non-diabetic CRC patients, regardless of the duration of metformin use [71]. Experimental and observational studies have yielded consistent conclusions, but most existing observational studies are subject to bias. Additionally, some studies have not demonstrated beneficial effects of metformin in cancer prevention and treatment. Currently, the evidence regarding the efficacy of metformin in the development of malignant tumors remains inconclusive, necessitating further prospective research and clinical trials to clarify its role.
Insulin
An early meta-analysis found a modestly increased risk of CRC associated with insulin use (OR: 1.11, 95% CI: 0.97-1.26) [71]. Insulin treatment is a risk factor for CRC in patients with T2DM (HR: 1.078, 95% CI: 1.027-1.131) [37]. In a 12-year cohort study with adjusted confounding factors, insulin use elevated the risk of CRC (HR: 1.86, 95% CI: 1.58-2.19) [72]. Insulin is considered to have a role in promoting cell proliferation and inhibiting apoptosis, acting as a potent growth factor and mitogen. It can increase the biological activity of IGF-1, thereby promoting tumor cell proliferation. Long-term use of exogenous insulin may elevate insulin levels in diabetic patients, thereby increasing the risk of CRC [73]. Therefore, diabetic patients receiving insulin treatment may require closer screening for CRC.
Interestingly, insulin analogs are associated with reduced CRC risk. Patients with T2DM using long-acting insulin analogs showed a reduction in the incidence of CRC (HR: 0.40, 95% CI: 0.24-0.69), as did those using a combination of rapid-acting and long-acting insulin (HR: 0.47, 95% CI: 0.25-0.88) [74]. However, in other studies, the use of long-acting insulin in patients with T2DM did not reduce the incidence of CRC (HR: 0.96, 95% CI: 0.70-1.34) [75,76]. It is clear that more research is needed to verify and further explore the impact of insulin analogs on the risk of CRC in T2DM patients. Clarifying this relationship will help to develop more accurate treatment plans and guidelines, as well as better understand the role of insulin analogs in cancer risk management.
Other hypoglycemic drugs
Studies on other glucose-lowering drugs have shown promising results. For instance, the use of statins can reduce the mortality rate of colorectal cancer (HR: 0.69, 95% CI: 0.54-0.89) [66]. An observational study involving 1,343,484 patients with T2DM found that acarbose reduces the incidence of CRC in T2DM patients in a dose-dependent manner [77]. In T2DM patients over 65 years old, the use of sitagliptin can reduce the risk of CRC, while the use of glimepiride and other sulfonylureas may increase the risk of CRC [78]. A meta-analysis of thiazolidinediones (TZDs) in T2DM patients showed that the CRC risk in TZD users reduced to a certain degree (estimated to be reduced by 9%) compared to non-TZD users [79]. Currently, the research on the relationship between acarbose, sulfonylureas, TZDs and CRC risk is still unsatisfactory, and further clinical studies are necessary to explore these relationships.
Perspectives
This review illustrates the specific mechanisms by which hyperglycemia affects the development of CRC, including cell proliferation and apoptosis, inflammatory response, oxidative stress, immune regulation, angiogenesis, and epigenetic changes in order to enhance our understanding of the association between hyperglycemia and CRC. Furthermore, it is evident that hyperglycemia has a definite impact on the prognosis of CRC, affecting the efficacy of anti-tumor treatments. Evaluating the effects of drug interventions targeting hyperglycemia (such as metformin, insulin, and other antidiabetic medications) on the risk of CRC can help in formulating more effective clinical management strategies.
Despite these findings, there are some limitations to consider. For instance, many studies have relied on observational designs, which may be subject to confounding factors and reverse causality, limiting the certainty of causal relationships. Although some mechanisms by which hyperglycemia affects the development of CRC have been proposed, a comprehensive understanding of these mechanisms is still limited, and more basic research is needed to clarify them. Differences in study designs, such as sample size, population characteristics, and methods of measuring and assessing glycemic control, may lead to inconsistencies in results. It is hoped that more prospective, randomized controlled trials, and Mendelian randomization studies will be conducted in the future to reduce bias and improve the accuracy of causal inferences. Exploring the sensitivity of different patient populations, such as those with diabetes and individuals with different genetic backgrounds, to the risks of hyperglycemia and CRC will help to develop personalized prevention and treatment strategies. Additionally, studies are needed as to how lifestyle factors (such as diet and exercise) interact with hyperglycemia and CRC risk, and how changes in lifestyle can reduce the risk.
In conclusion, we reviewed future research directions, and delved into the mechanisms and clinical applications of the correlation between hyperglycemia and CRC, hoping to provide more effective strategies for the prevention and treatment of CRC.
Acknowledgements
We express gratitude to those who have contributed to the research work, institutions or organizations.
Disclosure of conflict of interest
None.
References
- 1.Zeng H, Ran X, An L, Zheng R, Zhang S, Ji JS, Zhang Y, Chen W, Wei W, He J HBCR Working Group. Disparities in stage at diagnosis for five common cancers in China: a multicentre, hospital-based, observational study. Lancet Public Health. 2021;6:e877–e887. doi: 10.1016/S2468-2667(21)00157-2. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 3.Heisser T, Cardoso R, Niedermaier T, Hoffmeister M, Brenner H. Making colonoscopy-based screening more efficient: a “gateopener” approach. Int J Cancer. 2023;152:952–961. doi: 10.1002/ijc.34317. [DOI] [PubMed] [Google Scholar]
- 4.Spaander MCW, Zauber AG, Syngal S, Blaser MJ, Sung JJ, You YN, Kuipers EJ. Young-onset colorectal cancer. Nat Rev Dis Primers. 2023;9:21. doi: 10.1038/s41572-023-00432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kasprzak A. Insulin-like growth factor 1 (IGF-1) signaling in glucose metabolism in colorectal cancer. Int J Mol Sci. 2021;22:6434. doi: 10.3390/ijms22126434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sciacca L, Vigneri R, Tumminia A, Frasca F, Squatrito S, Frittitta L, Vigneri P. Clinical and molecular mechanisms favoring cancer initiation and progression in diabetic patients. Nutr Metab Cardiovasc Dis. 2013;23:808–815. doi: 10.1016/j.numecd.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Supabphol S, Seubwai W, Wongkham S, Saengboonmee C. High glucose: an emerging association between diabetes mellitus and cancer progression. J Mol Med (Berl) 2021;99:1175–1193. doi: 10.1007/s00109-021-02096-w. [DOI] [PubMed] [Google Scholar]
- 8.Vekic J, Zeljkovic A, Stefanovic A, Giglio RV, Ciaccio M, Rizzo M. Diabetes and colorectal cancer risk: a new look at molecular mechanisms and potential role of novel antidiabetic agents. Int J Mol Sci. 2021;22:12409. doi: 10.3390/ijms222212409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutiérrez-Salmerón M, Lucena SR, Chocarro-Calvo A, García-Martínez JM, Martín Orozco RM, García-Jiménez C. Metabolic and hormonal remodeling of colorectal cancer cell signalling by diabetes. Endocr Relat Cancer. 2021;28:R191–R206. doi: 10.1530/ERC-21-0092. [DOI] [PubMed] [Google Scholar]
- 10.Xu CX, Zhu HH, Zhu YM. Diabetes and cancer: associations, mechanisms, and implications for medical practice. World J Diabetes. 2014;5:372–380. doi: 10.4239/wjd.v5.i3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu B, Qu S. The relationship between diabetes mellitus and cancers and its underlying mechanisms. Front Endocrinol (Lausanne) 2022;13:800995. doi: 10.3389/fendo.2022.800995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peeters PJ, Bazelier MT, Leufkens HG, de Vries F, De Bruin ML. The risk of colorectal cancer in patients with type 2 diabetes: associations with treatment stage and obesity. Diabetes Care. 2015;38:495–502. doi: 10.2337/dc14-1175. [DOI] [PubMed] [Google Scholar]
- 13.Szablewski L. Diabetes mellitus: influences on cancer risk. Diabetes Metab Res Rev. 2014;30:543–553. doi: 10.1002/dmrr.2573. [DOI] [PubMed] [Google Scholar]
- 14.Ling S, Brown K, Miksza JK, Howells L, Morrison A, Issa E, Yates T, Khunti K, Davies MJ, Zaccardi F. Association of type 2 diabetes with cancer: a meta-analysis with bias analysis for unmeasured confounding in 151 cohorts comprising 32 million people. Diabetes Care. 2020;43:2313–2322. doi: 10.2337/dc20-0204. [DOI] [PubMed] [Google Scholar]
- 15.Sona MF, Myung SK, Park K, Jargalsaikhan G. Type 1 diabetes mellitus and risk of cancer: a meta-analysis of observational studies. Jpn J Clin Oncol. 2018;48:426–433. doi: 10.1093/jjco/hyy047. [DOI] [PubMed] [Google Scholar]
- 16.Goto A, Yamaji T, Sawada N, Momozawa Y, Kamatani Y, Kubo M, Shimazu T, Inoue M, Noda M, Tsugane S, Iwasaki M. Diabetes and cancer risk: a Mendelian randomization study. Int J Cancer. 2020;146:712–719. doi: 10.1002/ijc.32310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy N, Song M, Papadimitriou N, Carreras-Torres R, Langenberg C, Martin RM, Tsilidis KK, Barroso I, Chen J, Frayling TM, Bull CJ, Vincent EE, Cotterchio M, Gruber SB, Pai RK, Newcomb PA, Perez-Cornago A, van Duijnhoven FJB, Van Guelpen B, Vodicka P, Wolk A, Wu AH, Peters U, Chan AT, Gunter MJ. Associations between glycemic traits and colorectal cancer: a Mendelian randomization analysis. J Natl Cancer Inst. 2022;114:740–752. doi: 10.1093/jnci/djac011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao X, Wu X, Yi L, You F, Li X, Xiao C. Causal linkage between type 2 diabetes mellitus and inflammatory bowel disease: an integrated Mendelian randomization study and bioinformatics analysis. Front Endocrinol (Lausanne) 2024;15:1275699. doi: 10.3389/fendo.2024.1275699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh S, Earle CC, Bae SJ, Fischer HD, Yun L, Austin PC, Rochon PA, Anderson GM, Lipscombe L. Incidence of diabetes in colorectal cancer survivors. J Natl Cancer Inst. 2016;108:djv402. doi: 10.1093/jnci/djv402. [DOI] [PubMed] [Google Scholar]
- 20.Xiao Y, Wang H, Tang Y, Yan J, Cao L, Chen Z, Shao Z, Mei Z, Jiang Z. Increased risk of diabetes in cancer survivors: a pooled analysis of 13 population-based cohort studies. ESMO Open. 2021;6:100218. doi: 10.1016/j.esmoop.2021.100218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong X, He X, Wang Y, Hu Z, Huang H, Zhao S, Wei P, Li D. Warburg effect in colorectal cancer: the emerging roles in tumor microenvironment and therapeutic implications. J Hematol Oncol. 2022;15:160. doi: 10.1186/s13045-022-01358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bensard CL, Wisidagama DR, Olson KA, Berg JA, Krah NM, Schell JC, Nowinski SM, Fogarty S, Bott AJ, Wei P, Dove KK, Tanner JM, Panic V, Cluntun A, Lettlova S, Earl CS, Namnath DF, Vázquez-Arreguín K, Villanueva CJ, Tantin D, Murtaugh LC, Evason KJ, Ducker GS, Thummel CS, Rutter J. Regulation of tumor initiation by the mitochondrial pyruvate carrier. Cell Metab. 2020;31:284–300. e287. doi: 10.1016/j.cmet.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Shay JW. Multiple roles of APC and its therapeutic implications in colorectal cancer. J Natl Cancer Inst. 2017;109:djw332. doi: 10.1093/jnci/djw332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cha PH, Hwang JH, Kwak DK, Koh E, Kim KS, Choi KY. APC loss induces Warburg effect via increased PKM2 transcription in colorectal cancer. Br J Cancer. 2021;124:634–644. doi: 10.1038/s41416-020-01118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pate KT, Stringari C, Sprowl-Tanio S, Wang K, TeSlaa T, Hoverter NP, McQuade MM, Garner C, Digman MA, Teitell MA, Edwards RA, Gratton E, Waterman ML. Wnt signaling directs a metabolic program of glycolysis and angiogenesis in colon cancer. EMBO J. 2014;33:1454–1473. doi: 10.15252/embj.201488598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 27.Ho FM, Lin WW, Chen BC, Chao CM, Yang CR, Lin LY, Lai CC, Liu SH, Liau CS. High glucose-induced apoptosis in human vascular endothelial cells is mediated through NF-kappaB and c-Jun NH2-terminal kinase pathway and prevented by PI3K/Akt/eNOS pathway. Cell Signal. 2006;18:391–399. doi: 10.1016/j.cellsig.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Ning H, Qiu X, Baine L, Lin G, Lue TF, Lin CS. Effects of high glucose on human cavernous endothelial cells. Urology. 2012;80:1162, e7–11. doi: 10.1016/j.urology.2012.04.071. [DOI] [PubMed] [Google Scholar]
- 29.Tomas NM, Masur K, Piecha JC, Niggemann B, Zänker KS. Akt and phospholipase Cγ are involved in the regulation of growth and migration of MDA-MB-468 breast cancer and SW480 colon cancer cells when cultured with diabetogenic levels of glucose and insulin. BMC Res Notes. 2012;5:214. doi: 10.1186/1756-0500-5-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masur K, Vetter C, Hinz A, Tomas N, Henrich H, Niggemann B, Zänker KS. Diabetogenic glucose and insulin concentrations modulate transcriptome and protein levels involved in tumour cell migration, adhesion and proliferation. Br J Cancer. 2011;104:345–352. doi: 10.1038/sj.bjc.6606050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang YH, Yang HJ, Chen HW, Hsiao CW, Hsieh YC, Chan YW, Chang SW, Hwang WL, Chen WS, Cheng HH, Chou TY, Chang FP, Ho HL, Chu FY, Lo YL, Chen CJ, Tsai HF, Shiau MY. Characterization of collapsin response mediator protein 2 in colorectal cancer progression in subjects with diabetic comorbidity. Cells. 2022;11:727. doi: 10.3390/cells11040727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang B, Wang S, Wang W, Liu E, Guo S, Zhao C, Niu J, Zhang Z. Hyperglycemia promotes liver metastasis of colorectal cancer via upregulation of integrin αvβ6. Med Sci Monit. 2021;27:e930921. doi: 10.12659/MSM.930921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu J, Chen J, Xi Y, Wang F, Sha H, Luo L, Zhu Y, Hong X, Bu S. High glucose induces epithelial-mesenchymal transition and results in the migration and invasion of colorectal cancer cells. Exp Ther Med. 2018;16:222–230. doi: 10.3892/etm.2018.6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matějka VM, Fínek J, Králíčková M. Epithelial-mesenchymal transition in tumor tissue and its role for metastatic spread of cancer. Klin Onkol. 2017;30:20–27. doi: 10.14735/amko201720. [DOI] [PubMed] [Google Scholar]
- 35.Lu CC, Chu PY, Hsia SM, Wu CH, Tung YT, Yen GC. Insulin induction instigates cell proliferation and metastasis in human colorectal cancer cells. Int J Oncol. 2017;50:736–744. doi: 10.3892/ijo.2017.3844. [DOI] [PubMed] [Google Scholar]
- 36.Yang IP, Tsai HL, Huang CW, Lu CY, Miao ZF, Chang SF, Juo SH, Wang JY. High blood sugar levels significantly impact the prognosis of colorectal cancer patients through down-regulation of microRNA-16 by targeting Myb and VEGFR2. Oncotarget. 2016;7:18837–18850. doi: 10.18632/oncotarget.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JM, Lee KM, Kim DB, Ko SH, Park YG. Colorectal cancer risks according to sex differences in patients with type II diabetes mellitus: a Korean nationwide population-based cohort study. Clin Transl Gastroenterol. 2019;10:e00090. doi: 10.14309/ctg.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang SC, Yang WV. Hyperglycemia, tumorigenesis, and chronic inflammation. Crit Rev Oncol Hematol. 2016;108:146–153. doi: 10.1016/j.critrevonc.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Lisco G, Giagulli VA, De Pergola G, Guastamacchia E, Jirillo E, Triggiani V. Hyperglycemia-induced immune system disorders in diabetes mellitus and the concept of hyperglycemic memory of innate immune cells: a perspective. Endocr Metab Immune Disord Drug Targets. 2022;22:367–370. doi: 10.2174/1871530321666210924124336. [DOI] [PubMed] [Google Scholar]
- 40.Dey S, Murmu N, Mondal T, Saha I, Chatterjee S, Manna R, Haldar S, Dash SK, Sarkar TR, Giri B. Multifaceted entrancing role of glucose and its analogue, 2-deoxy-D-glucose in cancer cell proliferation, inflammation, and virus infection. Biomed Pharmacother. 2022;156:113801. doi: 10.1016/j.biopha.2022.113801. [DOI] [PubMed] [Google Scholar]
- 41.Deng R, Wu H, Ran H, Kong X, Hu L, Wang X, Su Q. Glucose-derived AGEs promote migration and invasion of colorectal cancer by up-regulating Sp1 expression. Biochim Biophys Acta Gen Subj. 2017;1861:1065–1074. doi: 10.1016/j.bbagen.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 42.Zhang P, Li T, Wu X, Nice EC, Huang C, Zhang Y. Oxidative stress and diabetes: antioxidative strategies. Front Med. 2020;14:583–600. doi: 10.1007/s11684-019-0729-1. [DOI] [PubMed] [Google Scholar]
- 43.Ighodaro OM. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed Pharmacother. 2018;108:656–662. doi: 10.1016/j.biopha.2018.09.058. [DOI] [PubMed] [Google Scholar]
- 44.Mihajlovic M, Gojkovic T, Vladimirov S, Miljkovic M, Stefanovic A, Vekic J, Zeljkovic D, Trifunovic B, Kotur-Stevuljevic J, Spasojevic-Kalimanovska V, Zeljkovic A. Changes in lecithin: cholesterol acyltransferase, cholesteryl ester transfer protein and paraoxonase-1 activities in patients with colorectal cancer. Clin Biochem. 2019;63:32–38. doi: 10.1016/j.clinbiochem.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Liu H, Liu X, Zhang C, Zhu H, Xu Q, Bu Y, Lei Y. Redox imbalance in the development of colorectal cancer. J Cancer. 2017;8:1586–1597. doi: 10.7150/jca.18735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi F, Wei B, Lan T, Xiao Y, Quan X, Chen J, Zhao C, Gao J. Low NLRP3 expression predicts a better prognosis of colorectal cancer. Biosci Rep. 2021;41:BSR20210280. doi: 10.1042/BSR20210280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Wang L, Pappan L, Galliher-Beckley A, Shi J. IL-1β promotes stemness and invasiveness of colon cancer cells through Zeb1 activation. Mol Cancer. 2012;11:87. doi: 10.1186/1476-4598-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shomali N, Mahmoudi J, Mahmoodpoor A, Zamiri RE, Akbari M, Xu H, Shotorbani SS. Harmful effects of high amounts of glucose on the immune system: an updated review. Biotechnol Appl Biochem. 2021;68:404–410. doi: 10.1002/bab.1938. [DOI] [PubMed] [Google Scholar]
- 49.Lv Y, Lin S, Liu M, Wang L, Wang X, Cui L, Xu J. Impacts of pre-existing diabetes mellitus on colorectal cancer in a mice model. Cancer Med. 2023;12:11641–11650. doi: 10.1002/cam4.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lega IC, Lipscombe LL. Review: diabetes, obesity, and cancer-pathophysiology and clinical implications. Endocr Rev. 2020;41:bnz014. doi: 10.1210/endrev/bnz014. [DOI] [PubMed] [Google Scholar]
- 51.Cepas V, Collino M, Mayo JC, Sainz RM. Redox signaling and advanced glycation endproducts (AGEs) in diet-related diseases. Antioxidants (Basel) 2020;9:142. doi: 10.3390/antiox9020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azizian-Farsani F, Abedpoor N, Hasan Sheikhha M, Gure AO, Nasr-Esfahani MH, Ghaedi K. Receptor for advanced glycation end products acts as a fuel to colorectal cancer development. Front Oncol. 2020;10:552283. doi: 10.3389/fonc.2020.552283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grzebyk E, Piwowar A. Inhibitory actions of selected natural substances on formation of advanced glycation endproducts and advanced oxidation protein products. BMC Complement Altern Med. 2016;16:381. doi: 10.1186/s12906-016-1353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vigorelli V, Resta J, Bianchessi V, Lauri A, Bassetti B, Agrifoglio M, Pesce M, Polvani G, Bonalumi G, Cavallotti L, Alamanni F, Genovese S, Pompilio G, Vinci MC. Abnormal DNA methylation induced by hyperglycemia reduces CXCR4 gene expression in CD34(+) stem cells. J Am Heart Assoc. 2019;8:e010012. doi: 10.1161/JAHA.118.010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biasci D, Smoragiewicz M, Connell CM, Wang Z, Gao Y, Thaventhiran JED, Basu B, Magiera L, Johnson TI, Bax L, Gopinathan A, Isherwood C, Gallagher FA, Pawula M, Hudecova I, Gale D, Rosenfeld N, Barmpounakis P, Popa EC, Brais R, Godfrey E, Mir F, Richards FM, Fearon DT, Janowitz T, Jodrell DI. CXCR4 inhibition in human pancreatic and colorectal cancers induces an integrated immune response. Proc Natl Acad Sci U S A. 2020;117:28960–28970. doi: 10.1073/pnas.2013644117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hjazi A, Nasir F, Noor R, Alsalamy A, Zabibah RS, Romero-Parra RM, Ullah MI, Mustafa YF, Qasim MT, Akram SV. The pathological role of C-X-C chemokine receptor type 4 (CXCR4) in colorectal cancer (CRC) progression; special focus on molecular mechanisms and possible therapeutics. Pathol Res Pract. 2023;248:154616. doi: 10.1016/j.prp.2023.154616. [DOI] [PubMed] [Google Scholar]
- 57.Al-Haddad R, Karnib N, Assaad RA, Bilen Y, Emmanuel N, Ghanem A, Younes J, Zibara V, Stephan JS, Sleiman SF. Epigenetic changes in diabetes. Neurosci Lett. 2016;625:64–69. doi: 10.1016/j.neulet.2016.04.046. [DOI] [PubMed] [Google Scholar]
- 58.Saini MK, Sanyal SN. Cell cycle regulation and apoptotic cell death in experimental colon carcinogenesis: intervening with cyclooxygenase-2 inhibitors. Nutr Cancer. 2015;67:620–636. doi: 10.1080/01635581.2015.1015743. [DOI] [PubMed] [Google Scholar]
- 59.Li H, Li C, Zhang B, Jiang H. Lactoferrin suppresses the progression of colon cancer under hyperglycemia by targeting WTAP/m(6)A/NT5DC3/HKDC1 axis. J Transl Med. 2023;21:156. doi: 10.1186/s12967-023-03983-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma YS, Yang IP, Tsai HL, Huang CW, Juo SH, Wang JY. High glucose modulates antiproliferative effect and cytotoxicity of 5-fluorouracil in human colon cancer cells. DNA Cell Biol. 2014;33:64–72. doi: 10.1089/dna.2013.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang YT, Tsai HL, Kung YT, Yeh YS, Huang CW, Ma CJ, Chiu HC, Wang JY. Dose-dependent relationship between metformin and colorectal cancer occurrence among patients with type 2 diabetes-a nationwide cohort study. Transl Oncol. 2018;11:535–541. doi: 10.1016/j.tranon.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang YJ, Chen YT, Huang CM, Kuo SH, Liao YY, Jhang WY, Wang SH, Ke CC, Huang YH, Cheng CM, Huang MY, Chuang CH. HIF-1α expression increases preoperative concurrent chemoradiotherapy resistance in hyperglycemic rectal cancer. Cancers (Basel) 2022;14:4053. doi: 10.3390/cancers14164053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown JC, Zhang S, Ou FS, Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, O’Neil BH, Shaw JE, Polite BN, Denlinger CS, Atkins JN, Goldberg RM, Ng K, Mayer RJ, Blanke CD, O’Reilly EM, Fuchs CS, Meyerhardt JA. Diabetes and clinical outcome in patients with metastatic colorectal cancer: CALGB 80405 (Alliance) JNCI Cancer Spectr. 2019;4:pkz078. doi: 10.1093/jncics/pkz078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miranda Baleiras M, Dias Domingues T, Severino E, Vasques C, Neves MT, Ferreira A, Vasconcelos de Matos L, Ferreira F, Miranda H, Martins A. Prognostic impact of type 2 diabetes in metastatic colorectal cancer. Cureus. 2023;15:e33916. doi: 10.7759/cureus.33916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mailliez A, Ternynck C, Duhamel A, Mailliez A, Ploquin A, Desauw C, Lemaitre M, Bertrand N, Vambergue A, Turpin A. Diabetes is associated with high risk of severe adverse events during chemotherapy for cancer patients: a single-center study. Int J Cancer. 2023;152:408–416. doi: 10.1002/ijc.34268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Erkinantti S, Hautakoski A, Sund R, Arffman M, Urpilainen E, Puistola U, Jukkola A, Peeter K, Läärä E. The association of metformin, other antidiabetic medications, and statins with the prognosis of colon cancer in patients with type 2 diabetes: a retrospective cohort study. Cancer Control. 2022;29:10732748221134090. doi: 10.1177/10732748221134090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ng CW, Jiang AA, Toh EMS, Ng CH, Ong ZH, Peng S, Tham HY, Sundar R, Chong CS, Khoo CM. Metformin and colorectal cancer: a systematic review, meta-analysis and meta-regression. Int J Colorectal Dis. 2020;35:1501–1512. doi: 10.1007/s00384-020-03676-x. [DOI] [PubMed] [Google Scholar]
- 68.De Santi M, Baldelli G, Diotallevi A, Galluzzi L, Schiavano GF, Brandi G. Metformin prevents cell tumorigenesis through autophagy-related cell death. Sci Rep. 2019;9:66. doi: 10.1038/s41598-018-37247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sugiura K, Okabayashi K, Seishima R, Ishida T, Shigeta K, Tsuruta M, Hasegawa H, Kitagawa Y. Metformin inhibits the development and metastasis of colorectal cancer. Med Oncol. 2022;39:136. doi: 10.1007/s12032-022-01722-y. [DOI] [PubMed] [Google Scholar]
- 70.Boutaud M, Auger C, Verdier M, Christou N. Metformin treatment reduces CRC aggressiveness in a glucose-independent manner: an in vitro and ex vivo study. Cancers (Basel) 2023;15:3724. doi: 10.3390/cancers15143724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh PP, Shi Q, Foster NR, Grothey A, Nair SG, Chan E, Shields AF, Goldberg RM, Gill S, Kahlenberg MS, Sinicrope FA, Sargent DJ, Alberts SR. Relationship between metformin use and recurrence and survival in patients with resected stage iii colon cancer receiving adjuvant chemotherapy: results from North Central Cancer Treatment Group N0147 (Alliance) Oncologist. 2016;21:1509–1521. doi: 10.1634/theoncologist.2016-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen CH, Lin CL, Hsu CY, Kao CH. Insulin enhances and metformin reduces risk of colorectal carcinoma in type-2 diabetes. QJM. 2020;113:194–200. doi: 10.1093/qjmed/hcz253. [DOI] [PubMed] [Google Scholar]
- 73.Yu GH, Li SF, Wei R, Jiang Z. Diabetes and colorectal cancer risk: clinical and therapeutic implications. J Diabetes Res. 2022;2022:1747326. doi: 10.1155/2022/1747326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin CM, Huang HL, Chu FY, Fan HC, Chen HA, Chu DM, Wu LW, Wang CC, Chen WL, Lin SH, Ho SY. Association between gastroenterological malignancy and diabetes mellitus and anti-diabetic therapy: a nationwide, population-based cohort study. PLoS One. 2015;10:e0125421. doi: 10.1371/journal.pone.0125421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pradhan R, Yin H, Yu OHY, Azoulay L. The use of long-acting insulin analogs and the risk of colorectal cancer among patients with type 2 diabetes: a population-based cohort study. Drug Saf. 2020;43:103–110. doi: 10.1007/s40264-019-00892-5. [DOI] [PubMed] [Google Scholar]
- 76.Wu JW, Filion KB, Azoulay L, Doll MK, Suissa S. Effect of long-acting insulin analogs on the risk of cancer: a systematic review of observational studies. Diabetes Care. 2016;39:486–494. doi: 10.2337/dc15-1816. [DOI] [PubMed] [Google Scholar]
- 77.Tseng YH, Tsan YT, Chan WC, Sheu WH, Chen PC. Use of an α-glucosidase inhibitor and the risk of colorectal cancer in patients with diabetes: a nationwide, population-based cohort study. Diabetes Care. 2015;38:2068–2074. doi: 10.2337/dc15-0563. [DOI] [PubMed] [Google Scholar]
- 78.Shin CM, Kim N, Han K, Kim B, Jung JH, Oh TJ, Lee DH. Anti-diabetic medications and the risk for colorectal cancer: a population-based nested case-control study. Cancer Epidemiol. 2020;64:101658. doi: 10.1016/j.canep.2019.101658. [DOI] [PubMed] [Google Scholar]
- 79.Liu Y, Jin PP, Sun XC, Hu TT. Thiazolidinediones and risk of colorectal cancer in patients with diabetes mellitus: a meta-analysis. Saudi J Gastroenterol. 2018;24:75–81. doi: 10.4103/sjg.SJG_295_17. [DOI] [PMC free article] [PubMed] [Google Scholar]


