Abstract
Liquid biopsy is an innovative approach that provides a more complete understanding of treatment response and prognosis in monitoring metastatic prostate cancer. It complements invasive tissue biopsy and involves the assessment of various biomarkers in body fluids such as blood, semen, and urine. Liquid biopsy analyzes circulating tumor cells, extracellular vesicles, circulating tumor DNA, and the secretome. This is particularly important given the heterogeneity of prostate cancer and the need for better prognostic biomarkers. Liquid biopsy can personalize the treatment of homonosensitive and castration-resistant metastatic prostate cancer by acting as a predictive and prognostic tool. This review discusses various biomarkers, assay techniques, and potential applications in daily clinical practice, highlighting the exciting possibilities that this emerging field holds for improving patient outcomes.
Keywords: Liquid biopsy, prostate cancer, circulating tumor DNA, circulating tumor cells, extracellular vesicles, secretome
Introduction
Prostate cancer (PCa) is a complex disease that is underdiagnosed using current conventional tests such as prostate-specific antigen (PSA), prostate biopsy, and/or imaging modalities, that do not reflect tumor heterogeneity [1,2]. In recent years, great progress has been made in understanding the complexity and variability of PCa, especially in advanced or metastatic stages [3]. Liquid biopsy (LB) represents a relevant advance in tumor characterization through a minimally invasive method that can analyze body fluids such as blood, saliva, semen, and urine (Figure 1). This allows the identification of biomarkers like circulating tumor cells (CTC), cell-free DNA (cfDNA), circulating tumor DNA (ctDNA), extracellular vesicles (EV), and the secretome [3,4].
Figure 1.
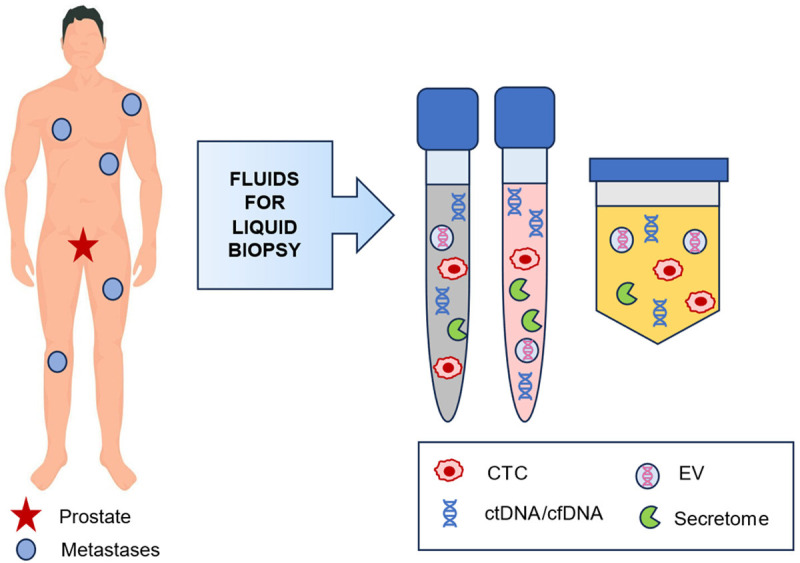
Fluids for liquid biopsy in metastatic prostate cancer: semen, blood, and urine. Biomarkers in liquid biopsy: CTC, ctDNA/cfDNA, EV, and secretome.
The real-time genomic, transcriptomic, and epigenomic information obtained by LB [5] allows continuous monitoring of tumor evolution, revealing dynamic changes that occur during active treatment or disease progression. It can be a useful tool for early detection of advanced disease, prognosis, treatment selection, response evaluation, and identification of resistance mechanisms or residual disease [1]. LB also overcomes certain limitations of solid biopsy, such as the lack of representation in terms of tumor heterogeneity, the low validity of the molecular characterization of tumor tissue processed at diagnosis, and the difficulty of sampling longitudinally or from multiple lesions in the case of tumor progression [6]. Nevertheless, the clinical application of LB is limited by the lack of standard protocols, the absence of prospective multicenter studies, and restrictions in terms of access and cost constraints [2,5,7,8].
Materials and methods
We performed a bibliographic search in Pubmed/Medline with different keyword combinations using the formula: (“liquid biopsy” AND “metastatic prostate cancer”) OR (“CTC” AND “metastatic prostate cancer”) OR (“ctDNA” AND “metastatic prostate cancer”) OR (“cfDNA” AND “metastatic prostate cancer”) OR (“extracellular vesicles” AND “metastatic prostate cancer”) OR (“secretome” AND “metastatic prostate cancer”). Through this search, we have identified the studies that informed on biomarkers for metastatic prostate cancer in LB up to October 2023.
Results
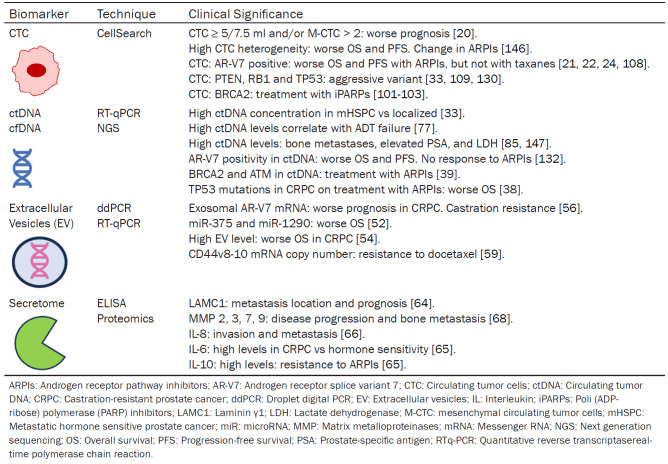
Types of analytes in liquid biopsy (Table 1)
Table 1.
Liquid biopsy biomarkers in metastatic prostate cancer. Detection techniques and clinical significance of the different analytes
CTC: circulating tumor cells
CTCs are an intermediate state between the primary tumor and metastases that can be evaluated in the blood as a marker of dissemination [9,10]. Unlike PSA, CTC detection is not dependent on androgen signaling pathways [11]. CTC quantification can outperform PSA as a response biomarker, regardless of the initial number [12]. The main limitation of CTCs is their low concentration in blood (1 CTC/million leukocytes) [13]. Therefore, techniques with high sensitivity and specificity are required for sample processing, isolation, and enumeration. Currently, available methods can be divided into two groups, EpCAM (epithelial cell adhesion molecule)-dependent and EpCAM-independent [14]. One of the most widely used is CellSearch® [15], which has been approved by the Food and Drug Administration (FDA) for clinical use in patients with metastatic castration-resistant PCa (mCRPC). The study by Bono et al. reported that a favorable CTC count (< 5 cells/7.5 ml) predicted significantly better progression-free survival (PFS) and overall survival (OS) than an unfavorable count (≥ 5 cells/7.5 ml) [16].
CTCs reflect more aggressive disease after acquiring a mesenchymal phenotype during the epithelial-mesenchymal transition [17]. CTCs can be detected using the CanPatrol CTC enrichment technique, which utilizes epithelial and mesenchymal biomarkers. These cells are divided into three types: E-CTC (epithelial), M-CTC (mesenchymal), and Bi-CTC (biphenotypic: mesenchymal and epithelial) [18,19]. A total CTC count of ≥ 5 and M-CTC ≥ 2 are independent predictors of early progression and shorter cancer-specific survival [20].
CTCs are also used as material for molecular studies. For example, in the detection of AR-V7 [21-25] and BRCA2 [26,27], CTCs enable characterization of the aggressive variant (PTEN, RB1 and TP53) [28,29] or sequencing to define tumor heterogeneity [28].
ctDNA and cfDNA
cfDNA is the total amount of circulating DNA in blood plasma, representing the total DNA shed by normal and tumor cells [30,31]. It has a short half-life (from minutes to hours), which is an advantage over other protein-based biomarkers that require several weeks to assess representative changes in tumor dynamics. In addition, these changes are independent of the androgen receptor (AR) pathway [32]. Chen et al. [33] observed an increase in plasma cfDNA concentration in patients with metastatic hormone-sensitive PCa (mHSPC) compared to patients with localized PCa and healthy individuals, establishing cfDNA as a biomarker of poor prognosis.
ctDNA is the fraction of cfDNA derived from tumor cells of the primary tumor, metastases, and CTCs, that is shed into the circulation and can range from 0.01% to 90% of total cfDNA [34]. Plasma levels can vary depending on tumor type, stage, and/or tumor burden. Higher levels are observed in metastatic cancer than in localized cancer, and also correlate with progression. Tumor progression can be monitored during therapy without the limitations of intratumoral heterogeneity in solid biopsies [1]. There are several methods for the quantitative and qualitative detection of ctDNA, the two most important being digital polymerase chain reaction (dPCR) [35], which has higher sensitivity and specificity (especially in the case of hotspot mutations), and next-generation sequencing (NGS). The latter covers a larger number of mutations and allows whole genome sequencing [36,37].
Characterization of genomic alterations from ctDNA can identify mutations (BRCA2, ATM, TP53) [38,39], copy number alterations [40], and structural rearrangements, which are useful as predictive and prognostic biomarkers [3]. Variations in DNA methylation, either global or locus-specific (GSTP-1, DOCK2, HALPN3, FBXO30), have been correlated with tumor burden, treatment response, and OS [6,40-42].
Extracellular vesicles (EV)
EVs are particles surrounded by a lipid bilayer. They include exosomes, microvesicles, and apoptotic bodies, all of which play a fundamental role in intercellular communication [43-45]. Through this protective layer, they can transport proteins, lipids, messenger RNA (mRNA), and microRNA (miRNA) to recipient cells, exchange genetic material, and modify the tumor microenvironment, thereby facilitating progression [46], metastasis and drug resistance [47]. They are secreted by all cell types and can reach all body fluids [48]. There is no universal method for their analysis, depending on the type of biological fluid, sample transport, and the molecule being studied. Some techniques include PCR, sequencing, western blotting, ELISA, and expression analysis [49]. Some advantages over other biomarkers are molecular stability, bioavailability, and therapeutic potential of the analysis [49]. They are some of the first biomarkers to be studied in PCa [50] and are a very active area of research. The main goal of these markers is to improve the detection of clinically significant disease and to aid in risk stratification decisions [51].
Several studies have reported changes in the expression levels of miRNAs isolated from patients with mCRPC that have prognostic value [52,53]. It should be noted that a higher concentration of EVs in plasma correlates with worse OS and castration resistance [54]. Expression of EV AR-V7 correlates with worse PFS and OS [55-58]. The number of mRNA copies predicts resistance to docetaxel [59]. The release of BRN4 and BRN2 mRNA from serum EV may modulate the progression of neuroendocrine PCa [60].
In addition, there is emerging evidence that DNA molecules in EVs may be superior to ctDNA as a biomarker in cancer [61,62].
Secretome
This term refers to the group of proteins secreted by tumor cells into the extracellular space (including proteinases, cytokines, and growth factors), that are involved in the processes of differentiation, invasion, metastasis, and angiogenesis [63].
The study of the metastatic PCa cell lines DU145 and PC3 identified a total of 598 secreted proteins such as laminin gamma 1 (LAMC1) and six mutated peptides capable of identifying different metastatic sites. These could help in the development of targeted therapy and become a new predictive and prognostic biomarker for metastasis [64].
IL-6 and IL-10 levels are consistently and significantly increased (P < 0.05) in patients with mCRPC who do not respond to abiraterone and/or enzalutamide [65]. In the CHAARTED trial, high levels of IL-8 in mHSPC before androgen deprivation therapy (ADT) predicted the development of bone metastases, castration resistance, and worse OS, independent of metastatic burden, time to metastasis, or docetaxel use (P < 0.001) [66]. IL-23 promotes the progression of mCRPC, so anti-IL-23 therapy could reverse castration resistance and improve the efficacy of enzalutamide [67].
Several studies have found increased levels of several metalloproteases (MMPs) such as MMP-2, -3, -7, -9, -13, -14, -15, and -26 in metastatic PCa, with a significant decrease in response to therapy [68]. Frieling et al. observed high levels of MMP-3 in patients with bone metastases [69], and Dhar et al. analyzed MMP-1, -2, -7, and -9 in mCRPC and found that clinical and biochemical responders had lower levels of MMP [70]. These results suggest that MMP could be used as a prognostic biomarker.
Cathepsins are another family of proteases that promote tumor progression. Cathepsin K expression is significantly higher in bone metastases than in primary PCa and is absent in normal prostate tissue [71].
High levels of insulin-like growth factor 1 (IGF-1) have been associated with an increased risk of disease progression and the development of bone metastases. Ongoing clinical trials are investigating this mechanism to improve survival [72].
mHSPC: prognostic and predictive value: (Table 2)
Table 2.
Studies of liquid biopsy in mHSPC: clinical applications
| Prognostic Value | |||
|
| |||
| Study | Analyte | N | Result |
|
| |||
| Reichert et al. [74] | CTC count | 58 | High CTC before ADT is more common in high tumor burden (P < 0.003) and correlated with no biochemical response at 7 months (P < 0.008). |
| Resel et al. [75] | CellSearch | 30 | CTC ≥ 4 worse OS and PFS (P < 0.001). |
| Yang et al. [76] | Can Patrol. M-CTC | 108 | > 2 M-CTC earlier development of CRPC and worse OS. |
| Yang et al. [20] | 54 | ≥ 5 CTC o > 2 M-CTC worse CSS and mCRPC-free survival (P < 0.05) in oligometastatic HSPC after radical prostatectomy. | |
| Vandekerkhove et al. [77] | ctDNA | 53 | ctDNA is increased in de novo mHSPC, especially in visceral versus bone/nodal metastases (P < 0.03). |
| Agarwal et al. [78] | NGS | 129 | ctDNA or AR aberrations (P < 0.05), or PI3K activation are associated with worse OS (P < 0.001). |
| Bjerre et al. [41] | ctDNA methylation: DOCK2, HALPN3 and FBXO30 | 65 | ctDNA methylation increases with high burden (P < 0.001) and is associated with a shorter time to mCRPC independent of tumor burden (P = 0.012). |
| MS-ddPCR | |||
| Cheng et al. [79] | miRNA | 50 | Baseline miR-375 levels are associated with PSA response at 28 weeks (P = 0.007). |
| RT-PCR | |||
|
| |||
| Predictive Value | |||
|
| |||
| Study | Analyte | N | Result |
|
| |||
| Goldkorn et al. [80] | CTC CellSearch | 523 | Undetectable CTC: almost 9 times more likely to achieve PSA ≤ 0.2 ng/ml at 7 months (P < 0.001) y 4 times more likely to achieve PFS > 2 years (P < 0.001). |
| Goodman et al. [81] | 33 | Favorable < 3 CTC/7.5 ml and unfavorable ≥ 3/7.5 ml for PSA response at 7 months (P ≤ 0.02). | |
| Kohli et al. [82] | ctDNA | 139 | Higher ctDNA predicted a shorter time to ADT failure (P = 0.02). |
| PCR | ATM, BRCA1, BRCA2, and CHEK2 mutations are associated with time to ADT failure and OS. | ||
| Harshman et al. [66] | IL-8 serum | 233 | High levels predict shorter OS (P = 0.001) and time to CPRC (P < 0.001). IL-8 > 9.3 pg/ml on ADT: worse OS (P = 0.007). |
AR: Androgen receptor; ADT: Androgen deprivation therapy; CTC: Circulating tumor cells; ctDNA: Circulating tumor DNA; mCRPC: metastatic castration-resistant prostate cancer; M-CTC: mesenchymal circulating tumor cells; MS-ddPCR: Methylation-specific droplet digital PCR; miRNA: microRNA; NGS: Next generation sequencing; RT-PCR: Reverse transcription polymerase chain reaction; PI3K: Phosphoinositide 3-kinase; mRNA: Messenger RNA; CSS: Cancer-specific survival; OS: Overall survival; PFS: Progression-free survival.
Prognostic value
An elevated CTC count correlates with a high tumor burden (52% vs 23%; P = 0.03) [73]. High tumor burden and pre-treatment CTC are independently associated with a lack of biochemical response at seven months (P = 0.005), which may indicate a need for treatment intensification [74]. A CTC count ≥ 4 is associated with shorter OS (24 vs 45 months) and PFS (7 vs 44 months) (P < 0,001) [75]. The subgroup with mCRPC and M-CTC experience progression earlier (10.5 vs 18 months, P = 0.003) and more frequently (P = 0.013) [76]. Total CTC count ≥ 5 and M-CTC ≥ 2 are independent predictors of early progression and shorter CSS in mCRPC (P < 0.001) [20].
The plasma ctDNA fraction is increased in de novo mHSPC, especially in patients with visceral metastases, but the exposure to ADT may compromise the utility of ctDNA [77]. In the TITAN trial, the presence of ctDNA or genomic aberrations before therapy (P < 0.05) and the activation of the PIK3 pathway after apalutamide were associated with worse OS (P < 0.001) [78]. ctDNA methylation of DOCK2, HALPN3, and FBXO30 is detected in 61.5% of patients with de novo mHSPC and is significantly increased in high burden compared to low burden PCa (89.3% vs 32.1%, P < 0.001). They are also significantly associated with a shorter time to castration resistance independent of tumor burden [41].
Studies of cfDNA fragmentation have correlated the presence of larger fragments, ≥ 142-170 pb, with a more aggressive type of mHSPC. Cheng et al. found that high levels of miR-141, miR-200a, and miR-375 were significantly associated with CTC count, and miR-375 correlated with PSA response at 28 weeks (P = 0.007) [79].
Predictive value
Two-year PFS is four times higher (P < 0.001) in patients with undetectable CTCs vs ≥ 5 CTC/7.5 ml [80]. Goodman et al. identified a threshold of 3 CTC/7.5 ml as a predictor of progression in mCRPC. In addition, CTC count predicts the duration and extent of response to ADT [81].
A high ctDNA fraction is predictive of early failure to ADT (P = 0.02). Mutations in DNA repair genes (ATM, BRCA1, BRCA2, CHEK2) are associated with time to failure of ADT and survival in mHSPC [82].
In the CHAARTED study, high pre-ADT IL-8 predicted worse OS (P = 0.007) and shorter time to castration resistance (P < 0.001), independent of docetaxel use, metastatic burden, or metachronous vs. synchronous presentation of metastatic disease [66].
mCRPC: prognostic and predictive value: (Table 3)
Table 3.
Studies of liquid biopsy in mCRPC: clinical applications
| Prognostic Value | |||
|
| |||
| Study | Analyte | N | Result |
|
| |||
| De Bono et al. [16] | CTC count. CellSearch | 231 | CTC ≥ 5/7.5 ml: Shorter OS. Change from unfavorable to favorable count improves prognosis. |
| Goldkorn et al. [84] | 263 | CTC < 5/7.5 ml and ≥ 5/7.5 ml correlate with OS (26 vs 13 months, respectively) in 1st line of docetaxel. | |
| Scher et al. [85] | 164 | High risk of death in patients starting chemotherapy: high LDH and CTC count. A predictive model for survival at 4, 8, or 12 weeks. | |
| Okegawa et al. [86] | 57 | Alkaline phosphatase and CTC count are independent prognostic factors for OS. | |
| CTC ≥ 5/7.5 ml after 3 cycles of docetaxel: worse OS. | |||
| Huang et al. [52] | Exosomal RNA sequencing with qRT-PCR | 100 | miR-1290 y miR-375 correlate with worse OS. |
| Del Re et al. [56] | Exosomal RNA for AR-V7 analysis through ddPCR | 36 | PFS and OS significantly worse in AR-V7 positive. |
|
| |||
| Predictive Value | |||
|
| |||
| Study | Analyte | N | Result |
|
| |||
| Antonarakis et al. [21] | CTC count y AR-V7 in CTC | 202 | Better response to abiraterone and enzalutamide: negative CTC counts better than by CTC positive/AR-V7 negative and then CTC positive/AR-V7 positive. |
| Heller et al. [90] | CTC count | 6081 | Baseline CTC > 0/7.5 ml a CTC 13th week = 0/7.5 ml: better OS. CTC count from unfavorable to favorable. |
| Lorente et al. [12] | CellSearch | 511 | Worse OS associated with CTC increase during the first weeks of abiraterone or docetaxel. |
| Conteduca et al. [96] | Number of AR copies and mutations in plasmatic DNA | 265 | An increase in AR copy number before enzalutamide or abiraterone correlates with worse OS and PFS, independent of the prior use of taxanes. |
| Goodall et al. [97] | cfDNA | 49 | A decrease in the allele frequency of somatic mutations in response to olaparib and > 50% decrease in cfDNA at 8 weeks independently correlate with better OS. |
| ctDNA | |||
| Torquato et al. [38] | cfDNA with NGS y number of AR copies and other 45 genes | 62 | High ctDNA concentration: worse PSA response, PFS, and OS in the setting of abiraterone or enzalutamide. Loss of TP53 and defects in the PIK3 pathway: worse OS. |
| Mehra et al. [98] | cfDNA | 571 | High baseline cfDNA is associated with shorter rPFS and OS after taxanes. Decrease in cfDNA during the first 9 weeks is associated with response to taxanes. |
| PROSELICA | |||
| FIRSTANA | |||
| Armstrong et al. [23] | mRNA AR-V7 CTC | 118 | AR-V7 correlates with shorter PFS and OS in patients treated with abiraterone or enzalutamide, independent of CTC count or clinical prognostic factors. |
| Scher et al. [22] | AR-V7 in CTC with Epic | 142 | Improved OS in high-risk AR-V7 positive patients treated with taxanes compared to patients treated with ARPIs. Improved OS in AR-V7 negative patients treated with ARPIs versus taxanes (19.8 vs 12.8 months, P = 0.05). |
| Annala et al. [39] | cfDNA with whole exome sequencing and deep sequencing | 202 | BRCA2 and ATM alterations: poor response to abiraterone or enzalutamide. |
| Somatic mutations in TP53 are independently associated with rapid resistance. | |||
| De Laere et al. [109] | CTC count | 168 | TP53 is a negative prognostic marker for ARPIs compared to any AR-derived biomarker. |
| RNA sequencing for CTC-ARV | |||
| cfDNA sequencing for AR | |||
| Peter et al. [110] | WGBS | 16 | cfDNA methylome dynamics during abiraterone/enzalutamide treatment. Methylation during treatment correlates with a longer time to clinical progression. |
| Mahon et al. [111] | PCR methylation | 600 | Methylated GSTP1 undetectable at baseline correlates with prolonged OS (P < 0.00001), and also after 2 cycles of docetaxel (P < 0.00001). |
| Hendriks et al. [42] | PCR methylation | 50 | Hypermethylation of GSTP1 and APC correlates with worse OS (P < 0.03). |
AR: androgen receptor; AR-V7: androgen receptor splice variant-7; ARPIs: Androgen receptor pathway inhibitors; cfDNA: cell-free DNA; CTC: circulating tumor cells; ctDNA: circulating tumor DNA; mCRPC: metastatic castration-resistant prostate cancer; GSTP1: glutathione S-transferase pi 1; LDH: Lactate dehydrogenase; miRNA: micro RNA; NGS: next-generation sequencing; RTq-PCR: quantitative reverse transcription polymerase chain reaction; PI3K: phosphoinositide 3-kinase; mRNA: messenger RNA; OS: overall survival; PFS: progression-free survival; rPFS: radiographic Progression-Free Survival; TP53: tumor protein 53; WGBS: Whole genome bisulfite sequencing.
Prognostic value
PSA is an imperfect biomarker of response. Up to 25% of patients experience clinical progression without an increase in PSA [83]. A baseline CTC count ≥ 5 CTC/7.5 ml correlates with worse OS (P < 0.0001) [16,84]. The prognosis improves with decreasing CTC (from 6.8 to 21.3 months) and worsens with increasing CTC (> 26 9.3 months) [16]. Baseline LDH and CTC are independent prognostic factors for OS, in contrast to PSA levels [85,86]. High levels of miR-1290 and miR-375 were associated with shorter OS (7.23 vs 19.3 months) [52]. In addition, PSMA-positive EVs are predominant in mCRPC [87] and correlate with worse OS [54].
TP53 aberrations are associated with poor prognosis and are observed in up to 50% of mCRPC [21,88]. This is also the case for androgen receptor aberrations (amplification or ≥ 2 mutations), with a median PFS of 1.9 versus 4.4 months compared to a single mutation (P = 0.035) [89]. Del Re et al. reported that AR-V7-positive patients have worse PFS and OS (P < 0.001) [56].
Predictive value
CTC-based parameters such as CTC = 0 at 13 weeks [90] and CTC conversion (≥ 5 CTC/7.5 ml at baseline, ≤ 4 CTC/7.5 ml at 13 weeks) [12] are significantly superior to PSA for assessing biochemical response and detecting early progression [14].
A rapid decrease in ctDNA (≥ 50%) is associated with prolonged PFS and OS [91-94], while an increase at 12 weeks increases the risk of early biochemical and radiographic progression (P < 0.001) [95-97]. Higher levels of cfDNA and ctDNA at baseline (≥ 30%) are independent predictors of shorter PFS and OS (P < 0.001) [39,91,95-98], with ctDNA considered a biomarker of response to androgen receptor pathway inhibitors (ARPIs) [38], poly (ADP-ribose) polymerase inhibitors (PARPi) [97], and taxanes [99,100]. Mutations in DNA repair genes of the homologous recombination (HR) pathway (BRCA1, BRCA2, ATM) are present in up to 27% of mCRPC and confer sensitivity to PARPi and platinum [101-103]. In addition, patients with mutations in mismatch repair genes (MLH1, MSH2, MSH6, PMS2), which account for approximately 2-3% of cases, respond to the PD-L1 inhibitor pembrolizumab [104,105], providing another therapeutic option.
In patients treated with PARPi, ctDNA studies have identified a heterogeneous scenario of subclonal mutations in BRCA2 or PALB2 that restore BRCA2 function at progression, and are absent at baseline [26,97].
Approximately half of patients have ctDNA aberrations in PIK3CA/B, PTEN, or AKT [106] which are associated with poor response to ARPIs and worse radiographic progression-free survival (rPFS) (P = 0.034) [39,107]. Loss of RB1 (P = 0.01) and upregulation of MET (P = 0.02) in ctDNA correlate significantly with PFS [29].
AR-V7 positivity is associated with worse outcome in terms of PSA response, PFS, and OS after ARPIs (P < 0.001) [21,23,108], but a better response to taxanes [22,24].
Loss of TP53 in ctDNA is predictive of worse response to ARPIs [39]. De Laere et al. stratified mCRPC according to this loss before ARPIs into two groups: poor prognosis (PFS ≤ 2.5 months) and good prognosis (PFS ≥ 14 months) [109].
Changes in methylation patterns in ctDNA and cfDNA in patients treated with ARPIs are indicative of rapid disease progression and may be associated with neuroendocrine markers that determine an aggressive pattern [110,111]. The combination of highly methylated GSTP1 + APC at baseline predicts OS (P < 0.02) and changes after treatment may define responders [42]. Bhagirath et al. [60], showed that enzalutamide increased the release of BRN4 and BRN2 mRNA by EVs and may modulate the progression of mCRPC to neuroendocrine PCa.
Future perspectives
Several lines of research are combining genomic and transcriptomic analyses to improve the accuracy of current tests and provide a more complete genomic profile [112].
Some of these studies are using biomarkers as part of adaptive cancer therapy based on different cellular subpopulations that form PCa and have different susceptibilities to antiandrogen therapy [113]. These strategies attempt to prolong treatment efficacy by maintaining a balance between sensitive and resistant subclones.
Emerging analytes are being explored, including lipids, glucans, and the microbiome [114-117]. Microbiome studies have defined distinctive signatures depending on the cancer type, demonstrating its potential as a complementary tool to ctDNA [118].
Technological advances such as mass cytometry will allow for the visualization of protein expression specific to PCa and related to progression and treatment response (PSA, PSMA, androgen receptors, EpCAM, B-catenin) [119]. cfDNA sequencing can identify novel associations between somatic mutations and response, improving personalized therapy [120,121]. Longitudinal cfDNA analysis can detect dynamic changes in methylation patterns that reflect tumor progression or treatment response [6,122-124]. Multiparametric analysis using multiple LB techniques can provide a deeper understanding of the disease [125].
Numerous ongoing studies in patients with metastatic PCa (Table 4) are analyzing different treatment strategies based on the profiling and monitoring of various biomarkers to predict and evaluate response to therapy as well as possible resistance mechanisms [1,51].
Table 4.
Ongoing studies of liquid biopsy in metastatic prostate cancer
| ClinicalTrials.gov | Status | Phase | Condition | Aim |
|---|---|---|---|---|
| NCT number | ||||
| NCT04015622 | Recruiting | Phase 2 | mCRPC | Optimization of PCa treatment by analysis of ctDNA in mCRPC after abiraterone. |
| PROTRACT | ||||
| NCT04601441 | Recruiting | Phase 4 | mHSPC | To evaluate changes in genomic alterations for 73 PCa driver genes during apalutamide treatment. |
| CUARTET | ||||
| NCT03228810 | Completed | NA | mPCa | To detect and calculate ctDNA about metastasis-directed radiation ± surgery, and ADT. |
| NCT05116579 | Recruiting | NA | mCRPC | To evaluate the value of personalized ctDNA monitoring for efficacy assessment and prediction during PARPi treatment. |
| NCT03903835 | Recruiting | Phase 3 | mCRPC | To prolong progression-free survival by measuring plasma ctDNA and adjusting the treatment accordingly. |
| ProBio | ||||
| NCT03385655 | Recruiting | Phase 2 | mCRPC | To evaluate whether cfDNA can predict which patients are most likely to respond to therapy. |
| NCT03601143 | Recruiting | NA | mCRPC | Optimal liquid biopsy approach to detect AR-V7 and explore novel approaches to best predict resistance to ARSi in mCRPC. |
| PEARL | ||||
| NCT05188911 | Recruiting | NA | mCRPC | To evaluate lesion heterogeneity and genomic alterations in mCRPC patients receiving abiraterone by incorporating dual-tracer PET/CT (PSMA and FDG) and ctDNA. |
| ANGELA | ||||
| NCT05415787 | Recruiting | NA | mPCa | To evaluate the technical feasibility of studying homologous recombination (HR) gene variants on ctDNA from patients with metastatic PCa. |
| PROMECI | ||||
| NCT04188275 | Recruiting | NA | mCRPC | To determine of AR-V7 splice variants on circulating tumor cells and evaluate circulating levels of miRNA during systemic treatment. |
| PRIMERA | ||||
| NCT05885009 | Recruiting | NA | mPCa | To evaluate the feasibility and impact of liquid biopsy-based genomic profiling on treatment decision making in patients with metastatic prostate cancer in Spain. |
| SOLTI-2102 (HOPE-PROSTATE) | ||||
| NCT04581109 | Recruiting | NA | mPCa | To detect of viable CTCs using EPIDROP technology. Demonstrate non-inferiority of EPIDROP to the CellSearch system. |
| EPIDROP | ||||
| NCT04489719 | Recruiting | Observational | mCRPC | To investigate the role of a DNA repair pathway in response to radium-223. |
ADT: Androgen Deprivation Therapy; ARSi: Androgen receptor signalling inhibitor; AR-V7: Androgen receptor splice variant-7; CTC: circulating tumor cells; ctDNA: circulating tumour DNA; mCRPC: metastatic castration-resistant prostate cancer; mHSPC: metastatic hormone-sensitive prostate cancer; NA: Not applicable; PARPi: Poly (ADP-ribose) polymerase inhibitors; PCa: prostate cancer; mPCa: metastatic prostate cancer.
Discussion
The identification of genomic biomarkers requires the analysis of primary tumor samples or a biopsy of a de novo metastasis [126]. However, sequencing errors can be as high as 30-40%. In addition, these samples do not reveal the diversity of competing tumor subclones present at different disease sites.
It is accepted that CTC better reflects inter- and intratumor heterogeneity compared to conventional biopsy [127]. A baseline count of ≥ 5 CTC/7.5 ml [16] is an unfavorable prognostic factor predicting worse PFS and OS. The mesenchymal phenotype is associated with more aggressive tumors [17]: the presence of M-CTC ≥ 2 is an independent predictor of early progression and shorter CSS in mCRPC [18-20].
The detection of AR-V7 in CTC is a useful biomarker for predicting response to first- or second-line androgen receptor-targeted therapy [21,24,25,128]. Molecular characterization of the aggressive variant (loss of PTEN, RB1 and TP53) in CTC correlates with worse PFS and OS, and higher genomic instability [28,129], and patients with mutations in PTEN, TP53, RB1, AR, SPOP, MYC, and ATM have a higher risk of early progression [33,130]. BRCA2 loss in CTCs is detected in up to 42% of cases, which is significantly higher than in tumor tissue analysis, thus improving the precision in detecting this alteration, which can be treated with PARPi in mCRPC [27,129]. However, CTC isolation and characterization are limited and costly using current platforms. Systems such as AdnaTest allow CTCs to be captured while preserving the quality of the mRNA. This facilitates further expression studies of biomarkers including AR-V7.
High cfDNA levels are a potential biomarker of poor prognosis that may be associated with shorter OS and rPFS in patients treated with taxanes or ARPIs [98,131-133]. A high ctDNA fraction (> 2%) is predictive of progression, castration resistance, and worse OS [134]. This may allow for patient selection for treatment intensification [135].
ctDNA analysis can assess genomic alterations involved in the development of metastases. The most common somatic mutations are TP53, APC, and androgen receptors. The most common amplifications are androgen receptors and MYC. These are associated with worse OS and metastasis-free survival (P < 0.01) [99,136,137]. In addition, DNA repair genes (BRCA1/2, ATM) are involved in resistance to ADT [138], early progression [139], and response to PARPi [103,140].
Finally, EVs actively regulate phenotypic changes including metabolism [141], proliferation [142], invasiveness/metastasis [143], stromal reprogramming [144] and treatment resistance [145]. Current procedures for EV isolation, storage, processing, and characterization need to be optimized and standardized for clinical applications [43]. The number of EVs has been associated with poor OS [54]. Furthermore, high levels of miR-1290 and miR-375 correlate with poorer OS and early recurrence [52,53]. Of note, enzalutamide increased the release of BRN4 and BRN2 mRNA from EVs and may modulate plasticity [60].
Conclusions
Liquid biopsy complements solid biopsy in the diagnosis and management of advanced PCa. The wide variety of definitions and platforms for analysis, as well as the cost and access to the necessary technology, limit the use of these biomarkers as routine tests. Many ongoing studies in this area will help to explain tumor heterogeneity and improve the clinical management of these patients.
Disclosure of conflict of interest
None.
References
- 1.He W, Xiao Y, Yan S, Zhu Y, Ren S. Cell-free DNA in the management of prostate cancer: current status and future prospective. Asian J Urol. 2023;10:298–316. doi: 10.1016/j.ajur.2022.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rzhevskiy AS, Kapitannikova AY, Butnaru DV, Shpot EV, Joosse SA, Zvyagin AV, Ebrahimi Warkiani M. Liquid biopsy in diagnosis and prognosis of non-metastatic prostate cancer. Biomedicines. 2022;10:3115. doi: 10.3390/biomedicines10123115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dathathri E, Isebia KT, Abali F, Lolkema MP, Martens JWM, Terstappen LWMM, Bansal R. Liquid biopsy based circulating biomarkers in metastatic prostate cancer. Front Oncol. 2022;12:863472. doi: 10.3389/fonc.2022.863472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puche-Sanz I, Rodríguez-Martínez A, Garrido-Navas MC, Robles-Fernández I, Vázquez-Alonso F, Álvarez Cubero MJ, Lorente-Acosta JA, Serrano-Fernández MJ, Cózar-Olmo JM. Liquid biopsy and prostate cancer. Current evidence applied to clinical practice. Actas Urol Esp (Engl Ed) 2020;44:139–47. doi: 10.1016/j.acuro.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Wang Z, Gang X, Wang G. Liquid biopsy in prostate cancer: current status and future challenges of clinical application. Aging Male. 2021;24:58–71. doi: 10.1080/13685538.2021.1944085. [DOI] [PubMed] [Google Scholar]
- 6.Silva R, Moran B, Baird AM, O’Rourke CJ, Finn SP, McDermott R, Watson W, Gallagher WM, Brennan DJ, Perry AS. Longitudinal analysis of individual cfDNA methylome patterns in metastatic prostate cancer. Clin Epigenetics. 2021;13:168. doi: 10.1186/s13148-021-01155-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lone SN, Nisar S, Masoodi T, Singh M, Rizwan A, Hashem S, El-Rifai W, Bedognetti D, Batra SK, Haris M, Bhat AA, Macha MA. Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments. Mol Cancer. 2022;21:79. doi: 10.1186/s12943-022-01543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catalano M, Generali D, Gatti M, Riboli B, Paganini L, Nesi G, Roviello G. DNA repair deficiency as circulating biomarker in prostate cancer. Front Oncol. 2023;13:1115241. doi: 10.3389/fonc.2023.1115241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danila DC, Pantel K, Fleisher M, Scher HI. Circulating tumors cells as biomarkers: progress toward biomarker qualification. Cancer J. 2011;17:438–50. doi: 10.1097/PPO.0b013e31823e69ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sölétormos G, Duffy MJ, Hayes DF, Sturgeon CM, Barak V, Bossuyt PM, Diamandis EP, Gion M, Hyltoft-Petersen P, Lamerz RM, Nielsen DL, Sibley P, Tholander B, Tuxen MK, Bonfrer JM. Design of tumor biomarker-monitoring trials: a proposal by the European Group on tumor markers. Clin Chem. 2013;59:52–9. doi: 10.1373/clinchem.2011.180778. [DOI] [PubMed] [Google Scholar]
- 11.Pantel K, Hille C, Scher HI. Circulating tumor cells in prostate cancer: from discovery to clinical utility. Clin Chem. 2019;65:87–99. doi: 10.1373/clinchem.2018.287102. [DOI] [PubMed] [Google Scholar]
- 12.Lorente D, Olmos D, Mateo J, Dolling D, Bianchini D, Seed G, Flohr P, Crespo M, Figueiredo I, Miranda S, Scher HI, Terstappen LWMM, de Bono JS. Circulating tumour cell increase as a biomarker of disease progression in metastatic castration-resistant prostate cancer patients with low baseline CTC counts. Ann Oncol. 2018;29:1554–60. doi: 10.1093/annonc/mdy172. [DOI] [PubMed] [Google Scholar]
- 13.Franklin WA, Glaspy J, Pflaumer SM, Jones RB, Hami L, Martinez C, Murphy JR, Shpall E. Incidence of tumor-cell contamination in leukapheresis products of breast cancer patients mobilized with stem cell factor and granulocyte colony-stimulating factor (G-CSF) or with G-CSF alone. Blood. 1999;94:340–7. [PubMed] [Google Scholar]
- 14.Casanova-Salas I, Athie A, Boutros PC, Del Re M, Miyamoto DT, Pienta KJ, Posadas EM, Sowalsky AG, Stenzl A, Wyatt AW, Mateo J. Quantitative and qualitative analysis of blood-based liquid biopsies to inform clinical decision-making in prostate cancer. Eur Urol. 2021;79:762–71. doi: 10.1016/j.eururo.2020.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 16.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–9. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 17.Liu W, Yin B, Wang X, Yu P, Duan X, Liu C, Wang B, Tao Z. Circulating tumor cells in prostate cancer: precision diagnosis and therapy. Oncol Lett. 2017;14:1223–32. doi: 10.3892/ol.2017.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Książkiewicz M, Markiewicz A, Żaczek AJ. Epithelial-mesenchymal transition: a hallmark in metastasis formation linking circulating tumor cells and cancer stem cells. Pathobiology. 2012;79:195–208. doi: 10.1159/000337106. [DOI] [PubMed] [Google Scholar]
- 19.Wu S, Liu S, Liu Z, Huang J, Pu X, Li J, Yang D, Deng H, Yang N, Xu J. Classification of circulating tumor cells by epithelial-mesenchymal transition markers. PLoS One. 2015;10:e0123976. doi: 10.1371/journal.pone.0123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang G, Xie J, Zhang S, Gu W, Yuan J, Wang R, Guo C, Ye L, Peng B, Yao X, Yang B. Clinical significance of mesenchymal circulating tumor cells in patients with oligometastatic hormone-sensitive prostate cancer who underwent cytoreductive radical prostatectomy. Front Oncol. 2022;11:812549. doi: 10.3389/fonc.2021.812549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Zhu Y, Silberstein JL, Taylor MN, Maughan BL, Denmeade SR, Pienta KJ, Paller CJ, Carducci MA, Eisenberger MA, Luo J. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J. Clin. Oncol. 2017;35:2149–56. doi: 10.1200/JCO.2016.70.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scher HI, Graf RP, Schreiber NA, Jayaram A, Winquist E, McLaughlin B, Lu D, Fleisher M, Orr S, Lowes L, Anderson A, Wang Y, Dittamore R, Allan AL, Attard G, Heller G. Assessment of the validity of nuclear-localized androgen receptor splice variant 7 in circulating tumor cells as a predictive biomarker for castration-resistant prostate cancer. JAMA Oncol. 2018;4:1179–86. doi: 10.1001/jamaoncol.2018.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armstrong AJ, Halabi S, Luo J, Nanus DM, Giannakakou P, Szmulewitz RZ, Danila DC, Healy P, Anand M, Rothwell CJ, Rasmussen J, Thornburg B, Berry WR, Wilder RS, Lu C, Chen Y, Silberstein JL, Kemeny G, Galletti G, Somarelli JA, Gupta S, Gregory SG, Scher HI, Dittamore R, Tagawa ST, Antonarakis ES, George DJ. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: the PROPHECY study. J. Clin. Oncol. 2019;37:1120–9. doi: 10.1200/JCO.18.01731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onstenk W, Sieuwerts AM, Kraan J, Van M, Nieuweboer AJ, Mathijssen RH, Hamberg P, Meulenbeld HJ, De Laere B, Dirix LY, van Soest RJ, Lolkema MP, Martens JW, van Weerden WM, Jenster GW, Foekens JA, de Wit R, Sleijfer S. Efficacy of cabazitaxel in castration-resistant prostate cancer is independent of the presence of AR-V7 in circulating tumor cells. Eur Urol. 2015;68:939–45. doi: 10.1016/j.eururo.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Scher HI, Lu D, Schreiber NA, Louw J, Graf RP, Vargas HA, Johnson A, Jendrisak A, Bambury R, Danila D, McLaughlin B, Wahl J, Greene SB, Heller G, Marrinucci D, Fleisher M, Dittamore R. Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncol. 2016;2:1441–1449. doi: 10.1001/jamaoncol.2016.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quigley D, Alumkal JJ, Wyatt AW, Kothari V, Foye A, Lloyd P, Aggarwal R, Kim W, Lu E, Schwartzman J, Beja K, Annala M, Das R, Diolaiti M, Pritchard C, Thomas G, Tomlins S, Knudsen K, Lord CJ, Ryan C, Youngren J, Beer TM, Ashworth A, Small EJ, Feng FY. Analysis of circulating cell-free DNA identifies multiclonal heterogeneity of BRCA2 reversion mutations associated with resistance to PARP inhibitors. Cancer Discov. 2017;7:999–1005. doi: 10.1158/2159-8290.CD-17-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnett ES, Schultz N, Stopsack KH, Lam ET, Arfe A, Lee J, Zhao JL, Schonhoft JD, Carbone EA, Keegan NM, Wibmer A, Wang Y, Solit DB, Abida W, Wenstrup R, Scher HI. Analysis of BRCA2 copy number loss and genomic instability in circulating tumor cells from patients with metastatic castration-resistant prostate cancer. Eur Urol. 2023;83:112–20. doi: 10.1016/j.eururo.2022.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malihi PD, Graf RP, Rodriguez A, Ramesh N, Lee J, Sutton R, Jiles R, Ruiz Velasco C, Sei E, Kolatkar A, Logothetis C, Navin NE, Corn P, Aparicio AM, Dittamore R, Hicks J, Kuhn P, Zurita AJ. Single-cell circulating tumor cell analysis reveals genomic instability as a distinctive feature of aggressive prostate cancer. Clin Cancer Res. 2020;26:4143–53. doi: 10.1158/1078-0432.CCR-19-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wyatt AW, Azad AA, Volik SV, Annala M, Beja K, McConeghy B, Haegert A, Warner EW, Mo F, Brahmbhatt S, Shukin R, Le Bihan S, Gleave ME, Nykter M, Collins CC, Chi KN. Genomic alterations in cell-free DNA and enzalutamide resistance in castration-resistant prostate cancer. JAMA Oncol. 2016;2:1598–606. doi: 10.1001/jamaoncol.2016.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moss J, Magenheim J, Neiman D, Zemmour H, Loyfer N, Korach A, Samet Y, Maoz M, Druid H, Arner P, Fu KY, Kiss E, Spalding KL, Landesberg G, Zick A, Grinshpun A, Shapiro AMJ, Grompe M, Wittenberg AD, Glaser B, Shemer R, Kaplan T, Dor Y. Comprehensive human cell-type methylation atlas reveals origins of circulating cell-free DNA in health and disease. Nat Commun. 2018;9:5068. doi: 10.1038/s41467-018-07466-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soda N, Rehm BHA, Sonar P, Nguyen NT, Shiddiky MJA. Advanced liquid biopsy technologies for circulating biomarker detection. J Mater Chem B. 2019;7:6670–704. doi: 10.1039/c9tb01490j. [DOI] [PubMed] [Google Scholar]
- 32.Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, Chin SF, Dunning MJ, Gale D, Forshew T, Mahler-Araujo B, Rajan S, Humphray S, Becq J, Halsall D, Wallis M, Bentley D, Caldas C, Rosenfeld N. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368:1199–209. doi: 10.1056/NEJMoa1213261. [DOI] [PubMed] [Google Scholar]
- 33.Chen E, Cario CL, Leong L, Lopez K, Márquez CP, Chu C, Li PS, Oropeza E, Tenggara I, Cowan J, Simko JP, Chan JM, Friedlander T, Wyatt AW, Aggarwal R, Paris PL, Carroll PR, Feng F, Witte JS. Cell-free DNA concentration and fragment size as a biomarker for prostate cancer. Sci Rep. 2021;11:5040. doi: 10.1038/s41598-021-84507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cimadamore A, Scarpelli M, Santoni M, Massari F, Tartari F, Cerqueti R, Lopez-Beltran A, Cheng L, Montironi R. Genitourinary tumors: update on molecular biomarkers for diagnosis, prognosis and prediction of response to therapy. Curr Drug Metab. 2019;20:305–12. doi: 10.2174/1389200220666190225124352. [DOI] [PubMed] [Google Scholar]
- 35.Chatfield-Reed K, Roche VP, Pan Q. cfDNA detection for HPV+ squamous cell carcinomas. Oral Oncol. 2021;115:104958. doi: 10.1016/j.oraloncology.2020.104958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keppens C, Dequeker EMC, Patton SJ, Normanno N, Fenizia F, Butler R, Cheetham M, Fairley JA, Williams H, Hall JA, Schuuring E, Deans ZC IQN Path ASBL. International pilot external quality assessment scheme for analysis and reporting of circulating tumour DNA. BMC Cancer. 2018;18:804. doi: 10.1186/s12885-018-4694-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Zheng Y, Wu L, Li J, Ji J, Yu Q, Dai W, Feng J, Wu J, Guo C. Current status of ctDNA in precision oncology for hepatocellular carcinoma. J Exp Clin Cancer Res. 2021;40:140. doi: 10.1186/s13046-021-01940-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torquato S, Pallavajjala A, Goldstein A, Toro PV, Silberstein JL, Lee J, Nakazawa M, Waters I, Chu D, Shinn D, Groginski T, Hughes RM, Simons BW, Khan H, Feng Z, Carducci MA, Paller CJ, Denmeade SR, Kressel B, Eisenberger MA, Antonarakis ES, Trock BJ, Park BH, Hurley PJ. Genetic alterations detected in cell-free DNA are associated with enzalutamide and abiraterone resistance in castration-resistant prostate cancer. JCO Precis Oncol. 2019;3:PO.18.00227. doi: 10.1200/PO.18.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Annala M, Vandekerkhove G, Khalaf D, Taavitsainen S, Beja K, Warner EW, Sunderland K, Kollmannsberger C, Eigl BJ, Finch D, Oja CD, Vergidis J, Zulfiqar M, Azad AA, Nykter M, Gleave ME, Wyatt AW, Chi KN. Circulating tumor DNA genomics correlate with resistance to abiraterone and enzalutamide in prostate cancer. Cancer Discov. 2018;8:444–57. doi: 10.1158/2159-8290.CD-17-0937. [DOI] [PubMed] [Google Scholar]
- 40.Conteduca V, Hess J, Yamada Y, Ku SY, Beltran H. Epigenetics in prostate cancer: clinical implications. Transl Androl Urol. 2021;10:3104–16. doi: 10.21037/tau-20-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bjerre MT, Nørgaard M, Larsen OH, Jensen SØ, Strand SH, Østergren P, Fode M, Fredsøe J, Ulhøi BP, Mortensen MM, Jensen JB, Borre M, Sørensen KD. Epigenetic analysis of circulating tumor DNA in localized and metastatic prostate cancer: evaluation of clinical biomarker potential. Cells. 2020;9:1362. doi: 10.3390/cells9061362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hendriks RJ, Dijkstra S, Smit FP, Vandersmissen J, Van de Voorde H, Mulders PFA, van Oort IM, Van Criekinge W, Schalken JA. Epigenetic markers in circulating cell-free DNA as prognostic markers for survival of castration-resistant prostate cancer patients. Prostate. 2018;78:336–42. doi: 10.1002/pros.23477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson J, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 45.Caruso S, Poon IKH. Apoptotic cell-derived extracellular vesicles: more than just debris. Front Immunol. 2018;9:1486. doi: 10.3389/fimmu.2018.01486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo Y, Ji X, Liu J, Fan D, Zhou Q, Chen C, Wang W, Wang G, Wang H, Yuan W, Ji Z, Sun Z. Effects of exosomes on pre-metastatic niche formation in tumors. Mol Cancer. 2019;18:39. doi: 10.1186/s12943-019-0995-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian S, Lei Z, Gong Z, Sun Z, Xu D, Piao M. Clinical implication of prognostic and predictive biomarkers for castration-resistant prostate cancer: a systematic review. Cancer Cell Int. 2020;20:409. doi: 10.1186/s12935-020-01508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murillo OD, Thistlethwaite W, Rozowsky J, Subramanian SL, Lucero R, Shah N, Jackson AR, Srinivasan S, Chung A, Laurent CD, Kitchen RR, Galeev T, Warrell J, Diao JA, Welsh JA, Hanspers K, Riutta A, Burgstaller-Muehlbacher S, Shah RV, Yeri A, Jenkins LM, Ahsen ME, Cordon-Cardo C, Dogra N, Gifford SM, Smith JT, Stolovitzky G, Tewari AK, Wunsch BH, Yadav KK, Danielson KM, Filant J, Moeller C, Nejad P, Paul A, Simonson B, Wong DK, Zhang X, Balaj L, Gandhi R, Sood AK, Alexander RP, Wang L, Wu C, Wong DTW, Galas DJ, Van Keuren-Jensen K, Patel T, Jones JC, Das S, Cheung KH, Pico AR, Su AI, Raffai RL, Laurent LC, Roth ME, Gerstein MB, Milosavljevic A. exRNA atlas analysis reveals distinct extracellular rna cargo types and their carriers present across human biofluids. Cell. 2019;177:463–477. e15. doi: 10.1016/j.cell.2019.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim CJ, Dong L, Amend SR, Cho YK, Pienta KJ. The role of liquid biopsies in prostate cancer management. Lab Chip. 2021;21:3263–88. doi: 10.1039/d1lc00485a. [DOI] [PubMed] [Google Scholar]
- 50.Nilsson J, Skog J, Nordstrand A, Baranov V, Mincheva-Nilsson L, Breakefield XO, Widmark A. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer. 2009;100:1603–7. doi: 10.1038/sj.bjc.6605058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramirez-Garrastacho M, Bajo-Santos C, Line A, Martens-Uzunova ES, de la Fuente JM, Moros M, Soekmadji C, Tasken KA, Llorente A. Extracellular vesicles as a source of prostate cancer biomarkers in liquid biopsies: a decade of research. Br J Cancer. 2022;126:331–50. doi: 10.1038/s41416-021-01610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang X, Yuan T, Liang M, Du M, Xia S, Dittmar R, Wang D, See W, Costello BA, Quevedo F, Tan W, Nandy D, Bevan GH, Longenbach S, Sun Z, Lu Y, Wang T, Thibodeau SN, Boardman L, Kohli M, Wang L. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur Urol. 2015;67:33–41. doi: 10.1016/j.eururo.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bryant RJ, Pawlowski T, Catto JW, Marsden G, Vessella RL, Rhees B, Kuslich C, Visakorpi T, Hamdy FC. Changes in circulating microRNA levels associated with prostate cancer. Br J Cancer. 2012;106:768–74. doi: 10.1038/bjc.2011.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nanou A, Miller MC, Zeune LL, de Wit S, Punt CJA, Groen HJM, Hayes DF, de Bono JS, Terstappen LWMM. Tumour-derived extracellular vesicles in blood of metastatic cancer patients associate with overall survival. Br J Cancer. 2020;122:801–11. doi: 10.1038/s41416-019-0726-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Del Re M, Crucitta S, Sbrana A, Rofi E, Paolieri F, Gianfilippo G, Galli L, Falcone A, Morganti R, Porta C, Efstathiou E, van Schaik R, Jenster G, Danesi R. Androgen receptor (AR) splice variant 7 and full-length AR expression is associated with clinical outcome: a translational study in patients with castrate-resistant prostate cancer. BJU Int. 2019;124:693–700. doi: 10.1111/bju.14792. [DOI] [PubMed] [Google Scholar]
- 56.Del Re M, Biasco E, Crucitta S, Derosa L, Rofi E, Orlandini C, Miccoli M, Galli L, Falcone A, Jenster GW, van Schaik RH, Danesi R. The detection of androgen receptor splice variant 7 in plasma-derived exosomal RNA strongly predicts resistance to hormonal therapy in metastatic prostate cancer patients. Eur Urol. 2017;71:680–7. doi: 10.1016/j.eururo.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 57.Nimir M, Ma Y, Jeffreys SA, Opperman T, Young F, Khan T, Ding P, Chua W, Balakrishnar B, Cooper A, De Souza P, Becker TM. Detection of AR-V7 in liquid biopsies of castrate resistant prostate cancer patients: a comparison of AR-V7 analysis in circulating tumor cells, circulating tumor RNA and exosomes. Cells. 2019;8:688. doi: 10.3390/cells8070688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woo HK, Park J, Ku JY, Lee CH, Sunkara V, Ha HK, Cho YK. Urine-based liquid biopsy: non-invasive and sensitive AR-V7 detection in urinary EVs from patients with prostate cancer. Lab Chip. 2018;19:87–97. doi: 10.1039/c8lc01185k. [DOI] [PubMed] [Google Scholar]
- 59.Kato T, Mizutani K, Kawakami K, Fujita Y, Ehara H, Ito M. CD44v8-10 mRNA contained in serum exosomes as a diagnostic marker for docetaxel resistance in prostate cancer patients. Heliyon. 2020;6:e04138. doi: 10.1016/j.heliyon.2020.e04138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhagirath D, Yang TL, Tabatabai ZL, Majid S, Dahiya R, Tanaka Y, Saini S. BRN4 is a novel driver of neuroendocrine differentiation in castration-resistant prostate cancer and is selectively released in extracellular vesicles with BRN2. Clin Cancer Res. 2019;25:6532–45. doi: 10.1158/1078-0432.CCR-19-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Möhrmann L, Huang HJ, Hong DS, Tsimberidou AM, Fu S, Piha-Paul SA, Subbiah V, Karp DD, Naing A, Krug A, Enderle D, Priewasser T, Noerholm M, Eitan E, Coticchia C, Stoll G, Jordan LM, Eng C, Kopetz ES, Skog J, Meric-Bernstam F, Janku F. Liquid biopsies using plasma exosomal nucleic acids and plasma cell-free DNA compared with clinical outcomes of patients with advanced cancers. Clin Cancer Res. 2018;24:181–8. doi: 10.1158/1078-0432.CCR-17-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wan Y, Liu B, Lei H, Zhang B, Wang Y, Huang H, Chen S, Feng Y, Zhu L, Gu Y, Zhang Q, Ma H, Zheng SY. Nanoscale extracellular vesicle-derived DNA is superior to circulating cell-free DNA for mutation detection in early-stage non-small-cell lung cancer. Ann Oncol. 2018;29:2379–83. doi: 10.1093/annonc/mdy458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karagiannis GS, Pavlou MP, Diamandis EP. Cancer secretomics reveal pathophysiological pathways in cancer molecular oncology. Mol Oncol. 2010;4:496–510. doi: 10.1016/j.molonc.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kwon OK, Jeon JM, Sung E, Na AY, Kim SJ, Lee S. Comparative secretome profiling and mutant protein identification in metastatic prostate cancer cells by quantitative mass spectrometry-based proteomics. Cancer Genomics Proteomics. 2018;15:279–90. doi: 10.21873/cgp.20086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pal SK, Moreira D, Won H, White SW, Duttagupta P, Lucia M, Jones J, Hsu J, Kortylewski M. Reduced T-cell numbers and elevated levels of immunomodulatory cytokines in metastatic prostate cancer patients de novo resistant to abiraterone and/or enzalutamide therapy. Int J Mol Sci. 2019;20:1831. doi: 10.3390/ijms20081831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harshman LC, Wang VX, Hamid AA, Santone G, Drake CG, Carducci MA, DiPaola RS, Fichorova RN, Sweeney CJ. Impact of baseline serum IL-8 on metastatic hormone-sensitive prostate cancer outcomes in the phase 3 CHAARTED trial (E3805) Prostate. 2020;80:1429–37. doi: 10.1002/pros.24074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Calcinotto A, Spataro C, Zagato E, Di Mitri D, Gil V, Crespo M, De Bernardis G, Losa M, Mirenda M, Pasquini E, Rinaldi A, Sumanasuriya S, Lambros MB, Neeb A, Lucianò R, Bravi CA, Nava-Rodrigues D, Dolling D, Prayer-Galetti T, Ferreira A, Briganti A, Esposito A, Barry S, Yuan W, Sharp A, de Bono J, Alimonti A. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature. 2018;559:363–9. doi: 10.1038/s41586-018-0266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gong Y, Chippada-Venkata UD, Oh WK. Roles of matrix metalloproteinases and their natural inhibitors in prostate cancer progression. Cancers (Basel) 2014;6:1298–327. doi: 10.3390/cancers6031298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frieling JS, Li T, Tauro M, Lynch CC. Prostate cancer-derived MMP-3 controls intrinsic cell growth and extrinsic angiogenesis. Neoplasia. 2020;22:511–21. doi: 10.1016/j.neo.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dhar M, Lam JN, Walser T, Dubinett SM, Rettig MB, Di Carlo D. Functional profiling of circulating tumor cells with an integrated vortex capture and single-cell protease activity assay. Proc Natl Acad Sci U S A. 2018;115:9986–91. doi: 10.1073/pnas.1803884115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liang W, Wang F, Chen Q, Dai J, Escara-Wilke J, Keller ET, Zimmermann J, Hong N, Lu Y, Zhang J. Targeting cathepsin K diminishes prostate cancer establishment and growth in murine bone. J Cancer Res Clin Oncol. 2019;145:1999–2012. doi: 10.1007/s00432-019-02950-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu G, Zhu M, Zhang M, Pan F. Emerging role of igf-1 in prostate cancer: a promising biomarker and therapeutic target. Cancers (Basel) 2023;15:1287. doi: 10.3390/cancers15041287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bryce AH, Chen YH, Liu G, Carducci MA, Jarrard DM, Garcia JA, Dreicer R, Hussain M, Eisenberger MA, Plimack ER, Vogelzang NJ, DiPaola RS, Harshman L, Sweeney CJ. Patterns of cancer progression of metastatic hormone-sensitive prostate cancer in the ECOG3805 CHAARTED trial. Eur Urol Oncol. 2020;3:717–24. doi: 10.1016/j.euo.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reichert ZR, Kasputis T, Nallandhighal S, Abusamra SM, Kasputis A, Haruray S, Wang Y, Williams S, Singhal U, Alva A, Cackowski FC, Caram MEV, Palmbos PL, Yentz SE, Smith DC, Alumkal JJ, Morgan TM. Multigene profiling of circulating tumor cells (CTCs) for prognostic assessment in treatment-naïve metastatic hormone-sensitive prostate cancer (mHSPC) Int J Mol Sci. 2021;23:4. doi: 10.3390/ijms23010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Resel Folkersma L, San José Manso L, Galante Romo I, Moreno Sierra J, Olivier Gómez C. Prognostic significance of circulating tumor cell count in patients with metastatic hormone-sensitive prostate cancer. Urology. 2012;80:1328–32. doi: 10.1016/j.urology.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 76.Yang YJ, Kong YY, Li GX, Wang Y, Ye DW, Dai B. Phenotypes of circulating tumour cells predict time to castration resistance in metastatic castration-sensitive prostate cancer. BJU Int. 2019;124:258–67. doi: 10.1111/bju.14642. [DOI] [PubMed] [Google Scholar]
- 77.Vandekerkhove G, Struss WJ, Annala M, Kallio HML, Khalaf D, Warner EW, Herberts C, Ritch E, Beja K, Loktionova Y, Hurtado-Coll A, Fazli L, So A, Black PC, Nykter M, Tammela T, Chi KN, Gleave ME, Wyatt AW. Circulating tumor DNA abundance and potential utility in de novo metastatic prostate cancer. Eur Urol. 2019;75:667–75. doi: 10.1016/j.eururo.2018.12.042. [DOI] [PubMed] [Google Scholar]
- 78.Agarwal N, Lucas J, Aguilar-Bonavides C, Thomas S, Gormley M, Chowdhury S, Merseburger A, Bjartell A, Uemura H. Genomic aberrations associated with overall survival (OS) in metastatic castration-sensitive prostate cancer (mCSPC) treated with apalutamide (APA) or placebo (PBO) plus androgen deprivation therapy (ADT) in TITAN. J. Clin. Oncol. 2022;40:5066. [Google Scholar]
- 79.Cheng HH, Plets M, Li H, Higano CS, Tangen CM, Agarwal N, Vogelzang NJ, Hussain M, Thompson IM Jr, Tewari M, Yu EY. Circulating microRNAs and treatment response in the Phase II SWOG S0925 study for patients with new metastatic hormone-sensitive prostate cancer. Prostate. 2018;78:121–7. doi: 10.1002/pros.23452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goldkorn A, Tangen C, Plets M, Morrison GJ, Cunha A, Xu T, Pinski JK, Ingles SA, Triche T, Harzstark AL, Kohli M, MacVicar GR, Vaena DA, Crispino AW, McConkey DJ, Lara PN Jr, Hussain MHA, Quinn DI, Vogelzang NJ, Thompson IM Jr, Agarwal N. Baseline circulating tumor cell count as a prognostic marker of PSA response and disease progression in metastatic castrate-sensitive prostate cancer (SWOG S1216) Clin Cancer Res. 2021;27:1967–73. doi: 10.1158/1078-0432.CCR-20-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goodman OB Jr, Symanowski JT, Loudyi A, Fink LM, Ward DC, Vogelzang NJ. Circulating tumor cells as a predictive biomarker in patients with hormone-sensitive prostate cancer. Clin Genitourin Cancer. 2011;9:31–8. doi: 10.1016/j.clgc.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 82.Kohli M, Tan W, Zheng T, Wang A, Montesinos C, Wong C, Du P, Jia S, Yadav S, Horvath LG, Mahon KL, Kwan EM, Fettke H, Yu J, Azad AA. Clinical and genomic insights into circulating tumor DNA-based alterations across the spectrum of metastatic hormone-sensitive and castrate-resistant prostate cancer. EBioMedicine. 2020;54:102728. doi: 10.1016/j.ebiom.2020.102728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bryce AH, Alumkal JJ, Armstrong A, Higano CS, Iversen P, Sternberg CN, Rathkopf D, Loriot Y, de Bono J, Tombal B, Abhyankar S, Lin P, Krivoshik A, Phung D, Beer TM. Radiographic progression with nonrising PSA in metastatic castration-resistant prostate cancer: post hoc analysis of PREVAIL. Prostate Cancer Prostatic Dis. 2017;20:221–7. doi: 10.1038/pcan.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goldkorn A, Ely B, Quinn DI, Tangen CM, Fink LM, Xu T, Twardowski P, Van Veldhuizen PJ, Agarwal N, Carducci MA, Monk JP 3rd, Datar RH, Garzotto M, Mack PC, Lara P Jr, Higano CS, Hussain M, Thompson IM Jr, Cote RJ, Vogelzang NJ. Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: a phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2014;32:1136–42. doi: 10.1200/JCO.2013.51.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scher HI, Jia X, de Bono JS, Fleisher M, Pienta KJ, Raghavan D, Heller G. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10:233–9. doi: 10.1016/S1470-2045(08)70340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Okegawa T, Itaya N, Hara H, Tambo M, Nutahara K. Circulating tumor cells as a biomarker predictive of sensitivity to docetaxel chemotherapy in patients with castration-resistant prostate cancer. Anticancer Res. 2014;34:6705–10. [PubMed] [Google Scholar]
- 87.Zavridou M, Strati A, Bournakis E, Smilkou S, Tserpeli V, Lianidou E. Prognostic significance of gene expression and DNA methylation markers in circulating tumor cells and paired plasma derived exosomes in metastatic castration resistant prostate cancer. Cancers (Basel) 2021;13:780. doi: 10.3390/cancers13040780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maughan BL, Guedes LB, Boucher K, Rajoria G, Liu Z, Klimek S, Zoino R, Antonarakis ES, Lotan TL. p53 status in the primary tumor predicts efficacy of subsequent abiraterone and enzalutamide in castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2018;21:260–8. doi: 10.1038/s41391-017-0027-4. [DOI] [PubMed] [Google Scholar]
- 89.Wyatt AW, Vandekerkhove G. Circulating tumour DNA as a biomarker source in metastatic prostate cancer. Société Internationale d’Urologie Journal. 2020;1:39–48. [Google Scholar]
- 90.Heller G, McCormack R, Kheoh T, Molina A, Smith MR, Dreicer R, Saad F, de Wit R, Aftab DT, Hirmand M, Limon A, Fizazi K, Fleisher M, de Bono JS, Scher HI. Circulating tumor cell number as a response measure of prolonged survival for metastatic castration-resistant prostate cancer: a comparison with prostate-specific antigen across five randomized phase III clinical trials. J. Clin. Oncol. 2018;36:572–80. doi: 10.1200/JCO.2017.75.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sumanasuriya S, Seed G, Parr H, Christova R, Pope L, Bertan C, Bianchini D, Rescigno P, Figueiredo I, Goodall J, Fowler G, Flohr P, Mehra N, Neeb A, Rekowski J, Eisenberger M, Sartor O, Oudard S, Geffriaud-Ricouard C, Ozatilgan A, Chadjaa M, Macé S, Lord C, Baxter J, Pettitt S, Lambros M, Sharp A, Mateo J, Carreira S, Yuan W, de Bono JS. Elucidating prostate cancer behaviour during treatment via low-pass whole-genome sequencing of circulating tumour DNA. Eur Urol. 2021;80:243–53. doi: 10.1016/j.eururo.2021.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goodall J, Assaf Z, Shi Z, Seed G, Zhang L, Lauffer B, Yuan W, Wongchenko M, Oliveira F, Carreira S, Gendreau S, de Bono J. Circulating tumor DNA (ctDNA) dynamics associate with treatment response and radiological progression-free survival (rPFS): analyses from a randomized phase II trial in metastatic castration-resistant prostate cancer (mCRPC) J. Clin. Oncol. 2020;38:5508. [Google Scholar]
- 93.Tolmeijer S, Boerrigter E, Sumiyoshi T, Ng S, Kwan E, Annala M, van Oort I, Schalken J, Van Erp N, Wyatt A, Mehra N. On-treatment plasma ctDNA fraction and treatment outcomes in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2022;40:5051. [Google Scholar]
- 94.Jayaram A, Wingate A, Wetterskog D, Wheeler G, Sternberg CN, Jones R, Berruti A, Lefresne F, Lahaye M, Thomas S, Gormley M, Meacham F, Garg K, Lim LP, Merseburger AS, Tombal B, Ricci D, Attard G. Plasma tumor gene conversions after one cycle abiraterone acetate for metastatic castration-resistant prostate cancer: a biomarker analysis of a multicenter international trial. Ann Oncol. 2021;32:726–35. doi: 10.1016/j.annonc.2021.03.196. [DOI] [PubMed] [Google Scholar]
- 95.Annala M, Fu S, Bacon JVW, Sipola J, Iqbal N, Ferrario C, Ong M, Wadhwa D, Hotte SJ, Lo G, Tran B, Wood LA, Gingerich JR, North SA, Pezaro CJ, Ruether JD, Sridhar SS, Kallio HML, Khalaf DJ, Wong A, Beja K, Schönlau E, Taavitsainen S, Nykter M, Vandekerkhove G, Azad AA, Wyatt AW, Chi KN. Cabazitaxel versus abiraterone or enzalutamide in poor prognosis metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase II trial. Ann Oncol. 2021;32:896–905. doi: 10.1016/j.annonc.2021.03.205. [DOI] [PubMed] [Google Scholar]
- 96.Conteduca V, Wetterskog D, Scarpi E, Romanel A, Gurioli G, Jayaram A, Lolli C, Tandefelt DG, Schepisi G, Casadei C, Wingate A, Matteucci F, Paganelli G, Gonzalez-Billalabeitia E, Demichelis F, De Giorgi U, Attard G. Plasma tumour DNA as an early indicator of treatment response in metastatic castration-resistant prostate cancer. Br J Cancer. 2020;123:982–7. doi: 10.1038/s41416-020-0969-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goodall J, Mateo J, Yuan W, Mossop H, Porta N, Miranda S, Perez-Lopez R, Dolling D, Robinson DR, Sandhu S, Fowler G, Ebbs B, Flohr P, Seed G, Rodrigues DN, Boysen G, Bertan C, Atkin M, Clarke M, Crespo M, Figueiredo I, Riisnaes R, Sumanasuriya S, Rescigno P, Zafeiriou Z, Sharp A, Tunariu N, Bianchini D, Gillman A, Lord CJ, Hall E, Chinnaiyan AM, Carreira S, de Bono JS TOPARP-A investigators. Circulating cell-free DNA to guide prostate cancer treatment with PARP inhibition. Cancer Discov. 2017;7:1006–17. doi: 10.1158/2159-8290.CD-17-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mehra N, Dolling D, Sumanasuriya S, Christova R, Pope L, Carreira S, Seed G, Yuan W, Goodall J, Hall E, Flohr P, Boysen G, Bianchini D, Sartor O, Eisenberger MA, Fizazi K, Oudard S, Chadjaa M, Macé S, de Bono JS. Plasma cell-free DNA concentration and outcomes from taxane therapy in metastatic castration-resistant prostate cancer from two phase III trials (FIRSTANA and PROSELICA) Eur Urol. 2018;74:283–91. doi: 10.1016/j.eururo.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wyatt AW, Annala M, Aggarwal R, Beja K, Feng F, Youngren J, Foye A, Lloyd P, Nykter M, Beer TM, Alumkal JJ, Thomas GV, Reiter RE, Rettig MB, Evans CP, Gao AC, Chi KN, Small EJ, Gleave ME. Concordance of circulating tumor DNA and matched metastatic tissue biopsy in prostate cancer. J Natl Cancer Inst. 2017;109:djx118. doi: 10.1093/jnci/djx118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hussain M, Corcoran C, Sibilla C, Fizazi K, Saad F, Shore N, Sandhu S, Mateo J, Olmos D, Mehra N, Kolinsky MP, Roubaud G, Özgüroğlu M, Matsubara N, Gedye C, Choi YD, Padua C, Kohlmann A, Huisden R, Elvin JA, Kang J, Adelman CA, Allen A, Poehlein C, de Bono J. Tumor genomic testing for >4,000 men with metastatic castration-resistant prostate cancer in the phase III trial PROfound (Olaparib) Clin Cancer Res. 2022;28:1518–30. doi: 10.1158/1078-0432.CCR-21-3940. [DOI] [PubMed] [Google Scholar]
- 101.Teyssonneau D, Margot H, Cabart M, Anonnay M, Sargos P, Vuong NS, Soubeyran I, Sevenet N, Roubaud G. Prostate cancer and PARP inhibitors: progress and challenges. J Hematol Oncol. 2021;14:51. doi: 10.1186/s13045-021-01061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Messina C, Cattrini C, Soldato D, Vallome G, Caffo O, Castro E, Olmos D, Boccardo F, Zanardi E. BRCA mutations in prostate cancer: prognostic and predictive implications. J Oncol. 2020;2020:4986365. doi: 10.1155/2020/4986365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, Chi KN, Sartor O, Agarwal N, Olmos D, Thiery-Vuillemin A, Twardowski P, Mehra N, Goessl C, Kang J, Burgents J, Wu W, Kohlmann A, Adelman CA, Hussain M. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382:2091–102. doi: 10.1056/NEJMoa1911440. [DOI] [PubMed] [Google Scholar]
- 104.Barata P, Agarwal N, Nussenzveig R, Gerendash B, Jaeger E, Hatton W, Ledet E, Lewis B, Layton J, Babiker H, Bryce A, Garje R, Stein C, Kiedrowski L, Saylor P, Sartor O. Clinical activity of pembrolizumab in metastatic prostate cancer with microsatellite instability high (MSI-H) detected by circulating tumor DNA. J Immunother Cancer. 2020;8:e001065. doi: 10.1136/jitc-2020-001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abida W, Cheng ML, Armenia J, Middha S, Autio KA, Vargas HA, Rathkopf D, Morris MJ, Danila DC, Slovin SF, Carbone E, Barnett ES, Hullings M, Hechtman JF, Zehir A, Shia J, Jonsson P, Stadler ZK, Srinivasan P, Laudone VP, Reuter V, Wolchok JD, Socci ND, Taylor BS, Berger MF, Kantoff PW, Sawyers CL, Schultz N, Solit DB, Gopalan A, Scher HI. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5:471–478. doi: 10.1001/jamaoncol.2018.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC, Attard G, Beltran H, Abida W, Bradley RK, Vinson J, Cao X, Vats P, Kunju LP, Hussain M, Feng FY, Tomlins SA, Cooney KA, Smith DC, Brennan C, Siddiqui J, Mehra R, Chen Y, Rathkopf DE, Morris MJ, Solomon SB, Durack JC, Reuter VE, Gopalan A, Gao J, Loda M, Lis RT, Bowden M, Balk SP, Gaviola G, Sougnez C, Gupta M, Yu EY, Mostaghel EA, Cheng HH, Mulcahy H, True LD, Plymate SR, Dvinge H, Ferraldeschi R, Flohr P, Miranda S, Zafeiriou Z, Tunariu N, Mateo J, Perez-Lopez R, Demichelis F, Robinson BD, Schiffman M, Nanus DM, Tagawa ST, Sigaras A, Eng KW, Elemento O, Sboner A, Heath EI, Scher HI, Pienta KJ, Kantoff P, de Bono JS, Rubin MA, Nelson PS, Garraway LA, Sawyers CL, Chinnaiyan AM. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sweeney C, Bracarda S, Sternberg CN, Chi KN, Olmos D, Sandhu S, Massard C, Matsubara N, Alekseev B, Parnis F, Atduev V, Buchschacher GL Jr, Gafanov R, Corrales L, Borre M, Stroyakovskiy D, Alves GV, Bournakis E, Puente J, Harle-Yge ML, Gallo J, Chen G, Hanover J, Wongchenko MJ, Garcia J, de Bono JS. Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2021;398:131–42. doi: 10.1016/S0140-6736(21)00580-8. [DOI] [PubMed] [Google Scholar]
- 108.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, Lotan TL, Zheng Q, De Marzo AM, Isaacs JT, Isaacs WB, Nadal R, Paller CJ, Denmeade SR, Carducci MA, Eisenberger MA, Luo J. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–38. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.De Laere B, Oeyen S, Mayrhofer M, Whitington T, van Dam PJ, Van Oyen P, Ghysel C, Ampe J, Ost P, Demey W, Hoekx L, Schrijvers D, Brouwers B, Lybaert W, Everaert EG, De Maeseneer D, Strijbos M, Bols A, Fransis K, Beije N, de Kruijff IE, van Dam V, Brouwer A, Goossens D, Heyrman L, Van den Eynden GG, Rutten A, Del Favero J, Rantalainen M, Rajan P, Sleijfer S, Ullén A, Yachnin J, Grönberg H, Van Laere SJ, Lindberg J, Dirix LY. TP53 outperforms other androgen receptor biomarkers to predict abiraterone or enzalutamide outcome in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2019;25:1766–73. doi: 10.1158/1078-0432.CCR-18-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peter MR, Bilenky M, Isserlin R, Bader GD, Shen SY, De Carvalho DD, Hansen AR, Hu P, Fleshner NE, Joshua AM, Hirst M, Bapat B. Dynamics of the cell-free DNA methylome of metastatic prostate cancer during androgen-targeting treatment. Epigenomics. 2020;12:1317–32. doi: 10.2217/epi-2020-0173. [DOI] [PubMed] [Google Scholar]
- 111.Mahon KL, Qu W, Lin HM, Spielman C, Cain D, Jacobs C, Stockler MR, Higano CS, de Bono JS, Chi KN, Clark SJ, Horvarth LG. Serum free methylated glutathione S-transferase 1 DNA levels, survival, and response to docetaxel in metastatic, castration-resistant prostate cancer: post hoc analyses of data from a phase 3 trial. Eur Urol. 2019;76:306–12. doi: 10.1016/j.eururo.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 112.Stelcer E, Konkol M, Głęboka A, Suchorska WM. Liquid biopsy in oligometastatic prostate cancer-a biologist’s point of view. Front Oncol. 2019;9:775. doi: 10.3389/fonc.2019.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cheng Q, Butler W, Zhou Y, Zhang H, Tang L, Perkinson K, Chen X, Jiang XS, McCall SJ, Inman BA, Huang J. Pre-existing castration-resistant prostate cancer-like cells in primary prostate cancer promote resistance to hormonal therapy. Eur Urol. 2022;81:446–55. doi: 10.1016/j.eururo.2021.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ionescu F, Zhang J, Wang L. Clinical applications of liquid biopsy in prostate cancer: from screening to predictive biomarker. Cancers (Basel) 2022;14:1728. doi: 10.3390/cancers14071728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Scott E, Munkley J. Glycans as biomarkers in prostate cancer. Int J Mol Sci. 2019;20:1389. doi: 10.3390/ijms20061389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Skotland T, Ekroos K, Kauhanen D, Simolin H, Seierstad T, Berge V, Sandvig K, Llorente A. Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur J Cancer. 2017;70:122–32. doi: 10.1016/j.ejca.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 117.Lin HM, Mahon KL, Weir JM, Mundra PA, Spielman C, Briscoe K, Gurney H, Mallesara G, MARX G, Stockler MR PRIMe Consortium. Parton RG, Hoy AJ, Daly RJ, Meikle PJ, Horvath LG. A distinct plasma lipid signature associated with poor prognosis in castration-resistant prostate cancer. Int J Cancer. 2017;141:2112–20. doi: 10.1002/ijc.30903. [DOI] [PubMed] [Google Scholar]
- 118.Poore GD, Kopylova E, Zhu Q, Carpenter C, Fraraccio S, Wandro S, Kosciolek T, Janssen S, Metcalf J, Song SJ, Kanbar J, Miller-Montgomery S, Heaton R, Mckay R, Patel SP, Swafford AD, Knight R. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature. 2020;579:567–74. doi: 10.1038/s41586-020-2095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gerdtsson E, Pore M, Thiele JA, Gerdtsson AS, Malihi PD, Nevarez R, Kolatkar A, Velasco CR, Wix S, Singh M, Carlsson A, Zurita AJ, Logothetis C, Merchant AA, Hicks J, Kuhn P. Multiplex protein detection on circulating tumor cells from liquid biopsies using imaging mass cytometry. Converg Sci Phys Oncol. 2018;4:015002. doi: 10.1088/2057-1739/aaa013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Faugeroux V, Lefebvre C, Pailler E, Pierron V, Marcaillou C, Tourlet S, Billiot F, Dogan S, Oulhen M, Vielh P, Rameau P, NgoCamus M, Massard C, Laplace-Builhé C, Tibbe A, Taylor M, Soria JC, Fizazi K, Loriot Y, Julien S, Farace F. An accessible and unique insight into metastasis mutational content through whole-exome sequencing of circulating tumor cells in metastatic prostate cancer. Eur Urol Oncol. 2020;3:498–508. doi: 10.1016/j.euo.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 121.Herberts C, Murtha AJ, Fu S, Wang G, Schönlau E, Xue H, Lin D, Gleave A, Yip S, Angeles A, Hotte S, Tran B, North S, Taavitsainen S, Beja K, Vandekerkhove G, Ritch E, Warner E, Saad F, Iqbal N, Nykter M, Gleave ME, Wang Y, Annala M, Chi KN, Wyatt AW. Activating AKT1 and PIK3CA mutations in metastatic castration-resistant prostate cancer. Eur Urol. 2020;78:834–44. doi: 10.1016/j.eururo.2020.04.058. [DOI] [PubMed] [Google Scholar]
- 122.Zhao SG, Sperger JM, Schehr JL, McKay RR, Emamekhoo H, Singh A, Schultz ZD, Bade RM, Stahlfeld CN, Gilsdorf CS, Hernandez CI, Wolfe SK, Mayberry RD, Krause HM, Bootsma M, Helzer KT, Rydzewski N, Bakhtiar H, Shi Y, Blitzer G, Kyriakopoulos CE, Kosoff D, Wei XX, Floberg J, Sethakorn N, Sharifi M, Harari PM, Huang W, Beltran H, Choueiri TK, Scher HI, Rathkopf DE, Halabi S, Armstrong AJ, Beebe DJ, Yu M, Sundling KE, Taplin ME, Lang LM. A clinical-grade liquid biomarker detects neuroendocrine differentiation in prostate cancer. J Clin Invest. 2022;132:e161858. doi: 10.1172/JCI161858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pavan N, Grassi G, Scaggiante B. An update of aberrant methylation detection on circulating cell-free DNA as a tool to improve prostate cancer diagnosis and prognosis. J Transl Genet Genom. 2021;5:173–81. [Google Scholar]
- 124.Taylor A, Sperger J, Sharifi M, Shi Y, Stahlfeld C, Schehr J, Emamekhoo H, Kyriakopoulos C, Armstrong A, Wei X, Taplin M, McKay R, Zhao S, Lang J. Association of emergent neuroendocrine prostate cancer detected by liquid biopsies with survival and treatment resistance. J. Clin. Oncol. 2023;41:247–247. [Google Scholar]
- 125.Hodara E, Morrison G, Cunha A, Zainfeld D, Xu T, Xu Y, Dempsey PW, Pagano PC, Bischoff F, Khurana A, Koo S, Ting M, Cotter PD, Moore MW, Gunn S, Usher J, Rabizadeh S, Danenberg P, Danenberg K, Carpten J, Dorff T, Quinn D, Goldkorn A. Multiparametric liquid biopsy analysis in metastatic prostate cancer. JCI Insight. 2019;4:e125529. doi: 10.1172/jci.insight.125529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Friedlander TW, Pritchard CC, Beltran H. Personalizing therapy for metastatic prostate cancer: the role of solid and liquid tumor biopsies. Am Soc Clin Oncol Educ Book. 2017;37:358–69. doi: 10.1200/EDBK_175510. [DOI] [PubMed] [Google Scholar]
- 127.Dago AE, Stepansky A, Carlsson A, Luttgen M, Kendall J, Baslan T, Kolatkar A, Wigler M, Bethel K, Gross ME, Hicks J, Kuhn P. Rapid phenotypic and genomic change in response to therapeutic pressure in prostate cancer inferred by high content analysis of single circulating tumor cells. PLoS One. 2014;9:e101777. doi: 10.1371/journal.pone.0101777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sharp A, Coleman I, Yuan W, Sprenger C, Dolling D, Rodrigues DN, Russo JW, Figueiredo I, Bertan C, Seed G, Riisnaes R, Uo T, Neeb A, Welti J, Morrissey C, Carreira S, Luo J, Nelson PS, Balk SP, True LD, de Bono JS, Plymate SR. Androgen receptor splice variant-7 expression emerges with castration resistance in prostate cancer. J Clin Invest. 2019;129:192–208. doi: 10.1172/JCI122819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gupta S, Hovelson DH, Kemeny G, Halabi S, Foo WC, Anand M, Somarelli JA, Tomlins SA, Antonarakis ES, Luo J, Dittamore RV, George DJ, Rothwell C, Nanus DM, Armstrong AJ, Gregory SG. Discordant and heterogeneous clinically relevant genomic alterations in circulating tumor cells vs plasma DNA from men with metastatic castration resistant prostate cancer. Genes Chromosomes Cancer. 2020;59:225–39. doi: 10.1002/gcc.22824. [DOI] [PubMed] [Google Scholar]
- 130.Hamid AA, Gray KP, Shaw G, MacConaill LE, Evan C, Bernard B, Loda M, Corcoran NM, Van Allen EM, Choudhury AD, Sweeney CJ. Compound genomic alterations of TP53, PTEN, and RB1 tumor suppressors in localized and metastatic prostate cancer. Eur Urol. 2019;76:89–97. doi: 10.1016/j.eururo.2018.11.045. [DOI] [PubMed] [Google Scholar]
- 131.Kubota Y, Hatakeyama S, Yoneyama T, Yoneyama MS, Hamano I, Konishi S, Okamoto T, Yamamoto H, Yoneyama T, Hashimoto Y, Ohyama C. Prognostic significance of total plasma cell-free DNA level and androgen receptor amplification in castration-resistant prostate cancer. World J Urol. 2021;39:3265–71. doi: 10.1007/s00345-021-03649-x. [DOI] [PubMed] [Google Scholar]
- 132.Fettke H, Kwan EM, Bukczynska P, Ng N, Nguyen-Dumont T, Southey MC, Davis ID, Mant A, Parente P, Pezaro C, Hauser C, Azad AA. Prognostic impact of total plasma cell-free DNA concentration in androgen receptor pathway inhibitor-treated metastatic castration-resistant prostate cancer. Eur Urol Focus. 2021;7:1287–91. doi: 10.1016/j.euf.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 133.Liu H, Gao Y, Vafaei S, Gu X, Zhong X. The prognostic value of plasma cell-free DNA concentration in the prostate cancer: a systematic review and meta-analysis. Front Oncol. 2021;11:599602. doi: 10.3389/fonc.2021.599602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sweeney C, Graf R, Fabrizio D, Yuen K, Wongchenko M, Gupta P, Hegde P, Oxnard G, Powles T. Circulating tumor DNA analysis of IMbassador250: association of ctDNA fraction, AR alterations and therapy outcome in mCRPC. J. Clin. Oncol. 2023;41:LBA249–LBA249. [Google Scholar]
- 135.Reichert ZR, Morgan TM, Li G, Castellanos E, Snow T, Dall’Olio FG, Madison RW, Fine AD, Oxnard GR, Graf RP, Stover DG. Prognostic value of plasma circulating tumor DNA fraction across four common cancer types: a real-world outcomes study. Ann Oncol. 2023;34:111–20. doi: 10.1016/j.annonc.2022.09.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sonpavde G, Agarwal N, Pond GR, Nagy RJ, Nussenzveig RH, Hahn AW, Sartor O, Gourdin TS, Nandagopal L, Ledet EM, Naik G, Armstrong AJ, Wang J, Bilen MA, Gupta S, Grivas P, Pal SK, Lanman RB, Talasaz A, Lilly MB. Circulating tumor DNA alterations in patients with metastatic castration-resistant prostate cancer. Cancer. 2019;125:1459–69. doi: 10.1002/cncr.31959. [DOI] [PubMed] [Google Scholar]
- 137.Lau E, McCoy P, Reeves F, Chow K, Clarkson M, Kwan EM, Packwood K, Northen H, He M, Kingsbury Z, Mangiola S, Kerger M, Furrer MA, Crowe H, Costello AJ, McBride DJ, Ross MT, Pope B, Hovens CM, Corcoran NM. Detection of ctDNA in plasma of patients with clinically localised prostate cancer is associated with rapid disease progression. Genome Med. 2020;12:72. doi: 10.1186/s13073-020-00770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fan L, Fei X, Zhu Y, Pan J, Sha J, Chi C, Gong Y, Du X, Zhou L, Dong B, Xue W. Comparative analysis of genomic alterations across castration sensitive and castration resistant prostate cancer via circulating tumor DNA sequencing. J Urol. 2021;205:461–9. doi: 10.1097/JU.0000000000001363. [DOI] [PubMed] [Google Scholar]
- 139.Yuan F, Liu N, Yang MZ, Zhang XT, Luo H, Zhou H. Circulating tumor DNA genomic profiling reveals the complicated olaparib-resistance mechanism in prostate cancer salvage therapy: a case report. World J Clin Cases. 2022;10:3461–71. doi: 10.12998/wjcc.v10.i11.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, Nava Rodrigues D, Robinson D, Omlin A, Tunariu N, Boysen G, Porta N, Flohr P, Gillman A, Figueiredo I, Paulding C, Seed G, Jain S, Ralph C, Protheroe A, Hussain S, Jones R, Elliott T, McGovern U, Bianchini D, Goodall J, Zafeiriou Z, Williamson CT, Ferraldeschi R, Riisnaes R, Ebbs B, Fowler G, Roda D, Yuan W, Wu YM, Cao X, Brough R, Pemberton H, A’Hern R, Swain A, Kunju LP, Eeles R, Attard G, Lord CJ, Ashworth A, Rubin MA, Knudsen KE, Feng FY, Chinnaiyan AM, Hall E, de Bono JS. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697–708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhao H, Yang L, Baddour J, Achreja A, Bernard V, Moss T, Marini JC, Tudawe T, Seviour EG, San Lucas FA, Alvarez H, Gupta S, Maiti SN, Cooper L, Peehl D, Ram PT, Maitra A, Nagrath D. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife. 2016;5:e10250. doi: 10.7554/eLife.10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Soekmadji C, Hill AF, Wauben MH, Buzás EI, Di Vizio D, Gardiner C, Lötvall J, Sahoo S, Witwer KW. Towards mechanisms and standardization in extracellular vesicle and extracellular RNA studies: results of a worldwide survey. J Extracell Vesicles. 2018;7:1535745. doi: 10.1080/20013078.2018.1535745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.El-Sayed IY, Daher A, Destouches D, Firlej V, Kostallari E, Maillé P, Huet E, Haidar-Ahmad N, Jenster G, de la Taille A, Abou Merhi R, Terry S, Vacherot F. Extracellular vesicles released by mesenchymal-like prostate carcinoma cells modulate EMT state of recipient epithelial-like carcinoma cells through regulation of AR signaling. Cancer Lett. 2017;410:100–11. doi: 10.1016/j.canlet.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 144.Webber JP, Spary LK, Sanders AJ, Chowdhury R, Jiang WG, Steadman R, Wymant J, Jones AT, Kynaston H, Mason MD, Tabi Z, Clayton A. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene. 2015;34:290–302. doi: 10.1038/onc.2013.560. [DOI] [PubMed] [Google Scholar]
- 145.Corcoran C, Rani S, O’Brien K, O’Neill A, Prencipe M, Sheikh R, Webb G, McDermott R, Watson W, Crown J, O’Driscoll L. Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS One. 2012;7:e50999. doi: 10.1371/journal.pone.0050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Oeyen S, Liégeois V, De Laere B, Buys A, Strijbos M, Dirix P, Meijnders P, Vermeulen P, Van Laere S, Dirix L. Automated enumeration and phenotypic characterization of CTCs and tdEVs in patients with metastatic castration resistant prostate cancer. Prostate Cancer Prostatic Dis. 2021;24:499–506. doi: 10.1038/s41391-020-00304-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Belic J, Graf R, Bauernhofer T, Cherkas Y, Ulz P, Waldispuehl-Geigl J, Perakis S, Gormley M, Patel J, Li W, Geigl JB, Smirnov D, Heitzer E, Gross M, Speicher MR. Genomic alterations in plasma DNA from patients with metastasized prostate cancer receiving abiraterone or enzalutamide. Int J Cancer. 2018;143:1236–48. doi: 10.1002/ijc.31397. [DOI] [PMC free article] [PubMed] [Google Scholar]