Abstract
We have previously shown that hepatitis B virus (HBV) replication is inhibited noncytopathically in the livers of transgenic mice following injection of HBV-specific cytotoxic T lymphocytes (CTLs) or infection with unrelated hepatotropic viruses, including lymphocytic choriomeningitis virus (LCMV) and adenovirus. These effects are mediated by gamma interferon (IFNγ), tumor necrosis factor alpha (TNFα), and IFNα/β. In the present study, we crossed HBV transgenic mice with mice genetically deficient for IFNγ (IFNγKO), the TNFα receptor (TNFαRKO), or the IFNα/β receptor (IFNα/βRKO) in order to determine the relative contribution of each cytokine to the antiviral effects observed in each of these systems. Interestingly, we showed that HBV replicates in unmanipulated IFNγKO and IFNα/βRKO mice at levels higher than those observed in control mice, implying that baseline levels of these cytokines control HBV replication in the absence of inflammation. We also showed that IFNγ mediates most of the antiviral effect of the CTLs while IFNα/β is primarily responsible for the early inhibitory effect of LCMV and adenovirus on HBV replication. In addition, we showed that the hepatic induction of IFNα/β observed after injection of poly(I · C) is sufficient to inhibit HBV replication and that a similar antiviral effect is achieved by systemic administration of very high doses of IFNα. We also compared the relative sensitivity of LCMV and adenovirus to control by IFNγ, TNFα, or IFNα/β in these animals. Importantly, IFNα/βRKO mice, and to a lesser extent IFNγKO mice, showed higher hepatic levels of LCMV RNA and adenovirus DNA and RNA than control mice, underscoring the importance of both interferons in controlling these other viral infections as well.
Hepatitis B virus (HBV) is a noncytopathic, enveloped virus that causes acute and chronic hepatitis and hepatocellular carcinoma (4). We have previously shown that the intrahepatic induction of gamma interferon (IFNγ), tumor necrosis factor alpha (TNFα), and IFNα/β downregulates HBV replication noncytopathically in the livers of transgenic mice (8, 9). This antiviral effect can be achieved by injecting transgenic mice with HBV-specific cytotoxic T lymphocytes (CTLs) (10) or infecting them with an unrelated hepatotropic virus, such as lymphocytic choriomeningitis virus (LCMV) or adenovirus (3, 7).
The CTL-dependent effect occurs within 24 h and appears to be mediated by both IFNγ and TNFα, since it is possible to block the regulatory effects of the CTLs by the prior administration of a cocktail of antibodies to these cytokines (10). Whether the antiviral cytokines are produced by the passively transferred CTLs or by host-derived cells is unknown.
The LCMV- and adenovirus-dependent effect occurs in two distinct phases. The first phase occurs within 12 to 24 h and is mediated by IFNα/β and/or TNFα induced by the infecting virus, since it is blocked by a cocktail of antibodies to these cytokines (3). The second phase occurs 5 to 7 days after infection and is associated with the intrahepatic induction of IFNα/β and TNFα as well as IFNγ produced during the cellular immune response to each virus (3).
We do not know whether these cytokines function independently or synergistically in this model. The facts that only a combination of antibodies to IFNγ and TNFα completely blocked the antiviral effect of the CTLs and that a cocktail of antibodies to TNFα and IFNα/β was needed to completely block the early LCMV- and adenovirus-induced inhibition of hepatic HBV replication suggest that these cytokines may cooperate by activating distinct regulatory pathways. In addition, the simultaneous administration of antibodies to TNFα and IFNα/β did not block the ability of LCMV or adenovirus to inhibit HBV replication during the late inflammatory phase of those infections, suggesting that other factors (e.g., IFNγ) may suppress HBV replication at that point.
In the present study, we crossed the HBV-transgenic mice with mice genetically deficient for IFNγ, TNFα receptor, or the IFNα/β receptor in order to (i) determine the relative contributions of transferred CTLs and host-derived inflammatory cells to the production of IFNγ and/or TNFα and the resultant inhibition of HBV replication; (ii) determine the relative contributions of IFNγ, TNFα, and IFNα/β to the inhibition of HBV replication after CTL injection and LCMV or adenovirus infection; (iii) assess the ability of these cytokines to inhibit LCMV and adenovirus infections in these animals; (iv) study the antiviral efficacy and mode of action of the IFNα/β inducer poly(I · C); and (v) compare the antiviral effect of systemic administration of IFNα with treatments that induce IFNα/β directly in the liver.
MATERIALS AND METHODS
Mice.
The HBV-transgenic mouse lineage 1.3.46 (inbred B10.D2) used in this study (official designation, Tg[HBV 1.3 genome]Chi46) has been described previously (11). HBV replicates at high levels in the livers of these mice without any evidence of cytopathology. Lineage 1.3.46 was crossed with three lineages of knockout mice that lack IFNγ (IFNγKO) (5), the TNFα receptor p55 (TNFαRKO) (18), or the IFNα/β receptor (IFNα/βRKO) (17). The knockout mice were provided by Timothy Stewart (Genentech, South San Francisco, Calif.) (IFNγKO), Tak Mak (University of Toronto, Toronto, Ontario, Canada) (TNFαRKO), and Michel Aguet (Genentech) (IFNα/βRKO). Heterozygous mice from lineage 1.3.46 were crossed with homozygous mice from each of the three knockout lineages to yield progeny whose sera were screened for the presence of the HBV e antigen (HBeAg) (using a commercially available kit from Abbott Laboratories, Abbott Park, Il.). HBeAg-positive progeny were screened for homozygosity of the null mutations by PCR exactly as described previously (5, 17, 18) and for homozygosity of the H-2d class I molecule (the restriction element utilized by HBV surface antigen [HBsAg]-specific CTL lines; see below) by fluorescence-activated cell sorter analysis as described previously (21). Mice that were homozygous for the H-2d class I molecule and either homozygous or heterozygous for the null mutation were matched for age (8 to 10 weeks), sex (male), and levels of HBeAg in their sera before the onset of experimental manipulations. All animals were housed in pathogen-free rooms under strict barrier conditions.
Injection of HBsAg-specific CTLs.
An IFNγ- and TNFα-producing HBsAg-specific, H-2d restricted, CD8+ CTL line was derived from spleen cells of nontransgenic B10.D2 mice immunized with 50 μg of plasmid pCMV-S2/S as described previously (21). The CTL line was maintained by weekly restimulation with irradiated P815 cells that stably express the HBV large envelope protein (ayw subtype) containing HBsAg, as previously described (1). Five days after the last stimulation, the cells were washed, counted, suspended in Hank's balanced salt solution containing 2% fetal calf serum, and injected intravenously into HBV-transgenic mice. Two days after injection, mice were sacrificed, and their livers either were harvested for histological and histochemical analyses or were snap frozen in liquid nitrogen and stored at −80°C for subsequent molecular analyses (see below).
LCMV isolates and infection of mice.
Clone 2.2 of the WE isolate of LCMV used in this study has been previously described (7). Stocks of virus were prepared by growth on BHK cells. All virus stocks were free of mycoplasma contamination as determined by Hoechst staining of cells growing in antibiotic-free medium 48 h after virus infection. The titers of the LCMV stocks, and also infectious virus titers in murine tissues, were determined by plaque assay on Vero cells as described previously (7). Adult male mice (8 to 10 weeks old) were infected by single intravenous inoculations (2 × 106 PFU) of LCMV WE clone 2.2, and they were sacrificed either 24 h or 7 days after infection, at which time their livers were processed exactly as described for the CTL-injected animals.
Adenovirus infection.
A recombinant, replication-deficient adenovirus designated Ad.CBlacZ (13) was kindly provided by James Wilson (University of Pennsylvania Medical Center, Philadelphia). Stocks of Ad.CBlacZ were grown in 293 cells and were purified by two rounds of CsCl density centrifugation as previously described (3). Viral titers were determined by plaque assay on 293 cells, and a single stock was used throughout this study. Adult male mice (8 to 10 weeks old) were infected by single intravenous inoculations (1.5 × 109 PFU) of Ad.CblacZ per mouse, and they were sacrificed either 24 h or 7 days after infection, at which time their livers were processed exactly as described for the CTL-injected animals.
Poly(I · C) treatment.
Mice were injected intravenously with single doses (200 μg/mouse) of poly(I · C) (Sigma Chemical, St. Louis, Mo.) and were sacrificed 24 h later, at which time their livers were processed exactly as described for the CTL-injected animals.
IFN-α treatment.
Mice were injected once intravenously with different doses (ranging from 5 × 103 to 5 × 106 U/mouse) of human IFNα (IFN A/D; kindly provided by Mary Graves, Roche Discovery, Welwyn, United Kingdom) and were sacrificed 24 h later, at which time their livers were processed exactly as described for the CTL-injected animals. Human IFNα (IFN A/D) has been previously shown to be functionally active on mouse cells both in vitro (20) and in vivo (22).
Tissue DNA and RNA analyses.
Frozen liver tissue was mechanically pulverized under liquid nitrogen, and total genomic DNA and RNA were isolated for Southern and Northern blot analyses exactly as previously described (11). Nylon membranes were analyzed for HBV DNA, HBV RNA, Ad.CBlacZ DNA and RNA, LCMV RNA, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and 2′,5′-oligoadenylate synthetase (2′,5′-OAS) as described previously (3). Quantitations of cytokines and of T-lymphocyte and macrophage marker mRNAs were performed by RNase protection assay exactly as previously described (10). IFNγKO mice produce a nonfunctional message for IFNγ that is detectable by RNase protection assay. The relative abundance of specific DNA and RNA molecules was quantitated by phosphorimaging analysis, using the Optiquant image analysis software (Packard, Meriden, Conn.).
Biochemical, histological, and immunohistochemical analyses.
The extent of hepatocellular injury was monitored by measuring serum alanine aminotransferase (sALT) activity at multiple time points after treatment with saline, CTLs, IFNα, or poly(I · C), or after infection with LCMV or adenovirus. sALT activity was measured in a Paramax chemical analyzer (Baxter Diagnostics Inc., McGaw Park, Ill.) exactly as previously described (10). For histological analysis, liver tissue samples were fixed in 10% zinc-buffered formalin (Anatech, Battle Creek, Mich.), embedded in paraffin, sectioned (3 μm thick), and stained with hematoxylin and eosin exactly as described elsewhere (10).
β-Galactosidase histochemistry.
The number of β-galactosidase-positive cells in the livers of Ad.CBlacZ-infected animals was quantitated exactly as described previously (3).
RESULTS
High levels of HBV replication in livers of mice genetically deficient for IFNγ or the IFNα/β receptor.
HBV-transgenic mice of lineage 1.3.46 (11) were crossed with mice genetically deficient for IFNγ, the TNFα receptor, or the IFNα/β receptor. Groups (six mice each) of age (8 to 10 weeks)-, sex (male)-, and serum HBeAg-matched animals that were either heterozygous or homozygous for the respective null mutation were sacrificed, and their livers were harvested. Following extraction, total hepatic RNA and DNA in each group were pooled and analyzed for HBV gene expression and replication by Northern and Southern blot analyses.
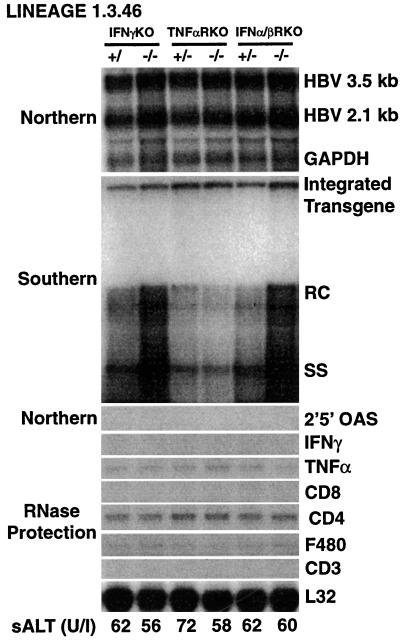
As shown in Fig. 1, HBV replicates in the livers of IFNα/βRKO and IFNγKO mice at levels that are about threefold higher than those of the respective heterozygous control littermates or the TNFαRKO mice (as measured by phosphorimaging analysis) (data not shown). In contrast, the intrahepatic levels of HBV RNA, including the pregenomic 3.5-kb RNA, were very similar. By phosphorimaging analysis, we calculated that the pregenomic RNA contents in the livers of the IFNα/βRKO and IFNγKO mice were, respectively, 1.07- and 1.26-fold higher than those of their heterozygous controls (data not shown). This indicates that the IFN-dependent effect on viral replication involves steps in the viral life cycle that follow accumulation of pregenomic RNA. The livers from all these animals were also tested for the expression of inflammatory cytokines (IFNγ, TNFα, and 2′,5′-OAS, a marker of IFNα/β induction) as well as T-cell and macrophage markers (CD8, CD4, CD3, and F480) by RNase protection and Northern blot analyses. The mRNAs for TNFα, CD4, and F480 were the only detectable RNA species (Fig. 1); all of these are products of the resident macrophages and are known to be expressed in the uninflamed liver (10, 12). The lack of inflammation in these livers was also underscored by the absence of sALT elevation (Fig. 1, bottom). The higher content of HBV replicative forms in the IFNα/βRKO and IFNγKO mice suggests that circulation of undetectable amounts of interferons may control HBV replication in the uninflamed livers of wild-type mice. IFNα/βRKO and IFNγKO mice have been monitored histologically for over 1 year, and despite their high levels of HBV replication, no pathological changes have been observed in any organ, including the liver (data not shown).
FIG. 1.
High levels of HBV replication in livers of mice genetically deficient for IFNγ or the IFNα/β receptor. Six age (8 to 10 weeks)-, sex (male)-, and serum HBeAg-matched mice that were either heterozygous (+/−) or homozygous (−/−) for the indicated null mutation were sacrificed, and their livers were harvested. Following extraction, total hepatic RNA and DNA in each group were pooled and analyzed for HBV gene expression and replication by Northern and Southern blot analyses. The membranes were hybridized with 32P-labeled HBV-, 2′,5′-OAS-, and GAPDH-specific DNA probes. Southern blot analysis was performed with 30 μg of total hepatic DNA. All DNA samples were RNAse treated before being subjected to gel electrophoresis. Bands corresponding to the integrated transgene and to relaxed-circular (RC) and single-stranded (SS) linear HBV DNA replicative forms are indicated. The integrated transgene can be used to normalize the amount of DNA bound to the membrane. The filter was hybridized with a 32P-labeled HBV-specific DNA probe. Total hepatic RNA (10 μg) from the same mice was also analyzed by RNase protection assay for the expression of IFNγ and TNFα transcripts and for the expression of CD3, CD4, CD8, and F480, as indicated. The mRNA encoding the ribosomal protein L32 was used to normalize the amount of RNA loaded in each lane. The mean sALT activity, measured at the time of autopsy, is indicated (bottom) for each group and is expressed in units per liter.
IFNγ produced by HBsAg-specific CTLs is sufficient to inhibit HBV replication in the livers of HBV-transgenic mice.
Next, we determined the relative contributions of IFNγ, TNFα, and IFNα/β to the antiviral effect of CTLs, and we examined the relative contributions of the transferred CTLs and host-derived inflammatory cells in the antiviral activity of IFNγ and/or TNFα. Six age-, sex-, and serum HBeAg-matched transgenic mice from the same groups described above were each injected intravenously with 2.5 × 107 lymphocytes derived from an IFNγ- and TNFα-producing CD8+, HBsAg-specific CTL line. Two days later, the mice were bled and sacrificed, and their livers were harvested.
As shown in Fig. 2 for two representative mice per group, HBV DNA replicative forms almost completely disappeared from the livers of all transgenic mice after CTL injection compared to the respective saline-injected controls. As measured by phosphorimaging analysis, the reductions of HBV DNA replicative forms were respectively about 15- and 20-fold in IFNγKO mice that were heterozygous or homozygous for the null mutation (IFNγKO+/− and IFNγKO−/− mice, respectively); about 12- and 10-fold in TNFαRKO+/− and TNFαRKO−/− mice, respectively; and about 12- and 21-fold in IFNα/βRKO+/− and IFNα/βRKO−/− mice, respectively. This effect was associated with the intrahepatic induction of IFNγ, TNFα, CD8, CD4, CD3, and F480 mRNAs. The expression of 2′,5′-OAS was barely detectable in CTL-injected livers with the exception of those that lack the IFNα/β receptor, in which it was undetectable. A relatively mild liver disease was revealed histologically (data not shown), as also indicated by the modest elevation in sALT level (Fig. 2, bottom).
FIG. 2.
IFN-γ produced by HBsAg-specific CTLs is sufficient to inhibit HBV replication in the livers of HBV-transgenic mice. Age-, sex-, and serum HBeAg-matched transgenic mice from the same groups described in the legend to Fig. 1 were injected intravenously with 2.5 × 107 CTLs and sacrificed 2 days later (d.2). Total hepatic RNA and DNA were analyzed for HBV gene expression and replication and for mRNAs for 2′,5′-OAS, IFNγ, TNFα, and T-cell (CD8, CD4, and CD3) and macrophage (F480) markers exactly as described in the legend to Fig. 1. Results were compared with those for livers pooled from 10 age-, sex-, and serum HBeAg-matched transgenic saline-injected controls (NaCl). The mean sALT activity, measured at the time of autopsy, is indicated (bottom) for each group and is expressed in units per liter. RC and SS, relaxed-circular and single-stranded linear HBV DNA replicative forms, respectively.
These results demonstrate that inhibition of HBV replication can occur in mice that do not respond to TNFα or IFNα/β, suggesting that TNFα and IFNα/β are not required to mediate the antiviral activity of CTLs. Since the antiviral effect of CTLs can also occur in mice that cannot produce IFNγ, it appears that the amount of IFNγ produced by passively transferred CTLs is sufficient to mediate the inhibitory effect on HBV replication.
These results also show that the hepatic expression of T-cell and macrophage markers differed considerably among the various groups of CTL-injected animals, while the levels of expression of IFNγ and TNFα were relatively similar. In particular, compared with wild-type controls or IFNγKO mice, we observed higher levels of CD3, CD8, and F480 RNA in TNFαRKO mice and higher levels of CD3, CD4, and F480 RNA in IFNα/βRKO mice. This suggests that TNFα and IFNα/β may inhibit the recruitment of CD8+ and CD4+ T cells, respectively, to the liver and that both cytokines may inhibit the recruitment of macrophages.
IFN-dependent inhibition of HBV and LCMV replication.
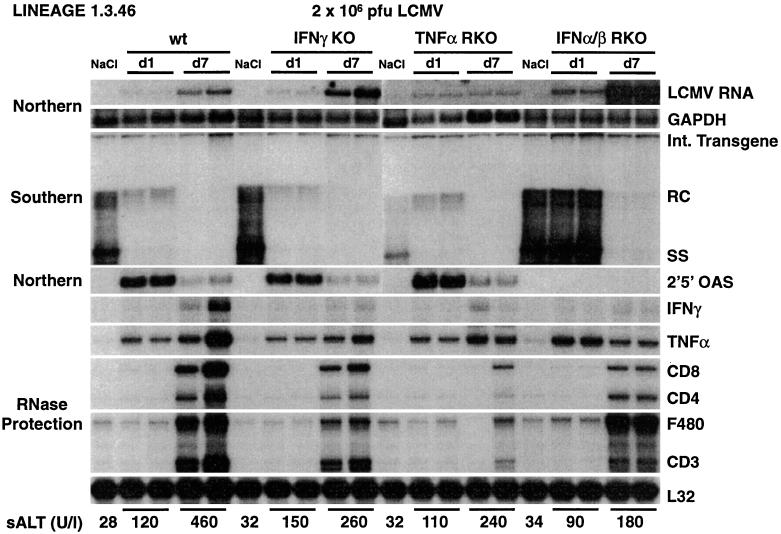
To determine the relative contributions of IFNγ, TNFα, and IFNα/β to the ability of LCMV to inhibit HBV replication, and to compare the relative sensitivities of HBV and LCMV to these cytokines, we monitored an acute LCMV infection in the same groups of animals described above. Animals indicated as wild type were heterozygous for the IFNα/β receptor null mutation (Fig. 3). Identical results were obtained in all heterozygous mouse strains (data not shown). sALT activity was only modestly elevated in all infected animals (Fig. 3, bottom), in keeping with the fact that LCMV clone WE 2.2 infects primarily macrophages and not hepatocytes (7). The induction of IFN-α/β (as monitored by the induction of 2′,5′-OAS) and TNFα at day 1 postinfection not only in wild-type mice but also in IFNγKO and TNFαRKO mice, was confirmed in this experiment, and this induction coincided with a profound decrease in HBV replication (Fig. 3). In contrast, HBV replication was not reduced in the animals that could not respond to IFN-α/β, despite a strong induction of TNFα, demonstrating that IFNα/β mediates the early antiviral effect of LCMV infection. Groups of mice were also sacrificed 7 days after infection, when the LCMV-specific T-cell response peaks in the liver (7) (Fig. 3) and when IFNγ and TNFα are induced (Fig. 3). At this time point, HBV replication was virtually abolished in all groups, including the IFNα/βRKO mice, indicating that IFNγ (and possibly TNFα) downregulates HBV replication by IFNα/β-independent pathways.
FIG. 3.
IFN-dependent inhibition of HBV and LCMV replication. Age-, sex-, and serum HBeAg-matched transgenic mice from the same groups of mice described in the legend to Fig. 1 were infected with LCMV WE clone 2.2 (2 × 106 PFU/mouse) and sacrificed either 24 h (d1) or 7 days (d7) after infection. Total hepatic RNA and DNA were analyzed for HBV gene expression and replication and for mRNAs for 2′,5′-OAS, IFNγ, TNFα, and T-cell (CD8, CD4, and CD3) and macrophage (F480) markers exactly as described in the legend to Fig. 1. Northern blot membranes were also hybridized with a 32P-labeled LCMV-specific DNA probe (top). Animals indicated as wild type (wt) were heterozygous for the IFNα/β receptor null mutation. Results were compared with those for livers pooled from 10 age-, sex-, and serum HBeAg-matched transgenic saline-injected controls (NaCl) that were sacrificed 1 day after NaCl injection. The mean sALT activity, measured at the time of autopsy, is indicated (bottom) for each group and is expressed in units per liter. Int., integrated; RC and SS, relaxed-circular and single-stranded linear HBV DNA replicative forms, respectively.
The extent of LCMV infection was monitored by Northern blot analysis of genomic LCMV RNA (Fig. 3, top). Importantly, IFNα/βRKO mice showed about threefold-higher hepatic levels of LCMV RNA (as measured by phosphorimaging analysis) (data not shown) on day 1 postinfection than wild-type controls and IFNγKO and TNFαRKO mice (Fig. 3, top). This indicates that LCMV, like HBV, is susceptible to the early antiviral effects of IFNα/β. In addition, the LCMV RNA contents in IFNα/βRKO and IFNγKO mice on day 7 were about 10- and 3-fold higher, respectively (as measured by phosphorimaging analysis) (data not shown), indicating that IFNγ can also inhibit LCMV replication.
IFN-dependent inhibition of HBV replication and of adenovirus entry and gene expression.
As shown in Fig. 4, similar results were obtained when the same groups of animals were infected intravenously with a dose (1.5 × 109 PFU/mouse) of a replication-deficient-, lacZ-expressing adenovirus (Ad.CblacZ) that rapidly infects all of the hepatocytes (as measured by β-galactosidase staining) (data not shown). One day after infection, high levels of 2′,5′-OAS were induced in the livers of all animals except the IFNα/βRKO mice, and this coincided with the inhibition of HBV replication (Fig. 4), demonstrating that IFNα/β mediates the early antiviral effect of adenovirus infection. In keeping with the modest elevation in sALT (Fig. 4, bottom), virtually no liver disease was observed histologically (data not shown). It is noteworthy that TNFα was not induced in these livers at this time point, in contrast to the LCMV infection system shown in Fig. 3. The lack of early TNFα induction may reflect the fact that Ad.CBlacZ infects exclusively hepatocytes (23), which are not a major source of TNFα (2), while LCMV clone WE 2.2 infects predominantly macrophages (7), which can produce high levels of TNFα upon activation (2). On day 7 after infection, T-cell and macrophage RNA was easily detectable in the liver of all lineages, and TNFα and IFNγ were induced along with IFNα/β. This was associated with a nearly complete inhibition of HBV replication in wild-type, IFNγKO, and TNFαRKO mice, while somewhat higher levels of HBV replication remained in the IFNα/βRKO mice, despite a relatively severe liver disease which was monitored histologically (data not shown) and biochemically (Fig. 4, bottom). As described previously for LCMV, these results indicate that IFNγ (and possibly TNFα) downregulates HBV replication by IFNα/β-independent pathways.
FIG. 4.
IFN-dependent inhibition of HBV replication and of adenovirus entry and gene expression. Age-, sex-, and serum HBeAg-matched transgenic mice from the groups described in the legend to Fig. 1 were infected with a dose (1.5 × 109 PFU/mouse) of a replication-deficient, lacZ-expressing adenovirus (Ad.CblacZ) that rapidly infects all of the hepatocytes. At 24 h (d1) or 7 days (d7) postinfection, the mice were sacrificed and total hepatic RNA and DNA were analyzed for HBV gene expression and replication and for mRNAs for 2′,5′-OAS, IFNγ, TNFα, and T-cell (CD8, CD4, and CD3) and macrophage (F480) markers exactly as described in the legend to Fig. 1. Northern and Southern blot membranes were also hybridized with a 32P-labeled lacZ-specific DNA probe (top). Animals indicated as wild type (wt) were heterozygous for the IFNα/β receptor null mutation. Results were compared with those for livers pooled from 10 age-, sex-, and serum HBeAg-matched transgenic saline-injected controls (NaCl) that were sacrificed 1 day after NaCl injection. The mean sALT activity, measured at the time of autopsy, is indicated (bottom) for each group and is expressed in units per liter. Int., integrated; RC and SS, relaxed-circular and single-stranded linear HBV DNA replicative forms.
The extents of adenovirus entry and gene expression were monitored by Southern blot analysis of Ad.CBlacZ DNA or Northern blot analysis of lacZ RNA (Fig. 4, top). Importantly, 1 day after infection, the hepatic contents of Ad.CBlacZ DNA and lacZ RNA were, respectively, about 7 and 10 times higher (as calculated by phosphorimaging analysis) (data not shown) in IFNα/βRKO mice than in wild-type controls (Fig. 4, top). This indicates that adenovirus entry, and possibly adenovirus-dependent gene expression, is also susceptible to the early antiviral activity of IFNα/β. The hepatic content of adenovirus DNA and RNA was also slightly higher (almost twofold) in IFNγKO and TNFαRKO mice than in wild-type controls (Fig. 4, top). On day 7 after infection, when the adenovirus-specific T-cell response peaks in the liver (3) (Fig. 4), the adenovirus DNA and RNA contents were, respectively, about 3- and 4-fold higher in IFNα/βRKO mice and about 1.2- and 2-fold higher in IFNγKO and TNFαRKO mice than in wild-type controls (Fig. 4, top), indicating that IFNα/β and, to a lesser extent, IFNγ and TNFα contribute to the disappearance of adenovirus DNA and RNA from the liver during the cellular antiviral immune response.
Time course of adenovirus infection in IFNα/βRKO mice.
To monitor the duration of the adenovirus effect in IFNα/βRKO mice, 12 age-, sex-, and serum HBeAg-matched transgenic mice that were either homozygous or heterozygous for the IFNα/β receptor null mutation were infected intravenously with 1.5 × 109 PFU of Ad.CBlacZ, and groups of 2 mice were sacrificed on days 1, 2, 3, 4, 5, and 7 after infection. The animals developed a liver disease that was detectable histologically (data not shown) and biochemically, as elevated sALT activity (Fig. 5, bottom), starting 3 to 5 days after infection.
FIG. 5.
Time course of adenovirus infection in IFNα/βRKO mice. Age-, sex-, and serum HBeAg-matched transgenic mice that were heterozygous (+/−) or homozygous (−/−) for the IFNα/β receptor null mutation were infected intravenously with Ad.CBlacZ (1.5 × 109 PFU/mouse) and sacrificed at the indicated time points (day 1 [d. 1] through day 5 [d. 5] plus day 7 [d. 7]). Results were compared with those for livers pooled from 10 uninjected age-, sex-, and serum HBeAg-matched transgenic controls (d.0). Total hepatic RNA and DNA were analyzed for HBV gene expression and replication and for mRNAs for 2′,5′-OAS, IFNγ, TNFα, and T-cell (CD8, CD4, and CD3) and macrophage (F480) markers exactly as described in the legend to Fig. 1. Northern and Southern blot membranes were also hybridized with a 32P-labeled lacZ-specific DNA probe (top). The mean sALT activity, measured at the time of autopsy, is indicated (bottom) for each group and is expressed in units per liter. RC and SS, relaxed-circular and single-stranded HBV DNA replicative forms.
As shown in Fig. 5 for one representative mouse per group, HBV replication was inhibited in heterozygous mice within 1 day of infection, coinciding with the induction of 2′,5′-OAS mRNA. This was followed by the partial reappearance of HBV replication on day 3 postinfection, coinciding with the disappearance of 2′,5′-OAS mRNA from the liver (Fig. 5). Finally, HBV replication was again suppressed between days 5 and 7 postinfection, coinciding with the reinduction of 2′,5′-OAS mRNA and the appearance of CD3, CD4, CD8, F480, IFNγ, and TNFα mRNAs (Fig. 5). In contrast, HBV replication in IFNα/βRKO mice was completely unaffected up to day 5 after adenovirus infection, and it was decreased on day 7 by about threefold (as calculated by phosphorimaging analysis) (data not shown). Interestingly, the induction of RNA for inflammatory cytokines and T-cell markers was delayed in the livers of homozygous IFNα/βRKO mice. As shown in Fig. 5, these messages were at most only slightly induced by day 5 in these mice compared with heterozygous controls, while similar levels of cytokine and T-cell marker RNAs were observed by day 7 in both groups of animals. These results suggest that IFNα/β may contribute to one or more steps of the cellular immune response to adenovirus (i.e., priming, clonal expansion, and/or homing) that ultimately results in the entry of activated T cells into the liver.
The extent of adenovirus entry and gene expression was monitored by Southern blot analysis of Ad.CBlacZ DNA or Northern blot analysis of lacZ RNA. Again, the hepatic contents of Ad.CBlacZ DNA and lacZ RNA were more abundant in IFNα/βRKO mice than in heterozygous controls, particularly on days 1 and 7 after infection (Fig. 5, top).
IFNα/β mediates the antiviral effect of poly(I · C).
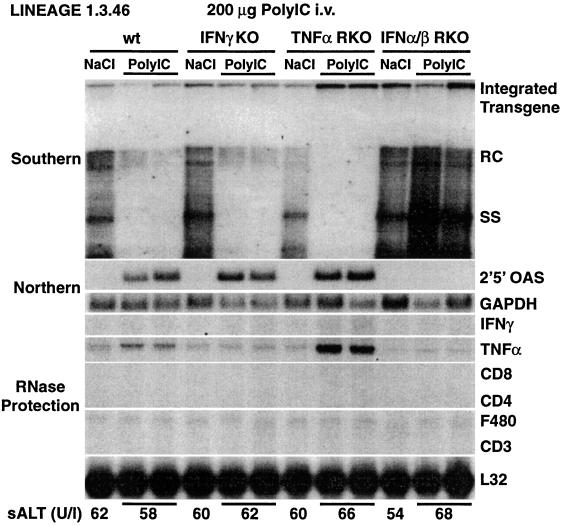
Next, we examined the antiviral effect of a single injection of the IFNα/β inducer poly(I · C) (14) in IFNγKO, TNFαRKO, and IFNα/βRKO mice. Groups of age-, sex-, and serum HBeAg-matched transgenic mice (four mice per group) were sacrificed 24 h after injection. No liver disease was observed histologically (data not shown) or biochemically (Fig. 6, bottom). As shown in Fig. 6 for two representative mice per group, a strong induction of 2′,5′-OAS occurred in all animals except those that lacked the IFNα/β receptor. As expected, the induction of 2′,5′-OAS coincided with a profound inhibition of HBV replication (Fig. 6), while virtually no change in the steady-state content of HBV RNA was observed (data not shown). In keeping with the absence of inflammation, no induction of IFNγ or of T-cell and macrophage marker RNAs was observed in the livers of poly(I · C)-injected mice (Fig. 6). These results demonstrate that IFNα/β mediates the antiviral effect of poly(I · C).
FIG. 6.
IFNα/β mediates the antiviral effect of poly(I · C). Age-, sex-, and serum HBeAg-matched transgenic mice from the same groups of mice described in the legend to Fig. 1 were sacrificed 24 h after receiving single injections of poly(I · C) (200 μg/mouse). Total hepatic RNA and DNA were analyzed for HBV gene expression and replication and for mRNAs for 2′,5′-OAS, IFNγ, TNFα, and T-cell (CD8, CD4, and CD3) and macrophage (F480) markers exactly as described in the legend to Fig. 1. Animals indicated as wild type (wt) were heterozygous for the IFNα/β receptor null mutation. Results were compared with those observed in livers pooled from 10 age-, sex-, and serum HBeAg-matched transgenic saline-injected controls (NaCl). The mean sALT activity, measured at the time of autopsy, is indicated (bottom) for each group and is expressed in units per liter. RC and SS, relaxed-circular and single-stranded HBV DNA replicative forms, respectively.
High doses of IFNα administered systemically inhibit HBV replication.
Based on the aforementioned results, it is clear that the local induction of IFNα/β is sufficient to inhibit HBV replication. It has been previously shown that repetitive injections of high doses of recombinant IFNα can effectively reduce viral titers in the sera of HBV-transgenic mice (15). In the present study, we compared the relative abilities of a single injection of IFNα versus a single injection of Poly-I/C or infection with LCMV or adenovirus to inhibit HBV replication in the livers of wild-type HBV-transgenic mice (lineage 1.3.46). Groups of age-, sex-, and serum HBeAg-matched transgenic mice (three mice per group) were injected intravenously, once per mouse, with 5 × 103, 1 × 104, 5 × 104, 1 × 105, 5 × 105, or 1 × 106 U of IFNα, and their livers were harvested 24 h after injection for Southern analysis of hepatic HBV DNA replicative forms. The results were compared with those of total hepatic DNA pooled from 10 matched HBV-transgenic control animals injected with saline. At all IFNα doses, sALT levels at the time of autopsy were identical to those detected before injection (50 to 70 U/liter). As shown in Fig. 7, a dose-dependent antiviral effect was observed, with a significant inhibition of HBV DNA replicative intermediates, at an IFNα dose of 5 × 105 U (or higher) (data not shown), while the content of HBV DNA replicative forms was unchanged at an IFNα dose of 105 U (or lower) (data not shown). As expected, the antiviral effect of IFNα was accompanied by a dose-dependent increase in the intrahepatic content of 2′,5′-OAS RNA, which also occurred in the animals treated with poly(I · C) or infected with LCMV or adenovirus, in which HBV replication was also inhibited (Fig. 7). These results indicate that systemic administration of only very high doses of IFNα inhibits HBV replication to the extent observed in the livers of transgenic mice in which IFNα/β is induced locally, following injection of poly(I · C) or infection with LCMV or adenovirus.
FIG. 7.
High doses of IFNα administered systemically inhibit HBV replication. Age-, sex-, and serum HBeAg-matched transgenic mice (lineage 1.3.46) were injected intravenously with various doses of IFNα, and their livers were harvested 24 h after injection for Southern analysis of HBV DNA replicative forms and Northern analysis of 2′,5′-OAS RNA exactly as described in the legend to Fig. 1. Results were compared with those for livers pooled from 10 age-, sex-, and serum HBeAg-matched transgenic saline-injected controls (NaCl). Results were also compared with those for livers harvested 24 h after injection with poly(I · C) (200 μg/mouse) or infection with LCMV (2 × 106 PFU/mouse) or adenovirus (1.5 × 109 PFU/mouse). RC and SS, relaxed-circular and single-stranded HBV DNA replicative forms, respectively.
DISCUSSION
We have previously shown that HBV-specific CTLs can abolish viral replication in the hepatocytes of HBV-transgenic mice by noncytopathic mechanisms that are mediated by IFNγ and TNFα (10). Cytokine-mediated antiviral events also occur in these animals during unrelated hepatotropic infections with LCMV or adenovirus, which inhibit HBV replication by inducing IFNα/β as well as IFNγ and TNFα (3, 7). Our goals in this study were to extend our previous observations by determining the relative contributions of the transferred CTLs and host-derived inflammatory cells to the antiviral activity of IFNγ and/or TNFα and to determine the relative contribution of each cytokine to the inhibitory effect on HBV replication observed after CTL injection and LCMV or adenovirus infection. Thus, we crossed the HBV-transgenic mice with mice genetically deficient for IFNγ, the TNFα receptor, or the IFNα/β receptor. In these animals, we also compared the relative sensitivity of LCMV and adenovirus to the antiviral activity of cytokines.
Several new observations have been made in this study. First, we have shown that in the livers of unmanipulated transgenic mice deficient for IFNγ or the receptor for IFNα/β, HBV replicates at levels that are about threefold higher than those of the respective controls. This effect occurs in the absence of liver disease and suggests that physiological concentrations of both interferons can partially control HBV replication.
Second, we have shown that the amount of IFNγ produced by passively transferred HBsAg-specific CTLs is sufficient to inhibit HBV replication in the livers of transgenic mice. This suggests that the host inflammatory cells recruited by the CTLs do not contribute to their antiviral activity; it also suggests that the CTL-derived IFNγ is sufficient to inhibit HBV replication. Furthermore, we have shown that TNFα and IFNα/β, respectively, may inhibit the recruitment of CD8+ and CD4+ T cells to the liver, and both cytokines may inhibit the recruitment of macrophages.
Third, we have shown that IFNα/β mediates the early inhibitory effect of LCMV and adenovirus infection on HBV replication, indicating that other cytokines, particularly TNFα, are dispensable for this process. Furthermore, reduced levels of DNA replicative forms were observed in all groups of animals (including IFNα/βRKO mice) 7 days after infection with LCMV or adenovirus, when the hepatic content of IFNγ reached maximal levels. These results indicate that IFNγ downregulates HBV replication by IFNα/β-independent pathways.
Fourth, we have shown that HBV replication in IFNα/βRKO mice is not inhibited by the adenovirus infection for at least 5 days. This observation is important because these animals will allow us to use adenovirus-based vectors to study the effect of novel gene products (including antiviral agents) on HBV replication in vivo as long as the expression of such genes will produce an experimental readout within 5 days of infection.
Fifth, we have shown that IFNα/β also mediates the inhibitory effect of poly(I · C) on HBV replication, suggesting that poly(I · C) may have therapeutic value as an antiviral agent for the treatment of chronic HBV infection. Indeed, poly(I · C) has been shown to inhibit viral replication in chimpanzees chronically infected with HBV (19), probably by the mechanism herein reported.
Sixth, we have shown that HBV replication in transgenic mice is inhibited by systemic administration of only very high doses of IFNα. Indeed, the minimal effective IFNα dose required to inhibit hepatic HBV replication in this model is between 1 × 105 and 5 × 105 U/mouse, while the standard IFNα regimen for chronically infected patients is about 3 × 106 U/day for 10 days. A dose of 3 × 106 U in humans corresponds to a dose of about 103 U in a 25-g mouse, an amount which would have no antiviral effect as a single dose in our model. It remains to be determined whether repetitive injections of low doses of IFNα would inhibit HBV replication in the transgenic mice. At any rate, since local induction of IFNα/β following a single nontoxic injection of poly(I · C) is very effective in our system, new therapeutic approaches aimed to induce antiviral cytokines at the site of the infection should be considered for the treatment of chronic HBV infection in man.
Last, we have shown that LCMV and adenovirus infect the livers of IFNα/βRKO mice and, to a lesser extent, IFNγKO mice more efficiently than they infect the livers respective control mice, indicating that interferons also play an important role in controlling these infections. Indeed, LCMV replication has been shown to be less efficiently controlled in mice that lack IFNγ and IFNα/β receptors (17) or that have been treated with IFN-specific antibodies (16). In the case of adenovirus, it has been reported that TNFα plays an important role in eliminating adenovirus vectors from the liver (6), most likely because TNFαRKO mice show a reduced infiltration of T cells into the liver 7 days after infection (6). We confirmed in our study that at 7 days after infection, the number of liver-infiltrating T cells (monitored by the detection of T-cell marker RNA in the liver) was smaller (and the adenovirus DNA and RNA contents were higher) in TNFαRKO mice than in wild-type controls (Fig. 4). To our knowledge, however, the role of IFNγ and IFNα/β in controlling infections of the liver by adenovirus vectors has not been investigated. Interestingly, we found that 7 days after infection, the adenovirus DNA and RNA contents were, respectively, about 3- and 4-fold higher in IFNα/βRKO mice and about 1.2- and 2-fold higher in IFNγKO mice than in wild-type controls (Fig. 4). This indicates that both IFNs independently contribute to the elimination of adenovirus vectors from the liver. This effect occurred without any reduction in the number of liver-infiltrating T cells at this time point (Fig. 4). On day 5, however, activated T cells were absent from the livers of IFNα/βRKO mice while they were present in the livers of control mice (Fig. 5), suggesting that IFNα/β regulates the entry of activated T cells into the liver. Even more importantly, the hepatic contents of adenovirus DNA and RNA 1 day after infection were, respectively, about 7 and 10 times more abundant in IFNα/βRKO mice and about twofold higher in IFNγKO mice than in wild-type controls (Fig. 4). These results suggest that adenovirus entry into the liver is also susceptible to the antiviral activity of IFNα/β and IFNγ. Along these lines, we have shown that 3 days following injection of the same dose of adenovirus vector, the hepatic content of adenovirus DNA is about 5 times higher in normal mice than in mice that are persistently infected with LCMV and constitutively produce high levels of IFNα/β in their livers (data not shown). Based on these results, coinjection of adenovirus vectors and antibodies to IFNα/β and/or IFNγ may increase the efficiency of adenovirus entry into the hepatocyte, thereby improving adenovirus-based strategies in gene therapy.
ACKNOWLEDGMENTS
We thank Timothy Stewart, Tak Mak, and Michel Aguet for providing IFNγKO, TNFαRKO, and IFNα/βRKO mice, respectively; Mary Graves for providing the recombinant human IFNα and Victoria Cavanaugh for the analysis of the effect of recombinant human IFNα in transgenic mice; James Wilson for providing the recombinant adenovirus Ad.CBlacZ; Persephone Borrow and Michael Oldstone for providing LCMV clone WE 2.2 (work supported by NIH grant AI09484); Monte Hobbs for providing the cytokine gene and T-cell marker probe sets used in the RNase protection assays; Jacquelyn Moorhead, Amy Brown, Christina Whitten, and Margie Chadwell for excellent technical assistance; and Jennifer Newmann for help with manuscript preparation.
This work was supported by grants AI40696 (L.G.G.) and CA40489 (F.V.C.) from the National Institutes of Health.
Footnotes
Manuscript no. 12745-MEM from the Scripps Research Institute.
REFERENCES
- 1.Ando K, Moriyama T, Guidotti L G, Wirth S, Schreiber R D, Schlicht H J, Huang S, Chisari F V. Mechanisms of class I restricted immunopathology. A transgenic mouse model of fulminant hepatitis. J Exp Med. 1993;178:1541–1554. doi: 10.1084/jem.178.5.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beutler B, Greenwald D, Hulmes J D, Chang M, Pan Y C, Mathison J, Ulevitch R, Cerami A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985;316:552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- 3.Cavanaugh V J, Guidotti L G, Chisari F V. Inhibition of hepatitis B virus replication during adenovirus and cytomegalovirus infections in transgenic mice. J Virol. 1998;72:2630–2637. doi: 10.1128/jvi.72.4.2630-2637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chisari F V, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 5.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 6.Elkon K B, Liu C C, Gall J G, Trevejo J, Marino M W, Abrahamsen K A, Song X, Zhou J L, Old L J, Crystal R G, Falck-Pedersen E. Tumor necrosis factor alpha plays a central role in immune-mediated clearance of adenoviral vectors. Proc Natl Acad Sci USA. 1997;94:9814–9819. doi: 10.1073/pnas.94.18.9814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guidotti L G, Borrow P, Hobbs M V, Matzke B, Gresser I, Oldstone M B A, Chisari F V. Viral cross talk: intracellular inactivation of the hepatitis B virus during an unrelated viral infection of the liver. Proc Natl Acad Sci USA. 1996;93:4589–4594. doi: 10.1073/pnas.93.10.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guidotti L G, Chisari F V. Cytokine-induced viral purging—role in viral pathogenesis. Curr Opin Microbiol. 1999;2:388–391. doi: 10.1016/s1369-5274(99)80068-x. [DOI] [PubMed] [Google Scholar]
- 9.Guidotti L G, Chisari F V. To kill or to cure: options in host defense against viral infection. Curr Opin Immunol. 1996;8:478–483. doi: 10.1016/s0952-7915(96)80034-3. [DOI] [PubMed] [Google Scholar]
- 10.Guidotti L G, Ishikawa T, Hobbs M V, Matzke B, Schreiber R, Chisari F V. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 11.Guidotti L G, Matzke B, Schaller H, Chisari F V. High-level hepatitis B virus replication in transgenic mice. J Virol. 1995;69:6158–6169. doi: 10.1128/jvi.69.10.6158-6169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guidotti L G, Rochford R, Chung L, Shapiro M, Purcell R, Chisari F V. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 13.Kozarsky K, Wilson J M. Gene therapy: adenovirus vectors. Curr Opin Genet Dev. 1993;3:499–503. doi: 10.1016/0959-437x(93)90126-a. [DOI] [PubMed] [Google Scholar]
- 14.Lampson G P, Tytell A A, Field A K, Nemes M M, Hilleman M R. Inducers of interferon and host resistance. I. Double-stranded RNA from extracts of Penicillium funiculosum. Proc Natl Acad Sci USA. 1967;58:782–789. doi: 10.1073/pnas.58.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrey J D, Sidwell R W. Transgenic mice as chemotherapeutic model for hepatitis B virus infection. Antivir Ther. 1998;3:165–174. [PubMed] [Google Scholar]
- 16.Moskophidis D, Battegay M, Bruendler M-A, Laine E, Gresser I, Zinkernagel R M. Resistance of lymphocytic choriomeningitis virus to alpha/beta interferon and to gamma interferon. J Virol. 1994;68:1951–1955. doi: 10.1128/jvi.68.3.1951-1955.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller U, Steinhoff U, Reis L F, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 18.Pfeffer K, Matsuyama T, Kündig T M, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi P S, Krönke M, Mak T W. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 19.Purcell R H, London W T, McAuliffe V J, Palmer A E, Kaplan P M, Gerin J L, Wagner J, Popper H, Lvovsky E, Wong D C, Levy H B. Modification of chronic hepatitis-B virus infection in chimpanzees by administration of an interferon inducer. Lancet. 1976;ii:757–761. doi: 10.1016/s0140-6736(76)90598-5. [DOI] [PubMed] [Google Scholar]
- 20.Samuel C E, Knutson G S. Mechanism of interferon action. Kinetics of induction of the antiviral state and protein phosphorylation in mouse fibroblasts treated with natural and cloned interferons. J Biol Chem. 1982;257:11791–11795. [PubMed] [Google Scholar]
- 21.Shimizu Y, Guidotti L G, Fowler P, Chisari F V. Dendritic cell immunization breaks cytotoxic T lymphocyte tolerance in hepatitis B virus transgenic mice. J Immunol. 1998;161:4520–4529. [PubMed] [Google Scholar]
- 22.Weck P K, Rinderknecht E, Estell D A, Stebbing N. Antiviral activity of bacteria-derived human alpha interferons against encephalomyocarditis virus infection of mice. Infect Immun. 1982;35:660–665. doi: 10.1128/iai.35.2.660-665.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y, Ertl H C J, Wilson J M. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]