Abstract
Herpes simplex virus (HSV) expresses a number of membrane glycoproteins, including gB, gD, and gH/gL, that function in both entry of virus particles and movement of virus from an infected cell to an uninfected cell (cell-to-cell spread). However, a complex of HSV glycoproteins gE and gI (gE/gI) is required for efficient cell-to-cell spread, especially between cells that form extensive cell junctions, yet it is not necessary for entry of extracellular virions. We previously showed that gE/gI has the capacity to localize specifically to cell junctions; the glycoprotein complex was found at lateral surfaces of cells in contact with other cells but not at those lateral surfaces not forming junctions or at apical surfaces. By virtue of these properties, gE/gI is an important molecular handle on the poorly understood process of cell-to-cell spread. Here, we show that the cytoplasmic domain of gE is important for the proper delivery of gE/gI to lateral surfaces of cells. Without this domain, gE/gI is found on the apical surface of epithelial cells, and more uniformly in the cytoplasm, although incorporation into the virion envelope is unaffected. However, even without proper trafficking signals, a substantial fraction of gE/gI retained the capacity to accumulate at cell junctions. Therefore, the extracellular domain of gE can mediate accumulation of gE/gI at cell junctions, if the glycoprotein can be delivered there, probably through interactions with ligands on the opposing cell. The role of phosphorylation of the cytoplasmic domain of gE was also studied. A second mutant HSV type 1 was constructed in which three serine residues that form a casein kinase II phosphorylation site were changed to alanine residues, reducing phosphorylation by 70 to 80%. This mutation did not affect accumulation at cell junctions or cell-to-cell spread.
The mechanisms by which animal viruses spread from an infected cell to a neighboring uninfected cell are poorly understood and often are distinct from pathways by which extracellular virus particles enter cells. The alphaherpesviruses are especially adept at moving from cell to cell in epithelial tissues and between neurons and other cells in the nervous system. Herpes simplex virus types 1 and 2 (HSV-1 and HSV-2, respectively), varicella-zoster virus (VZV), and pseudorabies virus (PrV) all express a heterodimeric complex, probably a dimer, composed of two glycoproteins, gE and gI, that can promote cell-to-cell spread (9, 22, 23, 25, 30). Without gE/gI, these alphaherpesviruses produce small plaques in cultured cells and spread poorly in various tissues in vivo (3, 8, 10, 11, 30, 31, 41). At least four other alphaherpesvirus glycoproteins, gB, gD, and gH/gL, are required for cell-to-cell spread, as well as for entry of extracellular or exogenous virus particles into cells (7, 17, 26, 36). However, in contrast to these glycoproteins, gE/gI plays no obvious role in entry of extracellular virus particles (3, 10). Thus, gE/gI apparently functions specifically in cell-to-cell spread, and we have exploited these observations in order to describe the molecular details of alphaherpesvirus cell-to-cell spread.
We previously observed that HSV-1 gE/gI can localize specifically to epithelial cell junctions, colocalizing with the adherens junction protein, β-catenin (12). gE/gI was not found on those lateral surfaces which were not in contact with another cell or on apical plasma membrane surfaces. This distribution of gE/gI was observed whether the glycoproteins were expressed after HSV infection or by using recombinant adenovirus vectors, i.e., in the absence of other HSV proteins. We concluded that gE/gI has the capacity to bind to cellular components of intercellular junctions and functions, either as a component of the virion envelope or of the plasma membrane, to facilitate movement of HSV across cell junctions. It is not clear at this point how this occurs. However, we have hypothesized that gE/gI can bind to cellular receptors that are components of cell junctions, and this interaction mediates movement of virions across cell junctions (12).
To begin to understand how gE/gI accumulates at cell junctions and mediates cell-to-cell spread, a series of mutant forms of gE are being constructed. One area of interest is the large cytoplasmic (CT) domains of gE and gI that contain tyrosine-based and dileucine motifs, as well as acidic domains surrounding phosphorylated residues (2, 34, 37, 38, 43). These domains cause endocytosis of gE/gI in transfected cells, as well as specific sorting directly to the trans-Golgi network (TGN). Presumably, phosphorylation can regulate these trafficking events (16, 24, 40). It was suggested that endocytosis of VZV gE/gI and concentration of the glycoprotein in the TGN or endosomes might facilitate envelopment of virions (18, 42, 43). However, the relative importance of endocytosis of these glycoproteins during virus infection of cells is not clear. Endocytosis of PrV gE/gI was observed in transfected cells, but there was little or no endocytosis of PrV gE/gI during virus infection of cells and endocytosed gE/gI did not become part of the virion envelope (38).
Our observations suggest that gE/gI functions at cell junctions and gE or gI mutants have a most profound phenotype in cells of epithelial or neuronal origin. Thus, it is important to study trafficking of gE/gI in polarized cells. The determinants for targeting of membrane proteins to basolateral domains of polarized cells are related or, in some cases, identical to the signals that mediate sorting into clathrin-coated vesicles in the TGN or entry into clathrin-coated pits leading to endocytosis at the cell surface. Basolateral targeting signals have been defined in low-density lipoprotein receptors, polymeric immunoglobulin G (IgG) receptors, and transferrin receptors and include motifs with critical tyrosine residues surrounded by acidic clusters or dileucine motifs, as well as signals that do not mediate endocytosis (reviewed in references 27 and 29). Thus, it was reasonable to hypothesize that the CT domains of gE and gI participate in sorting to the basolateral domain of the plasma membrane. Constituents of basolateral domains of polarized cells, such as E-cadherin and the Na+/K+ ATPase, can also be retained there by interactions with the cytoskeleton (19, 32) or by interactions with other cell adhesion molecules (CAMs) in the example of cadherins (reviewed in reference 1). We have previously shown that gE/gI is not bound tightly to the cytoskeleton and have proposed that retention or accumulation of gE/gI at cell junctions results from binding of the extracellular domain of gE/gI to some component of junctions (12). By this model, gE/gI acts like a CAM.
In this report we describe HSV-1 mutants in which gE lacks the entire CT tail (ΔCT) or where three serine residues (Ser) in the CT domain, sites of phosphorylation, were changed to alanine residues. Both mutant forms of gE were incorporated into the virion envelope. The Ser mutant mediated cell-to-cell spread as well as wild-type gE and localized normally to cell junctions. By contrast, the ΔCT gE mutant was unable to mediate cell-to-cell spread and localized to the apical, as well as basolateral, surface. However, even without the CT domain, gE ΔCT can accumulate at cell junctions and was less dense on free borders of cells, those lateral surfaces not in contact with other cells.
MATERIALS AND METHODS
Cells and viruses.
HEC-1A (human endometrial epithelial cells) (4) cells were grown in RPMI medium (Biowhitaker Inc., Walkersville, Md.) supplemented with 10% heat-inactivated fetal bovine serum (FBS; HyClone). Vero and R-970 cells were grown in Dulbecco's modified minimal essential medium (DMEM; Biowhitaker) supplemented with 4 to 5% FBS. ARPE-19 cells (American Type Culture Collection) were grown in DMEM/F12 media (50:50) supplemented with 10% FBS. HaCaT cells (a gift of N. E. Fusenig, Heidelberg, Germany) were grown in DMEM supplemented with 10% FBS. Camcell-1 cells (a gift of Margaret Stanley, University of Cambridge) were grown in DMEM supplemented with 10% FBS, 10 ng of epidermal growth factor (Clonetics, San Diego, Calif.) 0.5 μg per ml, of hydrocortisone (Clonetics) per ml, and 10−10 M cholera toxin (Gibco). HSV-1 strain F; F-US7kan, a virus unable to express gI; F-gEβ, a virus unable to express gE (10); and other HSV-1 gE mutants were propagated and titered on Vero cells.
Mutagenesis of the gE gene.
A 3.1-kbp fragment of HSV-1 DNA, encompassing all of the gE coding sequences, as well as the 3′ end of the gI gene and all of the US9 gene, was PCR amplified using a sample of DNA extracted from HSV-1 (strain F)-infected Vero cells. The following primer sequences were used: CCGGAATTCAGCATCGACCACGCCCTTC, which hybridizes 762 bp upstream of the gE ATG start codon, and CCCAAGCTTTAGCGGAGCAGCCACATC, which hybridizes 692 bp downstream of the gE TAA stop codon (nucleotides that deviate from the HSV sequence are in boldface throughout this section). The PCR product was digested with EcoRI and HindIII and ligated into pUC19 to produce a plasmid, pUC-US7/8, which was sequenced in both directions, and the sequence was compared with the published sequence for HSV-1 strain 17 (28). The F-gEΔCT and F-gESer mutant viruses were produced by PCR mutagenesis of pUC-US7/8 and rescue of the mutant sequences into HSV-1 strain F. A two-step PCR mutagenesis protocol was used. For construction of F-gEΔCT, two separate PCRs were performed involving (i) sense oligonucleotide, GCAGGCGGCCTCCGTCAATCTG (wild-type sequences corresponding to codons 340 to 346), and antisense oligonucleotide, CCCGTCATCAACGCCTGCGCCAA (mutant sequences corresponding to codons 445 to 452), and (ii) sense oligonucleotide, GGCGTTGATGACGGGCGGGTTAAA (mutant sequences corresponding to codons 448 to 455), and antisense oligonucleotide, CTGGCGGCGAGATTGATGCC (wild-type sequences corresponding to codons 523 to 529). For construction of F-gESer, two initial reactions were performed involving (i) sense oligonucleotide, GCAGGCGGCCTCCGTCAATCTG (wild-type sequences corresponding to codons 340 to 346), and antisense oligonucleotide, GGCGTCCGCGGCCCAGTCCG (mutant sequences corresponding to codons 474 to 481, and (ii) sense oligonucleotide, GGGCCGCGGACGCCGAGGGAGAAC (mutant sequences corresponding to codons 478 to 485), and antisense oligonucleotide, CTGGCGGCGAGATTGATGCC (wild-type sequences corresponding to 523 to 529). In each case, the two PCR products were diluted, mixed, and subjected to PCR amplification using wild-type oligonucleotides: GCAGGCGGCCTCCGTCAATCTG (wild-type sequences corresponding to codons 340 to 346) and CTGGCGGCGAGATTGATGCC (wild-type sequences corresponding to 523 to 529). This second PCR in each case produced a 570-bp PCR fragment corresponding to nucleotides 1017 to 1587 of the gE coding sequences, with the mutations indicated above. The PCR fragments were digested with MluI and BglII and inserted into pUC-US7/8 that had been digested with MluI and BglII. The PCR-generated sequences were entirely sequenced in both directions. pUC-US7/8 plasmids containing these two mutations or not having a mutation were linearized by digestion with XmnI and cotransfected with DNA extracted from F-gEβ-infected Vero cells by using the calcium phosphate precipitation technique (10). The transfection yield was titered on Vero cells, and then HSV able to express gE was screened by using black plaque staining using anti-gE monoclonal antibody (MAb) 3114 (20, 26).
The viruses expressing gE were plaque purified three times by centrifuging culture supernatants of infected cells at low speed and filtering these through 0.45-μm-pore-size filters before plaquing the virus isolates.
Radiolabeling of cells with [32P]orthophosphate and [35S]methionine/cysteine.
R-970 and HEC-1A cells were infected with HSV-1 (strain F) using 10 and 25 PFU/cell, respectively. Virus stocks were removed after 2 h, and the cells were incubated with DMEM containing 1% FBS. Five to 8 h after infection, the cells were washed three times with DMEM lacking methionine and cysteine or lacking phosphate and then incubated in this media for 15 to 60 min. Cells were radiolabeled with [35S]methionine-[35S]cysteine ([35S]methionine/cysteine) (25 μCi/ml) in media lacking cysteine and methionine or with [32P]orthophosphate (500 μCi/ml) in media lacking PO4 for 3 to 5 h. Alternatively, cells were pulse-labeled with 100 to 150 μCi of [35S]methionine/cysteine per ml for 20 to 30 min. The cells were washed in phosphate-buffered saline (PBS), and cell extracts were prepared using NP-40–deoxycholate (DOC) lysis buffer (100 mM NaCl, 50 mM Tris-HCl [pH 7.5], 1.0% NP-40, 0.5% DOC, 2 mg of bovine serum albumin [BSA] per ml, 1 mM phenylmethylsulfonyl fluoride) and frozen at −70°C. Cell extracts were clarified by high-speed centrifugation and mixed with antibodies specific for gB (17βB5), gC (a pool of MAbs specific for gC: C1, C3, C4), gD (DL6), or gE (MAb 3114 or II-481 [23]). In some instances the gE/gI complex was disrupted by addition of 50 mM NaCl and 0.1% sodium dodecyl sulfate (SDS) and heating cell extracts at 55°C for 5 to 10 min before addition of anti-gE MAb II-481. After 1 to 1.5 h, protein A agarose beads were added and incubated for 60 to 120 min before being washed three times with NP-40–DOC buffer. Precipitated proteins were eluted with 2× Laemmli sample buffer (4% SDS, 2% β-mercaptoethanol, 100 mM Tris-HCl [pH 6.8], 20% glycerol), boiled for 5 min, and loaded onto SDS–10% polyacrylamide gels.
Phosphoamino acid analysis.
HSV-1-infected R-970 cells were radiolabeled with [32P]orthophosphate and gE immunoprecipitated using MAb 3114. Proteins were subjected to electrophoresis using 10% polyacrylamide gels, gel dried, and exposed to X-ray film. The band corresponding to gE (the mature form of the glycoprotein) was excised and diced into small pieces, and the protein was eluted for 24 h at 37°C with constant shaking into a small volume of 0.05 M ammonium bicarbonate containing 0.1% SDS. The eluted proteins were precipitated using 10% trichloroacetic acid in the presence of 200 μg of BSA. The precipitate was washed twice with acetone and then subjected to hydrolysis in 5 M HCl at 110°C for 2 h. The hydrolysate was lyophilized and dissolved in a small volume of water and combined with nonradioactive phosphoserine, phosphotyrosine, and phosphothreonine (all from Sigma) as markers. Phosphoamino acids were separated by using cellulose thin-layer plates (20 by 20 cm, Polygam CEL300, 0.1 mm thick; Brinkmann) as previously described (6, 21). The positions of unlabeled phosphoamino acids were identified by ninhydrin staining, and those of the radiolabeled amino acids were identified by autoradiography.
Plaque assays of cell-to-cell spread.
Confluent 35-mm dishes of HEC-1A or other epithelial cells were infected for 2 h in the appropriate media supplemented with 1% FBS. After 2 h, the media were removed and fresh media containing 1% FBS and 0.3% human gamma globulin (a source of anti-HSV neutralizing antibodies; Baxter Healthcare, Glendale, Calif.) were added. After 48 h, cells were washed twice in PBS and fixed in PBS containing 4% paraformaldehyde for 20 min, and then they were washed once for 10 min and twice for 5 min with PBS containing 0.1% Tween 20. The fixed cells were incubated with a rabbit polyclonal anti-HSV-1 serum (Dako, Copenhagen, Denmark) for 2 h, washed three times each for 10 min with PBS-Tween 20, and then incubated for 1 h with donkey anti-rabbit IgG antibodies conjugated with horseradish peroxidase (Amersham, Arlington, Ill.). The cells were washed three times, each for 10 min with PBS-Tween 20, and plaques were visualized by the addition of the peroxidase substrate, 3,3′-diaminobenzidine tetrahydrochloride (Sigma). The area of each plaque was determined by capturing images using a Polaroid digital camera attached to an Olympus BX50 microscope and quantifying the size of 20 plaques per sample using National Institutes of Health Image software.
Confocal immunofluorescence microscopy.
HEC-1A cells were grown on glass coverslips for 2 days and were subconfluent monolayers; then the cells were infected using HSV-1 (25 PFU/cell). After 12 h, the cells were washed using PBS, fixed with 4% paraformaldehyde in PBS for 10 min, and washed three times with PBS. The cells were permeabilized using 0.2% Triton X-100 in PBS for 5 min and then incubated with blocking buffer (PBS containing 2% normal goat serum, 2% BSA, and 0.02% Tween 20) for 30 min. Samples were incubated for 2 h with MAb specific for gE (3114) or gD (DL6) and simultaneously with a rabbit anti-β-catenin serum (Sigma); then they were washed three times with PBS containing 0.02% Tween 20 before incubation with fluorescence-conjugated secondary antibodies: Cy-5-conjugated goat anti-rabbit IgG and fluorescein isothiocyanate-coupled goat anti-mouse IgG (Jackson ImmunoResearch, West Grove, Pa.). In the case of Cy-5-conjugated secondary antibodies, the blue signal was converted to a red signal. The cells were washed and mounted on microscope slides using Prolong (Molecular Probes, Eugene, Oreg.) and visualized using a Bio-Rad 1024 ES laser scanning confocal microscope on a Nikon eclipse TE300 inverted fluorescence microscope.
Incorporation of gE/gI into the virion.
Confluent 150-mm dishes of HEC-1A and R970 cells were infected using 25 and 10 PFU/cell, respectively. After 2 h, the virus was removed and media containing 1% FBS were added. After 22 to 24 h, the cell culture supernatants were removed and centrifuged at 3,750 rpm for 15 min in a Beckman GS6 rotor to remove cellular debris. Supernatants were overlaid onto 1.5 ml of 30% sucrose–1 mM sodium phosphate, pH 7.6, and centrifuged at 75,000 × g for 1 h in a Beckman SW41 rotor. Supernatant and sucrose were aspirated, and the pellet was resuspended in 50 μl of 1 mM phosphate buffer, pH 7.6, and then mixed with 50 μl of 2% NP-40–1% DOC–100 mM Tris-HCl [pH 7.5]–200 mM NaCl, incubated for 5 min on ice, and then centrifuged at 55,000 × g for 15 min. The supernatant was transferred to a fresh Eppendorf tube, and 25 μl of 4× Laemmli loading buffer (containing 8% SDS) was added. Samples were boiled for 5 min and subjected to electrophoresis on 10% polyacrylamide gels, and proteins were transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore, Bedford, Mass.). Membranes were blocked in 2% nonfat dry milk–1% BSA for 30 min and incubated for 1 h with anti-gE MAb 3114 or with anti-gD MAb DL6. The blots were washed three times with PBS-Tween 20 and then incubated for 1 h with horseradish peroxidase-conjugated goat anti-mouse IgG (Amersham). The blots were washed, and proteins were visualized by using an enhanced chemiluminescence kit (Amersham) and a Lumi Imager (Boehringer Mannheim) or by exposure to X-ray film.
RESULTS
The CT domain of gE and its phosphorylation.
We began to focus on the CT domain of HSV gE based on studies of the VZV and PrV gE homologues where the CT domain is critical for TGN sorting and endocytosis. Our studies focused on whether the CT domain affected basolateral sorting of gE/gI and accumulation at cell junctions and whether this domain was important for cell-to-cell spread. The CT domain of wild-type gE (strain F) is depicted in Fig. 1. There are 105 residues in the CT domain, beginning with three arginine residues (residues 447 to 449) proximal to the transmembrane domain (28). There are also two YXXØ motifs (underlined) that probably affect endocytosis by analogy with VZV gE and PrV gE (35, 37) and potentially determine basolateral targeting. Just downstream of these tyrosine motifs are three serine residues: 478, 479, and 481 that are flanked on the C-terminal side by a cluster of acidic residues (italics). These serine residues are potential sites of phosphorylation by casein kinase II (CKII), based on homology with other CKII sites and by analogy with VZV gE (33).
FIG. 1.
Amino acid sequences of the CT domains of wild-type HSV-1, F-gESer, and F-gEΔCT. A portion of the 105-residue CT domain of gE is depicted, including two YXXØ motifs (underlined) and three serine residues at positions 478, 479, and 481 (boldface) that are flanked on the C-terminal side by a cluster of acidic residues (italic). In F-gESer, the three serine residues were changed to alanine residues. F-gEΔCT contains two stop codons (stars) C terminal to three arginine residues that flank the transmembrane domain (TMD; outlined letters).
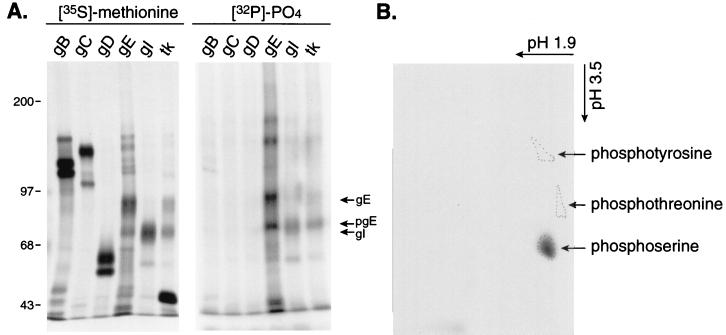
Edson et al. (15) previously analyzed the phosphate label in HSV-1 gE and found it to be alkaline labile, suggesting modification primarily on serine or threonine residues or both. Subsequently, Edson found that the homologous HSV-2 gE contained phosphoserine and no phosphothreonine (14). We further characterized the phosphorylation of HSV-1 gE and other viral proteins. Infected cells were radiolabeled with [32P]orthophosphate or, as a control, [35S]methionine, and several HSV proteins were immunoprecipitated with mouse MAbs or with polyclonal rabbit sera. Three of the major HSV glycoproteins, gB, gC, and gD, were not extensively phosphorylated, although there was a small amount of phosphate in gB (Fig. 2A). By contrast, gE and gI were substantially labeled with [32P]orthophosphate (Fig. 2A); in fact, these proteins were among the most highly phosphorylated proteins in HSV-infected cells (data not shown). The anti-thymidine kinase rabbit sera immunoprecipitated HSV thymidine kinase (≈43 kDa) which was not phosphorylated, and gE/gI as well, because gE/gI can bind to rabbit IgG (22, 23). To determine which amino acids were phosphorylated in gE phosphoamino acid, analyses were performed. These studies indicated that HSV-1 gE contains phosphoserine and little or no phosphothreonine or phosphotyrosine (Fig. 2B). Therefore, the three serine residues at positions 478, 479, and 481 appeared likely candidates as major sites of phosphorylation and, given their proximity to the YXXØ motifs and acidic cluster, might regulate targeting of gE to various membrane domains.
FIG. 2.
Phosphorylation of HSV-1 gE. (A) HSV-1-infected cells were labeled with [35S]methionine/cysteine or with [32P]orthophosphate, and then glycoproteins gB, gC, gD, gE, and gI or thymidine kinase was immunoprecipitated. Proteins were subjected to electrophoresis and exposed to X-ray film. The positions of gE, pgE (the immature form of gE), and gI are indicated, as well as molecular mass markers. (B) The band corresponding to gE was excised, eluted from the gel, hydrolyzed with 5 N HCl, and combined with nonradioactive phosphoserine, phosphotyrosine, and phosphothreonine as markers. Phosphoamino acids were separated using cellulose thin-layer plates. The positions of unlabeled phosphoamino acids were identified by ninhydrin staining, and those of the radiolabeled amino acids were identified by autoradiography.
We constructed mutant forms of gE in order to characterize the CT domain of gE. These mutations were built back into the genome of HSV-1 strain F. The published sequence of HSV-1 gE is that of strain 17 (28). Therefore, we sequenced the entire gE coding sequences, as well as the C-terminal half of gI and all of the US9 gene, in a 3.1-kb PCR fragment produced by using HSV-1 (strain F) DNA. The 3.1-kb fragment extended from the gI (US7) gene, approximately 762 bp upstream of the gE ATG, to the US9 gene 692 bp downstream of the gE stop codon and was inserted into pUC19, creating pUC-US7/8, which was then entirely and carefully sequenced in both directions. No differences were found in the predicted amino acid sequences of the gE CT domains of strains 17 (28) and F. However, gE from strain F contained two additional amino acids, E and G, inserted after residue 183 of the extracellular domain, and there were nucleotide changes that altered four residues in the extracellular domain: H130→Y (also Y in HSV-2 strain HG-52 gE), T241→A, S259→T, and V334→F. There were no differences in the US9 gene of strain 17 and that of strain F. Since the sequenced fragment was generated by PCR, several clones were compared and all were found to be identical in these regions. Moreover, the pUC-US7/8 plasmid was used to repair the gE mutant, F-gEβ, and the rescued virus could produce plaques similar to wild-type HSV-1 (see below).
Construction of gE ΔCT and Ser mutants and characterization of glycoproteins.
In order to delete the CT domain of gE, two adjacent stop codons were placed downstream of three arginine residues that are adjacent to the transmembrane domain (Fig. 1). In the Ser mutant, three serine residues (described above; residues 478, 479, and 481), which form potential sites of CKII, were all converted to alanine residues. Plasmids containing these two mutant forms of gE were constructed by PCR using plasmid pUC-US7/8 as a template. A 570-bp subfragment of the 3.1-kb fragment, bounded by MluI (5′) and BglII (3′) restriction sites, was generated by PCR, using degenerate oligonucleotides in the case of the mutant viruses, and this MluI/BglII subfragment was reinserted into the wild-type gE sequences in pUC-US7/8 by using restriction enzymes. In each case, the entire MluI/BglII fragment generated by PCR was sequenced in both directions to preclude the possibility of second site mutations. Vero cells were cotransfected with viral DNA derived from F-gEβ, which contains lacZ sequences in place of gE coding sequences (11) and one of three plasmids encoding wild-type gE (pUC-US7/8), ΔCT gE (pUC-US7/8 ΔCT), or Ser gE (pUC-US7/8Ser). Viruses that expressed gE were selected by staining viral plaques using anti-gE MAb 3114 in black plaque assays (11, 20). Thus, viruses expressing wild-type gE (F-gEβ-US7/8) or mutant forms of gE, F-gESer, and F-gEΔCT, were produced.
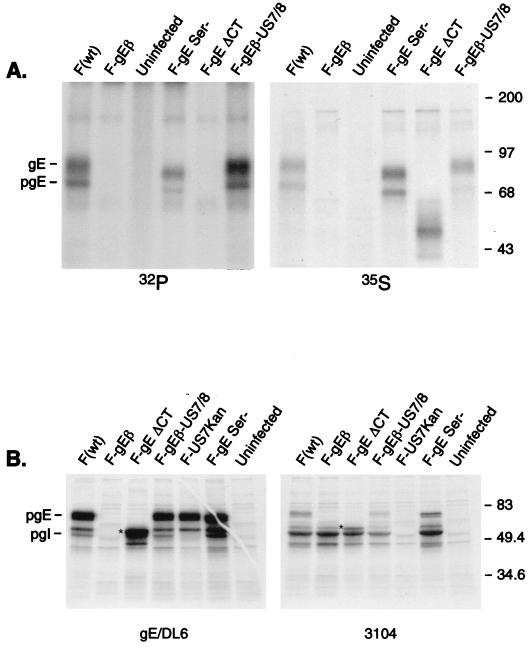
To characterize the gE expressed by mutant viruses, cells were infected with wild-type HSV-1 strain F, F-gEβ, F-gESer, or F-gEΔCT and then radiolabeled with [35S]methionine/cysteine or [32P]orthophosphate. Cell extracts were denatured to disrupt the gE/gI complex so that gI did not obscure the appearance of gE on SDS-polyacrylamide gels, and gE was immunoprecipitated using MAb II-481. As expected, the ΔCT mutant expressed a glycoprotein that was significantly smaller than wild-type gE and the Ser mutant (Fig. 3A). There was no phosphorylation of ΔCT gE, consistent with the notion that all of the phosphate is added to the CT domain. Analyses of [32P]PO4 incorporated into Ser gE using a phosphorimager and controlling the amount of gE using the [35S]methionine/cysteine signal indicated that the level of phosphorylation was approximately 20 to 30% of that observed with wild-type gE. Therefore, mutation of this CKII site reduces phosphorylation of gE by 70 to 80%; however, there are additional sites of serine phosphorylation in the CT tail of HSV-1 gE.
FIG. 3.
Expression of mutant forms of gE, binding to gI, and phosphorylation. (A) R-970 cells were infected with wild-type HSV-1 (strain F), F-gEβ, F-gESer, F-gEΔCT, or F-gEβ-US7/8 for 7 h. The cells were then radiolabeled for 3 h with [32P]orthophosphate or [35S]methionine/cysteine. Detergent extracts of the cells were made and gE was immunoprecipitated after a mild denaturation step (so that the gE/gI complex was disrupted) using anti-gE MAb II-481. (B) HEC-1A cells were infected for 7 h with the same viruses described for panel A, as well as F-US7Kan, a gI mutant. The cells were labeled for 30 min with [35S]methionine/cysteine and gE immunoprecipitated using anti-gE/DL6, an anti-gE rabbit serum, or gI immunoprecipitated using MAb 3104. The positions of gE and pgE, as well as molecular mass markers, are indicated. The ΔCT gE protein is indicated by a star, and gI migrates just slightly slower.
To determine whether the gE/gI complex formed with these mutants, infected cells radiolabeled with [35S]methionine/cysteine in a pulse format (in order to label the immature forms of gE and gI). Figure 3B shows that the gI-specific MAb 3104 immunoprecipitated gI, as well as gE from extracts of wild-type HSV-1-infected cells. In extracts from cells infected with F-gESer, gE was readily observed with the gI-specific MAb. In addition, a smaller form of gE was detected in extracts from F-gEΔCT-infected cells immunoprecipitated with the gI-specific MAb 3104; there was a band corresponding to the protein that migrated just slightly slower than gI (Fig. 3B). Similarly, we detected a band corresponding to gI when gE was immunoprecipitated using a polyclonal rabbit anti-gE serum. Moreover, rabbit IgG could precipitate a gE/gI complex from cells infected with F-gEΔCT (data not shown). Therefore, the ΔCT gE glycoprotein can bind to gI and to IgG.
Cell-to-cell spread of HSV-1 gE mutants.
Previously, we characterized cell-to-cell spread of HSV-1 gE and gI null mutants by measuring plaque sizes using normal human fibroblasts, HEC-1A cells, and neurons (10, 11), as well as a panel of transformed human and monkey cell lines (K. Dingwell, unpublished observations). To prevent virus movement through the extracellular compartment, HSV-neutralizing antibodies were incorporated into the cell culture media. The phenotype of gE and gI mutants was most profound on polarized epithelial cells or other cells that contain extensive cell junctions and was much less obvious with highly transformed cells such as HeLa or R-970 cells. To determine whether gE mutants behaved similarly on other epithelial or keratinocyte cell lines and in order to identify the most appropriate cells to study HSV cell-to-cell spread, we characterized a number of other cells: HaCaT cells, a keratinocyte cell line (5); Camcell-1 cells, a spontaneously transformed human cervical epithelial cell line (3); and ARPE-19 cells, retinal epithelial cells (13). Wild-type HSV-1 produced relatively larger plaques than the gE-negative mutant, F-gEβ, on all of these cell lines (Fig. 4A). Plaques formed on HEC-1A cells were significantly smaller, for both wild-type and mutant viruses (note that the bar is 100 μm), than the plaques formed on ARPE-19 and HaCaT cells (Fig. 4A) and on Camcell-1 cells (data not shown). On HEC-1A cells, the difference in the diameter of the plaques produced by the wild-type versus F-gEβ was approximately 2.5-fold, whereas this difference in plaque size was approximately 8-fold for HaCaT cells and 4.5- to 5-fold for Camcell-1 and ARPE-9 cells (Fig. 4B). The ΔCT mutant produced small plaques closely resembling those of F-gEβ on all these cells. By contrast, the Ser mutant produced large plaques similar to wild-type HSV-1. F-gEβ-US7/8, derived from F-gEβ and pUC-US7/8 (the plasmid containing PCR-amplified wild-type gE sequences), produced large plaques similar to those produced by wild-type strain F (Fig. 4B). Therefore, in all four of these cells, both the gE-negative and ΔCT mutants were deficient in cell-to-cell spread, whereas the Ser mutant functioned normally.
FIG. 4.
Plaques produced by wild-type HSV-1 and gE mutants. HEC-1A, ARPE-19, HaCaT, or Camcell-1 cells were infected with wild-type HSV-1 (strain F), F-gEβ, F-gEΔCT, F-gESer, or F-gE-US7/8 using 0.001 to 0.0001 PFU/cell. Two hours later the virus inoculum was removed and cells were overlaid with media containing 0.3% human gamma globulin, a source of anti-HSV-neutralizing antibodies. After 2 days the cells were fixed and stained for HSV antigens. (A) Photomicrograph of plaques formed on HEC-1A, ARPE-19, and HaCaT cells. (B) Twenty plaques produced by each virus were photographed and subjected to image analysis to quantify the area of the plaque.
Accumulation of gE/gI at cell junctions.
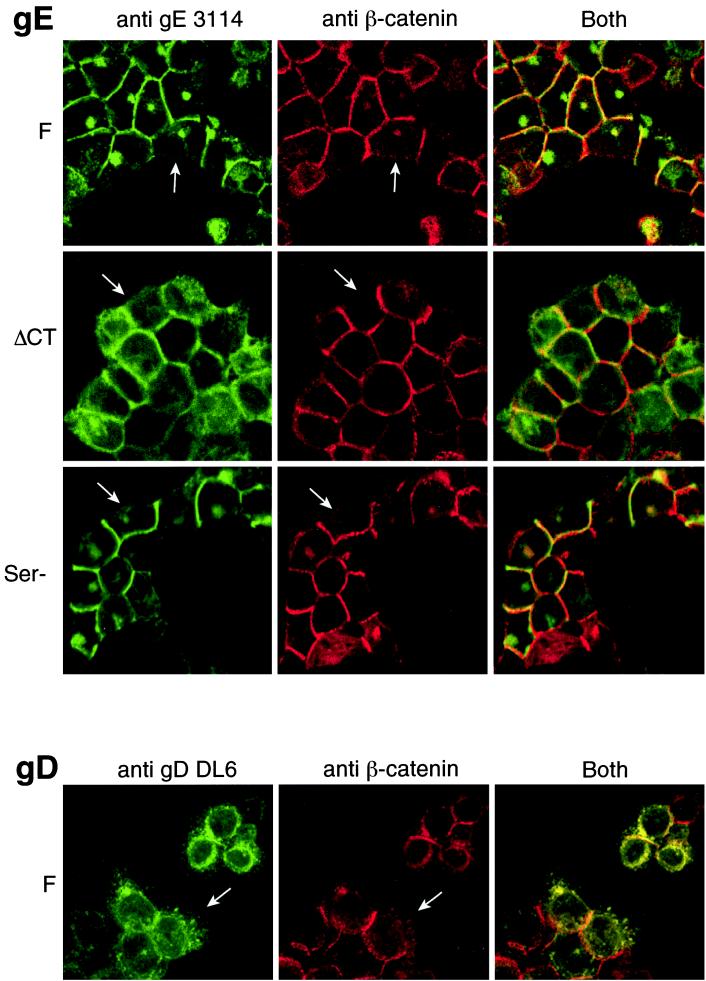
Previously, we found that gE/gI, when expressed by HSV-1 or by using recombinant adenovirus vectors, accumulated specifically at cell junctions (12). Therefore, we investigated whether the ΔCT and Ser mutants could traffic normally to basolateral domains and accumulate at cell junctions. HEC-1A epithelial cells were infected with wild-type strain F, F-gEΔCT, or F-gESer, and then the cells were simultaneously stained for gE and β-catenin. When monolayers of cells were infected with wild-type HSV-1 and viewed in the x-y plane (from above), gE was found predominately along the lateral surfaces of cells colocalizing with β-catenin, although there was a minor fraction of the protein in the cytoplasm, in a perinuclear location (Fig. 5, top panels). As a specific measure of the accumulation of gE at cell junctions, we observed very little gE at free borders, those lateral surfaces of cells not in contact with other cells (see white arrows in Fig. 5). A similar colocalization of gE and β-catenin was observed with the Ser mutant, and again there was little gE at free borders. The ΔCT gE was found more extensively in the cytoplasm, on the apical surfaces of cells, but was also clearly able to accumulate at cell junctions (colocalized with β-catenin). As with wild-type gE, there were much higher concentrations of the ΔCT gE at cell junctions when compared to those lateral surfaces not forming junctions or free borders (see white arrows in Fig. 5). Moreover, when compared with gD, which is found extensively in the cytoplasm, at the apical surfaces of cells and universally throughout the cell, the concentration of ΔCT gE at cell junctions was obvious.
FIG. 5.
Subcellular localization of gE and gD in epithelial cells. Subconfluent monolayers of HEC-1A cells were infected with HSV-1 wild type (strain F), F-gEΔCT, or F-gESer for 12 h and then were washed and fixed with paraformaldehyde. The cells were permeabilized using 0.2% Triton X-100, incubated with blocking buffer, and then incubated simultaneously with anti-gE MAb 3114 and a rabbit anti-β-catenin serum (upper three sets of panels) or with anti-gD MAb DL6 and anti-β-catenin serum (lower panels). The cells were washed, incubated with Cy-5-conjugated goat anti-rabbit IgG and fluorescein isothiocyanate-coupled goat anti-mouse IgG, washed again, and mounted on microscope slides. White arrows indicate lateral surfaces of the epithelial cells that are not in contact with other cells.
The distribution of these mutant gE molecules was further characterized by collecting a Z series of images (Fig. 6). In these images, the lateral surfaces of cells appear vertically and the apical surfaces appear horizontally along the top of the image. Wild-type gE was found predominately at cell junctions, those lateral surfaces of cells in contact with other cells, and was not on apical surfaces. A similar pattern was seen with the Ser gE mutant. However, the ΔCT gE mutant was present on apical surfaces of the cells, as well as accumulated at cell junctions. The distribution of ΔCT gE was distinctly different from that of gD, which was uniformly on the apical and lateral surfaces and much more extensively in the cytoplasm (Fig. 6, lower panel). Therefore, ΔCT gE retains the ability to accumulate at cell junctions, although the protein is mislocalized to the apical surface and other membranes to a much larger extent than wild-type gE.
FIG. 6.
z-axis analysis of gE and gD in epithelial cells. HEC-1A cells were infected and stained for gE or gD as described in the legend for Fig. 5. A Z series set of images was collected using a confocal microscope. The basal surface of the cell is at the bottom, lateral surfaces (cell junctions) appear vertically, and the apical surface extends horizontally along the top of the image.
Incorporation of mutant forms of gE into virus particles.
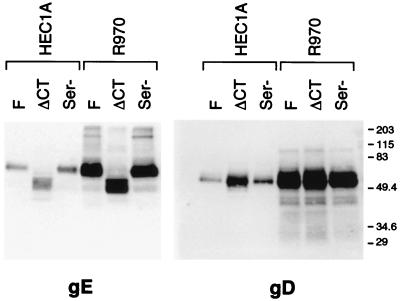
In order to promote cell-to-cell spread, it is possible that gE/gI must be incorporated into the virion envelope. To test whether the mutant gE proteins were incorporated into virus particles, HEC-1A or nonpolarized human R-970 cells were infected with wild-type strain F, F-gEΔCT, or F-gESer. Cell culture supernatants were harvested, viruses were pelleted through a sucrose cushion, and viral proteins were subjected to Western blotting. Based on the levels of both gD and gE present in purified virions, HEC-1A cells produced fewer virions, at least into the apical compartment, than R-970 cells (Fig. 7). This is consistent with our recent observations that the majority of virus particles produced by wild-type HSV-1 in HEC-1A are directed to cell junctions, and not on the apical surface (D. C. Johnson, unpublished results). By contrast, virus particles produced by nonpolarized R-970 cells were primarily found on the apical surface. However, the mutant forms of gE produced by F-gEΔCT and F-gESer were found in virions (Fig. 7). Analysis of blots by using a phosphorimager indicated that the ratio of gD:gE from wild-type HSV-1 and F-gESer virus particles was 1.4, whether the particles were produced by HEC-1A or R-970 cells. By contrast, F-gEΔCT particles produced in HEC-1A cells exhibited a gD:gE ratio of 2.7, yet F-gEΔCT particles from R-970 cells exhibited a gD:gE ratio of 1.4. Since the amounts of gE were normalized to gD (we assume gD would not be affected by this mutation) and the antibodies used in comparing the two virions were the same, we concluded that the amount of gE present in the F-gEΔCT virions is about half that in wild-type HSV-1 virions. Therefore, the mislocalization of ΔCT gE apparently causes reduced incorporation of this glycoprotein into virions, at least in virions collected from the apical compartment of these epithelial cells.
FIG. 7.
Incorporation of gE and gD into virions. HEC-1A epithelial cells or R-970 cells were infected with wild-type HSV-1 (strain F), F-gEΔCT, or F-gESer, and then cell culture supernatants from the cells were collected at 22 h, before the cells had rounded. Virus particles were collected from cell culture supernatants and centrifuged through a cushion of 30% sucrose, and detergent extracts of the virions were subjected to electrophoresis in polyacrylamide gels. Blots were incubated with anti-gE MAb 3114 or anti-gD MAb DL6, washed, incubated with horseradish peroxidase-conjugated goat anti-mouse IgG, and washed again, and proteins were visualized using enhanced chemiluminescence. The positions of molecular mass markers are shown along the right side of the gel.
DISCUSSION
We previously proposed that gE/gI acts to mediate cell-to-cell spread by binding to receptors concentrated at cell junctions (12). This hypothesis was based on two observations: (i) gE/gI acts in cell-to-cell spread and not in entry of extracellular virions (10) and (ii) gE/gI accumulates specifically at epithelial cell junctions and is absent from those lateral surfaces not in contact with another cell (12). The effects of gE/gI in promoting cell-to-cell spread in cultured cells have been observed not only with polarized epithelial cells but also with other cells, such as keratinocytes, fibroblasts, and neurons—cells that have extensive cell junctions but which may not be polarized in the classical sense (3, 10, 11, 44). By contrast, gE and gI mutants do not have an obvious phenotype, or have less of one, in highly transformed cells, such as HeLa, HEp-2, Vero, or R-970 cells-cells that form much less extensive junctions. Thus, there is a correlation between the requirement for gE/gI to promote cell-to-cell spread and the level of cell adhesion, e.g., adheren junctions but not necessarily cell polarization (tight junctions).
The studies described herein are consistent with the notion that the extracellular domain of gE, in conjunction with gI, is sufficient to mediate accumulation at cell junctions. A mutant form of gE lacking the CT tail and expressed in HSV-infected epithelial cells was mislocalized and found at apical surfaces, as well as in the cytoplasm, and at all lateral surfaces. In spite of this, a substantial fraction of the ΔCT gE was found at cell junctions, accumulating there in much higher concentrations than at free borders of cells where no junctions were formed. This may be related to the presence of the CT domain of gI, although gI cannot, on its own, accumulate at cell junctions (12; Dingwell, unpublished). Therefore, it appears that the extracellular domain of gE, in conjunction with gI, can mediate binding to ligands that are concentrated at cell junctions and, even if gE is transported to junction inefficiently, gE/gI accumulates there. However, this accumulation at cell junctions was not sufficient for cell-to-cell spread of HSV; F-gE ΔCT behaved like F-gEβ, a mutant lacking gE. It is possible that the concentration of gE present at junctions or in the virion envelope was insufficient to facilitate movement of virions across cell junctions. More likely, the CT domain of gE functions in some as yet uncharacterized process to promote movement of HSV across cell junctions.
The CT domains of gE and gI contain several interesting and, at least in VZV and PrV, well-studied motifs, e.g., YXXØ and dileucine, that can facilitate trafficking to the TGN or endocytosis of the glycoproteins (2, 34, 37, 38, 43). In most cases, these motifs have been uncovered or studied by transfecting cells with gE alone or with gI alone. Since a large fraction or perhaps a majority of gE and gI are found in the gE/gI complex, at least in HSV-infected cells (23), there may be some difficulties in interpreting the results of such studies. Signals in one glycoprotein may affect the complex as a whole or interact with signals in the partner glycoprotein. Moreover, studies of the trafficking or endocytosis of these proteins in the absence of other viral proteins, i.e., that rely exclusively on transfection, can also produce results different than that seen in virus-infected cells. For example, with PrV it is now clear that endocytosis does not occur during the majority of the virus infectious cycle, at least in PK15 cells (38).
Many of the signals in the CT domain of gE that have the potential to mediate endocytosis or TGN targeting, dileucine and tyrosine motifs, can also function as basolateral sorting signals when proteins are expressed in polarized epithelial cells (reviewed in references 27 and 29). Since gE/gI probably functions largely in polarized cells or in cells that produce extensive cell junctions, it is important to study the effects of these signals in these relevant cells. Our results show that, indeed, the CT domain of gE is important in directing gE/gI to the basolateral domain. Without the CT domain, gE was found more extensively on apical surfaces and in CT vesicles, though there was still a substantial fraction at basolateral surfaces. The ΔCT gE interacted with gI, and thus, the CT domain of gI was present in the gE/gI complex, and this domain contains a dileucine motif that can potentially serve, in a redundant fashion, as a basolateral sorting signal. When basolateral targeting sequences are totally eliminated from other cellular membrane proteins, these proteins are exclusively delivered to the apical surface, rather than becoming randomly distributed (29). Therefore, to test the effects of the CT domain of gI, it will be necessary to construct a mutant lacking the CT domains of both gI and gE.
Phosphorylation of both HSV-1 gE and gI is extensive, as these glycoproteins are among the most highly phosphorylated proteins in HSV-infected cells. Our studies and those of Edson (14) indicate that phosphorylation of gE is entirely on serine residues. This is in contrast to VZV gE, which has phosphoserine, phosphothreonine, and phosphotyrosine (14, 33). Phosphorylation of membrane proteins can regulate internalization and trafficking events in cells (16, 24, 40). The putative CKII site of gE is present within a cluster of acidic residues that, by analogy with VZV, could mediate targeting to the TGN (43) and perhaps also basolateral targeting. Mutation of the putative CKII phosphorylation site in HSV-1 gE, by alteration of all three serine residues, reduced phosphorylation by 70 to 80%. Therefore, there is probably phosphorylation of other serine residues in the CT domain of gE. One potential site of phosphorylation is the sequence 505SGFE, which can be recognized as having some limited homology to authentic CKII sites. However, there are also a total of 18 serines in the CT domain of gE, and it is not clear which of these may be phosphorylated in infected cells or whether this secondary phosphorylation involves CKII. Our F-gESer mutant behaved like wild-type HSV-1, with normal cell-to-cell spread and accumulation of gE/gI at cell junctions. Therefore, it is possible that the residual phosphorylation in gE (the 25% that remained) may be sufficient to promote normal trafficking of gE/gI. Related to this point, it is important to note that gE is largely bound to gI, which is also extensively phosphorylated. Our results are also consistent with the notion that phosphorylation of gE is not important in determining subcellular localization or in allowing gE/gI to function in cell-to-cell spread.
Whether gE/gI is directed exclusively to basolateral domains, in the case of wild-type HSV-1, or moves to both apical and basolateral surfaces, in the case of F-gEΔCT, there was obvious accumulation of gE/gI at cell junctions. This supports the hypothesis that the extracellular domain of gE is sufficient to mediate binding to cellular components of cell junctions, as with CAMs. At present, it is not clear whether substantial quantities of gE/gI found at cell junctions are integrated into the plasma membrane or whether most of this glycoprotein is part of the virion envelope. A perplexing but important question is whether gE/gI facilitates movement of virus across cell junctions as part of the virion envelope or as part of the plasma membrane. In order to address this question, we require mutants in which gE/gI is not incorporated into the virion but appears normal otherwise. Previously, Tirabassi et al. reported on a mutant, PRV25, which was proposed to lack the entire CT domain of PrV gE, and the mutant glycoprotein was not incorporated into the virion envelope (39). PRV25 was defective in cell-to-cell spread, and this supported the hypothesis that incorporation into the virus was important for cell-to-cell spread. Based on this, we were surprised to find that our HSV-1 ΔCT gE was incorporated into virions. However, subsequent studies with PRV25 demonstrated that gE contained an additional mutation upstream of the termination codon, creating a large, out-of-frame CT tail (37). A second mutant, PRV 107, which lacks the CT domain of PrV gE and which was incorporated into the virion, did not mediate cell-to-cell spread (37). Therefore, it remains to be determined whether gE/gI functions to mediate cell-to-cell spread as part of the virion envelope or as part of the plasma membrane. Other mutant forms of gE which do not become incorporated into the virion will be necessary to answer this question.
In summary, we propose that the CT domain of gE, probably acting in concert with the CT domain of gI, can promote sorting of gE/gI to the basolateral domain of polarized epithelial cells. However, the extracellular domain of gE/gI, even without the sorting signals associated with the gE CT domain, can promote accumulation of gE/gI specifically at cell junctions, perhaps by binding a component of cell junctions. This putative ligand of gE/gI must be cellular in nature, because localization to cell junctions occurs whether the opposing cell is infected or not.
ACKNOWLEDGMENTS
We are grateful to Aurelie Snyder for excellent technical assistance with confocal microscopy. We are grateful to Mary Huber and Tom McMillan for helpful discussions and for reviewing the manuscript. We thank Margaret Stanley of the University of Cambridge for Camcell-1 cells.
Support for this research was provided by a grant from the National Institutes of Health (CA73996).
REFERENCES
- 1.Adams C L, Nelson J W. Cytomechanics of cadherin-mediated cell-cell adhesion. Curr Opin Cell Biol. 1998;10:572–577. doi: 10.1016/s0955-0674(98)80031-8. [DOI] [PubMed] [Google Scholar]
- 2.Alconada A, Bauer U, Hoflack B. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 1996;15:6096–6110. [PMC free article] [PubMed] [Google Scholar]
- 3.Balan P, Davis-Poynter N, Bell S, Atkinson H, Browne H, Minson T. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoproteins gG, gE, gI or the putative gJ. J Gen Virol. 1994;75:1245–1258. doi: 10.1099/0022-1317-75-6-1245. [DOI] [PubMed] [Google Scholar]
- 4.Ball J M, Moldoveaunu Z, Melsen L R, Kozlowski P A, Jackson S, Mulligan M J, Mestecky J F, Compans R W. A polarized human endometrial cell line that binds and transports polymeric IgA. In Vitro Cell Dev Biol Anim. 1995;31:196–206. doi: 10.1007/BF02639434. [DOI] [PubMed] [Google Scholar]
- 5.Boukamp P, Petrussevska R T, Breitkreutz D, Hornung J, Markham A, Fusenig N E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Branton P E, Lassam N J, Graham F L, Mak S, Bayley S T. T-antigen-related protein kinase activity in cells infected and transformed with human adenoviruses 5 and 12. Cold Spring Harbor Symp Quant Biol. 1979;44:487–491. [Google Scholar]
- 7.Cai W Z, Person S, Warner S C, Zhou J H, DeLuca N A. Linker-insertion nonsense and restriction-site deletion mutations of the gB glycoprotein gene of herpes simplex virus type 1. J Virol. 1987;61:714–721. doi: 10.1128/jvi.61.3.714-721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Card J P, Whealy M E, Robbins A K, Enquist L W. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J Virol. 1992;66:3032–3041. doi: 10.1128/jvi.66.5.3032-3041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen J I, Nguyen H. Varicella-zoster virus glycoprotein I is essential for growth of virus in Vero cells. J Virol. 1997;71:6913–6920. doi: 10.1128/jvi.71.9.6913-6920.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dingwell K S, Brunetti C R, Hendricks R L, Tang Q, Tang M, Rainbow A J, Johnson D C. Herpes simplex virus glycoproteins E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J Virol. 1994;68:834–845. doi: 10.1128/jvi.68.2.834-845.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dingwell K S, Doering L C, Johnson D C. Glycoproteins E and I facilitate neuron-to-neuron spread of herpes simplex virus. J Virol. 1995;69:7087–7098. doi: 10.1128/jvi.69.11.7087-7098.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dingwell K S, Johnson D C. Herpes simplex virus gE/gI facilitates cell-to-cell spread and binds to components of cell junctions. J Virol. 1998;72:8933–8942. doi: 10.1128/jvi.72.11.8933-8942.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn K C, Aotaki K A, Putkey F R, Hjelmeland L M. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 14.Edson C M. Phosphorylation of neurotropic alphaherpesvirus envelope glycoproteins: herpes simplex virus type 2 gE2 and pseudorabies virus gI. Virology. 1993;195:268–270. doi: 10.1006/viro.1993.1372. [DOI] [PubMed] [Google Scholar]
- 15.Edson C M, Hosler B A, Waters D J. Varicella-zoster virus gpI and herpes simplex virus gE: phosphorylation and Fc binding. Virology. 1987;161:599–602. doi: 10.1016/0042-6822(87)90157-7. [DOI] [PubMed] [Google Scholar]
- 16.Fish K, Soderberg-Naucler C, Nelson J. Steady-state plasma membrane expression of human cytomegalovirus gB is determined by the phosphorylation state of ser900. J Virol. 1998;72:6657–6664. doi: 10.1128/jvi.72.8.6657-6664.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forrester A, Farrell H, Wilkinson G, Kaye J, Davis-Poynter N, Minson T. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J Virol. 1992;66:341–348. doi: 10.1128/jvi.66.1.341-348.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gershon A A, Sherman D L, Zhu Z, Gabel C A, Ambron R T, Gershon M D. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J Virol. 1994;68:6372–6390. doi: 10.1128/jvi.68.10.6372-6390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammerton R W, Krzeminski K A, Mays R W, Ryan T A, Wollner D A, Nelson W J. Mechanism for regulating cell surface distribution of Na+, K+-ATPase in polarized epithelial cells. Science. 1991;254:847–850. doi: 10.1126/science.1658934. [DOI] [PubMed] [Google Scholar]
- 20.Holland T C, Honxa F L, Marlin S D, Levine M, Glorioso J. Herpes simplex virus type 1 glycoprotein C-negative mutants exhibit multiple phenotypes, including secretion of truncated glycoproteins. J Virol. 1984;52:566–574. doi: 10.1128/jvi.52.2.566-574.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunter T, Sefton B M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci USA. 1980;77:1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson D C, Feenstra V. Identification of a novel herpes simplex virus type 1-induced glycoprotein which complexes with gE and binds immunoglobulin. J Virol. 1987;61:2208–2216. doi: 10.1128/jvi.61.7.2208-2216.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson D C, Frame M C, Ligas M W, Cross A M, Stow N D. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J Virol. 1988;62:1347–1354. doi: 10.1128/jvi.62.4.1347-1354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones B G, Thomas L, Molloy S S, Thulin C D, Fry M D, Walsh K A, Thomas G. Intracellular trafficking of furin is modulated by the phosphorylation state of a casein kinase II site in its cytoplasmic tail. EMBO J. 1995;14:5869–5883. doi: 10.1002/j.1460-2075.1995.tb00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimura H, Straus S E, Williams R K. Varicella-zoster virus glycoproteins E and I expressed in insect cells form a heterodimer that requires the N-terminal domain of glycoprotein I. Virology. 1997;233:382–391. doi: 10.1006/viro.1997.8625. [DOI] [PubMed] [Google Scholar]
- 26.Ligas M W, Johnson D C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988;62:1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matter K, Mellman I. Mechanisms of cell polarity: sorting and transport in epithelial cells. Curr Opin Cell Biol. 1994;6:545–554. doi: 10.1016/0955-0674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 28.McGeoch D J, Dolan A, Donald S, Rixon F J. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J Mol Biol. 1985;181:1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- 29.Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 30.Mettenleiter T C, Schreurs C, Zuckermann F, Ben-Porat T. Role of pseudorabies virus glycoprotein gI in virus release from infected cells. J Virol. 1987;61:2764–2769. doi: 10.1128/jvi.61.9.2764-2769.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mettenleiter T C, Zsak L, Kaplan A S, Ben-Porat T, Lomniczi B. Role of a structural glycoprotein of pseudorabies in virus virulence. J Virol. 1987;61:4030–4032. doi: 10.1128/jvi.61.12.4030-4032.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson W J, Shore E M, Wang A Z, Hammerton R W. Identification of a membrane-cytoskeletal complex containing the cell adhesion molecule uvomorulin (E-cadherin), ankyrin, and fodrin in Madin-Darby canine kidney epithelial cells. J Cell Biol. 1990;110:349–357. doi: 10.1083/jcb.110.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olson J K, Bishop G A, Grose C. Varicella-zoster virus Fc receptor gE glycoprotein: serine/threonine and tyrosine phosphorylation of monomeric and dimeric forms. J Virol. 1997;71:110–119. doi: 10.1128/jvi.71.1.110-119.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olson J K, Grose C. Complex formation facilitates endocytosis of the varicella-zoster virus gE:gI Fc receptor. J Virol. 1998;72:1542–1551. doi: 10.1128/jvi.72.2.1542-1551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olson J K, Grose C. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J Virol. 1997;71:4042–4054. doi: 10.1128/jvi.71.5.4042-4054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roop C, Hutchinson L, Johnson D C. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J Virol. 1993;67:2285–2297. doi: 10.1128/jvi.67.4.2285-2297.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tirabassi R S, Enquist L W. Mutation of the YXXL endocytosis motif in the cytoplasmic tail of pseudorabies virus gE. J Virol. 1999;73:2717–2728. doi: 10.1128/jvi.73.4.2717-2728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tirabassi R S, Enquist L W. Role of envelope glycoprotein gE endocytosis in the pseudorabies virus life cycle. J Virol. 1998;72:4571–4579. doi: 10.1128/jvi.72.6.4571-4579.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tirabassi R S, Townley R A, Eldridge M G, Enquist L W. Characterization of pseudorabies virus mutants expressing carboxy-terminal truncations of gE: evidence for envelope incorporation, virulence, and neurotropism domains. J Virol. 1997;71:6455–6464. doi: 10.1128/jvi.71.9.6455-6464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voorhees P, Diegnan E, van Donselaar E, Humphrey J, Marks M S, Peters P J, Bonifacino J S. An acidic sequence within the cytoplasmic tail domain of furin functions as a determinant of trans-Golgi network localization and internalization from the cell surface. EMBO J. 1995;14:4961–4975. doi: 10.1002/j.1460-2075.1995.tb00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whealy M E, Card J P, Robbins A K, Dubin J R, Rziha H J, Enquist L W. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. J Virol. 1993;67:3786–3797. doi: 10.1128/jvi.67.7.3786-3797.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Z, Gershon M D, Ambron R, Gabel C, Gershon A A. Infection of cells by varicella zoster virus: inhibition of viral entry by mannose 6-phosphate and heparin. Proc Natl Acad Sci USA. 1995;92:3546–3550. doi: 10.1073/pnas.92.8.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu Z, Hao Y, Gershon M D, Ambron R T, Gershon A A. Targeting of glycoprotein I (gE) of varicella-zoster virus to the trans-Golgi network by an AYRV sequence and an acidic amino acid-rich patch in the cytosolic domain of the molecule. J Virol. 1996;70:6563–6575. doi: 10.1128/jvi.70.10.6563-6575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zsak L, Zuckermann F, Sugg N, Ben-Porat T. Glycoprotein gI of pseudorabies virus promotes cell fusion and virus spread via direct cell-to-cell transmission. J Virol. 1992;66:2316–2325. doi: 10.1128/jvi.66.4.2316-2325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]