Abstract
Simple Summary
Breast cancer is one of the most diagnosed malignancies among women worldwide, affecting 18,000 women annually in the Netherlands. As life expectancy increases, monitoring quality of life (QoL) using Patient-Reported Outcome Measures (PROMs) becomes more important. The EQ-5D-5L and the EORTC QLQ-C30 are two widely used PROMs in the Netherlands. For utilizing PROMs in research and clinical care, internal responsiveness is key. Given the broad application of these PROMs in the Netherlands and the fact that internal responsiveness varies based on the disease, treatment regime, and geographical factors, this study assesses and compares the internal responsiveness of the EQ-5D-5L and the EORTC QLQ-C30 among Dutch breast cancer patients during the first year post-surgery. The results demonstrated that the EQ-5D-5L and the EORTC QLQ-C30 have small internal responsiveness (<0.5) at 6- and 12 months post-surgery. These findings provide crucial insights for interpreting outcomes from both PROMs in research and clinical practice.
Abstract
The EuroQoL 5-Dimension 5-Level questionnaire (EQ-5D-5L) and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core-30 (EORTC QLQ-C30) are commonly used Patient-Reported Outcome Measures (PROMs) for breast cancer. This study assesses and compares the internal responsiveness of the EQ-5D-5L and EORTC QLQ-C30 in Dutch breast cancer patients during the first year post-surgery. Women diagnosed with breast cancer who completed the EQ-5D-5L and EORTC QLQ-C30 pre-operatively (T0), 6 months (T6), and 12 months post-surgery (T12) were included. Mean differences of the EQ-5D-5L and EORTC QLQ-C30 between baseline and 6 months (delta 1) and between baseline and 12 months post-surgery (delta 2) were calculated and compared against the respective minimal clinically important differences (MCIDs) of 0.08 and 5. Internal responsiveness was assessed using effect sizes (ES) and standardized response means (SRM) for both deltas. In total, 333 breast cancer patients were included. Delta 1 and delta 2 for the EQ-5D-5L index and most scales of the EORTC QLQ-C30 were below the MCID. The internal responsiveness for both PROMs was small (ES and SRM < 0.5), with greater internal responsiveness for delta 1 compared to delta 2. The EQ-5D-5L index showed greater internal responsiveness than the EORTC QLQ-C30 Global Quality of Life scale and summary score. These findings are valuable for the interpretation of both PROMs in Dutch breast cancer research and clinical care.
Keywords: Breast Cancer, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core-30 (EORTC QLQ-C30), EuroQoL 5-Dimension 5-Level questionnaire (EQ-5D-5L), Patient-Reported Outcome Measures (PROMs), Responsiveness
1. Introduction
Breast cancer is one of the most commonly diagnosed malignancies among women worldwide [1,2]. Every year, about 18,000 women are diagnosed with breast cancer in the Netherlands and the incidence of invasive breast cancer is still increasing [3,4,5]. However, advancements in breast cancer treatments and early detection through nationwide screening programs have led to better prognoses for patients. While this improved prognosis is encouraging, breast cancer survivors may experience late-effect symptoms after diagnosis and completion of treatment, which can negatively affect their health status and health-related quality of life (HRQoL). Therefore, monitoring the health status and HRQoL of breast cancer patients throughout their disease trajectory and thereafter is important [5,6,7,8,9,10].
Patient-reported outcome measures (PROMs) are validated questionnaires that are used to measure HRQoL, health, and functional status of patients. They can be either generic or more disease-specific in nature [11,12]. Using PROMs in research and clinical care enables the monitoring of quality of care at a hospital level and provides clinicians with valuable information to better address the needs of each individual patient [13]. The EuroQoL 5-Dimension 5-Level questionnaire (EQ-5D-5L) and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core-30 (EORTC QLQ-C30) are commonly used PROMs in research and routine care for breast cancer patients in the Netherlands [14,15,16].
The EQ-5D-5L is a validated and standardized generic preference-based questionnaire that measures a patient’s health status using five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. This brief questionnaire is useful for measuring health status in a wide range of populations and can be immediately utilized for real-world cost-effectiveness analysis between different patient groups [12,14,17,18]. In contrast to the EQ-5D-5L, the EORTC QLQ-C30 is a cancer-specific measure of HRQoL. It consists of five functional scales, nine cancer symptom scales, and a global health/HRQoL scale [15]. The EORTC QLQ-C30 is more detailed compared to the EQ-5D-5L and focuses on specific domains that are relevant from the cancer patient’s perspective. Therefore, clinicians receive more detailed information about the HRQoL of their cancer patients that may not be covered by more generic questionnaires [12,15]. However, before EORTC QLQ-C30 scores can be used for cost-effectiveness analysis, a transformation of data is necessary [19,20,21,22].
For utilizing PROMs in research and clinical care, internal responsiveness is key. Internal responsiveness refers to the ability of a questionnaire to detect changes in a patient’s health status/HRQoL over time (including the changes due to received treatments). Internal responsiveness is crucial for informing clinical decisions and, to a lesser extent, enhancing quality management [23,24,25]. While the internal responsiveness of the EQ-5D-5L and the EORTC QLQ-C30 has been explored in various populations and countries, there remains a significant gap in studies focusing on Dutch breast cancer patients [26,27,28,29,30,31,32,33,34,35,36]. Given that both PROMs are widely used in the Netherlands and the fact that internal responsiveness can vary based on the type of disease, treatment regime, and geographical factors, this study specifically assesses and compares their internal responsiveness among Dutch breast cancer patients during the first year post-surgery [16,23,34]. The findings of this study will offer valuable insights, potentially informing adjustments in research approaches and patient care for breast cancer management in the Netherlands.
2. Materials and Methods
2.1. Data-Collection
All women diagnosed with invasive breast cancer who underwent surgical treatment with a curative intention at the Erasmus MC Cancer Institute in the Netherlands between 1 November 2015, and 1 January 2022 and who completed EQ-5D-5L and EORTC QLQ-C30 questionnaires pre-operatively (T0, baseline), 6 months post-surgery (T6), and 1-year post-surgery (T12) in the “patient data platform”, Erasmus MC’s online PROM collection tool, were included [16]. Patients who underwent proton therapy or palliative treatment, patients with recurrent breast cancer, and patients without treatment data were excluded. The Institutional Review Board was consulted and concluded that informed consent was not needed since the value-based healthcare strategy is considered the standard of care in Erasmus MC [16].
2.2. Surgical Treatment
Surgical interventions of the breast included breast-conserving surgery (BCS) or a mastectomy with or without a reconstruction. Additional chemotherapy, hormonal therapy, and/or (loco)regional radiotherapy were administered to patients following the Dutch national breast cancer guidelines [37,38].
2.3. Health Status/HRQoL Assessment
Health status/HRQoL was evaluated with the Dutch EQ-5D-5L (5-level version) and the EORTC QLQ-C30 (version 3.0) at T0, T6, and T12.
The EQ-5D-5L consists of two items: a descriptive system and the EQ visual analog scale (EQ VAS). Each dimension of the descriptive system (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) has five answer levels, ranging from no problems to extreme problems. The answers to the EQ-5D-5L questions can be transformed into a utility index using the Dutch value set. The EQ-5D-5L index values typically range from 0 to 1, with 0 representing death and 1 representing perfect health. Scores below 0 can occur, indicating that the person feels worse than being dead. The EQ VAS is used to rate the patient’s overall health on a scale of 0–100, with 0 being the best imaginable health and 100 being the worst imaginable health. The empirical reliability and validity of the EQ-5D-5L are documented in previously published literature [14,17,39].
The EORTC QLQ-C30 consists of five functional scales (physical, role, cognitive, emotional, and social), eight cancer symptom scales (fatigue, pain, nausea and vomiting, dyspnea, insomnia, appetite loss, constipation, and diarrhea), a global health/HRQoL scale, and a financial difficulties scale [15]. After linear transformation, all scales range in score from 0–100. For functional/global HRQoL scales, higher scores indicate better functioning; for symptom scales, higher scores indicate greater symptom severity. The empirical reliability and validity of the EORTC QLQ-C30 have previously been established [15,40,41]. The EORTC QLQ-C30 summary score can be calculated from the mean of 13 scales from the EORTC QLQ-C30, excluding the Global Quality of Life scale and the Financial Impact scale. Before determining the mean, symptom scales are reversed to ensure a consistent direction for all scales. Therefore, a higher summary score indicates higher HRQoL [42,43].
2.4. Statistical Analysis
Descriptive statistics were used to describe patient, tumor, and treatment characteristics. Means, medians, and standard deviations were used to describe the EQ-5D-5L and EORTC QLQ-C30 scores on T0, T6, and T12. The normality of all scores was assessed using the Kolmogorov–Smirnov and Shapiro–Wilk tests. If normally distributed, the parametric paired t-test was used to compare the scores between T0 and T6 and between T0 and T12. If not normally distributed, the non-parametric Wilcoxon signed-rank test was used.
Mean and median differences of the EQ-5D-5L and EORTC QLQ-C30 scores between T0 and T6 (delta 1) and between T0 and T12 (delta 2) were calculated to display within-group change. For the EQ-5D-5L scores and functional scales of the EORTC QLQ-C30, negative deltas indicate a decline in health status/HRQoL over time, while positive deltas indicate an increase in health status/HRQoL. For the symptom scales of the EORTC QLQ-C30, negative deltas indicate an improvement in HRQoL over time, while positive deltas indicate a decline in HRQoL. Mean deltas of the EQ-5D-5L index and EORTC QLQ-C30 Global Quality of Life scale were compared to the minimal clinically important difference (MCID) of the EQ-5D-5L index (0.08) and the EORTC QLQ-C30 (5) to determine clinical significance [44,45]. The MCID of the EQ-5D-5L index was based on the study of Pickard et al. as there are no MCID values available for cancer patients using the Dutch value set of the EQ-5D-5L [44].
To evaluate and compare the internal responsiveness of the two PROMs, effect sizes (ES) and standardized response means (SRM) were calculated. The ES was defined as the mean delta (e.g., the change in score from T0 to T6/T12), divided by the standard deviation of the T0 score. The SRM was calculated as the mean delta, divided by the standard deviation of the delta [23,46]. Both the ES and SRM were classified as large (≥0.8), moderate (0.5–0.79), or small (<0.5) based on previously established criteria [47,48,49]. The ES and SRM are standardized indicators of change in health status/HRQoL over time, regardless of the sample size [23,50].
To gain more insights into the internal responsiveness of the EQ-5D-5L and EORTC QLQ-C30 in different patient groups, a subgroup analysis was performed on patients receiving chemotherapy (neoadjuvant, adjuvant, or both) [37]. The internal responsiveness of the EQ-5D-5L and EORTC QLQ-C30 might be greater for patients receiving chemotherapy since chemotherapy has a significant effect on HRQoL [51].
All statistical analyses were performed using R statistical software version 4.2.2, and a two-sided p-value of 5% was considered significant [52].
3. Results
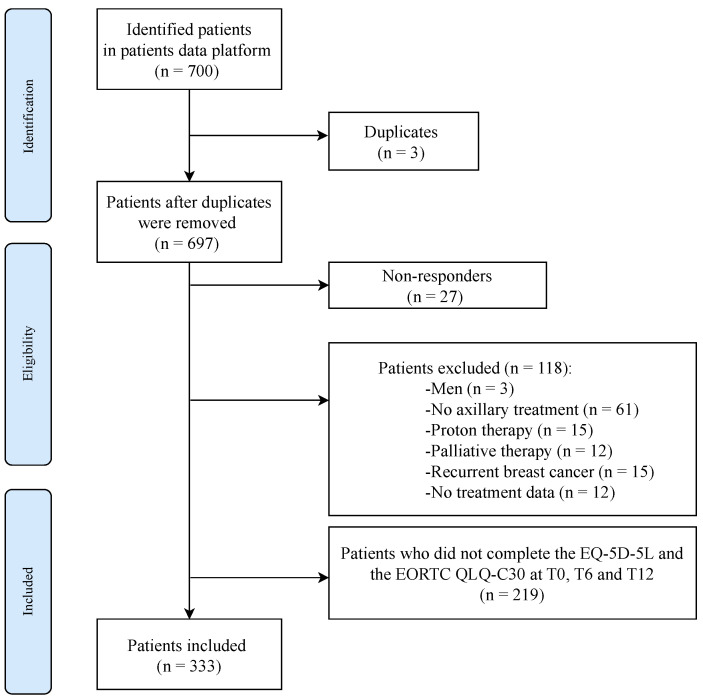
After removing 3 duplicates and 27 non-responders, 118 patients were further excluded based on the predefined exclusion criteria. Additionally, 219 patients were not included in the analysis due to incomplete responses on the EQ-5D-5L and EORTC QLQ-C30 questionnaires at baseline (T0), 6 months (T6), and 12 months (T12) post-surgery. A total of 333 patients were eligible for the statistical analysis (Figure 1).
Figure 1.
Flowchart. T0, baseline; T6, 6 months post-surgery; T12, 12 months post-surgery.
3.1. Patient Characteristics
The median age of the breast cancer patients was 54 years, ranging from 26.1 to 86.2 years, and 48.3% of the patients had a body mass index (BMI) below 25. Most of the patients were diagnosed with pT1 and pN0 breast cancer and had hormone receptor (HR) positive tumors (67.7%). Breast-conserving surgery (BCS) was the most frequently performed surgical procedure (56.2%). Hormonal therapy was received by 55.3% of the patients, and 41.1% were treated with chemotherapy. The most used axillary treatment was sentinel lymph node biopsy (SLNB) without additional axillary radiotherapy (Table 1).
Table 1.
Baseline characteristics of the study population.
| Characteristics | Patients (n = 333) |
|---|---|
| Age | |
| Mean (SD) | 54.0 (13.9) |
| Median [Min, Max] | 53.9 [26.1, 86.2] |
| BMI category | |
| <25 | 161 (48.3%) |
| 25–30 | 122 (36.6%) |
| >30 | 50 (15.0%) |
| Type of breast surgery | |
| Mastectomy | 91 (27.3%) |
| BCS | 187 (56.2%) |
| Mastectomy + reconstruction | 55 (16.5%) |
| Receptor status | |
| Triple negative | 47 (14.1%) |
| HER2 positive | 38 (11.4%) |
| HR positive and HER2 negative | 225 (67.6%) |
| Unknown | 23 (6.9%) |
| Primary tumor stage | |
| pT0 | 29 (8.7%) |
| pT1 | 187 (56.2%) |
| pT2 | 69 (20.7%) |
| pT3 | 9 (2.7%) |
| pT4 | 0 (0%) |
| pTis | 33 (9.9%) |
| pTmi | 6 (1.8%) |
| Regional lymph nodes stage | |
| pN0 | 249 (74.8%) |
| pN1 | 66 (19.8%) |
| pN2 | 14 (4.2%) |
| pN3 | 4 (1.2%) |
| Hormonal therapy | |
| No | 149 (44.7%) |
| Yes | 184 (55.3%) |
| Chemoimmunotherapy | |
| No | 196 (58.9%) |
| Yes | 137 (41.1%) |
| Axillary treatment | |
| SLNB/RISAS | 231 (69.4%) |
| ALND | 35 (10.5%) |
| SLNB/RISAS + Rtx | 27 (8.1%) |
| ALND + Rtx | 40 (12.0%) |
Abbreviations: ALND, axillary lymph node dissection; BCS, breast-conserving surgery; BMI, body mass index; HR, hormone receptor; RISAS, radioactive iodine seed localization in the axilla with sentinel node procedure; Rtx, radiotherapy; SLNB, sentinel lymph node biopsy. Note: HER2 positive, if HER2 is positive; HR-positive, estrogen receptor+ and/or progesterone receptor+ and HER2−; triple-negative, estrogen receptor- and progesterone receptor- and HER2−.
3.2. EQ-5D-5L and EORTC QLQ-C30 Scores over Time
The EQ-5D-5L index score at T6 (p < 0.001) and T12 (p = 0.006) is significantly lower than that at T0. The Global Quality of Life scale and the summary score of the EORTC QLQ-C30 decrease significantly between T0 and T6 (p = 0.010, p < 0.001, Table 2). The remaining functional and symptom scales of the EORTC QLQ-C30 tend to show significant differences more frequently between T0 and T6 than between T0 and T12 (Table 2).
Table 2.
EQ-5D-5L and EORTC QLQ-C30 scores pre-operatively, at 6- and 12 months post-surgery.
| T0 | T6 | T12 | p-Value (vs. T0) | ||
|---|---|---|---|---|---|
| HRQoL | (n = 333) | (n = 333) | (n = 333) | T6 | T12 |
| EQ-5D-5L | |||||
| Index | |||||
| Mean (SD) | 0.852 (0.143) | 0.799 (0.170) | 0.820 (0.192) | <0.001 | 0.006 |
| Median [Min, Max] | 0.883 [0.271, 1.00] | 0.818 [−0.182, 1.00] | 0.852 [−0.261, 1.00] | ||
| VAS score | |||||
| Mean (SD) | 78.0 (16.4) | 74.3 (15.7) | 76.1 (15.4) | <0.001 | 0.039 |
| Median [Min, Max] | 80.0 [5.00, 100] | 78.0 [8.00, 100] | 80.0 [2.00, 100] | ||
| EORTC QLQ-C30 | |||||
| Global Quality of Life scale | |||||
| Mean (SD) | 77.6 (17.3) | 75.1 (18.4) | 77.1 (18.2) | 0.010 | 0.783 |
| Median [Min, Max] | 83.3 [16.7, 100] | 83.3 [0, 100] | 83.3 [0, 100] | ||
| Emotional functioning | |||||
| Mean (SD) | 74.5 (21.1) | 78.9 (22.7) | 82.4 (20.1) | <0.001 | <0.001 |
| Median [Min, Max] | 83.3 [0, 100] | 83.3 [0, 100] | 91.7 [0, 100] | ||
| Role functioning | |||||
| Mean (SD) | 83.9 (24.3) | 74.6 (27.1) | 79.7 (26.6) | <0.001 | 0.007 |
| Median [Min, Max] | 100 [0, 100] | 83.3 [0, 100] | 83.3 [0, 100] | ||
| Physical functioning | |||||
| Mean (SD) | 90.1 (15.4) | 84.5 (16.4) | 86.9 (15.2) | <0.001 | <0.001 |
| Median [Min, Max] | 93.3 [20.0, 100] | 86.7 [26.7, 100] | 93.3 [20.0, 100] | ||
| Cognitive functioning | |||||
| Mean (SD) | 87.7 (16.4) | 80.0 (23.4) | 82.8 (20.5) | <0.001 | <0.001 |
| Median [Min, Max] | 100 [16.7, 100] | 83.3 [0, 100] | 83.3 [0, 100] | ||
| Social functioning | |||||
| Mean (SD) | 88.5 (20.2) | 81.7 (25.1) | 85.8 (22.2) | <0.001 | 0.122 |
| Median [Min, Max] | 100 [0, 100] | 100 [0, 100] | 100 [0, 100] | ||
| Fatigue | |||||
| Mean (SD) | 22.9 (21.8) | 31.8 (24.1) | 25.4 (22.4) | <0.001 | 0.076 |
| Median [Min, Max] | 22.2 [0, 100] | 22.2 [0, 100] | 22.2 [0, 100] | ||
| Nausea and vomiting | |||||
| Mean (SD) | 4.96 (12.3) | 4.71 (11.0) | 3.65 (10.1) | 0.709 | 0.187 |
| Median [Min, Max] | 0 [0, 83.3] | 0 [0, 83.3] | 0 [0, 100] | ||
| Pain | |||||
| Mean (SD) | 13.5 (19.0) | 20.9 (22.1) | 19.6 (21.7) | <0.001 | <0.001 |
| Median [Min, Max] | 0 [0, 83.3] | 16.7 [0, 100] | 16.7 [0, 100] | ||
| Dyspnea | |||||
| Mean (SD) | 7.41 (16.9) | 13.9 (23.6) | 9.91 (19.1) | <0.001 | 0.014 |
| Median [Min, Max] | 0 [0, 100] | 0 [0, 100] | 0 [0, 100] | ||
| Insomnia | |||||
| Mean (SD) | 27.7 (27.7) | 28.6 (30.0) | 25.8 (28.2) | 0.325 | 0.589 |
| Median [Min, Max] | 33.3 [0, 100] | 33.3 [0, 100] | 33.3 [0, 100] | ||
| Appetite loss | |||||
| Mean (SD) | 12.4 (21.8) | 7.61 (18.2) | 4.70 (13.7) | <0.001 | <0.001 |
| Median [Min, Max] | 0 [0, 100] | 0 [0, 100] | 0 [0, 100] | ||
| Constipation | |||||
| Mean (SD) | 7.41 (18.1) | 8.51 (20.0) | 8.21 (18.8) | 0.334 | 0.279 |
| Median [Min, Max] | 0 [0, 100] | 0 [0, 100] | 0 [0, 100] | ||
| Diarrhea | |||||
| Mean (SD) | 5.91 (15.1) | 4.10 (13.2) | 3.80 (12.9) | 0.134 | 0.099 |
| Median [Min, Max] | 0 [0, 100] | 0 [0, 66.7] | 0 [0, 66.7] | ||
| Financial difficulties | |||||
| Mean (SD) | 4.10 (14.6) | 8.61 (21.2) | 6.91 (18.7) | <0.001 | 0.01 |
| Median [Min, Max] | 0 [0, 100] | 0 [0, 100] | 0 [0, 100] | ||
| Summary score | |||||
| Mean (SD) | 86.3 (11.4) | 83.0 (13.5) | 85.9 (12.5) | <0.001 | 0.746 |
| Median [Min, Max] | 90.1 [32.6, 100] | 85.4 [27.9, 100] | 88.8 [28.6, 100] | ||
Abbreviations: T0, baseline; T6, 6 months post-surgery; T12, 12 months post-surgery. Note: EORTC QLQ-C30 functional/global HRQoL scales, higher scores indicate better functioning; symptom scales, higher scores indicate greater symptom severity. Bold indicates p < 0.05.
3.3. Deltas and MCID
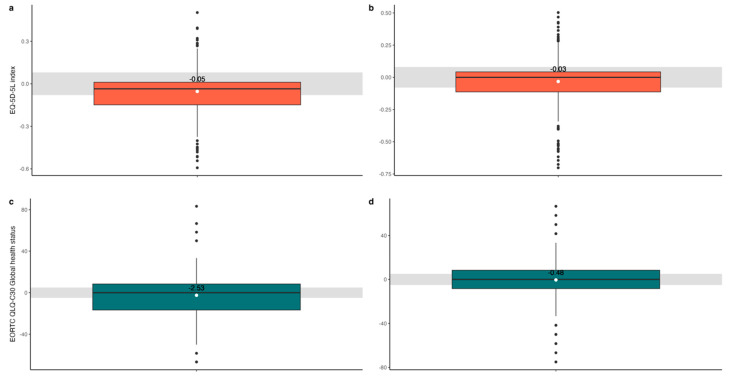
Mean delta 1 (e.g., the difference between T0 and T6) of the EQ-5D-5L index (−0.05) and the EORTC QLQ-C30 Global Quality of Life scale (−2.53) is negative, indicating that health status/HRQoL of patients with breast cancer is lower at T6 compared to T0. In addition, mean delta 2 (e.g., the difference between T0 and T12) of the EQ-5D-5L index (−0.03) and the EORTC QLQ-C30 Global Quality of Life scale (−0.48) is negative, indicating that health status/HRQoL of patients with breast cancer is lower at T12 compared to T0. Delta 1 and 2 of the EQ-5D-5L index and of the EORTC QLQ-C30 Global Quality of Life scale are smaller than the corresponding MCID: (0.08) and (5), respectively (Figure 2 and Appendix A, Table A1). Among the other scales of the EORTC QLQ-C30, only the delta 1 values for the functional scales exceed the MCID (Appendix A, Table A1).
Figure 2.
Boxplots of delta 1 (a) and delta 2 (b) for the EQ-5D-5L index (red) and of delta 1 (c) and delta 2 (d) for the EORTC QLQ-C30 Global health status (blue). The horizontal line in each boxplot represents the median value, while the white dot indicates the mean. The number within the boxplot represents the mean delta value. The grey interval represents the MCID of the EQ-5D-5L (0.08) and of the EORTC QLQ-C30 (5).
3.4. Internal Responsiveness
The ES and SRM for delta 1 and 2 of the EQ-5D-5L and the EORTC QLQ-C30 are all <0.5 (Table 3). Moreover, the ES and SRM tend to be greater for delta 1 compared to delta 2. In addition, the ES and SRM for the EQ-5D-5L index are greater compared to the ES and SRM for the Global Quality of Life scale and summary score of the EORTC QLQ-C30 (Table 3).
Table 3.
ES and SRM of delta 1 and delta 2.
| HRQoL | SRM: Delta 1 | SRM: Delta 2 | ES: Delta 1 | ES: Delta 2 |
|---|---|---|---|---|
| EQ-5D-5L | ||||
| Index | −0.339 | −0.176 | −0.374 | −0.224 |
| VAS score | −0.199 | −0.104 | −0.224 | −0.115 |
| EORTC QLQ-C30 | ||||
| Global Quality of Life scale | −0.133 | −0.024 | −0.146 | −0.028 |
| Physical functioning | −0.388 | −0.230 | −0.366 | −0.205 |
| Role functioning | −0.322 | −0.146 | −0.386 | −0.175 |
| Emotional functioning | 0.182 | 0.331 | 0.207 | 0.371 |
| Cognitive functioning | −0.352 | −0.231 | −0.468 | −0.297 |
| Social functioning | −0.273 | −0.11 | −0.337 | −0.131 |
| Fatigue | 0.354 | 0.109 | 0.407 | 0.115 |
| Nausea and vomiting | −0.017 | −0.093 | −0.020 | −0.106 |
| Pain | 0.295 | 0.261 | 0.389 | 0.321 |
| Dyspnea | 0.268 | 0.126 | 0.385 | 0.148 |
| Insomnia | 0.029 | −0.059 | 0.033 | −0.069 |
| Appetite loss | −0.194 | −0.340 | −0.221 | −0.354 |
| Constipation | 0.049 | 0.037 | 0.061 | 0.044 |
| Diarrhea | −0.099 | −0.119 | −0.119 | −0.139 |
| Financial difficulties | 0.222 | 0.145 | 0.308 | 0.192 |
| Summary score | −0.255 | −0.036 | −0.290 | −0.040 |
Abbreviations: SRM, standardized response mean; ES, effect size. Note: delta 1, difference in scores between T0 and T6; delta 2, difference in scores between T0 and T12.
3.5. Subgroup Analysis
For patients receiving chemotherapy, the ES and SRM for delta 1 and 2 of the EQ-5D-5L and the EORTC QLQ-C30 are generally below 0.5, except for cognitive functioning, pain, and appetite loss (EORTC QLQ-C30). The ES and SRM for delta 1 tend to be greater than those for delta 2 (Table 4). The ES and SRM for delta 1 tend to be greater for patients receiving chemotherapy compared to patients without chemotherapy. Delta 2 does not exhibit a consistent pattern regarding the ES and SRM between the two groups (Table 4 and Appendix A, Table A2).
Table 4.
Subgroup analysis: ES and SRM of delta 1 and delta 2 in patients receiving chemotherapy.
| HRQoL | SRM: Delta 1 | SRM: Delta 2 | ES: Delta 1 | ES: Delta 2 |
|---|---|---|---|---|
| EQ-5D-5L | ||||
| Index | −0.331 | −0.141 | −0.394 | −0.172 |
| VAS score | −0.221 | −0.050 | −0.247 | −0.049 |
| EORTC QLQ-C30 | ||||
| Global Quality of Life scale | −0.260 | 0.013 | −0.296 | 0.013 |
| Physical functioning | −0.436 | −0.149 | −0.399 | −0.140 |
| Role functioning | −0.381 | −0.079 | −0.452 | −0.097 |
| Emotional functioning | 0.126 | 0.300 | 0.149 | 0.341 |
| Cognitive functioning | −0.403 | −0.291 | −0.563 | −0.373 |
| Social functioning | −0.298 | −0.083 | −0.386 | −0.095 |
| Fatigue | 0.380 | 0.055 | 0.435 | 0.058 |
| Nausea and vomiting | −0.042 | −0.125 | −0.053 | −0.141 |
| Pain | 0.351 | 0.237 | 0.501 | 0.293 |
| Dyspnea | 0.294 | 0 | 0.475 | 0 |
| Insomnia | 0.008 | −0.112 | 0.009 | −0.139 |
| Appetite loss | −0.334 | −0.505 | −0.41 | −0.540 |
| Constipation | −0.021 | 0 | −0.022 | 0 |
| Diarrhea | −0.156 | −0.209 | −0.198 | −0.255 |
| Financial difficulties | 0.308 | 0.233 | 0.465 | 0.310 |
| Summary score | −0.236 | 0.060 | −0.282 | 0.067 |
Abbreviations: SRM, standardized response mean; ES, effect size. Note: delta 1, difference in scores between T0 and T6; delta 2, difference in scores between T0 and T12. Bold indicates SRM/ES > 0.5.
4. Discussion
This study aimed to evaluate and compare the internal responsiveness of the EQ-5D-5L and the EORTC QLQ-C30 among Dutch breast cancer patients during the first year post-surgery. The results revealed that the difference between T0 and T6/T12 of the EQ-5D-5L index value and most of the EORTC QLQ-C30 scales were lower than their respective MCIDs, suggesting that the changes in health status/HRQoL as captured by these PROMs may not be clinically meaningful for the patient. It is important to note that the range of reported MCID values for the EQ-5D and EORTC QLQ-C30 varies widely. This indicates that any conclusions regarding the clinical significance of these changes depend significantly on the chosen MCID threshold. The thresholds used in this study are relatively high, which makes clinically significant changes less likely to be observed [53,54,55,56,57].
Both the EQ-5D-5L and the EORTC QLQ-C30 have relatively small internal responsiveness (<0.5), measured by the ES and SRM, in Dutch breast cancer patients 6 and 12 months after surgery. Interestingly, the EQ-5D-5L index showed slightly greater internal responsiveness compared to the EORTC QLQ-C30 Global Quality of Life scale and summary score. Additionally, the results demonstrated that the internal responsiveness is greater for the period from baseline to 6 months (delta 1) than from baseline to 12 months post-surgery (delta 2), which aligns with previous research demonstrating the decreasing effect of diagnosis and treatment on health status/HRQoL over time and the tendency for health status/HRQoL to return to baseline levels [58,59].
The relatively low breast cancer stage of the patients included in this cohort may have contributed to the small changes in HRQOL/health status. Patients with more advanced breast cancer tend to experience a greater impact on their health status/HRQoL and therefore the internal responsiveness among advanced breast cancer patients may be greater than the internal responsiveness of both PROMs in the current patient cohort [60,61,62]. This is further supported by the subgroup analysis, which demonstrated that the internal responsiveness was slightly greater in patients receiving chemotherapy compared to patients without chemotherapy potentially due to the significant impact of chemotherapy on health status/HRQoL [51]. Patients receiving chemotherapy showed greater internal responsiveness for delta 1 compared to delta 2. This can be attributed to the fact that 6 months post-surgery, patients may still be undergoing chemotherapy and therefore experience chemotherapy-related symptoms, while at 12 months post-surgery, most patients have completed their chemotherapy treatment [37].
Overall, the internal responsiveness of both PROMs was small. However, greater responsiveness was observed at the expected time points and within the specific subgroups. The results of this study are consistent with some previous studies that have examined internal responsiveness in different study populations [26,27,28,29,30,31,32,33,34]. The study by Rundgren et al. found that the internal responsiveness of the EQ-5D was greater between baseline and 3- months post-surgery compared to the period between 3- and 12-months post-surgery in patients with a distal radius fracture [29]. Uwer et al. found that the EORTC QLQ-C30 was more responsive in colorectal cancer patients who received chemotherapy compared to patients who received radiotherapy and concluded that the internal responsiveness of the EORTC QLQ-C30 depends on the type of treatment given [28]. Previous studies that have been conducted in breast cancer patients demonstrated larger responsiveness of the EQ-5D and the EORTC QLQ-C30. It is important to note that these studies had a shorter follow-up period, included patients receiving advanced treatments, or did not analyze the internal responsiveness with the ES and/or SRM [31,32,33]. The study by Kimman et al. examined the internal responsiveness of the EQ-5D with the SRM in breast cancer patients during the first year after treatment. The study found that the EQ-5D was overall responsive, but not responsive enough to detect small changes in health status [30].
Given the variability in findings, where some studies report large internal responsiveness and others report small to moderate, it is evident that the internal responsiveness of health status/HRQoL instruments is influenced by factors such as the length of follow-up, the study population, and the type of intervention [26,27,28,29,30,31,32,33,34]. This underscores the necessity of conducting targeted research on internal responsiveness within specific disease cohorts to tailor insights more accurately. Furthermore, results from such studies must be interpreted with caution, considering the diverse factors that can influence outcomes.
4.1. Strengths and Limitations
To our knowledge, this is the first study assessing and comparing the internal responsiveness of the EQ-5D-5L and EORTC QLQ-C30 in Dutch breast cancer patients. As both PROMs are widely used in breast cancer research and clinical practice, the findings of this study provide crucial guidance for refining research methodologies and enhancing patient care strategies in the management of breast cancer [23,24,25]. Another key strength of this study is the availability of a relatively large sample size (more than 300 patients) and the longitudinal nature of the health status/HRQoL data. This is of significance as it is known that the health status/HRQoL of breast cancer patients is dynamic and may change over time [63]. Furthermore, a subgroup analysis was performed on patients undergoing chemotherapy [37]. This subgroup analysis provides insights into the internal responsiveness of both the EQ-5D-5L and EORTC QLQ-C30 in different patient groups. Moreover, given that MCIDs for the EQ-5D-5L and EORTC QLQ-C30 in breast cancer are not well defined, this study provides valuable data for future research to create more robust MCID guidelines [57]. Lastly, the results from this study could inform more accurate sample size calculations for future clinical trials in Dutch breast cancer patients that use these specific PROMs as endpoints.
One limitation of the current study is that only internal responsiveness was examined, which is one of a few measures to consider when selecting a particular PROM. The external responsiveness of the EQ-5D-5L and the EORTC QLQ-C30, which evaluates the degree to which changes in these PROMs correspond to changes in a reference measure of health status/HRQoL, was not assessed due to the absence of a gold standard for assessing health status/HRQoL. The external responsiveness of a PROM is important for clinical practice as this indicates whether the PROM correctly reflects the actual change in the HRQoL of patients [23]. Another limitation to consider is the interaction between the internal responsiveness of PROMs and the actual changes in a patient’s health status/HRQoL over time. When the actual changes in the patient’s health status/HRQoL are minimal, it has an impact on the responsiveness of the PROMs. This is particularly evident in breast cancer patients who maintain the highest stability in their health status/HRQoL compared to patients with other types of cancer [35]. This stability suggests that while PROMs are valuable, their responsiveness may be influenced by the inherent steadiness of health status/HRQoL in certain patient groups, necessitating careful interpretation of results in these contexts. Furthermore, the current study focused solely on the internal responsiveness of the EORTC QLQ-C30 and EQ-5D-5L. In a future study, it might be valuable to also examine and compare the internal responsiveness of commonly used breast cancer-specific PROMs such as the BREAST-Q and EORTC QLQ-BR23. The results will provide a better understanding of the internal responsiveness across a broader spectrum of PROMs and will improve the interpretation of those PROMs in clinical practice. Lastly, the subgroup analysis focused exclusively on chemotherapy while health status and HRQoL might also change depending on other patient and treatment characteristics or the baseline health status/HRQoL score of an individual. For instance, patients with initially low health status/HRQoL scores at baseline may have minimal changes in their health status/HRQoL following surgery, while patients with higher health status/HRQoL scores before surgery might experience significant reductions in their health status/HRQoL post-surgery. However, from a recent study, it is known that age, gender, and baseline HRQoL may not significantly impact the temporal stability of HRQoL measured with the EORTC QLQ-C30 in cancer patients [35].
4.2. Clinical Implications
The results of this study indicate that the internal responsiveness of the EQ-5D-5L and EORTC QLQ-C30 in breast cancer patients 6- and 12 months post-surgery is relatively small. Additional approaches to measuring health status/HRQoL in this specific patient cohort may be considered, such as administering PROMs at different or more time points to detect smaller changes in health status/HRQoL and using breast cancer-specific PROMs as recommended by the ICHOM Breast Cancer standard set to improve patient-centered healthcare and research in the future [35,36,64].
5. Conclusions
The results indicated that the observed mean differences between baseline and 6- and 12-months post-surgery of the EQ-5D-5L index and most of the EORTC QLQ-C30 scales were below their respective MCIDs at both time points. Internal responsiveness for the EQ-5D-5L and EORTC QLQ-C30 was small (<0.5), with greater responsiveness observed at 6 months compared to 12 months post-surgery. Additionally, the EQ-5D-5L index showed greater internal responsiveness than the Global Quality of Life scale and summary score of the EORTC QLQ-C30. These findings provide crucial insights for interpreting outcomes from both PROMs in Dutch breast cancer research and clinical practice.
Appendix A
Table A1.
Deltas EQ-5D-5L and EORTC QLQ-C30.
| HRQoL | Delta 1 (n = 333) |
Delta 2 (n = 333) |
|---|---|---|
| EQ-5D-5L | ||
| Index | ||
| Mean (SD) | −0.054 (0.158) | −0.032 (0.182) |
| Median [Min, Max] | −0.035 [−0.593, 0.503] | 0 [−0.702, 0.503] |
| VAS score | ||
| Mean (SD) | −3.69 (18.5) | −1.89 (18.2) |
| Median [Min, Max] | −1.00 [−69.0, 54.0] | 0 [−74.0, 55.0] |
| EORTC QLQ-C30 | ||
| Global Quality of Life scale | ||
| Mean (SD) | −2.53 (18.9) | −0.476 (19.9) |
| Median [Min, Max] | 0 [−66.7, 83.3] | 0 [−75.0, 66.7] |
| Emotional functioning | ||
| Mean (SD) | 4.36 (23.9) | 7.83 (23.6) |
| Median [Min, Max] | 8.33 [−75.0, 91.7] | 8.33 [−75.0, 83.3] |
| Role functioning | ||
| Mean (SD) | −9.36 (29.1) | −4.25 (29.1) |
| Median [Min, Max] | 0 [−100, 100] | 0 [−100, 100] |
| Physical functioning | ||
| Mean (SD) | −5.63 (14.5) | −3.14 (13.7) |
| Median [Min, Max] | −6.66 [−66.7, 60.0] | 0 [−66.7, 66.7] |
| Cognitive functioning | ||
| Mean (SD) | −7.66 (21.8) | −4.85 (21.0) |
| Median [Min, Max] | 0 [−100, 66.7] | 0 [−83.3, 66.7] |
| Social functioning | ||
| Mean (SD) | −6.81 (24.9) | −2.65 (24.1) |
| Median [Min, Max] | 0 [−100, 100] | 0 [−100, 66.7] |
| Fatigue | ||
| Mean (SD) | 8.88 (25.1) | 2.50 (23.0) |
| Median [Min, Max] | 11.1 [−88.9, 88.9] | 0 [−77.8, 77.8] |
| Nausea and vomiting | ||
| Mean (SD) | −0.250 (15.1) | −1.30 (13.9) |
| Median [Min, Max] | 0 [−66.7, 83.3] | 0 [−83.3, 50.0] |
| Pain | ||
| Mean (SD) | 7.41 (25.1) | 6.11 (23.4) |
| Median [Min, Max] | 0 [−83.3, 83.3] | 0 [−83.3, 100] |
| Dyspnea | ||
| Mean (SD) | 6.51 (24.3) | 2.50 (19.8) |
| Median [Min, Max] | 0 [−100, 100] | 0 [−100, 66.7] |
| Insomnia | ||
| Mean (SD) | 0.901 (31.3) | −1.90 (32.3) |
| Median [Min, Max] | 0 [−100, 100] | 0 [−100, 100] |
| Appetite loss | ||
| Mean (SD) | −4.80 (24.8) | −7.71 (22.6) |
| Median [Min, Max] | 0 [−100, 100] | 0 [−100, 66.7] |
| Constipation | ||
| Mean (SD) | 1.10 (22.6) | 0.801 (21.5) |
| Median [Min, Max] | 0 [−100, 100] | 0 [−100, 100] |
| Diarrhea | ||
| Mean (SD) | −1.80 (18.2) | −2.10 (17.7) |
| Median [Min, Max] | 0 [−100, 66.7] | 0 [−100, 66.7] |
| Financial difficulties | ||
| Mean (SD) | 4.50 (20.3) | 2.80 (19.3) |
| Median [Min, Max] | 0 [−100, 100] | 0 [−100, 100] |
| Summary score | ||
| Mean (SD) | −3.31 (13.0) | −0.459 (12.7) |
| Median [Min, Max] | −1.97 [−62.4, 40.9] | 0 [−48.0, 48.1] |
Note: delta 1, difference in scores between T0 and T6; delta 2, difference in scores between T0 and T12. Bold indicates delta > MCID of the EQ-5D-5L (0.08) or the EORTC QLQ-C30 (5).
Table A2.
Subgroup analysis: ES and SRM of delta 1 and delta 2 in patients without chemotherapy.
| HRQoL | SRM: Delta 1 | SRM: Delta 2 | ES: Delta 1 | ES: Delta 2 |
|---|---|---|---|---|
| EQ-5D-5L | ||||
| Index | −0.350 | −0.200 | −0.365 | −0.269 |
| VAS score | −0.182 | −0.138 | −0.208 | −0.168 |
| EORTC QLQ-C30 | ||||
| Global Quality of Life scale | −0.032 | −0.048 | −0.033 | −0.059 |
| Physical functioning | −0.351 | −0.307 | −0.341 | −0.258 |
| Role functioning | −0.276 | −0.203 | −0.343 | −0.247 |
| Emotional functioning | 0.236 | 0.360 | 0.262 | 0.409 |
| Cognitive functioning | −0.315 | −0.181 | −0.400 | −0.239 |
| Social functioning | −0.258 | −0.133 | −0.304 | −0.169 |
| Fatigue | 0.334 | 0.154 | 0.401 | 0.167 |
| Nausea and vomiting | 0.006 | −0.067 | 0.008 | −0.078 |
| Pain | 0.253 | 0.278 | 0.313 | 0.339 |
| Dyspnea | 0.253 | 0.252 | 0.312 | 0.269 |
| Insomnia | 0.044 | −0.017 | 0.050 | −0.019 |
| Appetite loss | −0.040 | −0.193 | −0.041 | −0.194 |
| Constipation | 0.101 | 0.079 | 0.148 | 0.091 |
| Diarrhea | −0.045 | −0.034 | −0.051 | −0.038 |
| Financial difficulties | 0.140 | 0.063 | 0.165 | 0.083 |
| Summary score | −0.283 | −0.124 | −0.318 | −0.145 |
Abbreviations: SRM, standardized response mean; ES, effect size. Note: delta 1, difference in scores between T0 and T6; delta 2, difference in scores between T0 and T12.
Author Contributions
Conceptualization, N.J.M.C.V.P., J.A.v.T., A.S.H., S.S., O.H. and L.B.K.; methodology, N.J.M.C.V.P.; formal analysis, N.J.M.C.V.P., J.A.v.T. and O.H.; data curation, L.B.K.; writing—original draft preparation, N.J.M.C.V.P.; writing—review and editing, N.J.M.C.V.P., J.A.v.T., A.S.H., S.S., O.H. and L.B.K.; visualization, N.J.M.C.V.P.; supervision, O.H. and L.B.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This is an observational study. The Erasmus MC Research Ethics Committee has confirmed that no ethical approval is required.
Informed Consent Statement
The Institutional Review Board was consulted and concluded that informed consent was not needed since the value-based healthcare strategy is considered the standard of care in Erasmus MC.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to privacy or ethical restrictions but are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
The authors received no financial support for this research. The involvement of Dr. Janine van Til was partly sponsored by a grant from the EuroHRQoL group (285-PHD).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Clegg L.X., Reichman M.E., Miller B.A., Hankey B.F., Singh G.K., Lin Y.D., Goodman M.T., Lynch C.F., Schwartz S.M., Chen V.W., et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: Selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20:417–435. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghoncheh M., Pournamdar Z., Salehiniya H. Incidence and Mortality and Epidemiology of Breast Cancer in the World. Asian Pac. J. Cancer Prev. 2016;17:43–46. doi: 10.7314/APJCP.2016.17.S3.43. [DOI] [PubMed] [Google Scholar]
- 3.(IKNL) IKN Incidentie Borstkanker. [(accessed on 21 December 2022)]. Available online: https://iknl.nl/kankersoorten/borstkanker/registratie/incidentie.
- 4.(IKNL) IKN Kerncijfers over Borstkanker uit de Nederlandse Kankerregistratie. 2020. [(accessed on 21 December 2022)]. Available online: https://iknl.nl/nieuws/2020/kerncijfers-over-borstkanker-uit-de-nederlandse.
- 5.(IKNL) IKN Borstkanker in Nederland 1989–2017: Hogere Incidentie; Betere Overleving. 2020. [(accessed on 21 December 2022)]. Available online: https://iknl.nl/nieuws/2020/borstkanker-in-nederland-1989-2017-hogere-incident.
- 6.Maajani K., Jalali A., Alipour S., Khodadost M., Tohidinik H.R., Yazdani K. The Global and Regional Survival Rate of Women With Breast Cancer: A Systematic Review and Meta-analysis. Clin. Breast Cancer. 2019;19:165–177. doi: 10.1016/j.clbc.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 7.RIVM Bevolkingsonderzoek Borstkanker. 2022. [(accessed on 18 November 2022)]. Available online: https://www.rivm.nl/bevolkingsonderzoek-borstkanker.
- 8.Heins M.J., de Ligt K.M., Verloop J., Siesling S., Korevaar J.C., PSCCR Group Adverse health effects after breast cancer up to 14 years after diagnosis. Breast. 2022;61:22–28. doi: 10.1016/j.breast.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Ligt K.M., Heins M., Verloop J., Ezendam N.P.M., Smorenburg C.H., Korevaar J.C., Siesling S. The impact of health symptoms on health-related quality of life in early-stage breast cancer survivors. Breast Cancer Res. Treat. 2019;178:703–711. doi: 10.1007/s10549-019-05433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biparva A.J., Raoofi S., Rafiei S., Kan F.P., Kazerooni M., Bagheribayati F., Masoumi M., Doustmehraban M., Sanaei M., Zarabi F., et al. Global quality of life in breast cancer: Systematic review and meta-analysis. BMJ Support. Palliat. Care. 2022;13:e528–e536. doi: 10.1136/bmjspcare-2022-003642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weldring T., Smith S.M.S. Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs) Health Serv. Insights. 2013;6:61–68. doi: 10.4137/HSI.S11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patrick D.L., Deyo R.A. Generic and disease-specific measures in assessing health status and quality of life. Med. Care. 1989;27((Suppl. S3)):S217–S232. doi: 10.1097/00005650-198903001-00018. [DOI] [PubMed] [Google Scholar]
- 13.Higginson I.J. Measuring quality of life: Using quality of life measures in the clinical setting. BMJ. 2001;322:1297–1300. doi: 10.1136/bmj.322.7297.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks R. EuroQol: The current state of play. Health Policy. 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 15.Aaronson N.K., Ahmedzai S., Bergman B., Bullinger M., Cull A., Duez N.J., Filiberti A., Flechtner H., Fleishman S.B., De Haes J.C.J.M., et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 16.Van Egdom L., Lagendijk M., van der Kemp M., van Dam J., Mureau M., Hazelzet J., Koppert L. Implementation of Value Based Breast Cancer Care. Eur. J. Surg. Oncol. 2019;45:1163–1170. doi: 10.1016/j.ejso.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Versteegh M.M., Vermeulen K.M., Evers S.M., De Wit G.A., Prenger R., Stolk E.A. Dutch Tariff for the Five-Level Version of EQ-5D. Value Health. 2016;19:343–352. doi: 10.1016/j.jval.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 18.National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013. National Institute for Health and Care Excellence (NICE); London, UK: 2013. [PubMed] [Google Scholar]
- 19.Kontodimopoulos N., Aletras V.H., Paliouras D., Niakas D. Mapping the Cancer-Specific EORTC QLQ-C30 to the Preference-Based EQ-5D, SF-6D, and 15D Instruments. Value Health. 2009;12:1151–1157. doi: 10.1111/j.1524-4733.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim S.H., Jo M.-W., Kim H.-J., Ahn J.-H. Mapping EORTC QLQ-C30 onto EQ-5D for the assessment of cancer patients. Health Qual. Life Outcomes. 2012;10:151. doi: 10.1186/1477-7525-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crott R., Briggs A. Mapping the QLQ-C30 quality of life cancer questionnaire to EQ-5D patient preferences. Eur. J. Health Econ. 2010;11:427–434. doi: 10.1007/s10198-010-0233-7. [DOI] [PubMed] [Google Scholar]
- 22.Kim E., Ko S.-K., Kang H.-Y. Mapping the cancer-specific EORTC QLQ-C30 and EORTC QLQ-BR23 to the generic EQ-5D in metastatic breast cancer patients. Qual. Life Res. 2012;21:1193–1203. doi: 10.1007/s11136-011-0037-y. [DOI] [PubMed] [Google Scholar]
- 23.Husted J.A., Cook R.J., Farewell V.T., Gladman D.D. Methods for assessing responsiveness: A critical review and recommendations. J. Clin. Epidemiol. 2000;53:459–468. doi: 10.1016/S0895-4356(99)00206-1. [DOI] [PubMed] [Google Scholar]
- 24.Deyo R.A., Centor R.M. Assessing the responsiveness of functional scales to clinical change: An analogy to diagnostic test performance. J. Chronic Dis. 1986;39:897–906. doi: 10.1016/0021-9681(86)90038-X. [DOI] [PubMed] [Google Scholar]
- 25.Guyatt G., Walter S., Norman G. Measuring change over time: Assessing the usefulness of evaluative instruments. J. Chronic Dis. 1987;40:171–178. doi: 10.1016/0021-9681(87)90069-5. [DOI] [PubMed] [Google Scholar]
- 26.Hu X., Jing M., Zhang M., Yang P., Yan X. Responsiveness and minimal clinically important difference of the EQ-5D-5L in cervical intraepithelial neoplasia: A longitudinal study. Health Qual. Life Outcomes. 2020;18:324. doi: 10.1186/s12955-020-01578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamu A.N., Björkman L., Hamre H.J., Alræk T., Musial F., Robberstad B. Validity and responsiveness of EQ-5D-5L and SF-6D in patients with health complaints attributed to their amalgam fillings: A prospective cohort study of patients undergoing amalgam removal. Health Qual. Life Outcomes. 2021;19:125. doi: 10.1186/s12955-021-01762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uwer L., Rotonda C., Guillemin F., Miny J., Kaminsky M.-C., Mercier M., Tournier-Rangeard L., Leonard I., Montcuquet P., Rauch P., et al. Responsiveness of EORTC QLQ-C30, QLQ-CR38 and FACT-C quality of life questionnaires in patients with colorectal cancer. Health Qual. Life Outcomes. 2011;9:70. doi: 10.1186/1477-7525-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rundgren J., Enocson A., Mellstrand Navarro C., Bergström G. Responsiveness of EQ-5D in Patients with a Distal Radius Fracture. Hand. 2018;13:572–580. doi: 10.1177/1558944717725378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimman M.L., Dirksen C.D., Lambin P., Boersma L.J. Responsiveness of the EQ-5D in breast cancer patients in their first year after treatment. Health Qual. Life Outcomes. 2009;7:11. doi: 10.1186/1477-7525-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conner-Spady B., Cumming C., Nabholtz J.M., Jacobs P., Stewart D. Responsiveness of the EuroQol in breast cancer patients undergoing high dose chemotherapy. Qual. Life Res. 2001;10:479–486. doi: 10.1023/A:1013018218360. [DOI] [PubMed] [Google Scholar]
- 32.Osoba D., Zee B., Pater J., Warr D., Kaizer L., Latreille J. Psychometric properties and responsiveness of the EORTC quality of Life Questionnaire (QLQ-C30) in patients with breast, ovarian and lung cancer. Qual. Life Res. 1994;3:353–364. doi: 10.1007/BF00451727. [DOI] [PubMed] [Google Scholar]
- 33.Lee C.F., Luo N., Ng R., Wong N.S., Yap Y.S., Lo S.K., Chia W.K., Yee A., Krishna L., Wong C., et al. Comparison of the measurement properties between a short and generic instrument, the 5-level EuroQoL Group’s 5-dimension (EQ-5D-5L) questionnaire, and a longer and disease-specific instrument, the Functional Assessment of Cancer Therapy-Breast (FACT-B), i. Qual. Life Res. 2013;22:1745–1751. doi: 10.1007/s11136-012-0291-7. [DOI] [PubMed] [Google Scholar]
- 34.Tordrup D., Mossman J., Kanavos P. Responsiveness of the EQ-5D to clinical change: Is the patient experience adequately represented? Int. J. Technol. Assess Health Care. 2014;30:10–19. doi: 10.1017/S0266462313000640. [DOI] [PubMed] [Google Scholar]
- 35.Hinz A., Schulte T., Rassler J., Zenger M., Geue K. Temporal stability of quality of life assessments in cancer patients. Sci. Rep. 2021;11:5191. doi: 10.1038/s41598-021-84681-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Payakachat N., Ali M.M., Tilford J.M. Can The EQ-5D Detect Meaningful Change? A Systematic Review. Pharmacoeconomics. 2015;33:1137–1154. doi: 10.1007/s40273-015-0295-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Federatie Medisch Specialisten . Borstkanker Richtlijnen. Federatie Medisch Specialisten; Utrecht, The Netherlands: 2022. [Google Scholar]
- 38.Nationaal Borstkanker Overleg Nederland . Borstkanker Richtlijn. Nationaal Borstkanker Overleg Nederland; Amsterdam, The Netherlands: 2020. [Google Scholar]
- 39.Herdman M., Gudex C., Lloyd A., Janssen M., Kind P., Parkin D., Bonsel G., Badia X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual. Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fayers P.M., Aaronson N.K., Bjordal K., Groenvold M., Curran D.B.A. The EORTC QLQ—C30 Scoring Manual. 3rd ed. European Organisation for Research and Treatment of Cancer; Brussels, Belgium: 2001. [Google Scholar]
- 41.Cocks K., King M.T., Velikova G., Martyn St-James M., Fayers P.M., Brown J.M. Evidence-Based Guidelines for Determination of Sample Size and Interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J. Clin. Oncol. 2011;29:89–96. doi: 10.1200/JCO.2010.28.0107. [DOI] [PubMed] [Google Scholar]
- 42.Giesinger J.M., Kieffer J.M., Fayers P.M., Groenvold M., Petersen M.A., Scott N.W., Sprangers M.A., Velikova G., Aaronson N.K. Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J. Clin. Epidemiol. 2016;69:79–88. doi: 10.1016/j.jclinepi.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Gundy C.M., Fayers P.M., Groenvold M., Petersen M.A., Scott N.W., Sprangers M.A.G., Velikova G., Aaronson N.K. Comparing higher order models for the EORTC QLQ-C30. Qual. Life Res. 2012;21:1607–1617. doi: 10.1007/s11136-011-0082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pickard A.S., Neary M.P., Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual. Life Outcomes. 2007;5:70. doi: 10.1186/1477-7525-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osoba D., Rodrigues G., Myles J., Zee B., Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J. Clin. Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 46.Kazis L.E., Anderson J.J., Meenan R.F. Effect sizes for interpreting changes in health status. Med. Care. 1989;27((Suppl. S3)):S178–S189. doi: 10.1097/00005650-198903001-00015. [DOI] [PubMed] [Google Scholar]
- 47.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Routledge; London, UK: 1988. [Google Scholar]
- 48.Beaton D.E., Hogg-Johnson S., Bombardier C. Evaluating changes in health status: Reliability and responsiveness of five generic health status measures in workers with musculoskeletal disorders. J. Clin. Epidemiol. 1997;50:79–93. doi: 10.1016/S0895-4356(96)00296-X. [DOI] [PubMed] [Google Scholar]
- 49.Liang M.H., Fossel A.H., Larson M.G. Comparisons of five health status instruments for orthopedic evaluation. Med. Care. 1990;28:632–642. doi: 10.1097/00005650-199007000-00008. [DOI] [PubMed] [Google Scholar]
- 50.Crosby R.D., Kolotkin R.L., Williams G.R. Defining clinically meaningful change in health-related quality of life. J. Clin. Epidemiol. 2003;56:395–407. doi: 10.1016/S0895-4356(03)00044-1. [DOI] [PubMed] [Google Scholar]
- 51.Hwang S.Y., Chang S.J., Park B.-W. Does chemotherapy really affect the quality of life of women with breast cancer? J. Breast Cancer. 2013;16:229–235. doi: 10.4048/jbc.2013.16.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2022. [(accessed on 21 December 2022)]. Available online: https://www.R-project.org/ [Google Scholar]
- 53.McClure N.S., Sayah F.A., Ohinmaa A., Johnson J.A. Minimally Important Difference of the EQ-5D-5L Index Score in Adults with Type 2 Diabetes. Value Health. 2018;21:1090–1097. doi: 10.1016/j.jval.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 54.Short H., Al Sayah F., Churchill K., Keogh E., Warner L., Ohinmaa A., Johnson J.A. The use of EQ-5D-5L as a patient-reported outcome measure in evaluating community rehabilitation services in Alberta, Canada. Health Qual. Life Outcomes. 2023;21:125. doi: 10.1186/s12955-023-02207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marks M., Grobet C., Audigé L. Validity, responsiveness and minimal important change of the EQ-5D-5L in patients after rotator cuff repair, shoulder arthroplasty or thumb carpometacarpal arthroplasty. Qual. Life Res. 2021;30:2973–2982. doi: 10.1007/s11136-021-02849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henry E.B., Barry L.E., Hobbins A.P., McClure N.S., O’Neill C. Estimation of an Instrument-Defined Minimally Important Difference in EQ-5D-5L Index Scores Based on Scoring Algorithms Derived Using the EQ-VT Version 2 Valuation Protocols. Value Health. 2020;23:936–944. doi: 10.1016/j.jval.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 57.Musoro J.Z., Coens C., Sprangers M.A., Brandberg Y., Groenvold M., Flechtner H.-H., Cocks K., Velikova G., Dirven L., Greimel E., et al. Minimally important differences for interpreting EORTC QLQ-C30 change scores over time: A synthesis across 21 clinical trials involving nine different cancer types. Eur. J. Cancer. 2023;188:171–182. doi: 10.1016/j.ejca.2023.04.027. [DOI] [PubMed] [Google Scholar]
- 58.King M.T., Kenny P., Shiell A., Hall J., Boyages J. Quality of life three months and one year after first treatment for early stage breast cancer: Influence of treatment and patient characteristics. Qual. Life Res. 2000;9:789–800. doi: 10.1023/A:1008936830764. [DOI] [PubMed] [Google Scholar]
- 59.Shimozuma K., Ganz P.A., Petersen L., Hirji K. Quality of life in the first year after breast cancer surgery: Rehabilitation needs and patterns of recovery. Breast Cancer Res. Treat. 1999;56:45–57. doi: 10.1023/A:1006214830854. [DOI] [PubMed] [Google Scholar]
- 60.Kwan M.L., Ergas I.J., Somkin C.P., Quesenberry C.P., Neugut A.I., Hershman D.L., Mandelblatt J., Pelayo M.P., Timperi A.W., Miles S.Q., et al. Quality of life among women recently diagnosed with invasive breast cancer: The Pathways Study. Breast Cancer Res. Treat. 2010;123:507–524. doi: 10.1007/s10549-010-0764-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Musoro J.Z., Coens C., Fiteni F., Katarzyna P., Cardoso F., Russell N.S., King M.T., Cocks K., Sprangers M.A., Groenvold M., et al. Minimally Important Differences for Interpreting EORTC QLQ-C30 Scores in Patients with Advanced Breast Cancer. JNCI Cancer Spectr. 2019;3:pkz037. doi: 10.1093/jncics/pkz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawahara T., Taira N., Shiroiwa T., Hagiwara Y., Fukuda T., Uemura Y., Mukai H. Minimal important differences of EORTC QLQ-C30 for metastatic breast cancer patients: Results from a randomized clinical trial. Qual. Life Res. 2022;31:1829–1836. doi: 10.1007/s11136-021-03074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park J.-H., Jung Y.S., Kim J.Y., Bae S.H. Trajectories of quality of life in breast cancer survivors during the first year after treatment: A longitudinal study. BMC Women’s Health. 2023;23:12. doi: 10.1186/s12905-022-02153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ong W.L., Schouwenburg M.G., Van Bommel A.C., Stowell C., Allison K.H., Benn K.E., Browne J.P., Cooter R.D., Delaney G.P., Duhoux F.P., et al. A Standard Set of Value-Based Patient-Centered Outcomes for Breast Cancer: The International Consortium for Health Outcomes Measurement (ICHOM) Initiative. JAMA Oncol. 2017;3:677–685. doi: 10.1001/jamaoncol.2016.4851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to privacy or ethical restrictions but are available from the corresponding author on reasonable request.