Abstract
The human polyomavirus JC virus (JCV) is the etiologic agent of a fatal central nervous system (CNS) demyelinating disease known as progressive multifocal leukoencephalopathy (PML). PML occurs predominantly in immunosuppressed patients and has increased dramatically as a result of the AIDS pandemic. The major target cell of JCV infection and lytic replication in the CNS is the oligodendrocyte. The mechanisms by which JCV initiates and establishes infection of these glial cells are not understood. The initial interaction between JCV and glial cells involves virus binding to N-linked glycoproteins containing terminal α(2-6)-linked sialic acids. The subsequent steps of entry and targeting of the viral genome to the nucleus have not been described. In this report, we compare the kinetics and mechanisms of infectious entry of JCV into human glial cells with that of the related polyomavirus, simian virus 40 (SV40). We demonstrate that JCV, unlike SV40, enters glial cells by receptor-mediated clathrin-dependent endocytosis.
JC virus (JCV) is a small, nonenveloped, double-stranded DNA containing virus belonging to the family Papovaviridae and the subfamily Polyomavirinae (23, 26). In vivo, JCV infection is restricted to oligodendrocytes, astrocytes, and B lymphocytes (17, 20). This highly restricted cell type specificity is also seen in vitro, as JCV infects primary cultures of human glial cells, human glial cell lines, and to a limited extent, primary human B cells and some B-cell lines (4, 17, 20, 28). The life cycle of JCV begins with virus attachment to a cell surface glycoprotein receptor containing α-(2-6)-linked sialic acid (15). Following attachment, the JCV virion must penetrate the plasma membrane and target its genome to the nucleus. Very little is known about the mechanisms of polyomavirus entry and nuclear targeting. Early work with the mouse polyomavirus and simian virus 40 (SV40) demonstrated that these virions were internalized into monopinocytotic vesicles which then accumulated at the nuclear membrane (12, 16). In some studies, viral particles were also seen in the nucleus, suggesting that the nucleus was the site of uncoating (7, 19, 21). More recent studies have shown that SV40 enters cells by receptor-mediated endocytosis into uncoated membrane-bound invaginations known as caveolae (1, 2, 24). An interaction between SV40 and major histocompatibility complex-encoded class I proteins induces the clustering of virus-receptor complexes into caveolin-rich membrane domains (5, 8, 24). Intracellular signals induced by SV40 binding to cells result in increased caveola-dependent endocytosis of the virus and delivery of the virions to the endoplasmic reticulum (9, 24). It is unclear how the viral genome is then targeted from the endoplasmic reticulum to the nucleus.
In this report, we studied the kinetics of JCV and SV40 infectious entry into human glial cells. Our results demonstrate that JCV rapidly enters glial cells and is completely internalized into a neutralizing antibody-resistant compartment within 30 min. SV40 entered glial cells with slightly delayed kinetics. We next asked whether JCV, like SV40, utilized caveolae to enter glial cells. Agents that disrupted caveola-dependent endocytosis significantly inhibited infection of glial cells by SV40 but had no effect on infectious entry of JCV. In contrast, agents that inhibited clathrin-dependent receptor-mediated endocytosis inhibited infection of glial cells by JCV but had no effect on SV40 infection. These results demonstrate that JCV and SV40 do not share similar mechanisms of internalization.
MATERIALS AND METHODS
Cells, virus, and antibody.
The human glial cell line SVG was established by transformation of human fetal glial cells by an origin-defective SV40 mutant and has been previously described (18). SVG cells were maintained in a humidified 37°C CO2 incubator in Eagle's minimum essential medium (Mediatech, Inc., Herndon, Va.) supplemented with 10% heat-inactivated fetal bovine serum (Mediatech, Inc.). The hybridoma PAB597, which produces an antibody to SV40 V antigen, was obtained from E. Harlow and maintained in RPMI 1640 Hybrimax medium (Sigma, St. Louis, Mo.) supplemented with 10% heat-inactivated fetal bovine serum (Mediatech, Inc.). The PAB597 monoclonal antibody has previously been shown to cross-react with JCV VP1 (6). Rabbit anti-SV40 antiserum and preimmune control antiserum were obtained from Lee Biomolecular Research, Inc. (San Diego, Calif.). Rabbit anti-JCV antiserum was prepared by injecting a New Zealand White rabbit with purified JCV in complete Freund's adjuvant (10). The rabbit was boosted twice with JCV in incomplete Freund's adjuvant. The antiserum was titered by enzyme-linked immunosorbent assay and a Western blot assay. Preimmune serum from this rabbit was used as a negative control. The hybrid Mad-1/SVEΔ virus was constructed by insertion of the regulatory region of SV40 into the regulatory region of the Mad-1 strain of JCV (Mad-1/SVE) (25). Propagation of Mad-1/SVE in human glial cells led to deletions and alterations exclusively in the regulatory region. The rearranged regulatory region contains the origin of replication, the TATA box and 78 bp of the first 98-bp repeat from JCV and one complete 72-bp repeat from SV40. Most of one of the 72-bp repeats and the 21-bp repeats from SV40 were deleted. The virus is termed Mad-1/SVEΔ to indicate this fact. A comparison of the restriction patterns of Mad-1/SVEΔ DNA with the prototype Mad-1 DNA were identical except for the regulatory region changes just discussed (25). No additional alterations were apparent following subsequent passage of Mad-1/SVEΔ in human fetal glial cells (25). We sequenced the VP1 gene of the chimeric virus, and it is identical to the published sequence of VP1 from the prototype Mad-1 strain (15).
Virus purification and labeling.
The preparation and labeling of JCV virions has been described (14). In brief, 108 SVG cells were infected with 3,200 hemagglutination units (HAU) of virus for 1 h at 37°C. At 3 weeks postinfection when the cells showed extensive cytopathic effect, they were removed from the dishes by scraping and pelleted by centrifugation at 960 × g for 30 min. The resulting cell pellet was suspended in 30 ml of the supernatant and subjected to three freeze-thaw cycles. Deoxycholic acid was then added to a concentration of 0.25%, and the suspension was incubated at 37°C for 1 h. Cell debris was removed by centrifugation at 1,960 × g, and the supernatants were layered on a cushion of cesium chloride (CsCl) (1.34 g/ml). Virus was banded by centrifugation for 24 h at 35,000 rpm in a SW55Ti rotor. This virus band was removed and dialyzed extensively against phosphate-buffered saline (PBS) (137 mM NaCl, 2.682 mM KCL, 8.1 mM Na2, HPO4, 1.47 mM KH2 PO4, [pH 7.2]). Purified virus was stored in 100-μl aliquots at −80°C. Virus titers were determined by hemagglutination assay. For virus labeling, 2.0 mg of gradient-purified JCV was dialyzed overnight in labeling buffer (0.05 M boric acid, 0.2 M NaCl [pH 9.2]). The virus was then incubated for 8 h at room temperature with 50 μl of a solution (5.0 mg/ml) of fluorescein isothiocyanate (FITC; Sigma) dissolved in dimethyl sulfoxide (DMSO; Sigma) (10). The FITC-labeled virus was purified by centrifugation over a cushion of cesium chloride. The FITC-labeled virus band was visualized with a handheld UV light, removed, and dialyzed extensively against PBS (pH 7.2). The ratio of FITC to protein was determined by spectrophotometry.
Kinetics of infectious entry.
SVG cells growing on coverslips were incubated with either 128 hemagglutination units (HAU) of JCV or 105 PFU/ml of SV40 for 30 min at 4°C. The cells were then washed three times in ice-cold PBS and shifted to 37°C in growth medium. Neutralizing concentrations of anti-JCV or anti-SV40 antiserum were then added to the cells at 0, 30, 60, 120, and 180 min following the shift to 37°C. Control coverslips were incubated with preimmune antiserum. At 3 days postinfection, the cells were fixed in acetone and the percentage of infected cells was scored by staining with an anti-V antigen monoclonal antibody.
Inhibition of infectious entry.
SVG cells growing on coverslips were incubated with phorbol 12-myristate 13-acetate (PMA) (10.0 μM in DMSO), nystatin (10 μg/ml in DMSO), or chlorpromazine (10 μg/ml in distilled water) for 45 min at 37°C. Control cells were incubated in medium with or without DMSO. The cells were then incubated with either JCV or SV40 for 4 h at 37°C in the continued presence of the drug. The cells were then washed three times in PBS and incubated with neutralizing concentrations of anti-JCV, anti-SV40, or preimmune control antiserum. Infected cells were scored at 3 days postinfection as described above.
Internalization of transferrin.
SVG cells were treated with PMA, nystatin, or chlorpromazine for 45 min at 37°C. The cells were then incubated with tetramethylrhodamine-conjugated transferrin (TRITC-transferrin) for 20 min at 37°C in the continued presence of the drug. Control cells were incubated with TRITC-transferrin in medium alone. The cells were then washed three times in PBS and fixed in PBS containing 2% paraformaldehyde for 1 h. The cells were washed twice in PBS and mounted onto slides with Vectashield mounting medium containing 4′,6′-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, Calif.). Fluorescence was visualized at a magnification of ×63 by laser-scanning confocal microscopy (LSM 410; Zeiss, Inc., Thornwood, N.Y.).
Colocalization of transferrin and JCV in endosomes.
SVG cells were incubated with TRITC-transferrin (35 μg/ml) and FITC-labeled JCV (FITC-JCV) for 20 min at 37°C. The cells were washed three times in PBS and fixed in PBS containing 2% paraformaldehyde. Cells were analyzed at a magnification of ×63 by laser-scanning confocal microscopy.
RESULTS
Kinetics of JCV and SV40 entry into human glial cells.
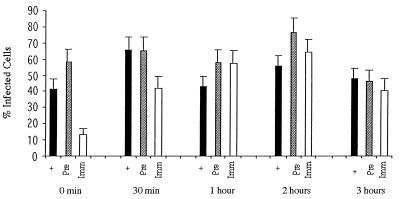
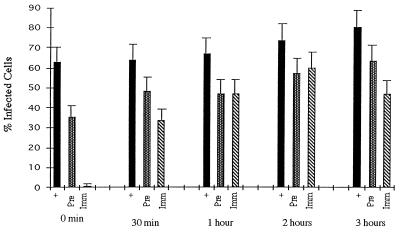
To study the kinetics of infectious viral entry, SVG cells were incubated with either JCV (128 HAU/ml; multiplicity of infection, approximately 10) or SV40 (105 PFU/ml; multiplicity of infection = 1.0) for 30 min at 4°C. Infection was initiated by warming the cells to 37°C. Neutralizing concentrations of anti-JCV, anti-SV40, or preimmune control serum was added to the cells at 0, 30, 60, 120, and 180 min following the shift to 37°C. Infection was scored at 3 days postinfection by indirect immunofluorescence analysis of V antigen-positive cells. The addition of neutralizing anti-JCV antiserum to the SVG cells at the time that they were shifted to 37°C resulted in a significant inhibition of infection (Fig. 1) (time zero). This same antiserum had a minimal inhibitory effect on infection when added 30 min following the shift to 37°C and had no significant effect if added at later time points (Fig. 1). Preimmune antiserum did not inhibit infection of SVG cells by JCV at any of the time points tested (Fig. 1). Similar to the results with JCV, neutralizing anti-SV40 antiserum inhibited infection of glial cells by SV40 if added immediately at the time of the shift to 37°C (Fig. 2) (time zero). This antiserum also inhibited infection if added at 30 min following the shift to 37°C but had little to no effect if added at later time points (Fig. 2). The preimmune antiserum had a slight inhibitory effect on infection of SVG cells by SV40 at all time points tested (Fig. 2).
FIG. 1.
Kinetics of JCV entry into human glial cells. JCV was preadsorbed to SVG cells for 30 min at 4°C, and then the cells were shifted to 37°C. At 0 min, 30 min, 1 h, 2 h, and 3 h following the shift to 37°C the cells were treated with either medium (+), preimmune antiserum (Pre), or anti-JCV antiserum (Imm). The percentage of infected cells were scored at 3 days postinfection by counting V antigen-positive cells by an indirect immunofluorescence assay. The error bars were derived by calculating the standard error of the mean of the average number of positive cells counted within one experiment. Experiments were repeated at least three times.
FIG. 2.
Kinetics of SV40 entry into human glial cells. SV40 was preadsorbed to SVG cells for 30 min at 4°C, and then the cells were shifted to 37°C. At 0 min, 30 min, 1 h, 2 h, and 3 h following the shift to 37°C the cells were treated with either medium (+), preimmune antiserum (Pre), or anti-SV40 antiserum (Imm). The percentage of infected cells were scored at 3 days postinfection by counting V antigen-positive cells by an indirect immunofluorescence assay. The error bars were derived by calculating the standard error of the mean of the average number of positive cells counted within one experiment. Experiments were repeated at least three times.
JCV infectious entry is inhibited by disruption of clathrin-mediated endocytosis but not by disruption of caveola-mediated endocytosis.
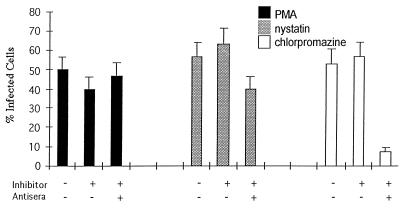
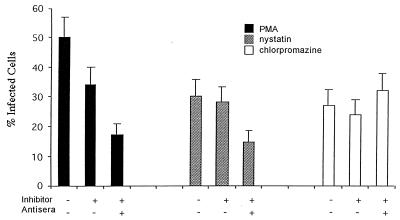
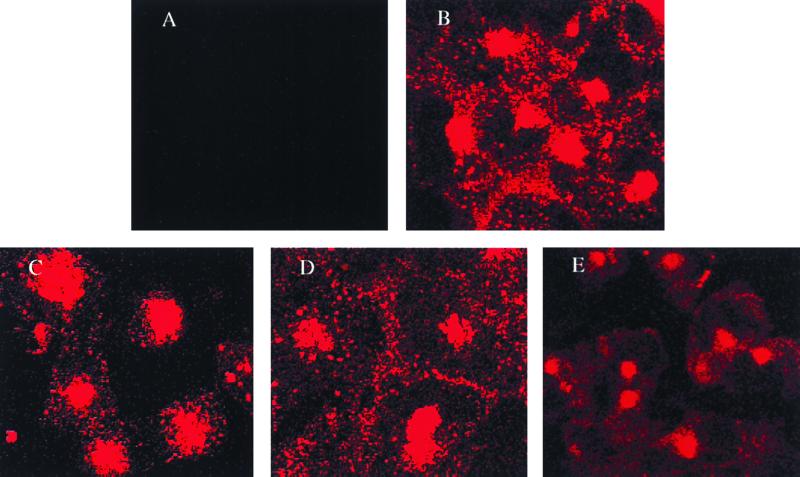
To determine whether JCV entered cells by a caveola-dependent pathway or by a clathrin-dependent pathway, SVG cells were treated with drugs that selectively inhibit each of these pathways. Treatment of the cells with PMA or nystatin, both of which inhibit caveola-dependent endocytosis, had no significant effect on infection of SVG cells by JCV (Fig. 3). In contrast, treatment of the cells with chlorpromazine, which inhibits clathrin-dependent endocytosis, significantly inhibited infection of the cells by JCV (Fig. 3). Chlorpromazine had no effect on JCV infection if added 24 h postinfection, indicating that the effect of chlorpromazine occurs at an early step in the life cycle of the virus (data not shown). In contrast to the results using JCV, nystatin and PMA both inhibited infection of SVG cells by SV40, whereas chlorpromazine was without effect (Fig. 4). As a control, we studied the effects of PMA, nystatin, and chlorpromazine on the internalization of TRITC-transferrin. Transferrin is known to be internalized by clathrin-dependent receptor-mediated endocytosis, which results in a characteristic punctuate pattern of staining in the cytoplasm. As expected, chlorpromazine, but not PMA or nystatin, inhibited the internalization of labeled transferrin (Fig. 5).
FIG. 3.
JCV infectious entry is inhibited by chlorpromazine but not by nystatin or PMA. SVG cells were either untreated or pretreated with PMA, nystatin, or chlorpromazine. The cells were then infected with JCV at 37°C for 4 h in the presence or absence of drug. JCV that remained bound to the cell surface was neutralized by the addition of anti-JCV antiserum. Preimmune antiserum was used as a negative control. The percentage of infected cells is indicated on the X axis. The error bars were derived by calculating the standard error of the mean of the average number of positive cells counted within one experiment. Experiments were repeated at least three times.
FIG. 4.
SV40 infectious entry is inhibited by nystatin and PMA, but not by chlorpromazine. SVG cells were either untreated or pretreated with PMA, nystatin, or chlorpromazine. The cells were then infected with JCV at 37°C for 4 h in the presence or absence of the drug. SV40 that remained bound to the cell surface was neutralized by the addition of anti-SV40 antiserum. Preimmune antiserum was used as a negative control. The percentage of infected cells is indicated on the x axis. The error bars were derived by calculating the standard error of the mean of the average number of positive cells counted within one experiment. Experiments were repeated at least three times.
FIG. 5.
Inhibition of the clathrin-dependent endocytic pathway by chlorpromazine. Clathrin-dependent receptor-mediated endocytosis of TRITC-transferrin was visualized in SVG cells that were either untreated or treated with PMA, nystatin, or chlorpromazine. (A) SVG cells alone; (B) SVG cells incubated with TRITC-transferrin in the absence of the drug; (C) SVG cells incubated with TRITC-transferrin in the presence of PMA; (D) SVG cells incubated with TRITC-transferrin in the presence of nystatin; (E) SVG cells incubated with TRITC-transferrin in the presence of chlorpromazine. Note the absence of endosomal staining (punctate cytoplasmic staining) in panel E compared to panels B to D.
Colocalization of JCV and transferrin in endosomes.
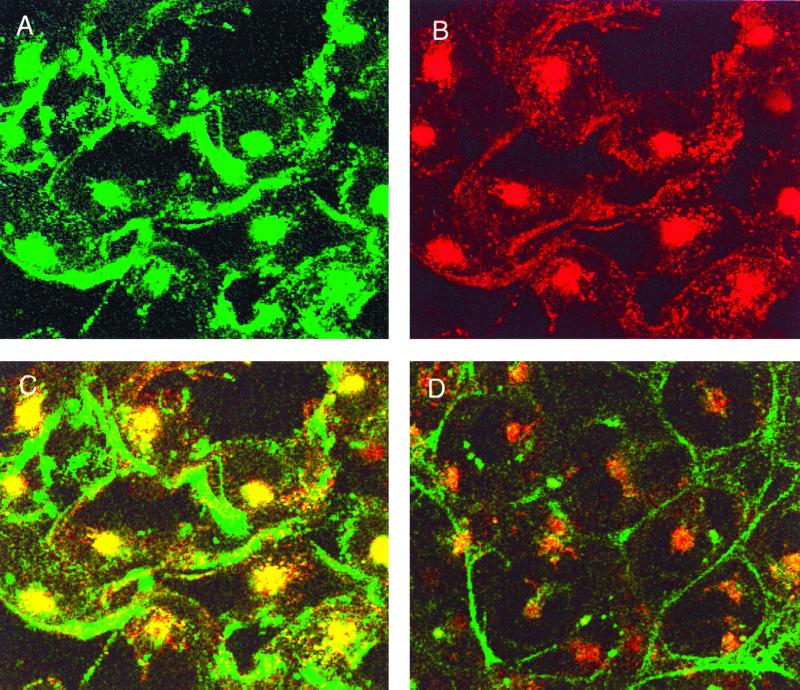
We next asked whether JCV and transferrin colocalize in endosomes. Cells were incubated with FITC-JCV and TRITC-transferrin for 20 min at 37°C. The cells were then fixed in 2% paraformaldehyde, mounted onto slides in mounting medium containing DAPI, and analyzed by conventional and laser-scanning confocal microscopy. FITC-JCV and TRITC-transferrin could both be seen in small punctate clusters within the cytoplasm as well as in larger clusters that were adjacent to the nucleus (Fig. 6, panels A and B). DAPI-stained nuclei were visualized using a conventional epifluorescent microscope (data not shown). An overlay of the red and green channels demonstrated that JCV colocalized with transferrin in endosomes (Fig. 6, yellow-orange color in panel C). As expected, colocalization was blocked by chlorpromazine (Fig. 6, panel D).
FIG. 6.
Colocalization of JCV and transferrin in endosomes. SVG cells were incubated with FITC-JCV and TRITC-transferrin for 20 min at 37°C. Cells were analyzed at a ×63 magnification by laser-scanning confocal microscopy. Images recorded under the FITC (green) (A) and TRITC (red) (B) channels, respectively, are shown. An overlay of the red and green channels to yield yellow where FITC-JCV and TRITC-transferrin colocalize is shown (C). An overlay of the red and green channels from cells that had been treated with chlorpromazine is shown (D).
DISCUSSION
To infect a glial cell, JCV must bind to specific cell surface receptors, penetrate the plasma membrane, and target its double-stranded DNA genome to the nucleus. We have previously characterized the first step in this process and have identified the JCV receptor as an N-linked glycoprotein containing terminal α(2-6)-linked sialic acids (15). To begin to address the subsequent steps of viral entry and nuclear targeting, we compared infectious entry of JCV with that of the related polyomavirus, SV40.
In our first experiment, we compared the kinetics of infectious entry of JCV and SV40. To do this, virus was allowed to adsorb to SVG cells for 30 min at 4°C, and then infection was initiated by warming the cells to 37°C. At various time points following the shift to 37°C, virus that remained at the cell surface was neutralized by the addition of anti-JCV or anti-SV40 antiserum. Infection was scored 3 days later by staining the cells with an anti-V antigen monoclonal antibody. This monoclonal was chosen as SVG cells constitutively express SV40 T antigen. Our results demonstrated that JCV entered into an antibody neutralization-resistant compartment within 30 min following the shift to 37°C. These data are consistent with our previous report showing that JCV enters into a trypsin-resistant compartment within 10 min postadsorption (28). SV40 entered cells with slightly delayed kinetics, which is consistent with other published studies (1).
We next used the antibody neutralization assay in conjunction with pharmacological agents to ask whether JCV infected cells by clathrin-dependent endocytosis or by caveola-dependent endocytosis. We used the drug chlorpromazine to inhibit clathrin-dependent endocytosis and PMA and nystatin to inhibit caveola-dependent endocytosis. Chlorpromazine is a cationic amphiphilic drug which prevents the recycling of clathrin and thus prevents endocytosis by clathrin-dependent mechanisms (11, 27). The phorbol ester PMA inhibits the caveola-dependent pathway by constitutively phosphorylating caveolin which is critical for internalization of these vesicles (3). The antibiotic nystatin is a known sterol binding agent and acts to remove membrane cholesterol, which is important for both the maintenance of caveolae and for the ability of caveolae to seal off from the plasma membrane (3, 13, 22). Note that PMA and nystatin had previously been shown to inhibit infectious entry of SV40 in monkey kidney cells (1). SVG cells that had been pretreated for 45 min with chlorpromazine, PMA, or nystatin were infected with either JCV or SV40 for 4 h at 37°C in the continued presence of the drug. The 4-h time point was chosen to allow sufficient time for both JCV and SV40 to completely internalize into an antibody neutralization-resistant compartment. Our data demonstrate that chlorpromazine, but not PMA or nystatin, inhibited infectious entry of JCV. In contrast, PMA and nystatin both inhibited infectious entry of SV40, whereas chlorpromazine was without effect. As both a positive and negative control for the specificity of the drugs, we tested whether chlorpromazine, PMA, or nystatin had any effect on the clathrin-dependent endocytosis of TRITC-transferrin. Only chlorpromazine inhibited uptake of labeled transferrin, which is consistent with its effects on clathrin-dependent endocytosis.
In a final experiment, FITC-JCV and TRITC-transferrin were colocalized in endosomes, indicating that JCV shares this clathrin-dependent pathway of endocytosis with transferrin. As expected, the colocalization of JCV and transferrin in endosomes was blocked by chlorpromazine.
Our data confirm that infectious entry of SV40 proceeds by caveola-dependent endocytosis and demonstrate that JCV, unlike SV40, infects cells by clathrin-dependent endocytosis. This is consistent with our previous data showing that JCV and SV40 do not share receptor specificity on human glial cells (14, 15). These results, when taken together, suggest that receptor usage dictates the endocytic pathway used by different polyomaviruses to penetrate the plasma membrane. The mechanisms that target the JCV genome to the nucleus are currently under investigation.
ACKNOWLEDGMENTS
This work was supported by Salomon Research grant 6-32263 and by Public Health Service grant CA71878 from the National Cancer Institute.
We thank Eugene O. Major for kindly providing the SVG cell line.
Mai T. Pho and Aarthi Ashok contributed equally to this work.
REFERENCES
- 1.Anderson H A, Chen Y, Norkin L C. Bound simian virus 40 translocates to caveolin enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol Biol Cell. 1996;7:1825–1834. doi: 10.1091/mbc.7.11.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson H A, Chen Y, Norkin L C. MHC class I molecules are enriched in caveolae but do not enter with simian virus 40. J Gen Virol. 1998;79:1469–1477. doi: 10.1099/0022-1317-79-6-1469. [DOI] [PubMed] [Google Scholar]
- 3.Anderson R G W. Caveolae: where incoming and outgoing messengers meet. Proc Natl Acad Sci USA. 1993;90:10909–10913. doi: 10.1073/pnas.90.23.10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atwood W J, Amemiya K, Traub R, Harms J, Major E O. Interaction of the human polyomavirus, JCV, with human B-lymphocytes. Virology. 1992;190:716–723. doi: 10.1016/0042-6822(92)90909-9. [DOI] [PubMed] [Google Scholar]
- 5.Atwood W J, Norkin L C. Class I major histocompatibility proteins as cell surface receptors for simian virus 40. J Virol. 1989;63:4474–4477. doi: 10.1128/jvi.63.10.4474-4477.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atwood W J, Wang L, Durham L C, Amemiya K, Traub R G, Major E O. Evaluation of the role of cytokine activation in the multiplication of JC virus (JCV) in human fetal glial cells. J Neurovirol. 1995;1:40–49. doi: 10.3109/13550289509111009. [DOI] [PubMed] [Google Scholar]
- 7.Barbanti-Brodano G, Swetly P, Koprowski H. Early events in the infection of permissive cells with simian virus 40: adsorption, penetration, and uncoating. J Virol. 1970;6:78–86. doi: 10.1128/jvi.6.1.78-86.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breau W C, Atwood W J, Norkin L C. Class I major histocompatibility proteins are an essential component of the simian virus 40 receptor. J Virol. 1992;66:2037–2045. doi: 10.1128/jvi.66.4.2037-2045.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Norkin L C. Extracellular simian virus 40 transmits a signal that promotes virus enclosure within caveolae. Exp Cell Res. 1999;246:83–90. doi: 10.1006/excr.1998.4301. [DOI] [PubMed] [Google Scholar]
- 10.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 11.Hunt R C, Marshall-Carlson L. Internalization and recycling of transferrin and its receptor: effect of trifluoperazine on recycling in human erythroleukemic cells. J Biol Chem. 1986;261:3681–3686. [PubMed] [Google Scholar]
- 12.Khare G, Consigli R. Multiplication of polyoma virus: use of selectively labeled (3H) virus to follow the course of infection. J Bacteriol. 1965;90:819–821. doi: 10.1128/jb.90.3.819-821.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lisanti M P, Tang Z, Sargiacomo M. Caveolin forms a hetero-oligomeric protein complex that interacts with an apical GPA-linked protein: implications for the biogenesis of caveolae. J Cell Biol. 1993;123:595–604. doi: 10.1083/jcb.123.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu C K, Hope A P, Atwood W J. The human polyomavirus, JCV, does not share receptor specificity with SV40 on human glial cells. J Neurovirol. 1998;4:49–58. doi: 10.3109/13550289809113481. [DOI] [PubMed] [Google Scholar]
- 15.Liu C K, Wei G, Atwood W J. Infection of glial cells by the human polyomavirus JC is mediated by an N-linked glycoprotein containing terminal α(2-6)-linked sialic acids. J Virol. 1998;72:4643–4649. doi: 10.1128/jvi.72.6.4643-4649.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackay R L, Consigli R A. Early events in polyoma virus infection: attachment, penetration, and nuclear entry. J Virol. 1976;19:620–636. doi: 10.1128/jvi.19.2.620-636.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Major E O, Amemiya K, Tornatore C S, Houff S A, Berger J R. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 1992;5:49–73. doi: 10.1128/cmr.5.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Major E O, Miller A E, Mourrain P, Traub R G, de Widt E, Sever J. Establishment of a line of human fetal glial cells that supports JC virus multiplication. Proc Natl Acad Sci USA. 1985;82:1257–1261. doi: 10.1073/pnas.82.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maul G G, Rovera A, Vorbrodt A, Abramczuk J. Membrane fusion as a mechanism for simian virus 40 entry into different cellular compartments. J Virol. 1978;28:936–944. doi: 10.1128/jvi.28.3.936-944.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monaco M G C, Atwood W J, Gravell M, Tornatore C S, Major E O. JCV infection of hematopoetic progenitor cells, primary B lymphocytes, and tonsillar stromal cells: implication for viral latency. J Virol. 1996;70:7004–7012. doi: 10.1128/jvi.70.10.7004-7012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishimura T, Kawai N, Kawai M, Notske K, Ichihara I. Fusion of SV40 induced endocytic vacuoles with the nuclear membrane. Cell Struct Funct. 1986;11:135–141. doi: 10.1247/csf.11.135. [DOI] [PubMed] [Google Scholar]
- 22.Rothberg K G, Ying Y S, Kamen B A, Anderson R G W. Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate. J Cell Biol. 1990;111:2931–2938. doi: 10.1083/jcb.111.6.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah K V. Polyomaviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2027–2043. [Google Scholar]
- 24.Stang E, Kartenbeck J, Parton R G. Major histocompatibility complex class I molecules mediate association of SV40 with caveolae. Mol Biol Cell. 1997;8:47–57. doi: 10.1091/mbc.8.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vacante D A, Traub R, Major E O. Extension of JC virus host range to monkey cells by insertion of a simian virus 40 enhancer into the JC virus regulatory region. Virology. 1989;170:353–361. doi: 10.1016/0042-6822(89)90425-x. [DOI] [PubMed] [Google Scholar]
- 26.Walker D L, Frisque R J. The biology and molecular biology of JC virus. In: Salzman N P, editor. The Papovaviridae. New York, N.Y: Plenum Press; 1986. pp. 327–377. [Google Scholar]
- 27.Wang L H, Rothberg K G, Anderson R G W. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993;123:107–117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei, G., C. K. Liu, and W. J. Atwood. JC virus binds to primary human glial cells, tonsillar stromal cells, and B-lymphocytes, but not to T-lymphocytes. J. Neurovirol., in press. [DOI] [PubMed]