Abstract
Simple Summary
Simple Summary: Wildlife in the Pantanal and Cerrado regions of Brazil face increasing threats, such as deforestation, urbanization, and road construction, which disrupt their natural habitats and increase the risk of diseases, including those that can spread to humans. The aim of this study was to characterize parasites affecting wild felids (large cats) in these areas, focusing on interactions among parasites, hosts, and the environment. The results provide a basis for the development of strategies to prevent the spread of disease and promote animal and human health. The use of advanced technology to monitor ecological changes and the importance of involving local communities in conservation efforts are emphasized. By integrating scientific research with public health measures and community engagement, this project aims to create sustainable solutions to protect biodiversity and public health. This is crucial for maintaining the ecosystem balance and ensuring the health of wildlife and nearby human populations.
Abstract
Environmental changes in the Brazilian Pantanal and Cerrado facilitate the spread of parasitic diseases in wildlife, with significant implications for public health owing to their zoonotic potential. This study aimed to examine the occurrence and diversity of gastrointestinal parasites in wild felids within these regions to assess their ecological and health impacts. We collected and analyzed helminth-positive samples from 27 wild felids using specific taxonomic keys. Diverse parasitic taxa were detected, including zoonotic helminths, such as Ancylostoma braziliense, Ancylostoma caninum, Ancylostoma pluridentatum, Toxocara cati, Toxocara canis, Dipylidium caninum, Taenia spp., Echinococcus spp., and Spirometra spp. Other nematodes, such as Physaloptera praeputialis and Physaloptera anomala, were identified, along with acanthocephalans from the genus Oncicola and a trematode, Neodiplostomum spp. (potentially the first record of this parasite in wild felids in the Americas). Human encroachment into natural habitats has profound effects on wild populations, influencing parasitic infection rates and patterns. This study underscores the importance of continuous monitoring and research on parasitic infections as a means of safeguarding both wildlife and human populations and highlights the role of wild felids as bioindicators of environmental health.
Keywords: biodiversity, conservation, helminthology, parasitism, public health, zoonosis
1. Introduction
The Cerrado and Pantanal are critical biomes in Brazil, renowned for their vast biodiversity. The Pantanal, an extensive wetland ecosystem, is distinguished by its seasonal flooding, which fosters a mosaic of aquatic and terrestrial habitats. This dynamic environment supports substantial populations of species that are rare or endangered elsewhere in South America. In contrast, the Cerrado is a conservation hotspot, characterized by its remarkable species richness and high levels of endemism. This expansive tropical savanna biome exhibits a complex mosaic of vegetation types, including grasslands, shrublands, and forests. The Cerrado’s sparse terrestrial vegetation and relatively dry climate further contribute to its unique ecological characteristics [1,2,3,4,5].
Human activities, notably urbanization and agricultural expansion, have profound effects on these biomes, leading to habitat fragmentation, and have substantial ecological consequences [6]. Fragmentation reduces the biodiversity and population sizes of native felid species; it impedes their ability to locate prey, suitable territories, and mating partners, thus intensifying intraspecific competition and affecting survival [7,8].
Anthropogenic activities, including deforestation, have been particularly rapid and aggressive in flat biomes, such as the Cerrado and Pantanal, endangering the viability of keystone species, such as the jaguar (Panthera onca) and ocelot (Leopardus pardalis). These species play critical roles in maintaining an ecological balance; however, they face severe survival challenges owing to environmental alterations [9,10].
Furthermore, human-induced environmental changes increase the incidence of diseases, notably zoonotic infections, with approximately 61% of human pathogens being zoonotic and 71.8% of emerging human diseases originating from wildlife [11,12,13,14]. Concurrently, the process of spillback or zooanthroponosis complicates wildlife conservation efforts, presenting a novel concern about transmission of pathogens from humans to wild animals [15].
In particular, urban, peri-urban and rural development in wild areas increase the risk of pathogen transmission between wildlife, domestic animals and humans. With the increasing interactions among humans, domestic animals, and wildlife, a greater “spillover” of diseases from domesticated animals to wildlife is expected to occur [16]. Furthermore, when these diseases spill over into wildlife, wild animals can become reservoirs and amplifiers of these diseases, posing a threat back to domestic animals and humans [17,18]. The increased prevalence of parasitic diseases necessitates studies of parasite distributions, occurrence, and transmission dynamics [16,19,20]. Although much of our understanding of disease spillover focuses on bacteria and viruses, larger parasites such as helminths are also important but have received less attention [21].
This study aimed to identify gastrointestinal helminths in wild felids of the Cerrado and Pantanal to enhance our understanding of the interactions between human activity and animal health, with a particular focus on parasites that have zoonotic potential. This study reveals that wild felids in the Brazilian Pantanal and Cerrado are hosts to a diverse range of gastrointestinal parasites, many of which have zoonotic potential and pose significant health risks to humans. Environmental changes driven by human activities, such as deforestation and urbanization, intensify these risks by altering natural habitats and facilitating increased interactions among wildlife, humans, and domestic animals. These findings highlight the urgent need for integrated wildlife conservation and public health strategies that address the ecological impacts of human encroachment to safeguard both ecosystem health and human well-being.
2. Materials and Methods
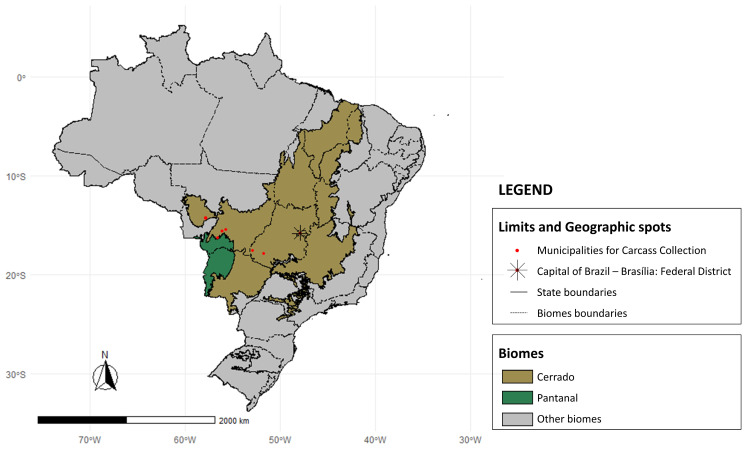
This study was performed in the Cerrado and Pantanal biomes of Brazil. Both biomes are located in central-western Brazil, with the Cerrado extending into the states of Goiás, Mato Grosso, Mato Grosso do Sul, Minas Gerais, Bahia, Maranhão, Piauí, Rondônia, Paraná, and São Paulo e Distrito Federal, while the Pantanal is primarily situated in Mato Grosso and Mato Grosso do Sul. The study area included the Cerrado regions within the states of Mato Grosso and Goiás, as well as the Pantanal region within Mato Grosso (Figure 1).
Figure 1.
Geographical distribution of carcass collection of wild felids sites and biomes in Brazil.
Wild felids from these biomes were analyzed after being retrieved from wildfires, from roadkill incidents on the peri-urban highways of southwest Goiás, or after dying during care at the Veterinary Hospital of the Federal University of Mato Grosso (UFMT) in 2020–2024. Some animals cared for at the Veterinary Hospital were from the UFMT Biological and Research Institute, which is located within the university campus (Cuiabá, Mato Grosso). The carcasses were subjected to parasitological necropsy, during which all gastrointestinal contents were sifted using a 0.2 mm mesh Tamis-type stainless-steel sieve. The filtrates were immediately inspected under a stereoscopic microscope to collect parasites. Helminth specimens were preserved in 70% ethanol.
For morphological identification, the nematodes were hydrated and clarified in either 50% glycerol or lactophenol solution for up to 24 h. For more robust helminths, a 90% phenol solution was used, according to the methodology outlined by Hoffmann [22]. Acanthocephalans and cestodes were stained with carmine acid, followed by decolorization in 0.5% hydrochloric acid (HCl) in 70% ethanol, sequential dehydration in a graded alcohol series, and clarification using eugenol (4-allyl-2-methoxyphenol), according to a modified protocol described by Amato [23].
Temporary microscopic slides were prepared and evaluated using an optical microscope (Zeiss Microscope AXIO Scope A1, Carl Zeiss, Oberkochen, Germany) at magnifications ranging from 100× to 400×. The morphological structures of the helminths were documented using TCapture Imaging software version 5.1.1.0. Taxonomic classification of the parasites relied on specific taxonomic keys and descriptions [24,25,26,27,28,29,30,31,32,33,34,35].
This study was approved by the Ethics and Animal Use Experimentation Committee at UFJ (CEUA/UFJ) under protocol number 004/2022 and by the Ethics Committee on Animal Research of the Federal University of Mato Grosso (CEUA protocol no. 23108.015878/2019-65). Additionally, procedures in this study were previously approved by the “Instituto Chico Mendes de Conservação da Biodiversidade” (ICMBio permit no. 84201-2 and 55104-1). This ensures compliance with ethical guidelines and registration standards, facilitating research aimed at enhancing our understanding of the parasitological effects on these endangered felid populations.
3. Results
3.1. Hosts and Municipalities
The animals included in this study represent a significant diversity of feline species inhabiting the Pantanal and Cerrado biomes. Leopardus pardalis (ocelot), Panthera onca (jaguar), Puma concolor (cougar), and Herpailurus yagouaroundi (jaguarundi) are native species to these biomes, each with distinct ecological habits and niches, providing a representative sample for the study of parasitic infestations in these environments. In total, four host species were studied. Brazil hosts 10 wild felid species, but two of these species do not occur in the Cerrado and Pantanal biomes.
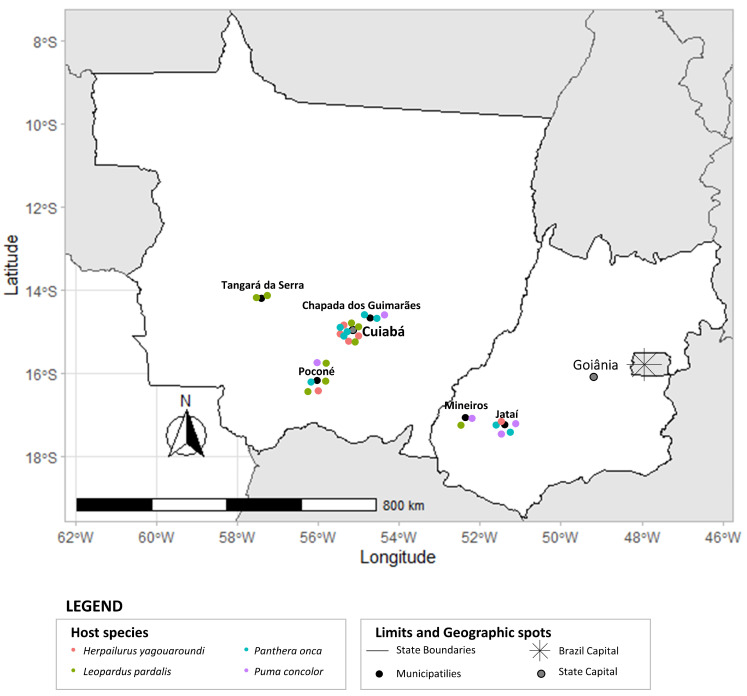
Infestations were detected in 27 wild felids, including L. pardalis (8), P. onca (8), P. concolor (5), and H. yagouaroundi (6), collected from peri-urban areas in the municipalities of Poconé (MT) in the Pantanal and Tangará da Serra (MT), Chapada dos Guimarães (MT), Cuiabá (MT), Jataí (GO), and Mineiros (GO) in the Cerrado biome (Figure 2).
Figure 2.
Municipalities in the Brazilian Cerrado and Pantanal biomes in which the remains of the wild felids were recovered for the analysis of gastrointestinal helminths are marked.
3.2. Parasite Identification and Load
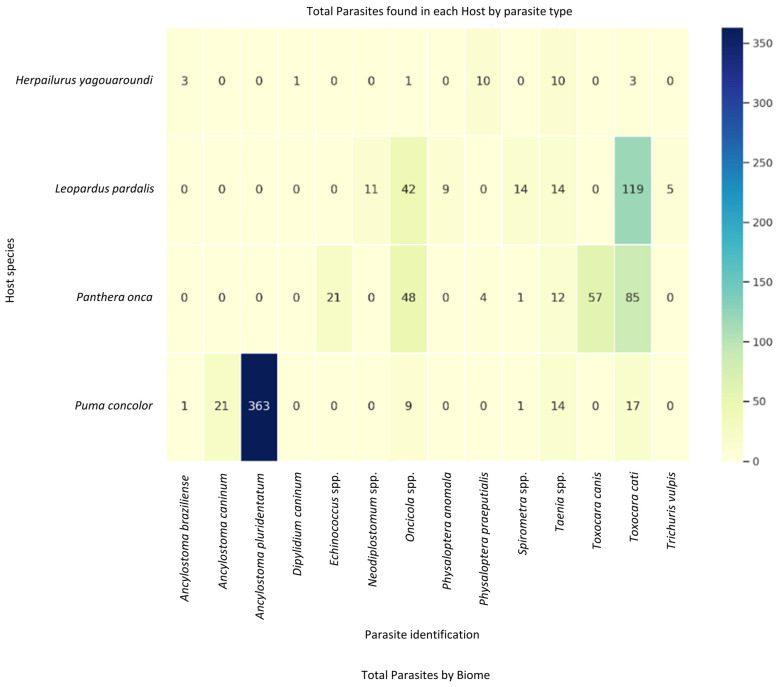
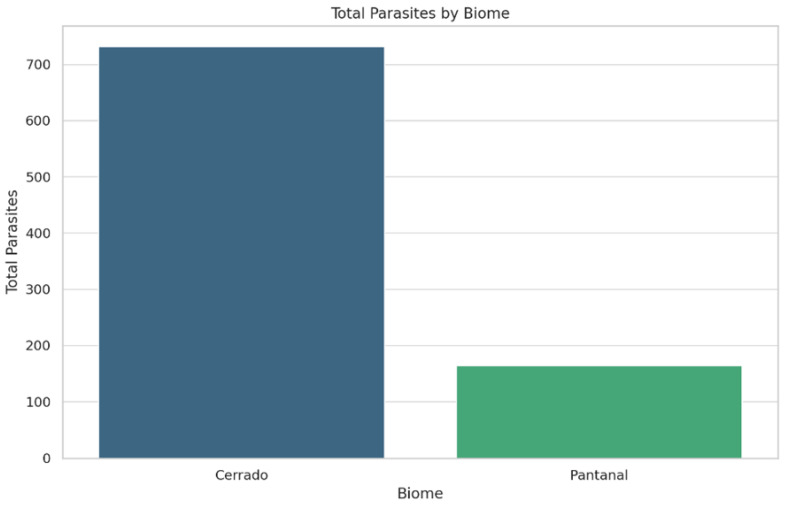
Comprehensive collection and taxonomic identification of 896 gastrointestinal helminth specimens from these felids revealed 14 species across 10 genera. The highest parasitic load was observed for Ancylostoma pluridentatum (n = 363) in H. yagouaroundi, followed by Toxocara cati in L. pardalis. In contrast, the lowest parasitic loads, with only one recovered specimen, were associated with Ancylostoma braziliense and Spirometra spp. in P. concolor, Dipylidium caninum and Oncicola spp. in H. yagouaroundi, and Spirometra spp. in P. onca (Figure 3). An analysis of the parasitic load distribution by biome indicated that parasite recovery was significantly higher in the Cerrado (n = 732 parasite by 21 hosts) than in the Pantanal (n = 164 by 6 hosts), and species diversity was higher in the Cerrado (n = 13 by 21 hosts) than in the Pantanal (n = 6 by 6 hosts; Figure 4).
Figure 3.
Heatmap of total parasites found in each host by parasite type and a bar plot of total parasites by biome. Light yellow indicates lowest range of parasite counts, indicating relatively minor infections, and dark blue represents the highest rang of parasite counts, indicating severe infections.
Figure 4.
Analysis of parasite–host–environment relationships. Total of parasite count by Biome (Cerrado and Pantanal).
3.3. Taxonomic Distribution
The genus Oncicola (Acanthocephala) was identified across all host species and in both studied biomes, with the highest parasitic loads in P. onca (n = 48) within Cerrado, and L. pardalis (n = 32) within the Pantanal. L. pardalis showed the highest frequency of parasitism by this genus (n = 4; Table 1).
Table 1.
Occurrence of parasitism in wild felids by host species, biome, and geographic locality.
| Host | Host ID | Biome | Location | Helminth Species | n(+) |
|---|---|---|---|---|---|
| Herpailurus yagouaroundi | UFJ-LPPV-156 | Pantanal | Poconé, MT | Physaloptera praeputialis | 10 |
| UFJ-LPPV-78 | Cerrado | Cuiabá, MT | Taenia spp. | 10 | |
| UFJ-LPPV-80 | Cerrado | Cuiabá, MT | Dipylidium caninum | 1 | |
| UFJ -LPPV-85 | Cerrado | Cuiabá, MT | Ancylostoma braziliense | 3 | |
| UFJ-LPPV-86 | Cerrado | Cuiabá, MT | Oncicola spp. | 1 | |
| UFJ-LPPV-304 | Cerrado | Jataí, GO | Toxocara cati | 3 | |
| Leopardus pardalis | UFJ-LPPV-133 | Pantanal | Poconé, MT | Spirometra spp. | 5 |
| Oncicola spp. | 4 | ||||
| UFJ-LPPV-154 | Pantanal | Poconé, MT | Toxocara cati | 5 | |
| UFJ-LPPV-155 | Pantanal | Poconé, MT | Physaloptera anomala | 9 | |
| Spirometra spp. | 9 | ||||
| Oncicola spp. | 32 | ||||
| UFJ-LPPV-216 | Cerrado | Tangará da Serra, MT | Oncicola spp. | 3 | |
| Taenia spp. | 14 | ||||
| UFJ-LPPV-303 | Cerrado | Cuiabá, MT | Toxocara cati | 6 | |
| Trichuris vulpis | 5 | ||||
| Neodiplostomum spp. | 11 | ||||
| UFJ-LPPV-302 | Cerrado | Cuiabá, MT | Toxocara cati | 75 | |
| UFJ-LPPV-301 | Cerrado | Cuiabá, MT | Toxocara cati | 24 | |
| Oncicola spp. | 3 | ||||
| UFJ-LPPV-75 | Cerrado | Mineiros, GO | Toxocara cati | 9 | |
| Panthera onca | UFJ-LPPV-140 | Pantanal | Poconé, MT | Toxocara cati | 62 |
| Toxocara canis | 51 | ||||
| UFJ -LPPV-77 | Cerrado | Chapada dos Guimarães, MT | Spirometra spp. | 1 | |
| UFJ-LPPV-79 | Cerrado | Cuiabá, MT | Taenia spp. | 12 | |
| UFJ-LPPV-82 | Cerrado | Jataí, GO | Toxocara cati | 10 | |
| UFJ-LPPV-81 | Cerrado | Jataí, GO | Toxocara cati | 5 | |
| Physaloptera praeputialis | 4 | ||||
| UFJ-LPPV-83 | Cerrado | Cuiabá, MT | Oncicola spp. | 48 | |
| UFJ-LPPV-84 | Cerrado | Chapada dos Guimarães, MT | Echinococcus spp. | 21 | |
| UFJ-LPPV-298 | Cerrado | Cuiabá, MT | Toxocara cati | 8 | |
| Toxocara canis | 6 | ||||
| Puma concolor | UFJ-LPPV-153 | Pantanal | Poconé, MT | Oncicola spp. | 9 |
| UFJ-LPPV-74 | Cerrado | Mineiros, GO | Toxocara cati | 1 | |
| Ancylostoma braziliense | 1 | ||||
| UFJ-LPPV-215 | Cerrado | Jataí, GO | Toxocara cati | 14 | |
| UFJ-LPPV-76 | Cerrado | Chapada dos Guimarães, MT | Ancylostoma pluridentatum | 363 | |
| Ancylostoma caninum | 21 | ||||
| UFJ-LPPV-299 | Cerrado | Jataí, GO | Taenia spp. | 14 | |
| Spirometra spp. | 1 | ||||
| Toxocara cati | 2 |
Nematodes, such as Ancylostoma, Toxocara, Physaloptera, and Trichuris, were also identified. Ancylostomatidae was found in one H. yagouaroundi and two P. concolor from the Cerrado, including A. braziliense and a co-infection involving A. pluridentatum and Ancylostoma caninum in P. concolor. Toxocara was the most frequently encountered helminth genus, with Toxocara cati or Toxocara canis present in all host species and biomes. T. cati was particularly pervasive and infected 12 hosts, whereas T. canis was observed exclusively in two P. onca. Physaloptera anomala, and Physaloptera praeputialis were detected on L. pardalis and H. yagouaroundi/P. onca, respectively. Trichuris vulpis was isolated from a single L. pardalis in the Cerrado (Table 1).
Cestodes were identified across three Cyclophyllidea genera (Echinococcus, Taenia, and Dipylidium) and one Pseudophyllidea genus (Spirometra), with occurrence noted in both biomes and across all studied host species. Spirometra spp. and Taenia spp. were present in four of the nine cestode-parasitized hosts, including a co-infection in P. concolor from the Cerrado. However, D. caninum and Echinococcus spp. were found only in one host from the Cerrado (Table 1). The only identified trematode genus, Neodiplostomum, was found in one L. pardalis sample from the Cerrado. The parasitic diversity across host species was uniform in L. pardalis, P. onca, and P. concolor, each hosting seven different parasite species, whereas H. yagouaroundi had six parasite species (Table 1).
3.4. Co-Infection Patterns
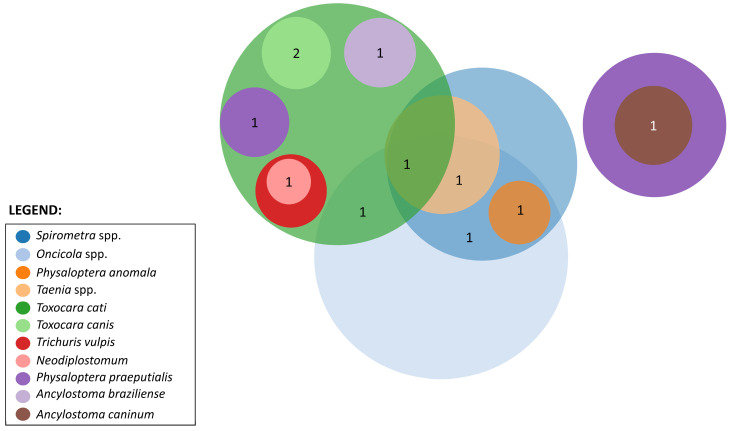
An analysis of the complex patterns of co-infection among wild felids revealed 10 unique co-infection schemas. Within the Cerrado biome, L. pardalis was susceptible to multiple parasitic combinations, including co-infections with T. cati, T. vulpis, and Neodiplostomum spp. as well as other associations, such as Oncicola spp., Taenia spp., and combinations of T. cati and Oncicola spp. Conversely, in the Pantanal biome, consistent co-infection patterns were observed in the same felid species, particularly Spirometra spp. and Oncicola spp. along with P. anomala and Spirometra spp. Different patterns of co-infections were also noted across other host species (Figure 5; Table 1).
Figure 5.
Venn diagram representation of helminth co-infections observed in the studied wild felids. Colors denote different helminth taxa, and the numbers indicate the frequency of observed co-infections among the studied wild felids.
Panthera onca exhibited two co-infections involving T. cati and T. canis in both biomes and one co-infection of T. cati and P. praeputialis in the Cerrado. Meanwhile, P. concolor showed co-infections of A. braziliense and T. cati; A. pluridentatum and A. caninum; and Taenia spp., Spirometra spp., and T. cati in the Cerrado. In contrast, no co-infections were observed in H. yagouaroundi (Figure 5; Table 1).
3.5. Morphological Insights
3.5.1. Acanthocephala
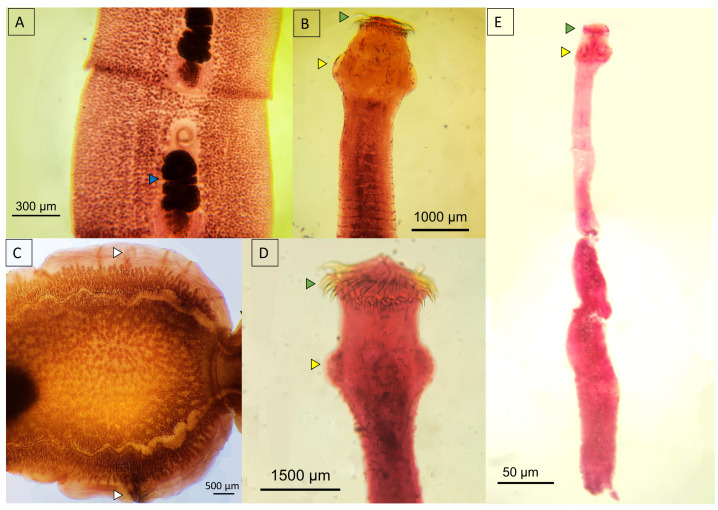
Oncicola spp. were characterized by a globular, cylindrical anterior trunk that elongates towards the rear and a retractable proboscis equipped with six rows containing six hooks each, devoid of a cervical collar, a feature that clearly separates them from the genus Prosthenorchis (Figure 6A). The proboscis of Oncicola spp. was anchored within a single-walled receptacle through a short neck. The lemnisci, which were long and tubular, extended towards the posterior trunk segment and were occasionally coiled (Figure 6B). In males, the anatomy included a copulatory bursa, two testicles (one anterior and one posterior), and cement glands (Figure 6A,B). Females were differentiated by a well-defined uterus that extended into a bell-shaped structure that progressed to the vagina and ultimately to the vulva.
Figure 6.
Micrograph of Oncicola spp. (A) Anterior portion of male Oncicola spp. recovered from Panthera onca; (B) posterior portion. Blue arrowhead—proboscis with hooks; black arrowhead—testis; yellow arrowhead—lemnisci; green arrowhead—cement glands.
3.5.2. Nematoda
In the genus Toxocara, the anterior segment was characterized by cervical alae, three prominent lips (Figure 7A–C), and as characteristic of the genera, it presents ventriculus that intercalated between the esophagus and the intestine, which is not present in the genus Toxascaris (Figure 7E,F). Sexual dimorphism was evident; males displayed externalized spicules that curved at the tail end and featured a digitiform process, whereas females possessed a tapered tail that lacked both curvature and a digitiform process (Figure 7D).
Figure 7.
Micrographs of Toxocara spp. detected in Panthera onca. (A) Cervical wing of Toxocara cati; (B) cervical wing of Toxocara canis; (C) anterior view of T. cati showing the three lips; (D) posterior view of a male T. canis with both spicules externalized. (E,F) Ventriculus that intercalated between the esophagus and the intestine in T. canis. Black arrowhead—lips; blue arrowhead—spicules; green arrowhead—digitiform process; white arrowhead—ventriculus.
Distinct morphological features among Toxocara spp. were primarily evident in the structure of the cervical alae. Toxocara canis exhibited narrower alae with gradual termination (Figure 7B), whereas T. cati had broader alae with abrupt, arrowhead-like terminations (Figure 7A). Shared traits were also observed, including digitiform processes in both species (Figure 7D).
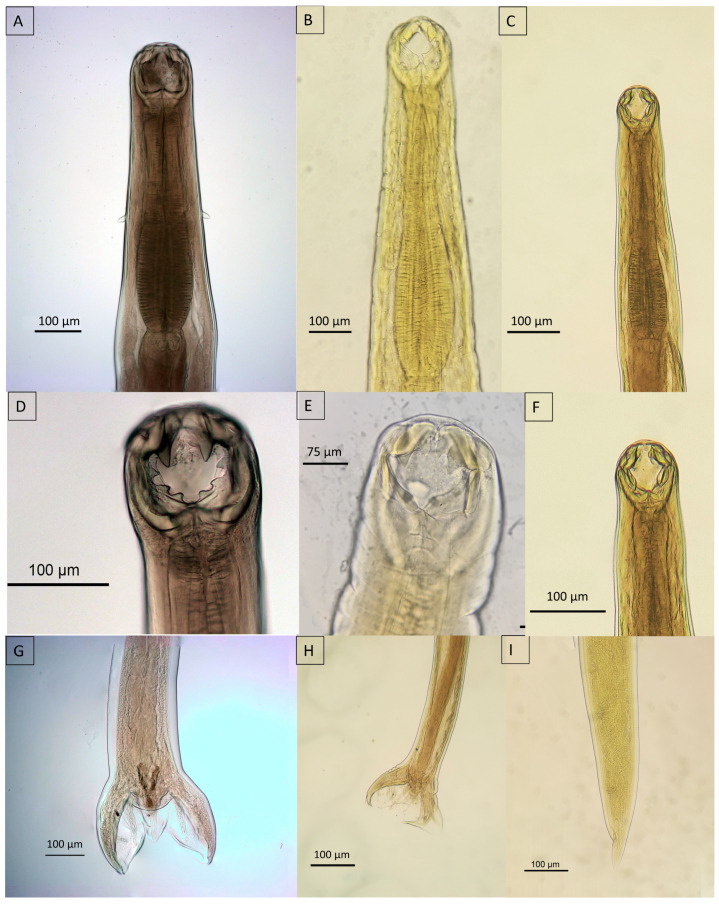
Ancylostomatids were characterized by well-defined buccal capsules and a muscular esophagus (Figure 8A–F). Sexual dimorphism within this genus was clearly observable; males exhibited a copulatory bursa and paired posterior spicules (Figure 8G,H), whereas females displayed a well-defined uterus, which may or may not contain eggs, and a tapered posterior end (Figure 8I). Differentiation among species within this genus is based on the placement, shape, and number of teeth within the buccal capsule as well as the characteristics of the dorsal rays in the male copulatory bursa.
Figure 8.
Micrograph of Ancilostomatids recovered from wild felids. (A,D) Anterior view of Ancylostoma pluridentatum from Puma concolor; (B,E) anterior view of Ancylostoma caninum from P. concolor; (C,F) anterior view of Ancylostoma braziliense from Herpailurus yagouarandi; (G) posterior view, male of A. pluridentatum; (H) posterior view, male of A. braziliense; (I) posterior view, female of A. caninum.
Ancylostoma pluridentatum was distinguished by the two pairs of ventral teeth that emerge from either side of the ventral dental plate. In the mature specimens, only the internal teeth were prominent at the opening of the buccal capsule. Moreover, this species featured three pairs of hook-shaped projections along the dorsal edge of the oral opening, distinguishing A. pluridentatum from other species within the genus (Figure 8A,D).
Ancylostoma caninum was characterized by a deep buccal capsule adorned with three pairs of teeth on each side of the ventral margin, complemented by a pair of triangular dorsal teeth. This morphological trait is common among ancylostomatids, including Ancylostoma buckleyi. However, A. caninum was uniquely identified by the presence of centrolateral teeth, which distinguished the species from its congeners (Figure 8B,E). Conversely, A. braziliense exhibited two dental plates, each bearing a single tooth (Figure 8C,F). A comparative analysis of the copulatory bursae revealed subtle variation among species, primarily in the length of the dorsal rays (Figure 8D,E).
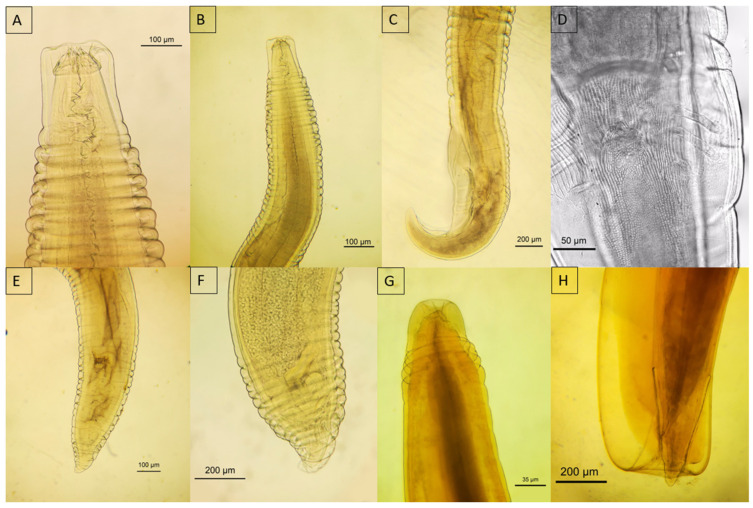
The genus Physaloptera was distinguished by two large triangular lateral lips equipped with teeth on the anterior extremity, and the anterior cuticle displayed a cephalic collar (Figure 9A,B). Sexual dimorphism within this genus was marked by the presence of caudal alae and sessile papillae in males (Figure 9C,D), whereas females exhibited well-defined uteri, which were prominently visible when containing eggs (Figure 9E,F).
Figure 9.
Micrograph of Physaloptera spp. recovered from wild felids. (A,B) Anterior portion of Physaloptera anomala from Leopardus pardalis; (C) posterior portion of male P. anomala; (D) showing the sessile papillae; (E) posterior portion of female P. anomala; (F) posterior end, part of the uterus filled with eggs; (G) anterior portion; (H) posterior portion of female showing the cuticular sheath of Physaloptera praeputialis in Herpailurus yagouarandi.
We detected P. praeputialis and P. anomala in H. yagouaroundi/P. onca and L. pardalis, respectively. Physaloptera praeputialis exhibited an anterior region featuring triangular lips with small teeth and a cuticular sheath that reflects forward at the anterior end, forming a preputial-like collar. The cuticle in both sexes extended posteriorly, forming a protective sheath that projects beyond the caudal terminus of the body (Figure 9G,H). This morphological configuration supports distinct ecological adaptations, facilitating survival and propagation of the parasite within its host.
Physaloptera anomala was characterized by the presence of three denticles on each lip (Figure 9A). Species differentiation was principally determined by the location and size of the sessile and pedunculate papillae in the posterior region of the male, together with the caudal bursa (Figure 9D). The arrangement of the pedunculated papillae included three pre-anal and one post-anal pair. Among the preanal papillae, the middle pair was positioned closest to the anus. The postanal region featured five pairs of papillae; the first and second pairs were small and aligned directly behind the anus, whereas the fourth and fifth pairs were larger and centrally located on the tail. The spatial distribution of these papillae was distinct; the gap between the third and fourth pairs was approximately four times that between the second and third pairs and double that between the fourth and fifth pairs.
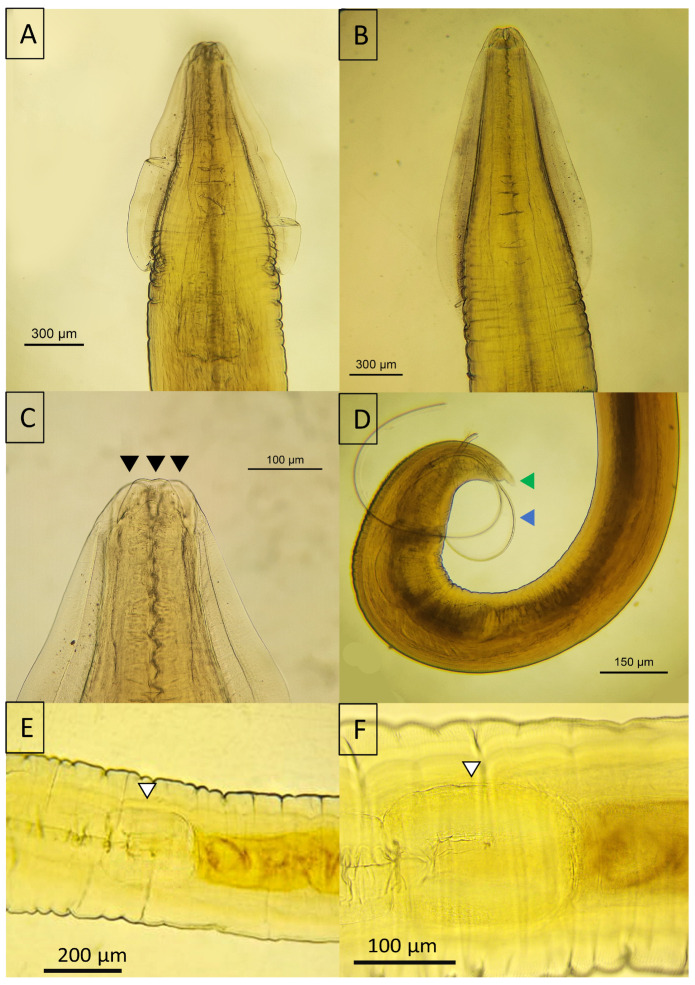
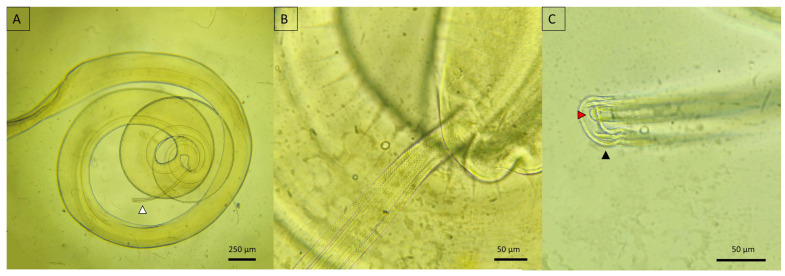
Trichuris vulpis exhibited a marked morphological disparity between its anterior and posterior segments, with the anterior part being significantly thinner and more elongated, resembling a whip. The cuticle of this region was marked by transverse striae with a longitudinal bacillary stripe along the ventral esophageal region. The posterior segment in males featured a spiraled configuration and was equipped with an evaginated spiny sheath (Figure 10). In females, the tail end was subtly curved, and gravid individuals displayed uteri laden with brownish eggs, each capped with an operculum at both ends.
Figure 10.
Micrograph of Trichuris vulpis recovered from wild felids. (A–C) Posterior portion of male T. vulpis from Leopardus pardalis; (B) highlighting the proximal portion of the sheath with spiny cuticle; (C) opening of the sheath with the tip of the spicule internalized. White arrowhead—spicule; black arrowhead—shealth; red arrowhead—tip of the spicule.
3.5.3. Cestoda
Spirometra spp. exhibited a dorsoventrally flattened scolex with attachment bothria and notably lacked hooks. The proglottids were organized such that the vagina is centrally located, and the uterus is uniform and spirally shaped, typically filled with eggs (Figure 11A).
Figure 11.
Micrograph of cestodes recovered from wild felids. (A) Mature proglottid of Spirometra sp. from Leopardus pardalis; (B,D) anterior portion of Taenia spp. from a Herpailurus yagouarandi and a Panthera onca, respectively; (C) gravid proglottid of Dipylidium caninum from H. yagouarandi; (E) Echinococcus sp. from P. onca. Blue arrowhead—uterus; yellow arrowhead—suckers; green arrowhead—hooks; white arrowhead—genital pore.
Dipylidium caninum was distinguished by its small scolex bearing four suckers and a rostellum equipped with multiple rows of hooks, enabling it to anchor firmly to the host’s intestinal mucosa. The proglottids of D. caninum were broader than they were long, and these dimensions were reversed during gravidity, adopting a barrel-like shape. Gravid proglottids were characterized by their segmentation into ovigerous sacs filled with eggs (Figure 11C), providing a clear indication of their reproductive status.
Species within the genus Taenia were distinguished by well-defined suckers on the scolex, and the examined specimens exhibited a rostellum equipped with hooks (Figure 11B,D). The body segments, or proglottids, were elongated and longer than they were wide, and each segment featured a hermaphroditic reproductive system that is characteristic of the genus. In contrast, Echinococcus spp. exhibited four suckers and a rostellum with hooks but included only three proglottids (Figure 11E), indicating a more streamlined morphological structure.
3.5.4. Trematoda
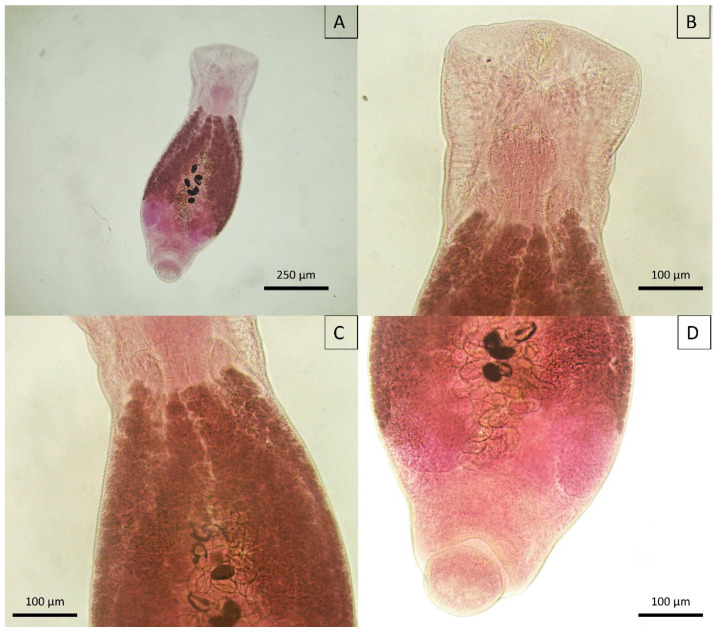
Neodiplostomum (syn. Fibricola) spp. displayed a dorsoventrally flattened body with a distinct morphological division; the posterior part was fusiform, and the anterior part was spatulate (Figure 12A–D). The cuticle was adorned with fine spines (Figure 12B) that tapered towards the posterior end. This species had two suckers: an oral sucker at the front of the body, used for attachment to the host, and a ventral sucker positioned further back. The distribution of vitelline follicles was limited and did not extend beyond the area between the ventral sucker and anterior testis (Figure 12B–D). The digestive system began with a small pre-pharynx, leading to a globular pharynx, which then split into a bifurcated esophagus, illustrating the specialized feeding structure of this trematode.
Figure 12.
Micrograph of Neodiplostomum (syn. Fibricola) spp. recovered from Leopardus pardalis. (A) Adult Neodiplostomum spp.; (B) anterior portion; (C) middle portion with eggs and vitelline glands; (D) posterior portion highlighting the testes.
4. Discussion
4.1. Parasite Occurrence and Consequences
Anthropogenic influences typically enhance the survival and proliferation of generalist helminths with direct life cycles, as these parasites are less dependent on a complex host system for life cycle completion than are specific helminths with heteroxenous cycles, which may be due to host scarcity [36]. This dynamic can be observed in our study, in which helminths from the genera Ancylostoma and Toxocara showed high frequencies.
Ancylostomatids, which are geohelminths with a direct lifecycle, are known to cause hemorrhagic gastroenteritis in carnivores. The primary mode of transmission is the oral–fecal route, which not only facilitates the spread of infection between hosts but also supports the occurrence of spillover and spillback events [37]. The detection of species such as A. braziliense and A. caninum in wild felids can possibly underscores their close interaction with human-altered landscapes that facilitates contacts of domestic and wild animals, highlighting the impact of anthropogenic activities on ecological health and parasite transmission dynamics [38,39,40].
These helminths are frequently found in both domestic and wild felids, indicating their broad zoonotic potential. Moreira et al. [41] documented the presence of Ancylostomatidae eggs in P. onca and L. pardalis in the Cerrado region of Brazil, underscoring the vulnerability of these wild species to parasites that are typically associated with domestic animals. Similar findings have been reported for other wild felids, such as P. concolor and Leopardus wiedii (margay), further illustrating the widespread nature of these infections [42,43].
The transmission of these parasites is often facilitated by environmental contamination through the dispersion of eggs from infected hosts [44]. For Toxocara spp., specifically T. canis and T. cati, which commonly infect wild carnivores, vertical transmission from the mother to offspring during gestation and via maternal milk plays a crucial role in maintaining the high prevalence and dissemination of these helminths [45,46]. Toxocara canis infection observed in felids may be correlated with the fact that these animals were from the UFMT Biological and Research Institute, where numerous dogs reside. This close interaction likely contributes to the occurrence and transmission of these helminths.
These infections have significant clinical implications. A. braziliense is frequently associated with dermatological conditions, whereas A. caninum is linked to eosinophilic enteritis and is a potential cause of diffuse unilateral subacute neuroretinitis in humans. These associations underline the close interrelationship between humans and domestic carnivores [47]. Infection with Toxocara spp. in humans can lead to granulomatous lesions in the eyes and neurological complications, including meningitis, encephalitis, myelitis, and cerebral vasculitis, as well as cutaneous and gastrointestinal issues [48,49,50]. Anthropization facilitates environmental interactions among dogs, cats, wildlife, and humans, thereby increasing the likelihood of cross-species transmission [51].
Physaloptera, a genus of parasitic nematodes within the order Spirurida and the family Physalopteridae, exhibits a broad geographic distribution and diverse hosts, including anteaters, jaguars, and pumas [52,53,54,55,56,57,58,59,60,61,62]. In Brazil, this parasite has been identified in native species, such as Cerdocyon thous (crab-eating fox) and Chrysocyon brachyurus (manned wolf) [43]. The life cycle of Physaloptera includes various arthropods as intermediate hosts, such as beetles, cockroaches, grasshoppers, and crickets [63,64,65], whereas reptiles, rodents, amphibians, and birds serve as paratenic hosts [66,67,68,69]. Wild carnivores may ingest these arthropods during periods of food scarcity or in response to hunting instincts triggered by arthropod movement [44,70]. Recent studies, such as Mendoza and Ortranto [71], suggest that Physaloptera infection in felids could represent a significant zoonotic risk, bridging the gap between wild and domestic species and potentially affecting humans through shared vectors or intermediate hosts. This highlights the need for ongoing research on parasite ecology and the implementation of preventive measures to curtail the risk of emerging zoonoses, particularly in areas where human habitation encroaches on natural habitats.
While T. vulpis is generally not considered a major zoonotic threat, its presence has been linked to visceral larva migrans syndrome and intestinal infections in humans [72,73,74,75]. Given that T. vulpis predominantly infects domestic animals, its detection in wild felids underscores the impact of human activities and the proximity of wild animals to peri-urban environments. This proximity leads to alterations in predator–prey dynamics, driving wild animals to encroach upon human settlements in search of food, thereby increasing exposure to parasites typically associated with domestic species [76].
Dipylidium caninum, a cestode affecting both domestic animals and humans, requires an arthropod in its life cycle. Infection in definitive hosts occurs through the ingestion of infected fleas, predominantly in the genera Ctenocephalides and Pulex, and lice in the genus Thricodectes [76,77]. Gravid proglottids of D. caninum, often found in the feces of hosts, are visually akin to rice grains during the shedding process [78]. Factors influencing the prevalence of D. caninum in domestic and wild carnivores include the host age and behaviors, such as shelter sharing, which can increase the susceptibility and severity of parasitism. In contrast, in wild carnivores, the prevalence of infection tends to decrease during periods of prey scarcity, particularly within migratory ecosystems, where fluctuations in prey availability are pronounced [79].
Research on the prevalence of gastrointestinal helminth infections in wild felids has demonstrated variation in infection rates. For instance, a study in London identified D. caninum as the second most prevalent helminth among 93 examined wild felids with a prevalence rate of 32.8% [80]. In contrast, a study in Australia reported a prevalence of only 2% in similar hosts [81]. Such variation may be attributed to the proximity of these animal habitats to urban environments, echoing the high prevalence rates among stray cats reported in studies of urban areas [82].
The genus Taenia presents significant zoonotic concerns. In wild felids, species such as Taenia taeniaeformis, Taenia pisiformis, Taenia omissa, Taenia macrocystis, and Taenia crassipoda have been documented in P. concolor, Leopardus geofroyi (Geofroy’s cat), and L. wiedii, among others [43,83,84,85]. Infection with these parasites can lead to cysticercosis in humans, highlighting the need for monitoring and preventive measures [8,86].
Within the genus Echinococcus, multiple species, including Echinococcus canadensis, Echinococcus felidis, Echinococcus multilocularis, Echinococcus oligarthrus, Echinococcus granulosus, Echinococcus shiquicus, and Echinococcus vogeli, have been identified in wild carnivores and possess significant zoonotic potential [87]. Notably, E. oligarthrus is prevalent among wild felids in South America, highlighting regional epidemiological trends that require ongoing monitoring and control efforts [43,88]. However, the morphological identification of E. oligarthrus and other species within genera Echinococcus is not precise, relying heavily on morphometric analysis, which itself can lack accuracy [86].
Although the life cycles and modes of transmission vary among species within the families Taeniidae and Diphyllobothriidae, they predominantly involve an indirect cycle linked to the ingestion of larval forms located in the musculature and subcutaneous tissues of intermediate hosts. This aspect underscores the integral role of carnivorous and hunting behaviors in facilitating parasitic infections in wild populations [86]. Several species within these groups have been implicated in parasitic zoonoses that pose significant health risks to humans and domestic animals [89].
Species in the genus Oncicola, including Oncicola campanulata, Oncicola chibigouzouensis, Oncicola oncicola, Oncicola lamacrurae, Oncicola venezuelensis, Oncicola magalhaesi, and Oncicola paracampanulata, are frequently found in wild felids [43,90,91,92]. A common challenge in the accurate identification of these species is the loss of characteristic hooks during parasite removal from the insertion tissue.
Oncicola spp. induce lesions within the small intestines of wild felids, potentially leading to substantial nutritional deficits. Under severe parasitism, coupled with food scarcity or weakened conditions, these infections may progress to serious disease or mortality [93,94]. Although the histopathological response to intestinal epithelial invasion by Oncicola spp. often involves minimal signs of inflammation, the frequent observation of collagenous tissue at the insertion sites indicates chronic infection stages [92].
The embedding of these parasites deep within the intestinal walls, penetrating the muscular layer, facilitates direct nutrient absorption through the body wall and lacunar channels in the hypodermis. The ecological and public health significance of Oncicola infections in wild felids is substantial and serves as an indicator of overall ecosystem health and biodiversity. Although these parasites are not directly transmissible to humans, their prevalence and severity in wild felids reflects broader environmental health dynamics [95].
Neodiplostomum, a genus associated with aquatic hosts, such as fish, birds, and certain small mammals, such as Hydromys chrysogaster (water rat or rakali), illustrates the impact of environmental changes, including deforestation and urbanization, on parasite–host dynamics [96,97,98]. These alterations influence the water distribution and, subsequently, the distribution of parasites. The observed morphological characteristics of this parasite in wild felids aligned with those of Neodiplostomum (syn. Fibricola) minor described by Dubois [35]. Molecular analyses are essential to confirm these findings; however, this observation may represent the first documented occurrence of this trematode in felids in the Americas.
Another species of Diplostomatidae, such as Alaria spp., has been described in felids of South America. However, the morphological traits of our helminth are not compatible with these species. Specifically, the forebody is no longer than the hindbody, there are no auricular pseudosuckers, the ovary is not located at the junction of the fore- and hindbody, and the vitellarium is not mainly confined to the forebody. Conversely, our helminth more closely resembles Neodiplostomum (syn. Fibricola) spp., which display a spatulate forebody and vitellarium usually located in the forebody but occasionally penetrating the hindbody [35].
4.2. Impacts of Environmental Changes and Co-Infections Considerations
In Brazil, wild felids are increasingly threatened by rapid environmental changes, including deforestation, urbanization, and road construction. These anthropogenic activities significantly alter ecological niches and reduce resource availability, affecting habitat stability and integrity [99,100,101]. As apex predators, felids play a fundamental role in controlling populations of their natural prey, thereby influencing the dynamics of the entire ecosystem [102]. Human-induced modifications to landscapes not only shrink natural habitats but also reduce the buffer zones between urban, rural, and wild areas, increasing the risk of interspecific parasite transmission, including those with zoonotic potential, and thereby altering parasite–host–environment dynamics [103,104].
Furthermore, the intersection of wild and human habitats, particularly in peri-urban zones, markedly increases the risk of zoonotic parasite transmission. This proximity enhances interactions between humans, domestic animals, and wildlife, consequently raising the potential for diseases such as echinococcosis (Echinococcus spp.), toxocariasis (Toxocara spp.), and other helminth infections [105,106]. Urban expansion further alters local ecological dynamics, influencing the distributions of hosts and vectors and reshaping patterns of parasitism [107].
Human activities may disrupt the ecological balance by facilitating the spread of vectors, intermediate hosts, and paratenic hosts of significant zoonotic parasites. This often results in higher concentrations of animal populations in confined areas, increasing the likelihood of completing the transmission cycles of these parasites [36].
Zhu et al. [108] investigated the influence of human population density and temperature variation on the prevalence of Toxoplasma gondii oocyst shedding by domestic and wild felids, illustrating how anthropogenic changes affect disease dynamics in these animal populations. This study highlights the complex interplay between environmental and anthropogenic factors that shape the epidemiological landscape of parasitic infections. Therefore, monitoring these parasites is critical to understanding ecological health and preventing zoonotic diseases. Comprehensive wildlife conservation and management strategies that prioritize ecosystem health are needed to mitigate the impacts of human encroachment and maintain biodiversity integrity.
The two biomes evaluated in this study, the Pantanal and the Cerrado, exhibit distinct ecological characteristics that support diverse parasitic communities. Environmental factors such as temperature, humidity, and seasonal variation significantly affect the prevalence and lifecycle dynamics of parasites [100]. In the Pantanal, periodic floods and drought distinctly modulate the occurrence of parasites, resulting in defined seasonal infection trends for species such as Spirometra spp. [100,106,109,110,111,112]. In contrast, the characteristics of the Cerrado influence the lifecycle of parasites that rely on direct contact with the soil or ingestion of infected prey, including Taenia spp. and Ancylostoma spp. [113,114,115].
The behaviors of wild felids, including their dietary habits and hunting ranges, determine their exposure to parasitic agents. Apex predators such as P. onca encounter a diverse array of parasites due to their varied diet, whereas smaller species such as H. yagouaroundi experience different parasitic risks due to their more restricted dietary habits, thus affecting the parasitic diversity observed within these hosts [13]. Immunological resilience and genetic diversity within feline populations are pivotal in determining their susceptibility or resistance to parasitic infections, potentially explaining the variability in the parasitic burden observed among hosts [116].
Co-infection with multiple parasitic species can significantly influence disease severity. The overall health impact of these infections can vary widely. Some combinations of parasites can even provide protective effects against severe disease manifestations, making it challenging to accurately assess the multispecies host–pathogen ecosystem [117,118,119,120,121]. However, it is important to note that our study relies on the necropsy of deceased animals, which could impact the results due to the decomposition of helminths. This may lead to an underestimation of infections and co-infections.
5. Conclusions
In our study, zoonotic species such as T. cati, T. canis, A. braziliense, A. caninum, D. caninum, and Echinococcus spp. were identified. Understanding the dynamics of parasitism in the Brazilian Pantanal and Cerrado requires a detailed examination of how parasites, hosts, and environmental factors interact. This interaction increases the potential for cross-species transmission of zoonotic pathogens, highlighting the urgent need for comprehensive monitoring programs. These programs should integrate animal health, public safety, and environmental conservation to effectively address the challenges posed by zoonotic diseases in these evolving ecosystems.
Author Contributions
Conceptualization, I.d.S.M., V.L.d.B.S., A.M.J., R.d.C.P. and D.G.d.S.R.; methodology, I.d.S.M., V.L.d.B.S., B.E.d.A.-S., A.P.N.G., N.F.d.U., V.B.A., R.d.S.G., K.V.A., Z.M.d.A.-S., L.F.d.S., E.A.Z., M.B.G., Í.A.B., K.C.S., E.M.C., A.M.J., R.d.C.P. and D.G.d.S.R.; validation, Í.A.B., K.C.S., E.M.C., A.M.J., R.d.C.P. and D.G.d.S.R.; formal analysis, I.d.S.M., V.L.d.B.S., B.E.d.A.-S., A.P.N.G., Í.A.B., K.C.S., E.M.C., A.M.J., R.d.C.P. and D.G.d.S.R.; investigation, I.d.S.M., V.L.d.B.S., B.E.d.A.-S., A.P.N.G., N.F.d.U., V.B.A., R.d.S.G., K.V.A., Z.M.d.A.-S., L.F.d.S., E.A.Z. and M.B.G.; resources, Í.A.B., K.C.S., E.M.C., A.M.J., R.d.C.P. and D.G.d.S.R.; writing—original draft preparation, I.d.S.M. and D.G.d.S.R.; writing—review and editing, I.d.S.M., Í.A.B., K.C.S., E.M.C., A.M.J., R.d.C.P. and D.G.d.S.R.; visualization, I.d.S.M.; supervision, A.M.J., R.d.C.P. and D.G.d.S.R.; project administration, D.G.d.S.R.; and funding acquisition, Í.A.B., K.C.S., E.M.C., A.M.J., R.d.C.P. and D.G.d.S.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was approved by the Ethics and Animal Use Experimentation Committee at UFJ (CEUA/UFJ) under protocol number 004/2022 and by the Ethics Committee on Animal Research of the Federal University of Mato Grosso (CEUA protocol no. 23108.015878/2019-65). Additionally, procedures in this study were previously approved by the “Instituto Chico Mendes de Conservação da Biodiversidade” (ICMBio permit no. 84201-2 and 55104-1). This ensures compliance with ethical guidelines and registration standards, facilitating research aimed at enhancing our understanding of the parasitological effects on these endangered felid populations.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for granting a scholarship to N.F.d.U., Z.M.d.A.S., A.M.J., and R.d.C.P., by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for granting a scholarship to I.d.S.M., V.L.d.B.S., B.E.A.d.S., R.d.S.G., and K.V.A, and by Fundação de Amparo à Pesquisa do Estado de Goiás (FAPEG) Grant number EQU2023101000018.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Junk W., Cunha C.N., Wantzen K., Petermann P., Strüssmann C., Marques M.I., Adis J. Biodiversity and its conservation in the Pantanal of Mato Grosso, Brazil. Aquat. Sci. 2006;68:278–309. doi: 10.1007/s00027-006-0851-4. [DOI] [Google Scholar]
- 2.Harris M.B., Tomas W., Mourão G., Silva C.J., Guimarães E., Sonoda F., Fachim E. Safeguarding the Pantanal Wetlands: Threats and Conservation Initiatives. Conserv. Biol. 2005;19:714–720. doi: 10.1111/j.1523-1739.2005.00708.x. [DOI] [Google Scholar]
- 3.Ratter J., Ribeiro J.F., Bridgewater S. The Brazilian Cerrado Vegetation and Threats to its Biodiversity. Ann. Bot. 1997;80:223–230. doi: 10.1006/anbo.1997.0469. [DOI] [Google Scholar]
- 4.Bortolotto I.M., Hiane P., Ishii I., Souza P.R., Campos R.P., Gomes R.B., Corrêa da Costa L.B.L. A knowledge network to promote the use and valorization of wild food plants in the Pantanal and Cerrado, Brazil. Reg. Environ. Chang. 2017;17:1329–1341. doi: 10.1007/s10113-016-1088-y. [DOI] [Google Scholar]
- 5.Lourival R., McCallum H., Grigg G., Arcângelo C., Machado R., Possingham H. A systematic evaluation of the conservation plans for the Pantanal wetland in Brazil. Wetlands. 2009;29:1189–1201. doi: 10.1672/08-118.1. [DOI] [Google Scholar]
- 6.Hernández-Camacho N., Zamora-Ledesma S. Mexican Fauna in the Anthropocene. Springer International Publishing; Berlin/Heidelberg, Germany: 2023. The potential of the parasite fauna as an indicator of ecosystem health in the anthropized environments of Mexico; pp. 569–579. [Google Scholar]
- 7.Fleschutz M.M., Gálvez N., Pe’er G., Davies Z.G., Henle K., Schüttler E. Response of a small felid of conservation concern to habitat fragmentation. Biodiv Conserv. 2016;25:1447–1463. doi: 10.1007/s10531-016-1118-6. [DOI] [Google Scholar]
- 8.Zanin M., Palomares F., Brito D. What we (don’t) know about the effects of habitat loss and fragmentation on felids. Oryx. 2015;49:96–106. doi: 10.1017/S0030605313001609. [DOI] [Google Scholar]
- 9.Eisenberg C., Eisenberg L. Jaguar (Panthera onca). The Carnivore Way: Coexisting with and Conserving North America’s Predators. Island Press; Washington, DC, USA: 2014. pp. 217–240. [Google Scholar]
- 10.Loarie S.R., Duffy P.B., Hamilton H., Asner G.P., Field C.B., Ackerly D.D. The velocity of climate change. Nature. 2009;462:1052–1055. doi: 10.1038/nature08649. [DOI] [PubMed] [Google Scholar]
- 11.Cui X., Fan K., Liang X., Gong W., Chen W., He B., Shen Y. Virus diversity, wildlife-domestic animal circulation and potential zoonotic viruses of small mammals, pangolins and zoo animals. Nat. Commun. 2023;14:2488. doi: 10.1038/s41467-023-38202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esposito M.M., Turku S., Lehrfield L., Shoman A. The impact of human activities on zoonotic infection transmissions. Animals. 2023;13:1646. doi: 10.3390/ani13101646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uribe M., Payán E., Brabec J., Vélez J., Taubert A., Chaparro-Gutiérrez J.J., Hermosilla C. Intestinal parasites of neotropical wild jaguars, pumas, ocelots, and jaguarundis in Colombia: Old friends brought back from oblivion and new insights. Pathogens. 2021;10:822. doi: 10.3390/pathogens10070822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perrucci S., Maestrini M., Coppola F., Di Marco M., Rosso A.D., Pacini M.I., Zintu P., Felicioli A. Gray wolf (Canis lupus italicus) and red fox (Vulpes vulpes) parasite survey in anthropized and natural areas of Central Italy. Vet. Sci. 2023;10:108. doi: 10.3390/vetsci10020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fagre A.C., Cohen L.E., Eskew E.A., Farrell M., Glennon E., Joseph M.B., Albery G.F. Assessing the risk of human-to-wildlife pathogen transmission for conservation and public health. Ecol. Lett. 2022;25:1534–1549. doi: 10.1111/ele.14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lempp C., Jungwirth N., Grilo M., Reckendorf A., Ulrich A., van Neer A., Bodewes R., Pfankuche V., Bauer C., Osterhaus A., et al. Pathological findings in the red fox (Vulpes vulpes), stone marten (Martes foina), and raccoon dog (Nyctereutes procyonoides), with special emphasis on infectious and zoonotic agents in Northern Germany. PLoS ONE. 2017;12:e0175469. doi: 10.1371/journal.pone.0175469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabozzi G., Bonizzi L., Crespi E., Somaruga C., Sokooti M., Tabibi R., Colosio C. Emerging Zoonoses: The “One Health Approach”. Saf. Health Work. 2012;3:77–83. doi: 10.5491/SHAW.2012.3.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackenstedt U., Jenkins D., Romig T. The role of wildlife in the transmission of parasitic zoonoses in peri-urban and urban areas. Int. J. Parasitol. Parasit. Wildl. 2015;4:71–79. doi: 10.1016/j.ijppaw.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devaux C.A., Mediannikov O., Medkour H., Raoult D. Infectious disease risk across the growing human population. Microb. Pathog. 2019;126:139–149. doi: 10.3389/fpubh.2019.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rainova I., Harizanov R., Kaftandjiev I., Tsvetkova N., Mikov O., Jordanova D. Zoonotic helminths in Bulgaria. Acta Zool. Bulg. 2019;71:279–283. [Google Scholar]
- 22.Hoffmann R.P. Diagnóstico de Parasitismo Veterinário. Porto Alegre; Sulina, Romania: 1987. [Google Scholar]
- 23.Amato J.F.R. Manual de Técnicas para a Preparacão de Coleções Zoológicas. Sociedade Brasileira de Zoologia; São Paulo, Brazil: 1985. 8. Platelmintos (temnocefálidos, trematódeos, cestóides, cestodários) e acantocéfalos. [Google Scholar]
- 24.Vicente J.J., Rodrigues H.O., Gomes D.C., Pinto R.M. Nematodes of Brazil. Part V: Nematodes of mammals. Rev. Bras. Zool. 1997;14((Suppl. 1)):1–452. doi: 10.1590/S0101-81751997000500001. [DOI] [Google Scholar]
- 25.Khalil L.F., Jones A., Bray R.A. Keys to the Cestode Parasites of Vertebrates. CABI International; London, UK: 1994. [Google Scholar]
- 26.Schmidt G.D. Revision of the Class Archiacanthocephala Meyer, 1931 (Phylum Acanthocephala), with Emphasis on Oligacanthorhynchidae Southwell et Macfie, 1925. J. Parasitol. 1972;58:290–297. doi: 10.2307/3278091. [DOI] [PubMed] [Google Scholar]
- 27.Gibson D.I., Jones A., Bray R.A. Keys to the Trematoda. Volume 2 CABI International; London, UK: 2002. [Google Scholar]
- 28.Anderson R.C., Chabaud A.G., Willmott S. Keys to the Nematode Parasites of Vertebrates. CABI International; London, UK: 2009. Archival Volume. [Google Scholar]
- 29.Gibbons L.M. Keys to the Nematode Parasites of Vertebrates. CABI International; London, UK: 2010. Supplementary Volume. [Google Scholar]
- 30.Thatcher V.E. Some hookworms of the genus Ancylostoma from Colombia and Panama. Proc. Helminthol. Soc. Wash. 1971;38:109–116. [Google Scholar]
- 31.Ortlepp M.A. The nematode genus Physaloptera. Rud. Proc. Zool. Soe. 1922;2:999–1107. [Google Scholar]
- 32.Ortlepp M.A. Some undescribed species ofthe nematode genus Physaloptera Rud., together with a key to the sufficiently known forms. Ondepstepoort J. Vet. Sei Anim. Indust. 1937;9:71–84. [Google Scholar]
- 33.Yevstafieva V.A., Kravchenko S.O., Gutyj B.V., Melnychuk V.V., Kovalenko P.N., Volovyk L.B. Morphobiological analysis of Trichuris vulpis (Nematoda, Trichuridae), obtained from domestic dogs. Regul. Mech. Biosyst. 2019;10:165–176. doi: 10.15421/021924. [DOI] [Google Scholar]
- 34.Yasuda F., Nishikawa H., Ujihashi M., Watanabe S. Studies on the life-history of Dipylidium caninum (Linnaeus, 1758). II. The ecology of the mature proglottids and the cellophane-tape method of diagnosis based on the biological characteristics of the proglottids. Bull. Nippon. Vet. Zootech. Coll. 1970;18:122–128. [Google Scholar]
- 35.Dubois G. Étude de quelques strigéidés d’Australie et notes sur le genre Fibricola Dubois 1932. Ann. Parasitol. Hum. Comp. 1937;15:231–247. doi: 10.1051/parasite/1937153231. [DOI] [Google Scholar]
- 36.Carrera-Játiva P.D., Acosta-Jamett G. Influence of habitat alteration on the structure of helminth communities in small mammals: A systematic review and critical appraisal of theory and current evidence. Parasitol. Res. 2023;122:1053–1070. doi: 10.1007/s00436-023-07804-8. [DOI] [PubMed] [Google Scholar]
- 37.Foster G.W., Cunningham M.W., Kinsella J.M., McLaughlin G., Forrester D.J. Gastrointestinal helminths of free-ranging Florida panthers (Puma concolor coryi) and the efficacy of the current anthelmintic treatment protocol. J. Wildl. Dis. 2006;42:402–406. doi: 10.7589/0090-3558-42.2.402. [DOI] [PubMed] [Google Scholar]
- 38.Quintana T., Johnson W.L., Ritchie D., Smith V., Martin K.A., McMahan K., Brewer M., Jesudoss Chelladurai J.J. Genetic characterization of the zoonotic parasite Ancylostoma caninum in the central and eastern United States. J. Helminthol. 2023;97:e37. doi: 10.1017/S0022149X23000159. [DOI] [PubMed] [Google Scholar]
- 39.Stocker T., Scott I., Šlapeta J. Unambiguous identification of Ancylostoma caninum and Uncinaria stenocephala in Australian and New Zealand dogs from faecal samples. Austral. Vet. J. 2023;101:373–376. doi: 10.1111/avj.13272. [DOI] [PubMed] [Google Scholar]
- 40.Evason M.D., Weese J.S., Polansky B.J., Leutenegger C.M., Evason M.D. Emergence of canine hookworm treatment resistance: Novel detection of Ancylostoma caninum anthelmintic resistance markers by fecal PCR in 11 dogs from Canada. Am. J. Vet. Res. 2023;84:ajvr.23.05.0116. doi: 10.2460/ajvr.23.05.0116. [DOI] [PubMed] [Google Scholar]
- 41.Moreira R.M.P., Aires C.G., Alves-Sobrinho A.V., Moraes I.S., Moreira C.N., Amaral A.V.C., Saturnino K.C., Braga Í.A., de Campos Pacheco R., de Souza Ramos D.G. Gastrointestinal parasites of wild carnivores from conservation institutions in the Cerrado of Goiás, Brazil. Braz. J. Vet. Parasitol. 2023;32:e004823. doi: 10.1590/s1984-29612023028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noronha D., Vicente J.J., Pinto R.M. A survey of new records for nematodes from mammals deposited in the Helminthological collection of the Institute Oswaldo Cruz (CHIOC) Rev. Bras. Zool. 2002;19:945–949. doi: 10.1590/S0101-81752002000300032. [DOI] [Google Scholar]
- 43.Vieira F.M., Luque J.L., Muniz-Pereira L.C. Checklist of helminth parasites in wild carnivore mammals from Brazil. Zootaxa. 2008;1721:1–23. doi: 10.11646/zootaxa.1721.1.1. [DOI] [Google Scholar]
- 44.Santos M.J., Pinto B.M., Santos-Reis M. Trophic niche partitioning between two native and two exotic carnivores in SW Portugal. Web. Ecol. 2007;7:53–62. doi: 10.5194/we-7-53-2007. [DOI] [Google Scholar]
- 45.Coati N., Schnieder T., Epe C. Vertical transmission of Toxocara cati Schrank 1788 (Anisakidae) in the cat. Parasitol. Res. 2004;92:142–146. doi: 10.1007/s00436-003-1019-y. [DOI] [PubMed] [Google Scholar]
- 46.Maciag L., Morgan E.R., Holland C. Toxocara: Time to let cati ‘out of the bag’. Trends Parasitol. 2022;38:280–289. doi: 10.1016/j.pt.2021.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Bowman D.D., Montgomery S.P., Zajac A.M., Eberhard M.L., Kazacos K.R. Hookworms of dogs and cats as agents of cutaneous larva migrans. Trends Parasitol. 2010;26:162–167. doi: 10.1016/j.pt.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Gavignet B., Piarroux R., Aubin F., Millon L., Humbert P. Manifestações cutâneas da toxocaríase humana. J. Am. Acad. Dermatol. 2008;59:1031–1042. doi: 10.1016/j.jaad.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 49.Ma G., Holland C.V., Wang T., Hofmann A., Fan C.K., Maizels R.M., Hotez P.J., Gasser R.B. Human toxocariasis. Lancet Infect. Dis. 2018;18:e14–e24. doi: 10.1016/S1473-3099(17)30331-6. [DOI] [PubMed] [Google Scholar]
- 50.Axelerad A.D., Stroe A.Z., Gogu A.E., Pusztai A., Jianu D.C., Daniel D., Axelerad D.D. Espectro clínico de sintomas em toxocaríase cerebral. Exper. Ther. Med. 2020;21:1–11. [Google Scholar]
- 51.Chihai O., Rusu Ş., Tălămbuţă N., Nistreanu V., Larion A., Savin A., Naforniţa N. Parasite fauna diversity in Red Fox (Vulpes vulpes) from natural and anthropized ecosystems of the Republic of Moldova. Sustainable Use and Protection of Animal World in the Context of Climate Change; Proceedings of the Xth International Conference of Zoologists; Chisinau, Moldova. 16–17 September 2021; pp. 180–186. [Google Scholar]
- 52.Hayasaki M., Ohishi I., Munakata A. Incidence of stomach worm, Physaloptera praeputialis von Linstow, 1889, in two cats and a dog in Tokyo, Japan. JAPN J. Parasitol. 1982;31:499–506. [Google Scholar]
- 53.Rausch R.L., Maser C., Hoberg E.P. Gastrointestinal helminths of the cougar, Felis concolor L., in northeastern Oregon. J. Wildl. Dis. 1983;19:14–19. doi: 10.7589/0090-3558-19.1.14. [DOI] [PubMed] [Google Scholar]
- 54.Scholz T., Uhlirova M., Ditrich O. Helminth parasites of cats from the Vientiane province, Laos, as indicators of the occurrence of causative agents of human parasitosis. Parasite. 2003;10:343–350. doi: 10.1051/parasite/2003104343. [DOI] [PubMed] [Google Scholar]
- 55.Naem S., Farshid A.A., Marand V.T. Pathological findings on natural infection with Physaloptera praeputialis in cats. Vet. Arch. 2006;76:315–321. [Google Scholar]
- 56.Guerrero J.H.M., Solis M.E.P., Ramos J.J.Z., Alferez F.R., Casio H.H. Report of Physaloptera praeputialis (Von Linstow) in mountain lion (Puma concolor, Linneaus, 1771) J. Animal. Vet. Adv. 2010;9:601–603. [Google Scholar]
- 57.Borji H., Razmi G., Ahmadi A., Karami H., Yaghfoori S., Abedi V. A survey on endoparasites and ectoparasites of stray cats from Mashhad (Iran) and association with risk factors. J. Parasit. Dis. 2011;35:202–206. doi: 10.1007/s12639-011-0057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cantó G.J., García M.P., García A., Guerrero M.J. The prevalence and abundance of helminth parasites in stray dogs from the city of Queretaro in central Mexico. J. Helminthol. 2011;85:263–269. doi: 10.1017/S0022149X10000544. [DOI] [PubMed] [Google Scholar]
- 59.Torres F.D., Otranto D. Dogs, cats, parasites, and humans in Brazil: Opening the black box. Parasite Vectors. 2014;7:22. doi: 10.1186/1756-3305-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quadros R.M., Marques S.M.T., Moura A.B.D., Antonelli M. First arasitosthe nematode Physaloptera praeputialis parasitizing a Jaguarandi. Neotrop. Biol. Conserv. 2014;9:186–189. [Google Scholar]
- 61.Hajipour N., Imani Baran A., Yakhchali M., Banan Khojasteh S.M., Sheikhzade Hesari F., Esmaeilnejad B., Arjmand J. A survey study on gastrointestinal parasites of stray cats in Azarshahr (East Azerbaijan province, Iran) J. Parasit. Dis. 2016;40:1255–1260. doi: 10.1007/s12639-015-0663-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramos D.G.S., Santos A.R.G.L.O., Freitas L.C., Correa S.H.R., Kempe G.V., Morgado T.O., Aguiar D.M., Wolf R.W., Rossi R.V., Sinkoc A.L., et al. Endoparasites of wild animals from three biomes in the state of Mato Grosso, Brazil. Braz. J. Vet. Animal Sci. 2016;68:571–578. doi: 10.1590/1678-4162-8157. [DOI] [Google Scholar]
- 63.Hobmaier M. Extramammalian phase of Physaloptera maxillaris Molin, 1860 (Nematoda) J. Parasitol. 1941;27:233. doi: 10.2307/3273016. [DOI] [Google Scholar]
- 64.Petri L.H., Ameel D.J. Studies on the life cycle of Physaloptera rara Hall and Wigdor, 1918 and Physaloptera praeputialis Linstow, 1889. J. Parasitol. 1950;36:40. [Google Scholar]
- 65.Goldberg S.R., Bursey C.R. Gastrointestinal helminths of seven gekkonid lizard species (Sauria: Gekkonidae) from Oceania. J. Nat. Hist. 2002;36:2249–2264. doi: 10.1080/00222930110069069. [DOI] [Google Scholar]
- 66.Widmer E.A. Development of third-stage Physaloptera larvae from Crotalus viridis Rafinesque, 1818 in cats with notes on pathology of the larvae in the reptile. J. Wildl. Dis. 1970;6:89–93. doi: 10.7589/0090-3558-6.2.89. [DOI] [PubMed] [Google Scholar]
- 67.Cawthorn R.J., Anderson R.C. Development of Physaloptera maxillaris (Nematoda: Physalopteroidea) in skunk (Mephitis mephitis) and the role of paratenic and other hosts in its life cycle. Can. J. Zool. 1976;54:313–323. doi: 10.1139/z76-035. [DOI] [PubMed] [Google Scholar]
- 68.Velikanov V.P., Sharpillo V.P. Experimental identification of Physaloptera praeputialis and Pterygodermatites cahirensis larvae (Nematoda, Spirurata) from paratenic hosts. Vestn. Zool. 2002;6:25–29. (In Russian) [Google Scholar]
- 69.Kalyanasundaram A., Henry C., Brym M.Z., Kendall R.J. Molecular identification of Physaloptera sp. from wild northern bobwhite (Colinus virginianus) in the Rolling Plains ecoregion of Texas. Parasitol. Res. 2018;117:2963–2969. doi: 10.1007/s00436-018-5993-5. [DOI] [PubMed] [Google Scholar]
- 70.Seibold S., Gossner M.M., Simons N.K., Blüthgen N., Müller J., Ambarli D., Ammer C., Bauhus J., Fischer M., Habel J.C., et al. Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature. 2019;574:671–674. doi: 10.1038/s41586-019-1684-3. [DOI] [PubMed] [Google Scholar]
- 71.Mendoza Roldan J., Otranto D. Zoonotic parasites associated with predation by dogs and cats. Parasite Vectors. 2023;16:55. doi: 10.1186/s13071-023-05670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hall J.E., Sonnenberg B. An apparent case of human infection with the whipworm of dogs, Trichuris vulpis (Froelich 1789) J. Parasitol. 1956;42:197–199. doi: 10.2307/3274735. [DOI] [PubMed] [Google Scholar]
- 73.Dunn J.J., Columbus S.T., Aldeen W.E., Davis M., Carroll K.C. Trichuris vulpis recovered from a patient with chronic diarrhea and five dogs. J. Clin. Microbiol. 2002;40:2703–2704. doi: 10.1128/JCM.40.7.2703-2704.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kirkova Z., Georgieva D., Raychev E. Study on the prevalence of trichurosis in different categories of dogs and wild carnivores. Bulg. J. Vet. Vet. Med. 2006;9:141–147. [Google Scholar]
- 75.Traversa D. Are we paying too much attention to cardio-pulmonary nematodes and neglecting old-fashioned worms like Trichuris vulpis? Parasit. Vectors. 2011;4:32. doi: 10.1186/1756-3305-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bartsocas C.S., Von Graevenitz A., Blodgett F. Dipylidium infection in a 6-month-old infant. J. Pediatr. 1966;69:814–815. doi: 10.1016/S0022-3476(66)80132-4. [DOI] [PubMed] [Google Scholar]
- 77.Neira O.P., Jofré M.L., Muñoz S.N. Infection by Dipylidium caninum: Case presentation and literature review. Chil. J. Infectol. 2008;25:465–471. [PubMed] [Google Scholar]
- 78.Benitez-Bolivar P., Rondón S., Ortiz M., Díaz-Díaz J., León C., Riveros J., Molina H., González C. Morphological and molecular characterization of the parasite Dipylidium caninum infecting an infant in Colombia: A case report. Parasit. Vectors. 2022;15:463. doi: 10.1186/s13071-022-05573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.East M.L., Kurze C., Wilhelm K., Benhaiem S., Hofer H. Factors influencing Dipylidium sp. infection in a free-ranging social carnivore, the spotted hyena (Crocuta crocuta) Int. J. Parasitol. Parasites Wildl. 2013;2:257–265. doi: 10.1016/j.ijppaw.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nichol S., Ball S.J., Snow K.R. Prevalence of intestinal parasites in feral cats in some urban areas of England. Vet. Parasitol. 1981;9:107–110. doi: 10.1016/0304-4017(81)90028-5. [DOI] [PubMed] [Google Scholar]
- 81.Coman B.J., Jones E.H., Driesen M.A. Helminth parasites and arthropods of feral cats. Austral. Vet. J. 1981;57:324–327. doi: 10.1111/j.1751-0813.1981.tb05837.x. [DOI] [PubMed] [Google Scholar]
- 82.Waap H., Gomes J., Nunes T. Parasite communities in stray cat populations from Lisbon, Portugal. J. Helminthol. 2014;88:389–395. doi: 10.1017/S0022149X1300031X. [DOI] [PubMed] [Google Scholar]
- 83.Gomez-Puerta L.A.L., Mayor P. The red brocket deer (Mazama americana) as a new intermediate host of Taenia omissa (Taeniidae) Parasitol. Int. 2021;85:102439. doi: 10.1016/j.parint.2021.102439. [DOI] [PubMed] [Google Scholar]
- 84.Chinchilla-Carmona M., Valerio-Campos I., Gutiérrez-Espeleta G.A., Soto-Fournier S., Vanegas-Pissa J.C., Salom-Pérez R., Arroyo-Arce S. Intestinal parasites found in fecal samples of wild cats of Costa Rica. Int. J. Vet. Sci. 2020;9:153–156. [Google Scholar]
- 85.Bally A., Francis-Charles S., Ackbar T., Beharrylal Y., Charles R., Basu A., Suepaul R. The prevalence of gastrointestinal parasites and seroprevalence of Toxoplasma gondii in captive ocelots (Leopardus pardalis) in Trinidad, West Indies. Vet. Med. Int. 2021;2021:1–5. doi: 10.1155/2021/8820548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arrabal J.P., Arce L.F., Macchiaroli N., Kamenetzky L. Ecological and molecular associations between Neotropical wild felids and Taenia (Cestoda: Taeniidae) in the Atlantic Forest: A new report for Taenia omissa. Parasitol. Res. 2023;122:2999–3012. doi: 10.1007/s00436-023-07989-y. [DOI] [PubMed] [Google Scholar]
- 87.Carmena D., Cardona G.A. Equinococose em espécies carnívoras selvagens: Epidemiologia, diversidade genotípica e implicações para a saúde pública veterinária. Vet. Parasitol. 2014;202:69–94. doi: 10.1016/j.vetpar.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 88.Schwantes J.B., de Souza Quevedo P., D’Ávila M.F., De Paula A.A., Fortes V.B., Sganzerla Graichen D.A. Another piece of the puzzle: Echinococcus oligarthrus recorded in jaguarundis (Herpailurus yagouaroundi) in southern Brazil. J. Wildl. Dis. 2021;57:936–941. doi: 10.7589/JWD-D-20-00208. [DOI] [PubMed] [Google Scholar]
- 89.Kakundi E.M. Doctoral Dissertation. University of Nairobi; Nairobi, Kenya: 2020. Molecular Epidemiology of Echinococcus and Taenia Species in Dogs from Cystic Echinococcosis Endemic Areas in Kenya. [Google Scholar]
- 90.Santos E.G.N., Chame M., Chagas-Moutinho V.A., Santos C.P. Morphology and molecular analysis of Oncicola venezuelensis (Acanthocephala: Oligacanthorhynchidae) from the ocelot Leopardus pardalis in Brazil. J. Helminthol. 2017;91:605–612. doi: 10.1017/S0022149X16000651. [DOI] [PubMed] [Google Scholar]
- 91.Sianto L., de Souza M.V., Chame M., da Luz M.D.F., Guidon N., Pessis A.M., Araújo A. Helminths in feline coprolites up to 9000 years in the Brazilian Northeast. Parasitol. Int. 2014;63:851–857. doi: 10.1016/j.parint.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 92.Palmer J.P.S., Dib L.V., Lobão L.F., Pinheiro J.L., Ramos R.C.F., Uchoa C.M.A., Bastos O.M.P., Silva M.E.M., Nascimento J.L., Pissinatti A., et al. Oncicola venezuelensis (Marteau, 1977) (Acanthocephala: Oligacanthorhynchidae) in Puma concolor in Rio de Janeiro, Brazil. Braz. J. Vet. Parasitol. 2020;29:e009620. doi: 10.1590/s1984-29612020046. [DOI] [PubMed] [Google Scholar]
- 93.Richardson D.J. Acanthocephala. In: Wiley InterScience, editor. Encyclopedia of Life Sciences. John Wiley & Sons; Chichester, UK: 2013. [Google Scholar]
- 94.Núñez V., Drago F. Phylum Acanthocephala. In: Drago F.B., editor. Macroparasites: Parasitology and Biology. National University of La Plata; La Plata, Argentina: 2017. pp. 112–127. [Google Scholar]
- 95.Becker A.A.M.J., Rajeev S., Freeman M.A., Beierschmitt A., Savinon V., Wulcan J.M., Bolfa P. Acanthocephalan extraintestinal Oncicola venezuelensis (Oligacanthorhynchidae) em mangustos indianos pequenos (Herpestes auropunctatus) e macacos-verdes africanos (Chlorocebus aethiops sabaeus) Vet. Pathol. 2019;56:794–798. doi: 10.1177/0300985819848502. [DOI] [PubMed] [Google Scholar]
- 96.Achatz T.J., Pulis E.E., Woodyard E.T., Rosser T.G., Martens J.R., Weinstein S., Fecchio A., McAllister C.T., Carrion Bonilla C., Tkach V. Molecular phylogenetic analysis of Neodiplostomum and Fibricola (Digenea, Diplostomidae) does not support host-based systematics. Parasitology. 2022;149:542–554. doi: 10.1017/S003118202100216X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bogan J., Garner M., Childress A., Wellehan J. Larval Neodiplostomum americanum in the lung of a sugar glider (Petaurus breviceps) J. Exot. Pet. Med. 2019;30:72–75. doi: 10.1053/j.jepm.2018.12.003. [DOI] [Google Scholar]
- 98.Izrailskaia A.V., Besprozvannykh V. Neodiplostomum cf. seoulense (Seo, Rim, Lee, 1964) sensu Pyo et al., 2014 (Trematoda: Diplostomidae Poirier, 1886): Morphology, life cycle, and phylogenetic relationships. J. Helminthol. 2023;97:e61. doi: 10.1017/S0022149X23000408. [DOI] [PubMed] [Google Scholar]
- 99.Alho C.J.R. Concluding remarks: Overall impacts on biodiversity and future perspectives for conservation in the Pantanal biome. Braz. J. Biol. 2011;71:337–341. doi: 10.1590/S1519-69842011000200013. [DOI] [PubMed] [Google Scholar]
- 100.Santos A.L.P.G., Rosa C.A., Bager A. Seasonal variation of wildlife roadkill on highway MG 354, South of Minas Gerais—Brazil. Biotemas. 2012;25:73–79. [Google Scholar]
- 101.Clevenger A.P., Chruszcz B., Gun S.K. Spatial patterns and factors influencing small vertebrate fauna road-kill aggregations. Biol. Conserv. 2003;109:15–26. doi: 10.1016/S0006-3207(02)00127-1. [DOI] [Google Scholar]
- 102.Pitman M.R.P.L., Oliveira T.G., Paula R.C., Indrusiak C. Manual de Identificação, Prevenção e Controle de Predação por Carnívoros. IBAMA; Brasília, Brazil: 2002. 83p [Google Scholar]
- 103.Holmes J.C. Parasites as threats to biodiversity in shrinking ecosystems. Biodiv. Conserv. 1996;5:975–983. doi: 10.1007/BF00054415. [DOI] [Google Scholar]
- 104.Cleaveland S., Haydon D.T., Taylor L. Overviews of pathogen emergence: Which pathogens emerge, when and why? Wildlife and emerging zoonotic diseases: The biology, circumstances and consequences of cross-species transmission. Curr. Top. Microbiol. Immunol. 2007;315:85–111. doi: 10.1007/978-3-540-70962-6_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mukarati N.L., Vassilev G.D., Tagwireyi W.M., Tavengwa M. Occurrence, prevalence and intensity of internal parasite infections in African lions (Panthera leo) in enclosures at a recreation park in Zimbabwe. J. Zoo Wildl. Med. 2013;44:686. doi: 10.1638/2012-0273R.1. [DOI] [PubMed] [Google Scholar]
- 106.Clark N.J., Seddon J.M., Šlapeta J., Wells K. Parasite spread at the domestic animal—Wildlife interface: Anthropogenic habitat use, phylogeny and body mass drive risk of cat and dog flea (Ctenocephalides spp.) infestation in wild mammals. Parasit. Vectors. 2018;11:8. doi: 10.1186/s13071-017-2564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liang H., He Y.-D., Theodorou P., Yang C.-F. The effects of urbanization on pollinators and pollination: A meta-analysis. Ecol. Lett. 2023;26:1629–1642. doi: 10.1111/ele.14277. [DOI] [PubMed] [Google Scholar]
- 108.Zhu S., VanWormer E., Shapiro K. More people, more cats, more parasites: Human population density and temperature variation predict prevalence of Toxoplasma gondii oocyst shedding in free-ranging domestic and wild felids. PLoS ONE. 2023;18:e0286808. doi: 10.1371/journal.pone.0286808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Almeida G.G., Coscarelli D., Melo M.N., Melo A.L., Pinto H.A. Identificação molecular de Spirometra spp. em alguns animais selvagens do Brasil. Parasitol. Int. 2016;65:428–431. doi: 10.1016/j.parint.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 110.Oda F.H., Borteiro C., da Graca R.J., Tavares L.E.R., Crampet A., Guerra V., Lima F.S., Bellay S., Karling L.C., Castro L., et al. Parasitism by larval cestodes of the genus Spirometra in South American amphibians and reptiles: New records from Brazil and Uruguay. Acta Tropica. 2016;164:150–164. doi: 10.1016/j.actatropica.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 111.Liu W., Gong T., Chen S., Liu Q., Zhou H., He J., Wu Y., Li F., Liu Y. Epidemiology, diagnosis, and prevention of sparganosis in Asia. Animals. 2022;12:1578. doi: 10.3390/ani12121578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mamede S.B., Alho C.J.R. Response of wild mammals to the seasonal shrinking and expansion of habitats due to the flooding regime of the Pantanal, Brazil. Braz. J. Biol. 2006;66:991–998. doi: 10.1590/S1519-69842006000600006. [DOI] [PubMed] [Google Scholar]
- 113.Maracahipes-Santos L., Lenza E., Santos J.O., Mews H.A., Oliveira B. Effects of soil and space on the woody species composition and vegetation structure of three Cerrado phytophysiognomies in the Cerrado-Amazon transition. Braz. J. Biol. 2017;77:830–839. doi: 10.1590/1519-6984.02016. [DOI] [PubMed] [Google Scholar]
- 114.Queiroz M.S., Pontes M.R., Neto M.C., Campião K.M., Anjos L.A. Helminths of eight anuran species from a remnant riparian forest in the Cerrado biome, Brazil. Herpetol. Notes. 2020;13:463–478. [Google Scholar]
- 115.Oliveira S.S., Sousa Machado H.T., Castro Araújo K., Sousa Silva C., Ávila R.W. Nematodes associated with Leptodactylus cf. mystaceus (Anura: Leptodactylidae) in agricultural landscapes of Ibiapaba plateau, Ceará state, Brazil. Caldasia. 2024;46 doi: 10.15446/caldasia.v46n2.101535. in press . [DOI] [Google Scholar]
- 116.Maizels R.M. Parasite immunomodulation and polymorphisms of the immune system. J. Biol. 2009;8:62. doi: 10.1186/jbiol166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Petney T.N., Andrews R.H. Multiparasite communities in animals and humans: Frequency, structure and pathogenic significance. Int. J. Parasitol. 1998;28:377–393. doi: 10.1016/S0020-7519(97)00189-6. [DOI] [PubMed] [Google Scholar]
- 118.Nkuo-Akenji T.K., Chi P.C., Cho J.F., Ndamukong K.K., Sumbele I. Malaria and helminth co-infection in children living in a malaria endemic setting of Mount Cameroon and predictors of anemia. J. Parasitol. 2006;96:1191–1195. doi: 10.1645/GE-895R.1. [DOI] [PubMed] [Google Scholar]
- 119.Boel M., Carrara V.I., Rijken M., Proux S., Nacher M., Pimanpanarak M., McGready R. Complex interactions between soil-transmitted helminths and malaria in pregnant women on the Thai-Burmese border. PloS Negl. Trop. Dis. 2010;4:e887. doi: 10.1371/journal.pntd.0000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ezenwa V., Jolles A. From host immunity to pathogen invasion: The effects of helminth coinfection on the dynamics of microparasites. Integr. Comp. Biol. 2011;51:540–551. doi: 10.1093/icb/icr058. [DOI] [PubMed] [Google Scholar]
- 121.Griffiths E.C., Pedersen A.B., Fenton A., Petchey O.L. The nature and consequences of coinfection in humans. J. Infect. 2013;63:200–206. doi: 10.1016/j.jinf.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.