Abstract
Simple Summary
Antibiotic-resistant pathogens from overuse or misuse of antibiotics in livestock and poultry to treat human and animal diseases have become a global threat. Therefore, alternatives to the use of antibiotics have become imperative. The aim of the study was the in vitro antibacterial investigation of eight drinking water additives based on essential oils phytogenics (Phyto CSC Liquide B, AEN 350 B Liquid), acid-based eubiotics (Salgard liquid, Intesti-Flora) and blends of essential oils and organic acids (ProPhorceTM SA Exclusive, Herbal acid, Rigosol-N and Eubisan 3000) against Gram-negative bacteria such as Campylobacter spp., Escherichia coli, Salmonella Typhimurium and Gram-positive bacteria such as Staphylococcus aureus and Listeria spp. The results showed that most of the tested products had promising antibacterial activity. Specifically, the products categorized as “Blends of essential oils and organic acids” performed the highest antibacterial capacity, followed by the “acid-based eubiotics”, while products within the “essential oil-based phytogenics” category performed the lowest antibacterial capacity. Concluding, phytogenic and acid-based eubiotics, as well as their combination, could be good candidates for pathogen control on poultry farms and for the reduction of antimicrobial resistance.
Abstract
The aim of the study was to investigate in vitro the antibacterial activity of 8 commercial drinking water additives against major zoonotic poultry pathogens (Campylobacter spp., Escherichia coli, Salmonella Typhimurium, Staphylococcus aureus and Listeria spp.). We tested two essential oil-based phytogenics (Phyto CSC Liquide B, AEN 350 B Liquid), two acid-based eubiotics (Salgard® liquid, Intesti-Flora), and four blends of essential oils and organic acids (ProPhorceTM SA Exclusive, Herbal acid, Rigosol-N and Eubisan 3000). The antibacterial activity was determined by estimating the minimum inhibitory concentration (MIC) using a microdilution method. The MICs of the products against Campylobacter spp. ranged from 0.071% to 0.568% v/v, in which Herbal acid, a blend rich in lactic and phosphoric acids, also containing thyme and oregano oils, exhibited the highest efficacy (MIC: 0.071% v/v) against all the tested strains. The MICs of the tested products against Escherichia coli ranged between 0.071% and 1.894% v/v. Specifically, the MIC of Rigosol-N, a blend of high concentrations of lactic and acetic acid, was 0.142% v/v for both tested strains, whereas the MICs of Intesti-Flora, a mixture rich in lactic and propionic acid, ranged from 0.284% to 0.568% v/v. The MICs of the products against Salmonella Typhimurium were between 0.095% and 1.894% v/v. Specifically, the MIC of Eubisan 3000, a blend rich in oregano oil, was 0.284% v/v. The MICs against Staphylococcus aureus were between 0.142% and 9.090% v/v. The MICs of Phyto CSC Liquide B, which is rich in trans-cinnamaldehyde, were between 3.030% and 9.090% v/v, showing the highest MIC values of all tested products. Finally, the MIC values of the tested commercial products against Listeria spp. were 0.095% to 3.030% v/v. The MICs of ProPhorceTM SA Exclusive, a highly concentrated blend of formic acid and its salts, were 0.095–0.142% v/v against Listeria spp., while the MICs of AEN 350 B Liquid were between 0.284% and 1.894% exhibiting high Listeria spp. strain variability. In conclusion, all the selected commercial products exhibited more or less antibacterial activity against pathogenic bacteria and, thus, can be promising alternatives to antibiotics for the control of zoonotic poultry pathogens and the restriction of antimicrobial-resistant bacteria.
Keywords: poultry, zoonotic bacteria, phytogenic additives, acid-based eubiotics, antimicrobial activity, minimum inhibitory concentration (MIC)
1. Introduction
Poultry meat is a significant source of high-quality animal protein without any societal, cultural, or religious constraints. According to OECD-FAO 2023, it is estimated that 41% of global human protein consumption is predicted to originate from poultry meat by 2032 [1]. However, higher production requires a greater concentration of poultry in crowded conditions on large intensive livestock farms. This may lead to a rise in the incidence and spread of viral and bacterial diseases, including zoonoses, according to EFSA-EMA 2017 [2].
Currently, the most prevalent and noteworthy foodborne zoonotic poultry pathogens are Campylobacter spp., Salmonella spp., E. coli, Staphylococcus spp., and Listeria spp. [3,4]. In terms of public health, these pathogenic poultry bacteria may induce fever, stomach cramps, diarrhea, and nausea, whereas infections by Listeria spp. can also induce stiff neck, confusion, loss of balance, convulsions, hospitalization, and fatality [5,6,7]. Poultry products are among the most common vehicles for human transmission of Salmonella spp. and Campylobacter spp., with the latter accounting for the majority of bacterial gastroenteritis cases in Europe [8]. Additionally, human intake of undercooked, contaminated meat, as well as inadequate storage and sanitary practices across the food chain, have been associated with E. coli and S. aureus infections [4,9,10]. Moreover, ready-to-eat poultry meat products can easily get contaminated with Listeria spp. during processing or prolonged refrigerated storage [11].
In poultry, Campylobacter spp., Salmonella spp., E. coli, Staphylococcus spp., and Listeria spp. have been associated with little-to-severe clinical signs, gross lesions, productivity loss, and welfare issues, all depending on the infected strain, the type of bird hybrid, immunization level, feed composition, and management practices. For Campylobacter spp., infections may lead to diarrhea and mucous-tinted dropping, as well as a decrease in body weight and production, while the prevalence rate in poultry flocks is higher than 50% [4]. On the other hand, Salmonella spp. infections are usually more severe, leading to anorexia, watery diarrhea, decreased egg production, and a high mortality rate of 80% or even higher, while the financial losses due to Salmonella contamination in poultry products is accounting for USD 2.8 billion annually [12,13]. In addition, avian pathogenic E. coli (APEC) infections are frequently correlated with perihepatitis, pericarditis, and airsacculitis, accompanied by septicemia. Furthermore, in the U.S., losses in the broiler sector due to E. coli can reach up to USD 40 million per year just from carcass condemnation [14]. Finally, S. aureus is usually involved in arthritis, synovitis, chondronecrosis, osteomyelitis, gangrenous dermatitis, subdermal abscesses (bumblefoot), and septicemia, leading to economic losses due to lower productivity [15,16].
In previous decades, the abuse or misuse of antibiotics in poultry has hastened the development of antimicrobial resistance (AMR) in foodborne zoonotic poultry pathogens. Particularly, Campylobacter spp. may be resistant to macrolides (erythromycin), penicillins (ampicillin), tetracycline, fluoroquinolones (nalidixic acid), ciprofloxacin, trimethoprim/sulfonamides (trimethoprim/sulfamethoxazole), aminoglycosides (streptomycin and gentamicin) and cephalosporins (cephalothin) [17]. The most common resistance observed in Salmonella is against fluoroquinolones (nalidixic acid), β-lactams (ampicillin, amoxicillin/clavulanic acid), aminoglycosides (streptomycin) and trimethoprim/sulfonamides (trimethoprim/sulfamethoxazole) [18]. Moreover, E. coli has high resistance to fluoroquinolones (nalidixic acid), β-lactams (penicillins) and tetracycline has been recorded [19]. Resistance to antibiotics, including amoxicillin, amoxicillin-clavulanic acid, ampicillin, cefoxitin, kanamycin, penicillin and tetracycline has frequently been reported for S. aureus isolated from poultry [16], whereas Listeria spp. shows resistant or reduced susceptibility to oxacillin, cefoxitin, cefotaxime, cefepime, rifampicin, ciprofloxacin, enrofloxacin, and nitrofurantoin [20]. Thus, AMR in foodborne zoonotic poultry pathogens is regarded as a serious global health threat.
It was recently suggested that reducing pathogens colonization in poultry at the primary production level may be an important tool to control the incidence of human infections [21]. Among others, water can be an excellent vector for the dissemination of poultry bacterial pathogens [22]. As a result, a variety of natural additives, including phytogenics and acid-based eubiotics, are employed in the drinking water of poultry farms, aiming to reduce or even prevent the spread of infectious diseases [23,24]. Particularly, phytogenics are plant-based additives derived from herbs, spices, and extracts such as essential oils (EOs) [25] that are frequently utilized in poultry nutrition to improve performance and health through their potent antimicrobial, antioxidant, anti-inflammatory, immunomodulatory, and digestion stimulating properties [24]. On the other hand, acid-based eubiotics, such as organic acids and their salts, are naturally occurring substances that possess acidic characteristics [26]. They enhance birds’ health and performance by improving nutrient digestibility, stimulating gastric secretions and the intestinal immune system, reducing pathogen load, and promoting beneficial bacteria [27,28]. Recently, blends of EOs and organic acids have been reported as an efficient formula due to their synergic or additive effect on broilers’ performance and intestinal health [29,30].
Several studies have reported the antimicrobial activity of individual/or blends of essential oils or organic acids [31,32,33,34]. However, the antimicrobial activity of commercial products containing “blends” of the above-mentioned categories is rarely reported. Therefore, the aim of this study was the in vitro investigation of the minimum inhibitory concentration (MIC) of eight commercial water “essential oil-based phytogenics”, “acid-based eubiotics” and “blends of EOs and organic acids” (Phyto CSC Liquide B, AEN 350 B Liquid, Salgard® liquid, Intesti-Flora, ProPhorceTM SA Exclusive, Herbal acid, Rigosol-N and Eubisan 3000) against major foodborne zoonotic poultry pathogens, including Campylobacter spp., E. coli, Salmonella spp., Staphylococcus spp., and Listeria spp. The commercial products were selected due to their wide application in primary poultry production.
2. Materials and Methods
2.1. Products under Examination
Eight different commercial products used in drinking water on poultry farms and authorized by the EU were tested for their antimicrobial activity against major foodborne zoonotic poultry pathogens. The products were divided into three categories based on their synthesis:
Essential oil-based phytogenics: Phyto CSC Liquide B (Phytosynthese, Mozac, France), AEN 350 B Liquid (Phytosynthese, Mozac, France)
Acid-based eubiotics: Salgard® liquid (Anpario plc, Manton Wood Enterprise Park, Worksop, Nottinghamshire, UK), Intesti-Flora (Kanters, Lieshout, The Netherlands)
Blends of essential oils and organic acids: ProPhorceTM SA Exclusive (©Perstorp, Malmö, Sweden), Herbal acid (Pancosma, Rolle, Switzerland), Rigosol-N (Panaroma EPE, Kilkis, Greece), Eubisan 3000 (MIRAVIT®, Münster, Germany)
All the commercial products were in liquid form at ambient temperature. The active compounds of each product and the recommended dosage by the manufacturers are listed in Table 1.
Table 1.
Information on the synthesis and the recommended dose of the selected commercial products.
| Category | Tested Product | Active Ingredients | r. Dosage Range 1 |
|---|---|---|---|
| Essential oil-based phytogenics | Phyto CSC Liquide B | Trans-cinnamaldehyde (43.93%), Thymol (29.83%), Carvacrol (10.56%) | 0.030–0.060% |
| AEN 350 B Liquid | Trans-cinnamaldehyde (87.12%), Eugenol (10.83%), (E)-caryophyllene (1.40%) | 0.030–0.100% | |
| Acid-Based Eubiotics | Salgard® liquid | Ammonium formate (20%), Propionic acid (5.2%), Ammonium Propionate (1%) and Carrier | 0.100–0.200% |
| Intesti-Flora | Lactic acid, Propionic acid, Sorbic acid Copper-chelates of glycine, Oligofructose syrup | 0.020–0.100% | |
| Blends of essential oils and organic acids | ProPhorceTM SA Exclusive | Formic acid (50–60%), Sodium formate (20–30%), L-(+)-lactic acid (5–10%), Cinnamaldehyde (1–5%) | 0.080–0.100% |
| Herbal acid | Lactic acid 30%, Phosphoric acid 20%, Formic acid 15%, Acetic acid 5%, Citric acid 1%, Malic acid 1%, Xtract anabasis (Thyme oil, Oregano oil) 0.5% | 0.050–0.600% | |
| Rigosol-N | Lactic acid 60–80%, Acetic acid 20–10%, Propionic acid 5–10%, Benzoic acid 1–2%, Oregano oil 5% | 0.040–0.060% | |
| Eubisan 3000 | Oregano oil (80,000 mg), Cinnamon oil (3000 mg), Aniseed oil (3000 mg), Citric acid | 0.020–0.025% |
1: Recommended dosage range by the manufacturer (when animals are present).
The pH of each concentration of the product in the tested medium was determined to evaluate the effect of it on different concentrations of the commercial products under examination, with the use of a digital pH meter (pH 315i, WTW Wissenschaftlich-Technische Werkstatten, Weilheim, Germany).
2.2. Tested Bacterial Strains
Thirteen strains of poultry-associated pathogenic bacteria, 7 gram-negative (G−) and 6 gram-positive (G+), were tested in this study.
Gram-negative strains: Campylobacter jejuni S1 (S1: Strain 1) and Campylobacter coli S1 were collected between 2018 and 2019 as part of epidemiological surveillance. Both were field isolates of avian and porcine origin, respectively, obtained from the Campylobacter strain collection of the Greek National Reference Laboratory for Campylobacter in Ioannina, Greece. Campylobacter jejuni S2 (S2: Strain 2) was isolated from a commercial poultry slaughterhouse during a routine surveillance program and detected by PCR in 2019. Escherichia coli ATCC 25922 [35], a reference strain of clinical provenance, is recommended for antimicrobial susceptibility testing by the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST), while Escherichia coli ATCC 11303 [36], strain obtained from a culture collection, is used as a bacteriophage host and as a model organism for Escherichia coli experimental protocols. Salmonella Typhimurium DT 120, a pathogenic strain isolated during a salmonellosis outbreak in Denmark after consumption of turkey meat. Salmonella Typhimurium U292 is a pathogenic strain isolated during an outbreak in Denmark from a patient with gastrointestinal symptoms [33].
Gram-positive strains: Staphylococcus aureus DSM 102262 was isolated from a patient in North Korea, while Staphylococcus aureus DSM 25629’s origin is unknown. Both staphylococci strains are methicillin and multidrug-resistant pathogens. Staphylococcus aureus S1 was a wild strain isolated from a commercial poultry slaughterhouse. Listeria monocytogenes Scott A, utilized as a reference pathogenic strain, is a serovar 4b clinical strain that was initially isolated in 1983 during an outbreak in Massachusetts, USA. Listeria innocua ATCC 33090 [37] is another reference strain that is used as a quality control strain in microbiological testing. It was originally isolated from a cow brain. Finally, Listeria monocytogenes S1, a serotype 1/2a wild strain, was isolated from raw chicken meat [33].
2.3. Preparation of the Tested Inoculum
The tested strains were reconstituted from 13% glycerol broth maintained at −80 °C. Fresh cultures were prepared in Brain Heart Infusion broth (BHI, CM1135, Oxoid Ltd., Basingstoke, UK) and incubated according to the conditions specified for each bacteria strain. Particularly, E. coli, S. Typhimurium, S. aureus, and Listeria spp. were incubated under aerobic conditions at 37 °C for 24 h, whereas C. jejuni and C. coli were incubated at 42 °C under microaerophilic conditions for 48 h. Each strain was plated on BHI or cation-adjusted Mueller Hinton agar (MHA, B11438, Becton Dickinson, Franklin Lakes, NJ, USA). Finally, Campylobacter strains were cultured on MHA + 5% horse blood (Oxoid, CM 129), in microaerophilic conditions, approximately 5% O2, 10% CO2, and 85% N2 (Thermo Scientific™ Oxoid™ CampyGen™ 2.5 L Sachet, Oxoid, Basingstoke, UK). Prior to the preparation of the second culture (working culture), all strain cultures were verified according to their morphological and biochemical characteristics. After inoculation, colonies from each working culture were utilized to generate a 0.5 McFarland suspension in Mueller-Hinton broth (MHB, CM0405, Oxoid Ltd., Basingstoke, UK), using a densitometer (Densimat, Biomerieux, Knutsford, UK) which was then diluted to yield a working solution of 106 CFU of bacteria/mL.
2.4. MIC Assay
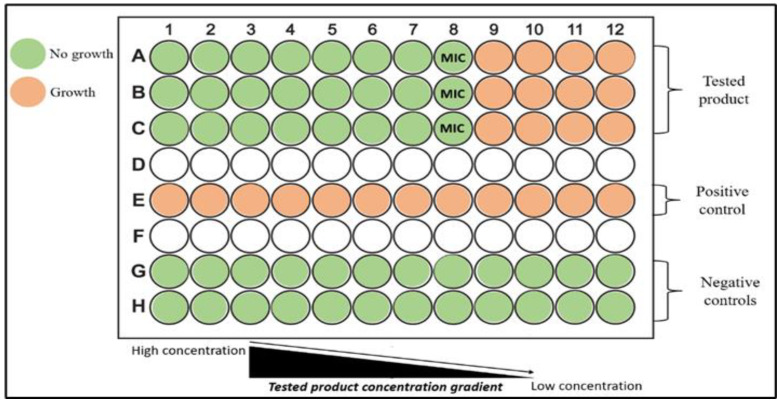
The determination of the MIC of the tested products was performed according to CLSI standards M07-A10 [38] and M100-S28 [39], with modifications. Each product was tested against all the selected strains in concentrations ranging from 0.0044 to 9.090% v/v, using a microdilution MIC method (micro-MIC). Particularly, for each bacterial strain, individual wells in a flat-bottomed 96-well polystyrene microtiter plate (83.3924, TC-Platte 96 Well, Standard, F) were used and filled with cation-adjusted MH broth. Each product was added to the microtiter plates using the two-fold serial dilution method, followed by inoculation of the bacterial strain into each well. This process was applied in triplicate for all the products and all the tested bacterial strains. One strip of wells in each microplate served as the negative control (addition of the sterile culture medium only) and another as the positive control (addition of the bacterial inoculum only), as presented in Figure 1. Each microplate was sealed and incubated for 24 h at 37 °C, under aerobic conditions, apart from the plates containing the Campylobacter strains, which were incubated for 48 h at 42 °C under microaerophilic conditions (Thermo Scientific™ Oxoid™ CampyGen™ 2.5 L Sachet, Oxoid, Basingstoke, UK). After incubation, the plates were examined for bacterial growth indicated by turbidity in the broth of the wells. The last dilution that inhibited visual growth was expressed as the MIC of each product against a specific bacterial strain.
Figure 1.
Schematic representation of the microdilution MIC method used to evaluate the antibacterial activity of the commercial drinking water additives against major zoonotic poultry bacteria. The numbers 1–12 referred to the gradient concentrations of the tested products while letters A–C referred to the treatment groups, letter E to positive control, letters G and H to the negative controls, and letters D and F were blank.
2.5. Statistical Analysis
The raw data from the MIC measurements were analyzed with SPSS 28.0 (BM SPSS Statistics for Windows®, Version 28.0. Armonk, NY, USA: IBM Corp.). The MIC value of each product against each tested pathogen was interpreted as the mean MIC value (% v/v ± standard deviation) of the individual value of each triplicate.
3. Results
The MIC values of the selected commercial poultry products against the tested bacterial strains are summarized in Table 2, Figure 2 and Figure 3.
Table 2.
The minimum inhibitory concentrations (v/v *) of the tested commercial products on the selected bacterial strains (x ± SD).
| Phytogenics | Acid-Based Eubiotics | Blends of Essential Oils and Organic Acids | ||||||
|---|---|---|---|---|---|---|---|---|
| Tested Strains | Phyto CSC Liquide B | AEN 350 B Liquid | Salgard® Liquid | Intesti-Flora | ProPhorceTM SA Exclusive | Herbal Acid | Rigosol-N | Eubisan 3000 |
| Gram-negative bacteria | ||||||||
| C. jejuni S1 | 0.142 ± 0.000 | 0.142 ± 0.000 | 0.568 ± 0.000 | 0.284 ± 0.000 | 0.142 ± 0.000 | 0.071 ± 0.000 | 0.071 ± 0.000 | 0.118 ± 0.041 |
| C. coli S1 | 0.142 ± 0.000 | 0.071 ± 0.000 | 0.568 ± 0.000 | 0.142 ± 0.000 | 0.071 ± 0.000 | 0.071 ± 0.000 | 0.071 ± 0.000 | 0.142 ± 0.000 |
| C. jejuni S2 | 0.142 ± 0.000 | 0.071 ± 0.000 | 0.568 ± 0.000 | 0.284 ± 0.000 | 0.142 ± 0.000 | 0.071 ± 0.000 | 0.142 ± 0.000 | 0.118 ± 0.041 |
| E. coli ATCC 25922 | 1.894 ± 0.657 | 1.136 ± 0.000 | 1.136 ± 0.000 | 0.568 ± 0.000 | 0.142 ± 0.000 | 0.142 ± 0.000 | 0.142 ± 0.000 | 0.379 ± 0.164 |
| E. coli ATCC 11303 | 0.379 ± 0.164 | 1.136 ± 0.000 | 0.568 ± 0.000 | 0.284 ± 0.000 | 0.071 ± 0.000 | 0.142 ± 0.000 | 0.142 ± 0.000 | 0.284 ± 0.000 |
| S. Typhimurium DT120 | 1.894 ± 0.657 | 1.136 ± 0.000 | 1.136 ± 0.000 | 0.568 ± 0.000 | 0.142 ± 0.000 | 0.142 ± 0.000 | 0.142 ± 0.000 | 0.284 ± 0.000 |
| S. Typhimurium U292 | 1.136 ± 0.000 | 1.136 ± 0.000 | 1.136 ± 0.000 | 0.568 ± 0.000 | 0.095 ± 0.041 | 0.142 ± 0.000 | 0.142 ± 0.000 | 0.284 ± 0.000 |
| Gram-positive bacteria | ||||||||
| S. aureus DSM 102262 | 9.090 ± 0.000 | 2.273 ± 0.000 | 1.136 ± 0.000 | 0.568 ± 0.000 | 0.142 ± 0.000 | 0.284 ± 0.000 | 0.142 ± 0.000 | 0.568 ± 0.000 |
| S. aureus DSM 25629 | 9.090 ± 0.000 | 4.545 ± 0.000 | 1.136 ± 0.000 | 0.284 ± 0.000 | 0.142 ± 0.000 | 0.284 ± 0.000 | 0.142 ± 0.000 | 0.284 ± 0.000 |
| S. aureus S1 | 3.030 ± 1.312 | 1.894 ± 0.657 | 1.894 ± 0.657 | 0.237 ± 0.082 | 0.284 ± 0.000 | 0.142 ± 0.000 | 0.142 ± 0.000 | 0.758 ± 0.328 |
| L. monocytogenes Scott A | 2.273 ± 0.000 | 1.894 ± 0.657 | 0.568 ± 0.000 | 0.568 ± 0.000 | 0.142 ± 0.000 | 0.142 ± 0.000 | 0.142 ± 0.000 | 0.379 ± 0.164 |
| L. innocua ATCC 33090 | 3.030 ± 1.312 | 1.136 ± 0.000 | 1.136 ± 0.000 | 1.136 ± 0.000 | 0.142 ± 0.000 | 0.284 ± 0.000 | 0.379 ± 0.164 | 1.136 ± 0.000 |
| L. monocytogenes S1 | 0.568 ± 0.000 | 0.284 ± 0.000 | 1.136 ± 0.000 | 0.568 ± 0.000 | 0.095 ± 0.041 | 0.142 ± 0.000 | 0.118 ± 0.411 | 0.189 ± 0.082 |
* MIC values are presented as %v/v concentration of the product in the Mueller-Hinton broth.
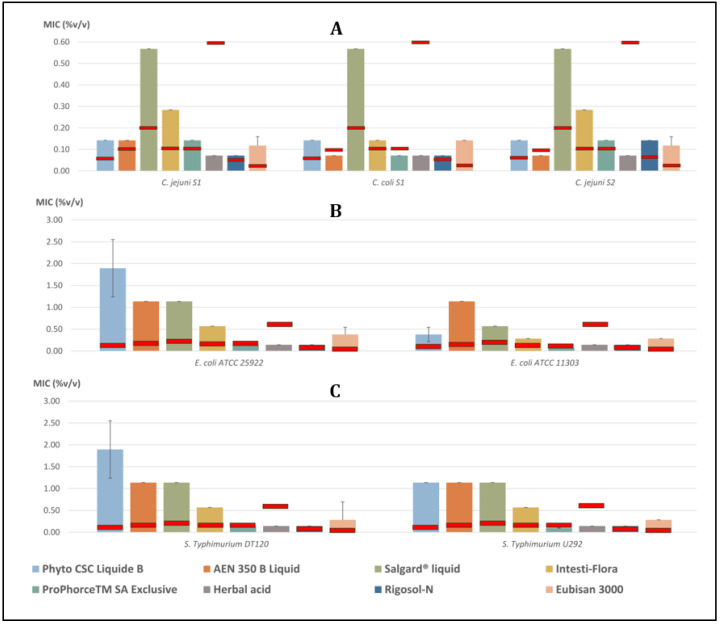
Figure 2.
Graphs of recorded MIC values (columns; mean %v/v ± SD) of the tested commercial products on the tested (A) Campylobacter, (B) E.coli and (C) S. Typhimunium strains.
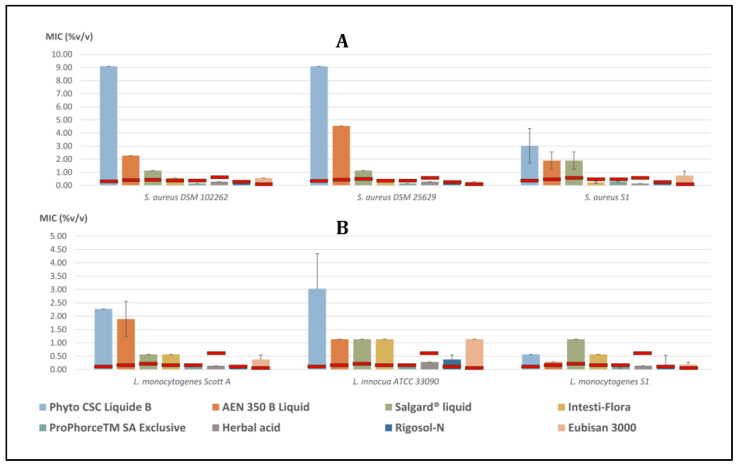
Figure 3.
Graphs of recorded MIC values (columns; mean %v/v ± SD) of the tested commercial products on the tested (A) S. aureus and (B) Listeria spp. strains.
3.1. Gram-Negative Bacteria
3.1.1. Campylobacter spp.
The MIC value of Herbal acid and Rigosol-N for C. jejuni S1 was 0.071% v/v. The MIC values for Eubisan 3000, Intesti-Flora, and Salgard® liquid were 0.118% v/v, 0.284% v/v, and 0.568% v/v, respectively. Furthermore, the MIC values for Phyto CFC Liquide B, AEN 350 B Liquid, and ProPhorceTM SA Exclusive against C. jejuni S1 were 0.142% v/v.
The MIC value for AEN 350 B Liquid, ProPhorceTM SA Exclusive, Herbal acid, and Rigosol-N against C. coli S1 was 0.071% v/v. Moreover, the MIC value for Phyto CFC Liquide B, Intesti-Flora, and Eubisan 3000 was 0.142% v/v. The MIC value for Salgard® liquid against C. coli S1 was 0.568% v/v.
The MIC value of AEN 350 B Liquid and Herbal acid was 0.071% v/v, whereas the MIC value of Phyto CFC Liquide B, ProPhorceTM SA Exclusive, and Rigosol-N was 0.142% v/v for C. jejuni S2. The MIC values for Eubisan 3000, Intesti-Flora and Salgard® liquid against C. jejuni S2 were 0.118% v/v, 0.284% v/v, and 0.568% v/v, respectively.
3.1.2. Escherichia coli
The MIC value of ProPhorceTM SA Exclusive, Herbal acid, and Rigosol-N against E. coli ATCC 25922 was 0.142% v/v. Furthermore, the MIC values for Eubisan 3000, Intesti-Flora, and Phyto CSC Liquide B were 0.379% v/v, 0.568% v/v and 1.894% v/v, respectively. The MIC value for AEN 350 B Liquid and Salgard® liquid against E. coli ATCC 25922 was 1.136% v/v.
The MIC values for ProPhorceTM SA Exclusive, Phyto CSC Liquide B, Salgard® liquid, and AEN 350 B Liquid against E. coli ATCC 11303 were 0.071% v/v, 0.379% v/v, 0.568% v/v and 1.136% v/v, respectively. Moreover, the MIC value of Herbal acid and Rigosol-N was 0.142% v/v, whereas that of Intesti-Flora and Eubisan 3000 was 0.284% v/v for E. coli ATCC 11303.
3.1.3. Salmonella Typhimurium
The MIC value of ProPhorceTM SA Exclusive, Herbal acid, and Rigosol-N for S. Typhimurium DT120 was 0.142% v/v. The MIC values for Eubisan 3000, Intesti-Flora, and Phyto CSC Liquide B were 0.284% v/v, 0.568% v/v and 1.894% v/v, respectively. The MIC value for Salgard® liquid and AEN 350 B Liquid against S. Typhimurium DT120 was 1.136% v/v.
The MIC values for ProPhorceTM SA Exclusive, Eubisan 3000, and Intesti-Flora against S. Typhimurium U292 were 0.095% v/v, 0.284% v/v and 0.568% v/v, respectively. The MIC value of Herbal acid and Rigosol-N against S. Typhimurium U292 was 0.142% v/v, while Phyto CSC Liquide B, Salgard® liquid, and AEN 350 B Liquid had MIC values of 1.136% v/v.
3.2. Gram-Positive Bacteria
3.2.1. Staphylococcus aureus
The MIC value of ProPhorceTM SA Exclusive, and Rigosol-N for S. aureus DSM 102262 was 0.142% v/v. Moreover, the MIC values for Eubisan 3000 and Intesti-Flora against S. aureus DSM 102262 were 0.568% v/v. The MIC values for Herbal acid, Salgard® liquid, AEN 350 B Liquid and Phyto CSC Liquide B were 0.284% v/v, 1.136% v/v, 2.273% v/v and 9.090% v/v, respectively.
The MIC value of ProPhorceTM SA Exclusive, and Rigosol-N for S. aureus DSM 25629 was 0.142% v/v. Furthermore, the MIC values for Intesti-Flora, Herbal acid and Eubisan 3000 against S. aureus DSM 25629 were 0.284% v/v. The MIC values for Salgard® liquid, AEN 350 B Liquid and Phyto CSC Liquide B were 1.136% v/v, 4.545% v/v and 9.090% v/v, respectively.
Herbal acid and Rigosol-N had a MIC value of 0.142% v/v for S. aureus S1. Moreover, the MIC values for Intesti-Flora, ProPhorceTM SA Exclusive, Eubisan 3000, and Phyto CSC Liquide B against S. aureus S1 were 0.237% v/v, 0.284% v/v, 0.758% v/v and 3.030% v/v. The MIC value for Salgard® liquid and AEN 350 B Liquid was 1.894% v/v.
3.2.2. Listeria spp.
The MIC value of ProPhorceTM SA Exclusive, Herbal acid, and Rigosol-N for L. monocytogenes Scott A was 0.142% v/v. The MIC values for Salgard® liquid and Intesti-Flora were 0.568% v/v. The MIC values for Eubisan 3000, AEN 350 B Liquid, and Phyto CSC Liquide B against L. monocytogenes Scott A were 0.379% v/v, 1.894% v/v and 2.273% v/v, respectively.
The MIC values for ProPhorceTM SA Exclusive, Herbal acid, Rigosol-N, and Phyto CSC Liquide B against L. innocua ATCC 33090 were 0.142% v/v, 0.284% v/v, 0.379% v/v and 3.030% v/v, respectively. Moreover, the MIC values for AEN 350 B Liquid, Salgard® liquid, Intesti-Flora and Eubisan 3000 against L. innocua ATCC 33090 were 1.136% v/v.
The MIC values for ProPhorceTM SA Exclusive, Rigosol-N, Herbal acid, Eubisan 3000, AEN 350 B Liquid and Salgard® liquid against L. monocytogenes S1 were 0.095% v/v, 0.118% v/v, 0.142% v/v, 0.189% v/v, 0.284% v/v and 1.136% v/v, respectively. Furthermore, the MIC value for Phyto CSC Liquide B and Intesti-Flora against L. monocytogenes S1 was 0.568% v/v.
Phyto CFC Liquid B altered the pH of the culture medium from 7.25 to 7.47 units during higher tested concentrations but did not change the pH in its lowest concentrations. The pH of the culture medium for the MICs at which Phyto CFC Liquid B was effective against the tested bacterial strains ranged from 7.25 to 7.47 units. AEN 350 B Liquid changed the pH of the growth medium from 7.10 to 7.47 units at the concentrations studied. The pH of the culture medium for the MIC values at which AEN 350 B Liquid was effective against the investigated bacterial strains ranged between 7.19 and 7.47 units. Furthermore, Salgard® liquid decreased the pH of the culture medium at the tested concentrations from 4.58 to 7.43. The pH of the growth medium at which the specific product was able to inhibit bacterial growth for all the tested strains was between 5.02 and 5.30. Intesti-Flora altered the pH of the culture medium from 2.92 to 7.41 units during the tested concentrations. The pH of the culture medium for the MICs at which Intesti-Flora was effective against the tested bacterial strains ranged from 4.43 to 6.79 units. Moreover, ProPhorceTM SA Exclusive changed the pH of the growth medium from 3.12 to 7.40 units at the concentrations studied. The pH of the culture medium for the MIC values at which ProPhorceTM SA Exclusive was effective against the investigated bacterial strains ranged between 3.97 and 4.35 units. Herbal acid decreased the pH of the culture medium at the tested concentrations from 2.07 to 7.39. The pH of the growth medium at which the specific product was able to inhibit bacterial growth for all the tested strains was between 4.27 and 5.93 units. Rigosol-N changed the pH of the growth medium from 2.55 to 7.39 units at the concentrations studied. The pH of the culture medium for the MIC values at which Rigosol-N was effective against the investigated bacterial strains ranged between 4.91 and 5.73 units. Eubisan 3000 decreased the pH of the culture medium at the tested concentrations from 3.33 to 7.42 units. The pH of the culture medium for the MIC values at which Eubisan 3000 was effective against the investigated bacterial strains ranged between 4.77 and 6.95 units. Finally, the negative control wells had the same pH at all tested concentrations. The pH values of the dilutions of the commercial products in the appropriate medium are presented in Table 3.
Table 3.
pH values of culture medium used for the determination of the MICs according to the concentration of the commercial products examined.
| Two-Fold Serial Dilutions | 1 | 1/2 | 1/4 | 1/8 | 1/16 | 1/32 | 1/64 | 1/128 | 1/256 | 1/512 | 1/1024 | 1/2048 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phyto CSC Liquide B | 7.25 | 7.30 | 7.35 | 7.39 | 7.45 | 7.46 | 7.47 | 7.47 | 7.47 | 7.47 | 7.48 | 7.48 |
| AEN 350 B Liquid | 7.10 | 7.19 | 7.28 | 7.37 | 7.42 | 7.46 | 7.47 | 7.47 | 7.47 | 7.47 | 7.47 | 7.47 |
| Salgard® liquid | 4.58 | 4.63 | 4.74 | 5.02 | 5.30 | 6.20 | 7.00 | 7.21 | 7.31 | 7.37 | 7.41 | 7.43 |
| Intesti-Flora | 2.92 | 3.27 | 3.82 | 4.43 | 5.01 | 5.52 | 6.79 | 7.14 | 7.28 | 7.36 | 7.40 | 7.41 |
| ProPhorceTM SA Exclusive | 3.12 | 3.23 | 3.39 | 3.58 | 3.81 | 3.97 | 4.00 | 4.35 | 5.10 | 5.90 | 6.60 | 7.40 |
| Herbal acid | 2.07 | 2.41 | 2.83 | 3.28 | 3.75 | 4.27 | 4.91 | 5.93 | 6.76 | 7.12 | 7.29 | 7.39 |
| Rigosol-N | 2.55 | 2.83 | 3.13 | 3.49 | 3.87 | 4.34 | 4.91 | 5.73 | 6.69 | 7.14 | 7.31 | 7.39 |
| Eubisan 3000 | 3.33 | 3.78 | 4.27 | 4.77 | 5.39 | 6.35 | 6.95 | 7.14 | 7.31 | 7.37 | 7.42 | 7.42 |
| Negative control 1 | 7.48 | 7.48 | 7.48 | 7.48 | 7.48 | 7.48 | 7.48 | 7.48 | 7.48 | 7.48 | 7.48 | 7.48 |
Grey coloring cells represent the two-fold serial dilutions at which each product inhibited the growth of the tested bacterial strains. 1: MH broth only.
4. Discussion
Antimicrobial resistance has reduced the effectiveness of various antibiotic classes and led the poultry sector to search for alternative compounds to control major poultry pathogens. The widespread adoption of natural alternatives such as phytogenic additives and acid-based eubiotics is advised because they are safe for the ecosystem, promote antibacterial activity, reduce the prevalence of antibiotic resistance aid in the production of organic chicken meat and are safe for human consumption [40]. Additionally, in the last decades, numerous in vitro studies demonstrated the efficacy of these compounds against poultry pathogens. However, the majority of these studies have focused on investigating the antimicrobial activity of individual essential oils or organic acids, with only a few examining the antibacterial activity of commercial products incorporating blends of the aforementioned categories [41,42,43].
Poultry serves as the primary reservoir for thermotolerant Campylobacter spp., with poultry meat being the primary vehicle for transmitting these microorganisms to humans [44]. The European Food Safety Authority (EFSA) has suggested that incorporating feed and/or water additives containing organic acids and/or essential oils during poultry production could reduce the prevalence of Campylobacter-positive flocks, thereby reducing the risk of human infection [45]. However, there is limited data available regarding the anti-Campylobacter activity of commercially available products. In the present study, all the products exhibited anti-Campylobacter spp. activity in concentrations ranging from 0.071% to 0.568% v/v. Particularly, Herbal acid, a product with large concentrations of lactic and phosphoric acids, also containing thyme and oregano essential oils, demonstrated efficacy by inhibiting the growth of the tested Campylobacter strains at a rather low concentration (0.071% v/v). This high efficacy of Herbal acid may be attributed to the synergic anti-Campylobacter activity of oregano oil and lactic acid, as previously reported by Navarro et al., 2015 [46]. Furthermore, the main active compounds in oregano and thyme oils, carvacrol and thymol, respectively, are known for their anti-biofilm properties and their ability to inhibit bacterial motility, as well as to alter bacterial cell membrane permeability, ultimately leading to cell death [32,47,48,49,50].
In contrast, Salgard® liquid, a product rich in ammonium formate, required the highest concentration (0.568% v/v) among all tested products to inhibit the growth of Campylobacter spp. This finding is consistent with an in vitro antimicrobial efficacy investigation of sodium formate against C. jejuni, which revealed low antimicrobial activity [31]. Campylobacter species grow at an optimal pH range of 6.5–7.5, with survival even at pH levels close to 5.5 [51]. This could explain why Salgard® liquid required such a high concentration to inhibit the growth of the tested strains, as in this concentration, the medium was under a pH of 5.3, which could be challenging for the growth of the microorganism, according to previous studies [31,51]. However, it is worth noting that despite its in vitro performance, Salgard® liquid demonstrated efficacy in vivo when applied continuously in the drinking water of experimentally challenged by C. jejuni broiler chicks by reducing C. jejuni counts in the ceca of birds by 0.7log10 CFU/g [52].
Organic acid salts, such as those found in Salgard® liquid, offer several advantages over free organic acids. They are less expensive, easier to handle in diets, less corrosive for the equipment, odorless to birds, and more water-soluble [53]. Additionally, salts can effectively bypass the stomach and undergo free acid forms in the lower parts of the gastrointestinal tract (GIT), whereas free organic acids are often metabolized and absorbed in the upper GIT of birds [54]. However, a major disadvantage of salts is that when the H+ ion is replaced by other cations, such as NH+, they do not lower the environmental pH to the same extent as free organic acids [52].
Escherichia coli is an omnipresent intestinal bacteria in humans and animals, which commonly develops antimicrobial resistance (AMR), further increasing its pathogenicity and highlighting the importance of exploring alternative antimicrobial agents. In the present study, all the tested commercial products recorded MIC values ranging from 0.071% to 1.894% v/v against the two strains of E. coli used (ATCC 25922, ATCC 11303). Particularly, Rigosol-N, a product based on oregano oil and lactic and acetic acids, exhibited antimicrobial activity against both tested strains of E. coli in the concentration of 0.142% v/v. Carvacrol, the primary active component of oregano oil, has been demonstrated to be an efficient agent against the growth of E. coli [55]. The antibacterial action of carvacrol against E. coli has been attributed to the downregulation of virulence genes, such as stx genes encoding Shiga toxin production [56]. Furthermore, the hydrophobic nature of carvacrol and other essential oils’ components allows them to interfere with bacterial lipid membrane synthesis, distributing enzymes and proteins and increasing membrane permeability, ultimately leading to bacterial cell death [57]. Moreover, acetic acid, in addition to lactic acid (both concentrated in Rigosol-N), is widely used in the food sector to reduce or control pathogenic bacteria, including E. coli [58,59]. It has been demonstrated that both lactic and acetic acids induce alterations in the metabolic processes of the bacteria [60]. A study found that a blend of lactic and acetic acid had a strong inhibitory effect on the biological processes within the cytoplasm of E. coli O157:H7 [61]. Additionally, a study reported that the combination of acetic acid and oregano oil inhibited the growth of E. coli O157:H7 on fresh lettuce [62].
Intesti-Flora exhibited higher MIC values (ranging from 0.284% to 0.568% v/v) for the tested strains of E. coli compared to other commercial products. Intesti-Flora is composed of both short-chain fatty acids (SCFAs), such as propionic and lactic acid, as well as medium-chain fatty acids (MCFAs), like sorbic acid. Additionally, it contains copper chelates and oligofructose syrup ingredients aimed at enhancing its effectiveness [33]. Propionic acid is known for its potent inhibitory effects against bacteria, yeasts, and molds [28]. The proliferation of the most pH-sensitive bacteria, such as E. coli, Salmonella spp., and C. perfringens, is minimized below a pH value of 5.0, whereas acid-tolerant bacteria survive [53]. To that end, a correlation between the antimicrobial activity of the specific product and the pH of the culture medium was noted. Specifically, the pH of the culture medium ranged between 5.0 and 5.5 units in the MICs, which is the product exhibited against E. coli. As a result, the use of acid-based eubiotics to lower pH, as attributed to the undissociated forms of the acids, may exhibit a direct inhibitory effect on bacteria. Acid-based eubiotics can easily cross the bacterial cell membrane and enter the cytoplasm, lowering intracellular pH, disrupting the cell membrane, and inhibiting enzymatic processes and nutrient transport systems [53].
Foodborne zoonotic Salmonella infections remain a major public health concern worldwide. Therefore, global efforts are required to decrease Salmonella surveillance, targeting poultry, which consists of a major natural reservoir [63]. Blends of essential oils and organic acids have gained interest in the last decades due to their synergic effect on growth performance, intestinal health, and antimicrobial activity [29]. Eubisan 3000, a blend of oregano, cinnamon, aniseed oil and citric acid, exhibited notable antibacterial efficacy against both strains of S. Typhimurium, with MIC values of 0.284% v/v. Previous studies have reported the in vitro antibacterial activity of compounds contained in Eubisan 3000 against several pathogenetic bacteria [64,65,66]. Two in vivo experiments utilizing combinations of organic acids and EOs in the nutrition of S. Enteritis-infected broiler chicks reduced Salmonella load in the ceca [67,68]. The primary mechanism underlying this synergism appears to be that hydrophobic molecules, such as EOs, increase the permeability of the bacterial cell membrane, allowing organic acids to permeate into the cells and alter the regulated efflux/influx of the bacteria cell wall. On the other hand, organic acids lower the intracellular bacterial pH, increasing the hydrophobicity of Eos, allowing them to penetrate the cell wall more easily and dissolve in the lipids, causing alterations in the bacterial membrane rigidity and fluidity [69].
Staphylococcus aureus is a widespread commensal and opportunistic pathogen found in humans and animals. In our study, all tested commercial products demonstrated inhibitory activity against the tested S. aureus strains, recording MICs varying from 0.142% to 9.090% v/v. Notably, Phyto CSC Liquide B, a highly concentrated blend of trans-cinnamaldehyde (the primary compound of cinnamon oil) and thymol (the primary compound of thyme oil), exhibited the highest MIC values against S. aureus strains in concentrations ranging from 3.030% to 9.090% v/v. This result could be ascribed to the biofilm formation of S. aureus, which makes the bacterium resistant to essential oils or to the low solubility and limited volatility of EOs [70].
Listeria spp., including pathogenic species like L. monocytogenes, pose a significant risk to human health due to their presence in raw and processed foods, as well as their ability to survive in various stress conditions (sanitization, pH, water activity, temperature), by forming biofilms on surfaces [71]. In this study, all the tested products demonstrated inhibitory efficacy against all the strains of Listeria spp. recording MICs ranging from 0.095% to 3.030% v/v. Particularly, ProPhorceTM SA Exclusive, a blend of formic acid and sodium formate, demonstrated potent inhibitory activity against all strains of Listeria spp. at relatively low concentrations (0.095% to 0.142% v/v). The effectiveness of ProPhorceTM SA Exclusive may be attributed to its ability to lower the pH of the broth medium in its specific concentrations, ranging from 4.0 to 4.3 units (Table 3), which is less than the growth limit of Listeria spp., which is 4.4 units [72]. Previous in vitro and in vivo studies have also reported the high antibacterial activity of ProPhorceTM SA Exclusive against a variety of bacteria [31,52]. Formic acid and its salts, both concentrated in ProPhorceTM SA Exclusive, are SCFAs. The capacity of SCFAs to lower pH has been reported to be determined by chemical parameters, such as the acid constant (pKa), dissociation constant (Ka) numbers, undissociated form concentration, and organic acid form concentration. Thus, the lower the pKa value, the more successful the acid is at lowering the pH of the medium. Most of the acids used as feed additives have pKa values ranging from 3.0 to 5.0 [53]. On the other hand, AEN 350 B Liquid, which is highly concentrated in trans-cinnamaldehyde, required relatively higher concentrations (0.284–1.894% v/v) to inhibit the growth of Listeria spp. strains. Previous studies have also reported the antimicrobial activity of trans-cinnamaldehyde against Listeria spp. [73,74]. However, research indicates L. monocytogenes can modulate its membrane fatty acid content to counteract the membrane fluidizing effect of trans-cinnamaldehyde [75].
Our investigation demonstrated that commercial essential oil-based phytogenic products, such as Phyto CSC Liquide B and AEN 350 B Liquid, exhibited higher MIC values against the gram-positive bacteria than gram-negative bacteria. This finding is aligned with previous in vitro studies that evaluated phytogenic natural compounds against bacteria [76,77,78]. The higher resistance of gram-positive bacteria to phytogenic products might be attributed to their thicker peptidoglycan coating cell wall, which restricts essential oils from entering the bacterial cell. Furthermore, in normal conditions, bacteria cells have a negative surface charge due to the presence of anionic groups (e.g., carboxyl and phosphate) in the membrane. A study demonstrated that gram-positive bacteria were more resistant to the reduction of the negative surface charge than gram-negative bacteria following exposure to EO compounds [77]. However, most research suggests that EOs and their components are more effective against gram-positive bacteria, which are mostly composed of a peptidoglycan layer. Gram-negative bacteria, on the other hand, have an outer membrane (OM) that contains hydrophilic lipopolysaccharides (LPS), preventing hydrophobic molecules such as EOs from penetrating the cell wall [69]. Finally, it must be highlighted that the comparison of the results of different studies is often difficult due to the use of different approaches, inoculum preparation techniques, inoculum size, growth medium, incubation conditions, and determination of endpoints.
The results of our study also showed a substantial strain-to-strain variability from some tested bacteria, such as Listeria spp., S. aureus and E. coli. In a previous investigation that included 62 clinical and food-associated L. monocytogenes isolates, several moderate differences in virulence and stress-associated genes were observed between the strains [79]. In addition, it has been suggested that the type of strain, the origin and the environmental conditions may impact the level of biofilm production by L. monocytogenes independently of the planktonic growth rate [80]. On the other hand, the genotypic and phenotypic characteristics of S. aureus strains may be responsible for the variability in biofilm formation [81]. Furthermore, a wide range of virulence pathways related to infectivity, toxin generation and antimicrobial resistance are associated with the strain variation of the bacteria [82].
5. Conclusions
The MIC values of eight commercial products commonly used in the poultry industry for water application against major zoonotic poultry pathogens, including Campylobacter spp., Escherichia coli, Salmonella Typhimurium, Staphylococcus aureus, and Listeria spp. were investigated. Our results indicate that all the tested products exhibited antimicrobial activity against the majority of the pathogens investigated. Notably, the products categorized as “Blends of essential oils and organic acids” (ProPhorceTM SA Exclusive, Herbal acid, Rigosol-N and Eubisan 3000) demonstrated higher antimicrobial activity. Following closely were products classified as “Acid-based eubiotics” (Salgard® liquid, Intesti-Flora), while products within the “essential oil-based phytogenics” category (Phyto CSC Liquide B, AEN 350 B Liquid) displayed the lowest antimicrobial capacity, against the tested pathogens.
Our in vitro findings shed light on the potential of natural antimicrobials as safe alternatives for preventing major zoonotic poultry pathogens and combating the emergence and spread of antimicrobial resistance. While our investigation provides promising insights, further research remains crucial to fully comprehend the mechanisms of action of these natural antimicrobials. Standardization of their effects on the health, welfare and performance of birds are essential avenues for future inquiry. Combinations of phytogenic additives and acid-based eubiotics emerge as particularly promising strategies, potentially surpassing the effectiveness of individual components akin to antibiotics. Leveraging optimal blends alongside impeccable management and husbandry practices will be paramount in reducing antibiotic usage in the poultry industry while maintaining or even enhancing production outcomes.
Acknowledgments
The authors express their gratitude to Natalia Mavromati, Dimitrio Chrysochoou, and Chrysanthi Bellou for their excellent collaboration and assistance.
Author Contributions
Each author has made substantial contributions to the conception of the work, has approved the submitted version, and agrees to be personally accountable for the author’s own contributions and for ensuring that questions related to the accuracy or integrity of any part of the work, even sections in which the author was not personally involved, are appropriately investigated, resolved, and documented in the literature. In particular: conceptualization, K.K., T.M., and V.T.; methodology, K.K., T.M., V.E., V.T., A.T., and E.P.; investigation, K.K., T.M., A.T., V.E., A.P., I.A., I.G., and V.T.; writing—original draft preparation, K.K., T.M., and V.T.; writing—review and editing, K.K., TM., V.T., V.E., E.P., P.F., I.G., and G.A.P.; supervision, K.K., and V.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
In this study, the antibacterial activity of each product was investigated by the in vitro, micro-MIC method. Additionally, in this investigation, neither humans nor animals were employed. So, there was no need for ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
None of the data presented were deposited in an official repository.
Conflicts of Interest
Apostolos Patsias is employed by Agricultural Poultry Cooperation of Ioannina “PINDOS”. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Funding Statement
This study is a part of doctoral research and received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.OECD (Economic Co-operation and Development) FAO (Food and Agriculture Organization) OECD-FAO Agricultural Outlook 2023–2032. Food and Agriculture Organization of the United Nations; Rome, Italy: 2023. pp. 1–90. [Google Scholar]
- 2.EMA Committee for Medicinal Products for Veterinary Use (CVMP) EFSA Panel on Biological Hazards (BIOHAZ) EMA and EFSA Joint Scientific Opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union, and the resulting impacts on food safety (RONAFA) EFSA J. 2017;15:e04666. doi: 10.2903/j.efsa.2017.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonçalves-Tenório A., Silva B., Rodrigues V., Cadavez V., Gonzales-Barron U. Prevalence of Pathogens in Poultry Meat: A Meta-Analysis of European Published Surveys. Foods. 2018;7:69. doi: 10.3390/foods7050069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hafez H.M., Hauck R. Zoonoses: Infections Affecting Humans and Animals. Springer International Publishing; Cham, Switzerland: 2022. Zoonoses Transmitted by Poultry: Risks Related to Poultry Rearing and Eating Poultry Products Zoonoses; pp. 1–24. [Google Scholar]
- 5.EFSA Panel on Biological Hazards (BIOHAZ) EFSA Panel on Contaminants in the Food Chain (CONTAM) EFSA Panel on Animal Health and Welfare (AHAW) Scientific Opinion on the public health hazards to be covered by inspection of meat (poultry) EFSA J. 2012;10:2741 [Google Scholar]
- 6.Heredia N., García S. Animals as sources of food-borne pathogens: A review. Anim. Nutr. 2018;4:250–255. doi: 10.1016/j.aninu.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woyda R., Oladeinde A., Abdo Z. Chicken Production and Human Clinical Escherichia coli Isolates Differ in Their Carriage of Antimicrobial Resistance and Virulence Factors. Appl. Environ. Microbiol. 2023;89:e0116722. doi: 10.1128/aem.01167-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Food Safety Authority. European Centre for Disease Prevention and Control The European Union One Health 2021 Zoonoses Report. EFSA J. 2022;20:7666. doi: 10.2903/j.efsa.2022.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arsenos G., Giannenas I. Sustainable Use of Feed Additives in Livestock: Novel Ways for Animal Production. Springer International Publishing; Cham, Switzerland: 2023. pp. 1–969. [Google Scholar]
- 10.Abreu R., Semedo-Lemsaddek T., Cunha E., Tavares L., Oliveira M. Antimicrobial Drug Resistance in Poultry Production: Current Status and Innovative Strategies for Bacterial Control. Microorganisms. 2023;11:953. doi: 10.3390/microorganisms11040953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dos Santos J.S., Biduski B., Dos Santos L.R. Listeria monocytogenes: Health risk and a challenge for food processing establishments. Arch. Microbiol. 2021;203:5907–5919. doi: 10.1007/s00203-021-02590-2. [DOI] [PubMed] [Google Scholar]
- 12.Scharff L.R. Food Attribution and Economic Cost Estimates for Meat- and Poultry-Related Illnesses. J. Food Prot. 2020;83:959–967. doi: 10.4315/JFP-19-548. [DOI] [PubMed] [Google Scholar]
- 13.Tariq S., Samad A., Hamza M., Ahmer A., Muazzam A., Ahmad S., Amhabj AM A. Salmonella in Poultry; An Overview. Int. J. Multidiscip. Sci. Arts. 2022;1:80–84. doi: 10.47709/ijmdsa.v1i1.1706. [DOI] [Google Scholar]
- 14.Joseph J., Zhang L., Adhikari P., Evans J.D., Ramachandran R. Avian Pathogenic Escherichia coli (APEC) in Broiler Breeders: An Overview. Pathogens. 2023;12:1280. doi: 10.3390/pathogens12111280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Żbikowska K., Michalczuk M., Dolka B. The Use of Bacteriophages in the Poultry Industry. Animals. 2020;10:872. doi: 10.3390/ani10050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Mesquita Souza Saraiva M., Lim K., Do Monte DF M., Givisiez PE N., Alves LB R., De Freitas Neto O.C., Kariuki S., Júnior A.B., De Oliveira CJ B., Gebreyes W.A. Antimicrobial resistance in the globalized food chain: A One Health perspective applied to the poultry industry. Braz. J. Microbiol. 2022;53:465–486. doi: 10.1007/s42770-021-00635-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hlashwayo D.F., Sigaúque B., Bila C.G. Epidemiology and antimicrobial resistance of Campylobacter spp. in animals in Sub-Saharan Africa: A systematic review. Heliyon. 2020;6:e03537. doi: 10.1016/j.heliyon.2020.e03537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castro-Vargas R.E., Herrera-Sánchez M.P., Rodríguez-Hernández R., Rondón-Barragán I.S. Antibiotic resistance in Salmonella spp. isolated from poultry: A global overview. Vet. World. 2020;13:2070–2084. doi: 10.14202/vetworld.2020.2070-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S., Kim H., Kim Y., Kim M., Kwak H., Ryu S. Antimicrobial Resistance of Escherichia coli from Retail Poultry Meats in Korea. J. Food Prot. 2020;83:1673–1678. doi: 10.4315/JFP-20-150. [DOI] [PubMed] [Google Scholar]
- 20.Panera-Martínez S., Rodríguez-Melcón C., Serrano-Galán V., Alonso-Calleja C., Capita R. Prevalence, quantification and antibiotic resistance of Listeria monocytogenes in poultry preparations. Food Control. 2022;135:108608. doi: 10.1016/j.foodcont.2021.108608. [DOI] [Google Scholar]
- 21.European Food Safety Authority (EFSA) European Centre for Disease Prevention and Control (ECDC) The European Union One Health 2018 Zoonoses Report. EFSA J. 2019;17:e05926. doi: 10.2903/j.efsa.2019.5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerba C.P. Environmental Microbiology. 2nd ed. Elsevier; Amsterdam, The Netherlands: 2009. Environmentally Transmitted Pathogens; pp. 445–484. Chapter 22. [Google Scholar]
- 23.Ricke S. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poult. Sci. 2003;82:632–639. doi: 10.1093/ps/82.4.632. [DOI] [PubMed] [Google Scholar]
- 24.Al-Mnaser A., Dakheel M., Alkandari F., Woodward M. Polyphenolic phytochemicals as natural feed additives to control bacterial pathogens in the chicken gut. Arch. Microbiol. 2022;204:253. doi: 10.1007/s00203-022-02862-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdelli N., Solà-Oriol D., Pérez J.F. Phytogenic Feed Additives in Poultry: Achievements, Prospective and Challenges. Animals. 2021;11:3471. doi: 10.3390/ani11123471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang X., Xin H., Yang C., Yang X. Impact of essential oils and organic acids on the growth performance, digestive functions and immunity of broiler chickens. Anim. Nutr. 2018;4:388–393. doi: 10.1016/j.aninu.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao C.-Q., Shi H.-Q., Xie W.-Y., Zhao L.-H., Zhang J.-Y., Ji C., Ma Q.-G. Dietary supplementation with acidifiers improves the growth performance, meat quality and intestinal health of broiler chickens. Anim. Nutr. 2021;7:762–769. doi: 10.1016/j.aninu.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vu Thuy Hong Loan N., Trung Thong H., Nu Anh Thu L., Viet Duc H. Feed Additives—Recent Trends in Animal Nutrition. IntechOpen; London, UK: 2023. Acidifiers as Alternatives for Antibiotics Reduction and Gut Health Improvement for Poultry and Swine. [DOI] [Google Scholar]
- 29.Liu Y., Yang X., Xin H., Chen S., Yang C., Duan Y., Yang X. Effects of a protected inclusion of organic acids and essential oils as antibiotic growth promoter alternative on growth performance, intestinal morphology and gut microflora in broilers. Anim. Sci. J. 2017;88:1414–1424. doi: 10.1111/asj.12782. [DOI] [PubMed] [Google Scholar]
- 30.Stefanello C., Rosa D.P., Dalmoro Y.K., Segatto A.L., Vieira M.S., Moraes M.L., Santin E. Protected Blend of Organic Acids and Essential Oils Improves Growth Performance, Nutrient Digestibility, and Intestinal Health of Broiler Chickens Undergoing an Intestinal Challenge. Front. Vet. Sci. 2020;6:491. doi: 10.3389/fvets.2019.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kovanda L., Zhang W., Wei X., Luo J., Wu X., Atwill E.R., Vaessen S., Li X., Liu Y. In Vitro Antimicrobial Activities of Organic Acids and Their Derivatives on Several Species of Gram-Negative and Gram-Positive Bacteria. Molecules. 2019;24:3770. doi: 10.3390/molecules24203770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milagres De Almeida J., Crippa B.L., Martins Alencar De Souza V.V., Perez Alonso V.P., Da Motta Santos Júnior E., Siqueira Franco Picone C., Prata A.S., Cirone Silva N.C. Antimicrobial action of Oregano, Thyme, Clove, Cinnamon and Black pepper essential oils free and encapsulated against foodborne pathogens. Food Control. 2023;144:109356. doi: 10.1016/j.foodcont.2022.109356. [DOI] [Google Scholar]
- 33.Mantzios T., Tsiouris V., Kiskinis K., Economou V., Petridou E., Tsitsos A., Patsias A., Apostolou I., Papadopoulos G.A., Giannenas I., et al. In Vitro Investigation of the Antibacterial Activity of Nine Commercial Water Disinfectants, Acidifiers, and Glyceride Blends against the Most Important Poultry Zoonotic Bacteria. Pathogens. 2023;12:381. doi: 10.3390/pathogens12030381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merino N., Berdejo D., Pagán E., Girard C., Kerros S., Spinozzi E., Pagán R., García-Gonzalo D. Phenotypic and Genotypic Comparison of Antimicrobial-Resistant Variants of Escherichia coli and Salmonella Typhimurium Isolated from Evolution Assays with Antibiotics or Commercial Products Based on Essential Oils. Pharmaceuticals. 2023;16:1443. doi: 10.3390/ph16101443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Standard ATCC 25922. [(accessed on 25 May 2024)]. Available online: https://www.atcc.org/products/25922.
- 36.Standard ATCC 11303. [(accessed on 25 May 2024)]. Available online: https://www.atcc.org/products/11303?matchtype=&network=x&device=c&adposition=&keyword=&gad_source=1&gclid=EAIaIQobChMI9777zJ2whgMVdZKDBx02azKTEAAYAiAAEgLRofD_BwE.
- 37.Standard ATCC 33090. [(accessed on 25 May 2024)]. Available online: https://www.atcc.org/products/33090.
- 38.Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Approved Standard, 10th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2015. [Google Scholar]
- 39.Performance Standards for Antimicrobial Susceptibility Testing. Twenty-Eighth Informational Supplement. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2018. CLSI Document. [Google Scholar]
- 40.Abd El-Hack M.E., El-Saadony M.T., Salem H.M., El-Tahan A.M., Soliman M.M., Youssef G.B.A., Taha A.E., Soliman S.M., Ahmed A.E., El-Kott A.F., et al. Alternatives to antibiotics for organic poultry production: Types, modes of action and impacts on bird’s health and production. Poult. Sci. 2022;101:101696. doi: 10.1016/j.psj.2022.101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beier R., Byrd J., Caldwell D., Andrews K., Crippen T., Anderson R., Nisbet D. Inhibition and Interactions of Campylobacter jejuni from Broiler Chicken Houses with Organic Acids. Microorganisms. 2019;7:223. doi: 10.3390/microorganisms7080223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mutlu-Ingok A., Tasir S., Seven A., Akgun N., Karbancioglu-Guler F. Evaluation of the single and combined antibacterial efficiency of essential oils for controlling Campylobacter coli, Campylobacter jejuni, Escherichia coli, Staphylococcus aureus, and mixed cultures. Flavour. Fragr. J. 2019;34:280–287. doi: 10.1002/ffj.3501. [DOI] [Google Scholar]
- 43.Peh E., Kittler S., Reich F., Kehrenberg C. Antimicrobial activity of organic acids against Campylobacter spp. and development of combinations—A synergistic effect? PLoS ONE. 2020;15:e0239312. doi: 10.1371/journal.pone.0239312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ammar A.M., El-Naenaeey E.-S.Y., Abd El-Hamid M.I., El-Gedawy A.A., Elmalt R.M.S. Campylobacter as a major foodborne pathogen: A review of its characteristics, pathogenesis, antimicrobial resistance and control. J. Microbiol. Biotechnol. Food Sci. 2021;10:609–619. doi: 10.15414/jmbfs.2021.10.4.609-619. [DOI] [Google Scholar]
- 45.EFSA Panel on Biological Hazards (BIOHAZ) Koutsoumanis K., Allende A., Alvarez-Ordóñez A., Bolton D., Bover-Cid S., Davies R., De Cesare A., Herman L., Hilbert F., et al. Update and review of control options for Campylobacter in broilers at primary production. EFSA J. 2020;18:e06090. doi: 10.2903/j.efsa.2020.6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Navarro M., Stanley R., Cusack A., Sultanbawa Y. Combinations of plant-derived compounds against Campylobacter in vitro. J. Appl. Poult. Res. 2015;24:352–363. doi: 10.3382/japr/pfv035. [DOI] [Google Scholar]
- 47.De Souza G.T., De Carvalho R.J., De Sousa J.P., Tavares J.F., Schaffner D., De Souza E.L., Magnani M. Effects of the Essential Oil from Origanum vulgare L. on Survival of Pathogenic Bacteria and Starter Lactic Acid Bacteria in Semihard Cheese Broth and Slurry. J. Food Prot. 2016;79:246–252. doi: 10.4315/0362-028X.JFP-15-172. [DOI] [PubMed] [Google Scholar]
- 48.Albano M., Crulhas B.P., Alves F.C.B., Pereira A.F.M., Andrade B.F.M.T., Barbosa L.N., Furlanetto A., Lyra L.P.D.S., Rall V.L.M., Júnior A.F. Antibacterial and anti-biofilm activities of cinnamaldehyde against S. epidermidis. Microb. Pathog. 2019;126:231–238. doi: 10.1016/j.micpath.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 49.Ashrafudoulla M., Mizan M.F.R., Ha A.J., Park S.H., Ha S.-D. Antibacterial and antibiofilm mechanism of eugenol against antibiotic resistance Vibrio parahaemolyticus. Food Microbiol. 2020;91:103500. doi: 10.1016/j.fm.2020.103500. [DOI] [PubMed] [Google Scholar]
- 50.Rathod N.B., Kulawik P., Ozogul F., Regenstein J.M., Ozogul Y. Biological activity of plant-based carvacrol and thymol and their impact on human health and food quality. Trends Food Sci. Technol. 2021;116:733–748. doi: 10.1016/j.tifs.2021.08.023. [DOI] [Google Scholar]
- 51.Elmi A., Nasher F., Dorrell N., Wren B., Gundogdu O. Revisiting Campylobacter jejuni Virulence and Fitness Factors: Role in Sensing, Adapting, and Competing Front. Cell. Infect. Microbiol. 2021;10:607704. doi: 10.3389/fcimb.2020.607704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mantzios T., Tsiouris V., Papadopoulos G.A., Economou V., Petridou E., Brellou G.D., Giannenas I., Biliaderis C.G., Kiskinis K., Fortomaris P. Investigation of the Effect of Three Commercial Water Acidifiers on the Performance, Gut Health, and Campylobacter jejuni Colonization in Experimentally Challenged Broiler Chicks. Animals. 2023;13:2037. doi: 10.3390/ani13122037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pearlin B.V., Muthuvel S., Govidasamy P., Villavan M., Alagawany M., Ragab Farag M., Dhama K., Gopi M. Role of acidifiers in livestock nutrition and health: A review. Anim. Physiol. Nutr. 2020;104:558–569. doi: 10.1111/jpn.13282. [DOI] [PubMed] [Google Scholar]
- 54.Hamed D.M., Hassan A.M.A. Acids Supplementation to Drinking Water and Their Effects on Japanese Quails Experimentally Challenged with Salmonella Enteritidis. Res. Zool. 2013;3:15–22. [Google Scholar]
- 55.Hao Y., Kang J., Yang R., Li H., Cui H., Bai H., Tsitsilin A., Li J., Shi L. Multidimensional exploration of essential oils generated via eight oregano cultivars: Compositions, chemodiversities, and antibacterial capacities. Food Chem. 2022;374:131629. doi: 10.1016/j.foodchem.2021.131629. [DOI] [PubMed] [Google Scholar]
- 56.Mith H., Clinquart A., Zhiri A., Daube G., Delcenserie V. The impact of oregano (Origanum heracleoticum) essential oil and carvacrol on virulence gene transcription by Escherichia coli O157:H7. FEMS Microbiol. Lett. 2015;362:1–7. doi: 10.1093/femsle/fnu021. [DOI] [PubMed] [Google Scholar]
- 57.Kachur K., Suntres Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020;60:3042–3053. doi: 10.1080/10408398.2019.1675585. [DOI] [PubMed] [Google Scholar]
- 58.Dan S.D., Mihaiu M., Reget O., Oltean D., Tăbăran A. Pathogens Contamination Level Reduction on Beef Using Organic Acids Decontamination Methods. Bull. Univ. Agric. Sci. Veter-Med. Cluj-Napoca. Veter-Med. 2017;74:212–217. doi: 10.15835/buasvmcn-vm:0052. [DOI] [Google Scholar]
- 59.Li Y., Huang T.-Y., Ye C., Chen L., Liang Y., Wang K., Liu J. Formation and Control of the Viable but Non-Culturable State of Foodborne Pathogen Escherichia coli O157:H7. Front. Microbiol. 2020;11:1202. doi: 10.3389/fmicb.2020.599739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McDermott A., Whyte P., Brunton N., Lyng J., Fagan J., Bolton D.J. The effect of organic acid and sodium chloride dips on the shelf-life of refrigerated Irish brown crab (Cancer pagurus) meat. LWT. 2018;98:141–147. doi: 10.1016/j.lwt.2018.08.039. [DOI] [Google Scholar]
- 61.Wang J., Lei Y., Yu Y., Yin L., Zhang Y. Use of Acetic Acid to Partially Replace Lactic Acid for Decontamination against Escherichia coli O157:H7 in Fresh Produce and Mechanism of Action. Foods. 2021;10:2406. doi: 10.3390/foods10102406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ijabadeniyi O., Mbedla A., Ajayeoba T. Microbiological quality and antimicrobial efficacy of combined oregano essential oil and acetic acid on fresh lettuce. Ital. J. Food Sci. 2020;32:399–409. [Google Scholar]
- 63.Galán-Relaño Á., Valero Díaz A., Huerta Lorenzo B., Gómez-Gascón L., Mena Rodríguez M.Á., Carrasco Jiménez E., Pérez Rodríguez F., Astorga Márquez R.J. Salmonella and Salmonellosis: An Update on Public Health Implications and Control Strategies. Animals. 2023;13:3666. doi: 10.3390/ani13233666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou F., Ji B., Zhang H., Jiang H., Yang Z., Li J., Li J., Ren Y., Yan W. Synergistic Effect of Thymol and Carvacrol Combined with Chelators and Organic Acids against Salmonella Typhimurium. J. Food Prot. 2007;70:1704–1709. doi: 10.4315/0362-028X-70.7.1704. [DOI] [PubMed] [Google Scholar]
- 65.Zheng L., Bae Y.-M., Jung K.-S., Heu S., Lee S.-Y. Antimicrobial activity of natural antimicrobial substances against spoilage bacteria isolated from fresh produce. Food Control. 2013;32:665–672. doi: 10.1016/j.foodcont.2013.01.009. [DOI] [Google Scholar]
- 66.Walker K. Antimicrobial Effectiveness of Citrus Essential Oils and Organic Acids against Salmonella and Listeria monocytogenes. West Virginia University Libraries; Morgantown, WV, USA: 2015. pp. 1–67. [Google Scholar]
- 67.Machado P.C., Beirão B.C.B., Filho T.F., Lourenço M.C., Joineau M.L., Santin E., Caron L.F. Use of blends of organic acids and oregano extracts in feed and water of broiler chickens to control Salmonella Enteritidis persistence in the crop and ceca of experimentally infected birds. J. Appl. Poult. Res. 2014;23:671–682. doi: 10.3382/japr.2014-00979. [DOI] [Google Scholar]
- 68.Hu Z., Liu L., Guo F., Huang J., Qiao J., Bi R., Huang J., Zhang K., Guo Y., Wang Z. Dietary supplemental coated essential oils and organic acids mixture improves growth performance and gut health along with reduces Salmonella load of broiler chickens infected with Salmonella Enteritidis. J. Anim. Sci. Biotechnol. 2023;14:95. doi: 10.1186/s40104-023-00889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nazzaro F., Fratianni F., De Martino L., Coppola R., De Feo V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals. 2013;6:1451–1474. doi: 10.3390/ph6121451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vázquez-Sánchez D., Cabo M.L., Ibusquiza P.S., Rodríguez-Herrera J.J. Biofilm-forming ability and resistance to industrial disinfectants of Staphylococcus aureus isolated from fishery products. Food Control. 2014;39:8–16. doi: 10.1016/j.foodcont.2013.09.029. [DOI] [Google Scholar]
- 71.Mejía L., Espinosa-Mata E., Freire A.L., Zapata S., González-Candelas F. Listeria monocytogenes, a silent foodborne pathogen in Ecuador. Front. Microbiol. 2023;14:1278860. doi: 10.3389/fmicb.2023.1278860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Engstrom S.K., Anderson K.M., Glass K.A. Effect of Commercial Protective Cultures and Bacterial Fermentates on Listeria monocytogenes Growth in a Refrigerated High-Moisture Model Cheese. J. Food Prot. 2021;84:772–780. doi: 10.4315/JFP-20-247. [DOI] [PubMed] [Google Scholar]
- 73.Upadhyay A., Johny A.K., Amalaradjou M.A.R., Ananda Baskaran S., Kim K.S., Venkitanarayanan K. Plant-derived antimicrobials reduce Listeria monocytogenes virulence factors in vitro, and down-regulate expression of virulence genes. Int. J. Food Microbiol. 2012;157:88–94. doi: 10.1016/j.ijfoodmicro.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 74.Corrêa J.A.F., Santos J.V.G.D., Evangelista A.G., Pinto A.C.S.M., Macedo R.E.F.D., Luciano F.B. Combined application of phenolic acids and essential oil components against Salmonella Enteritidis and Listeria monocytogenes in vitro and in ready-to-eat cooked ham. LWT. 2021;149:111881. doi: 10.1016/j.lwt.2021.111881. [DOI] [Google Scholar]
- 75.Rogiers G., Kebede B.T., Van Loey A., Michiels C.W. Membrane fatty acid composition as a determinant of Listeria monocytogenes sensitivity to trans-cinnamaldehyde. Res. Microbiol. 2017;168:536–546. doi: 10.1016/j.resmic.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 76.Borges A., Simões L.C., Saavedra M.J., Simões M. The action of selected isothiocyanates on bacterial biofilm prevention and control. Int. Biodeterior. Biodegrad. 2014;86:25–33. doi: 10.1016/j.ibiod.2013.01.015. [DOI] [Google Scholar]
- 77.Lopez-Romero J.C., González-Ríos H., Borges A., Simões M. Antibacterial Effects and Mode of Action of Selected Essential Oils Components against Escherichia coli and Staphylococcus aureus. Evid. -Based Complement. Altern. Med. 2015;2015:795435. doi: 10.1155/2015/795435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oulkheir S., Aghrouch M., Mourabit F.E., Dalha F., Graich H., Amouch F., Ouzaid K., Moukale A., Chadli S. Antibacterial Activity of Essential Oils Extracts from Cinnamon, Thyme, Clove and Geranium against a Gram Negative and Gram Positive Pathogenic Bacteria. J. Dis. Med. Plants. 2017;3:1–5. [Google Scholar]
- 79.Muchaamba F., Eshwar A.K., Stevens M.J.A., Stephan R., Tasara T. Different Shades of Listeria monocytogenes: Strain, Serotype, and Lineage-Based Variability in Virulence and Stress Tolerance Profiles. Front. Microbiol. 2022;12:792162. doi: 10.3389/fmicb.2021.792162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lianou A., Nychas G.-J.E., Koutsoumanis K.P. Strain variability in biofilm formation: A food safety and quality perspective. Food Res. Int. 2020;137:109424. doi: 10.1016/j.foodres.2020.109424. [DOI] [PubMed] [Google Scholar]
- 81.Meléndez-Carmona M.Á., Mancheño-Losa M., Ruiz-Sorribas A., Muñoz-Gallego I., Viedma E., Chaves F., Van Bambeke F., Lora-Tamayo J. Strain-to-strain variability among Staphylococcus aureus causing prosthetic joint infection drives heterogeneity in response to levofloxacin and rifampicin. J. Antimicrob. Chemother. 2022;77:3265–3269. doi: 10.1093/jac/dkac311. [DOI] [PubMed] [Google Scholar]
- 82.Ferreira M.A., Bernardo L.G., Neves L.S., Campos M.R.H., Lamaro-Cardoso J., André M.C.P. Virulence profile and genetic variability of Staphylococcus aureus isolated from artisanal cheese. J. Dairy Sci. 2016;99:8589–8597. doi: 10.3168/jds.2015-10732. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
None of the data presented were deposited in an official repository.