Abstract
Simple Summary
Pre-surgical evaluation of a sonographically indeterminate adnexal mass is a complex process, and surgical referral relies on subjective evaluation of multiple clinical parameters. The requirement to mitigate malignancy risk means that the vast majority of post-surgical diagnoses are ultimately benign. Conversely, less than half of cancer patients receive a primary referral to an oncology expert, which can adversely affect their long-term outcome. In this study, we highlight the combination of transvaginal ultrasound with a multi-biomarker panel (MMP) to accurately identify and differentiate benign from all stages of malignant disease. The MMP index combined with transvaginal ultrasound (TVU) was superior to TVU plus CA125, particularly for the identification of early-stage malignancies. Incorporation of the MMP index into TVU-driven workflows can provide improved accuracy for the pre-surgical assessment of an adnexal mass, enabling greater confidence for subsequent patient triage.
Abstract
Pre-surgical clinical assessment of an adnexal mass typically relies on transvaginal ultrasound for comprehensive morphological assessment, with further support provided by biomarker measurements and clinical evaluation. Whilst effective for masses that are obviously benign or malignant, a large proportion of masses remain sonographically indeterminate at surgical referral. As a consequence, post-surgical diagnoses of benign disease can outnumber malignancies up to 9-fold, while less than 50% of cancer cases receive a primary referral to a gynecological oncology specialist. We recently described a blood biomarker signature (multi-marker panel—MMP) that differentiated patients with benign from malignant ovarian disease with high accuracy. In this study, we have examined the use of the MMP, both individually and in combination with transvaginal ultrasound, as an alternative tool to CA-125 for enhanced decision making in the pre-surgical referral process.
Keywords: ovarian, cancer, CXCL10, biomarker, triage, malignant, benign, diagnostic
1. Introduction
Current 5-year survival rates for ovarian cancer languish below 50%, making it one of the most lethal gynecological malignancies [1]. Early diagnosis prior to extra-ovarian spread provides the greatest benefit to patients, with above-90% 5-year survival [1]. The initial identification of ovarian cancer presents a significant clinical challenge; however, due to its low incidence, non-specific presentation, and lack of effective diagnostic testing. Whilst population-based screening may ultimately reduce overall mortality, there is currently no effective screening modality to identify early, low-volume malignant disease.
Critical for effective clinical management is the initial assessment and risk stratification for patients with a suspected adnexal mass. The standard work-up includes an evaluation of symptoms, a physical examination, family and medical history, transvaginal ultrasound (TVU), and biomarker measurements. Among these, TVU is recognized as the most effective approach for first-pass investigation [2,3], and subjective evaluation by an expert sonographer (with appropriate training and experience in gynecology) is particularly useful for the differentiation of benign from malignant adnexal masses before surgery. For the identification of ovarian malignancies, ultrasound provides a more effective first-pass evaluation than CA125 [4]. Initial TVU risk assessment is typically based on visible morphological features (e.g., cystic or solid regions, vascularity, presence of papillary projections or peritoneal ascites [5]) and classifies masses as “almost certainly benign”, “suspicious for malignancy” or “indeterminate” [6]. Whilst TVU generally provides an accurate evaluation, the subjective nature of assessment means that interpretation can vary between practitioners [6,7]. As the majority of adnexal masses observed by transvaginal ultrasound (TVU) in postmenopausal women will require surgical intervention [2], it is important to establish an accurate risk of malignancy early and triage patients appropriately.
In the absence of a universally adopted classification system, several approaches have been proposed for the imaging-based classification of adnexal masses. Prominent amongst these are the International Ovarian Tumour Analysis (IOTA) Simple Rules [8,9], which classify lesions visualized by ultrasound into one of five categories according to the presence of benign “B” or malignant “M” features. Recent meta-analysis suggests the IOTA simple rules can identify malignancy with high sensitivity (93% [95%CI, 83–97%]) and good specificity (82% [95%CI 62–93%]) [10]. IOTA simple rules are generally used in pre-menopausal patients [11]. A related imaging-based scoring system is the Ovarian-Adnexal Reporting and Data System (O-RADS) [12], which encompasses all risk categories and malignant schemes. IOTA scoring also forms the basis of the Assessment of Different Neoplasias in the Adnexa (ADNEX) model [13], which incorporates the IOTA simple rules, Cancer Antigen 125 (CA125) serum titer, age, and menopausal status to define the likelihood of malignancy. The O-RADS, IOTA, and ADNEX models all provide similar sensitivity/specificity characteristics for the pre-surgical identification of a benign versus malignant adnexal mass [10,14,15,16]. However, more than 20% of the masses remain indeterminate about following these approaches.
The Risk of Malignancy Index (RMI) [5,17] also combines the ultrasound score, CA125 serum titer and menopausal status to assess the risk of malignancy, and is the only internationally recognized scoring system recommended in medical guidelines [2,3,18]. RMI is most useful in post-menopausal women, where a threshold score of 200 is the cut-off for referral to a gynecological oncology specialist [2]. With specificity at 90%, RMI (200) achieves sensitivity between 64% (pre-menopausal) and 72.3% (postmenopausal) for the detection of cancer [15,16]. Similarly, an RMI score between 25 and 200 is considered “intermediate” risk [3,19] and requires a subjective judgement to be made for the next step in the referral pathway. Whilst rapid and direct referral to a gynecological oncology specialist has the greatest benefit for cancer patients [20,21,22,23,24], RMI does not provide sufficient accuracy for the reliable identification of malignancy—particularly in pre-menopausal women and in those with early-stage, low-volume disease [25,26]. As a consequence, less than 50% of cancer patients receive a primary referral to a gynecologic oncology specialist [6,27]. Patients with benign disease may also benefit from a more conservative treatment approach, particularly one focused on fertility preservation [28,29]. However, the clinical requirement to effectively manage risk means that in the US, around nine benign cases are currently diagnosed for every cancer [23,30,31]. Exploratory surgery for ultimately benign masses carries complication rates between 2 and 15% [32], highlighting the need for accurate diagnosis to minimize potentially harmful interventions and improve the referral process [32].
Biomarker-based testing is an adjunct to TVU for pre-surgical triage, and the potential benefit of multimodal tests is recognized in the American College of Obstetricians and Gynaecologists (ACOG) guidelines [2]. Whilst the Risk of Malignancy Algorithm (ROMA) and OVA1TM tests are suggested as potential alternatives to CA125, neither is recommended for use. We recently identified a multi-marker panel (MMP) that provided high sensitivity and specificity for the differentiation of benign from malignant adnexal masses and that could correctly identify the majority of early-stage and low-CA125 tumours [26,33]. However, its performance against TVU—the primary tool used for referral of patients with a suspicious adnexal mass [2,3]—was not assessed. In this study, we have evaluated the use of the multi-marker panel, both individually and in combination with first look TVU, for its potential to provide improved identification of malignancy for pre-surgical triage.
2. Materials and Methods
2.1. Patient Cohort
Data were obtained from a recently published retrospective cohort analysis, assembled from multiple centres in Victoria, Australia, between 2007 and 2021 [26]. Relevant details of the cohort are provided in Table 1. A total of 169 samples, for which ultrasound, CA125 titer, RMI2 score, and Multi-Marker Panel (MMP) Index scores were available, were eligible for inclusion in this study. All patients included in the study provided informed written consent (ethical approval numbers: HREC #06032C, #02031B; Southern Health Human Research Ethics Committee). In brief, samples of EDTA-chelated plasma were collected from chemo-naïve patients who underwent surgery in specialist gynecological oncology clinics following referral for suspected malignancy. All patients were anaesthetized at the time of blood collection. Post-surgical diagnoses were confirmed from hospital records, and a review of ultrasound imaging according to the RMI2 schedule [34] was performed and scored by a gynecological oncology specialist. All other details were as previously described [26].
Table 1.
Cohort characteristics of patient samples included in this study. All patients were triaged to a gynecological oncology specialist centre for secondary evaluation and surgery.
| All | Pre-Menopausal | Post-Menopausal | ||
|---|---|---|---|---|
| # Participants (Total) | n = 169 | n = 66 | n = 103 | |
| Age at diagnosis (years) | median | 54 | 44.6 | 62.6 |
| IQ range | 47–65 | 40–49 | 56–69 | |
| Pathology (n=) | benign | 113 | 57 | 56 |
| malignant | 56 | 9 | 47 | |
| Tumour type (n=) | Serous | 44 | 4 | 40 |
| endometroid | 4 | 1 | 3 | |
| clear cell | 3 | 2 | 1 | |
| mixed epithelial | 5 | 2 | 3 | |
| Grade (n=) | 1 | 2 | 1 | 1 |
| 2 | 5 | 1 | 4 | |
| 3 | 49 | 7 | 42 | |
| Stage (n=) | I | 7 | 4 | 3 |
| II | 3 | nil | 3 | |
| III–IV | 46 | 5 | 41 | |
| Genetic Predisposition (n=) | BRCA1 | 29 | 16 | 13 |
| BRCA2 | 33 | 17 | 16 | |
| other (lynch, BRIP1+, PALB+, VUS) | 18 | 10 | 8 | |
| wild type | 19 | 7 | 12 | |
| unknown | 70 | 16 | 54 | |
| Ultrasound score (n=) | 1 | 101 | 50 | 51 |
| 4 | 68 | 16 | 52 | |
2.2. Predictive Scoring and Cut-Off Values
Predictive scores for transvaginal ultrasound imaging (1 or 4), CA125 titer, calculated RMI2, and the recently published MMP index [26] were obtained from Stephens et al. [26]. The cutoff values used for CA125 (post-menopausal > 35 U/mL; pre-menopausal > 200) were based on the most recent American College of Gynecology (ACOG) guidelines [2]. Ultrasound scoring was performed by a gynecological oncology specialist. The risk of Malignancy Index (RMI) was calculated as previously described [35] using the formula:
| RMI = ultrasound score × menopausal status × serum CA125 |
where the ultrasound score is 1 or 4, menopausal status is 1 (pre) or 4 (post), and serum CA125 is in units/mL. The cutoff value for RMI (>200) was used as defined in the published literature [17]. The MMP index was calculated using a multivariate logistic regression model [26], which combines 5 individual biomarker measurements into a single score. A cutoff score of <3.684 that provided 95% sensitivity/specificity for discrimination between samples from patients with benign versus malignant disease was previously determined [26].
2.3. Statistical Analyses
All statistical analyses, including logistic regression, Receiver-Operator Curve (ROC) generation, determination of odds ratios (OR), classification tables, predictive power, and t-tests, were performed using GraphPad Prism v10.0.3 (275) (Boston, MA, USA). Logistic regression was used to estimate the pooled sensitivity, specificity, positive and negative likelihood ratios (PLRs and NLRs, respectively), diagnostic odds ratio (DOR), and their respective 95% confidence intervals (CIs). Receiver operator characteristic (ROC) curves and the area under the curve (AUC) method were used to estimate diagnostic performance. Sensitivity, specificity, and positive and negative predictive values (PPV and NPV) were calculated as described [36].
3. Results
3.1. Cohort Features
The characteristics of the cohort data accessed for this study are provided in Table 1 (additional details can be found in [26]). A total of 169 patient samples were recovered with complete biomarker and ultrasound information and were eligible for inclusion in the study. All patients were from a retrospectively collected cohort, previously triaged to a gynecological oncology specialist centre for suspected ovarian malignancy following suspicious ultrasound findings. Within this cohort were a total of 56 ovarian malignancies (33%) and 113 benign (67%) cases, with 39% derived from pre- and 61% from post-menopausal women, respectively (Table 1). Malignancy was more commonly diagnosed in post-menopausal women (~46% of samples) compared to pre-menopausal (~14% of samples). The vast majority of cancer diagnoses in post-menopausal patients were of serous epithelial pathology (87%), whilst pre-menopausal patients tended to have other types (endometroid, clear cell), but nearly all cancers diagnosed were high grade (grades 2–3) in any case. Amongst the samples included were 7 (~4%) stage I ovarian cancers, which were more likely to be diagnosed in pre-menopausal (44%) than post-menopausal (6%) women. The ultrasound score was proportionally higher in post-menopausal patients (50% with ultrasound score = 4) than pre-menopausal patients (24% with ultrasound score = 4). Overall, 47% of the cohort (80 of 169) had known genetic abnormalities at the time of diagnosis, with pre-menopausal patients more likely (~65%) than post-menopausal patients (~36%) to have a known mutation, although over half (52%) of post-menopausal patients had an “unknown” mutational status, compared with only 24% of pre-menopausal patients.
3.2. Scoring Distribution between Disease Groupings
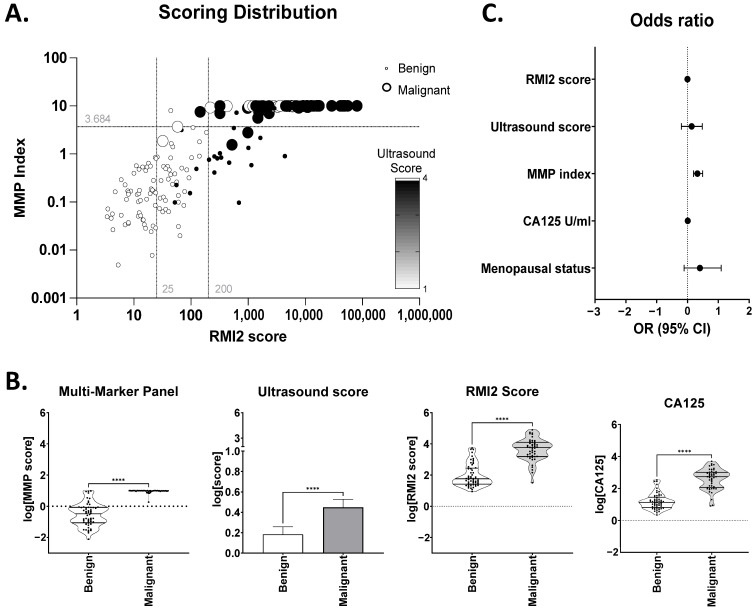
The relationship between ultrasound score (used for initial triage of patients to the specialist centre and assigned on review by the specialist consultant), calculated RMI2 score, and MMP index [26] for the combined cohort is shown in Figure 1. A largely linear relationship between RMI2 and the MMP index was observed (note: the MMP index has a maximum score of 10), indicating a good correlation between the two. Ultrasound score alone, a major component of initial patient triage, was less useful; an ultrasound score of 4 (indicating malignancy) was associated with ~35% of benign cases, whilst ~25% of malignant cases had ultrasound scores of 1 (Figure 1A). Whilst mean scores for each of the MMP index, RMI2, ultrasound, or CA125, were significantly different between groups (Figure 1B), significant overlap was evident between benign and malignant cases for RMI2, CA125 and ultrasound. Only the MMP index provided a clear separation between the groups.
Figure 1.
Relationship between Ultrasound, RMI2 score and MMP index. (A) MMP index vs. RMI score were plotted for each patient. Each data point was coloured according to ultrasound score of 1 (white) or 4 (black). Circle size indicates benign (small) or malignant (large) status. Grey lines indicate RMI score cut-off values at 25 and 200. (B) Comparison of each MMP index, ultrasound score, RMI2, and CA125 between benign and malignant samples. **** p ≤ 0.0001. (C) Odds ratio +/− 95% confidence intervals for parameters considered in logistic regression modelling.
Odds ratios were calculated to assess the relative contribution of variables and their predictive power for differentiation between benign and malignant groups (Figure 1C). Age at diagnosis, menopausal status, BMI (<30 or >30), and genetic risk factors (BRCA1+. BRCA2+, Lynch Syndrome) were all considered, in addition to CA125 and the previously calculated scores for ultrasound, RMI2, and MMP index. Amongst the parameters included in the model, only the MMP index (OR 2.09; 95%CI 1.57–3.09; p < 0.0001) was significant. Menopausal status (OR 2.53; 95%CI 0.78–12.48; p = 0.1) was not significant in this small dataset but trended towards a positive impact on the differentiation of benign from malignant disease (Figure 1C). Neither ultrasound score (OR 1.37; 95%CI 0.67–3.04; p = 0.42), RMI2 score (OR 1.0; 95%CI 0.99–1.00; p = 0.13) nor CA125 (OR 1.02; 95%CI 1.00–1.05; p = 0.09) contributed significantly to differentiation between groups (Figure 1C), reflecting the “high risk” nature of this cohort already triaged to a gynecological oncology clinic based on elevated RMI and CA125 scores [26]. No significant discrimination was evident for age (OR 0.93; 95%CI 0.83–1.03), genetic status (OR 0.06; 95%CI 0.06–6.87), or BMI (OR 0.18; 95%CI 0.01–1.16) between groups although BMI > 30 showed a trend towards a negative impact on group differentiation, suggesting a BMI < 30 may be more commonly associated with benign status.
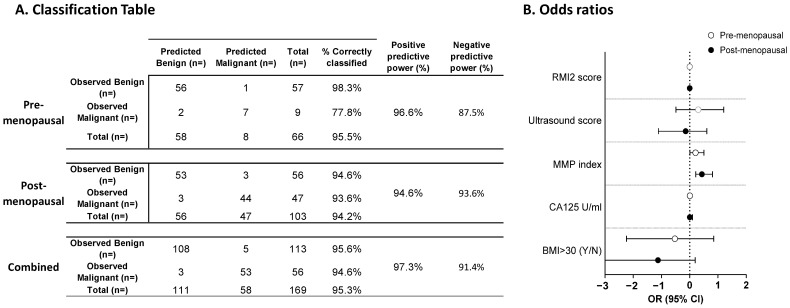
As menopausal status is related to disease risk, regression analyses and odds ratios were re-calculated for each of the pre- and post-menopausal groups separately (Figure 2). Age and genetic status were omitted from the model, as they had no clear influence on the outcome. Between 94 and 97% of patients could be correctly classified regardless of menopausal status and achieved similar negative predictive powers (Figure 2A). Positive predictive power was slightly lower in pre-menopausal patients (87.5%) than post-menopausal (93.6%) patients, although this may reflect low numbers in the pre-menopausal cohort; interestingly, ~44% of cancers in the pre-menopausal cohort were stage 1, suggesting reasonable performance for early-stage cancer detection in this group (Figure 2A). The calculation of odds ratios (Figure 2B) again highlighted the MMP index as an important variable for classification (pre-menopausal OR 1.61 95%CI 1.01–3.20, p = 0.047); post-menopausal OR 2.71 95%CI 1.63–6.93, p = 0.003). Again, no other variables contributed significantly to the classification. Overall, ~95% of samples could be correctly classified with a negative predictive power of 97.3% and a positive predictive power of 91.4% (Figure 2A).
Figure 2.
Results of logistic regression analysis. (A) Classification table according to logistic regression. Analyses were performed on all samples, or on samples separated by menopausal status. (B) Odds ratios +/− 95% confidence intervals associated with pre-menopausal (white circle) or post-menopausal (black circle) status.
3.3. MMP Index Out-Performs RMI2 Score for Differentiation of Benign from Malignant Disease
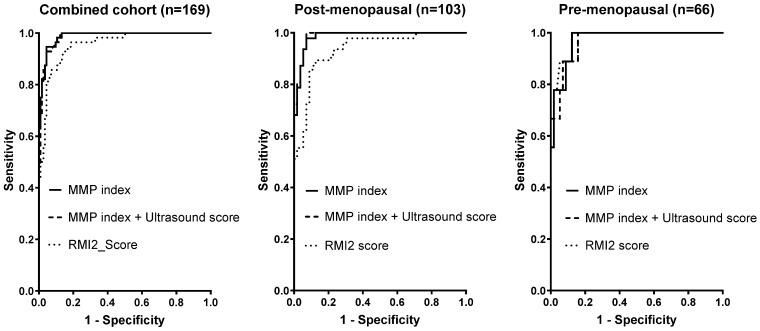
The diagnostic performances of the MMP index, RMI2 score, and ultrasound for discrimination between benign and malignant samples were compared using ROC analysis (Figure 3). As ultrasound imaging is currently the clinical gold standard for patient triage, the MMP index was also assessed in combination with ultrasound. Data were evaluated using both the combined cohort and samples separated by pre- or post-menopausal status. Scoring thresholds for ultrasound (1 or 4), RMI2 (>200), and the MMP index (<3.684) were used as previously [17,26]. Metrics for comparison included the area under the curve (AUC), sensitivity/specificity, and negative/positive predictive values (Table 2).
Figure 3.
Relative performance for each of the MMP index, RMI score, MMP index, and TVU. ROC curves were individually calculated for each classifier. Cohort details (either total, pre-menopausal, or post-menopausal) are indicated.
Table 2.
Diagnostic parameters for each classifier model according to cohort. AUC, sensitivity, specificity, and PPV/NPV were calculated for each predictor using published cut-off values.
| Predictor | Published Cut-Off | Menopausal Status | n= | AUC (95%CI) | Sensitivity % | Specificity % | PPV % | NPV % |
|---|---|---|---|---|---|---|---|---|
| Ultrasound | combined | 169 | 0.77 (0.70–0.85) | 63.2% | 87.1% | 76.8% | 77.9% | |
| 1 or 4 | pre | 66 | 0.87 (0.74–10.0) | 50.0% | 98.0% | 88.9% | 86.0% | |
| post | 103 | 0.72 (0.62–0.82) | 67.3% | 76.5% | 74.5% | 69.6% | ||
| RMI2 score | combined | 169 | 0.95 (0.93–0.98) | 90.2% | 85.2% | 66.1% | 96.5% | |
| ≥200 | pre | 66 | 0.98 (0.94–1.00) | 85.7% | 94.9% | 66.7% | 98.2% | |
| post | 103 | 0.94 (0.89–0.98) | 88.9% | 77.6% | 68.1% | 92.9% | ||
| combined | 169 | 0.99 (0.98–1.00) | 91.1% | 95.6% | 91.1% | 95.6% | ||
| MMP index | ≥3.648 | pre | 66 | 0.97 (0.94–1.00) | 85.7% | 94.9% | 66.7% | 98.2% |
| post | 103 | 0.99 (0.97–1.00) | 92.0% | 98.1% | 97.9% | 92.9% | ||
| MMP Index + Ultrasound | combined | 169 | 0.98 (0.97–1.00) | 91.2% | 96.4% | 92.9% | 95.6% | |
| n/a | pre | 66 | 0.97 (0.93–1.00) | 85.7% | 94.9% | 66.7% | 98.2% | |
| post | 103 | 0.99 (0.97–1.00) | 92.0% | 98.1% | 97.9% | 92.9% |
Receiver operator characteristic (ROC) curves were generated for each of RMI, MMP index, and the combination of MMP index and ultrasound score (Figure 3; note that ultrasound alone was omitted from ROC curves for clarity). In the combined cohort, the MMP index, either alone or in combination with ultrasound, achieved a clear increase in overall efficacy compared to the RMI2 score (Figure 3). When separated by menopausal status, the improvement of MMP index over RMI2 was more pronounced in post-menopausal patients; in the pre-menopausal cohort, there was no clear difference between MMP index, RMI2, or the combination of MMP index with ultrasound (Figure 3). The AUC, sensitivity/specificity, positive and negative predictive values (PPV/NPV) for each score and in each cohort subset are presented in Table 2. Overall AUCs were between 0.94 and 0.99 (Table 2), with the exception of ultrasound alone (0.72–0.87). When pre-menopausal patients were considered separately, ultrasound alone provided the greatest specificity and positive predictive value, although it only achieved a sensitivity of 50% (Table 2). There was no obvious advantage identified between the scoring systems for the pre-menopausal cohort.
In post-menopausal patients, the MMP index (AUC 0.99) had a clear advantage over the use of RMI2 (AUC 0.94). In particular, sensitivity/specificity were substantially higher using the MMP index (92.0%/98.1%) than RMI2 (89.9%/77.6%). Whilst the NPV was similar across both measurements, the PPV obtained by the MMP index was also substantially higher than the PPV for RMI2 (97.9% vs. 68.1%, respectively). The combination of MMP index and ultrasound score resulted in a minor improvement in sensitivity, specificity, and positive predictive value, which was evident in the combined cohort only. Overall, however, MMP index alone or in combination with ultrasound achieved the highest level of sensitivity, specificity, PPV, and NPV in this cohort for the discrimination of benign from malignant disease and exceeded the performance of the clinical gold standard RMI score.
3.4. Classification Performance for Early-Stage Cancers
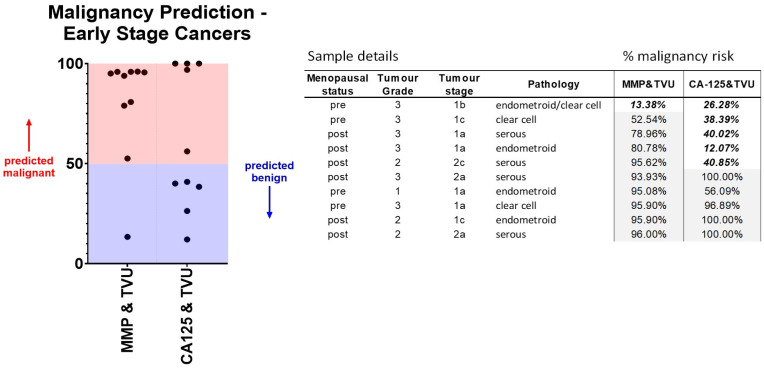
Of particular importance in the early triage process is the accurate identification of early-stage or low-volume cancers. Within the cohort studied, 10 patients were diagnosed with either stage I (n = 7) or stage II (n = 3) epithelial ovarian cancers. The predicted probabilities calculated by logistic regression associated with each sample using RMI, MMP index, or MMP index plus TVU are provided in Table 2. Whilst 50% of early-stage samples were predicted as unlikely to be malignant (score < 0.5) using RMI2, almost all were correctly predicted using the MMP index. The addition of ultrasound scores to the MMP index further increased the prediction scores to encompass 90% of early-stage samples (Figure 4).
Figure 4.
A combination of MMP index with TVU improves classification of stage I–II ovarian malignancy compared to CA125 with TVU. Predicted probabilities for all “early” stage (stages I or II) cancers within the cohort were obtained following logistic regression. Pathology details and the calculated malignancy risk for each sample are shown. A threshold value > 50% suggests malignancy.
4. Discussion
The management of an adnexal mass at first clinical presentation is a complex and multi-faceted process. Whilst a combination of radiological imaging, biomarkers, patient history, and symptoms underlie the ultimate judgement on whether surgical referral is warranted [6], it is transvaginal ultrasound that provides the most effective tool for the early characterization of an adnexal mass [2,3]. The primary goal of early evaluations is to exclude malignancy, and regardless of the interpretation system used, all patients are categorized into groups with a “low”, “intermediate”, or “high” risk of malignancy and triaged accordingly [6]. The majority of adnexal masses in postmenopausal women will require surgical intervention [2], but following pre-surgical triage, up to 90% of patients are subsequently diagnosed with benign disease [9,37]. In the US alone, there are ~428,000 admissions and ~231,000 surgeries annually for adnexal masses [6,37,38]; yet only ~22,000 malignancies are identified. For the remaining patients, exploratory surgery for benign disease carries between 2 and 15% risk of complications [39,40]. It is therefore critical not only to improve the early identification and referral of patients with a malignant mass but also to eliminate those non-malignant cases where patients may benefit from a more conservative intervention.
Our data demonstrate that the calculation of the MMP index provides substantially improved differentiation of benign from malignant disease compared to the “gold standard” combination of TVU with CA125. Whilst RMI score and MMP index both achieved similar sensitivity and NPVs within this cohort, the specificity and PPV of the MMP index (95.6% and 91.1%) were substantially better than the RMI score (85.2% and 66.1%). This is particularly promising as this study evaluated “high-risk” individuals—already triaged to a gynecological oncology centre based on elevated CA125 and suspicious TVU findings—representing a biased patient group skewed towards high RMI scores.
The combination of MMP index with TVU also achieved an incremental improvement over MMP index alone, highlighting its potential as an alternative to CA125 for improved pre-surgical triage of adnexal masses. Adjunct biomarker testing for CA125 is recommended in all clinical guidelines for adnexal mass assessment [2,3,18] and is a critical component of both the ADNEX [13] and RMI [5,17] scoring models. Interestingly, however, ultrasound classifications are not significantly impacted by CA125 [41]. Recent analyses suggest RMI should be replaced for pre-surgical triage [16,42], a proposal consistent with indications in the current ACOG guidelines that multi-modal biomarker panels could provide an alternative to CA125 [2]. Our data strongly suggest that the MMP index has high potential to outperform RMI in a non-selected patient cohort, particularly in combination with TVU, as an alternative biomarker test to CA125.
An observation of particular importance was a significant improvement in the identification of early-stage (FIGO stages I–II) ovarian cancers using the MMP index. Compared to the RMI score, which identified 50% of early-stage cancers, the combination of the MMP index with TVU (instead of CA125) in this cohort successfully identified 90% of early cancer cases. Whilst several meta-analyses have confirmed the good overall performance of RMI, ROMA, and ADNEX [16,42], they typically perform poorly for the identification of stage I disease [43,44,45]. Early-stage ovarian cancers rarely display elevated CA125 [46,47], and our previous analyses highlighted that the MMP index could efficiently identify and classify low CA125 malignancies [33]. Taken together, our data demonstrate that the MMP index can provide an effective triage test to correctly identify ovarian cancers at all stages and differentiate them from benign disease.
TVU operator expertise is critical in the pre-surgical determination of malignancy risk [6]. The availability of such expertise varies widely, however [6,12], and is a limiting factor in the overall predictive capacity afforded by TVU. Misclassification of adnexal masses due to interpretative error can be as high as 75% [48]; whilst in post-menopausal women recruited to the UKTOCS study, TVU-based identification of normal ovaries had a specificity of only 47.5% [49]. Standardized scoring systems, including the IOTA simple rules [8,9] and O-RADS-US [12], proposed to maintain consistency in TVU interpretation, typically provide ~80% specificity [4,10,50,51]. Nevertheless, a substantial fraction of adnexal lesions cannot be definitively stratified following TVU [28,52,53]. To improve the characterization of sonographically indeterminate lesions, magnetic resonance imaging (MRI) as part of the O-RADS MRI scoring scheme has been suggested [48]. MRI improves the characterization of adnexal masses and is useful in the diagnosis of malignancy when a sonographically indeterminate mass is observed by TVU [10,54]. Most benign masses will require extended follow-up, however, so the broader application of O-RADS MRI may be minimized due to the morbidity associated with repeated exposure to contrast dye as well as economic considerations [54]. Biomarker testing may provide a more cost-effective option, without a similar risk of morbidity.
This study also confirmed our previous reports [26,33] highlighting the strong negative predictive value of the MMP index to exclude benign cases. Pre-surgical identification of benign masses is essential to exclude malignancy and develop conservative management strategies to minimize morbidity associated with surgical interventions [28,29,32], particularly since most benign cysts do not progress to malignancy [55,56]. Whilst repeat TVU screening for benign disease is usually indicated, the nature and extent of follow-up are not well defined. Current ACOG guidelines recommend follow-up for cysts up to 10 cm in size [2], whilst O-RADS guidelines suggest cysts of less than 3 cm do not require repeat screening [12]. Similarly, the Society of Radiologists in Ultrasound (SRU) only recommends follow-up for cysts over 7 cm in pre-menopausal women, provided “good ultrasound characterization is available” [32]. Recent studies have also suggested extended follow-up for benign adnexal cysts until resolution, potentially up to 8 years after initial identification [57]. An accurate, biomarker-based panel for the early identification of benign disease will not only minimize the large number of false positive cases that proceed to surgery [23,30,31]; but may also provide a far more cost-effective means for ongoing monitoring of non-malignant disease.
5. Conclusions
Our data demonstrate superior performance of the MMP index over the current clinical workflow using TVU and CA125 and highlight the combination of the MMP index with TVU to substantially improve the identification of malignancy at an early stage. Incorporation of the MMP index into TVU-driven workflows is likely to improve the clinical management of adnexal masses, particularly for the rapid triage of early-stage cancers as well as the exclusion of non-malignant cases. Prospective cohort studies are now underway to further establish the suitability of the MMP index as a pre-surgical triage panel.
6. Patents
Aspects of this study are covered by granted patent 2020404453 and provisional patent 540674PRV.
Acknowledgments
The authors acknowledge the Ovarian Cancer Biobank manager Rhiannon Dudley for data recovery and collation of information used in this study, and the Ovarian cancer Research Foundation of Australia (https://www.ocrf.com.au), which funds the tissue-banking program maintained at the Hudson Institute of Medical Research.
Author Contributions
Conceptualisation and methodology, A.N.S. and S.-W.K.; data curation, validation, and formal analysis, A.N.S., S.-W.K. and S.J.H.; writing—original draft preparation, A.N.S., R.A. and S.J.H.; writing—review and editing, A.N.S., S.-W.K., S.J.H., R.A., M.K.O. and T.W.J.; funding acquisition, A.N.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Monash Human Research Ethics Committee (HREC) (protocol code: #HREC06032C; approval date: 14 June 2006).
Informed Consent Statement
Informed consent was obtained from all the subjects in the study.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
A.N.S. and R.A. are employees and executive board members for Cleo Diagnostics and declare a financial conflict of interest. T.W.J. is a non-executive board member for Cleo Diagnostics and declares a financial conflict of interest. S.J.H. was independently contracted by Cleo Diagnostics to perform statistical analyses and declares a financial conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was supported by funding from the Ovarian Cancer Research Foundation of Australia (#OCRF-GA-2018-11), the National Health and Medical Research Council (#APP1099375), and the Victorian Government’s Operational Infrastructure Support Program.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Cabasag C.J., Fagan P.J., Ferlay J., Vignat J., Laversanne M., Liu L., van der Aa M.A., Bray F., Soerjomataram I. Ovarian cancer today and tomorrow: A global assessment by world region and Human Development Index using GLOBOCAN 2020. Int. J. Cancer. 2022;151:1535–1541. doi: 10.1002/ijc.34002. [DOI] [PubMed] [Google Scholar]
- 2.American College of Obstetricians. Gynecologists’ Committee on Practice Bulletins Practice Bulletin No. 174: Evaluation and Management of Adnexal Masses. Obstet. Gynecol. 2016;128:e210–e226. doi: 10.1097/AOG.0000000000001768. [DOI] [PubMed] [Google Scholar]
- 3.Royal College of Obstetricians and Gynaecologists . Management of Suspected Ovarian Masses in Premenopausal Women. RCOG/BSGE Joint Guideline; London, UK: 2011. Green–Top Guideline No. 62. [Google Scholar]
- 4.Ashmore A.A., Gnanachandran C., Luqman I., Horrocks K. One-stop clinic for patients with suspected ovarian cancer: Results from a retrospective outcome study of the referral pathway. BMC Womens Health. 2021;21:429. doi: 10.1186/s12905-021-01540-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tingulstad S., Hagen B., Skjeldestad F.E., Onsrud M., Kiserud T., Halvorsen T., Nustad K. Evaluation of a risk of malignancy index based on serum CA125, ultrasound findings and menopausal status in the pre-operative diagnosis of pelvic masses. Br. J. Obstet. Gynaecol. 1996;103:826–831. doi: 10.1111/j.1471-0528.1996.tb09882.x. [DOI] [PubMed] [Google Scholar]
- 6.Glanc P., Benacerraf B., Bourne T., Brown D., Coleman B.G., Crum C., Dodge J., Levine D., Pavlik E., Timmerman D., et al. First International Consensus Report on Adnexal Masses: Management Recommendations. J. Ultrasound Med. 2017;36:849–863. doi: 10.1002/jum.14197. [DOI] [PubMed] [Google Scholar]
- 7.Levine D., Brown D.L., Andreotti R.F., Benacerraf B., Benson C.B., Brewster W.R., Coleman B., Depriest P., Doubilet P.M., Goldstein S.R., et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2010;256:943–954. doi: 10.1148/radiol.10100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timmerman D., Ameye L., Fischerova D., Epstein E., Melis G.B., Guerriero S., Van Holsbeke C., Savelli L., Fruscio R., Lissoni A.A., et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: Prospective validation by IOTA group. BMJ. 2010;341:c6839. doi: 10.1136/bmj.c6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Timmerman D., Van Calster B., Testa A., Savelli L., Fischerova D., Froyman W., Wynants L., Van Holsbeke C., Epstein E., Franchi D., et al. Predicting the risk of malignancy in adnexal masses based on the Simple Rules from the International Ovarian Tumor Analysis group. Am. J. Obstet. Gynecol. 2016;214:424–437. doi: 10.1016/j.ajog.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q., Dai X., Li W. Systematic Review and Meta-Analysis of O-RADS Ultrasound and O-RADS MRI for Risk Assessment of Ovarian and Adnexal Lesions. AJR Am. J. Roentgenol. 2023;221:21–33. doi: 10.2214/AJR.22.28396. [DOI] [PubMed] [Google Scholar]
- 11.Yeoh M. Investigation and management of an ovarian mass. Aust. Fam. Physician. 2015;44:48–52. [PubMed] [Google Scholar]
- 12.Andreotti R.F., Timmerman D., Strachowski L.M., Froyman W., Benacerraf B.R., Bennett G.L., Bourne T., Brown D.L., Coleman B.G., Frates M.C., et al. O-RADS US Risk Stratification and Management System: A Consensus Guideline from the ACR Ovarian-Adnexal Reporting and Data System Committee. Radiology. 2020;294:168–185. doi: 10.1148/radiol.2019191150. [DOI] [PubMed] [Google Scholar]
- 13.Van Calster B., Van Hoorde K., Valentin L., Testa A.C., Fischerova D., Van Holsbeke C., Savelli L., Franchi D., Epstein E., Kaijser J., et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: Prospective multicentre diagnostic study. BMJ. 2014;349:g5920. doi: 10.1136/bmj.g5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S., Lee J.E., Hwang J.A., Shin H. O-RADS US: A Systematic Review and Meta-Analysis of Category-specific Malignancy Rates. Radiology. 2023;308:e223269. doi: 10.1148/radiol.223269. [DOI] [PubMed] [Google Scholar]
- 15.Davenport C., Rai N., Sharma P., Deeks J.J., Berhane S., Mallett S., Saha P., Champaneria R., Bayliss S.E., Snell K.I., et al. Menopausal status, ultrasound and biomarker tests in combination for the diagnosis of ovarian cancer in symptomatic women. Cochrane Database Syst. Rev. 2022;7:CD011964. doi: 10.1002/14651858.CD011964.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davenport C.F., Rai N., Sharma P., Deeks J., Berhane S., Mallett S., Saha P., Solanki R., Bayliss S., Snell K., et al. Diagnostic Models Combining Clinical Information, Ultrasound and Biochemical Markers for Ovarian Cancer: Cochrane Systematic Review and Meta-Analysis. Cancers. 2022;14:3621. doi: 10.3390/cancers14153621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs I., Oram D., Fairbanks J., Turner J., Frost C., Grudzinskas J.G. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. Br. J. Obstet. Gynaecol. 1990;97:922–929. doi: 10.1111/j.1471-0528.1990.tb02448.x. [DOI] [PubMed] [Google Scholar]
- 18.National Institute for Health and Care Excellence . Ovarian Cancer: The Recognition and Initial Management of Ovarian Cancer. National Institute for Health and Care Excellence; Cardiff, UK: 2011. Guidelines. [Google Scholar]
- 19.Royal College of Obstetricians and Gynaecologists . Ovarian Cysts in Postmenopausal Women. Royal College of Obstetricians and Gynaecologists; London, UK: 2016. Green-Top Guideline No. 34. [Google Scholar]
- 20.Chan J.K., Kapp D.S., Shin J.Y., Husain A., Teng N.N., Berek J.S., Osann K., Leiserowitz G.S., Cress R.D., O’Malley C. Influence of the gynecologic oncologist on the survival of ovarian cancer patients. Obstet. Gynecol. 2007;109:1342–1350. doi: 10.1097/01.AOG.0000265207.27755.28. [DOI] [PubMed] [Google Scholar]
- 21.Earle C.C., Schrag D., Neville B.A., Yabroff K.R., Topor M., Fahey A., Trimble E.L., Bodurka D.C., Bristow R.E., Carney M., et al. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J. Natl. Cancer Inst. 2006;98:172–180. doi: 10.1093/jnci/djj019. [DOI] [PubMed] [Google Scholar]
- 22.Engelen M.J., Kos H.E., Willemse P.H., Aalders J.G., de Vries E.G., Schaapveld M., Otter R., van der Zee A.G. Surgery by consultant gynecologic oncologists improves survival in patients with ovarian carcinoma. Cancer. 2006;106:589–598. doi: 10.1002/cncr.21616. [DOI] [PubMed] [Google Scholar]
- 23.Giede K.C., Kieser K., Dodge J., Rosen B. Who should operate on patients with ovarian cancer? An evidence-based review. Gynecol. Oncol. 2005;99:447–461. doi: 10.1016/j.ygyno.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Vernooij F., Heintz P., Witteveen E., van der Graaf Y. The outcomes of ovarian cancer treatment are better when provided by gynecologic oncologists and in specialized hospitals: A systematic review. Gynecol. Oncol. 2007;105:801–812. doi: 10.1016/j.ygyno.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 25.Arora T., Mullangi S., Lekkala M.R. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2023. Ovarian Cancer. [Google Scholar]
- 26.Stephens A.N., Hobbs S.J., Kang S.W., Bilandzic M., Rainczuk A., Oehler M.K., Jobling T.W., Plebanski M., Allman R. A Novel Predictive Multi-Marker Test for the Pre-Surgical Identification of Ovarian Cancer. Cancers. 2023;15:5267. doi: 10.3390/cancers15215267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carney M.E., Lancaster J.M., Ford C., Tsodikov A., Wiggins C.L. A population-based study of patterns of care for ovarian cancer: Who is seen by a gynecologic oncologist and who is not? Gynecol. Oncol. 2002;84:36–42. doi: 10.1006/gyno.2001.6460. [DOI] [PubMed] [Google Scholar]
- 28.Froyman W., Landolfo C., De Cock B., Wynants L., Sladkevicius P., Testa A.C., Van Holsbeke C., Domali E., Fruscio R., Epstein E., et al. Risk of complications in patients with conservatively managed ovarian tumours (IOTA5): A 2-year interim analysis of a multicentre, prospective, cohort study. Lancet Oncol. 2019;20:448–458. doi: 10.1016/S1470-2045(18)30837-4. [DOI] [PubMed] [Google Scholar]
- 29.Canis M., Rabischong B., Houlle C., Botchorishvili R., Jardon K., Safi A., Wattiez A., Mage G., Pouly J.L., Bruhat M.A. Laparoscopic management of adnexal masses: A gold standard? Curr. Opin. Obstet. Gynecol. 2002;14:423–428. doi: 10.1097/00001703-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Gottschau M., Rosthoj S., Settnes A., Aalborg G.L., Viuff J.H., Munk C., Jensen A., Kjaer S.K., Mellemkjaer L. Long-Term Health Consequences After Ovarian Removal at Benign Hysterectomy: A Nationwide Cohort Study. Ann. Intern. Med. 2023;176:596–604. doi: 10.7326/M22-1628. [DOI] [PubMed] [Google Scholar]
- 31.Hassan H., Allen I., Sofianopoulou E., Walburga Y., Turnbull C., Eccles D.M., Tischkowitz M., Pharoah P., Antoniou A.C. Long-term outcomes of hysterectomy with bilateral salpingo-oophorectomy: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2023;230:44–57. doi: 10.1016/j.ajog.2023.06.043. [DOI] [PubMed] [Google Scholar]
- 32.Rose S.L. When Less Is More: Using Ultrasound Guidelines to Reduce Unnecessary Follow-Up for Ovarian Cysts. Obstet. Gynecol. 2023;142:1291–1292. doi: 10.1097/AOG.0000000000005436. [DOI] [PubMed] [Google Scholar]
- 33.Stephens A.N., Hobbs S.J., Kang S.W., Oehler M.K., Jobling T.W., Allman R. Reclassification of patients with ambiguous CA125 for optimised pre-surgical triage. Diagnostics. 2024;14:671. doi: 10.3390/diagnostics14070671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karimi-Zarchi M., Mojaver S.P., Rouhi M., Hekmatimoghaddam S.H., Moghaddam R.N., Yazdian-Anari P., Teimoori S. Diagnostic Value of the Risk of Malignancy Index (RMI) for Detection of Pelvic Malignancies Compared with Pathology. Electron. Physician. 2015;7:1505–1510. doi: 10.19082/1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore R.G., McMeekin D.S., Brown A.K., DiSilvestro P., Miller M.C., Allard W.J., Gajewski W., Kurman R., Bast R.C., Jr., Skates S.J. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol. Oncol. 2009;112:40–46. doi: 10.1016/j.ygyno.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trevethan R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front. Public Health. 2017;5:307. doi: 10.3389/fpubh.2017.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2015. CA Cancer J. Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 38.Whiteman M.K., Kuklina E., Jamieson D.J., Hillis S.D., Marchbanks P.A. Inpatient hospitalization for gynecologic disorders in the United States. Am. J. Obstet. Gynecol. 2010;202:541.e1–541.e6. doi: 10.1016/j.ajog.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 39.Menon U., Gentry-Maharaj A., Hallett R., Ryan A., Burnell M., Sharma A., Lewis S., Davies S., Philpott S., Lopes A., et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: Results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) Lancet Oncol. 2009;10:327–340. doi: 10.1016/S1470-2045(09)70026-9. [DOI] [PubMed] [Google Scholar]
- 40.Buys S.S., Partridge E., Greene M.H., Prorok P.C., Reding D., Riley T.L., Hartge P., Fagerstrom R.M., Ragard L.R., Chia D., et al. Ovarian cancer screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial: Findings from the initial screen of a randomized trial. Am. J. Obstet. Gynecol. 2005;193:1630–1639. doi: 10.1016/j.ajog.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Pelayo M., Pelayo-Delgado I., Sancho-Sauco J., Sanchez-Zurdo J., Abarca-Martinez L., Corraliza-Galan V., Martin-Gromaz C., Pablos-Antona M.J., Zurita-Calvo J., Alcazar J.L. Comparison of Ultrasound Scores in Differentiating between Benign and Malignant Adnexal Masses. Diagnostics. 2023;13:1307. doi: 10.3390/diagnostics13071307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chacon E., Dasi J., Caballero C., Alcazar J.L. Risk of Ovarian Malignancy Algorithm versus Risk Malignancy Index-I for Preoperative Assessment of Adnexal Masses: A Systematic Review and Meta-Analysis. Gynecol. Obstet. Investig. 2019;84:591–598. doi: 10.1159/000501681. [DOI] [PubMed] [Google Scholar]
- 43.Yue X., Zhong L., Wang Y., Zhang C., Chen X., Wang S., Hu J., Hu J., Wang C., Liu X. Value of Assessment of Different Neoplasias in the Adnexa in the Differential Diagnosis of Malignant Ovarian Tumor and Benign Ovarian Tumor: A Meta-analysis. Ultrasound Med. Biol. 2022;48:730–742. doi: 10.1016/j.ultrasmedbio.2022.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Phinyo P., Patumanond J., Saenrungmuaeng P., Chirdchim W., Pipanmekaporn T., Tantraworasin A., Tongsong T., Tantipalakorn C. Early-Stage Ovarian Malignancy Score versus Risk of Malignancy Indices: Accuracy and Clinical Utility for Preoperative Diagnosis of Women with Adnexal Masses. Medicina. 2020;56:702. doi: 10.3390/medicina56120702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Czekierdowski A., Stachowicz N., Smolen A., Lozinski T., Guzik P., Kluz T. Performance of IOTA Simple Rules Risks, ADNEX Model, Subjective Assessment Compared to CA125 and HE4 with ROMA Algorithm in Discriminating between Benign, Borderline and Stage I Malignant Adnexal Lesions. Diagnostics. 2023;13:885. doi: 10.3390/diagnostics13050885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skates S.J., Mai P., Horick N.K., Piedmonte M., Drescher C.W., Isaacs C., Armstrong D.K., Buys S.S., Rodriguez G.C., Horowitz I.R., et al. Large prospective study of ovarian cancer screening in high-risk women: CA125 cut-point defined by menopausal status. Cancer Prev. Res. 2011;4:1401–1408. doi: 10.1158/1940-6207.CAPR-10-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sopik V., Rosen B., Giannakeas V., Narod S.A. Why have ovarian cancer mortality rates declined? Part III. Prospects for the future. Gynecol. Oncol. 2015;138:757–761. doi: 10.1016/j.ygyno.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 48.Thomassin-Naggara I., Belghitti M., Milon A., Abdel Wahab C., Sadowski E., Rockall A.G., EURAD Study Group O-RADS MRI score: Analysis of misclassified cases in a prospective multicentric European cohort. Eur. Radiol. 2021;31:9588–9599. doi: 10.1007/s00330-021-08054-x. [DOI] [PubMed] [Google Scholar]
- 49.Stott W., Campbell S., Franchini A., Blyuss O., Zaikin A., Ryan A., Jones C., Gentry-Maharaj A., Fletcher G., Kalsi J., et al. Sonographers’ self-reported visualization of normal postmenopausal ovaries on transvaginal ultrasound is not reliable: Results of expert review of archived images from UKCTOCS. Ultrasound Obstet. Gynecol. 2018;51:401–408. doi: 10.1002/uog.18836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Auekitrungrueng R., Tinnangwattana D., Tantipalakorn C., Charoenratana C., Lerthiranwong T., Wanapirak C., Tongsong T. Comparison of the diagnostic accuracy of International Ovarian Tumor Analysis simple rules and the risk of malignancy index to discriminate between benign and malignant adnexal masses. Int. J. Gynaecol. Obstet. 2019;146:364–369. doi: 10.1002/ijgo.12891. [DOI] [PubMed] [Google Scholar]
- 51.Expert Panel on Women’s Imaging. Atri M., Alabousi A., Reinhold C., Akin E.A., Benson C.B., Bhosale P.R., Kang S.K., Lakhman Y., Nicola R., et al. ACR Appropriateness Criteria((R)) Clinically Suspected Adnexal Mass, No Acute Symptoms. J. Am. Coll. Radiol. 2019;16:S77–S93. doi: 10.1016/j.jacr.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 52.Meys E.M., Kaijser J., Kruitwagen R.F., Slangen B.F., Van Calster B., Aertgeerts B., Verbakel J.Y., Timmerman D., Van Gorp T. Subjective assessment versus ultrasound models to diagnose ovarian cancer: A systematic review and meta-analysis. Eur. J. Cancer. 2016;58:17–29. doi: 10.1016/j.ejca.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 53.Sadowski E.A., Paroder V., Patel-Lippmann K., Robbins J.B., Barroilhet L., Maddox E., McMahon T., Sampene E., Wasnik A.P., Blaty A.D., et al. Indeterminate Adnexal Cysts at US: Prevalence and Characteristics of Ovarian Cancer. Radiology. 2018;287:1041–1049. doi: 10.1148/radiol.2018172271. [DOI] [PubMed] [Google Scholar]
- 54.Lee S.I., Kang S.K. MRI Improves the Characterization of Incidental Adnexal Masses Detected at Sonography. Radiology. 2023;307:e222866. doi: 10.1148/radiol.222866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith-Bindman R., Poder L., Johnson E., Miglioretti D.L. Risk of Malignant Ovarian Cancer Based on Ultrasonography Findings in a Large Unselected Population. JAMA Intern. Med. 2019;179:71–77. doi: 10.1001/jamainternmed.2018.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greenlee R.T., Kessel B., Williams C.R., Riley T.L., Ragard L.R., Hartge P., Buys S.S., Partridge E.E., Reding D.J. Prevalence, incidence, and natural history of simple ovarian cysts among women >55 years old in a large cancer screening trial. Am. J. Obstet. Gynecol. 2010;202:373.e1–373.e9. doi: 10.1016/j.ajog.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lasher A., Harris L.E., Solomon A.L., Harbin L.M., Raby L., Dietrich C.S., Kryscio R.J., van Nagell J.R., Pavlik E.J. Variables Associated with Resolution and Persistence of Ovarian Cysts. Obstet. Gynecol. 2023;142:1293–1301. doi: 10.1097/AOG.0000000000005411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.