Abstract
Snakehead rhabdovirus (SHRV) affects warm water fish in Southeast Asia and belongs to the genus Novirhabdovirus by virtue of its nonvirion gene (NV). Because SHRV grows best at temperatures between 28 and 31°C, we were able to use the T7 expression system to produce viable recombinant SHRV from a cloned cDNA copy of the viral genome. Expression of a positive-strand RNA copy of the 11,550-nucleotide SHRV genome along with the viral nucleocapsid (N), phosphoprotein (P), and polymerase (L) proteins resulted in the generation of infectious SHRV in cells preinfected with a vaccinia virus vector for T7 polymerase expression. Recombinant virus production was verified by detection of a unique restriction site engineered into the SHRV genome between the NV and L genes. Since we were now able to begin examining the function of the NV gene, we constructed a recombinant virus containing a nonsense mutation located 22 codons into the coding sequence of the NV protein. The NV knockout virus was produced at a concentration as high as that of wild-type virus in cultured fish cells, and the resulting virions appeared to be identical to the wild-type virions in electron micrographs. These initial studies suggest that NV has no critical function in SHRV replication in cultured fish cells.
The ability to manipulate directly the genomic sequence of a virus provides a powerful and specific means of elucidating the relationship between genetic information and biological function. For RNA viruses, correlating biological function with specific sequence changes can be done only if infectious virus particles can be recovered from viral cDNA clones. Because the RNA particle of a positive-strand RNA virus can often serve both as the genomic template and as the viral mRNA, recovery of infectious virus requires only the RNA genome. However, with negative-strand RNA viruses, the viral RNA genome is infectious only if it is introduced along with all the viral proteins required for transcription and replication.
In 1994, Schnell et al. (18) reported the first recovery of a recombinant negative-strand RNA virus, rabies virus, entirely from cDNA clones. This report was followed closely by two other reports from groups able to repeat the work using vesicular stomatitis virus (12, 20). The strategy employed by Schnell et al. (18) involved expressing a full-length positive-strand (antigenomic) RNA copy of the rabies virus genome under the control of a T7 RNA polymerase promoter. The genome was expressed in a virus-susceptible tissue culture cell line along with the viral nucleocapsid (N), polymerase (L), and phosphoprotein (referred to here as P but also known as NS or M1) proteins. High levels of cytoplasmic T7 RNA polymerase were induced by preinfecting cells with the recombinant vaccinia virus vTF7-3 (7), which is designed to overexpress the T7 RNA polymerase. In the years since this first report, similar methodologies have been used successfully to produce several other recombinant segmented and nonsegmented negative-strand RNA viruses (for reviews, see references 4 and 17). To date, only mammalian RNA viruses have been recovered successfully with these procedures.
Snakehead rhabdovirus (SHRV) is a rhabdovirus of warm water fish, and it was isolated from a diseased snakehead fish (Ophicephalus struatus) during an epizootic outbreak in Thailand (9). The disease caused characteristic necrotic ulcerations and was seen in both wild and pond-cultured fish throughout Southeast Asia. SHRV tentatively belongs to the Rhabdovirus genus Novirhabdovirus (8) and is related to two of the world's most economically important fish pathogens, viral hemorrhagic septicemia virus, and infectious hematopoietic necrosis virus (IHNV) (GenBank accession no. AF147498). Although SHRV is not the prototype Novirhabdovirus, it is a better representative for reverse genetics experiments, as it is the only member which can replicate at the optimal temperatures for vaccinia virus infection and T7 RNA polymerase transcription.
A defining feature of all viruses belonging to the genus Novirhabdovirus is a small gene located between the viral glycoprotein (G) and polymerase (L) genes. The gene is always approximately 500 nucleotides long and encodes a single open reading frame (ORF) which is approximately 120 codons long. The putative protein encoded by the NV gene is not found in the virions, hence the name nonvirion (NV) protein. The genus name Novirhabdovirus is derived from the NV gene. The NV gene was first described for a Novirhabdovirus in 1985 (10) and has since been extensively studied at both the mRNA and protein level, yet the function of the NV gene is still not known.
We report here the generation of recombinant SHRV entirely from cDNA clones. This marks the first time reverse genetics has been performed on a nonmammalian negative-strand RNA virus. A viable SHRV was created which contained a truncated NV ORF, suggesting that the NV protein is not required for viral replication in cells in culture.
MATERIALS AND METHODS
Oligonucleotide primers.
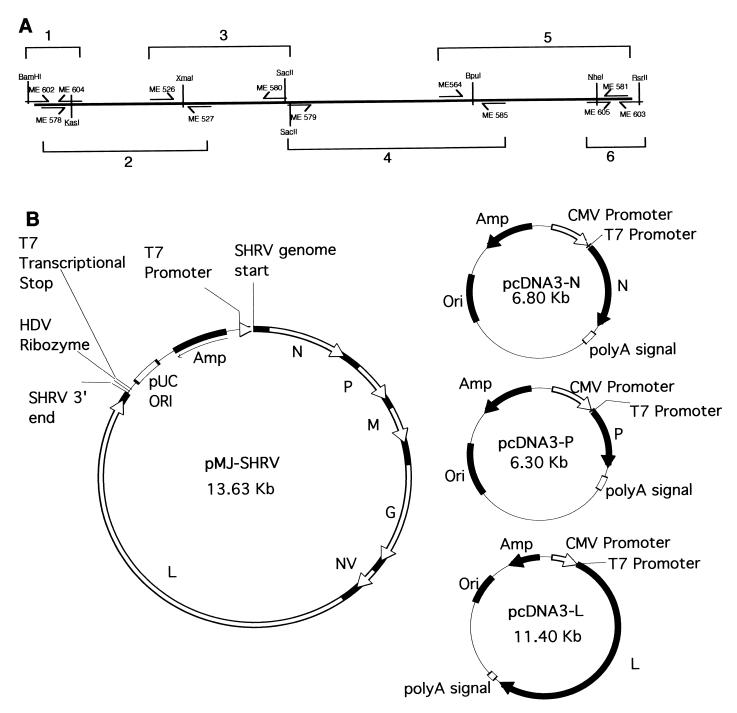
The oligonucleotide primers used in this study are listed in Table 1. The locations of several of these primers within the genome are shown in Fig. 1A.
TABLE 1.
Primers used in this studya
| Primer | Sequenceb | Positions (nucleotides) |
|---|---|---|
| SHRV primers | ||
| ME 602 | CCGGATCCTTGTAATACGACTCACTATAGGG | 1–30 |
| GTATCAAAAAAGATGATGATACTTGGAAGA | ||
| ME 575 | GGAGAGATATCAAGAATCAAACCGAG | 137–162 |
| ME 578 | GGACCGGGGCTGATACTGATGGAAACACC | 280–308 |
| ME 568 | CGAGAGTGCTTAAATCTTTGAAACCC | 1522–1547 |
| ME 526 | AAGGCTGAACTCACAAAGTGAC | 2230–2251 |
| ME 624c | CTCTCTCGCGACCCTTAAGACTGCATCC | 4948–4975 |
| ME 531 | ATATATGAAGACAACGACTCCTGGTAGA | 5239–5266 |
| ME 579 | CCGCGGTGGCATTCAATCC | 5459–5484 |
| CCCGACTGAAACGGAC | ||
| ME 564 | GCACTCACAAAAAAGGCGCT | 8205–8224 |
| ME 605 | CACGGTACCGGGCTAGCACAGACAGCATC | 11143–11162 |
| ME 604 | CACGGTACCGGCGCCTCAATCCCCGAACTT | 497–476 |
| ME 540 | TCCTCCTGAAGGTTCTCTTGTGTCTTGG | 1588–1561 |
| ME 569 | CTCCTGTCAGGATTTTAGCACTCTC | 2325–2304 |
| ME 527 | GTTTCATGTTTGGGAGCGTC | 3224–3205 |
| ME 580 | CCGCGGCGTTGTGGGTTGATGGAGGGCCTAGT | 5451–5424 |
| ME 554 | CAAATATCTCGTGGACTCGTG | 7299–7279 |
| ME 585 | ACCATGGACCGGATCAATTTCTCGATTC | 8275–8248 |
| ME 581 | TTCTCGTTTGTCCTGATCAGCGATCCCCC | 11457–11429 |
| ME 603 | AGGTCGGACCGCGAGGAGGTGGAGATGCCATGCC | 11550–11521 |
| GACCCGTATAGAAAAAGATGATATATTTTTTTCTA | ||
| T7 primers | ||
| ME 550 | CCGGATCCATGAACACGATTAACATCGCT | |
| ME 551 | CGAATTCTTACGCGAACGCGAAGTCC | |
| pMJ construction primers | ||
| ME 520 | CCGACGTCCGATGACGATAAGGATCCGA | |
| ME 521 | CCACATGTGGATATAGTTCCTCCTTTCAGCA | |
| ME 522 | AATTCGGTCCGACCTGGGCATCCGAAGGAGGACGTCG | |
| TCCACTCGGATGGCTAAGGGAGAGCA | ||
| ME 523 | AGCTTGCTCTCCCTTAGCCATCCGAGTGGACGACGTCC | |
| TCCTTCGGATGCCCAGGTCGGACCG |
Some primers contain engineered sequences.
SHRV sequences are underlined.
mutagenesis primer.
FIG. 1.
Vector construction. (A) Locations of the six PCR fragments used to construct the full-length SHRV genomic clone and relevant restriction sites. Restriction site lines (vertical lines) which pass through the primer (arrow lines) indicate restriction sites that were designed into the primer and lines which pass through the representative genome line indicate sites naturally occurring in the genomic clone. (B) Schematic of the four plasmids used to produce recombinant SHRV in vTF7-3-preinfected EPC cells. Amp, ampicillin resistance gene; HDV, hepatitis delta virus; CMV, cytomegalovirus; Ori, origin.
Cells and viruses.
Epithelioma papulosum cyprini (EPC) cells (6) were used for virus propagation. The cells were grown at room temperature in minimal essential medium (MEM) supplemented with 10% fetal calf serum and 2 mM l-glutamine (MEM-10). The SHRV was obtained from John Fryer, Oregon State University. EPC cells were infected with SHRV at a multiplicity of infection (MOI) of 0.5 and incubated at room temperature until there was 70% cytopathic effect (CPE) (20 h). The infected cells were collected and used in subsequent RNA purification.
RNA purification.
Total cellular RNA was extracted from infected EPCs with RNAzol (Tel-Test, Inc., Friendswood, Tex.) according to the manufacturer's instructions.
PCR and cDNA.
SHRV cDNA was prepared by mixing 1 μg of total RNA with 50 pmol of random hexamers, 20 nmol each deoxynucleoside triphosphate, 4 μl of 5× avian myeloblastosis virus (AMV) reverse transcriptase (RT) buffer (250 mM Tris-HCl [pH 8.3], 250 mM KCl, 50 mM MgCl2, 2.5 mM spermidine, 50 mM dithiothreitol) (Promega, Madison, Wis.), 10 U of RNasin (Promega), and 10 U of AMV RT (Promega) in a total volume of 20 μl and incubated for 1 h at 42°C. Following reverse transcription, cDNA was mixed with 900 μl of H2O, and 1 μl from the cDNA reaction mixture was used as the template for each of the subsequent PCR amplifications. Amplifications were performed in an Amplitron II programmable heating block (Barnstead/Thermolyne, Dubuque, Iowa). All PCRs were performed with the same basic parameters; 30 cycles of PCR were done, with 1 cycle consisting of 10 s at 95°C, 1 min at 55°C, and 3 min at 72°C. Extension times were varied, depending on the PCR size.
DNA sequence analysis.
All DNA sequence analyses were performed by the Oregon State University Center for Gene Research and Biotechnology Central Services Facility on a 373 DNA sequencer (DuPont Applied Biosystems, Boston, Mass.). To avoid PCR error, all regions of the SHRV genome were sequenced at least three times.
Luciferase assays.
Luciferase assays were performed with either 6-well (10-cm2) or 98-well (1-cm2) monolayers of EPC cells. Cells were transfected with the appropriate vectors using the Lipofectamine Plus Reagent (Life Technologies, Gaithersburg, Md.) and assayed at 24 h posttransfection using the Luciferase Assay System (Promega), according to the manufacturer's instructions.
Plasmids.
The plasmid pcDNA3-N was created by PCR amplification of the SHRV N gene from SHRV cDNA using Pfu polymerase (Stratagene, La Jolla, Calif.) with primers ME 575 and ME 540, and blunt end cloning the fragment into the EcoRV site of pcDNA3 (InVitrogen, San Diego, Calif.). The plasmid pcDNA3-P was made in the same manner as pcDNA3-N except primers ME 568 and ME 569 were used. Plasmid pcDNA3-T7 was created by PCR amplification of the T7 RNA polymerase gene from the T7-expressing bacterial cell line BL21 LysE (Novagen, Madison, Wis.) using Platinum Taq HF (Life Technologies), with primers ME 550 and ME 551, and subcloning the fragment into the BamHI and EcoRI sites of pcDNA3 using restriction sites designed into the primers. The plasmid pcDNA-L was created in a four-step cloning process. First, the 5′ and 3′ ends of SHRV-L were PCR amplified from SHRV cDNA using Platinum Taq HF with primers ME 579 and ME 585 and primers ME 564 and ME 581 (PCR products 4 and 5 [Fig. 1A]), respectively. The two fragments were then subcloned into pCRXLTopo (InVitrogen) using the manufacturer's instructions, creating pCRXLTopo-3′L and pCRXLTopo-5′L. Next, the 5′ end of SHRV-L was subcloned from pCRXLTopo-5′L into pcDNA3 using the vector's unique HindIII and ApaI sites, creating pcDNA3-5′L. Finally, the 3′ end of SHRV-L was added by partially digesting pCRXLTopo-3′L with BpuI and SpeI and subcloning the 3.2-kb fragment into the unique BpuI and XbaI sites of pcDNA3-5′L, creating pcDNA3-L. Plasmid pTM1-Luc was created by subcloning the luciferase gene from pGL2-Basic (Promega) into the multiple cloning site of pTM1 (5).
Creation of the plasmid pMJ-SHRV was a nine-step cloning process. First, the T7 transcriptional stop site and part of the multiple cloning site of vector pRSET-B (InVitrogen) was PCR amplified using Pfu polymerase, with primers ME 520 and ME 521, and subcloned into the AflIII and AatII sites of pUC19 using restriction sites designed into the primers, creating pUC19+. Second, a linker containing part of the hepatitis delta virus (HDV) ribozyme sequence (16), created by annealing oligonucleotide primers ME 522 and ME 523, was cloned into the EcoRI and HindIII sites of pUC19+, creating pMJ. Third, the 3′ end (positive strand) of the SHRV genome was PCR amplified from SHRV cDNA (PCR product 6 [Fig. 1A]) using Platinum Taq HF, with primers ME 605 and ME 603, and subcloned into the KpnI and RsrII sites of pMJ using sites incorporated in the primers, creating pMJ+3′. Fourth, the 5′ end of the SHRV genome was PCR amplified from SHRV cDNA using Platinum Taq HF, with primers ME 602 and ME 604 (PCR product 1 [Fig. 1A]), and subcloned into the KpnI and BamHI sites of pMJ+3′ using sites incorporated in the primers, creating pMJ+3′+5′. Fifth and sixth, the 5′ half of the genome was PCR amplified from SHRV cDNA using Platinum Taq HF in two PCR amplifications: the first with primers ME 578 and ME 527 and the second with primers ME 526 and ME 580 (PCR amplifications 2 and 3 [Fig. 1A]). Both products were subcloned into pCRXLTopo, creating pCRXLTopo-578/527 and pCRXLTopo-526/580. Seventh, vector pCRXLTopo-526/580 was digested with XmaI, and the 4.8-kb fragment was subcloned into the 3.8-kb fragment of pCRXLTopo-578/527 partially digested with XmaI, creating pCRXLTopo-578/580. Eighth, pCRXLTopo-578/580 and pcDNA3-L were digested with ApaI and SacII, and the 6-kb fragment from pcDNA3-L was cloned into the 9-kb fragment from pCRXLTopo-578/580, creating pCRXLTopo-578/581. Finally, pCRXLTopo-578/581 and pMJ were digested with KasI and NheI, and the 11-kb fragment of pCRXLTopo-578/581 was subcloned into pMJ, creating pMJ-SHRV. Several errors found in the completed genome, which presumably occurred as a result of PCR error, were fixed either by oligonucleotide-directed mutagenesis or by cutting and pasting in error-free fragments. The construction of the entire plasmid was verified by DNA sequencing.
The plasmid pMJ-SHRV-NV− was created by PCR amplifying a portion of the NV gene from pMJ-SHRV using the primers ME 624 and ME 580. The primer ME 624 incorporated the restriction site NruI and contained two altered nucleotides which created the restriction site AflII. The two-nucleotide change in ME 624 also changed the 23rd codon of NV's ORF from an arginine to a stop codon. The ME 624/ME 580 PCR fragment was subcloned, digested with restriction enzymes NruI and SacII, and cloned back into the NruI and SacII sites of pMJ-SHRV to create the plasmid pMJ-SHRV-NV−.
Viral recovery.
Recovery of infectious virus from cells transfected with the cDNA clones was performed in 6-well (10-cm2) plates seeded with EPC cells at a concentration of approximately 5 × 106 cells/well at 16 h before the transfection. At the time of transfection, the cell monolayers were 70 to 90% confluent. Transfections were performed using the Lipofectamine Plus Reagent (Life Technologies) according to the manufacturer's instructions. In experiments where the cells were preinfected with vaccinia virus, 30 min before the cells were overlaid with the transfection media, they were infected with vaccinia virus vTF7-3 (7), which was kindly provided by Doug Grosenbach (Oregon State University). The infection was carried out at an MOI of 5 in 200 μl of MEM-0. Thirty minutes after infection, the cells were rinsed twice with MEM without serum (MEM-0) and overlaid with transfection medium. Following transfection, the cells were incubated at 31°C.
Verification of the recombinant SHRV.
RNA from the first passage of the presumed recombinant SHRV was collected as described above. Two reverse transcription reactions were prepared. The first reaction mixture contained 1 μg of total RNA with 50 pmol of random hexamers, 20 nmol each deoxynucleoside triphosphate, 4 μl of 5× AMV RT buffer, 10 U of RNasin (Promega), and 10 U of AMV RT (Promega) in a total volume of 20 μl. The second mixture included the same ingredients except it received 1 μl of H2O instead of AMV RT. Both reaction mixtures were incubated for 1 h at 42°C, after which they were diluted with 80 μl of H2O and 1 μl was used in the subsequent PCR. PCR was performed using Platinum Taq HF with primers ME 531 and ME 554. Three PCRs were performed, two with the reverse transcription reaction just described and a third with wild-type SHRV cDNA. Standard PCR amplification was performed except the reaction was carried out for only 25 cycles. The PCR products were analyzed by agarose gel electrophoresis and direct sequencing with primer ME 531.
Plaque assay.
Plaque assays were used to determine the titers of the wild-type SHRV and recombinant SHRV (3). Infected cells were incubated at 31°C for 20 h, after which they were fixed with crystal violet stain, which contained 3.5% formaldehyde and 0.1% crystal violet in H2O.
Electron microscopy.
Tissue culture supernatant fluid from infected cells were filtered through a 0.22-μm-pore-size Acrodisc filter (Gelman, Ann Arbor, Mich.) and then ultracentrifuged for 2 h in a SW 50.1 rotor (Beckman, Fullerton, Calif.). The virus pellets were resuspended in 40 μl of distilled water and layered onto an electron microscopy grid. The grids were stained with phosphotungstic acid for 1 min and examined with a transmission electron microscope (Philips).
RESULTS
Expression of T7 in fish cells.
The first challenge in developing a recombinant cDNA system for producing SHRV was finding a way of expressing T7 RNA polymerase in the cytoplasm of EPC cells. Expression of the T7 RNA polymerase gene mediated by either a transfected plasmid or by a recombinant vaccinia virus vector was evaluated. By using the first method, transfection with a plasmid capable of constitutive expression of the T7 polymerase was examined. The T7 polymerase gene was PCR amplified from the T7-expressing bacterial cell line BL21 LysE (Novagen) and subcloned into the vector pcDNA3 (InVitrogen). To test the pcDNA3-T7 vector, a reporter vector with luciferase under T7 promoter control was constructed. This reporter vector was constructed by cloning the luciferase gene from pGL2-Basic (Promega) into the T7 promoter expression vector pTM1 (5), creating pTM1-Luc. Upon cotransfection of the pcDNA3-T7 with pTM1-Luc (Fig. 2A), we saw a 10- to 20-fold increase in luciferase production over that of cells cotransfected with pTM1-Luc and pcDNA3.
FIG. 2.
T7 luciferase reporter assays. (A) Luciferase activity from duplicate samples of cells transfected with pTM1-Luc and pcDNA3 and cells transfected with pTM1-Luc and pcDNA3-T7. Transfections were carried out in 6-well (10-cm2) tissue culture plates. (B) Luciferase activity from duplicate samples of cells that were mock infected and then transfected with pTM1-Luc and cells that were infected with vTF7-3 and then transfected with pTM1-Luc. Transfections were carried out in 98-well (1-cm2) tissue culture plates. Note that the transfection conditions in panels A and B were not identical and thus cannot be compared with one another directly.
In the second method, T7 polymerase expression was mediated through a recombinant vaccinia vector. Vaccinia virus is a promiscuous virus which is able to infect fish cells, but is unable to complete a productive infection (D. Hruby and C. Franke, personal communication). To determine whether the recombinant T7 RNA polymerase expressing virus vTF7-3 (7) could produce active T7 RNA polymerase in EPC cells, the cells were either infected with vTF7-3 or mock infected and subsequently transfected with T7 expression vector pTM1-Luc (Fig. 2B). Cells preinfected with vTF7-3 expressed approximately 10-fold-higher levels of luciferase. At 24 h postinfection, the EPC cells preinfected with vTF7-3 did show some obvious signs of the viral infection, including cell rounding and some cell death, but the majority of infected cells remained attached to the monolayer.
N-, P-, and L-expression plasmids.
Several vectors were constructed for the expression of the viral proteins N, P, and L. These proteins are presumably required for SHRV transcription and replication, and previous studies with other rhabdoviruses showed that these viral genes were required for recovery of viable virus from cDNA clones. These genes were all amplified by PCR (see Materials and Methods) and subcloned into the expression vector pcDNA3 (Fig. 1B). The vector, pcDNA3, was particularly well suited for this project as it contains both a cytomegalovirus promoter, which has been proven to be highly active in fish cells (1), and a T7 promoter.
Next, a vector was constructed which encoded the entire SHRV genome. The details of the construction of this vector (pMJ-SHRV) are given in Materials and Methods. To summarize, the basic features of the constructed vector are as follows: a pUC19 backbone containing the origin of replication and an ampicillin resistance gene, a T7 promoter positioned so that the first transcribed nucleotide corresponded exactly with the first nucleotide of the positive strand of the SHRV genome, the full-length 11,550-nucleotide SHRV genome, the hepatitis delta virus ribozyme sequence, which is positioned to self-cleave the RNA at exactly the last nucleotide of SHRV's genome, and a T7 transcriptional stop site (Fig. 1B). A single alteration was made in the genome of SHRV. Between the NV and L genes, at the overlap of primers ME 579 and ME 580 (nucleotide 5451), a SacII restriction site was added.
Recombinant virus recovery.
Initial attempts to obtain recombinant SHRV were performed with the pcDNA3-T7 expression vector. Five wells containing EPCs were transfected with the following: 0.2 μg of pcDNA3-T7, 0.4 μg of pMJ-SHRV, 0.2 μg of pcDNA3-N, 0.15 μg of pcDNA3-P, or 0.05 of μg of pcDNA3-L. The ratio of expression vectors used in the transfection studies was designed to approximate the ratio of the different viral proteins produced in a wild-type viral infection. Similar ratios have been used in the production of recombinant vesicular stomatitis virus (12, 20). A sixth well was transfected with just pcDNA3. After 6 days, no CPE was seen. Cells were then trypsinized and subjected to three rounds of rapid freeze-thawing, and the supernatant was placed on fresh EPC monolayers. After 6 days, there was still no visible CPE. The transfection was repeated on fresh cells, but again no CPE was seen.
The second set of experiments to produce recombinant SHRV was carried out by preinfecting EPC cells with vaccinia virus vTF7-3 and then transfecting those cells with the individual SHRV gene plasmid vectors. In this experiment, cells in three wells were preinfected with vTF7-3 and then transfected with 0.5 μg of pMJ-SHRV, 0.25 μg of pcDNA3-N, 0.20 μg of pcDNA3-P, and 0.5 μg of pcDNA3-L. Cells in a fourth well were preinfected with vTF7-3 and transfected with 1 μg of the parental plasmid pcDNA3. Cells in a fifth well that were not treated with vaccinia virus received 1 μg of the parental plasmid pcDNA3 only. Within 48 h of the transfection, total CPE, i.e., all cells were nonadherent, was observed in all three experimental wells, while the cells in both negative-control wells were still in complete monolayers. The supernatant fluids from all five wells were collected into individual tubes at this time, and a portion from each sample tube was diluted 1:100 in MEM-10 and placed on a fresh monolayer. Within 48 h, cells overlaid with diluted supernatant from the initial experiment had undergone cytopathic changes characteristic of an SHRV lytic infection. Cells exposed to the supernatant fluids from negative-control wells remained unchanged.
Verification of recombinant SHRV production.
In order to verify the recombinant nature of the infectious agents, the engineered restriction sites in the genomes were verified. To do this, RNA was collected from virus after the first passage, of viruses and the RNA was either reverse transcribed or mock reverse transcribed. Following the reverse transcription, the cDNA from both reactions was PCR amplified using primers which flank the engineered restriction site. The expected PCR product of 2,041 bp was observed in all samples which received reverse transcriptase, but not in the controls, verifying that there was no residual plasmid DNA (data not shown). The PCR products were then sequenced, and all of the altered nucleotide sequences were identified to show that recombinant virus had indeed been produced from the pMJ-SHRV plasmid. The recombinant virus contained the engineered restriction site SacII between their NV and L genes. A separate reverse transcription PCR and sequencing was performed with the NV knockout virus to verify that the engineered NV stop codon was intact.
Recombinant NV knockout.
The finding that infectious, viable SHRV could be produced in tissue culture cells from a cDNA copy of the virus provided us with an important tool for examining the functions of nonstructural and regulatory sequences in the SHRV genome. The NV gene, which always contains a single ORF, is present in all known novirhabdoviruses, but the function of this gene is not known. We were very interested in examining the role of the NV gene, and all of our previous attempts to uncover its function had failed. With the recombinant cDNA expression system, we were now able to evaluate the importance of the NV ORF with an SHRV cDNA expression vector engineered to contain a truncated NV ORF. The vector pMJ-SHRV-NV− was created with a two-nucleotide alteration in the NV ORF. This mutation introduced a stop codon 23 amino acids into the NV protein (Fig. 3).
FIG. 3.
Schematic of the mutations introduced into pMJ-SHRV-NV−.
The recombinant virus recovery experiment was repeated as before using either the unmodified genome (pMJ-SHRV) or a second vector which had a truncated NV protein (pMJ-SHRV-NV−). In both cases, total CPE was achieved within 48 h. The final viral titer of both the unmodified virus and the virus with a truncated NV protein was >107 PFU/ml of supernatant, which is approximately the same titer achieved by wild-type SHRV.
Comparison of plaque formation and virus morphology.
The replication efficiency of the recombinant viruses appeared to be no different from that of the parental, wild-type virus. In our initial assessment of the replication competency of the recombinant viruses, we found no apparent difference between wild-type SHRV and unmodified recombinant SHRV or SHRV-NV− in the amount of virus produced and in the cell CPE. We also examined the plaque morphology produced by each virus. EPC cells were infected with wild-type, unmodified recombinant, or truncated NV recombinant SHRV and incubated for 20 h. The cells were then stained with crystal violet, and the plaque sizes and morphology were compared (Fig. 4). The plaques from all three viruses had approximately the same size and appearance, indicating that the biological activity of recombinant viruses in tissue culture cells were not substantially altered.
FIG. 4.
Viral plaques of wild-type SHRV (A), pMJ-SHRV recombinant virus (B), and pMJ-SHRV-NV− recombinant virus (C).
To assess whether the alterations in the genome of SHRV had any effect on viral morphology, virus particles were concentrated and viewed by negative staining with an electron microscope (Fig. 5). There were no obvious morphological differences between the three viruses.
FIG. 5.
Electron micrographs of virions of wild-type SHRV (A), pMJ-SHRV recombinant virus (B), and pMJ-SHRV-NV− recombinant virus (C). Bars, 100 nm.
DISCUSSION
The function of the novirhabdovirus NV protein has long been the subject of much speculation among fish virologists. The original report of NV indicated that it was present in high concentrations in IHNV-infected cells (10). Subsequent reports have shown that the NV protein is present in very low or undetectable levels in [35S]methionine-labeled cells infected with either IHNV, viral hemorrhagic septicemia virus, or hirame rhabdovirus (2, 14, 15, 19). Most recently, we have reported that we can no longer detect NV in IHNV-infected cells (3a). Because of the difficulty in detecting the NV protein in vitro, it has been suggested that the NV protein may not be essential to viral infection or may be required only in catalytic amounts (2). The NV mRNA has been shown to be stably expressed in the infected cell (3a), a finding that suggests that NV has an important role in replication. Nevertheless, we have clearly observed that the viral replication cycle in cell culture takes place in the absence of detectable amounts of the NV protein by pulse labeling. To address this issue of whether NV has a function in novirhabdovirus replication, we sought a method for constructing an NV knockout virus. We did this with SHRV and the mammalian T7 expression system.
Two systems for producing active T7 RNA polymerase within fish tissue culture cells were evaluated: transfection of cells with a T7 expression plasmid and infection of cells with a recombinant T7 expression vaccinia virus. Although both methods were successful in producing active T7 in transient reporter assays, only the vaccinia virus system was capable of mediating the production of recombinant virus. This inconsistency is probably the result of variable transfection efficiencies. Transfection efficiencies using eukaryotic expression vectors in EPC cells usually reach a maximum of 1 to 10%, whereas the vaccinia virus expression system presumably induced expression in virtually every cell. It is also possible that the two systems produced variable T7 levels within individual cells. Although the reporter assays indicated that both of the expression systems yielded a 10- to 20-fold induction of the reporter protein, this induction reflects the amount of induction within the total monolayer and not necessarily the T7 concentration within individual cells.
The yield of recombinant SHRV produced in this study appeared to be quite high, approximately 107 particles from a 10-cm2 well containing 106 cells or approximately 10 particles/cell. We believe that this yield is likely due to the amplification of a few bona fide recombinant viruses, since complete CPE was not attained in 24 h, which is the typical period for complete CPE at a MOI of 10 to 100 with SHRV in EPC cells. Rather, it took 48 h for the cell monolayer to exhibit complete CPE, and we estimate approximately 10 to 100 infectious particles were created per 106 cells. The high rate of production for recombinant SHRV may result from the fact that vaccinia virus infection of fish cells is nonproductive and nonlytic (Hruby and Franke, personal communication), and this may permit the cells to produce more recombinant virus.
The identity of the recombinant viruses was verified by reverse transcription PCR and sequencing. Negative-control PCRs were performed with recombinant virus RNA which had not been reverse transcribed. Because amplification was achieved only after reverse transcription, we concluded that the PCR product resulted from RNA and not from residual plasmid DNA. Analysis of the sequence of PCR products verified both that the RNA was indeed SHRV and that the modified nucleotide positions were maintained. This study marks the first occasion in which reverse genetics has been performed on a nonmammalian negative-strand virus.
On the basis of these findings, we proceeded to construct an SHRV genomic cDNA clone with two-nucleotide changes in the NV ORF that resulted in a stop codon early in the NV coding region. The virus produced from this template appeared to be no different from wild-type virus in infectivity, plaque and virion morphology, and presumably virus production. The NV mutant was designed so that the changes in the SHRV genome were minimal. The total sizes of the SHRV genome and the NV mRNA transcript, and presumably, the mRNA secondary structure remained unchanged. Only a single stop codon was introduced into this first NV knockout mutant virus, and we cannot be certain that there was not read-through the stop codon. However, these findings are consistent with the results of others who found that detectable quantities of NV protein was not required for virus replication (2, 3a). The study reported here constitutes a preliminary assessment of the NV gene in novirhabdoviruses. What is needed next is a set of experiments with deletion and insertion mutants of NV as well as experiments that examine one-step virus growth from plaque-cloned NV knockout virus. We must also examine the role of NV in virus pathogenesis in fish.
The NV gene is a perplexing phenomenon. Because rhabdoviruses have relatively small genomes, generally around 12 kb, little of the coding capacity is wasted. For most rhabdovirus genomes, greater than 90% of the nucleotides are used to code for protein. For this reason, it is reasonable to assume that an additional gene which has been retained in this genus must have a critical function. There are points of similarity and difference among the NV genes from the four known novirhabdoviruses (11). The most obvious similarity is conservation of location and size of the genes. All four NV genes are located between the glycoprotein and polymerase gene on the viral genome, and all four genes are approximately 500 nt long. In addition, all four genomes retain the NV gene with the transcriptional regulatory flanking sequences required for RNA initiation and termination. This would indicate that the NV mRNAs from all four viruses are produced. Finally, NV mRNAs from all four viruses encode a single ORF encoding a potential protein between 110 and 122 amino acids long. However, despite the conservation in size of the NV proteins, there is little if any sequence homology among the NV proteins. Although the five other proteins encoded by novirhabdoviruses contain between 30 to 60% amino acid identity among the four viruses, less than 20% amino acid identity exists among the NV proteins (13). Again, these observations suggest that NV protein is not important in virus replication, and we should consider experiments which alter the NV mRNA structure or size.
Although no obvious change in the growth of the NV knockout virus was observed in tissue-cultured cells, it is possible that the NV gene has a role in the virus pathogenesis in the whole animal. Unfortunately, no animal model for SHRV pathogenesis exists. The virus was isolated from snakehead fish in Thailand and found to be associated with chronic mortality among fish reared at high densities. When the virus was injected back into snakehead fish, it did not produce any disease symptoms. The current hypothesis is that SHRV will kill fish only when the fish are stressed and when other secondary pathogens, e.g., Aeromonas hydrophila, are present. We are trying to develop the reverse genetics system for IHNV in rainbow trout. For this particular virus, the disease model is well established and we may be able to address the question of whether NV has a role in virus pathogenesis in the whole animal.
ACKNOWLEDGMENTS
We thank Doug Grosenbach, Scott Hansen, and the rest of the Dennis Hruby laboratory for assistance with the vaccinia virus system.
This research was supported in part by the U.S. Department of Agriculture (the Western Regional Aquaculture Center) (grant 92-38500-7195, project no. 92080441), by an Oregon Sea Grant with funds from the Office of Sea Grant, National Oceanic and Atmospheric Administration, Department of Commerce (grant NA89AA-D-SG108, project R/FSD-16; grant NA36RG451, project R/FSD-23, and amendment no. 2), and by a grant from the USDA-NRI competitive grants program (grant CO140A).
Footnotes
Technical paper 11623 of the Oregon State University Agricultural Experiment Station.
REFERENCES
- 1.Anderson E D, Mourich D V, Leong J A. Gene expression in rainbow trout (Oncorhynchus mykiss) following intramuscular injection of DNA. Mol Mar Biol Biotechnol. 1996;5:105–113. [PubMed] [Google Scholar]
- 2.Basurco B, Benmansour A. Distant strains of the fish rhabdovirus VHSV maintain a sixth functional cistron which codes for a nonstructural protein of unknown function. Virology. 1995;212:741–745. doi: 10.1006/viro.1995.1534. [DOI] [PubMed] [Google Scholar]
- 3.Burke J A, Mulcahy D. Plaquing procedure for infectious hematopoietic necrosis virus. Appl Environ Microbiol. 1980;39:872–876. doi: 10.1128/aem.39.4.872-876.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Chiou P. Ph.D. thesis. Corvallis: Oregon State University; 1997. [Google Scholar]
- 4.Conzelmann K K. Genetic manipulation of non-segmented negative-strand RNA viruses. J Gen Virol. 1996;77:381–389. doi: 10.1099/0022-1317-77-3-381. [DOI] [PubMed] [Google Scholar]
- 5.Elroy-Stein O, Fuerst T R, Moss B. Cap-independent translation of mRNA conferred by encephalomyocarditis virus 5′ sequence improves the performance of the vaccinia virus/bacteriophage T7 hybrid expression system. Proc Natl Acad Sci USA. 1989;86:6126–6130. doi: 10.1073/pnas.86.16.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fijan N, Sulimanovic D, Bearzotti M, Muzinic D, Zwillenberg L O, Chilmonczyk S, Vautherot J F, de Kinkelin P. Some properties of the Epithelioma papulosum cyprini (EPC) cell line from carp Cyprinus carpio. Ann Virol (Paris) 1983;134:207–220. [Google Scholar]
- 7.Fuerst T R, Niles E G, Studier F W, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson M C, Maxwell J M, Leong J-A C. Molecular characterization of the glycoproteins from two warm water rhabdoviruses: snakehead rhabdovirus (SHRV) and rhabdovirus of penaeid shrimp (RPS)/spring viremia of carp virus (SVCV) Virus Res. 1999;64:95–106. doi: 10.1016/s0168-1702(99)00071-4. [DOI] [PubMed] [Google Scholar]
- 9.Kasornchandra J, Engelking H M, Lannan C N, Rohovec J S, Fryer J L. Characterization of three rhabdoviruses from snakehead fish Ophicephalus striatus. Dis Aquat Org. 1992;13:89–94. [Google Scholar]
- 10.Kurath G, Leong J C. Characterization of infectious hematopoietic necrosis virus mRNA species reveals a nonvirion rhabdovirus protein. J Virol. 1985;53:462–468. doi: 10.1128/jvi.53.2.462-468.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurath G, Higman K H, Bjorklund H V. Distribution and variation of NV genes in fish rhabdoviruses. J Gen Virol. 1997;78:113–117. doi: 10.1099/0022-1317-78-1-113. [DOI] [PubMed] [Google Scholar]
- 12.Lawson N D, Stillman E A, Whitt M A, Rose J K. Recombinant vesicular stomatitis viruses from DNA. Proc Natl Acad Sci USA. 1995;92:4477–4481. doi: 10.1073/pnas.92.10.4477. . (Erratum, 92:9009.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morzunov S P, Winton J R, Nichol S T. The complete genome structure and phylogenetic relationship of infectious hematopoietic necrosis virus. Virus Res. 1995;38:175–192. doi: 10.1016/0168-1702(95)00056-v. [DOI] [PubMed] [Google Scholar]
- 14.Nishizawa T, Yoshimizu M, Winton J R, Kimura T. Characterization of structural proteins of hirame rhabdovirus, HRV. Dis Aquat Org. 1991;10:167–172. [Google Scholar]
- 15.Nishizawa T, Yoshimizu M, Winton J R, Kimura T. Comparison of genome size and synthesis of structural proteins of hirame rhabdovirus, infectious hematopoietic necrosis virus, and viral hemorrhagic septicemia virus. Gyobyo Kenkyu. 1991;26:77–81. [Google Scholar]
- 16.Perrotta A T, Been M D. A pseudoknot-like structure required for efficient self-cleavage of hepatitis delta virus RNA. Nature. 1991;350:434–436. doi: 10.1038/350434a0. [DOI] [PubMed] [Google Scholar]
- 17.Rose J K. Positive strands to the rescue again: a segmented negative-strand RNA virus derived from cloned cDNAs. Proc Natl Acad Sci USA. 1996;93:14998–15000. doi: 10.1073/pnas.93.26.14998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schnell M J, Mebatsion T, Conzelmann K K. Infectious rabies viruses from cloned cDNA. EMBO J. 1994;13:4195–4203. doi: 10.1002/j.1460-2075.1994.tb06739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schutze H, Enzmann P-J, Kuchling R, Mundt E, Niemann H, Mettenleiter T C. Complete genomic sequence of the fish rhabdovirus infectious hematopoietic necrosis virus. J Gen Virol. 1995;76:2519–2527. doi: 10.1099/0022-1317-76-10-2519. [DOI] [PubMed] [Google Scholar]
- 20.Whelan S P, Ball L A, Barr J N, Wertz G T. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc Natl Acad Sci USA. 1995;92:8388–8392. doi: 10.1073/pnas.92.18.8388. [DOI] [PMC free article] [PubMed] [Google Scholar]