Abstract
We present the case of a 70-year-old never-smoking female patient with epidermal growth factor receptor (EGFR) p.L858R-mutated metastatic non-small cell lung cancer (NSCLC). After three months of first-line treatment with erlotinib, progression occurred and platinum/pemetrexed was initiated, followed by a response for more than two years. After the progression, the molecular testing of a vertebral metastasis revealed a ROS proto-oncogene 1 (ROS1) translocation and a human epidermal growth factor receptor 2 (HER2) p.S310F mutation, in addition to the known EGFR p.L858R mutation. Crizotinib then led to a durable response of 17 months. The molecular retesting of the tumour cells obtained from the recurrent pleural effusion revealed the absence of the ROS1 translocation, whereas the EGFR and HER2 mutations were still present. Afatinib was added to the crizotinib, and the combination treatment resulted in another durable response of more than two years. The patient died more than 7 years after the initial diagnosis of metastatic NSCLC. This case demonstrates that the repeated molecular testing of metastatic NSCLC may identify new druggable genomic alterations that can impact the patient management and improve the patient outcome.
Keywords: NSCLC, EGFR-mutated NSCLC, targeted therapies, drug combinations, molecular pathology
1. Introduction
The guidelines recommend repeated genomic tumour testing in metastatic non-small cell lung cancer (NSCLC) to guide the treatment decisions during the course of the disease [1,2]. The detection of targetable driver mutations ideally results in the treatment with targeted therapies. There is limited knowledge regarding the impact of the co-alterations that may alter the response to targeted therapies. Therefore, careful decision-making is crucial in this setting.
2. Case Presentation
A 70-year-old never-smoking female presented with a cough and dyspnoea during exercise in October 2015. Computed tomography (CT) and 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) showed a spiculated nodule in the lingula of the lung, additional bilateral pulmonary nodules and enlarged locoregional lymph nodes. Primary adenocarcinoma of the lung was diagnosed by bronchoalveolar lavage (BAL) and biopsy of a bronchial mass (TNM staging (7th edition): cT4, cN3, cM1a, stage IV). The next-generation sequencing (NGS; Ion AmpliSeq Colon and Lung Cancer Research Panel v2) of the tumour DNA revealed a classical epidermal growth factor receptor (EGFR) mutation in exon 21 (p.L858R; c.2573T > G). Additionally, a MET amplification (mean gene copy number 9; MET/CEN7-ratio 2.4) was detected by fluorescence in situ hybridization (FISH).
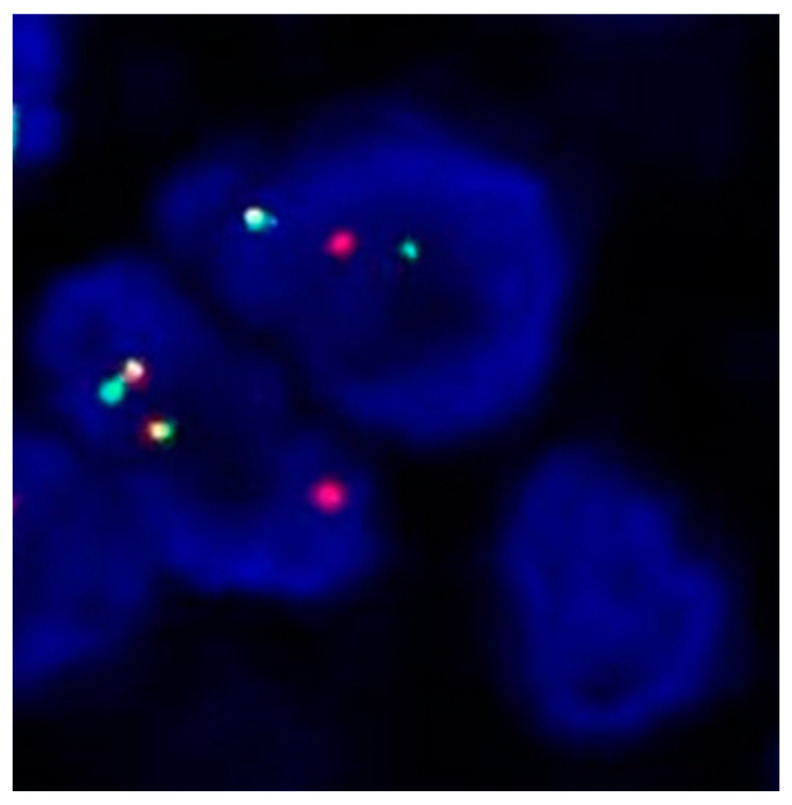
The patient was started on first-line erlotinib (150 mg daily) in November 2015, which led to a stable disease. In February 2016, the patient complained about increasing cough and dyspnoea due to pulmonary progression. The molecular testing of the carcinoma cells obtained by BAL showed no EGFR T790M mutation, and she was started on carboplatin and pemetrexed followed by pemetrexed maintenance, resulting in a partial remission for 32 months. In November 2018, the patient underwent spinal surgery for a pathological fracture of the 12th thoracic vertebra. Molecular testing (Oncomine Focus Assay) was performed on the epidural tumour tissue that was removed at the time of surgery. In addition to the initial EGFR p.L858R mutation, a human epidermal growth factor receptor 2 (HER2) mutation (p.S310F, c.929C > T) was discovered. Break-apart ROS proto-oncogene 1 (ROS1) FISH was positive for ROS1 translocation (split signals in 39% of tumour cell nuclei, Figure 1). Furthermore, FISH revealed persistence of the MET amplification (mean gene copy number 4.6; MET/CEN7 ratio 2.2).
Figure 1.
FISH analysis of NSCLC cells using ROS1 dual colour break-apart probe. Nuclei show a signal pattern characterized by separate red and green signals indicative of ROS1 translocation.
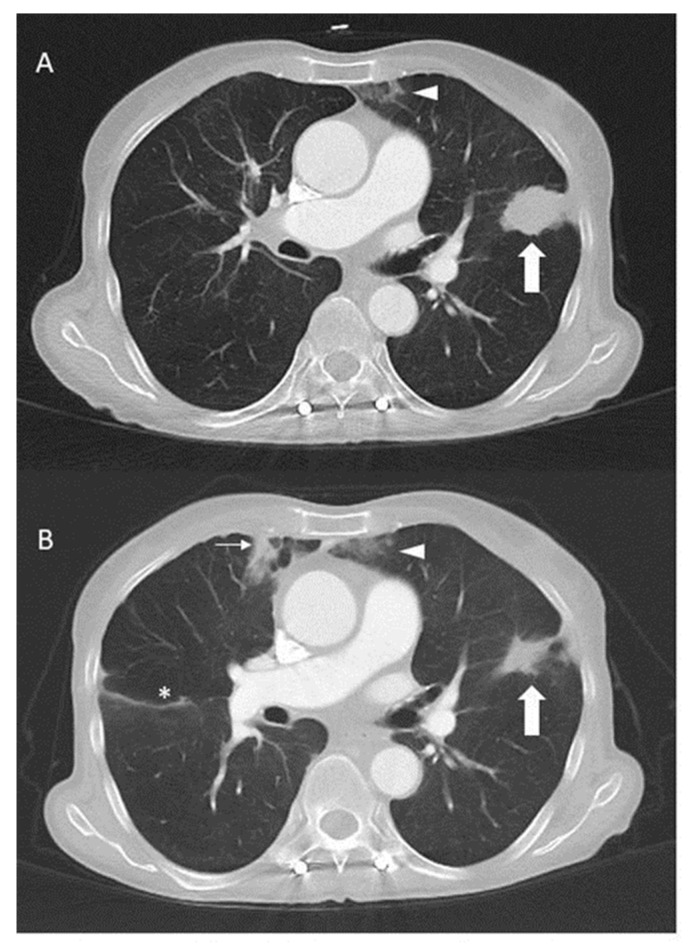
Third-line crizotinib monotherapy (250 mg twice per day) was started in February 2019, resulting in a partial remission lasting 17 months until July 2020, when the disease again progressed in the lungs and pleura. The molecular testing (Oncomine Focus Assay) of the tumour cells from the pleural effusion showed persistent EGFR p.L858R and HER2 p.S310F mutations, but no ROS1 fusion transcripts. In addition, the ROS1 FISH test was also negative. Due to the stable lesions interpreted as a response to the treatment outside of the thorax, crizotinib was continued and the pan-HER-inhibitor afatinib 30 mg daily was added to target EGFR and HER2 in October 2020. Crizotinib subsequently had to be reduced to 200 mg every other day due to oedema and afatinib to 20 mg at 2 out of 3 days due to skin toxicity. This time, a partial response with a clinical benefit could be observed for two years (Figure 2).
Figure 2.
Computed tomography of the thorax with contrast before (A) and three months after the addition (B) of crizotinib to afatinib in October 2020. The mass (thick arrow) in the lingula responded to therapy. Incidental findings include subpleural consolidations (thin arrow) and dystelectasis (arrowheads) and slight interlobar effusion (*) on the right side.
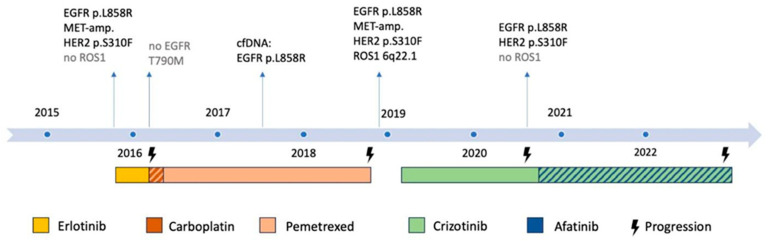
Unfortunately, the patient deteriorated in November 2022 due to progressive disease and confusion as a result of likely paraneoplastic multiple infarct dementia and died in December 2022, more than seven years after the initial diagnosis of metastatic NSCLC. The molecular findings in the course of the disease are summarized in Figure 3.
Figure 3.
Overview of the molecular testing results and treatments during the course of disease (including important results from retrospective testing). The hatchings indicate the treatment with a drug combination.
3. Discussion
In this case report, we describe the history of a never-smoking female patient with metastatic adenocarcinoma of the lung with an EGFR p.L858R mutation, a HER2 p.S310F mutation and a MET amplification developing a ROS1 translocation during the course of her disease. Our case illustrates the importance of repeated molecular testing upon disease progression in NSCLC patients treated with targeted therapy, which is also recommended in the international guidelines [1,2].
EGFR mutations occur in 10–20% of the lung adenocarcinomas in Western populations [3,4,5]. In our patient, the duration of disease control was rather short with erlotinib, a selective EGFR tyrosine kinase inhibitor (TKI). Although the outcome of the patients with the EGFR p.L858R mutation is inferior compared with EGFR exon 19 deletions, first-line therapy with erlotinib typically results in a progression-free survival in the range of 12 months [6,7,8]. One reason for the short duration of the response to erlotinib may have been the de novo MET amplification at the first diagnosis. MET amplifications are reported in approximately 5% of the patients with acquired resistance to first-generation EGFR TKIs [9,10,11] but are also rarely encountered as co-alterations in EGFR TKI-naïve patients [12,13]. The HER2 p.S310F mutation may also have contributed to the reduced response to erlotinib in our patient [14]. This mutation had already been present at the time of diagnosis, as demonstrated by the post hoc NGS analysis using a larger gene panel.
After the progression on EGFR TKI, the patient had a prolonged response to pemetrexed-based chemotherapy for more than two years. Surprisingly, at the time of the progression on pemetrexed, a ROS1 translocation was found by FISH (39% of the tumour cells with a split signal) upon molecular NSCLC retesting. However, ROS1 fusion transcripts could not be detected in a post hoc analysis of the tumour RNA using the FusionPlex Expanded Sarcoma Assay. The ROS1 translocation had not been present upon the initial diagnosis, which has been confirmed by a recently performed post hoc test using the tumour cells obtained at the time of the first diagnosis. More favourable outcomes with pemetrexed have been observed in ROS1-altered NSCLC compared to ALK- or EGFR-positive disease [15]. Co-alterations of the ROS1 and EGFR genes are exquisitely rare [16,17,18,19].
Based on the positive ROS1 FISH result, a systemic therapy with crizotinib, a TKI inhibiting ROS1, MET and ALK, was started [20,21,22,23]. Crizotinib has been investigated in the PROFILE 1001 trial and shown to be effective in ROS1-altered NSCLC with durable responses (median duration of response 24.7 months; 95% confidence interval (CI) 15.2–45.3) [21]. Our patient was able to clinically benefit for 2 years as well, suggesting that, indeed, ROS1, rather than MET, was likely the driver of the tumour progression. The patient developed new pleural effusions under the systemic treatment with crizotinib after approximately 15 months. Interestingly, the ROS1 translocation disappeared, whereas the known EGFR and HER2 mutations were still present. The addition of afatinib, a pan-HER-inhibitor, to crizotinib with the intention to target the HER2 and EGFR mutation led to another prolonged response of over 2 years [24]. Afatinib shows some activity not only against classical EGFR but also HER2-mutated tumours [25,26]. In addition, afatinib has been used successfully in a patient with metastatic lung adenocarcinoma harbouring an EGFR p.L858R mutation co-occurring with the same HER2 p.S310F mutation [27]. The preferred approach of the concurrent inhibition of all the detected alterations with a combination therapy rather than a single-agent therapy against the postulated resistance mutation has been recently shown in the INSIGHT 2 study [28,29]. In this study, patients with EGFR-mutated NSCLC and a MET amplification as the resistance mechanism showed a much higher response rate (ORR) with the combination of osimertinib and tepotinib compared to tepotinib alone (54.5% [95% CI 32.2–75.6] vs. 8.3% [95% CI 0.2–38.5]) [28].
According to this observation, nowadays, one would have preferably treated this patient with the combination of afatinib and crizotinib from the beginning of the detection of the resistance mutations.
Our case underlines the importance of repeated molecular testing in NSCLC treated with targeted therapies in order to individualise the systemic treatment in the course of the disease [1,2]. Due to tumour heterogeneity, mixed responses and oligoprogression on the EGFR TKIs may occur when subclonal tumour cells harbouring different EGFR or other gene alterations co-exist with tumour cells still harbouring sensitive EGFR mutations [30,31,32].
With broader and deeper molecular testing, the presence of co-mutations and the emergence of novel resistance mutations during TKI therapy are becoming increasingly challenging in clinical practice. Nowadays, the first-line treatment of patients with lung cancer harbouring a classical EGFR mutation (exon 19 deletion or L858R mutation in exon 21) is usually osimertinib, a third-generation irreversible EGFR TKI, with or without chemotherapy as the first-line therapy [33,34]. The emergence of ROS1 rearrangement as a resistance mechanism is extremely rare, although it has also been reported in this context [35].
4. Conclusions
Our case presentation underlines the importance of repeated testing for molecular alterations in the case of disease progression. The combination of afatinib and crizotinib in a patient with EGFR-, HER2-, ROS1- and MET-altered NSCLC led to a durable response and was well-tolerated after dose modification.
Author Contributions
Writing—Original Draft Preparation, E.P., M.F. and K.-L.K.; Writing—Review and Editing, all authors; Visualization, E.P., A.L. and W.J. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical approval is not required for this study in accordance with local guidelines.
Informed Consent Statement
Informed consent was obtained from the patient for publication of the details of her medical case and the accompanying images.
Data Availability Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Conflicts of Interest
Eva Plomer declares no conflict of interest. Martin Früh: advisory role: BMS, MSD, Astra Zeneca, Boehringer Ingelheim, Roche, Takeda, Pfizer, Janssen, Daiichi-Sankyo, Pharmamar; unrestricted grants to institution: BMS, Astra Zeneca. Arno Lauber declares no conflict of interest. Izadora Demmer declares no conflict of interest. Wolfram Jochum: advisory role: Astra Zeneca, Janssen, Merck, Stemline; research funding: Astra Zeneca (institution), Thermo Fisher Scientific (institution). Kira-Lee Koster: support for attending meetings and travel (paid to institution) from Janssen-Cilag and Takeda.
Funding Statement
There was no funding for this study.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hendriks L.E., Kerr K.M., Menis J., Mok T.S., Nestle U., Passaro A., Peters S., Planchard D., Smit E.F., Solomon B.J., et al. Oncogene-Addicted Metastatic Non-Small-Cell Lung Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023;34:339–357. doi: 10.1016/j.annonc.2022.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Hanna N.H., Robinson A.G., Temin S., Baker S., Brahmer J.R., Ellis P.M., Gaspar L.E., Haddad R.Y., Hesketh P.J., Jain D., et al. Therapy for Stage IV Non–Small-Cell Lung Cancer with Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. J. Clin. Oncol. 2021;39:1040–1091. doi: 10.1200/JCO.20.03570. [DOI] [PubMed] [Google Scholar]
- 3.Burnett H., Emich H., Carroll C., Stapleton N., Mahadevia P., Li T. Epidemiological and Clinical Burden of EGFR Exon 20 Insertion in Advanced Non-Small Cell Lung Cancer: A Systematic Literature Review. PLoS ONE. 2021;16:e0247620. doi: 10.1371/journal.pone.0247620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dearden S., Stevens J., Wu Y.-L., Blowers D. Mutation Incidence and Coincidence in Non Small-Cell Lung Cancer: Meta-Analyses by Ethnicity and Histology (MutMap) Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013;24:2371–2376. doi: 10.1093/annonc/mdt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordan E.J., Kim H.R., Arcila M.E., Barron D., Chakravarty D., Gao J., Chang M.T., Ni A., Kundra R., Jonsson P., et al. Prospective Comprehensive Molecular Characterization of Lung Adenocarcinomas for Efficient Patient Matching to Approved and Emerging Therapies. Cancer Discov. 2017;7:596–609. doi: 10.1158/2159-8290.CD-16-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosell R., Carcereny E., Gervais R., Vergnenegre A., Massuti B., Felip E., Palmero R., Garcia-Gomez R., Pallares C., Sanchez J.M., et al. Erlotinib versus Standard Chemotherapy as First-Line Treatment for European Patients with Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer (EURTAC): A Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 7.Zhou C., Wu Y.-L., Chen G., Feng J., Liu X.-Q., Wang C., Zhang S., Wang J., Zhou S., Ren S., et al. Erlotinib versus Chemotherapy as First-Line Treatment for Patients with Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer (OPTIMAL, CTONG-0802): A Multicentre, Open-Label, Randomised, Phase 3 Study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 8.Zhou C., Wu Y.L., Chen G., Feng J., Liu X.-Q., Wang C., Zhang S., Wang J., Zhou S., Ren S., et al. Final Overall Survival Results from a Randomised, Phase III Study of Erlotinib versus Chemotherapy as First-Line Treatment of EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer (OPTIMAL, CTONG-0802) Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015;26:1877–1883. doi: 10.1093/annonc/mdv276. [DOI] [PubMed] [Google Scholar]
- 9.Sequist L.V., Waltman B.A., Dias-Santagata D., Digumarthy S., Turke A.B., Fidias P., Bergethon K., Shaw A.T., Gettinger S., Cosper A.K., et al. Genotypic and Histological Evolution of Lung Cancers Acquiring Resistance to EGFR Inhibitors. Sci. Transl. Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu H.A., Arcila M.E., Rekhtman N., Sima C.S., Zakowski M.F., Pao W., Kris M.G., Miller V.A., Ladanyi M., Riely G.J. Analysis of Tumor Specimens at the Time of Acquired Resistance to EGFR-TKI Therapy in 155 Patients with EGFR-Mutant Lung Cancers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013;19:2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelman J.A., Zejnullahu K., Mitsudomi T., Song Y., Hyland C., Park J.O., Lindeman N., Gale C.-M., Zhao X., Christensen J., et al. MET Amplification Leads to Gefitinib Resistance in Lung Cancer by Activating ERBB3 Signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 12.Lu C., Kang J., Chen H.-J., Tu H.-Y., Zhou Q., Ye J.-Y., Wu Y.-L., Yang J.-J. Co-Occurring Alterations in Driver Genes Impact on EGFR-Targeted Therapy among Patients with EGFR-Mutant Advanced Non–Small Cell Lung Cancer. Ann. Oncol. 2018;29:ix158–ix159. doi: 10.1093/annonc/mdy425.025. [DOI] [Google Scholar]
- 13.Clement M.S., Ebert E.B.F., Meldgaard P., Sorensen B.S. Co-Occurring MET Amplification Predicts Inferior Clinical Response to First-Line Erlotinib in Advanced Stage EGFR-Mutated NSCLC Patients. Clin. Lung Cancer. 2021;22:e870–e877. doi: 10.1016/j.cllc.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Nagasaka M., Singh V., Baca Y., Sukari A., Kim C., Mamdani H., Spira A.I., Uprety D., Bepler G., Kim E.S., et al. The Effects of HER2 Alterations in EGFR Mutant Non-Small Cell Lung Cancer. Clin. Lung Cancer. 2022;23:52–59. doi: 10.1016/j.cllc.2021.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y.-F., Hsieh M.-S., Wu S.-G., Chang Y.-L., Yu C.-J., Yang J.C.-H., Yang P.-C., Shih J.-Y. Efficacy of Pemetrexed-Based Chemotherapy in Patients with ROS1 Fusion-Positive Lung Adenocarcinoma Compared with in Patients Harboring Other Driver Mutations in East Asian Populations. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2016;11:1140–1152. doi: 10.1016/j.jtho.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 16.Uguen A., Schick U., Quéré G. A Rare Case of ROS1 and ALK Double Rearranged Non-Small Cell Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2017;12:e71–e72. doi: 10.1016/j.jtho.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Lambros L., Guibourg B., Uguen A. ROS1-Rearranged Non-Small Cell Lung Cancers With Concomitant Oncogenic Driver Alterations: About Some Rare Therapeutic Dilemmas. Clin. Lung Cancer. 2018;19:e73–e74. doi: 10.1016/j.cllc.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Wiesweg M., Eberhardt W.E.E., Reis H., Ting S., Savvidou N., Skiba C., Herold T., Christoph D.C., Meiler J., Worm K., et al. High Prevalence of Concomitant Oncogene Mutations in Prospectively Identified Patients with ROS1-Positive Metastatic Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2017;12:54–64. doi: 10.1016/j.jtho.2016.08.137. [DOI] [PubMed] [Google Scholar]
- 19.Van Der Steen N., Mentens Y., Ramael M., Leon L.G., Germonpré P., Ferri J., Gandara D.R., Giovannetti E., Peters G.J., Pauwels P., et al. Double Trouble: A Case Series on Concomitant Genetic Aberrations in NSCLC. Clin. Lung Cancer. 2018;19:35–41. doi: 10.1016/j.cllc.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Christensen J.G., Zou H.Y., Arango M.E., Li Q., Lee J.H., McDonnell S.R., Yamazaki S., Alton G.R., Mroczkowski B., Los G. Cytoreductive Antitumor Activity of PF-2341066, a Novel Inhibitor of Anaplastic Lymphoma Kinase and c-Met, in Experimental Models of Anaplastic Large-Cell Lymphoma. Mol. Cancer Ther. 2007;6:3314–3322. doi: 10.1158/1535-7163.MCT-07-0365. [DOI] [PubMed] [Google Scholar]
- 21.Shaw A.T., Riely G.J., Bang Y.-J., Kim D.-W., Camidge D.R., Solomon B.J., Varella-Garcia M., Iafrate A.J., Shapiro G.I., Usari T., et al. Crizotinib in ROS1-Rearranged Advanced Non-Small-Cell Lung Cancer (NSCLC): Updated Results, Including Overall Survival, from PROFILE 1001. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019;30:1121–1126. doi: 10.1093/annonc/mdz131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ou S.-H.I. Crizotinib: A Novel and First-in-Class Multitargeted Tyrosine Kinase Inhibitor for the Treatment of Anaplastic Lymphoma Kinase Rearranged Non-Small Cell Lung Cancer and Beyond. Drug Des. Devel. Ther. 2011;5:471–485. doi: 10.2147/DDDT.S19045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies K.D., Le A.T., Theodoro M.F., Skokan M.C., Aisner D.L., Berge E.M., Terracciano L.M., Cappuzzo F., Incarbone M., Roncalli M., et al. Identifying and Targeting ROS1 Gene Fusions in Non-Small Cell Lung Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012;18:4570–4579. doi: 10.1158/1078-0432.CCR-12-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li D., Ambrogio L., Shimamura T., Kubo S., Takahashi M., Chirieac L.R., Padera R.F., Shapiro G.I., Baum A., Himmelsbach F., et al. BIBW2992, an Irreversible EGFR/HER2 Inhibitor Highly Effective in Preclinical Lung Cancer Models. Oncogene. 2008;27:4702–4711. doi: 10.1038/onc.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sequist L.V., Yang J.C.-H., Yamamoto N., O’Byrne K., Hirsh V., Mok T., Geater S.L., Orlov S., Tsai C.-M., Boyer M., et al. Phase III Study of Afatinib or Cisplatin Plus Pemetrexed in Patients With Metastatic Lung Adenocarcinoma with EGFR Mutations. J. Clin. Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 26.Schuler M., Yang J.C.-H., Park K., Kim J.-H., Bennouna J., Chen Y.-M., Chouaid C., De Marinis F., Feng J.-F., Grossi F., et al. Afatinib beyond Progression in Patients with Non-Small-Cell Lung Cancer Following Chemotherapy, Erlotinib/Gefitinib and Afatinib: Phase III Randomized LUX-Lung 5 Trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016;27:417–423. doi: 10.1093/annonc/mdv597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia Y., Ali S.M., Saad S., Chan C.A., Miller V.A., Halmos B. Successful Treatment of a Patient with Li-Fraumeni Syndrome and Metastatic Lung Adenocarcinoma Harboring Synchronous EGFR L858R and ERBB2 Extracellular Domain S310F Mutations with the Pan-HER Inhibitor Afatinib. Cancer Biol. Ther. 2014;15:970–974. doi: 10.4161/cbt.29173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazieres J., Kim T.M., Lim B.K., Wislez M., Dooms C., Finocchiaro G., Hayashi H., Liam C.K., Raskin J., Tho L.M., et al. LBA52 Tepotinib + Osimertinib for EGFRm NSCLC with MET Amplification (METamp) after Progression on First-Line (1L) Osimertinib: Initial Results from the INSIGHT 2 Study. Ann. Oncol. 2022;33:S1419–S1420. doi: 10.1016/j.annonc.2022.08.054. [DOI] [Google Scholar]
- 29.Tan D.S.-W., Kim T.M., Guarneri V., VOON P.J., Lim B.K., Wislez M., Huang C., Liam C.K., Mazieres J., Tho L.M., et al. Tepotinib + Osimertinib for EGFR Mutant (EGFRm) NSCLC with MET Amplification (METamp) after First-Line (1L) Osimertinib. J. Clin. Oncol. 2023;41:9021. doi: 10.1200/JCO.2023.41.16_suppl.9021. [DOI] [Google Scholar]
- 30.Chen Z.-Y., Zhong W.-Z., Zhang X.-C., Su J., Yang X.-N., Chen Z.-H., Yang J.-J., Zhou Q., Yan H.-H., An S.-J., et al. EGFR Mutation Heterogeneity and the Mixed Response to EGFR Tyrosine Kinase Inhibitors of Lung Adenocarcinomas. The Oncol. 2012;17:978–985. doi: 10.1634/theoncologist.2011-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taniguchi K., Okami J., Kodama K., Higashiyama M., Kato K. Intratumor Heterogeneity of Epidermal Growth Factor Receptor Mutations in Lung Cancer and Its Correlation to the Response to Gefitinib. Cancer Sci. 2008;99:929–935. doi: 10.1111/j.1349-7006.2008.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohsaka S., Petronczki M., Solca F., Maemondo M. Tumor Clonality and Resistance Mechanisms in EGFR Mutation-Positive Non-Small-Cell Lung Cancer: Implications for Therapeutic Sequencing. Future Oncol. Lond. Engl. 2019;15:637–652. doi: 10.2217/fon-2018-0736. [DOI] [PubMed] [Google Scholar]
- 33.Ramalingam S.S., Vansteenkiste J., Planchard D., Cho B.C., Gray J.E., Ohe Y., Zhou C., Reungwetwattana T., Cheng Y., Chewaskulyong B., et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2020;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 34.Planchard D., Jänne P.A., Cheng Y., Yang J.C.-H., Yanagitani N., Kim S.-W., Sugawara S., Yu Y., Fan Y., Geater S.L., et al. Osimertinib with or without Chemotherapy in EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2023;389:1935–1948. doi: 10.1056/NEJMoa2306434. [DOI] [PubMed] [Google Scholar]
- 35.Xu C., Li D., Duan W., Tao M. TPD52L1-ROS1 Rearrangement as a New Acquired Resistance Mechanism to Osimertinib That Responds to Crizotinib in Combination With Osimertinib in Lung Adenocarcinoma. JTO Clin. Res. Rep. 2020;1:100034. doi: 10.1016/j.jtocrr.2020.100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.