Abstract
Despite the high quality of soybean protein, raw soybeans and soybean meal cannot be directly included in animal feed mixtures due to the presence of Kunitz (KTi) and Bowman–Birk protease inhibitors (BBis), which reduces animal productivity. Heat treatment can substantially inactivate trypsin and chymotrypsin inhibitors (BBis), but such treatment is energy-intensive, adds expense, and negatively impacts the quality of seed proteins. As an alternative approach, we have employed CRISPR/Cas9 gene editing to create mutations in BBi genes to drastically lower the protease inhibitor content in soybean seed. Agrobacterium-mediated transformation was used to generate several stable transgenic soybean events. These independent CRISPR/Cas9 events were examined in comparison to wild-type plants using Sanger sequencing, proteomic analysis, trypsin/chymotrypsin inhibitor activity assays, and qRT-PCR. Collectively, our results demonstrate the creation of an allelic series of loss-of-function mutations affecting the major BBi gene in soybean. Mutations in two of the highly expressed seed-specific BBi genes lead to substantial reductions in both trypsin and chymotrypsin inhibitor activities.
Keywords: soybean, Glycine max (L. (Merr)), antinutritional factors, Bowman–Birk inhibitor, CRISPR-Cas9 enabled mutagenesis
1. Introduction
Soybean Glycine max (L.) Merr) is grown worldwide as a rotation partner for grain crops, particularly maize, due to an ability to fix atmospheric nitrogen, high seed yield potential, relatively few major biotic pathogens, and relatively low input costs [1,2]. Soybean seeds contain far more protein than other legumes (on average 40%), and soybean meal is often the first choice for swine and poultry feed mixtures due to a balanced (albeit suboptimal) amino acid profile. Soybean meal, produced after extraction of seed oil, has a relatively low cost and is of high quality compared to other competing protein meal sources [3].
Despite the high quality of soybean protein, raw soybeans and soybean meal cannot be directly included in animal feed mixtures due to anti-nutritional compounds that interfere with digestion, cause pancreatic inflammation, and effectively reduce animal weight gain. Soybean seeds contain two major classes of proteinaceous anti-nutritional factors [4], which were named after the scientists that discovered them: (1) Kunitz trypsin inhibitors [5] (KTis), which inactivate the digestive enzyme trypsin, and (2) Bowman–Birk inhibitors [6] (BBis), which inactivate both trypsin and chymotrypsin animal digestive enzymes [7]. In soybean, three KTi isoforms (KTi1, KTi2, and KTi3) have been reported [8,9,10]. There are at least 11 different BBi-encoding genes present in the soybean genome; however, expression analysis of RNAseq data reveals that only three of them are expressed at appreciable levels in developing seeds (Figure S1).
Experiments have conclusively shown that ingestion of high levels of active protease inhibitors is responsible for reduced animal weight gain and can even result in hypoglycemia, pancreatic hypertrophy, or liver damage [11]. To avoid these issues and permit the inclusion of soybean meal at high levels in animal feed, KTi and BBi anti-nutritional proteins in typical soybean seeds must be inactivated by heat treatment, which has been shown to reduce, but not eliminate, ANF activity [7]. Heat inactivation allows soybean meal to be included at moderately high levels in chicken and swine feed mixtures. While this step meets the needs of the industry, such treatment is energy-intensive, adds expense, and requires a balance between sufficiently reducing ANF activity and negatively impacting the quality of seed proteins [3,11].
One success story in genetically reducing soybean’s trypsin inhibitor activity was the identification and analysis of a soybean accession (PI 157740), which features a marked reduction (~40%) in trypsin inhibitor activity. This genetic source has been examined through both genetic analysis and animal feeding trials. Raw extruded protein meal lacking KTi3 protein was found to result in superior animal weight gain compared with raw soybeans [12] but not to a degree that could eliminate the need for heat inactivation. In our previous research, a soybean line (PI 68679) with reductions in trypsin inhibition due to a mutation affecting the KTi1 gene was identified [13]. Using hybridization, the KTi-1 mutation (PI 68679) was combined with the previously identified KTi-3 mutation (PI 542044), to yield soybean lines that lack both KTi1 and KTi3 protein. These soybean lines have lower trypsin inhibitor activity than the currently available KTi-3 mutant [13]. Unfortunately, all lines that have reduced KTi levels unexpectedly had unacceptable levels of trypsin inhibitor activity due to overaccumulation of BBi proteins in seeds of all mutant lines (KTi1, KTi3, and KTi1/KTi3).
To develop soybean lines that require extremely low or no heat-inactivation processing, we hypothesize that both KTi and BBi gene expression in seeds must be reduced or ideally eliminated. Naturally occurring BBi mutants have been identified in perennial soybean collections [14]. However, to the best of our knowledge, no naturally occurring G. max mutant or germplasm entry has been reported with substantively reduced BBi accumulation or activity. However, one report indicated that transgenic expression of a modified protein can lower BBi activity and resulted in viable plants that were able to produce seeds [15].
CRISPR/Cas9 gene editing [16,17] is now a routine practice for inducing targeted loss-of-function mutations in soybean [17,18,19]. This method could be leveraged to induce mutations of soybean seed BBi genes and decrease or eliminate their accumulation in seeds. In this study, we report on the creation of an allelic series of loss-of-function mutations affecting the major BBi seed-expressed genes in soybean, and mutation of two genes was shown to lead to substantial reductions in both trypsin and chymotrypsin inhibitor activities due to loss-of-function mutations affecting BBi genes.
2. Results
2.1. Generation of BBi Mutants by CRISPR/Cas9 Gene Editing
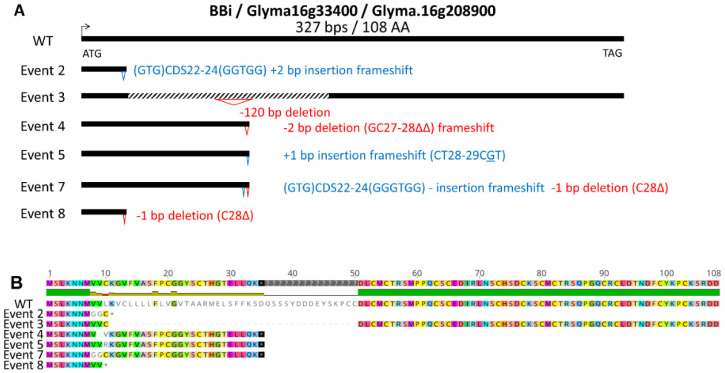
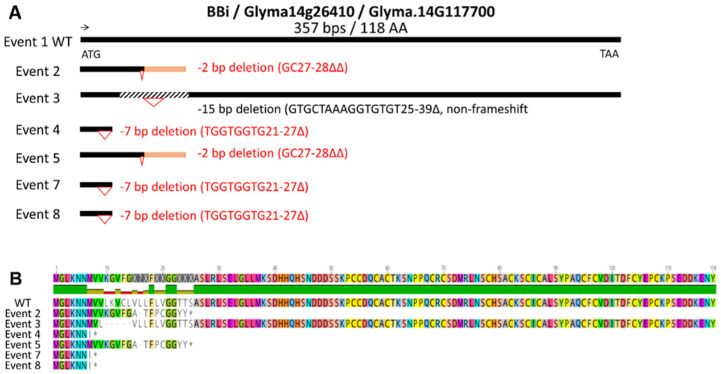
At least 11 BBi-encoding genes exist in the soybean genome; however, transcriptome analysis implicates only one major and two minor seed-specific BBi genes in developing soybean seeds (Supplementary Figure S1) [20]. Because BBi genes share extensive sequence homology, we designed target sites that enabled simultaneous knockout of all three seed-expressed genes, and two CRISPR/Cas9 expression cassettes were designed, which differed in use of either single or double guideRNA (Supplementary Figure S2). Utilizing these two CRISPR/Cas9 expression cassettes, we generated several independent transgenic events by Agrobacterium-mediated soybean transformation (Figure 1A). Six transgenic events were grown in the greenhouse, and leaf tissue from these plants was utilized for the isolation of genomic DNA. Sanger sequencing of PCR products generated by utilizing primers that are specific for the most abundantly expressed BBi gene (Glyma.16g208900) identified several mutations in this gene. Figure 1A shows a schematic summary of mutations identified in these six independent events leading to deletions, insertions, and frameshift mutations on the amino acid sequence (Figure 1B and Figure S3). Similarly, we also sequenced PCR products generated by utilizing primers that are specific for the second-most abundantly expressed BBi gene (Glyma.14G117700). This analysis revealed several mutations that introduced deletions, insertions, and frameshift mutations on the amino acid sequence (Figure 2A,B and Figure S3).
Figure 1.
CRISPR-Cas9 mutations identified in Glyma.16g208900, the most highly expressed BBi isoform. (A) Summary of mutations identified in six independent events. (B) Effect of CRISPR—CAS9-induced deletions, insertions, and frameshift mutations on the amino acid sequence. * = stop codon.
Figure 2.
CRISPR-Cas9 mutations identified in Glyma.14G117700, the second-most highly expressed BBi isoform. (A) Summary of mutations identified in six independent events. (B) Effect of CRISPR—CAS9-induced deletions, insertions, and frameshift mutations on the amino acid sequence. * = stop codon.
2.2. SDS-PAGE Analysis of Seed Proteins of CRISPR/Cas9 Transgenic Plants
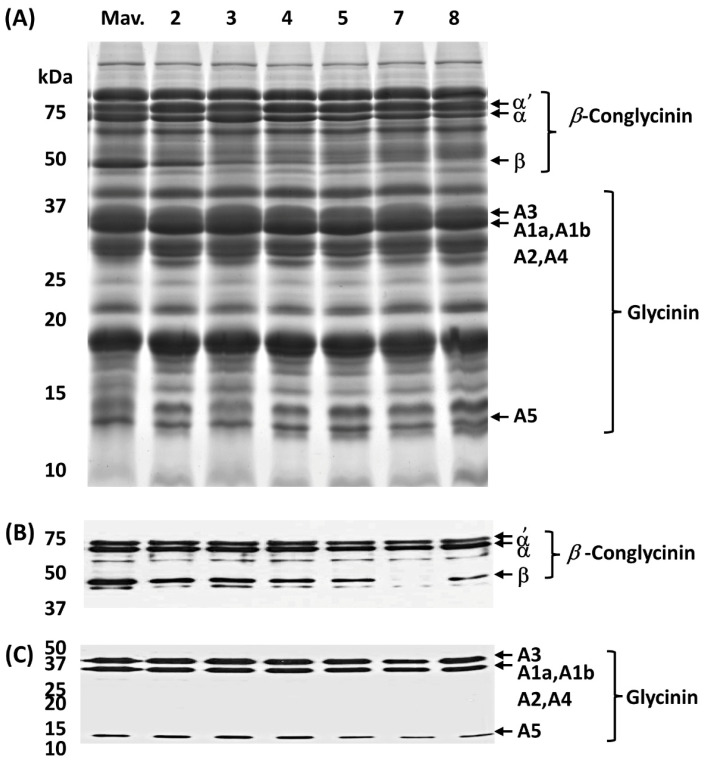
First, we wanted to compare the total seed protein profile between the wild-type and CRISPR/Cas9 transgenic plants. For this purpose, we first isolated total seed proteins from the wild-type and the six independent transgenic events and resolved them by SDS-PAGE (Figure 3A). A visual examination of the total seed protein profile between wild-type and transgenic events did not reveal any drastic alterations between them (Figure 3A). All soybean seeds examined accumulated the two most abundant seed storage proteins (7S β-conglycinin and 11S glycinin). However, the accumulation of the β-subunit of β-conglycinin, a 52 kDa glycoprotein, in all transgenic events was noticeably lower when compared to the wild type (Figure 3A). To substantiate these changes, we performed immunoblot analysis using antibodies raised against the 7S β-conglycinin and 11S glycinin antibodies. The 7S β-conglycinin antibody reacted against all three subunits. The accumulation pattern of the α′ and α subunits of β-conglycinin was similar between the wild-type and the six CRISPR/Cas9 transgenic events (Figure 3B). In contrast, the accumulation of the β-subunit of β-conglycinin was much lower in all transgenic events when compared to the wild type, and for one transgenic event (Figure 3B, lane 7) only an extremely faint band was detected. We also performed immunoblot analysis to quantify any changes in the 11S glycinin accumulation between wild-type and CRISPR/Cas9 transgenic events. The glycinin antibody recognized three glycinin species (A3; A1a, A1b, A2, and A4; A5). In contrast to the β-subunit of β-conglycinin, the accumulation of 11S glycinin showed little variation in abundance between the wild-type and the CRISPR/Cas9 transgenic events (Figure 3C).
Figure 3.
Proteomic analysis of total seed proteins from CRISPR-Cas9 BBi mutant and control lines. (A) Sodium dodecyl sulfate polyacrylamide gel electrophoresis of total seed proteins visualized with Coomassie Blue; (B) immunoblot using β-conglycinin-specific antibodies; (C) immunoblot using glycinin-specific antibodies. Samples in order are ‘Maverick’ (Mav., non-transgenic control); 2 = Event 2; 3 = Event 3; 4 = Event 4; 5 = Event 5; 7 = Event 7; 8 = Event 8.
2.3. Immunoblot Analysis Reveals the Absence of BBi in the Seeds of Transgenic BBi Mutants
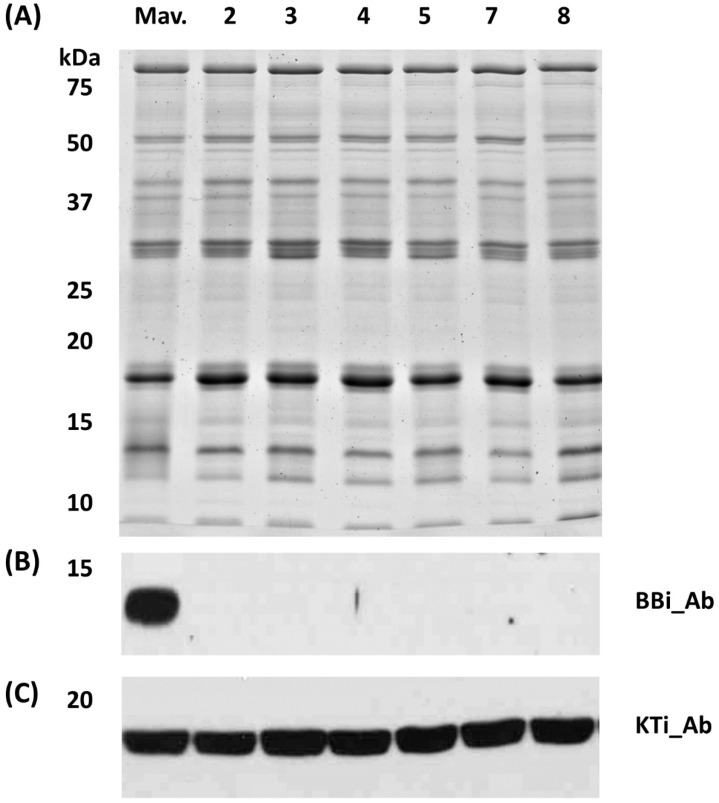
To examine the accumulation of BBi in seeds of the CRISPR/Cas9 transgenic events, we performed Western blot analysis utilizing BBi-specific peptide antibodies [21]. A protein fraction enriched for protease inhibitors obtained by the calcium fractionation method [22] was utilized for the immunoblot analysis and transferred to a nitrocellulose membrane. The nitrocellulose membrane when challenged with soybean BBi-specific antibodies failed to detect the accumulation of this protein in all transgenic events. In contrast, the BBi antibody reacted against a 11–12 kDa protein in the wild-type plant (Figure 4B). We also examined the accumulation of KTi, which also contributes significantly to the overall protease inhibitor of soybean seed, by immunoblot analysis, via antibodies raised against soybean KTi [9]. Immunoblot analysis clearly demonstrated normal accumulation of KTi proteins in all the plants examined in our study (Figure 4C).
Figure 4.
Proteomic analysis of soluble seed proteins from CRISPR-Cas9 BBi mutant and control lines. (A) Sodium dodecyl sulfate polyacrylamide gel electrophoresis of calcium fractionated soluble seed proteins visualized with Coomassie Blue; (B) immunoblot using Bowman–Birk inhibitor (BBi)-specific antibodies; (C) immunoblot using KTi-specific antibodies (KTis). Samples in order are ‘Maverick’ (Mav., non-transgenic control); 2 = Event 2; 3 = Event 3; 4 = Event 4; 5 = Event 5; 7 = Event 7; 8 = Event 8.
2.4. Chymotrypsin and Trypsin Inhibitor Activities Are Drastically Reduced in CRISPR/Cas9 Gene-Edited Transgenic BBi Mutants
Since our immunoblot analysis clearly showed the absence of BBi protein accumulation in the CRISPR/Cas9 gene-edited transgenic BBi mutants, we also wanted to examine protease inhibitor activities in these plants. The chymotrypsin inhibitor activity (CIU) was measured using N-Succinyl-Ala-Ala-Pro-Phe p-nitroanilide as a substrate and is expressed as CIU /mg seed powder. The wild-type soybean seed contained 17.2 CIU, while in transgenic seeds the activity ranged from 2.89 to 15.9 CIU. Out of the six transgenic events examined in this study, four had drastic reductions; we saw a 2.8–6.0-fold reduction in CIU compared to the wild type (Table 1). Since soybean BBi proteins can inhibit both chymotrypsin and trypsin, we also measured the trypsin inhibitor activity (TIU) using the same seed extracts (Table 1), with Nα-Benzoyl-DL-arginine 4-nitroanilide hydrochloride as a substrate. The trypsin inhibitor activity in the wild-type soybean seeds averaged 57.25 TIU/mg seed powder, whereas we saw reduced activities in mutant lines ranging from a low of 14.46 (Event 8) to a high of 35.7 TIU (Event 5) for different transgenic BBi events. This corresponds to a 1.6–4.0-fold reduction in TIU activities. TIU activity in all examined transgenic BBi mutants was significantly lower than in wild-type seeds. Moreover, in four of the transgenic events (Events 2, 4, 7, and 8; Table 1) we observed drastic reductions in the TIU activities ranging from 25.3 to 41.9% of the TIU activities found in wild-type seeds. Interestingly, the reduction in TIU activity in these BBi mutants is more significant than we observed for a naturally occurring soybean frameshift KTi-3 mutant (Table 1). CIU and TIU activities in seeds of the mutant and the control line were fully concordant when activities were expressed as TIU/mg seed powder or as TIU/mg seed protein (Table 1).
Table 1.
Analysis of trypsin inhibitor activity and chymotrypsin inhibitor activities in seeds from transgenic CRISPR-Cas9BBi-mutagenized and control plants. Tukey’s HSD indicates the results of a Tukey–Shapiro Honest Square Difference test; presence of the same letter indicates insignificant differences between genotypes.
| Seed Powder | Seed Protein | ||||
|---|---|---|---|---|---|
| Genotype | n= | Mean ± SD CIU/mg | Tukey’s HSD | Mean ± SD CIU/mg | Tukey’s HSD |
| ‘Maverick’ (WT) | 3 | 17.2 ± 1.91 | A | 56.93 ± 6.32 | A |
| KTi-3 | 3 | 18.55 ± 0.42 | A | 60.97 ± 1.39 | A |
| Event 5 | 3 | 15.93 ± 1.66 | A | 56.45 ± 5.89 | A |
| Event 3 | 3 | 15.35 ± 1.63 | A | 51.34 ± 5.44 | A |
| Event 4 | 3 | 6.2 ± 1.77 | B | 21.15 ± 6.03 | B |
| Event 2 | 3 | 4.19 ± 1.03 | B | 15.28 ± 3.77 | B |
| Event 7 | 3 | 3.9 ± 1.24 | B | 16.85 ± 5.38 | B |
| Event 8 | 3 | 2.89 ± 0.04 | B | 11.54 ± 0.17 | B |
| Seed Powder | Seed Protein | ||||
| Genotype | n= | Mean ± SD TIU/mg | Tukey’s HSD | Mean ± SD TIU/mg | Tukey’s HSD |
| ‘Maverick’ (WT) | 3 | 57.25 ± 0.35 | A | 189.48 ± 1.17 | A |
| KTi-3 | 3 | 36.45 ± 1.63 | B | 119.8 ± 5.35 | BC |
| Event 5 | 3 | 35.7 ± 1.27 | B | 126.55 ± 4.51 | B |
| Event 3 | 3 | 31.96 ± 1 | C | 106.88 ± 3.35 | C |
| Event 4 | 3 | 24 ± 0.59 | D | 81.87 ± 2.01 | D |
| Event 2 | 3 | 21.38 ± 0.06 | DE | 77.97 ± 0.21 | D |
| Event 7 | 3 | 19.75 ± 1.89 | E | 85.35 ± 8.15 | D |
| Event 8 | 3 | 14.46 ± 1.71 | F | 57.74 ± 6.82 | E |
2.5. qRT-PCR Analysis Confirms the Absence of Expression of the Abundant BBi Genes in CRISPR/Cas9 Gene-Edited Transgenic BBi Mutants
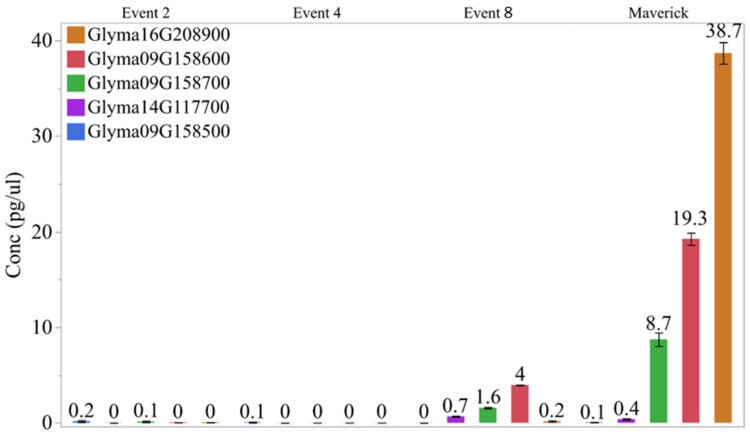
We also examined the expression of the two most abundantly expressed BBi genes (Glyma.16G208900 and Glyma.14G117700) along with a few other BBi genes (Glyma.09G158500, Glyma.09G158600, and Glyma.09G158700) in the CRISPR/Cas9 gene-edited transgenic BBi mutants. Total RNA was isolated from mid-stage (35 days after flowering) developing T1 soybean seeds and used for qRT-PCR analysis. The gene-specific primers used in our study are shown in Table S1. In the wild-type soybean seeds, we could detect the expression of most of these BBi genes. At this stage of seed development, we observed high level expression of Glyma.16G208900, Glyma.14G117700, and Glyma.09G158700 (Figure 5). In contrast, the expression of these abundantly expressed BBi genes was essentially not detected in two of the transgenic events (events 2 and 4), while several-fold lower expression was detected in one of the mutants (event 8).
Figure 5.
RT-PCR analysis of T1 seeds with CRISPR-Cas9 BBi mutant and control lines.
3. Discussion
In this study, we generated several stable transgenic soybean lines that exhibit the lowest levels of trypsin and chymotrypsin inhibitor activities that have been reported in the literature. Several of our CRISPR-Cas9 BBi lines have much lower trypsin inhibitor activity (62% to 75% lower) when compared to control plants. The relative abundance of KTi proteins in soybean seeds is higher than that of BBis [23]. It has been reported that BBi proteins are more heat stable than KTi proteins since the BBi contains multiple cysteine residues leading to conformational change in the BBi during heating [24]. Since our CRISPR-Cas9 BBi lines contain undetectable levels of BBi proteins, as evidenced by immunoblot analysis, one would expect that these newly developed BBi mutant lines may require only minimal heat treatment.
Until now, no soybean null mutants with reduced or ablated BBi proteins have been identified in G. max germplasm. However, BBi mutants have been identified in several perennial Glycine spp. [14]. Unfortunately, substantial hybridization barriers exist between perennial Glycine spp. and G. max, which has hampered the development of G. max BBi mutants by traditional breeding. Attempts have been made to lower the protease inhibitor activity in soybean seeds through overexpression of a mutated BBi gene [15]. The transgenic seeds generated in that study revealed a 20–50% reduction in protease inhibitory activity. In contrast, we have developed several CRISPR-Cas9-mutated BBi lines that showed remarkable reduction (ranging from 63 to 84%) in chymotrypsin inhibitor activity when compared to the wild-type seeds. Even though our CRISPR-Cas9 constructs were targeted to knockout seed-specific BBi genes, their pronounced effect on lowering the trypsin inhibitor is striking. This drastic reduction in trypsin inhibitor activity is not mediated by a reduction in the accumulation of KTi proteins in the CRISPR-Cas9 BBi lines. Our immunoblot analysis (Figure 4) clearly demonstrates that KTi proteins accumulate to the similar levels in BBi mutant lines as in wild-type seeds. Thus, despite the presence of functional versions of all three KTi seed-expressed genes, we saw a significant reduction in the trypsin inhibitor activity. This reduction is solely because of BBi mutations and unexpectedly revealed that BBi contributes significantly to overall trypsin inhibitor activity in soybean seeds.
Our immunoblot analysis (Figure 4) and qRT-PCR analysis (Figure 5) confirmed that the gRNA we employed in our study to knockout the seed-specific BBi genes was very effective. In addition to eliminating the expression of the two most abundant BBi genes (Glyma.16G208900 and Glyma.14G117700), other BBi genes (Glyma.09G158500, Glyma.09G158600, and Glyma.09G158700) were also eliminated in some the CRISPR-Cas9 BBi-induced mutant lines. This was an expected result as BBi genes share extensive sequence homology, and constructs were designed to target multiple BBi genes. The full number of mutated genes is unknown and will likely require creation and analysis of segregated populations to discern the number of mutated genes and their relative contributions to trypsin and chymotrypsin inhibition activities. One intriguing prospect is the combination of null mutations for both seed-expressed KTi genes and our newly identified null mutations affecting all seed-expressed BBi genes.
Our study also reveals proteome alteration due to knockout of BBi genes. One-dimensional gel analysis of total seed proteins from BBi mutant lines reveals lower accumulation of the β-subunit of β-conglycinin. The accumulation of this protein is influenced by the nitrogen and sulfur status of the plant [25,26]. The accumulation of the β subunit of β-conglycinin, a protein that is deficient in sulfur containing amino acids, is elevated when the plants are grown under limited sulfur supply conditions [27,28,29]. In the current study, there is a drastic reduction in the accumulation of BBis in all CRISPR-Cas9-mutated BBi lines. BBi contains 14 cysteine residues and acts as a main sink for sulfur storage in the seed [30,31]. In the absence of BBi accumulation, cysteine that would be normally incorporated in BBis will be freely available. We speculate that the reduction in the accumulation of the β-subunit of β-conglycinin could be related to an increase in the availability of cysteine in the seeds, which would be incorporated into other, normally lower abundance seed-expressed proteins. Our suggestion for this possibility is strengthened by previous reports that has shown that an increased availability of sulfur amino acids in transgenic soybean plants reduces the accumulation of β subunit of β-conglycinin [32]. However, this hypothesis remains to be rigorously tested through greenhouse and/or field studies. Although we clearly demonstrate the creation of an allelic series of BBi null mutations through CRISPR Cas9 mutagenesis, we acknowledge that we do not currently know what impact, if any, such mutations (or a combination of mutations) will have on field agronomic performance or seed yield. Similar work with KTi-3 mutant lines did not identify any deleterious impacts on whole-plant agronomic traits or seed yield [33].
Our results open the possibility that breeding lines could be developed that require substantially less heat treatment, which would save both money and energy used to heat treat soybeans. One study performed with KTi-3 and lectin-free seeds demonstrated that partial loss of trypsin/chymotrypsin inhibitors resulted in superior animal weight gain performance as compared to conventional soybean meal; however, both were inferior to heat-processed soybean meal [11]. Given our finding that our BBi mutants had reduced trypsin inhibitor activity equivalent to the KTi-3 mutation (Table 1), the BBi allelic series we have created may open the door to even greater reductions in trypsin and chymotrypsin inhibitor content in animal feed mixtures and for human food products, such as tofu and soymilk, while also reducing the energy required for heat processing.
4. Materials and Methods
4.1. Chemicals
Most chemicals and reagents used in this study were of analytical grade. Chemicals for electrophoresis, including acrylamide, bis acrylamide, SDS, TEMED, and ammonium persulfate, were purchased from GE healthcare (Piscataway, NJ, USA). Tris-HCL, trypsin, chymotrypsin, β-mercaptoethanol, Nα-Benzoyl-DL-arginine p-nitroanilide hydrochloride, and N-Succinyl-Ala-Ala-Pro-Phe p-nitroanilide were purchased from Sigma-Aldrich (St. Louis, MO, USA).
4.2. Analysis of Expression of BBi Genes Using Public Data Repositories
Soybase.org is a repository for data from multiple experimental datasets for soybean. We identified protein coding genes in the ‘Williams 82’ genome soybean with the annotation PFAM PF00228 (Bowman–Birk serine protease inhibitor family) as targets. Previously collected data from the ‘RNA-Seq Atlas’ [20] was used to prioritize target BBi genes for CRISPR-Cas9 constructs, gene sequencing, and RT-PCR analysis. In developing seeds, two genes, Glyma.14g117700 and Glyma.16g208900, represent between 17.0 and 31.3% and 68.5 and 82.8% of the total detectable BBi gene expression from seeds samples between 21 and 42 days after flowering.
4.3. Generation of CRISPR/CAS9-Induced BBi Knockout Soybean Mutants
For CRISPR/Cas9-mediated mutagenesis of the BBi genes in soybean, two single gRNAs were designed to target the two major expressed BBi genes and three other BBi genes. gRNA1: (5′-GAACAACATGGTGGTGCTAAAGG-3′) was a common target in five BBi genes (Glyma.09G158500; Glyma.14G117700; Glyma.09G158600; Glyma.09G158700; Glyma.16G202800), and gRNA2 (5′-CACATGCAGAGATCACAGCATGG-3′, reverse complimentary of the sense strand sequence 5′-CCATGCTGTGATCTCTGCATGTG-3′) was a common target in only 3 BBi genes (Glyma.09G158600; Glyma.09G158700; Glyma.16G202800). Two plasmid vectors were constructed: the first plasmid contained only gRNA1, and the second plasmid contained both gRNA1 and gRNA2. For cloning of the BBi gRNAs in the T-DNA vector, a two-step Golden Gate Assembly was performed firstly in the module vector pMOD_B2103 and then subsequently in the destination T-DNA vector pTRANS230d as described previously [18,34]. Recombinant pMOD_B2103_BBi gRNAs plasmids harboring BBi gRNAs were verified by Sanger sequencing of the gRNA cassette with primers flanking the gRNAs: the forward primer TC320 (5′-CTAGAAGTAGTCAAGGCGGC-3′) and reverse primer TC089R (5′-GGAACCCTAATTCCCTTATCTGG-3′), respectively. The second Gloden Gate Assembly reaction was composed of the recombinant pMOD_B2103_BBi gRNAs, pMOD_A0521, pMOD_C2906, and a destination T-DNA vector pTRANS230d. Final CRISPR/Cas9_BBi gRNA constructs in the destination T-DNA vector were designated as pTRANS230/BBi_1gRNA and pTRANS230/BBi_2gRNA, respectively. The CRISPR constructs were mobilized into a disarmed Agrobacterium tumefaciens strain EHA105 by a heat shock procedure.
4.4. Soybean Transformation
The soybean genotype ‘Maverick’ [35] was transformed using Agrobacterium-mediated transformation of half-seed explants. The transformation protocol was modified from Zeng et al. [36]. The process began by germinating sterilized seeds for 16 h in Petri dishes on germination media. Then, half seed explants were made from the seeds and treated with an Agrobacterium culture [37]. After 5 days of co-cultivation, the explants were transferred to shoot induction medium. Subsequently, the explants underwent two rounds of subcultures before being moved into shoot elongation medium. Elongated shoots were then cut from the explants and used for rooting. After 2 weeks, the rooted plants were transferred into soil. To identify putative transgenic soybean plants, a leaf paginating assay was performed with 100 mg L−1 glufosinate–ammonium solution on leaves at three stages. Herbicide-resistant plants were cultivated in greenhouses under controlled conditions, with a 16 h day at 28 °C and 8 h night at 24 °C until T1 seeds were harvested.
4.5. Protein Extraction and 1-D Electrophoresis
Total soybean seed protein extraction and subsequent separation of proteins by 1-D SDS-PAGE were carried out as previously described [38]. Briefly, dry soybean seeds were ground to a fine power with a mortar and pestle and extracted with 1 mL of sodium dodecyl sulfate (SDS) sample buffer (60 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol and 5% 2-mercaptoethanol). Tubes were vortexed (Vortex Genie, Scientific Industries, San Diego, CA, USA) for 10 min at room temperature and then heated to 100 °C for 5 min followed by centrifugation (Eppendorf, Beckman Coulter, Inc., Indianapolis, IN, USA) at 15,800× g for 5 min. The supernatant is the total seed protein fraction. This fraction was resolved by 13.5% SDS-PAGE gels using a Hoefer SE 260 minigel apparatus (Amersham Biosciences, Piscataway, NJ, USA). Electrophoretic separation of proteins was achieved with 20 mA per gel (constant current). SDS-PAGE-separated proteins were visualized using Coomassie Blue R-250 solution (0.3% Coomassie Brilliant Blue R-250, 45% methanol, and 10% glacial acetic acid).
4.6. Real-Time qRT-PCR Analysis of BBi Genes in Developing Soybean Seeds
Total RNA from 35 days after anthesis soybean seeds was extracted using TRIzol reagent (Invitrogen, Waltham, MA, USA) according to the manufacturer’s instructions. Real-time qRT-PCR was conducted using a LightCycler 480 II instrument (Roche, Indianapolis, IN, USA). Each qRT-PCR reaction was conducted with two replications in 20 µL using the QuantiNova SYBR Green RT-PCR Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. The cycling condition of absolute quantification analysis were 10 min at 50 °C then 2 min 95 °C for cDNA synthesize followed by 40 cycles of 20 s at 95 °C and 40 s at 60 °C, with the data acquired at 60 °C in the green channel. The reference gene (F-box protein, Glyma.12G051100) was amplified by RT-PCR from Maverick soybean seed total RNA with a gene-specific primer set (Supplementary Table S1). The amplified reference gene RT-PCR fragment was purified from agarose gel, and the standard was calculated based on the purified fragment concentration and molecular weight. The reference gene was subjected to a 10-fold serial dilution (1.00 × 101 to 1.00 × 10−10) with DEPC. For absolute quantification analysis, five standard dilutions from 1.00 × 10−5 to 1.00 × 10−9 for a standard curve were used for quantifying both target and reference gene expression in cDNA samples.
4.7. Screening of Putative Transgenic Plants and Identification of CRISPR-CAS9-Induced Mutations
Twenty seeds from 10 individual T0 BBi events were planted in the Sears Plant greenhouse in 2-gallon pots containing Pro-Mix® HP Mycorrhizae™ (Premier Tech, Quakertown, PA, USA). The plants were fertilized with Osmocote Plus (Scotts, Marysville, OH, USA) according to the manufacturer’s recommendations. Greenhouse settings were 16 h daylength and 30 °C/18 °C day/night temperatures. For every plant that germinated, fresh tissue was collected, and genomic DNA isolated from ~20–30 mg lyophilized leaf tissue using a Maxwell Promega robot with the Maxwell RSC PureFood GMO and Authentication Kit (Promega, Madison, WI, USA).
PCR primer pairs were designed to bear at least two gene-specific SNP differences as compared to homologous genes. Primers used for PCR amplification and sequencing are detailed in Supplementary Table S1. We blasted all primers against the unmasked Glycine max ‘Williams 82’ genomic sequence (E-value cut off = 10.0) to ensure primer specificity (www.soybase.org, accessed on 15 November 2021). PCR amplification was performed using Titanium taq according to the manufacturer’s recommendations (Takara, Kusatsu, Japan) in a PTC-200 thermocycler (MJ Research/Bio-Rad, Hercules, CA, USA), using these conditions: 95 °C for an initial 5 min denaturation, followed by 40 cycles of 95 °C for 30 s, followed by 60 °C for 30 s, and an extension step at 72 °C for 1 min/kilobase of the target sequence. PCR products were separated on a 1% agarose gel and compared to DNA ladder size standards to ensure appropriate size. PCR products were purified through use of a QIAquick PCR purification kit (Qiagen, Hilden, Germany). After PCR purification, products were Sanger-sequenced at the DNA Core Facility of the University of Missouri-Columbia.
PCR product sequencing traces were imported into Geneious Primer software, version 11.0.11+9 (Invitrogen); trimmed; and aligned to gene sequences from the G275_W82.a2.v1 assembly (http://www.phytozome.net/soybean, accessed on 12 February 2022) using the Geneious Primer software, version 11.0.11+9 (Invitrogen). Disagreements were manually assessed in comparison to the G275_W82.a2.v1 assembly [39]. Putative indels and polymorphisms were verified by a minimum of two independent PCR reactions.
4.8. Immunoblot Analysis
Total seed proteins isolated from wild-type soybean cultivar ‘Maverick’ and T1:2 seeds of BBi-mutated CRISPR/CAS9 lines were resolved on 13.5% SDS-PAGE gels. Resolved proteins were transferred to nitrocellulose membranes and incubated with TBS (10 mM Tris-HCl, pH 7.5, 500 mM NaCl) containing 5% non-fat dry milk for 1 h at room temperature. Following this step, the nitrocellulose membranes were washed 3X with TBST (15 min each) and incubated overnight with 1:10,000 diluted antibodies raised either against purified soybean Kunitz trypsin or Bowman–Birk protease inhibitors. The 7S β-conglycinin and 11S glycinin-specific antibodies were used at 1:50,000 dilution. Specifically bound primary antibodies were detected using anti-rabbit IgG-horseradish peroxidase conjugates and a SuperSignal West Pico kit (Pierce, Rockford, IL, USA) as previously described [21].
4.9. Trypsin and Chymotrypsin Inhibitor Assay
Trypsin inhibitor activity in different BBi-mutated CRISPR/CAS9 lines was measured following the procedure described by [40]. Briefly, 20 mg of dry soybean seed powder was placed in a 2 mL Eppendorf centrifuge tube. To this, 1 mL of 10 mM NaOH solution was added and vigorously agitated on a vortex mixer for 10 min at room temperature. The resulting slurry was clarified by centrifugation at 16,000× g for 10 min. Clear supernatant was utilized for measuring KTi activity. Trypsin inhibitor activity was expressed as trypsin units inhibited (TUI) per mg of sample, and results are expressed as the mean ± SD from three biological replicates.
Chymotrypsin inhibitor activity in soybean seed extracts were measured N-Succinyl-Ala-Ala-Pro-Phe p-nitroanilide (AAPF) as the substrate. Soybean seed extract, which resulted in 40 to 60% chymotrypsin inhibition range, was added to a 1.5 mL Eppendorf tube containing 900 µL of assay buffer (100 mM-Tris-HCL, pH 8.0), 8 µL of α-chymotrypsin (Sigma-Aldrich Company) dissolved in 1 mM HCl solution (0.1 mg/mL), and seed extract (8 to 10 µL) and incubated at 37 °C for 5 min. Following this step, 80 µL of AAPF (1 mg/mL) was added and incubated for an additional 10 min at 37 °C. The assay was stopped by adding 500 µL of 30% acetic acid. Absorbance at 410 nm was measured, and chymotrypsin inhibitor units were calculated as the amount of inhibitor that reduced the absorbance at 410 nm by 0.1 optical density. The results are expressed as the mean ± SD from three biological replicates. Trypsin and chymotrypsin inhibitor assay data were visualized and compared using JMP statistical software 16.0 (SAS Institute Inc., Cary, NC, USA). One-way ANOVA was performed, and significant differences (α = 0.05) between means were determined using the Tukey–Kramer HSD test.
Acknowledgments
The authors would like to thank Nathan Oehrle for his excellent technical assistance. Mention of a trademark, vendor, or proprietary product does not constitute a guarantee or warranty of the product by the USDA and does not imply its approval to the exclusion of other products or vendors that may also be suitable. The US Department of Agriculture, Agricultural Research Service, Midwest Area, is an equal opportunity, affirmative action employer and all agency services are available without discrimination.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25115578/s1.
Author Contributions
H.B.K., W.-S.K. and J.D.G. conceptualized the original project. Experimental work was performed by W.-S.K., S.K., J.D.G., J.L., M.R.J., H.B.K., R.M.S. and J.L. contributed new reagents and CRISPR/Cas9 constructs. The original manuscript was written by H.B.K. and J.D.G. All authors were involved in reviewing and editing the manuscript. The project administration was led by H.B.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data supporting the findings of this study are provided in the manuscript and Supplementary Information.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
Funding for this research was provided by United Soybean Board (2020-152-0114 and 2335-203-0101) and Agricultural Research Service, USDA (8042-21220-234-00D) and Minnesota Soybean Research and Promotion Council (3006-11028-00103891).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bullock D.G. Crop rotation. Crit. Rev. Plant Sci. 1992;11:309–326. doi: 10.1080/07352689209382349. [DOI] [Google Scholar]
- 2.Mohammad Sohidul I., Imam M., Islam M.R., Hasan M.K., Hafeez A.S.M.G., Hosen M.M., Saneoka H., Ueda A., Liu L., Naz M., et al. Soybean and Sustainable Agriculture for Food Security. In: Takuji O., Yoshihiko T., Norikuni O., Takashi S., Sayuri T., editors. Soybean. IntechOpen; Rijeka, Croatia: 2022. Chapter 1. [DOI] [Google Scholar]
- 3.Ruiz N., Parsons C., Stein H., Coon C., Eys J., Miles R. A Review: 100 Years of Soybean Meal. ADM; Chicago, IL, USA: 2020. [Google Scholar]
- 4.Vorster J., van der Westhuizen W., du Plessis G., Marais D., Sparvoli F., Cominelli E., Camilli E., Ferrari M., Le Donne C., Marconi S., et al. In order to lower the antinutritional activity of serine protease inhibitors, we need to understand their role in seed development. Front. Plant Sci. 2023;14:1252223. doi: 10.3389/fpls.2023.1252223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunitz M. Crystalline Soybean Trypsin Inhibitor: II. General Properties. J. Gen. Physiol. 1947;30:291–310. doi: 10.1085/jgp.30.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowman D.E. Fractions derived from soy beans and navy beans which retard tryptic digestion of casein. Proc. Soc. Exp. Biol. Med. 1944;57:139–140. doi: 10.3181/00379727-57-14731P. [DOI] [Google Scholar]
- 7.DiPietro C.M., Liener I.E. Heat inactivation of the Kunitz and Bowman-Birk soybean protease inhibitors. J. Agric. Food Chem. 1989;37:39–44. doi: 10.1021/jf00085a010. [DOI] [Google Scholar]
- 8.Jofuku K.D., Schipper R.D., Goldberg R.B. A frameshift mutation prevents Kunitz trypsin inhibitor mRNA accumulation in soybean embryos. Plant Cell. 1989;1:427–435. doi: 10.1105/tpc.1.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnan H.B. Characterization of a soybean [Glycine max (L.) Merr.] mutant with reduced levels of Kunitz trypsin inhibitor. Plant Sci. 2001;160:979–986. doi: 10.1016/S0168-9452(01)00346-6. [DOI] [PubMed] [Google Scholar]
- 10.Orf J., Hymowitz T. Inheritance of the Absence of the Kunitz Trypsin Inhibitor in Seed Protein of Soybeans 1. Crop Sci. 1979;19:107–109. doi: 10.2135/cropsci1979.0011183X001900010026x. [DOI] [Google Scholar]
- 11.Palacios M., Easter R., Soltwedel K., Parsons C., Douglas M., Hymowitz T., Pettigrew J. Effect of soybean variety and processing on growth performance of young chicks and pigs. J. Anim. Sci. 2004;82:1108–1114. doi: 10.2527/2004.8241108x. [DOI] [PubMed] [Google Scholar]
- 12.Bernard R.L., Hymowitz T., Cremeens C.R. Registration of ‘Kunitz’ Soybean. Crop Sci. 1991;31:232–233. doi: 10.2135/cropsci1991.0011183X003100010059x. [DOI] [Google Scholar]
- 13.Gillman J.D., Kim W.-S., Krishnan H.B. Identification of a new soybean kunitz trypsin inhibitor mutation and its effect on bowman− birk protease inhibitor content in soybean seed. J. Agric. Food Chem. 2015;63:1352–1359. doi: 10.1021/jf505220p. [DOI] [PubMed] [Google Scholar]
- 14.Kollipara K.P., Singh R.J., Hymowitz T. Inheritance of Protease Inhibitors in Glycine tomentella Hayata (2n = 38), a Perennial Relative of Soybean. J. Hered. 1996;87:461–463. doi: 10.1093/oxfordjournals.jhered.a023038. [DOI] [Google Scholar]
- 15.Livingstone D., Beilinson V., Kalyaeva M., Schmidt M.A., Herman E.M., Nielsen N.C. Reduction of protease inhibitor activity by expression of a mutant Bowman-Birk gene in soybean seed. Plant Mol. Biol. 2007;64:397–408. doi: 10.1007/s11103-007-9163-x. [DOI] [PubMed] [Google Scholar]
- 16.Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N., Hsu P.D., Wu X., Jiang W., Marraffini L.A. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mali P., Yang L., Esvelt K.M., Aach J., Guell M., DiCarlo J.E., Norville J.E., Church G.M. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J., Gunapati S., Mihelich N.T., Stec A.O., Michno J.M., Stupar R.M. Genome Editing in Soybean with CRISPR/Cas9. Methods Mol. Biol. 2019;1917:217–234. doi: 10.1007/978-1-4939-8991-1_16. [DOI] [PubMed] [Google Scholar]
- 19.Sun X., Hu Z., Chen R., Jiang Q., Song G., Zhang H., Xi Y. Targeted mutagenesis in soybean using the CRISPR-Cas9 system. Sci. Rep. 2015;5:10342. doi: 10.1038/srep10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Severin A.J., Woody J.L., Bolon Y.-T., Joseph B., Diers B.W., Farmer A.D., Muehlbauer G.J., Nelson R.T., Grant D., Specht J.E., et al. RNA-Seq Atlas of Glycine max: A guide to the soybean transcriptome. BMC Plant Biol. 2010;10:160. doi: 10.1186/1471-2229-10-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnan H.B., Kim S., Pereira A.E., Jurkevich A., Hibbard B.E. Adenanthera pavonina, a potential plant-based protein resource: Seed protein composition and immunohistochemical localization of trypsin inhibitors. Food Chem. X. 2022;13:100253. doi: 10.1016/j.fochx.2022.100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnan H.B., Oehrle N.W., Natarajan S.S. A rapid and simple procedure for the depletion of abundant storage proteins from legume seeds to advance proteome analysis: A case study using Glycine max. Proteomics. 2009;9:3174–3188. doi: 10.1002/pmic.200800875. [DOI] [PubMed] [Google Scholar]
- 23.Dipietro C., Liener I. Soybean protease inhibitors in foods. J. Food Sci. 1989;54:606–609. doi: 10.1111/j.1365-2621.1989.tb04663.x. [DOI] [Google Scholar]
- 24.He H., Li X., Kong X., Hua Y., Chen Y. Heat-induced inactivation mechanism of soybean Bowman-Birk inhibitors. Food Chem. 2017;232:712–720. doi: 10.1016/j.foodchem.2017.04.061. [DOI] [PubMed] [Google Scholar]
- 25.Sexton P., Naeve S., Paek N., Shibles R. Sulfur availability, cotyledon nitrogen: Sulfur ratio, and relative abundance of seed storage proteins of soybean. Crop Sci. 1998;38:983–986. doi: 10.2135/cropsci1998.0011183X003800040017x. [DOI] [Google Scholar]
- 26.Paek N.C., Imsande J., Shoemaker R.C., Shibles R. Nutritional control of soybean seed storage protein. Crop Sci. 1997;37:498–503. doi: 10.2135/cropsci1997.0011183X003700020031x. [DOI] [Google Scholar]
- 27.Holowach L.P., Thompson J.F., Madison J.T. Storage protein composition of soybean cotyledons grown in vitro in media of various sulfate concentrations in the presence and absence of exogenous L-methionine. Plant Physiol. 1984;74:584–589. doi: 10.1104/pp.74.3.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gayler K.R., Sykes G.E. Effects of nutritional stress on the storage proteins of soybeans. Plant Physiol. 1985;78:582–585. doi: 10.1104/pp.78.3.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujiwara T., Hirai M.Y., Chino M., Komeda Y., Naito S. Effects of sulfur nutrition on expression of the soybean seed storage protein genes in transgenic petunia. Plant Physiol. 1992;99:263–268. doi: 10.1104/pp.99.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke E., Wiseman J. Developments in plant breeding for improved nutritional quality of soya beans I. Protein and amino acid content. J. Agric. Sci. 2000;134:111–124. doi: 10.1017/S0021859699007431. [DOI] [Google Scholar]
- 31.BIRK Y. The Bowman-Birk inhibitor. Trypsin-and chymotrypsin-inhibitor from soybeans. Int. J. Pept. Protein Res. 1985;25:113–131. doi: 10.1111/j.1399-3011.1985.tb02155.x. [DOI] [PubMed] [Google Scholar]
- 32.Kim W.-S., Sun-Hyung J., Oehrle N.W., Jez J.M., Krishnan H.B. Overexpression of ATP sulfurylase improves the sulfur amino acid content, enhances the accumulation of Bowman–Birk protease inhibitor and suppresses the accumulation of the β-subunit of β-conglycinin in soybean seeds. Sci. Rep. 2020;10:14989. doi: 10.1038/s41598-020-72134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orf J.H. Modifying soybean composition by plant breeding; Proceedings of the Soybean Utilization Alternatives Conference, University of Minnesota; Minneapolis, NM, USA. 16–18 February 1988. [Google Scholar]
- 34.Čermák T., Curtin S.J., Gil-Humanes J., Čegan R., Kono T.J.Y., Konečná E., Belanto J.J., Starker C.G., Mathre J.W., Greenstein R.L., et al. A Multipurpose Toolkit to Enable Advanced Genome Engineering in Plants. Plant Cell. 2017;29:1196–1217. doi: 10.1105/tpc.16.00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sleper D.A., Nickell C.D., Noel G.R., Cary T.R., Thomas D.J., Clark K.M., Rao Arelli A.P. Registration of ‘Maverick’ Soybean. Crop Sci. 1998;38:549–550. doi: 10.2135/cropsci1998.0011183X003800020072x. [DOI] [Google Scholar]
- 36.Zeng P., Vadnais D., Zhang Z., Polacco J. Refined glufosinate selection in Agrobacterium-mediated transformation of soybean [Glycine max (L.) Merrill] Plant Cell Rep. 2004;22:478–482. doi: 10.1007/s00299-003-0712-8. [DOI] [PubMed] [Google Scholar]
- 37.Paz M.M., Martinez J.C., Kalvig A.B., Fonger T.M., Wang K. Improved cotyledonary node method using an alternative explant derived from mature seed for efficient Agrobacterium-mediated soybean transformation. Plant Cell Rep. 2006;25:206–213. doi: 10.1007/s00299-005-0048-7. [DOI] [PubMed] [Google Scholar]
- 38.Song B., Oehrle N.W., Liu S., Krishnan H.B. Development and Characterization of a Soybean Experimental Line Lacking the α’ Subunit of β-Conglycinin and G1, G2, and G4 Glycinin. J. Agric. Food Chem. 2018;66:432–439. doi: 10.1021/acs.jafc.7b05011. [DOI] [PubMed] [Google Scholar]
- 39.Schmutz J., Cannon S.B., Schlueter J., Ma J., Mitros T., Nelson W. Genome sequence of the palaeopolyploid soybean. Nature. 2010;463:178–183. doi: 10.1038/nature08670. [DOI] [PubMed] [Google Scholar]
- 40.Kim S., Krishnan H.B. Chapter Seven—A fast and cost-effective procedure for reliable measurement of trypsin inhibitor activity in soy and soy products. In: Jez J., editor. Methods in Enzymology. Volume 680. Academic Press; Cambridge, MA, USA: 2023. pp. 195–213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are provided in the manuscript and Supplementary Information.