Abstract
Background: Malignant pleural effusion (MPE) affects up to 15% of patients with malignancy, and the prevalence is increasing. Non-expandable lung (NEL) complicates MPE in up to 30% of cases. However, it is not known if patients with malignant pleural effusion and NEL are more symptomatic in activities of daily living compared to patients with MPE with expandable lung. Methods: This was an observational study on consecutively recruited patients with MPE from our pleural clinic. Before thoracentesis, patients completed patient-reported outcomes on cancer symptoms (ESAS), health-related quality of life (5Q-5D-5L), and dyspnoea scores. Following thoracentesis, patients scored dyspnoea relief and symptoms during thoracentesis. Data on focused lung ultrasound and pleural effusion biochemistry were collected. The non-expandable lung diagnosis was made by pleural experts based on radiological and clinical information. Results: We recruited 43 patients, including 12 with NEL (28%). The NEL cohort resembled those from previous studies concerning ultrasonography, pleural fluid biochemistry, and fewer cases with high volume thoracentesis. Patients with and without NEL were comparable concerning baseline demography. The 5Q-5D-5L utility scores were 0.836 (0.691–0.906) and 0.806 (0.409–0.866), respectively, for patients with and without NEL. We observed no between-group differences in symptom burden or health-related quality of life. Conclusion: While the presence of NEL affects the clinical management of recurrent MPE, the presence of NEL seems not to affect patients’ overall symptom burden in patients with MPE.
Keywords: non-expandable lung, malignant pleural effusion, patient-reported outcomes, survival
1. Introduction
Up to 15% of all cancer patients develop malignant pleural effusion (MPE) during the course of their disease [1,2,3]. The number of patients diagnosed with and surviving malignancy is increasing, and thus the incidence of MPE is increasing too [4]. MPE is a serious condition as it denotes metastatic disease in almost all cases. Furthermore, MPE can be complicated by the inability of the lung to fully expand (non-expandable lung (NEL)), with the inability of the pleural surfaces to attain apposition [5,6]. NEL is caused by bronchial tumour obstruction, chronic atelectasis, or reduced elasticity of the visceral pleura, and affects up to 30% of patients with MPE [6,7,8,9,10]. There is no available treatment for NEL in the vast majority of patients, so therapeutic management focuses on the palliation of the MPE and underlying disease [3,6,11,12].
MPE is associated with a significant symptom burden [13,14]. Compared to MPE with expandable lung, MPE with NEL is clinically associated with lower pleural effusion volumes at thoracentesis, thoracentesis-related pain, and cough, and biochemically with transudative rather than exudative effusions [15,16,17]. In patients with pleural mesothelioma, NEL is found to be associated with increased symptom burden and poorer survival [18]. These findings may not be extrapolated to non-mesothelioma malignancies, where the presence of MPE—in contrast to mesothelioma—by definition denotes disseminated disease (stage IV) [19]. Therefore, we aimed to investigate whether NEL confers additional symptoms to those related to disseminated malignancy in the presence of MPE [20].
2. Materials and Methods
A prospective single-centre study was performed at a respiratory clinic with a dedicated outpatient pleural service (Pleural Clinic, Department of Internal and Respiratory Medicine, Zealand University Hospital, Naestved, Denmark). Patients were eligible if >18 years, had MPE, and needed a thoracentesis due to an effusion larger than 2 cm (ultrasound measurement). Exclusion criteria were life expectancy shorter than 3 months, inability to understand written or spoken Danish, pregnancy, and contradictions to therapeutic thoracentesis.
Following inclusion, baseline data and patient-reported outcome measures (PROMs) were collected, followed by thoracic ultrasound (TUS), TUS-guided thoracentesis, and then post-thoracentesis TUS and PROMs.
2.1. Non-Expandable Lung (NEL) Definition
As initially proposed by Lan et al. [8] and refined in the study by Salamonsen et al. [21], we defined NEL based on a combination of clinical observations and post-thoracentesis radiology (Supplementary Materials S1). Two independent interventional pulmonologists subspecialised in pleural disease (AH and JSS) assessed study data after four months of follow-up and classified cases as non-expandable lung (NEL), expandable lung (EL), or “Unable to determine”. In the event of disagreement between assessors, a third subspecialised interventional pulmonologist (UB) assessed study information and consensus was achieved. The assessors were blinded to the decision of the fellow assessor, and were provided with underlying diagnoses, relevant clinical observations during thoracentesis (e.g., chest tightness or severe coughing alleviated or not by thoracentesis pause, air in the chest tube, and undrainable pleural effusion despite unblocked chest tube), and available radiology reports and images (before and after thoracentesis) as well as performed PROMs.
2.2. Baseline Data
Basic demographics, including sex, smoking history, comorbidity, existing radiology reports and images, blood and pleural biochemistry (protein, albumin, and lactate dehydrogenase (LDH)) where available, and final cause of pleural effusion were collected at study inclusion and at the four-month follow-up. Survival status was registered at the four-month follow-up where a final diagnosis was made, and again twelve months later.
2.3. Patient-Reported Outcome Measures (PROMs)
PROMs were screened for validation on patients with NEL and in Danish but could only be found for the latter. We identified PROMs that were well studied in patients with malignancy (or dyspnoea with minimal important differences specified in patient groups with malignancy) and validated in Danish.
2.3.1. Pre-Thoracentesis PROMs
PROMs were completed by the patient after initial study inclusion was completed and while sitting ready for planned thoracentesis.
EuroQol EQ-5D-5L measures health-related quality of life [22]. The descriptive system comprises five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) scored by the patient on a 5-level Likert scale (no/slight/moderate/severe/extreme problems). Responses were converted to a utility score utilising Danish national validated scores [23], with 1 being least possible symptoms. It also contains a 100 VAS score on self-rated health, with higher scores indicating better quality of life.
The Edmonton Symptom Assessment Score (ESAS) measures cancer-related disease burden [24]. It comprises nine predefined symptoms and one patient-defined symptom scored by the patient on an 11-point Likert scale (0 = no symptoms, 10 = worst symptoms imaginable). Scores were dichotomized into “none-to-mild” (score 0–3) or “moderate-to-severe” (score 4–10) [24,25,26].
The Medical Research Council dyspnoea scale (modified version) (mMRC) measures dyspnoea on a 5-point Likert scale completed by the patient (0 = I only get breathless with strenuous exercise, 4 = I am too breathless to leave the house, or I am breathless when dressing/undressing). Additionally, the ESAS dyspnoea symptom score was included to ascertain dyspnoea burden.
The Borg Rating of Perceived Exertion (MBS) scale (modified) measures the severity of perceived exertion on an 11-point Likert scale completed by the patient (0 = no effort at all, 10 = very, very hard). Scores were dichotomized as mild–moderate (score 0–3) or severe (score 4–10 ) [27].
2.3.2. Post-Thoracentesis PROMs
Following thoracentesis and immediately prior to leaving the outpatient clinic (having dressed), patients were asked to describe the following symptoms:
Symptoms during thoracentesis, evaluating experienced chest tightness or pain relating to thoracentesis on a 4-point Likert scale (no/slight/moderate/marked symptoms) completed by the patient.
Symptom change in dyspnoea and in wellbeing after thoracentesis, each evaluated on a 6-point Likert scale (“markedly better”, “slightly to moderately better”, “unchanged”, “slightly worse”, “moderately worse”, and “markedly worse”) completed by the patient. “Markedly better” and “slightly-to-moderately better” defined a “clinical benefit” in the present study.
2.4. Thoracentesis and TUS-Protocol
Two physicians (JKP and KF) performed all procedures. Both were trained and certified in TUS and thoracentesis according to national guidelines of the Danish Respiratory Society [28]. Ultrasound assessments were performed using LOGIQ S8 (GE Healthcare, Wauwatosa, USA) using the C1-6-D curved (2–5 Mhz) transducer in abdominal preset. Thoracentesis was performed with both a diagnostic and therapeutic intent, aiming for optimal patient symptom relief. Thoracentesis was aborted when more than 2 L of pleural fluid was accumulated or patient symptoms of severe cough, chest pain, or general discomfort became clinically significant.
TUS was performed before and after thoracentesis. TUS initially assessed both hemithoraces with measurement performed on the area of primary effusion and adjacent lung zones where thoracentesis was planned. A full ultrasound protocol has been previously published [29].
Large-volume thoracentesis was defined as more than 760 mL of pleural fluid being removed during thoracentesis as per the study by Mishra and colleagues [30].
Pre-thoracentesis TUS assessment [31] included the evaluation of visible atelectasis (yes/no) pleural, parietal pleural thickening over 1 cm (yes/no), septations (yes/no), lung sliding apically of the effusion (yes/no), and swirling sign(31) (yes/no).
Post-thoracentesis TUS assessment: Following thoracentesis, the hemithorax was assessed using ultrasound for complete drainage status (less than 0.1 L fluid assessed by visual estimation) and pleural apposition.
2.5. Follow-Up Data
All patients had a chest X-ray (CXR) performed after thoracentesis, except when a same-day chest computer tomography (CT) was booked by the treating oncologist or physician.
Final diagnosis was recorded 4 months from thoracentesis using the electronic patient records.
3. Results
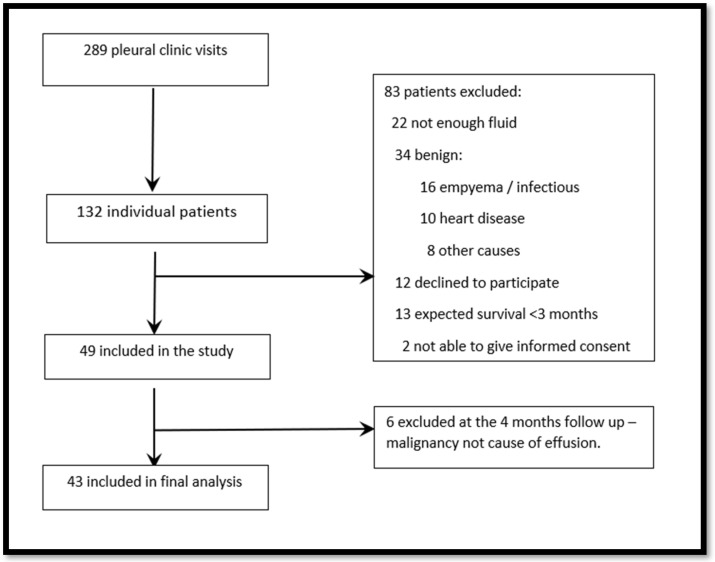
Between May 2021 and December 2021, 132 patients were screened at 289 visits, and 49 patients were included in the study (see flowchart, Figure 1). Six patients with a final diagnosis of non-malignant disease were excluded, and one patient (NEL) was unable to complete all questionnaires due to fatigue. Thus, the study included 43 patients with complete PROMs data from 42 patients.
Figure 1.
Patient inclusion.
A total of 12/43 patients were diagnosed with NEL (29%). Inter-rater agreement on probable/definite NEL vs. probable/definite EL was “very good” with a Kappa of 0.93 (SE 0.16), and with an overall weighted Kappa of 0.79 (SE 0.13) on the non-dichotomised assessments. Table 1 shows basic demographics stratified by NEL status.
Table 1.
Demographic and key clinical variables.
| Non-Expandable Lung NEL (n = 12) | Expandable Lung EL (n = 31) |
p-Value | |
|---|---|---|---|
| Age, mean (SD) | 75 (8) | 71 (8) | 0.11 * |
| Male, n (%) | 5 (42%) | 17 (55%) | 0.44 # |
| Smoking status | 0.15 # | ||
| Current, n (%) | 3 (25%) | 5 (16%) | |
| Former, n (%) | 9 (75%) | 18 (58%) | |
| Never, n (%) | 0 (0%) | 8 (26%) | |
| Tobacco packyears, | 32 (16) | 29 (17) | 0.62 * |
| Cancer diagnosis | 0.077 # | ||
| Lung, n (%) | 6 (50%) | 14 (45%) | |
| Mesothelioma, n (%) | 4 (33%) | 0 (0%) | |
| Breast, n (%) | 2 (17%) | 8 (26%) | |
| Kidney, n (%) | 0 (0%) | 2 (6%) | |
| Female reproductive, n (%) | 0 (0%) | 2 (6%) | |
| Melanoma, n (%) | 0 (0%) | 1 (3%) | |
| Prostate, n (%) | 0 (0%) | 1 (3%) | |
| Lymph, n (%) | 0 (0%) | 1 (3%) | |
| Other, n (%) | 0 (0%) | 2 (6%) | |
| Lung cancers | 0.37 # | ||
| Adenocarcinoma, n (%) | 4 (67%) | 11 (85%) | |
| Squamous-cell carcinoma, n (%) | 1 (17%) | 1 (8%) | |
| Small-cell lung cancer, n (%) | 0 (0%) | 1 (8%) | |
| Other, n (%) | 1 (17%) | 0 (0%) | |
| Heart failure, n (%) | 0 (0%) | 3 (10%) | 0.26 # |
| Liver failure, n (%) | 0 (0%) | 0 (0%) | - |
| Kidney failure, n (%) | 0 (0%) | 1 (3%) | 0.53 # |
| Tuberculosis, n (%) | 0 (0%) | 0 (0%) | - |
| Performance status ≥2, n (%) | 5 (42%) | 14 (45%) | 0.84 # |
| Preceding thoracenteses, median n (range) |
3 (2–7) | 2 (1–5) | 0.28 ¤ |
| Time since last thoracentesis, median days (range) | 42 (7–81) | 22 (7–41) | 0.35 ¤ |
| Pleural fluid volume drained, mean mL (SD) |
990 (621) | 1283 (515) | 0.13 * |
| Complete drainage at TUS, n (%) | 2 (18%) | 19 (63%) | 0.010 # |
| Death before follow-up, n (%) | 5 (42%) | 11 (35%) | 0.71 # |
| Survival from first thoracentesis, median days (range) |
76 (59–111) | 116 (59–287) | 0.23 ¤ |
| Time from cancer diagnosis to first thoracentesis, median days (range) | 3 (−3–236) | 84 (0–743) | 0.049 ¤ |
| Death at 12 months past follow-up, n(%) | 10 (83%) | 17 (55%) | 0.083 # |
| Survival from cancer diagnosis Median days (range) |
348 (78–510) | 527 (341–1335) | 0.11 ¤ |
* Two-sample t-test; # Pearson Chi-square; ¤ Wilcoxon rank-sum test.
3.1. Primary Outcome
Dyspnoea before thoracentesis did not consistently differ between groups regardless of PROMs used (Table 2). Furthermore, no inter-group difference in dyspnoea relief following thoracentesis was observed (p = 0.14). In the subgroup with large thoracentesis volumes (predefined as >760 mL), patients with EL reported more dyspnoea relief than patients with NEL (p = 0.047).
Table 2.
Baseline dyspnoea scores and change in dyspnoea and wellbeing scores after thoracentesis.
| Non-Expandable Lung n =12 |
Expandable Lung n =31 |
p-Value | |
|---|---|---|---|
| Post-thoracentesis | |||
| Change in dyspnoea | 0.14 | ||
| Much better | 4 (36%) | 7 (23%) | |
| Somewhat better | 1 (9%) | 11 (35%) | |
| Unchanged | 4 (36%) | 12 (39%) | |
| Somewhat worse | 1 (9%) | 1 (3%) | |
| Moderately worse | 1 (9%) | 0 (0%) | |
| Wellbeing after thoracentesis | 0.25 | ||
| Much better | 3 (27%) | 3 (10%) | |
| Somewhat better | 2 (18%) | 7 (23%) | |
| Unchanged | 5 (45%) | 20 (65%) | |
| Somewhat worse | 0 (0%) | 1 (3%) | |
| Much worse | 1 (9%) | 0 (0%) | |
| Pain in or tightness of chest during thoracentesis | 0.83 | ||
| None | 8 (73%) | 24 (77%) | |
| Light | 2 (18%) | 3 (10%) | |
| Moderate | 1 (9%) | 2 (6%) | |
| Severe | 0 (0%) | 2 (6%) | |
| Benefit of large volume drainage | 0.047 | ||
| Much better | 2 (29%) | 7 (26%) | |
| Somewhat better | 0 (0%) | 9 (33%) | |
| Unchanged | 3 (43%) | 11 (41%) | |
| Somewhat worse | 1 (14%) | 0 (0%) | |
| Moderately worse | 1 (14%) | 0 (0%) | |
| Pre-thoracentesis | |||
| MRC | 0.80 | ||
| Breathless only with strenuous exercise | 2 (18%) | 2 (6%) | |
| Short of breath when hurrying on level ground or up a slight hill | 2 (18%) | 6 (19%) | |
| Slower than most people of the same age on a level surface or have to stop when walking at my own pace on the level | 0 (0%) | 1 (3%) | |
| Stop for breath walking 100 m or after walking a few minutes at my own pace on level ground | 2 (18%) | 5 (16%) | |
| Too breathless to leave the house | 5 (45%) | 17 (55%) | |
| MBS at rest | 0.26 | ||
| Mild-to-moderate (0.5–3) | 11 (92%) | 25 (74%) | |
| Severe (>4) | 1 (8%) | 8 (26%) | |
| MBS in activity | 1.00 | ||
| Mild-to-moderate (0.5–3) | 2 (17%) | 5 (16%) | |
| Severe (>4) | 10 (83%) | 26 (84%) | |
| Shortness of breath (ESAS) | 5 (2–8) | 6 (4–8) | 0.41 |
Large volume drainage pre-defined as >760 mL [30].
3.2. Secondary Outcomes
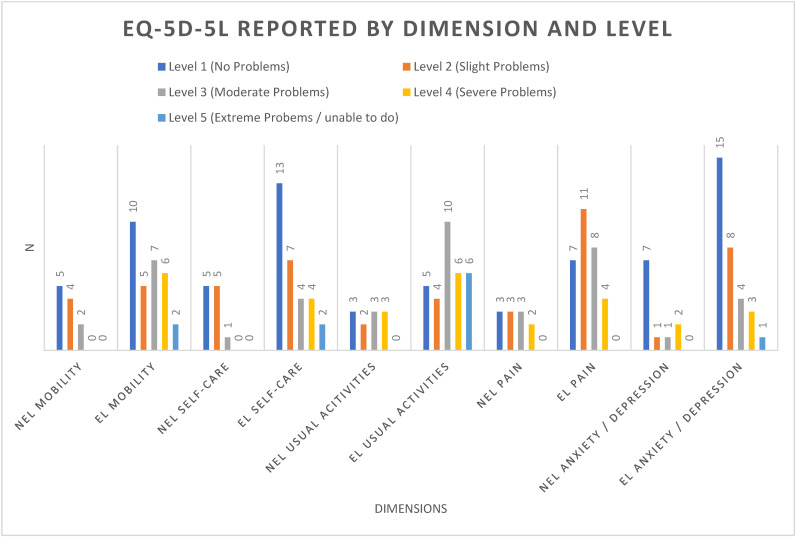
Neither overall symptom burden (Table 3) nor health-related QoL (Figure 2) was significantly worse in patients with NEL compared to EL. EQ-5D-5L median utility scores showed no between-group difference (0.836 & 0.806).
Table 3.
Prevalence of moderate-to-severe symptom burden as assessed with ESAS (score 4–10) or EQ-5D-5L (score moderate or worse).
| Non-Expandable Lung n = 12 |
Expandable Lung n = 31 |
p-Value | |
|---|---|---|---|
| ESAS | n = 12 | n = 31 | |
| Pain, n (%) | 3 (25%) | 12 (39%) | 0.49 |
| Tiredness, n (%) | 6 (50%) | 21 (68%) | 0.31 |
| Nausea, n (%) | 1 (8%) | 8 (26%) | 0.40 |
| Drowsiness, n (%) | 4 (33%) | 15 (48%) | 0.50 |
| Appetite, n (%) | 4 (33%) | 19 (61%) | 0.17 |
| Dyspnoea, n (%) | 9 (75%) | 25 (81%) | 0.69 |
| Depression, n (%) | 4 (33%) | 13 (42%) | 0.73 |
| Anxiety, n (%) | 4 (33%) | 13 (45%) | 0.73 |
| Wellbeing, n (%) | 5 (42%) | 21 (68%) | 0.17 |
| EQ-5D-5L | |||
| Mobility, n (%) | 3 (23%) | 18 (53%) | 0.065 |
| Self-care, n (%) | 1 (8%) | 12 (35%) | 0.058 |
| Usual Activities, n (%) | 8 (62%) | 25 (71%) | 0.51 |
| Pain/discomfort, n (%) | 7 (54%) | 14 (41%) | 0.43 |
| Anxiety/depression, n (%) | 3 (23%) | 9 (26%) | 0.85 |
| Health, mean VAS score (SD) | 64 (28) | 58 (26) | 0.49 |
| Combined utility score (IQR) | 0.836 (0.691–0.906) | 0.806 (0.409–0.866) | 0.37 |
Figure 2.
EQ-5D-5L reported by dimension and level.
Biochemical and ultrasound findings are listed in Table 4.
Table 4.
Pleural fluid biochemistry (and blood/pleural fluid ratios), and pre-thoracentesis ultrasonographic findings.
| Non-Expandable Lung n =12 |
Expandable Lung n =31 |
p-Value | |
|---|---|---|---|
| Biochemistry | |||
| Pleural fluid protein, g/L | 34.6 (11.5) | 40.7 (10.9) | 0.22 |
| Pleural fluid albumin, g/L | 17.1 (9.5) | 23.1 (6.2) | 0.058 |
| Plasma albumin, g/L | 31.0 (8.1) | 28.5 (4.4) | 0.30 |
| Albumin gradient, g/L | 10.8 (2.3) | 6.3 (5.8) | 0.080 |
| Pleural fluid LDH, U/L | 360 (284–1724) | 165 (121–225) | 0.007 |
| Serum LDH, U/L | 189 (180–190) | 200 (170–270) | 0.16 |
| LDH gradient, U/L | 7.3 (8.6) | 1.2 (1.3) | 0.015 |
| Pre-thoracentesis ultrasound | |||
| Pleural fluid swirling, n (%) | 3 (25%) | 18 (58%) | 0.052 |
| Pleural fluid septations, n (%) | 6 (50%) | 3 (10%) | 0.004 |
| Pleural thickening, n (%) | 10 (83%) | 20 (65%) | 0.23 |
| Pleural nodules, n (%) | 3 (25%) | 8 (26%) | 0.96 |
| Atelectasis/consolidation, n (%) | 7 (58%) | 16 (52%) | 0.21 |
| Lung sliding, n (%) | 2 (18%) | 20 (65%) | 0.008 |
| Post-thoracentesis ultrasound | |||
| Apposition of pleural lines, n (%) | 1 (9%) | 22 (73%) | <0.001 |
| US assessed full drainage, n (%) | 2 (18%) | 19 (63%) | 0.010 |
Data are presented as mean (SD) or median (IQR). LDH; Lactate Dehydrogenase. Gradient (blood concentration minus pleural fluid concentration).
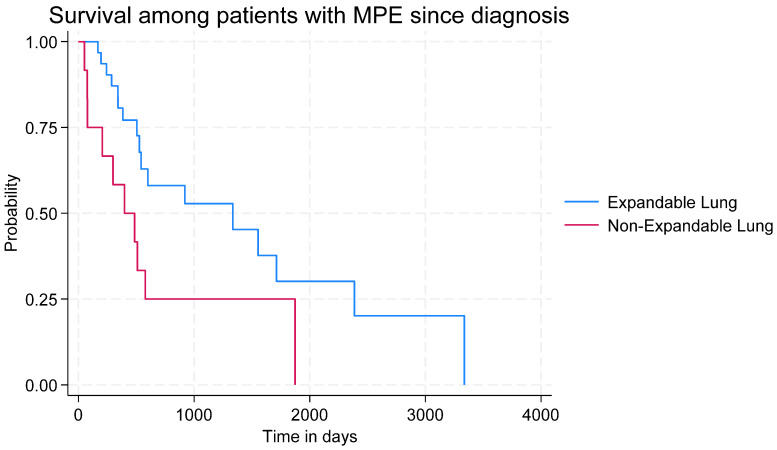
All patients with NEL had undergone a previous thoracentesis, compared to 74% of patients with EL (p = 0.043). Effusions in the NEL group were characterised by higher levels of LDH (360 U/L vs. 165 U/L, p = 0.007) and a higher ratio between pleural fluid and plasma LDH (7.3 U/L vs. 1.2 U/L, p = 0.015). Thoracic ultrasound in the NEL group more often demonstrated pleural septations and rarely showed lung sliding. Median survival from first thoracentesis was numerically lower in the NEL group but the difference did not reach significance (NEL: 76 (IQ range 59–111) vs. EL: 116 (IQ range 59–287) days, p = 0.23). For overall survival from the diagnosis of malignancy, the Kaplan–Meier survival curve (Figure 3) showed a significant between-group difference (p = 0.024)
Figure 3.
Kaplan–Meier survival graph.
4. Discussion
This is the first prospective study to describe symptom burden in patients with NEL in the context of MPE in comparison to those without NEL. We found no differences in dyspnoea or symptom burden between patients with and without NEL despite using a comprehensive collection of recognised PROMs concerning malignancy or pleural effusions. The NEL group displayed typical characteristics shown in earlier studies such as lower pleural volume removed [32], lower symptoms relief with large thoracentesis volumes [30], and a greater degree of incompletely drained effusions, as well as lower levels of pleural fluid albumin and protein, and elevated lactate dehydrogenase (LDH) [32]. This would support our NEL cohort being comparable to previous studies [32,33]. We expected a higher degree of baseline symptom burden in patients with NEL and MPE, as these patients—in addition to diaphragm immobility induced by pleural effusion—also suffer from impaired lung movement due to NEL [34].
We investigated if living with NEL is associated with a greater subjective symptom burden before having a planned thoracentesis. EQ-5D-5L utility scores were calculated (high scores equal low symptom burden), and compared to national reference values. Scores were calculated from a baseline of 1, with each trajectory subtracting a standard reference value according to the severity of the given trajectory. EQ-5D-5L median utility scores (0.836 and 0.806) did not differ significantly and reflect the VAS scores from the questionnaire. For reference, Danish reference values have been calculated at 0.90 [35]. Our median scores are markedly higher than those found in a comparable population with MPE (0.55–0.71) from the UK [36]. This may reflect cultural differences or more likely that the basic reference values (used to adjust the domains) may be markedly different—these are currently under re-evaluation [37].
Though our study seems to suggest that NEL in MPE is not associated with an overall excess of symptoms, the presence of NEL in MPE poses a variety of challenges to the clinician. This includes confusion with iatrogenic pneumothorax after drainage, the drainage of lower effusion volumes due to patient discomfort, the lower efficacy of large thoracentesis volumes, the lower efficacy of talc pleurodesis, and the delayed insertion of indwelling pleural catheter (IPC). All these are likely to affect patients’ quality of life during MPE management. Thus, the correct and early identification of NEL is crucial for optimal MPE management.
Our study is one of the few prospective studies focusing on patients with MPE and stratified by NEL status. We found that about half of the patients experience symptom relief following thoracentesis. Psallidas et al. [38] found that 71% (10/14) of patients with NEL experienced dyspnoea relief following thoracentesis versus 89% (39/44) of patients with EL. Possible differences in NEL definition or population may account for the observed effect difference, but neither NEL definition or descriptive data on the NEL population is available in that paper [38].
NEL has no “gold standard” diagnostic test and is usually recognized from patient symptoms and radiological findings. It has been suggested that pleural manometry could play a diagnostic role, but studies have shown significant differences in initial pleural pressure and elastance in patients with and without NEL [8,39].
Our study has several strengths and weaknesses. Firstly, we consider it as a strength to assess the overall symptom burden associated with malignancy and impaired health, as this acknowledges the huge variability in symptoms imposed by cancer [40]. Secondly, the consecutive inclusion of a broad variety of patients with suspected or known MPE is a strength as our patients represent the heterogeneous population living with MPE. Thirdly, PROMs being answered just before thoracentesis should represent the potentially most symptomatic phase, without confusing responses with thoracentesis-related discomforts. Further, most patients were familiar with the procedure, so bias due to pre-procedure anxiety is expected to be low. Fourthly, our NEL cohort presented several of the known characteristics of NEL known from previous studies, and thus we consider our cohort to be representative. Lastly, the majority of patients (33/43) had a volume drained exceeding 760 mL, which Mishra et al. identified as the mean volume to induce a minimal important difference in dyspnoea measured with a visual analogue scale [30].
The major limitation is the sample size, which hampers firm conclusions. We were unable to perform a sample size calculation due to the lack of previous studies on dyspnoea relief specifically in MPE-related NEL. Despite the lack of a proper sample size calculation, our cohort was large enough to show significant between-group differences concerning biochemistry and ultrasonography, and thus is likely to be also large enough to detect a signal on major differences in symptom burden between NEL and EL. Between-group differences were limited, but it should be noted that there was a numeric difference in the NEL subgroup with metastatic disease that did not achieve statistical significance (p = 0.77) We excluded patients with very short life expectancy to avoid confusing patient-reported outcomes with performance, symptoms, and challenges related to overwhelming cancer burden and imminent end-of-life palliative needs. This may have led to selection bias, yet we are unaware if NEL is markedly more prevalent in patients with a very short life span. Likewise, we did not collect data on time living with malignant pleural effusion before study inclusion, so our data on survival may be influenced by lead-time bias. However, the insignificant yet increasing gap in survival curves between NEL and EL (Figure 3) suggests that NEL may represent a later phase in the MPE trajectory.
Several dyspnoea scales exist but no single tool encompasses all dimensions of the complexity of dyspnoea [30,41]. No scale on MPE-related dyspnoea has been linguistically validated in Danish, and therefore several scales were used to measure and compare dyspnoea. Our negative results may thus be due to an inherent inability to measure MPE-related dyspnoea in our cohort via the chosen PROMs. This highlights the need for domestic translation-validated dyspnoea scales for patients with MPE to assess dyspnoea either as a unidimensional VAS-scale [30] or a multidimensional scale such as the Cancer Dyspnoea Scale [42].
Finally, there is currently no consensus on optimal time point for the measurement of change in dyspnoea following thoracentesis. In some studies, measurement is performed immediately following thoracentesis while others find that optimal time at two days post thoracentesis [12,34]. We chose to use same-day time points, as we were worried that the ongoing COVID pandemic would impair our data collection if a time point 2 days later were chosen. We consider it unlikely that our patients were in phase with few symptoms at the time of study inclusion, since patients book an appointment for out-patient therapeutic thoracentesis within 1–2 days in our unit, when the patients experience significant discomfort due to recurrent pleural effusion.
The Kaplan–Meier survival graph showed a significant difference between groups, with worse survival in the NEL group. As symptoms between groups are comparable, we suggest that NEL may especially affect overall survival due to lessened respiratory physiological reserves and advanced dissemination, making patients more vulnerable to infection and further disease dissemination. Shunting in the pulmonary arterial system or pulmonary arterial hypertension may play pathophysiological roles, but this needs further scientific assessment.
5. Conclusions
The overall symptom burden (including dyspnoea) in patients with MPE is high but appears not to be higher in patients with non-expandable lung. The Kaplan–Meier survival graph suggests that NEL negatively affects overall survival.
Acknowledgments
A humble thank you to all participating patients and clinical staff of the Pleural Clinic at Naestved Hospital.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics14111176/s1, Supplementary Material S1: Study data are protected under the Danish GDPR legislation. An enquiry into data availability via Region Zealand Data committee can be made to share anonymised data.
Author Contributions
Conceptualization, J.K.P., K.F, P.F.C., C.B.L., R.B. and U.B.; methodology, J.K.P., K.F, C.B.L., R.B. and U.B.; software, J.K.P. and D.B.R.; validation, D.B.R.; formal analysis, J.K.P. and D.B.R.; investigation, J.K.P., K.F., J.S.S. and A.H.; resources, J.K.P., K.F. and G.A.; data curation, J.K.P., G.A., J.S.S. and A.H.; writing—original draft preparation, J.K.P.; writing—review and editing, K.F, G.A., P.F.C., C.B.L., R.B. and U.B.; visualization, J.K.P.; supervision, R.B., C.B.L. and U.B.; project administration, U.B.; funding acquisition, U.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. All patients provided informed consent. The study was approved by the Regional Ethics Committee (approval nr. SJ-891, approved 23 April 2021) and Region Zealand Data Committee (REG-001-2021).
Informed Consent Statement
All patients provided informed consent.
Data Availability Statement
All data from the study are presented in this manuscript or in the online Supplementary Material. For questions concerning data, please contact the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
The study was part of a PhD project supported by grants from NSR Hospitals (Region Zealand, Denmark) and the Danish Cancer Plan 4.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Porcel J.M., Gasol A., Bielsa S., Civit C., Light R.W., Salud A. Clinical features and survival of lung cancer patients with pleural effusions. Respirology. 2015;20:654–659. doi: 10.1111/resp.12496. [DOI] [PubMed] [Google Scholar]
- 2.Arnold D.T., De Fonseka D., Perry S., Morley A., Harvey J.E., Medford A., Brett M., Maskell N.A. Investigating unilateral pleural effusions: The role of cytology. Eur. Respir. J. 2018;52:1801254. doi: 10.1183/13993003.01254-2018. [DOI] [PubMed] [Google Scholar]
- 3.Bibby A.C., Dorn P., Psallidas I., Porcel J.M., Janssen J., Froudarakis M., Subotic D., Astoul P., Licht P., Schmid R., et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur. Respir. J. 2018;52:1800349. doi: 10.1183/13993003.00349-2018. [DOI] [PubMed] [Google Scholar]
- 4.Bodtger U., Hallifax R.J. Pleural Diseases ERS Monograph. ERS; Sheffield, UK: 2020. Epidemiology: Why is pleural disease becoming more common? [Google Scholar]
- 5.Dresler C.M., Olak J., Herndon J.E., Richards W.G., Scalzetti E., Fleishman S.B., Kernstine K.H., Demmy T., Jablons D.M., Kohman L., et al. Phase III Intergroup Study of Talc Poudrage vs. Talc Slurry Sclerosis for Malignant Pleural Effusion. Chest. 2005;127:909–915. doi: 10.1378/chest.127.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muruganandan S., Azzopardi M., Fitzgerald D.B., Shrestha R., Kwan B.C.H., Lam D.C.L., De Chaneet C.C., Ali M.R.S.R., Yap E., Tobin C.L., et al. Aggressive versus symptom-guided drainage of malignant pleural effusion via indwelling pleural catheters (AMPLE-2): An open-label randomised trial. Lancet Respir. Med. 2018;6:671–680. doi: 10.1016/s2213-2600(18)30288-1. [DOI] [PubMed] [Google Scholar]
- 7.Thomas R., Fysh E.T.H., Smith N.A., Lee P., Kwan B.C.H., Yap E., Lee Y.G. Effect of an indwelling pleural catheter vs. talc pleurodesis on hospitalization days in patients with malignant pleural effusion: The AMPLE randomized clinical trial. JAMA—J. Am. Med. Assoc. 2017;318:1903–1912. doi: 10.1001/jama.2017.17426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lan R.-S., Lo S.K., Chuang M.-L., Yang C.-T., Tsao T.C.-Y., Lee C.-H. Elastance of the Pleural Space: A Predictor for the Outcome of Pleurodesis in Patients with Malignant Pleural Effusion. Ann. Intern. Med. 1997;126:768–774. doi: 10.7326/0003-4819-126-10-199705150-00003. [DOI] [PubMed] [Google Scholar]
- 9.Davies H.E., Mishra E.K., Kahan B.C., Wrightson J.M., Stanton A.E., Guhan A., Rahman N.M. Effect of an indwelling pleural catheter vs. chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: The TIME2 randomized controlled trial. JAMA—J. Am. Med. Assoc. 2012;307:2383–2389. doi: 10.1001/jama.2012.5535. [DOI] [PubMed] [Google Scholar]
- 10.Halford P.J., Bhatnagar R., White P., Haris M., Harrison R.N., Holme J., Sivasothy P., West A., Bishop L.J., Stanton A.E., et al. Manometry performed at indwelling pleural catheter insertion to predict unexpandable lung. J. Thorac. Dis. 2020;12:1374–1384. doi: 10.21037/jtd.2020.02.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feller-Kopman D.J., Reddy C.B., DeCamp M.M., Diekemper R.L., Gould M.K., Henry T., Iyer N.P., Lee Y.C.G., Lewis S.Z., Maskell N.A., et al. Management of Malignant Pleural Effusions An Official ATS/STS/STR Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018;198:839–849. doi: 10.1164/rccm.201807-1415ST. [DOI] [PubMed] [Google Scholar]
- 12.Muruganandan S., Azzopardi M., Thomas R., Fitzgerald D.B., Kuok Y.J., Cheah H.M., Read C.A., Budgeon C.A., Eastwood P.R., Jenkins S., et al. The Pleural Effusion and Symptom Evaluation (PLEASE) study of breathlessness in patients with a symptomatic pleural effusion. Eur. Respir. J. 2020;55:1900980. doi: 10.1183/13993003.00980-2019. [DOI] [PubMed] [Google Scholar]
- 13.Sivakumar P., Fitzgerald D.B., Ip H., Rao D., West A., Noorzad F., Wallace D., Haris M., Prudon B., Hettiarachchi G., et al. The impact of outpatient versus inpatient management on health-related quality of life outcomes for patients with malignant pleural effusion: The OPTIMUM randomised clinical trial. Eur. Respir. J. 2024;63:2201215. doi: 10.1183/13993003.01215-2022. [DOI] [PubMed] [Google Scholar]
- 14.Mishra E.K., Muruganandan S., Clark A., Bhatnagar R., Maskell N., Lee G., Rahman N.M. Breathlessness Predicts Survival in Patients with Malignant Pleural Effusions Meta-analysis of Individual Patient Data from Five Randomized Controlled Trials. Chest. 2021;160:351–357. doi: 10.1016/j.chest.2021.02.052. [DOI] [PubMed] [Google Scholar]
- 15.Divietro M., Huggins J., Doelken P., Gurung P., Kaiser L., Sahn S. Prevalence and Causes of Unexpandable Lung Over a Ten Year Period. Chest. 2011;140:700A. doi: 10.1378/chest.1118822. [DOI] [Google Scholar]
- 16.Feller-Kopman D. Therapeutic thoracentesis: The role of ultrasound and pleural manometry. Curr. Opin. Pulm. Med. 2007;13:312–318. doi: 10.1097/mcp.0b013e3281214492. [DOI] [PubMed] [Google Scholar]
- 17.Lester M.G., Feller-Kopman D., Maldonado F. Pleural Diseases ERS Monograph. ERS; Sheffield, UK: 2020. Pleural physiology: What do we understand and what should we measure in clinical practice? [Google Scholar]
- 18.Bibby A.C., Halford P., de Fonseka D., Morley A.J., Smith S., Maskell N.A. The prevalence and clinical relevance of nonexpandable lung in malignant pleural mesothelioma: A prospective, single-center cohort study of 229 patients. Ann. Am. Thorac. Soc. 2019;16:1273–1279. doi: 10.1513/AnnalsATS.201811-786OC. [DOI] [PubMed] [Google Scholar]
- 19.Detterbeck F.C., Boffa D.J., Kim A.W., Tanoue L.T. The Eighth Edition Lung Cancer Stage Classification. Chest. 2017;151:193–203. doi: 10.1016/j.chest.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Hooper C., Lee Y.C.G., Maskell N. Investigation of a unilateral pleural effusion in adults: British Thoracic Society pleural disease guideline 2010. Thorax. 2010;65((Suppl. S2)):ii4–ii17. doi: 10.1136/thx.2010.136978. [DOI] [PubMed] [Google Scholar]
- 21.Salamonsen M.R., Lo A.K.C., Ng A.C.T., Bashirzadeh F., Wang W.Y.S., Fielding D.I.K. Novel Use of Pleural Ultrasound Can Identify Malignant Entrapped Lung Prior to Effusion Drainage. Chest. 2014;146:1286–1293. doi: 10.1378/chest.13-2876. [DOI] [PubMed] [Google Scholar]
- 22.Janssen M.F., Pickard A.S., Golicki D., Gudex C., Niewada M., Scalone L., Swinburn P., Busschbach J. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: A multi-country study. Qual. Life Res. 2013;22:1717–1727. doi: 10.1007/s11136-012-0322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen C.E., Sørensen S.S., Gudex C., Jensen M.B., Pedersen K.M., Ehlers L.H. The Danish EQ-5D-5L Value Set: A Hybrid Model Using cTTO and DCE Data. Appl. Health Econ. Health Policy. 2021;19:579–591. doi: 10.1007/s40258-021-00639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbera L., Seow H., Howell D., Sutradhar R., Earle C., Liu Y., Stitt A., Husain A., Sussman J., Dudgeon D. Symptom burden and performance status in a population-based cohort of ambulatory cancer patients. Cancer. 2010;116:5767–5776. doi: 10.1002/cncr.25681. [DOI] [PubMed] [Google Scholar]
- 25.Pain S. What Should be the Optimal Cut Points for Mild, Moderate, and Severe Pain? J. Palliat. Med. 2007;10:1338–1346. doi: 10.1089/jpm.2007.0087. [DOI] [PubMed] [Google Scholar]
- 26.Selby D., Cascella A., Gardiner K., Do R., Moravan V., Myers J., Chow E. A Single Set of Numerical Cutpoints to Define Moderate and Severe Symptoms for the Edmonton Symptom Assessment System. J. Pain Symptom Manag. 2010;39:241–249. doi: 10.1016/j.jpainsymman.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Fjaellegaard K., Petersen J.K., Rasmussen D.B., Clementsen P.F., Laursen C.B., Bhatnagar R., Bodtger U. Prediction of Time to Next Therapeutic Thoracentesis and Identification of Risk Factors of Rapid Pleural Fluid Recurrence: A Prospective Observational Study. Respiration. 2023;102:333–340. doi: 10.1159/000528558. [DOI] [PubMed] [Google Scholar]
- 28.Pietersen P.I., Davidsen J.R., Skaarup S.H., Schultz H.H.L., Jeschke K.N., Bodtger U., Laursen C.B. Fokuseret Lungeultralydsskanning. 2020. [(accessed on 27 February 2023)]. Available online: https://lungemedicin.dk/fokuseret-lungeultralydskanning/
- 29.Petersen J.K., Fjaellegaard K., Rasmussen D.B., Alstrup G., Høegholm A., Sidhu J.S., Sivapalan P., Gerke O., Bhatnagar R., Clementsen P.F., et al. Ultrasound in the Diagnosis of Non-Expandable Lung: A Prospective Observational Study of M-Mode, B-Mode, and 2D-Shear Wave Elastography. Diagnostics. 2024;14:204. doi: 10.3390/diagnostics14020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mishra E.K., Corcoran J.P., Hallifax R.J., Stradling J., Maskell N.A., Rahman N.M. Defining the Minimal Important Difference for the Visual Analogue Scale Assessing Dyspnea in Patients with Malignant Pleural Effusions. PLoS ONE. 2015;10:e0123798. doi: 10.1371/journal.pone.0123798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bugalho A., Ferreira D., Dias S.S., Schuhmann M., Branco J.C., Gomes M.J.M., Eberhardt R. The Diagnostic Value of Transthoracic Ultrasonographic Features in Predicting Malignancy in Undiagnosed Pleural Effusions: A Prospective Observational Study. Respiration. 2014;87:270–278. doi: 10.1159/000357266. [DOI] [PubMed] [Google Scholar]
- 32.Trovisco R., Freitas C., Serino M., Ferreira P., Martins B., Coelho D., Melo N., Fernandes G., Magalhães A., Bastos H. Predictors of lung entrapment in malignant pleural effusion. Pulmonology. 2022 doi: 10.1016/j.pulmoe.2022.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Huggins J.T., Doelken P., Sahn S.A. The unexpandable lung. F1000 Med. Rep. 2010;2:77. doi: 10.3410/m2-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas R., Jenkins S., Eastwood P.R., Lee Y.G., Singh B. Physiology of breathlessness associated with pleural effusions. Curr. Opin. Pulm. Med. 2015;21:338–345. doi: 10.1097/mcp.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jensen M.B., Jensen C.E., Gudex C., Pedersen K.M., Sørensen S.S., Ehlers L.H. Danish population health measured by the EQ-5D-5L. Scand. J. Public Health. 2023;51:241–249. doi: 10.1177/14034948211058060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhatnagar R., Piotrowska H.E., Laskawiec-Szkonter M., Kahan B.C., Luengo-Fernandez R., Pepperell J.C., Maskell N.A. Effect of Thoracoscopic Talc Poudrage vs. Talc Slurry via Chest Tube on Pleurodesis Failure Rate among Patients with Malignant Pleural Effusions: A Randomized Clinical Trial. JAMA—J. Am. Med. Assoc. 2020;323:60–69. doi: 10.1001/jama.2019.19997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.EuroQol Website. [(accessed on 30 May 2024)]. Available online: https://euroqol.org/information-and-support/resources/value-sets/
- 38.Psallidas I., Yousuf A., Talwar A., Hallifax R.J., Mishra E.K., Corcoran J.P., Ali N., Rahman N.M. Assessment of patient-reported outcome measures in pleural interventions. BMJ Open Respir. Res. 2017;4:e000171. doi: 10.1136/bmjresp-2016-000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villena V., López-Encuentra A., Pozo F., De-Pablo A., Martín-Escribano P. Measurement of Pleural Pressure during Therapeutic Thoracentesis. Am. J. Respir. Crit. Care Med. 2000;162:1534–1538. doi: 10.1164/ajrccm.162.4.9907047. [DOI] [PubMed] [Google Scholar]
- 40.Raijmakers N.J.H., Zijlstra M., van Roij J., Husson O., Oerlemans S., van de Poll-Franse L.V. Health-related quality of life among cancer patients in their last year of life: Results from the PROFILES registry. Support. Care Cancer. 2018;26:3397–3404. doi: 10.1007/s00520-018-4181-6. [DOI] [PubMed] [Google Scholar]
- 41.Bausewein C., Farquhar M., Booth S., Gysels M., Higginson I. Measurement of breathlessness in advanced disease: A systematic review. Respir. Med. 2007;101:399–410. doi: 10.1016/j.rmed.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka K., Akechi T., Okuyama T., Nishiwaki Y., Uchitomi Y. Development and validation of the Cancer Dyspnoea Scale: A multidimensional, brief, self-rating scale. Br. J. Cancer. 2000;82:800–805. doi: 10.1054/bjoc.1999.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data from the study are presented in this manuscript or in the online Supplementary Material. For questions concerning data, please contact the corresponding author.