Abstract
Simple Summary
In spontaneous tumor regression, tumors shrink and disappear without conventional treatments. This phenomenon challenges the view that cancer is an irreversible genetic disease and that the only treatment option is to kill cancer cells or surgically remove them. In tumor reversion, cancer cells have been shown to return to normal cells when they are transplanted into a normal cellular environment. Additionally, people consuming a Western diet ingest excessive amounts of dietary phosphate, and a dysregulated oversupply of phosphate can be transported into cells, stimulating the cellular growth that forms tumors. Based on reviewed evidence, this paper proposes that reducing excessive dietary phosphate potentially activates tumor regression and reversion, as components of cancer cells are self-digested. Furthermore, fevers and fasting-mimicking diets are associated with tumor regression, which also may be initiated by reduced phosphate intake. Studies are needed to test dietary phosphate reduction in tumor regression and reversion to improve cancer patient survival.
Abstract
Tumors that spontaneously shrink from unknown causes in tumor regression, and that return to normal cells in tumor reversion, are phenomena with the potential to contribute new knowledge and novel therapies for cancer patient survival. Tumorigenesis is associated with dysregulated phosphate metabolism and an increased transport of phosphate into tumor cells, potentially mediated by phosphate overload from excessive dietary phosphate intake, a significant problem in Western societies. This paper proposes that reduced dietary phosphate overload and reregulated phosphate metabolism may reverse an imbalance of kinases and phosphatases in cell signaling and cellular proliferation, thereby activating autophagy in tumor regression and reversion. Dietary phosphate can also be reduced by sickness-associated anorexia, fasting-mimicking diets, and other diets low in phosphate, all of which have been associated with tumor regression. Tumor reversion has also been demonstrated by transplanting cancer cells into a healthy microenvironment, plausibly associated with normal cellular phosphate concentrations. Evidence also suggests that the sequestration and containment of excessive phosphate within encapsulated tumors is protective in cancer patients, preventing the release of potentially lethal amounts of phosphate into the general circulation. Reducing dietary phosphate overload has the potential to provide a novel, safe, and effective reversion therapy for cancer patients, and further research is warranted.
Keywords: spontaneous tumor regression, tumor reversion, dietary phosphate overload, kinases and phosphatases, dysregulated phosphate metabolism, tumor microenvironment, tumor encapsulation, fasting-mimicking diet, sickness-associated anorexia, autophagy
1. Introduction
A handful of recently published papers on the spontaneous regression and reversion of tumors has revived interest in unexplained tumor disappearance, a historical phenomenon observed over many centuries in plants, animals, and humans [1,2,3,4,5,6,7,8]. The spontaneous regression of cancer in humans was defined in 1956 by Dr. Warren H. Cole and Dr. Tilden C. Everson as “the partial or complete disappearance of a malignant tumor in the absence of all treatment, or in the presence of therapy which is considered inadequate to exert a significant influence on neoplastic disease” [9]. Uncovering the elusive cause of spontaneous tumor regression is tantamount to solving a biomedical whodunit. Even more mysteriously, tumor reversion has appeared to magically transform cancer cells to normal cells under experimental conditions [1]. Irrefutable evidence of tumor regression and reversion challenges the somatic mutation theory, which proposes that cancer is an irreversible genetic disease [1]. The somatic mutation theory is based on the assumption that “a somatic cell in the adult organism would undergo successive DNA mutations, and these mutations would be responsible for the cancer phenotype” [10]. Yet, only 30–40% of cancer cells contain genetic mutations, and similar mutations occur in normal cells without cancer [2].
The assumed irreversibility of cancer has guided the research and development of treatments that destroy cancer cells in patients, including chemotherapy, immunotherapy, radiotherapy, and surgery. Yet, current treatments do not remove the underlying cause of cancer. “Cancer therapy is still based on the millenary paradigm that establishes that in order to achieve a cure, the complete eradication of cancer cells must be achieved”, but critics have called for the development of cancer treatments that incorporate a more holistic approach toward patients’ needs [11]. “Knowledge of how a tumor heals itself would be immensely helpful in developing more effective therapeutic modalities” [4]. Additionally, studying the causes of the spontaneous regression and reversion of tumors could contribute to a paradigm shift in cancer therapy and improve the survival and welfare of cancer patients.
Rising cancer incidence in people under 50 years of age is of immediate concern [12], and new approaches are needed to reverse this alarming trend, including a greater focus on nutrition and diet as advised by the World Cancer Research Fund/American Institute for Cancer Research [13]. A healthful diet supplies the body with building materials and fuel for energy, but nutrients and related metabolites also help manage many cellular processes in the body, including protein modification, cell signaling, and gene expression [14]. The dysregulation of these cellular processes leads to dysregulated cellular function in diseases like cancer, implying the involvement of dietary nutrients. For example, chronic nutrient overload can burden cell organelles with an excessive supply of metabolites that overactivate signaling pathways for energy storage and tissue biosynthesis [15]. These types of findings suggest that cancer is a metabolic disease caused by dysregulated metabolic functions [16].
The evidence reviewed in the present paper investigates the associations of spontaneous regression and reversion of tumors with the dietary mineral phosphorus, an essential nutrient which is acquired from the diet in the form of phosphate (PO43−) [17]. A grounded theory literature review method [18] was used to search concepts in Google, Google Scholar, Pub Med, and Scopus, related to diet, cancer, and the spontaneous regression and reversion of tumors, including concepts involving dietary phosphate overload, dysregulated phosphate metabolism in tumorigenesis, and cellular autophagy. All data from credible sources are included in this grounded theory study regardless of the type and date of the data. A comparative analysis of reviewed findings was used to inductively group concepts into categories and themes, which were iteratively synthesized into an explanatory grounded theory of tumor regression and reversion. The paper’s narrative review presents new knowledge and novel insights and proposes that the spontaneous regression and reversion of tumors is potentially associated with reduced dietary phosphate and reregulated phosphate metabolism.
Importantly, the article does not suggest that patients should replace conventional therapies for cancer with a low-phosphate diet for tumor regression/reversion. Clinical evidence demonstrating a causative effect of reduced dietary phosphate on tumor regression is currently lacking, and other factors may be involved. The aim of the review is to synthesize fragmentary pieces of available evidence from the literature into a coherent rationale for conducting further preclinical research and testing. The long-term objective proposed by the paper is to increase options for adjuvant therapies available to cancer patients based on reliable clinical trial evidence. This paper intends to provide an important foundational starting point towards those goals.
2. Tumor Regression
In their 1956 paper, doctors Cole and Everson clarified that spontaneous regression need not imply complete tumor disappearance, “nor that spontaneous regression is synonymous with cure” [9]. Furthermore, the authors mentioned cases in which a spontaneous regression of a tumor in one area did not affect tumors that “flourished unchecked in other areas of the body or reappeared at a later time”, implying the potential involvement of a systemic causative mechanism. Doctors Cole and Everson also proposed factors that are plausibly responsible for the spontaneous regression of tumors, including acute infection and fever (immune responses), endocrine factors, removal of a “carcinogenic agent”, and “interference of the nutrition of the tumor”.
After serving as President of the American Cancer Society in 1959 [19], Dr. Cole wrote in the Annals of the New York Academy of Sciences in 1974 that the use of the word “spontaneous” in cancer regression is a misnomer [20]. He explained that such cases of cancer regression have an unknown cause, and therefore “idiopathic” cancer regression is a more appropriate term to describe this phenomenon. Dr. Cole also pointed out the difficulty of accurately estimating the incidence rate of spontaneous cancer regression, which he suggested is far more frequent than 1 out of 80,000 to 100,000 cancer patients. Spontaneous regression has most often been documented for lymphomas, neuroblastomas, melanomas, renal cell carcinomas, and testicular malignancies, and less often for breast cancer and lung cancer [3]. However, Papac noted the reason that the spontaneous regression of breast cancer is rarely reported is because it is so commonly treated [21]. The spontaneous regression of gastrointestinal malignancies was also more than twice as common in men compared to women in a recent review of 390 cases [22].
Dr. Cole hypothesized that “regression occurs when the factors that cause the growth are no longer present” [20]. In further discussing the elimination of a carcinogen as a cancer-regression factor, Dr. Cole presented evidence in which bladder cancer regression was induced when patients’ ureters were detached from the bladder and transplanted to the colon. He wrote, “there appears to have been something in the urine either responsible for the development of, or the continuing growth of, the bladder cancer”,—i.e., a carcinogenic substance eliminated by the kidneys. The present paper proposes that a potential growth-promoting factor fitting Dr. Cole’s prescient hypothesis is the renal excretion of dietary phosphate, which is a metabolite associated with tumorigenesis when consumed in excessive amounts—a factor also known as dietary phosphate overload.
2.1. Dietary Phosphate Overload
Phosphate overload in the human body is defined as “a state where the phosphorus load is higher than is physiologically necessary”, and causes of phosphate overload include a diet that is high in phosphorus and/or insufficient renal function to regulate phosphate serum levels [23]. Serum phosphate is regulated by an endocrine system consisting of the kidneys, bones, parathyroid glands, and intestines, which is coordinated by endocrine hormones: fibroblast growth factor 23 (FGF23), klotho, parathyroid hormone (PTH), and 1,25-dihydroxyvitamin D3. Excess phosphate is excreted in the urine and is also temporarily stored in a reservoir “pool” within the body, mostly as hydroxyapatite (calcium phosphate) in long bones and in extracellular fluid as inorganic phosphate (Pi) [24]. When intracellular and extracellular levels of phosphate rise too high, a condition known as phosphate toxicity can negatively affect most of the major organ systems of the body [25].
Dietary phosphate overload from excessive phosphate intake is increasingly associated with chronic diseases including cancer. Importantly, chronic kidney disease (CKD) is associated with increased cancer risk [26]. A murine model of CKD showed that dietary phosphate overload induced inflammation, vascular calcification, malnutrition, and premature death [27]. Researchers advise that “dietary strategies are required to reduce dietary phosphate overload and improve human health” [28]. The natural whole foods that are highest in phosphorus are meats, poultry, seafood, dairy, cheese, eggs, nuts and seeds, whole grains, and legumes [29]. Table A2 in Appendix B lists the food items highest in phosphorus. Phosphate additives are also prevalent in processed foods including baked goods, colas, processed meats, and fast foods. Refined lipids, on the other hand, have most minerals removed, as do refined carbohydrates. Among whole unrefined foods, fresh fruits and vegetables are generally lowest in phosphate. A list of foods lowest in phosphorus is included in Table A3 in Appendix C. Dietary reference intakes for the phosphorus needed by the population are listed in Table A1 in Appendix A. Surveys show that the average adult consumes approximately double the 700 mg of phosphorus per day recommended by the Institute of Medicine [30].
A 2020 systematic review and meta-analysis investigated fruit and vegetable consumption associated with prognosis of cancer survivors who are at increased risk for a second primary tumor [31]. The researchers found that fruit and vegetable intake was associated with reduced mortality in patients with ovarian cancer, and vegetable intake was also associated with reduced mortality in patients with head and neck cancers. Additionally, a study of the BioBank Japan Project found that low intake of green leafy vegetables was associated with higher mortality in male patients with colorectal cancer [32]. Overall, observational studies show that reduced cancer risk is associated with plant-based diets that are higher in fruits and vegetables and lower in animal-based foods [33]. These findings generally imply that fruits and vegetables, in addition to providing important nutrients, tend to lower the overall intake of dietary phosphate.
2.2. Immunotherapy, Fevers, and Fasting
In 1976, Dr. Warren H. Cole delivered an address at the John Hopkins Medical Institutions on the importance of finding the cause for the spontaneous regression of cancer [34]. He stated, “If we are going to make progress in finding answers, I suspect we will have to reach outside the realm of accepted facts about cancer”.
2.2.1. Immunotherapy
In his final article on efforts to explain the spontaneous regression of cancer in 1981, Dr. Cole wrote that he was “convinced stimulation of the immune process is the most important factor”, and he was enthusiastic about interferon therapy for cancer [35]. Nevertheless, Dr. Cole also reiterated that “elimination of carcinogens appears important”, based on the findings of bladder cancer regression associated with the diversion of a potential carcinogen in urine. Since then, immunotherapies have continued to face challenges to meet expectations for cancer treatment efficacy [36,37], and tumors’ evasion of attacks from the immune system is now well recognized [38]. These research findings highlight the need for investigators to refocus on the elimination of carcinogens in the spontaneous regression of cancer, including eliminating dietary factors like phosphate overload.
Fortunately, Dr. Cole’s comments on bladder cancer have since been corroborated by additional evidence involving phosphorus. For example, the 2011 Belgian case–control study of bladder cancer found that the highest intake of dietary phosphate was associated with a statistically significant two-fold increased odds of bladder cancer in older adults [39]. More recently, phosphate in urine from patients with bladder cancer was associated with greater toxicity and altered cell proliferation [40]. As previously mentioned, phosphate is dysregulated in CKD, and a higher risk of bladder cancer is associated with CKD in non-dialysis patients [41].
2.2.2. Fevers
In their review of the spontaneous regression of cancer, Radha and Lopus mentioned that tumor regression following fevers and acute infections were reported by the ancient Egyptians [4]. The authors also described how, in 1891, Dr. William Bradley Coley induced fevers in cancer patients using injections of bacteria, but that subsequent tumor regression was inconsistent, and the treatment had adverse effects. Similar responses to infection have been suggested to account for spontaneous tumor regression following a systemic reaction to the COVID-19 vaccination with “intense reactogenicity” [42]. Although these cases of tumor regression are associated with the stimulation of the immune system, other related factors should be considered. For example, loss of appetite and reduced dietary intake during acute infections and febrile conditions, including reduced intake of dietary phosphate, should be investigated.
Importantly, appetite suppression or sickness-associated anorexia in infected humans and other species is part of an immune response that defends the survival of the infected host—a nutritional immunology factor [43]. Studies have shown that feeding during sickness could aggravate the illness and that underfeeding can be beneficial. Sickness-associated anorexia also promotes autophagy. Derived from the Greek words for “self” and “eat”, autophagy is defined by Ebrahimi et al. as a “process by which eukaryotic cells degrade large cellular components into substrates that subsequently can be either used as fuel source, or utilized for the synthesis of critical cellular components” [44]. The researchers also found that dietary phosphate restriction activates autophagy in yeast, implying that the association of the spontaneous regression of tumors with autophagy and sickness-associated anorexia is potentially mediated by dietary phosphate restriction.
In addition to associations with the regression of solid tumors, fever was associated with spontaneous remission in over 90% of acute myeloid leukemia cases in a 2014 review [45]. The spontaneous remission of acute myelomonocytic leukemia includes lowered blood neutrophil and platelet counts without chemotherapy, and remission was recently documented in a case study of a woman with fever and a severe COVID-19 infection [46]. However, the authors noted that spontaneous remission usually does not endure in cases of acute myeloid leukemia, and remission lasted only six months in their patient.
2.2.3. Fasting
Cancer cells appear to be more vulnerable to nutrient deprivation than normal cells [47], plausibly related to a weaker blood supply to cancer cells due to the highly abnormal structure and function of the tumor vasculature [48]. Consequently, nutrient deprivation through fasting can reduce levels of growth factors and metabolites and create “environments that can reduce the capability of cancer cells to adapt and survive” [47]. Additionally, autophagy “plays a critical role in regulating cellular nutrient status in a fasted state” [44], and fasting-mimicking diets are associated with tumor regression [49]. Fasting-mimicking diets have also improved clinical outcomes in cancer patients in combination with conventional antineoplastic treatments [50,51].
Originally developed in 2015 by Brandhorst et al., a fasting-mimicking diet was shown to reduce cancer incident in C57BL/6 mice, and the diet lowered cancer risk factors and biomarkers in humans [52]. In the pilot clinical study of the diet, participants were randomized to receive a plant-based low-protein fasting-mimicking diet for five days a month and a total of three months. The diet consisted of approximately 1090 calories consumed on the first day and 725 calories consumed on each of the next four days. The researchers designed the diet “to provide 34–54% of the normal caloric intake with a composition of at least 9–10% proteins, 34–47% carbohydrates, and 44–56% fat”. The participants consumed their usual diet during the rest of the study.
Future research of dietary phosphate restriction associated with autophagy and fasting therapy for cancer regression is warranted, including investigations of effects from nutrients other than phosphorus. Additionally, appetite loss is a common side effect of cancer treatments [53], and research should investigate sickness-associated anorexia and autophagy as confounding factors associated with reduced dietary phosphate in tumor regression during cancer treatments.
3. Autophagy and Phosphate in Tumor Regression and Reversion
The National Cancer Institute noted that autophagy may protect cancer cells “by providing extra nutrients to them or by keeping anticancer drugs or other substances from destroying them”, but no mention was made of autophagy in tumor regression and reversion [54]. Gluconeogenesis keeps cancer cells supplied with glucose, which is derived from lactate and amino acids [55], and autophagy is a necessary mechanism in gluconeogenesis for maintenance of tumors [56]. In addition to promoting tumor growth in the later stages of cancer, autophagy plays a role in tumor prevention by suppressing cancer initiation in the early stages of tumorigenesis [57]. Although these autophagy mechanisms affect tumor growth factors, they do not fully explain autophagy in idiopathic tumor regression. Hypothetically, cancer cells may degrade intracellular components by applying the same basic principles of autophagy as other cells.
In a review of the basic principles of autophagy by Kundu and Thompson, the authors mentioned the role of lysosomes in autophagy as part of the “process by which cellular components are sequestered within the vesicular system and delivered to lysosomes for degradation and recycling of bioenergetic components” [58]. An important distinction between autophagy and autolysis is that enzymes in lysosomes are used to degrade intracellular components in autophagy, whereas the same enzymes are used to destroy the entire cell in autolysis [59]. Evidence of cancer cell reversion in the present paper shows that the cell is not destroyed, suggesting that autophagy rather than autolysis is the main mechanism involved in tumor reversion.
Kundu and Thompson described the steps of autophagy, beginning with the selection and packaging of cargo for degradation, with the subsequent exportation and recycling of “metabolic building blocks” [58]. For example, bioenergetic and biosynthetic phosphate substrates are recycled through autophagy for incorporation into adenosine triphosphate (ATP) and nucleotides, which support the cell survival from energy starvation and depleted nucleotide pools [60]. Kundu and Thompson also noted that the type of stimulus determines if autophagy targets nonspecific or specific contents of the cytoplasm for degradation. The authors added that autophagy can be an “adaptive response” to a stimulus, and that a change in the availability of nutrients signals the initiation of nonselective autophagy. Additionally, the authors listed cellular stresses that dramatically induce autophagy, including “growth factor withdrawal”. These principles of autophagy appear consistent with the withdrawal of phosphate overload in tumor regression and reversion.
In addition to recycling substrates, an additional function of autophagy is the elimination of intracellular macromolecules [61]. A mechanism, by which autophagy targets specific contents in the cytoplasm of cancer cells, involves acid phosphatases contained in lysosomes which break apart (lyse) macromolecules during autophagy [62]. Intracellular phosphate is stored as macromolecules of inorganic polyphosphate in some types of cancer cells [63], and phosphatase has been shown to catalyze the breakdown or hydrolysis of polyphosphate [64]. Studies should investigate the potential suppression of acid phosphatase in lysosomes as polyphosphate accumulates in cancer cells during tumorigenesis, as well as the activation of acid phosphatase during autophagy that breaks apart polyphosphate stored in cancer cells. Summing up, the withdrawal of phosphate overload is proposed to stimulate autophagy in cancer cells, including the specific hydrolysis of polyphosphate macromolecules. Also, bioenergetic and biosynthetic phosphate substrates may be eliminated or recycled.
4. Experimental Evidence of Tumor Reversion
In 1959, Pierce and Dixon used a mouse model of testicular teratocarcinoma to restore a normal phenotype in malignant embryonic cells by modifying the cell microenvironment [65]. In 1975, Mintz and Illmensee demonstrated that malignant embryonic cells in a mouse model produced normal functioning cells when transplanted into a normal environment [66]. Mintz implied that tumorigenesis was due to the cell environment, not to autonomous changes in the cell itself [67]. Other research confirmed that an embryonic microenvironment has anti-tumor effects on malignant cells [68], plausibly related to the tighter regulation of biochemical factors conducive to normal growth in an embryonic microenvironment compared to dysregulated growth in a tumor microenvironment.
Notably, a “normal” cellular microenvironment in tumor reversion implies normal concentrations of phosphate. Generally, phosphate levels within mammals have been shown to range from 0.5 to 5 mM, but higher concentrations of Pi, up to 10 mM, can stimulate cell proliferation [69]. Studies are needed to investigate phosphate concentrations in the cellular microenvironment associated with facilitation of tumor reversion.
In their 2023 literature review, Pensotti, Bertolaso, and Bizzarri summarized conclusions from experimental evidence supporting tumor reversion [2], shown in Table 1.
Table 1.
Conclusions supporting tumor reversion [2].
| “(a) Cancer cells display relevant plasticity, and their fate is not ‘irreversibly’ determined”. |
| “(b) It is possible to inhibit the phenotypic expression of the malignant characteristics of cancer cells mostly through epigenetic processes, although other mechanisms are likely to participate”. “(c) Depending on the tumor type and stage, some context-dependent conditions/constraints (such as those pertaining to the microenvironment of specific embryogenesis stages) can induce a phenotypic reversion of malignant cancer cells”. “(d) Gene mutations do not play a ‘causative’ role as the somatic mutation theory (SMT) posits, albeit they can be associated throughout the process of cancer development”. |
5. Dysregulated Phosphate Metabolism and Tumorigenesis
The research literature on cancer describes the cancer cell’s demand for nutrients to support autonomous growth [70], yet evidence associates tumor cell growth with adaptation to cellular nutrient overload—a reflexive response dependent on an externally imposed oversupply in contrast to an independent internal demand. As a potential carcinogenic and epigenetic factor, the toxic effects of nutrient overload are well recognized in nutritional epidemiology, and cancer is also strongly associated with diet [71]. “Metabolic reprogramming of cancer cells may result in strong dependencies on nutrients that could be exploited for therapy”, and dietary interventions restricting nutrients required for tumor growth have been proposed for cancer therapy [72].
In a review of dietary phosphate consumption and tumorigenesis, Arnst and Beck Jr. noted that changes in intracellular and extracellular levels of Pi affect glucose metabolism, inflammation, and oxidative stress, which are associated with tumorigenesis and cancer progression [73]. Studies cited by the authors showed that tumors in mice and humans had greater Pi uptake and retention compared to healthy tissue. The authors also reviewed evidence showing that Pi activates cell-signaling pathways for cellular growth, and the authors noted “the possibility that Pi consumption can be manipulated to control cell growth, particularly in rapidly dividing cells”.
Importantly, Arnst and Beck Jr. emphasized “the paradox of how an element critical to essential cellular functions can, when available in excess, influence and promote a cancer phenotype”. Furthermore, the stealth ability of phosphate to hide in plain sight as an essential nutrient for cell growth, and then surreptitiously turn into a carcinogenic agent during dietary phosphate overload could explain how the association of phosphate with tumorigenesis has evaded detection by most cancer researchers. Moreover, this mechanism is potentially related to tumor regression and reversion under circumstances of reduced dietary phosphate. Although Arnst and Beck Jr. did not review regression and reversion of tumors, they suggested that dietary phosphate restriction has potential as a novel therapeutic approach to control tumorigenesis [73].
Sullivan and Vander Heiden wrote that “proliferation requires that cells accumulate sufficient biomass to grow and divide. Cancer cells within tumors must acquire a variety of nutrients, and tumor growth slows or stops if necessary metabolites are not obtained in sufficient quantities” [74]. Befittingly, the dietary mineral phosphorus is a primary nutrient for the growth of human cellular biomass [75]. As a growth factor, phosphate nutrient overload is a potential carcinogenic agent for cancer-cell proliferation within the tumor microenvironment, and a target for phosphate reduction in tumor regression and reversion.
5.1. Nutrient Toxicity
Excessive amounts of nutrients can become toxic, according to a 1981 article in Nutrition Reviews by Campbell, Allison, and Fisher [76]. The authors explained that the intake of a nutrient on a dose–response curve spans across ranges indicating deficiency, adequacy, and toxicity. They further explained that the original objective of developing guidelines for the recommended nutrient intake was to reduce the risk of deficiency diseases, but some nutrients may be consumed in amounts much greater than recommended. “The public appears to understand the possibility of adverse effects resulting from excessive consumption of calories, salt, protein, refined sugar, fat and cholesterol, and alcohol: but this concept is not extended as easily to vitamins and essential minerals”, including dietary phosphorus.
Compared to toxicity from a single megadose of nutrients, there is “greater public health concern about chronic nutrient toxicity where the effects develop more subtly and slowly” [76]. For example, Table A1 in Appendix A shows that the estimated average requirement (EAR) for phosphorus is 580 mg/day for adults, which increases to a recommended dietary allowance (RDA) of 700 mg/day to meet the needs of 97.5% of the adult population [77]. The tolerable upper intake level (UL) for phosphorus in most adults without causing harmful health effects is 4000 mg. Yet, although mean phosphorus intake within the United States adult population is well below the UL considered toxic [30], an intake of more than twice the RDA is nevertheless sufficient to associate cancer with chronic nutrient toxicity from excessive dietary phosphate intake. For instance, middle-aged women who consumed >1800 mg phosphorus were recently found to have over twice the relative risk of breast cancer incidence compared to women consuming 800–1000 mg phosphorus [78].
In their review on dietary phosphate and cancer [73], Arnst and Beck Jr. included contradictory findings of increased cancer risk in a study of mice consuming a low-phosphate diet [79]. This contradiction prompted Arnst and Beck Jr. to speculate that the association of dietary phosphate levels and cancer risk could follow a U-shaped curve. However, the cited mouse study did not appear to control for the high amount of phosphate in casein fed to the mice, as advised by the American Institute of Nutrition’s (AIN) final report of the AIN-93 rodent diet [80]. Consequently, equal amounts of casein (200 g/kg diet) were inadvertently fed to both the normal and low-phosphate groups of the study. In comparison with casein, fat in cow’s milk contains no phosphorus, which could contribute to the “anticarcinogenic” effects of the fat components of cow’s milk in animal experiments [81]. Interestingly, neuroblastoma has a high propensity for spontaneous regression and is much more frequent in infants [82,83], perhaps related to the variable intake of cow’s milk which is high in phosphorus—by comparison, breast milk is approximately six times lower in phosphorus [84]. More studies are needed to investigate changes in dietary phosphate overload and the spontaneous regression of neuroblastoma in infants.
5.2. Phosphate in the Tumor Microenvironment
During tumorigenesis, excess phosphate in the tumor microenvironment upregulates the expression of sodium-dependent Pi transporters, NaPi2b, which sequester phosphate into cancer cells [85]. High extracellular levels of Pi in breast cancer cells (MDA-MB-231 cell line) were shown to stimulate H+-independent Pi transporters with five-fold more Pi transport than NaPi2b transporters [86].
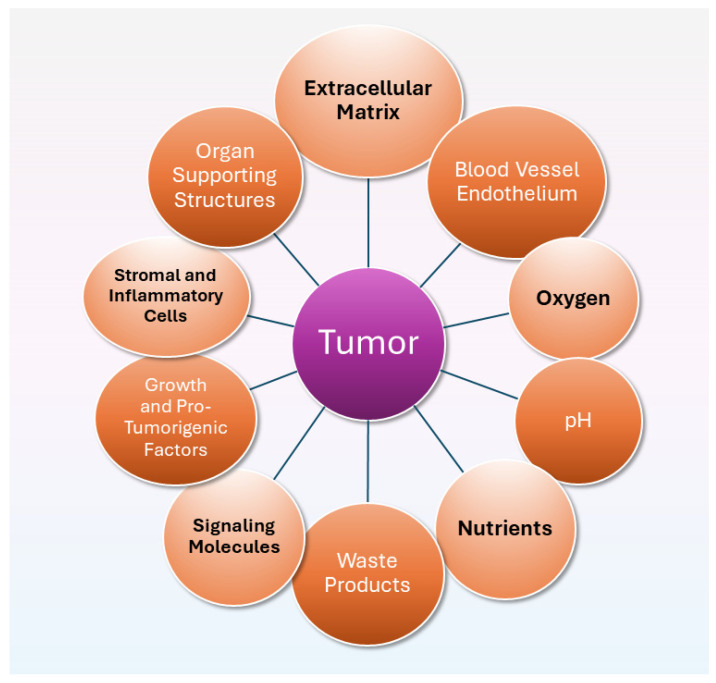
In 2017, West Virginia University researchers used an in vivo electron paramagnetic resonance technique in mouse models of cancer to investigate components of the tumor microenvironment, which included the extracellular matrix, blood vessel endothelium, and other heterogeneous components shown in Figure 1 [87]. The researchers found that higher levels of interstitial Pi within the tumor microenvironment were associated with metastasizing tumors. This important finding implies that metastasis is potentially a systemic disorder associated with dysregulated phosphate metabolism—providing an alternative to the seed and soil theory that proposes that metastasis spreads to secondary sites as cancer cells escape from a tumor [88]. More recently in 2023, the West Virginia University researchers used the same in vivo technique in a mouse breast-cancer model to investigate cancer biomarkers within the tumor microenvironment, including oxygen concentration and extracellular pH [89]. The researchers found that only interstitial Pi, a biomarker of deviated metabolic status, was consistently elevated during all stages of tumorigenesis up to and including malignancy.
Figure 1.
Schematic drawing of heterogeneous components within the tumor microenvironment, based on Bobko et al. [87].
Additionally, in 2021, South American researchers used micro X-ray fluorescence to measure phosphorus in samples of tumor tissue from the mammary glands of BALB/c mice, and the researchers found a strong correlation of phosphorus levels with active adenocarcinoma cells [90]. Other researchers exposed cancer cell samples to extracellular Pi and found that concentrations above 16 mM caused cancer cell death by apoptosis [69].
5.3. Kinases and Phosphatases
The phosphorylation of a protein by kinase enzymes is a post-translational modification that binds a negatively charged phosphate group to the protein’s amino acids (serine/threonine, and tyrosine) [91]. This reaction modifies the protein’s polarity and changes its configuration as it interacts with other biomolecules in cell signaling. Importantly, activation of this cell-signaling mechanism is reversible through the dephosphorylation by phosphatase enzymes. Furthermore, “the uncontrolled activation of kinases and the suppression of phosphatases has been frequently observed in cancer” [92].
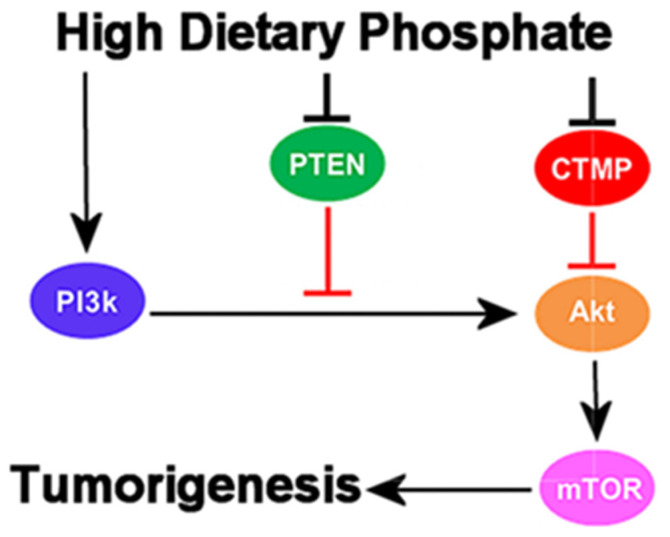
Over 1000 variations have been found in protein kinases in human tumors [91]. Yet, clinical trials of kinase inhibitors have been mostly unsuccessful in treating solid tumors, and some researchers suggest the necessity of simultaneously activating phosphatases such as Protein Phosphatase 2A (PP2A) [93]. However, high dietary phosphate in a mouse model of lung cancer was sufficient to inhibit expression of the tumor suppressors phosphatase and tensin homolog (PTEN) and carboxyl-terminal modulator protein (CTMP), while also activating the Akt signaling pathway (protein kinase B) [94]. These findings, illustrated in Figure 2, imply that the problem with treating cancer patients with kinase inhibitors and phosphatase activators may lie in the failure to control dietary phosphate intake, which regulates the balance of kinases and phosphatases. Moreover, experiments have shown that intestinal alkaline phosphatase activity and the expression of alkaline phosphatase 3 protein levels in the intestines of mice were increased with a low-phosphate diet compared to higher phosphate intake [95]. Based on the preceding findings, this paper proposes reducing dietary phosphate overload and reregulating phosphate metabolism to reverse uncontrolled kinase activation and phosphatase inhibition in cancer-cell signaling, thereby inducing tumor regression and reversion.
Figure 2.
High dietary phosphate and cancer-cell signaling [96]. This flowchart shows that high dietary phosphate activates the phosphoinositide 3-kinase (PI3K)/Akt/mTOR signaling pathway while deactivating tumor suppression by PTEN and CTMP, increasing cell growth in tumorigenesis.
5.4. Nutrient Metabolism in Cancer
Although scientists have experimentally demonstrated tumor reversion by transplanting malignant cells into healthy cellular microenvironments, an understanding of the causative mechanisms of tumor reversion and regression remains elusive. Altea-Manzano et al. summarized several factors that influence nutrient metabolism in cancer [97]. These factors apply to dysregulated phosphate metabolism in tumorigenesis with potential applications to tumor regression and reversion (Table 2).
Table 2.
Nutrient metabolism factors in cancer [97] compared with dysregulated phosphate metabolism.
| Nutrient Metabolism Factor in Cancer | Dysregulated Phosphate Metabolism | |
|---|---|---|
| “(i) diet, the primary source of bodily nutrients which influences circulating metabolite levels”; | (i) dietary phosphate is the primary nutrient source of the circulating metabolite Pi; | |
| “(ii) tissue of origin, which can influence the tumor’s reliance on specific nutrients to support cell metabolism and growth”; | (ii) phosphate influences most tissue types and stages of tumor growth; | |
| “(iii) local microenvironment, which dictates the accessibility of nutrients to tumor cells”; | (iii) excessive extracellular Pi in local microenvironment is transported into tumor cells; | |
| “(iv) tumor heterogeneity, which promotes metabolic plasticity and adaptation to nutrient demands”; | (iv) adaptation to oversupply of phosphate promotes tumorigenesis; | |
| “(v) functional demand, which intensifies metabolic reprogramming to fuel the phenotypic changes required for invasion, growth, or survival”. | (v) dietary phosphate overload which may be reduced in tumor regression and reversion. |
5.5. Tumor Metabolic-Reversal Model
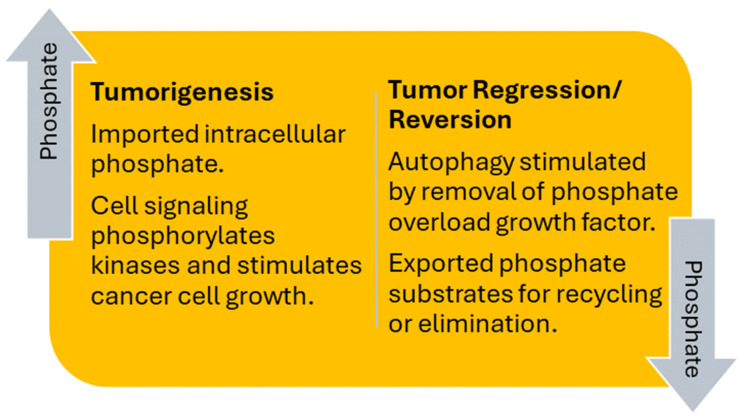
The following tumor metabolic-reversal model is based on a biological framework for cancer research developed by Schipper et al., which posits that cancer is a metabolic disease and may be reversable [98]. The model, shown in Figure 3, incorporates the metabolic principles of tumorigenesis and tumor regression/reversion, which are stimulated by high and low levels of phosphate overload, respectively. In tumorigenesis, imported intracellular phosphate increases cell signaling by phosphorylating kinases, which stimulates tumor cell growth. In tumor regression/reversion, autophagy is stimulated by the removal of the tumor growth factor when phosphate overload is reduced. Phosphate substrates from autophagy are subsequently exported from the cell and are either eliminated or recycled in the body for the bioenergetic and biosynthetic formation of new compounds (e.g., ATP and nucleotides).
Figure 3.
Tumor Metabolic-Reversal Model. The model shows that tumorigenesis from imported intracellular phosphate and cell signaling is reversed by removal of phosphate overload, which stimulates autophagy and recycles or eliminates phosphate substrates.
6. Tumor Encapsulation and Phosphate Toxicity
Tumors that are encapsulated with a surrounding band of connective tissue are considered benign tumors, although not all benign tumors are encapsulated [99]. By contrast, a malignant tumor continues to grow without a well-formed capsule [100]. The foreign body hypothesis proposes that “capsule formation represents an attempt by the host to contain the tumour” in a fashion analogous to “encapsulation of a foreign body” [99]. Encapsulated tumors are associated with a protective effect in cancer patients. For example, 72 cases of encapsulated hepatocellular carcinoma resulted in “significantly better disease-free and actuarial survival times” compared to nonencapsulated cases [101]. The researchers noted that “lower extensive tumor invasiveness” was a significant factor contributing to better prognosis in encapsulated cases, and similar results were found in women with encapsulated hepatocellular carcinoma [102]. Another study using magnetic resonance imaging found that complete tumor encapsulation in patients with a large solitary hepatocellular carcinoma was a “predictor for favorable biology” [103].
Importantly, the tumor contains products of nutrient metabolism that are toxic to the system, including excessive phosphate. Breaching toxin containment by destroying the tumor releases these intracellular toxins into the blood stream, causing phosphate toxicity in a life-threatening oncological emergency known as tumor lysis syndrome [104]. The effects of tumor lysis syndrome produce “acute kidney injury, uremia, and systemic end-organ damage, including renal failure and liver failure, potentially causing seizures, cardiac dysrhythmias, and death”. Notable electrolyte irregularities in tumor lysis syndrome include hyperphosphatemia and hypocalcemia, secondary to elevated serum phosphate.
Although considered a rare event, the mortality rate of tumor lysis syndrome for solid tumors is as high as 40% [105], demonstrating the potential importance of toxin containment in encapsulated tumors as a protective effect. Future studies need to investigate how changes in dietary phosphate overload affect encapsulation in tumorigenesis. The overall evidence reviewed in this paper suggests that increased expression of phosphate transporters sequesters excessive extracellular Pi during the promotion of tumorigenesis, and tumor encapsulation potentially prevents efflux of contained Pi ions from reentering the general circulation causing phosphate toxicity. A recent review demonstrated that a mouse model of tumor suppression produced cancer cachexia effects of sarcopenia, organ atrophy, bone disorders, and premature death, similar to effects of phosphate toxicity [106].
7. Future Studies
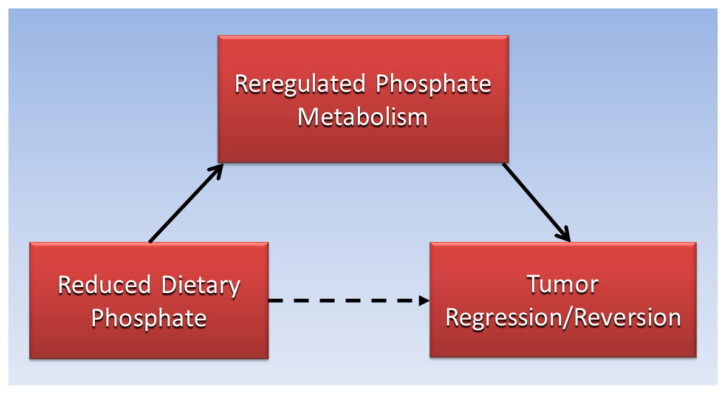
The present paper proposes that reversing the transport and containment of an overload of Pi in tumors by reducing dietary phosphate intake has the potential to stimulate tumor regression and reversion. Figure 4 is a directed acyclic graph proposing that tumor regression and reversion is associated with reduced dietary phosphate through mediation by reregulated phosphate metabolism. Factors that potentially reduce dietary phosphate include sickness-associated anorexia [44], fasting-mimicking diets [52], and other diets low in phosphate such as certain types of ketogenic and plant-based diets. Phosphate binders also reduce the bioavailability of dietary phosphate absorbed in the intestines [107], and preclinical studies should investigate dietary phosphate intake levels associated with all of these dietary factors in tumor regression and reversion. Cancer reversion also occurs in malignant embryonic cells when transplanted into a healthy microenvironment [66], and studies should explore the effects of different concentrations of Pi in the microenvironment on malignant cell reversion. The uncontrolled activation of kinases is common in cancer [92], and studies are needed to prevent cancer initiation by restoring a proper balance of phosphorylated kinases and activated phosphatases using a reduced dietary phosphate load. Finally, autophagy is stimulated by the withdrawal of growth factors [58], and autophagy activated by the withdrawal of phosphate overload is a potential factor in tumor regression and reversion that warrants further study.
Figure 4.
Proposed association of reduced dietary phosphate with tumor regression/reversion, mediated by reregulated phosphate metabolism.
8. Conclusions
The spontaneous regression and reversion of tumors challenges the assumption that cancer is an irreversible genetic disease. Among the factors potentially responsible for tumor regression, the removal of a carcinogen by reducing elevated phosphate levels in the tumor microenvironment may be possible through reduced dietary phosphate intake. Tumor reversion depends on a healthy cellular microenvironment, which implies normal levels of cellular phosphate and a proper balance of kinases and phosphatases to reduce cell signaling in cancer. Fasting-mimicking diets and sickness-associated anorexia appear to stimulate the autophagy of tumors, and this effect may be due to a lower dietary phosphate intake. Encapsulated tumors have a protective effect potentially related to containment of toxic levels of phosphate. Preclinical studies of a low-phosphate diet for the regression and reversion of tumors in cancer patients are warranted.
Appendix A
Table A1.
Phosphorus Dietary Reference Intakes (mg/day). Source: Institute of Medicine (2006) [108].
| Age | EAR * | RDA * | AI * | UL * |
|---|---|---|---|---|
| Infants 0–6 months | — | — | 100 | — |
| Infants 7–12 months | — | — | 275 | — |
| Children 1–3 years | 380 | 460 | — | 3000 |
| Children 4–8 years | 405 | 500 | — | 3000 |
| Children 9–18 years | 1055 | 1250 | — | 4000 |
| Adults 19–70 years | 580 | 700 | — | 4000 |
| Adults over 70 years | 580 | 700 | — | 3000 |
| Pregnancy 18 years and under | 1055 | 1250 | — | 3500 |
| Pregnancy 19 years and over | 580 | 700 | — | 3500 |
| Lactation 18 years and under | 1055 | 1250 | — | 4000 |
| Lactation 19 years and over | 580 | 700 | — | 4000 |
* AI = Adequate Intake. * EAR = Estimated Average Requirement. * RDA = Recommended Dietary Allowance. * UL = Tolerable Upper Intake Level (food, water, and supplements).
Appendix B
Table A2.
Foods high in phosphorus. Source: U.S. Department of Agriculture (USDA), FoodData Central. https://fdc.nal.usda.gov/, accessed on 29 May 2024.
| Food (100 g) | Phosphorus (mg) |
|---|---|
| Leavening agents, baking powder, low-sodium | 6870 |
| Seeds, hemp seed, hulled | 1650 |
| Beverages, protein powder whey-based | 1320 |
| Gelatin desserts, dry mix, reduced calories, with aspartame | 1290 |
| Egg yoke, dried | 1040 |
| Fish, cod, Atlantic, dried and salted | 950 |
| Cheese spread, American or cheddar cheese base, reduced fat | 931 |
| Beverages, cocoa mix, no sugar added, powder | 893 |
| Wheat germ, crude | 842 |
| Cheese, Swiss, low fat | 605 |
| Biscuits, plain or buttermilk, dry mix | 585 |
| Wheat flout, white, all-purpose, self-rising, enriched | 595 |
| Beans, dry, light red kidney (0% moisture) | 549 |
| Pork, cured, bacon, cooked, broiled, pan-fried | 533 |
| Nuts, cashew nuts, raw | 532 |
| Flour, almond | 512 |
| Wheat, durum | 508 |
| Peanuts, Virginia, oil-roasted, with salt | 506 |
| Beef, variety meats and by-products, liver, cooked, braised | 497 |
| Cheese, provolone | 496 |
| Fish, sardine, Atlantic, canned in oil, drained solids with bone | 490 |
| Breast, cornbread, dry mix, enriched (includes corn muffin mix) | 489 |
| Cereals, QUAKER, instant oatmeal organic, regular | 474 |
| Cereals ready-to-eat, POST GRAPE-NUTS Cereal | 467 |
| Quinoa, uncooked | 457 |
| Cereals ready-to-eat, granola, homemade | 431 |
| Waffles, plain, frozen, ready-to-heat, toasted | 429 |
| Macaroni or noodles with cheese, made from reduced fat packaged mix, unprepared | 403 |
| Egg, yolk, raw, fresh | 390 |
| Lentils, dry | 374 |
| DENNY’S mozzarella cheese sticks | 358 |
| Fish, salmon, red, (sockeye), canned, smoked (Alaska Native) | 350 |
| Restaurant, Mexican, cheese quesadilla | 347 |
| Vegetarian meatloaf or patties | 344 |
| Pasta, whole-wheat, dry | 343 |
| Cheese, feta | 337 |
| Peanut butter, smooth style, without salt | 335 |
| Crackers, rye, wafers, plain | 334 |
| Beef, loin, tenderloin steak, boneless, separable lean only, trimmed to 0 fat, choice, cooked, grilled | 321 |
| Pork, fresh, loin, top loin (chops), boneless, separable lean only, with added solution, cooked, pan-broiled | 320 |
| School lunch, pizza, cheese topping, thin crust, whole grain, frozen, cooked | 316 |
| Chocolate, dark, 70–85% cacao solids | 308 |
| Ham, turkey, sliced, extra lean, prepackaged or deli | 304 |
| Bread, whole-wheat, commercially prepared, toasted | 303 |
| Chicken, broiler or fryers, giblets, cooked, simmered | 289 |
| Snacks, potato chips, white, restructured, baked | 274 |
| Doughnuts, cake-type, plain (included unsugared, old-fashioned) | 260 |
| Milk, canned, condensed, sweetened | 253 |
| Beef, ground, 85% lean meat/15% fat, crumbles, cooked, pan-browned | 238 |
| KFC, fried chicken, ORIGINAL RECIPE, thigh, meat and skin with breading | 230 |
| Rolls, dinner, whole-wheat | 224 |
| Bread, whole-wheat, commercially prepared | 212 |
| Nuts, macadamia nuts, raw | 188 |
| Cookies, sugar, refrigerated dough, baked | 187 |
| Bologna, beef, low fat | 178 |
| Turkey breast, low salt, prepackaged or deli, luncheon meat | 162 |
| Snacks, potato chips, lightly salted | 148 |
| Cake, chocolate, commercially prepared with chocolate frosting in-store bakery | 137 |
| Fast foods, potato, French fried in vegetable oil | 125 |
| Milk shakes, thick vanilla | 115 |
Appendix C
Table A3.
Foods low in phosphorus. Source: USDA, FoodData Central. https://fdc.nal.usda.gov/, accessed on 29 May 2024.
| Food (100 g) | Phosphorus (mg) |
|---|---|
| Oils: avocado; coconut; almond; olive; vegetable; soybean; peanut; canola; corn; sunflower; safflower; cottonseed; palm | 0 |
| Beverages, tea, herb, brewed, chamomile | 0 |
| Beverages, carbonated, ginger ale | 0 |
| Egg, white, raw, frozen, pasteurized | 0 |
| Beverages, coffee, brewed, prepared with tap water, decaffeinated | 1 |
| Salad dressing, French, cottonseed oil, home recipe | 3 |
| Soup, chicken broth or bouillon, dry, prepared with water | 3 |
| Beverages, coffee, instant, regular, prepared with water | 3 |
| Olives, ripe, canned | 3 |
| Alcoholic beverage, distilled, all (gin, rum, vodka, whiskey) 94 proof | 4 |
| Honey; sugars, brown: vinegar, distilled | 4 |
| Pineapple, raw | 5 |
| Blueberries, frozen, sweetened; apple juice, canned or bottled, unsweetened, without added ascorbic acid | 7 |
| Grapefruit, raw, white, all areas | 8 |
| Apples, red delicious, with skin, raw | 9 |
| Raw fruits: papaya; pears, bartlett | 10 |
| Syrups, table blends, pancake, with 2% maple, with added potassium | 10 |
| Melons, honeydew, raw | 11 |
| Milk, human, mature, fluid (for reference only) | 14 |
| Raw fruits: mangos; figs | 14 |
| Squash, winter, butternut, frozen, cooked, boiled, with salt | 14 |
| Melons, cantaloupe, raw | 15 |
| Alcoholic beverage, wine, light | 15 |
| Eggplant, cooked, boiled, drained, with salt | 15 |
| Beets, pickled, canned, solids and liquids | 17 |
| Orange juice, chilled, includes from concentrate, with added calcium and vitamins A, D, E | 17 |
| Cereals, CREAM OF WHEAT, instant, prepared with water, without salt | 18 |
| Beans, snap, green, canned, regular pack, solids and liquids | 18 |
| Vinegar, balsamic | 19 |
| Radishes, raw; Fast foods, coleslaw; sauerkraut, canned solids and liquids | 20 |
| Raw fruits: cherries, sweet: jackfruit: clementines | 21 |
| Raw fruits: bananas; peaches, yellow; blackberries; grapes, green, seedless | 22 |
| Nuts, coconut cream, canned, sweetened | 22 |
| Raw fruits: oranges, navels; apricots; strawberries | 23 |
| Raw vegetables: celery; cucumber with peel; tomatoes, red, ripe; peppers, sweet, yellow | 24 |
| Butter, whipped, with salt | 24 |
| Noodles, Japanese, soba, cooked | 25 |
| Prune juice, canned | 25 |
| Summer squash, zucchini, includes skin, frozen, cooked, boiled, drained, without salt | 25 |
| Onions, sweet, raw; collards, frozen, chopped, unprepared | 27 |
| Kale, frozen, unprepared | 29 |
| Mushrooms, shiitake, cooked, without salt | 29 |
| Carrots, cooked, boiled, drained, without salt | 30 |
| Lettuce, cos or romaine, raw | 30 |
| Cabbage, cooked, boiled, drained, without salt | 33 |
| Rice, white, steamed, Chinese restaurant | 33 |
| Sauce, pasta, spaghetti/marinara, ready-to-serve | 34 |
| Beans, snap, yellow, cooked, boiled, drained, with salt | 39 |
| Potatoes, boiled, cooked, without skin, flesh, with salt | 40 |
| Cauliflower, raw | 44 |
| Peas and carrots, frozen, cooked, boiled, drained, without salt | 49 |
| Spinach, raw | 49 |
| Broccoli, frozen, chopped, cooked, boiled, drained, with salt | 49 |
| Arugula, raw | 52 |
| Sweet potato, cooked, baked in skin, flesh, without salt | 54 |
| Avocados, raw, California | 54 |
| Brussels sprouts, cooked, boiled, drained, with salt | 56 |
| Dates, deglet noor, medjool | 62 |
| Figs, dried, uncooked | 67 |
| Candies, MARS SNACKFOOD US, 3 MUSKETEERS bar | 69 |
| DENNY’S hash browns | 71 |
| Sour cream, reduced fat | 85 |
| Yogurt, vanilla, non-fat | 88 |
| Corn, sweet, yellow, raw | 89 |
| Milk, producer, fluid, 3.7% milkfat | 93 |
| Milk, nonfat, fluid, with added vitamin A and vitamin D (fat free or skim) | 107 |
| Nuts, coconut meat, raw | 113 |
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The author declares no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Pensotti A., Bizzarri M., Bertolaso M. The phenotypic reversion of cancer: Experimental evidences on cancer reversibility through epigenetic mechanisms (Review) Oncol. Rep. 2024;51:48. doi: 10.3892/or.2024.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pensotti A., Bertolaso M., Bizzarri M. Is Cancer Reversible? Rethinking Carcinogenesis Models-A New Epistemological Tool. Biomolecules. 2023;13:733. doi: 10.3390/biom13050733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Alessandris N., Santoro A., Arciuolo D., Angelico G., Valente M., Scaglione G., Sfregola S., Carlino A., Navarra E., Mulè A., et al. What Can Trigger Spontaneous Regression of Breast Cancer? Diagnostics. 2023;13:1224. doi: 10.3390/diagnostics13071224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radha G., Lopus M. The spontaneous remission of cancer: Current insights and therapeutic significance. Transl. Oncol. 2021;14:101166. doi: 10.1016/j.tranon.2021.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qureshi A., Gollamudi S., Qureshi S., Sondhi N., Nabi S., Genato R., Xiao P., Asarian A. The phenomenon of spontaneous tumor regression in breast cancer. J. Surg. Case Rep. 2023;2023:rjad651. doi: 10.1093/jscr/rjad651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin D., Cho K.-H. Critical transition and reversion of tumorigenesis. Exp. Mol. Med. 2023;55:692–705. doi: 10.1038/s12276-023-00969-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joo J.I., Park H.-J., Cho K.-H. Normalizing Input–Output Relationships of Cancer Networks for Reversion Therapy. Adv. Sci. 2023;10:2207322. doi: 10.1002/advs.202207322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dou X., Tong P., Huang H., Zellmer L., He Y., Jia Q., Zhang D., Peng J., Wang C., Xu N., et al. Evidence for immortality and autonomy in animal cancer models is often not provided, which causes confusion on key issues of cancer biology. J. Cancer. 2020;11:2887–2920. doi: 10.7150/jca.41324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole W.H., Everson T.C. Spontaneous regression of cancer: Preliminary report. Ann. Surg. 1956;144:366–383. doi: 10.1097/00000658-195609000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonnenschein C., Soto A.M. Somatic mutation theory of carcinogenesis: Why it should be dropped and replaced. Mol. Carcinog. 2000;29:205–211. doi: 10.1002/1098-2744(200012)29:4<205::aid-mc1002>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 11.Galmarini C.M. Lessons from Hippocrates: Time to Change the Cancer Paradigm. Int. J. Chronic Dis. 2020;2020:4715426. doi: 10.1155/2020/4715426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ledford H. Why are so many young people getting cancer? What the data say. Nature. 2024;627:258–260. doi: 10.1038/d41586-024-00720-6. [DOI] [PubMed] [Google Scholar]
- 13.WCRF/AICR . Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. World Cancer Research Fund/American Institute for Cancer Research; London, UK: 2018. [Google Scholar]
- 14.Chen Y., Michalak M., Agellon L.B. Importance of Nutrients and Nutrient Metabolism on Human Health. Yale J. Biol. Med. 2018;91:95–103. [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu H., Schlegel V. Impact of nutrient overload on metabolic homeostasis. Nutr. Rev. 2018;76:693–707. doi: 10.1093/nutrit/nuy023. [DOI] [PubMed] [Google Scholar]
- 16.Gyamfi J., Kim J., Choi J. Cancer as a Metabolic Disorder. Int. J. Mol. Sci. 2022;23:1155. doi: 10.3390/ijms23031155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner C.A. The basics of phosphate metabolism. Nephrol. Dial. Transpl. 2024;39:190–201. doi: 10.1093/ndt/gfad188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolfswinkel J.F., Furtmueller E., Wilderom C.P.M. Using grounded theory as a method for rigorously reviewing literature. Eur. J. Inf. Syst. 2013;22:45–55. doi: 10.1057/ejis.2011.51. [DOI] [Google Scholar]
- 19.American College of Surgeons Warren H. Cole, MD, FACS, 1899–1990. [(accessed on 10 March 2024)]. Available online: https://www.facs.org/about-acs/archives/past-highlights/colehighlight/
- 20.Cole W.H. Spontaneous regression of cancer: The metabolic triumph of the host? Ann. N. Y. Acad. Sci. 1974;230:111–141. doi: 10.1111/j.1749-6632.1974.tb14441.x. [DOI] [PubMed] [Google Scholar]
- 21.Papac R.J. Spontaneous regression of cancer: Possible mechanisms. In Vivo. 1998;12:571–578. [PubMed] [Google Scholar]
- 22.Minacapelli C.D., Leuszkiewicz P., Patel A., Catalano C., Abdelsayed G., Lalos A., Rustgi V. The Spontaneous Regression of Primary Gastrointestinal Malignancies: An Observational Review. Cureus. 2022;14:e32970. doi: 10.7759/cureus.32970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang M., Zhang J., Kalantar-Zadeh K., Chen J. Focusing on Phosphorus Loads: From Healthy People to Chronic Kidney Disease. Nutrients. 2023;15:1236. doi: 10.3390/nu15051236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacerda-Abreu M.A., Meyer-Fernandes J.R. Extracellular Inorganic Phosphate-Induced Release of Reactive Oxygen Species: Roles in Physiological Processes and Disease Development. Int. J. Mol. Sci. 2021;22:7768. doi: 10.3390/ijms22157768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Razzaque M.S. Phosphate Metabolism: From Physiology to Toxicity. In: Razzaque M.S., editor. Phosphate Metabolism: From Physiology to Toxicity. Springer International Publishing; Cham, Switzerland: 2022. pp. 1–6. [Google Scholar]
- 26.Hu M., Wang Q., Liu B., Ma Q., Zhang T., Huang T., Lv Z., Wang R. Chronic Kidney Disease and Cancer: Inter-Relationships and Mechanisms. Front. Cell Dev. Biol. 2022;10:868715. doi: 10.3389/fcell.2022.868715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada S., Tokumoto M., Tatsumoto N., Taniguchi M., Noguchi H., Nakano T., Masutani K., Ooboshi H., Tsuruya K., Kitazono T. Phosphate overload directly induces systemic inflammation and malnutrition as well as vascular calcification in uremia. Am. J. Physiol.-Ren. Physiol. 2014;306:F1418–F1428. doi: 10.1152/ajprenal.00633.2013. [DOI] [PubMed] [Google Scholar]
- 28.Erem S., He P., Razzaque M.S. Chapter 23—Regulation of phosphate in health and disease. In: Faul C., Gutiérrez O.M., White K.E., editors. Fibroblast Growth Factor 23. Elsevier; Amsterdam, The Netherlands: 2021. pp. 343–355. [Google Scholar]
- 29.Julson E. Top 12 Foods That Are High in Phosphorus. [(accessed on 15 March 2024)]. Available online: https://www.healthline.com/nutrition/foods-high-in-phosphorus.
- 30.Office of Dietary Supplements/National Institutes of Health Phosphorus—Fact Sheet for Health Professionals. [(accessed on 15 March 2024)]; Available online: https://ods.od.nih.gov/factsheets/Phosphorus-HealthProfessional/
- 31.Hurtado-Barroso S., Trius-Soler M., Lamuela-Raventós R.M., Zamora-Ros R. Vegetable and Fruit Consumption and Prognosis Among Cancer Survivors: A Systematic Review and Meta-Analysis of Cohort Studies. Adv. Nutr. 2020;11:1569–1582. doi: 10.1093/advances/nmaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamakoshi A., Nakamura K., Ukawa S., Okada E., Hirata M., Nagai A., Matsuda K., Kamatani Y., Muto K., Kiyohara Y., et al. Characteristics and prognosis of Japanese colorectal cancer patients: The BioBank Japan Project. J. Epidemiol. 2017;27:S36–S42. doi: 10.1016/j.je.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeClercq V., Nearing J.T., Sweeney E. Plant-Based Diets and Cancer Risk: What is the Evidence? Curr. Nutr. Rep. 2022;11:354–369. doi: 10.1007/s13668-022-00409-0. [DOI] [PubMed] [Google Scholar]
- 34.Cole W. Spontaneous regression of cancer and the importance of finding its cause. Natl. Cancer Inst. Monogr. 1976;44:5–9. [PubMed] [Google Scholar]
- 35.Cole W.H. Efforts to explain spontaneous regression of cancer. J. Surg. Oncol. 1981;17:201–209. doi: 10.1002/jso.2930170302. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Z., Zheng L., Chen W., Weng W., Song J., Ji J. Delivery strategies of cancer immunotherapy: Recent advances and future perspectives. J. Hematol. Oncol. 2019;12:126. doi: 10.1186/s13045-019-0817-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bai R., Chen N., Li L., Du N., Bai L., Lv Z., Tian H., Cui J. Mechanisms of Cancer Resistance to Immunotherapy. Front. Oncol. 2020;10:1290. doi: 10.3389/fonc.2020.01290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim S.K., Cho S.W. The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Front. Pharmacol. 2022;13:868695. doi: 10.3389/fphar.2022.868695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brinkman M.T., Buntinx F., Kellen E., Dagnelie P.C., Van Dongen M.C., Muls E., Zeegers M.P. Dietary intake of micronutrients and the risk of developing bladder cancer: Results from the Belgian case-control study on bladder cancer risk. Cancer Causes Control. 2011;22:469–478. doi: 10.1007/s10552-010-9718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao H., Tse R.T., Cheng C.K., Wong C.Y., Kong A.W., Chan R.C., Chiu P.K., Ng C.F., Teoh J.Y. In vitro cytotoxicity of human urine and its potential toxic parameters towards bladder cancer cells. PLoS ONE. 2022;17:e0276127. doi: 10.1371/journal.pone.0276127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lees J.S., Elyan B.M.P., Herrmann S.M., Lang N.N., Jones R.J., Mark P.B. The ‘other’ big complication: How chronic kidney disease impacts on cancer risks and outcomes. Nephrol. Dial. Transplant. 2022;38:1071–1079. doi: 10.1093/ndt/gfac011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sousa L.G., McGrail D.J., Li K., Marques-Piubelli M.L., Gonzalez C., Dai H., Ferri-Borgogno S., Godoy M., Burks J., Lin S.Y., et al. Spontaneous tumor regression following COVID-19 vaccination. J. Immunother. Cancer. 2022;10:e004371. doi: 10.1136/jitc-2021-004371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Niekerk G., Isaacs A.W., Nell T., Engelbrecht A.M. Sickness-Associated Anorexia: Mother Nature’s Idea of Immunonutrition? Mediat. Inflamm. 2016;2016:8071539. doi: 10.1155/2016/8071539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ebrahimi M., Habernig L., Broeskamp F., Aufschnaiter A., Diessl J., Atienza I., Matz S., Ruiz F.A., Büttner S. Phosphate Restriction Promotes Longevity via Activation of Autophagy and the Multivesicular Body Pathway. Cells. 2021;10:3161. doi: 10.3390/cells10113161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rashidi A., Fisher S.I. Spontaneous remission of acute myeloid leukemia. Leuk. Lymphoma. 2015;56:1727–1734. doi: 10.3109/10428194.2014.970545. [DOI] [PubMed] [Google Scholar]
- 46.Peñuela R., Hernandez I., Fernandes-Pineda M., Cortina L., Zapata D., Urrego O., Herrera J., Saenz I., Orduz R., Mejia F., et al. Spontaneous remission without treatment of acute myelomonocytic leukemia associated with COVID-19 infection. Hematol. Transfus. Cell Ther. 2023 doi: 10.1016/j.htct.2023.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Nencioni A., Caffa I., Cortellino S., Longo V.D. Fasting and cancer: Molecular mechanisms and clinical application. Nat. Rev. Cancer. 2018;18:707–719. doi: 10.1038/s41568-018-0061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagy J.A., Chang S.H., Dvorak A.M., Dvorak H.F. Why are tumour blood vessels abnormal and why is it important to know? Br. J. Cancer. 2009;100:865–869. doi: 10.1038/sj.bjc.6604929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caffa I., Spagnolo V., Vernieri C., Valdemarin F., Becherini P., Wei M., Brandhorst S., Zucal C., Driehuis E., Ferrando L., et al. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature. 2020;583:620–624. doi: 10.1038/s41586-020-2502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vernieri C., Fucà G., Ligorio F., Huber V., Vingiani A., Iannelli F., Raimondi A., Rinchai D., Frigè G., Belfiore A., et al. Fasting-Mimicking Diet Is Safe and Reshapes Metabolism and Antitumor Immunity in Patients with Cancer. Cancer Discov. 2022;12:90–107. doi: 10.1158/2159-8290.Cd-21-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ligorio F., Fucà G., Provenzano L., Lobefaro R., Zanenga L., Vingiani A., Belfiore A., Lorenzoni A., Alessi A., Pruneri G., et al. Exceptional tumour responses to fasting-mimicking diet combined with standard anticancer therapies: A sub-analysis of the NCT03340935 trial. Eur. J. Cancer. 2022;172:300–310. doi: 10.1016/j.ejca.2022.05.046. [DOI] [PubMed] [Google Scholar]
- 52.Brandhorst S., Choi I.Y., Wei M., Cheng C.W., Sedrakyan S., Navarrete G., Dubeau L., Yap L.P., Park R., Vinciguerra M., et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015;22:86–99. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.National Cancer Institute Appetite Loss and Cancer Treatment. [(accessed on 18 March 2024)]; Available online: https://www.cancer.gov/about-cancer/treatment/side-effects/appetite-loss.
- 54.National Cancer Institute Autophagy. [(accessed on 29 May 2024)]; Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/autophagy.
- 55.Grasmann G., Smolle E., Olschewski H., Leithner K. Gluconeogenesis in cancer cells—Repurposing of a starvation-induced metabolic pathway? Biochim. Biophys. Acta Rev. Cancer. 2019;1872:24–36. doi: 10.1016/j.bbcan.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karsli-Uzunbas G., Guo J.Y., Price S., Teng X., Laddha S.V., Khor S., Kalaany N.Y., Jacks T., Chan C.S., Rabinowitz J.D., et al. Autophagy is required for glucose homeostasis and lung tumor maintenance. Cancer Discov. 2014;4:914–927. doi: 10.1158/2159-8290.Cd-14-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li X., He S., Ma B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer. 2020;19:12. doi: 10.1186/s12943-020-1138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kundu M., Thompson C.B. Autophagy: Basic principles and relevance to disease. Annu. Rev. Pathol. 2008;3:427–455. doi: 10.1146/annurev.pathmechdis.2.010506.091842. [DOI] [PubMed] [Google Scholar]
- 59.Hyun J.J., Chun H.J., Keum B., Seo Y.S., Kim Y.S., Jeen Y.T., Lee H.S., Um S.H., Kim C.D., Ryu H.S., et al. Autolysis: A plausible finding suggestive of long ESD procedure time. Surg. Laparosc. Endosc. Percutan Tech. 2012;22:e115-117. doi: 10.1097/SLE.0b013e318247c347. [DOI] [PubMed] [Google Scholar]
- 60.Guo J.Y., Teng X., Laddha S.V., Ma S., Van Nostrand S.C., Yang Y., Khor S., Chan C.S., Rabinowitz J.D., White E. Autophagy provides metabolic substrates to maintain energy charge and nucleotide pools in Ras-driven lung cancer cells. Genes Dev. 2016;30:1704–1717. doi: 10.1101/gad.283416.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mizushima N. Autophagy: Process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 62.Kirkegaard T., Jäättelä M. Lysosomal involvement in cell death and cancer. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2009;1793:746–754. doi: 10.1016/j.bbamcr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 63.Boyineni J., Sredni S.T., Margaryan N.V., Demirkhanyan L., Tye M., Johnson R., Gonzalez-Nilo F., Hendrix M.J.C., Pavlov E., Soares M.B., et al. Inorganic polyphosphate as an energy source in tumorigenesis. Oncotarget. 2020;11:4613–4624. doi: 10.18632/oncotarget.27838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang R., Wan B., Hultz M., Diaz J.M., Tang Y. Phosphatase-Mediated Hydrolysis of Linear Polyphosphates. Environ. Sci. Technol. 2018;52:1183–1190. doi: 10.1021/acs.est.7b04553. [DOI] [PubMed] [Google Scholar]
- 65.Pierce G.B., Dixon F.J., Jr. Testicular teratomas. I. Demonstration of teratogenesis by metamorphosis of multipotential cells. Cancer. 1959;12:573–583. doi: 10.1002/1097-0142(195905/06)12:3<573::aid-cncr2820120316>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 66.Mintz B., Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc. Natl. Acad. Sci. USA. 1975;72:3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmeck H.M., Jr. Cancer Cells in Experiment Give Rise to Normal Tissue. The New York Times. Feb 13, 1979.
- 68.Hendrix M.J.C., Seftor E.A., Seftor R.E.B., Kasemeier-Kulesa J., Kulesa P.M., Postovit L.-M. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat. Rev. Cancer. 2007;7:246–255. doi: 10.1038/nrc2108. [DOI] [PubMed] [Google Scholar]
- 69.He P., Mann-Collura O., Fling J., Edara N., Hetz R., Razzaque M.S. High phosphate actively induces cytotoxicity by rewiring pro-survival and pro-apoptotic signaling networks in HEK293 and HeLa cells. FASEB J. 2020;35:e20997. doi: 10.1096/fj.202000799RR. [DOI] [PubMed] [Google Scholar]
- 70.Hanahan D., Weinberg R.A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 71.Giovannucci E. Nutritional epidemiology and cancer: A Tale of Two Cities. Cancer Causes Control. 2018;29:1007–1014. doi: 10.1007/s10552-018-1088-y. [DOI] [PubMed] [Google Scholar]
- 72.Garcia-Bermudez J., Williams R.T., Guarecuco R., Birsoy K. Targeting extracellular nutrient dependencies of cancer cells. Mol. Metab. 2020;33:67–82. doi: 10.1016/j.molmet.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arnst J.L., Beck G.R., Jr. Modulating phosphate consumption, a novel therapeutic approach for the control of cancer cell proliferation and tumorigenesis. Biochem. Pharmacol. 2021;183:114305. doi: 10.1016/j.bcp.2020.114305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sullivan M.R., Vander Heiden M.G. Determinants of nutrient limitation in cancer. Crit. Rev. Biochem. Mol. Biol. 2019;54:193–207. doi: 10.1080/10409238.2019.1611733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bird R.P., Eskin N.A.M. Chapter Two—The emerging role of phosphorus in human health. In: Eskin N.A.M., editor. Advances in Food and Nutrition Research. Volume 96. Academic Press; Cambridge, MA, USA: 2021. pp. 27–88. [DOI] [PubMed] [Google Scholar]
- 76.Campbell T.C., Allison R.G., Fisher K.D. Nutrient Toxicity. Nutr. Rev. 1981;39:249–256. doi: 10.1111/j.1753-4887.1981.tb07453.x. [DOI] [PubMed] [Google Scholar]
- 77.Chang A.R., Anderson C. Dietary Phosphorus Intake and the Kidney. Annu. Rev. Nutr. 2017;37:321–346. doi: 10.1146/annurev-nutr-071816-064607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown R.B., Bigelow P., Dubin J.A., Mielke J.G. High Dietary Phosphorus Is Associated with Increased Breast Cancer Risk in a U.S. Cohort of Middle-Aged Women. Nutrients. 2023;15:3735. doi: 10.3390/nu15173735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu C.X., Jin H., Lim H.T., Ha Y.C., Chae C.H., An G.H., Lee K.H., Cho M.H. Low dietary inorganic phosphate stimulates lung tumorigenesis through altering protein translation and cell cycle in K-ras(LA1) mice. Nutr. Cancer. 2010;62:525–532. doi: 10.1080/01635580903532432. [DOI] [PubMed] [Google Scholar]
- 80.Reeves P.G., Nielsen F.H., Fahey G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 81.Parodi P.W. Cows’ Milk Fat Components as Potential Anticarcinogenic Agents1. J. Nutr. 1997;127:1055–1060. doi: 10.1093/jn/127.6.1055. [DOI] [PubMed] [Google Scholar]
- 82.Brodeur G.M., Bagatell R. Mechanisms of neuroblastoma regression. Nat. Rev. Clin. Oncol. 2014;11:704–713. doi: 10.1038/nrclinonc.2014.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brodeur G.M. Spontaneous regression of neuroblastoma. Cell Tissue Res. 2018;372:277–286. doi: 10.1007/s00441-017-2761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pietrzak-Fiećko R., Kamelska-Sadowska A.M. The Comparison of Nutritional Value of Human Milk with Other Mammals’ Milk. Nutrients. 2020;12:1404. doi: 10.3390/nu12051404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.D’Arcangelo M., Brustugun O., Xiao Y., Choi Y., Behrens C., Solis L., Wang Y., Firestein R., Boyle T., Lund-Iversen M. 194P prevalence and prognostic significance of sodium-dependent phosphate transporter 2B (NAPI2B) protein expression in non-small cell lung cancer (NSCLC) Ann. Oncol. 2014;25:iv66–iv67. doi: 10.1093/annonc/mdu326.28. [DOI] [Google Scholar]
- 86.Lacerda-Abreu M.A., Russo-Abrahão T., Cosentino-Gomes D., Nascimento M.T.C., Carvalho-Kelly L.F., Gomes T., Rodrigues M.F., König S., Rumjanek F.D., Monteiro R.Q., et al. H(+)-dependent inorganic phosphate transporter in breast cancer cells: Possible functions in the tumor microenvironment. Biochim. Biophys. Acta Mol. Basis Dis. 2019;1865:2180–2188. doi: 10.1016/j.bbadis.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 87.Bobko A.A., Eubank T.D., Driesschaert B., Dhimitruka I., Evans J., Mohammad R., Tchekneva E.E., Dikov M.M., Khramtsov V.V. Interstitial inorganic phosphate as a tumor microenvironment marker for tumor progression. Sci. Rep. 2017;7:41233. doi: 10.1038/srep41233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;133:571–573. doi: 10.1016/S0140-6736(00)49915-0. [DOI] [PubMed] [Google Scholar]
- 89.Eubank T.D., Bobko A.A., Hoblitzell E.H., Gencheva M., Driesschaert B., Khramtsov V.V. In Vivo Electron Paramagnetic Resonance Molecular Profiling of Tumor Microenvironment upon Tumor Progression to Malignancy in an Animal Model of Breast Cancer. Mol. Imaging Biol. 2023 doi: 10.1007/s11307-023-01847-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Falchini G.E., Malezan A., Poletti M.E., Soria E., Pasqualini M., Perez R.D. Analysis of phosphorous content in cancer tissue by synchrotron micro-XRF. Radiat. Phys. Chem. 2021;179:109157. doi: 10.1016/j.radphyschem.2020.109157. [DOI] [Google Scholar]
- 91.Ardito F., Giuliani M., Perrone D., Troiano G., Lo Muzio L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (Review) Int. J. Mol. Med. 2017;40:271–280. doi: 10.3892/ijmm.2017.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Turdo A., D’Accardo C., Glaviano A., Porcelli G., Colarossi C., Colarossi L., Mare M., Faldetta N., Modica C., Pistone G., et al. Targeting Phosphatases and Kinases: How to Checkmate Cancer. Front. Cell Dev. Biol. 2021;9:690306. doi: 10.3389/fcell.2021.690306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Westermarck J. Targeted therapies don’t work for a reason; the neglected tumor suppressor phosphatase PP2A strikes back. FEBS J. 2018;285:4139–4145. doi: 10.1111/febs.14617. [DOI] [PubMed] [Google Scholar]
- 94.Jin H., Xu C.-X., Lim H.-T., Park S.-J., Shin J.-Y., Chung Y.-S., Park S.-C., Chang S.-H., Youn H.-J., Lee K.-H. High dietary inorganic phosphate increases lung tumorigenesis and alters Akt signaling. Am. J. Respir. Crit. Care Med. 2009;179:59–68. doi: 10.1164/rccm.200802-306OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sasaki S., Segawa H., Hanazaki A., Kirino R., Fujii T., Ikuta K., Noguchi M., Sasaki S., Koike M., Tanifuji K., et al. A Role of Intestinal Alkaline Phosphatase 3 (Akp3) in Inorganic Phosphate Homeostasis. Kidney Blood Press. Res. 2018;43:1409–1424. doi: 10.1159/000493379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brown R.B., Bigelow P., Dubin J.A., Neiterman E. Breast cancer, alcohol, and phosphate toxicity. J. Appl. Toxicol. 2023;44:17–27. doi: 10.1002/jat.4504. [DOI] [PubMed] [Google Scholar]
- 97.Altea-Manzano P., Cuadros A.M., Broadfield L.A., Fendt S.M. Nutrient metabolism and cancer in the in vivo context: A metabolic game of give and take. EMBO Rep. 2020;21:e50635. doi: 10.15252/embr.202050635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schipper H., Turley E.A., Baum M. A new biological framework for cancer research. Lancet. 1996;348:1149–1151. doi: 10.1016/S0140-6736(96)06184-3. [DOI] [PubMed] [Google Scholar]
- 99.Barr L.C. The encapsulation of tumours. Clin. Exp. Metastasis. 1989;7:277–282. doi: 10.1007/BF01753680. [DOI] [PubMed] [Google Scholar]
- 100.Jang S.J., Gardner J.M., Ro J.Y. Diagnostic approach and prognostic factors of cancers. Adv. Anat. Pathol. 2011;18:165–172. doi: 10.1097/PAP.0b013e31820cb39e. [DOI] [PubMed] [Google Scholar]
- 101.Ng I.O., Lai E.C., Ng M.M., Fan S.T. Tumor encapsulation in hepatocellular carcinoma. A pathologic study of 189 cases. Cancer. 1992;70:45–49. doi: 10.1002/1097-0142(19920701)70:1<45::aid-cncr2820700108>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 102.Ng I.O., Ng M.M., Lai E.C., Fan S.T. Better survival in female patients with hepatocellular carcinoma. Possible causes from a pathologic approach. Cancer. 1995;75:18–22. doi: 10.1002/1097-0142(19950101)75:1<18::aid-cncr2820750105>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 103.Lu D.S., Siripongsakun S., Kyong Lee J., Wei S.H., Cheng P.M., Sabounchi S., Lee J.S., Raman S., Tong M.J., Busuttil R.W., et al. Complete tumor encapsulation on magnetic resonance imaging: A potentially useful imaging biomarker for better survival in solitary large hepatocellular carcinoma. Liver Transpl. 2013;19:283–291. doi: 10.1002/lt.23597. [DOI] [PubMed] [Google Scholar]
- 104.Rivera-Gamma S., Davis M.E. CE: Tumor Lysis Syndrome: An Oncologic Emergency. Am. J. Nurs. 2023;123:30–35. doi: 10.1097/01.NAJ.0000920996.75505.c2. [DOI] [PubMed] [Google Scholar]
- 105.Weber C.M., Cohen D.A., Daugherty K.M. Case Report: Spontaneous Tumor Lysis Syndrome in Untreated Ovarian Cancer. Am. J. Med. 2023;136:e201. doi: 10.1016/j.amjmed.2023.06.005. [DOI] [PubMed] [Google Scholar]
- 106.Brown R.B. Cancer Cachexia and Dysregulated Phosphate Metabolism: Insights from Mutant p53 and Mutant Klotho Mouse Models. Metabolites. 2022;12:1284. doi: 10.3390/metabo12121284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Floege J. Phosphate binders in chronic kidney disease: An updated narrative review of recent data. J. Nephrol. 2020;33:497–508. doi: 10.1007/s40620-019-00689-w. [DOI] [PubMed] [Google Scholar]
- 108.Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. The National Academies Press; Washington, DC, USA: 2006. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.