Abstract
Transmission of simian immunodeficiency virus SIVmac239Δnef (Δnef) to macaques results in attenuated replication of the virus in most animals and ultimately induces protection against challenge with some pathogenic, wild-type SIV strains. It has been difficult, however, to identify a culture system in which the replication of Δnef is severely reduced relative to that of the wild type. We have utilized a primary culture system consisting of blood-derived dendritic cells (DCs) and autologous T cells. When the DCs were fully differentiated or mature, the DC–CD4+ T-cell mixtures supported replication of both the parental SIV strain, 239 (the wild type), and its mutant with nef deleted (Δnef), irrespective of virus dose and the cell type introducing the virus to the coculture. In contrast, when immature DCs were exposed to Δnef and cocultured with T cells, virus replication was significantly lower than that of the wild type. Activation of the cultures with a superantigen allowed both Δnef and the wild type to replicate comparably in immature DC–T-cell cultures. Immature DCs, which, it has been hypothesized, capture and transmit SIV in vivo, are deficient in supporting replication of Δnef in vitro and may contribute to the reduced pathogenicity of Δnef in vivo.
The simian immunodeficiency virus (SIV) nef gene potentiates viral load and pathogenicity of the virus in macaques (28, 55). Initial studies indicated that rhesus monkeys vaccinated with SIVmac239Δnef (Δnef) were protected against challenge by intravenous inoculation of pathogenic SIV (10, 11, 55). Recent studies, however, have shown that the vaccine effect in rhesus macaques is not fully protective (4, 51). Several individuals that were infected with forms of human immunodeficiency virus type 1 (HIV-1) with nef deleted have maintained low viral loads for more than a decade (12, 29), but recently it has been reported that some HIV-1Δnef-infected humans are showing signs of immune dysfunction (25).
The importance of nef in HIV replication in primary cells in vitro has been demonstrated (1, 9, 14, 21, 34, 53, 59). HIV-1Δnef replicates poorly in CD4+ cells stimulated postinfection, when the virus dose is low (53). Furthermore, the HIV nef gene can replace the SIV nef gene to a large extent in vivo to produce a pathogenic infection (2, 30). SIV nef has been shown to confer a positive growth advantage to SIV in both human (34) and macaque peripheral blood mononuclear cells (PBMCs) (50) that were activated either before or after virus infection. nef also promotes virus growth in a macaque T-cell line (3). However, a primary in vitro culture system that demonstrates the deficient replication of Δnef in resting macaque cells more akin to cellular environments encountered in vivo is lacking.
We and others have used the dendritic cell (DC) system to study HIV and SIV replication and showed that the DC–T-cell environment promotes the growth of HIV and SIV in vitro (7, 22, 27, 40, 43–45, 47, 61). It has been hypothesized that DCs are the initial targets for HIV infection (5, 31, 62, 63). Some evidence exists that DCs in the mucosa are a site for HIV (18, 19, 36, 41) and SIV (26, 32, 33, 54) replication. However, more recent work has shown that T cells are the major cells that can be detected producing virus at the early stages of infection (56, 64). Interestingly, virus-positive cells are not detected in the first day or two following infection. Therefore, even though it is difficult to find virus-positive DCs, they could still be signaling the T cells (and other cell types) and thereby be amplifying virus replication in this milieu.
DCs at body surfaces, including the skin (Langerhans cells) and several mucosal surfaces, as well as in the blood (35, 38, 61) are present in an immature state. A characteristic of immature DCs is their ability to endocytose antigens. DCs express CD4 (24, 39) and chemokine receptors (13, 24, 48, 52). In particular, immature DCs express CCR5 (24, 48, 52, 65) and selectively capture and replicate macrophagetropic (R5) strains of HIV-1 (22, 47), which predominate during early stages after virus transmission in humans (16, 49, 60). When the DCs mature and traffic to the lymph nodes, the infected cells could efficiently spread virus to CD4+ T cells, since it has been shown that mature DCs initiate vigorous HIV-1 (8, 22, 23, 44, 46) and SIV (27, 45) replication with T cells in vitro.
We have investigated the requirements for the replication of Δnef in cultures of DCs and T cells. The replication of Δnef was dependent on the maturation status of the DCs. In mixtures of immature DCs and T cells the level of replication of Δnef was significantly lower than that of the wild type. In contrast, in cultures of mature DCs and T cells, SIV wild-type and Δnef replication rates were similar. A similar phenomenon could take place in vivo, wherein during the initial stages of infection immature DCs are present to capture virus and Δnef replication follows at low levels.
MATERIALS AND METHODS
Animals.
Adult macaques (Macaca mulatta) were housed in the Tulane Regional Primate Research Center. Prior to use, all animals used in this study tested negative for antibodies to SIV, type D retroviruses, and simian T-cell leukemia virus. Male and female adult macaques were used for this study.
Culture medium.
RPMI 1640 (Cellgro; Fisher Scientific, Springfield, N.J.) was supplemented with 2 mM l-glutamine (GIBCO-BRL Life Technologies, Grand Island, N.Y.), 50 μM 2-mercaptoethanol (Sigma Chemical Company, St. Louis, Mo.), 10 mM HEPES (GIBCO-BRL Life Technologies), penicillin (100 U/ml)–streptomycin (100 μg/ml) (GIBCO-BRL Life Technologies), and 1% human plasma (heparinized).
Isolation of PBMCs and generation of DCs.
DCs were generated from PBMCs as previously described (37). In brief, peripheral blood was collected by standard venipuncture from healthy SIV-seronegative rhesus macaques (M. mulatta). The mononuclear cell fraction was isolated by Ficoll-Hypaque density gradient, and PBMCs were plated at 12 × 106 to 15 × 106 cells in 3 ml of culture medium and allowed to adhere for 60 min at 37°C. Nonadherent cells, which included a greater relative number of T and B cells, were washed off with warm phosphate-buffered saline (PBS) and cultured at a concentration of 0.5 × 107 to 1 × 107 cells/ml in medium. The adherent fraction of the PBMCs was then cultured in the presence of 100 U of recombinant human interleukin 4 (IL-4) (R&D Systems, Minneapolis, Minn.) per ml and 1,000 U of recombinant human granulocyte macrophage-colony-stimulating factor (GM-CSF) (Immunex, Seattle, Wash.) per ml to generate DCs. The cells were fed every 2 days with 1,000 U of GM-CSF per ml and 100 U of IL-4 per ml. After 7 to 9 days in culture, the immature DCs were harvested for infection. To generate mature DCs 50% of the medium was substituted with monocyte-condition medium for two additional days in culture. Monocyte-conditioned medium was generated as previously described (15).
DCs were further purified by magnetic separation. The cells were stained with anti-human CD2 (Dako Corporation, Carpinteria, Calif.) and anti-human CD20 (Becton Dickinson Immunocytometry Systems, San Jose, Calif.) monoclonal antibodies (mAbs). The T and B cells were removed by using three rounds of goat anti-mouse immunoglobulin-coated magnetic bead (Dynal, A.S, Oslo, Norway) depletion, following the manufacturer's instructions. The phenotypes of immature and mature DCs and their purity were monitored by flow cytometry in each experiment. DCs (104) were resuspended in PBS–5% fetal calf serum–0.1% sodium azide (staining buffer) containing conjugated mAbs. The cells were incubated in v-bottomed 96-well plates with anti-HLA-DR–fluorescein isothiocyanate (Becton Dickinson [BD]) combined with phycoerythrin-conjugated (PE-conjugated) anti-CD25–PE (BD), -CD86 (PharMingen), or -CD83 (Immunotech) for 20 min at 4°C. Cells were then washed, fixed, and examined by flow cytometry using a FACScan (BD). Immature DCs express moderate levels of HLA-DR, little or no CD83 or CD25, and moderate levels of CD86 on their surface. Mature DCs express high levels of HLA-DR and increased levels of CD25, CD83, and CD86 (37). The maturation state and purity of each population used in these experiments were confirmed in this manner.
Isolation of T cells.
The nonadherent fraction was cultured at 0.5 × 107 to 1 × 107 cells/ml for 7 to 9 days, and cells were further purified by negative selection with magnetic beads (Dynal, A.S.). The cells were incubated at 4°C for 30 min with murine mAbs specific for CD8 and HLA-DR (Becton Dickinson Immunocytometry Systems). The cells were washed and subjected to three rounds of goat anti-mouse immunoglobulin-coated magnetic bead depletion at 4°C. The resulting cell preparations were at least 98% viable by trypan blue dye exclusion. The purity of the cells was verified by direct staining flow cytometry for membrane expression of CD8 and HLA-DR.
SIV isolates.
The previously described (28) cloned viruses SIVmac239 (the wild type) and Δnef were generously provided by Preston Marx. The viruses were grown as previously described (45).
nef PCR.
Sequences spanning the deleted region of nef were amplified by nested PCR using a slight modification of a previously published protocol (10). CEMx174 cells were infected with the wild type or Δnef, and genomic DNA was extracted 5 days postinfection. Cell lysates were prepared by transferring the cells into a 0.5-ml microcentrifuge tube (National Scientific, San Rafael, Calif.) and centrifuged for 2 to 3 min at 3,000 rpm (MicroSpin 125 Sorvall instruments; DuPONT). The supernatant was aspirated, and the cells were washed in cold PBS by centrifugation for 2 to 3 min at 3,000 rpm. The supernatant was carefully aspirated, and the pellet was resuspended in 50 μl of hypotonic lysis buffer (10 mM Tris [pH 8.0], 1 mM EDTA, 0.001% Triton-X 100–sodium dodecyl sulfate in sterile double-distilled H2O) containing 600 μg of proteinase K (Boehringer Mannheim, Mannheim, Germany) per ml, per 105 cells. The cells were then incubated for 1 h at 56°C and then for 15 min at 95°C, to inactivate the protease. After lysis, the DNA was stored at −20°C. DNA (equivalent to 4 × 103 cells) was added to a PCR mixture containing (in a total volume of 50 μl) the following: 20 pmol of each of the outer primers, 10× reaction buffer (Promega, Madison, Wis.), 3 mM MgCl2, a 200 μM concentration of each deoxynucleoside triphosphate, double-distilled H2O, and 2 U of Taq DNA polymerase (Promega). Two drops of mineral oil were added to the samples, and amplification was carried out in a DNA Thermal cycler (Perkin-Elmer Cetus, Norwalk, Conn.) for 35 cycles of the following program: 94°C for 1 min, 60°C for 30 s, and 72°C for 45 s. A 5-μl volume of the first-round product was transferred to a new reaction mixture containing the inner primers. Amplification was carried out for 35 additional cycles under the same conditions as described above. PCR products were separated on a 1% agarose gel and visualized by ethidium bromide staining.
In vitro infection of DC–T-cell cultures.
DCs, T cells, or cocultures of DCs with T cells were infected with 5 × 103 50% tissue culture infective doses (TCID50) per 105 cells of the wild type or Δnef for 1.5 h at 37°C. For coculture infection, the cells were infected in 96-well round-bottomed trays. For the infection of pure DCs or T cells, the cells were pulsed in Eppendorf tubes as previously described (46). In most experiments CD4+-enriched cells were used. They were enriched by magnetic depletion of HLA-DR+ and CD8+ cells from nonadherent cells. After infection the cells were washed with medium to remove excess virus and cultured at a ratio of 1 DC per 10 T cells in aliquots of 105 cells per well in a 96-well round-bottomed well tray (ICN Biomedicals, Inc., Aurora, Ohio) for up to 15 days. Every 2 days 50-μl aliquots were collected and analyzed for the content of p27 using a p27 enzyme-linked immunosorbent assay (ELISA) (Cellular Products Inc., Buffalo, N.Y.). The cutoff point of the ELISA is 0.5 ng/ml. For activation of the DC–T-cell cultures with superantigen, Staphylococcus aureus enterotoxin B (SEB) was added at a concentration of 5 ng/ml and 10% IL-2 (Boehringer Mannheim) was added every 3 days.
Analysis of viral phenotype using the 221 cell line.
221 cells, maintained as described previously (3), were used to verify the defective replicative capacity of our Δnef virus stocks. Cells (106 cells in a 1-ml volume) were infected with the wild type and Δnef at a dose of 5 × 102 TCID50 per 105 cells in a 48-well tray and cultured with and without the external addition of 10% IL-2. Virus replication was monitored over 8 days by collecting cell culture supernatants and analyzing the release of p27 by ELISA. Samples were collected every 2 days, and fresh medium containing 5% FCS with or without 10% IL-2 was added.
Statistical analysis.
The paired t test was applied to determine the statistical significance between Δnef and wild-type replication in all 22 experiments performed comparing Δnef and wild-type replication in immature DC–T-cell cultures.
RESULTS
Analysis of virus stocks.
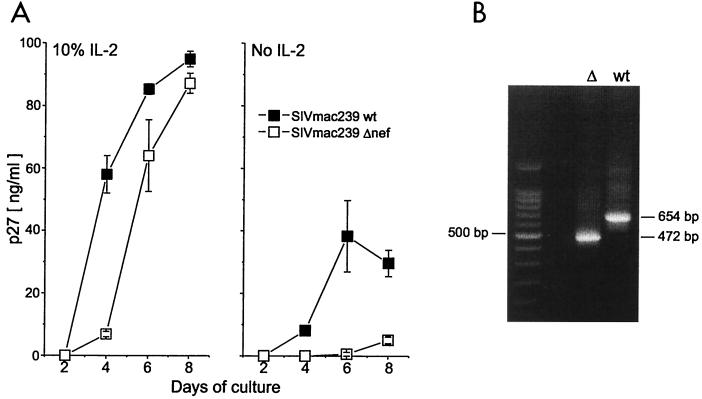
To confirm the authenticity of the virus stocks used in our experiments, the phenotype (Fig. 1A) and genotype (Fig. 1B) were analyzed. For the phenotype, we used the macaque T-cell line 221, which depends on IL-2 for proliferation. It has been shown that, in the absence of IL-2, Δnef replication in this cell line is reduced compared to that of the wild type. Infection with the wild type leads to IL-2 synthesis by 221 cells and increased levels of virus replication (3). Stocks of wild-type virus replicated in the absence of IL-2, with a peak virus production of 40 ng of p27/ml, whereas Δnef showed no virus replication until day 8, then only reaching 5 ng of p27/ml (Fig. 1A). In the presence of IL-2, both viruses replicated well with increased kinetics, reaching concentrations of around 90 ng of p27/ml at day 8. Δnef showed a slight delay in virus replication. Our results confirm the published results of Alexander et al., who also used this cell line (3).
FIG. 1.
Analysis of virus stocks. (A) Replication of SIVmac239 wild type (solid squares) and Δnef (open squares) in 221 cells. 221 cells (106) were infected (5 × 102 TCID50 per 105 cells) with wild-type or Δnef virus and cultured in a 48-well plate in medium with or without 10% IL-2. To monitor virus replication, 50 μl of the culture supernatants were collected every 2 days and the amount of p27 in the medium was analyzed using a p27 ELISA. Results shown are means ± standard deviations (error bars) of triplicate cultures. (B) PCR analysis of the nef gene. CEMx174 cells were infected (5 × 103 TCID50 per 105 cells) with SIVmac239 wild type (wt) or Δnef (▵). The cells were harvested 5 days after infection. Proviral DNA was analyzed by nested PCR, using primers to amplify the nef gene product. The amplified product of the nef gene from the Δnef virus stock is 472 bp and from the wild-type virus stock is 654 bp (shown, respectively, in the two rightmost lanes). The molecular weight marker is shown in the leftmost lane.
To rule out the possibility that the stocks were contaminated with trace amounts of wild-type virus, we analyzed the virus genotype by PCR. The permissive cell line, CEMx174 (provided by the National Institutes of Health AIDS Research and Reference Program), was infected with either the wild type or Δnef, and 5 days later the cells were harvested for analysis of proviral DNA by nested PCR, using primers to amplify the nef gene product (Fig. 1B). The PCR product of the Δnef virus stock, at 472 bp, was smaller than the amplified product of the wild-type virus stock, which yielded a 654-bp fragment, correlating with previously published results (51). This confirmed that the nef gene in the Δnef stock had the expected 182-bp deletion (28) and that contamination with wild-type virus was undetectable.
SIV replication in mature DC–T-cell cultures.
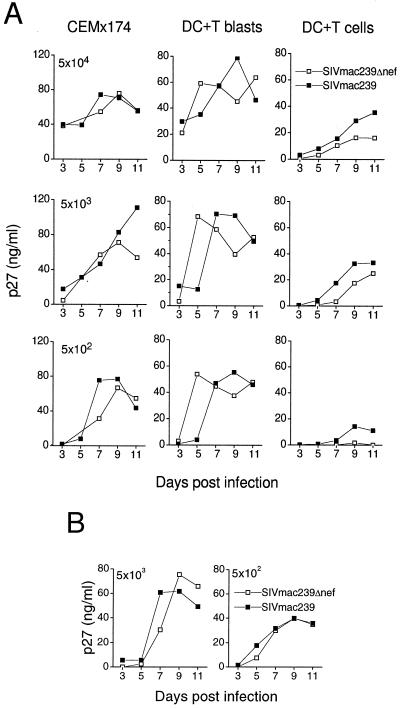
To investigate the impact of nef gene expression on SIV replication in primary DC–T-cell cocultures, we infected mature DC-T-cell cocultures with Δnef or the wild type. Virus replication in DCs cultured with resting T cells was compared to that in DCs cultured with T cells that had been activated 2 days before infection (Fig. 2A). The T-cell line CEMx174 was infected as a control. Prior reports on HIV-1Δnef replication in CD4+ cells (53) and PBMCs (34) described a dramatic difference in virus replication between HIV-1Δnef and the wild type at low virus doses. Therefore, graded doses of Δnef or wild type were used for infection. Both virus isolates replicated in these cultures, even at the low virus dose of 5 × 102 TCID50 per 105 cells (Fig. 2A). In contrast to findings for human CD4+ T cells (53) or PBMCs (34), we found that replication of both viruses in cultures of mature DCs with either resting or activated T cells was similar. The wild-type replication was at most twofold higher in the resting DC–T-cell coculture (DC plus T cells). The absolute levels of replication in DC cultures with activated T cells (DC plus T blasts) were comparable to those of the CEMx174 cells, whereas in cultures of DCs with resting T cells the level of virus replication was lower.
FIG. 2.
SIVmac239 and Δnef replication in mature DC–T-cell cultures. (A) Cells of the cell line CEMx174, mature DC–T-cell cocultures, and mature DCs cocultured with preactivated T cells (T blasts, activated with SEB [5 ng/ml for 48 h]) were infected with graded doses of virus, as follows: 5 × 104 (top row), 5 × 103 (middle row), or 5 × 102 (bottom row) TCID50 per 105 cells of SIVmac239 (solid squares) or Δnef (open squares). Every 2 days cell culture supernatants were collected and assayed for p27 production. (B) Mature DC–CD4+ T-cell cocultures were infected with 5 × 103 or 5 × 102 TCID50 per 105 cells of SIVmac239 (solid squares) or Δnef (open squares). The infection was monitored by collecting supernatant samples every 2 days and analyzing them by p27 ELISA.
The T cells used in these initial experiments were bulk T cells, which contained CD8+ T cells and some B cells. It has been previously shown that it is the CD4+ T cells that promote HIV (8, 23, 44) and SIV (M. Pope, unpublished observations) replication in culture with DCs. The presence of large numbers of CD8+ T cells in the bulk T-cell population could influence and potentially impair virus replication, especially in the DC–resting T-cell coculture infected with lower doses of virus. In macaque blood around 50% of the bulk T-cell population can be CD8+ T cells (6). Therefore, we depleted the T-cell population of CD8+ and HLA-DR+ cells (activated T cells, B cells, monocytes, and DCs) by magnetic beading. The purified CD4+ T-cell population was mixed with mature DCs and infected with 5 × 103 and 5 × 102 TCID50 per 105 cells (Fig. 2B). As a consequence of CD8+ cell depletion, virus replication was enhanced in the DC–resting T-cell milieu, even when a low virus dose was used. No difference between Δnef and wild-type replication could be observed. CD8-depleted T-cell populations were used for all future experiments.
Δnef replication in DC–T-cell cultures is influenced by maturation state of the DCs.
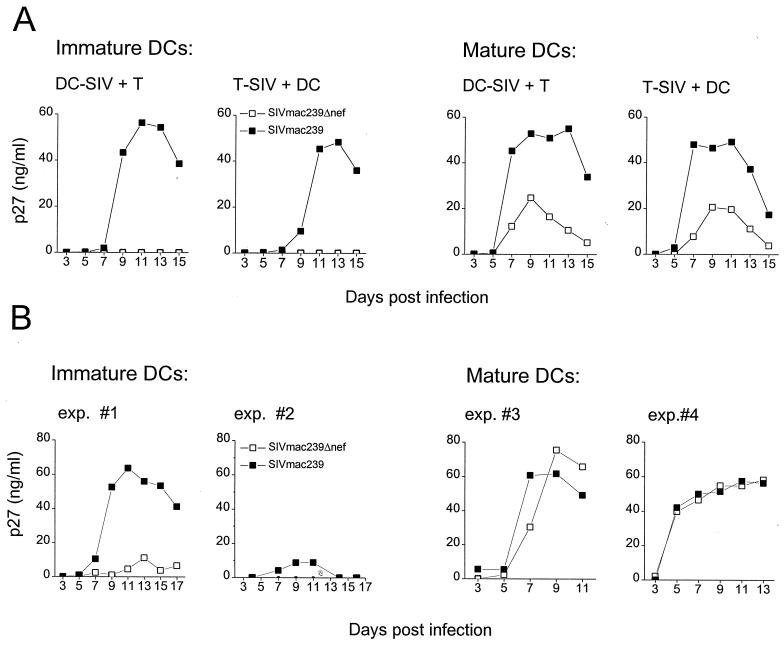
To identify a deficit in the replicative capacity of Δnef as has been described for virus replication in vivo, we investigated the replication of Δnef in the presence of immature DCs. It is postulated that immature DCs pick up SIV and HIV at body surfaces and spread infection to surrounding T cells in the lymph node. We compared the replication of the wild type and Δnef in cultures of immature DCs or mature DCs with CD4+ T cells (Fig. 3). We also analyzed whether virus replication was influenced by the cell type that introduced the virus to the culture (Fig. 3A).
FIG. 3.
SIVmac239 and Δnef replication in immature and mature DC–T-cell cultures. (A) Either immature or mature DCs isolated from the same macaque blood (DC-SIV) or the CD4+ T cells (T-SIV) were infected with 5 × 103 TCID50 per 105 cells of the wild type (solid squares) or Δnef (open squares). After infection the cells were washed and mixed with CD4+ T cells (DC-SIV + T) or DCs (T-SIV + DC), respectively, at a ratio of 1 DC/10 T cells. Every 2 days, 50 μl of the supernatant was collected and exchanged with fresh medium and the supernatant was assayed for p27 content. (B) Immature DCs from two different macaques or mature DCs from two different macaques were mixed with autologous CD4+ T cells and infected with 5 × 103 TCID50 per 105 cells of the wild type (solid squares) or Δnef (open squares). These results are representative of experiments with cells isolated from four different monkeys. Infection was monitored as described for panel A.
As shown in Fig. 3A immature and mature DCs from the same blood were assayed for Δnef and wild-type replication in cocultures with T cells. Due to the limitation of the amount of blood that can be taken from the animals, the number of generated DCs can be very low, and, therefore, comparison of immature and mature DCs from the same animal is difficult. Hence, many of the experiments were performed with either immature or mature DCs (Fig. 3B).
Virus replication of both the wild type and Δnef in autologous cocultures of mature DCs with resting T cells showed no significant differences (Fig. 2 and 3B). The greatest observed difference between wild-type and Δnef levels of replication in mature DC–T-cell coculture was twofold and is shown in Fig. 3A. In other experiments with mature DC–T-cell cultures, two examples of which are shown in Fig. 3B, Δnef and wild-type replication levels were identical. SIV replication is independent of whether DCs or T cells introduced the virus to the culture (Fig. 3A).
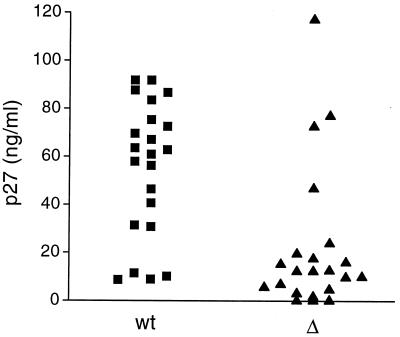
In contrast, Δnef replication in cultures of immature DCs with CD4+ T cells was significantly impaired compared to that of the wild type (Fig. 3A). Again, this did not depend on which cell type introduced the virus to the culture. However, SIV wild-type replication in the immature DC environment showed a 2- to 4-day delay compared to mature DC–T-cell cultures but eventually supported peak values of p27 production similar to those found in the mature DC environment (Fig. 3A). In 22 experiments, with cells isolated from different macaques, replication of the SIV wild type was significantly greater than that of Δnef in the immature DC–T-cell mixtures. The largest difference observed is shown in Fig. 3A, where Δnef replication could not be detected in immature DC–T-cell cultures, whereas wild-type replication peaked around 60 ng of p27/ml. The variation between experiments using cells from different monkeys is illustrated in Fig. 3B, which shows representative results with two immature DC–T-cell cultures. In some experiments, a sixfold increase in the peak level of SIV wild-type replication compared to the level of Δnef replication in immature DC–T-cell cultures was seen (Fig. 3B). In other experiments wild-type replication was low (10 ng of p27/ml) but Δnef replication was undetectable (Fig. 3B). The p27 production at the peak of virus replication in wild-type- and Δnef-infected cultures in all 22 experiments is shown in Fig. 4. The difference between wild-type and Δnef replication levels was calculated by the difference in p27 production between the wild type and Δnef in each experiment at the peak of virus replication using the paired t test (P = 0.0001). The difference between Δnef and wild-type virus replication levels is highly statistically significant.
FIG. 4.
p27 levels released in wild-type- and Δnef-infected immature DC–T-cell cultures at the peak of virus replication. The p27 values at the peak of wild-type (wt) and Δnef (▵) replication for all 22 experiments are shown. The paired t test was used to analyze the statistical significance of the difference between wild-type and Δnef replication levels and revealed a P value of 0.0001.
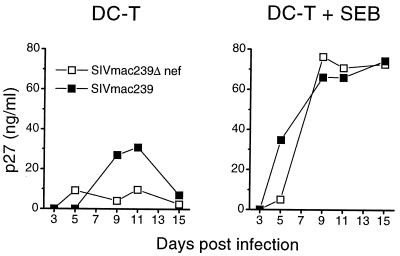
To confirm that infectious virus was present in the immature DC–T-cell mixtures, we activated the cocultures with the SEB (Fig. 5). SEB activation augmented the replication of both viruses and led to identical replication and kinetics of Δnef and wild-type SIV.
FIG. 5.
SEB activation of SIV wild-type- and Δnef-infected immature DC–T-cell cultures. Immature DC–CD4+ T-cell cocultures were infected with 5 × 103 TCID50 per 105 cells of the wild type (solid squares) or Δnef (open squares). After infection, the cells were washed and cultured in medium (DC-T) or 5 ng of SEB per ml was added to the culture (DC-T + SEB). IL-2 (10%) was added every 4 days to the SEB-containing cultures. Infection was monitored as described for previous figures.
DC and T-cell phenotypes are not modulated by wild-type SIV.
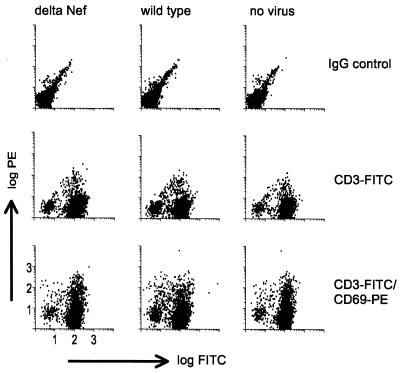
We investigated whether nef induced changes in the immature DC or T-cell phenotype, which would help explain the increased replication of the wild type. Infected cocultures were monitored for various T-cell activation markers by fluorescence-activated cell sorter. The expression of the early activation marker CD69 (Fig. 6) shows that there was no significant alteration of the T-cell phenotype in 6-day cocultures infected with either Δnef or wild-type virus compared to that in noninfected control cultures. This was true even at the earlier time points of 6, 48, and 72 h, and for other T-cell activation markers like CD25 and HLA-DR (data not shown).
FIG. 6.
SIV does not induce up-regulation of T-cell activation markers on the cell surface. Immature DC–CD4+ T-cell cultures were infected with either the wild type or Δnef and compared to uninfected cultures (no virus) as a control. Six days after infection the T cells were analyzed by staining with anti-CD3–FITC and, for activation, PE-conjugated anti-CD69.
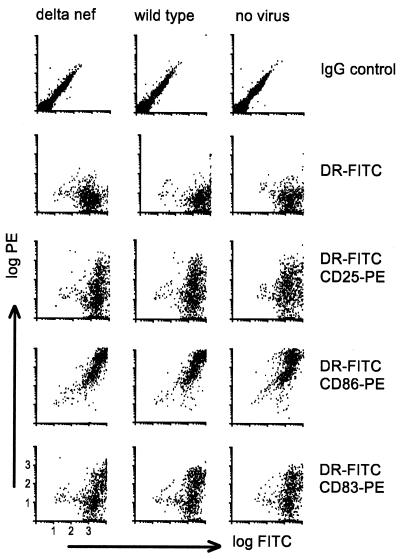
Alternatively, the increased replication levels of wild-type virus in immature DC–T-cell mixtures could result from nef inducing DC maturation. Immature DC–T-cell mixtures were cultured alone or in the presence of the wild type or Δnef. Three days after infection the cultures were monitored for standard DC maturation markers by FACS, as follows: HLA-DR versus CD25, CD86, and CD83 (Fig. 7). The samples were analyzed after 3 days because of the 2- to 4-day delay in replication of wild-type virus in immature DC–T-cell cultures and since DCs typically require at least 2 days to mature following exposure to maturation stimuli. Wild-type virus did not induce maturation of DCs. Therefore, it appears that the difference in virus replication was not due to changes in the DC or T-cell phenotype.
FIG. 7.
FACS analysis of SIV-infected immature DC–CD4+ T-cell cultures. Cocultures of immature DCs with T cells were infected with the wild type or Δnef. Noninfected cultures were used as a control. After 1.5 h, the cocultured cells were washed and fresh medium was added. Three days after infection, the DCs were assayed for maturation by staining with FITC-conjugated anti-HLA-DR and PE-conjugated anti-CD25, -CD86, and -CD83. T and B leukocytes were excluded by gating only on the large cells.
DISCUSSION
This work has demonstrated deficient replication of Δnef in immature DC–CD4+ T-cell cultures, which were used as a potentially biologically relevant system for the replication of SIV and the induction of antiviral immune responses in vivo. We analyzed the replication of SIVmac239 and Δnef, the latter having been used as a vaccine strain in macaques and shown to induce protection in a majority of monkeys (10, 11, 55). We found a primary culture system of DCs and T cells in which replication of Δnef is dependent on the maturation state of the DCs. Replication of both viruses in cocultures of mature DCs and T cells was comparable, whereas immature DC–T-cell cultures showed restricted Δnef replication. The severely compromised replication of Δnef occurred irrespective of which cell type delivered the virus to the coculture. The low levels of Δnef replication in our immature DC–T-cell cultures correlate with published results for wild-type and nef-mutated HIV-1 isolates of NL4-3, which demonstrated a significant reduction in growth rates and maximal titers of nef-mutated viruses compared to those of wild-type virus in resting CD4+ cells and CD4+ T cells activated after infection (53). Furthermore, it has been shown that in preactivated human PBMCs (34) and PBMCs activated after infection, the replication levels of nef-mutated HXB2 were considerably lower than those of the wild type (14, 34). Similar observations have been made in activated macaque PBMCs (50). Also, YU2Δnef replication in alveolar macrophages was low compared to that of the wild type (34). Immature DC–T-cell cultures resemble PBMCs in that the DCs present in freshly isolated PBMCs are primarily immature (38, 39, 61). The difference between wild-type and Δnef replication in immature DC-T cell cultures, therefore, might resemble the findings for nonactivated PBMCs.
When immature DC–T-cell cultures were polyclonally activated with the superantigen SEB after infection, both viruses replicated with identical levels and kinetics. In contrast, in PBMCs (34) or CD4+ T cells (53) activated pre- and postinfection, Δnef showed reduced levels of replication. The difference in our finding could be due to the presence of the larger numbers of DCs in our cultures (i.e., DC/T-cell ratio = 1:10, which is 10% compared to <1% in PBMCs), suggesting that higher numbers of DCs, even though immature, might provide additional signals to compensate for the nef defect in the setting of T-cell activation. On the other hand, the type of activation could play a role. We used SEB, whereas the other studies were carried out with phorbol myristate acetate–IL-2.
As described previously (27), members of our group were unable to detect virus production in isolated SIV-infected mature DC or T-cell populations. In addition, we were also unable to detect infection in immature DC suspensions with SIVmac239 wild type or Δnef (data not shown). This is in contrast to observations made for the human system in which virus replication has been demonstrated in immature human DCs infected with a macrophagetropic (R5) HIV-1 (22, 47). This discrepancy could be due to inherent differences between human and monkey immature DCs or to the virus strain used.
We propose that, in the macaque system, the interaction of an immature DC with a T cell is insufficient to support vigorous replication in the absence of nef and that the presence of nef is required for cell signaling and virus replication. Since exogenous activation of the cultures with SEB enables Δnef replication, this suggests that the necessary signals were not triggered in the immature DC–T-cell milieu alone. SEB also cross-links the DCs and T cells together via major histocompatibility complex and the T-cell receptor. This would allow tight contact, which itself could increase the cell-to-cell spread of virus as well as possibly signaling the cells to amplify virus replication. In contrast, mature DCs override the need for nef seen with the immature DCs and signal sufficiently to promote Δnef replication. Mature DCs express higher levels of costimulatory and adhesion molecules (17, 20, 42), which could facilitate binding between the DCs and T cells without the need for nef or SEB in this setting. nef has been shown to have superantigenlike qualities (57, 58), and it could be acting in a similar way to SEB in our cultures to drive virus replication. The possibility of activation of intracellular pathways by nef in the minor fraction of infected cells in our DC–T-cell mixtures, even in the absence of phenotypic changes in the total population (Fig. 6 and 7), is under investigation.
The immature DC–T-cell coculture system allows us to study the requirements for virus replication in a biologically relevant environment without the need for exogenous activation. It is important to understand the factors that are activated or changed in the mature DC–T-cell cultures where nef is not required for virus replication. A better understanding of the mechanism involved in virus replication in the immature DC–T-cell and mature DC–T-cell environments could reveal factors critical for virus replication and/or spread. We are currently investigating those requirements.
Our hypothesis is that, in vivo, immature DCs are one type of leukocyte likely to encounter virus, especially at mucosal surfaces. In the presence of wild-type virus, efficient transmission of infection and high levels of virus replication would ensue when the DCs encounter CD4+ T cells, whereas Δnef carried by immature DCs would replicate and spread much less efficiently in this environment. However, the restricted replication of Δnef might provide sufficient antigen for the induction of immune responses, while not overwhelming the immune system, and thereby afford some protective capacity to the animal. DCs and T cells contribute two important but opposing aspects to pathogenesis, providing not only a site in which robust replication can occur but also the environment in which protection against virus infection can be induced.
ACKNOWLEDGMENTS
We thank Agegnehu Gettie for providing blood samples, Preston A. Marx for providing SIVmac239 and Δnef, Ron Desrosiers for providing the 221 cells, and Ruth Connor for advice and primers for nef PCR. We also thank Heidi Cleven and John Mealey for technical assistance and Judy Adams for help with the graphics. We are grateful to Bradley Messmer for critical review and helpful discussions on the manuscript.
This work was supported by funding provided by American Foundation for AIDS Research, NIH grants AI 44335 and AI 42129, the Campbell Foundation, the Dorothy Schiff Foundation, and the Irma Hirschl Trust.
REFERENCES
- 1.Aiken C, Trono D. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J Virol. 1995;69:5048–5056. doi: 10.1128/jvi.69.8.5048-5056.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander L, Du Z, Howe A Y M, Czajak S, Desrosiers R C. Induction of AIDS in rhesus monkeys by a recombinant simian immunodeficiency virus expressing nef of human immunodeficiency virus type 1. J Virol. 1999;73:5814–5825. doi: 10.1128/jvi.73.7.5814-5825.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander L, Du Z, Rosenzweig M, Jung J U, Desrosiers R C. A role for natural simian immunodeficiency virus and human immunodeficiency virus type 1 Nef alleles in lymphocyte activation. J Virol. 1997;71:6094–6099. doi: 10.1128/jvi.71.8.6094-6099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baba T W, Liska V, Khimani A H, Ray N B, Dailey P J, Penninck D, Bronson R, Greene M F, McClure H M, Martin L N, Ruprecht R M. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat Med. 1999;5:194–203. doi: 10.1038/5557. [DOI] [PubMed] [Google Scholar]
- 5.Blauvelt A. The role of skin dendritic cells in the initiation of human immunodeficiency virus infection. Am J Med. 1997;102:16–20. doi: 10.1016/s0002-9343(97)00055-7. [DOI] [PubMed] [Google Scholar]
- 6.Buijs L, Bogers W M, Eichberg J W, Heeney J L. CD8+ cell-mediated immune responses: relation to disease resistance and susceptibility in lentivirus-infected primates. J Med Primatol. 1997;26:129–138. doi: 10.1111/j.1600-0684.1997.tb00044.x. [DOI] [PubMed] [Google Scholar]
- 7.Cameron P U, Forsum U, Teppler H, Granelli-Piperno A, Steinman R M. During HIV-1 infection most blood dendritic cells are not productively infected and can induce allogeneic CD4+ T cells clonal expansion. Clin Exp Immunol. 1992;88:226–236. doi: 10.1111/j.1365-2249.1992.tb03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron P U, Freudenthal P S, Barker J M, Gezelter S, Inaba K, Steinman R M. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992;257:383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- 9.Chowers M Y, Spina C A, Kwoh T J, Fitch N J S, Richman D D, Guatelli J C. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J Virol. 1994;68:2906–2914. doi: 10.1128/jvi.68.5.2906-2914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connor R I, Montefiori D C, Binley J M, Moore J P, Bonhoeffer S, Gettie A, Fenamore E A, Sheridan K E, Ho D D, Dailey P J, Marx P A. Temporal analyses of virus replication, immune responses, and efficacy in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J Virol. 1998;72:7501–7509. doi: 10.1128/jvi.72.9.7501-7509.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniel M D, Kirchhoff F, Czajak S C, Sehgal P K, Desrosiers R C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 12.Deacon N J, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker D J, McPhee D A, Greenway A L, Ellett A, Chatfield C, et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 13.Delgado E, Finkel V, Baggiolini M, Mackay C R, Steinman R M, Granelli-Piperno A. Mature dendritic cells respond to SDF-1, but not to several beta-chemokines. Immunobiology. 1998;198:490–500. doi: 10.1016/s0171-2985(98)80073-9. [DOI] [PubMed] [Google Scholar]
- 14.de Ronde A, Klaver B, Keulen W, Smit L, Goudsmit J. Natural HIV-1 NEF accelerates virus replication in primary human lymphocytes. Virology. 1992;188:391–395. doi: 10.1016/0042-6822(92)90772-h. [DOI] [PubMed] [Google Scholar]
- 15.Dhodapkar M V, Steinman R M, Sapp M, Desai H, Fossella C, Krasovsky J, Donahoe S M, Dunbar P R, Cerundolo V, Nixon D F, Bhardwaj N. Rapid generation of broad T-cell immunity in humans after a single injection of mature dendritic cells. J Clin Investig. 1999;104:173–180. doi: 10.1172/JCI6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 17.Fischer H, Dohlsten M, Lindvall M, Sjogren H O, Carlsson R. Binding of staphylococcal enterotoxin A to HLA-DR on B cell lines. J Immunol. 1989;142:3151–3157. [PubMed] [Google Scholar]
- 18.Frankel S S, Tenner-Racz K, Racz P, Wenig B M, Hansen C H, Heffner D, Nelson A M, Pope M, Steinman R M. Active replication of HIV-1 at the lymphoepithelial surface of the tonsil. Am J Pathol. 1997;151:89–96. [PMC free article] [PubMed] [Google Scholar]
- 19.Frankel S S, Wenig B M, Burke A P, Mannan P, Thompson L D, Abbondanzo S L, Nelson A M, Pope M, Steinman R M. Replication of HIV-1 in dendritic cell-derived syncytia at the mucosal surface of the adenoid. Science. 1996;272:115–117. doi: 10.1126/science.272.5258.115. [DOI] [PubMed] [Google Scholar]
- 20.Gascoigne N R, Ames K T. Direct binding of secreted T-cell receptor beta chain to superantigen associated with class II major histocompatibility complex protein. Proc Natl Acad Sci USA. 1991;88:613–616. doi: 10.1073/pnas.88.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glushakova S, Grivel J-C, Suryanarayana K, Meylan P, Lifson J D, Desrosiers R, Margolis L. Nef enhances human immunodeficiency virus replication and responsiveness to interleukin-2 in human lymphoid tissue ex vivo. J Virol. 1999;73:3968–3974. doi: 10.1128/jvi.73.5.3968-3974.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granelli-Piperno A, Delgado E, Finkel V, Paxton W, Steinman R M. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J Virol. 1998;72:2733–2737. doi: 10.1128/jvi.72.4.2733-2737.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Granelli-Piperno A, Finkel V, Delgado E, Steinman R M. Virus replication begins in dendritic cells during the transmission of HIV-1 from mature dendritic cells to T cells. Curr Biol. 1999;9:21–29. doi: 10.1016/s0960-9822(99)80043-8. [DOI] [PubMed] [Google Scholar]
- 24.Granelli-Piperno A, Moser B, Pope M, Chen D, Wei Y, Isdell F, O'Doherty U, Paxton W, Koup R, Mojsov S, Bhardwaj N, Clark-Lewis I, Baggiolini M, Steinman R M. Efficient interaction of HIV-1 with purified dendritic cells via multiple chemokine coreceptors. J Exp Med. 1996;184:2433–2438. doi: 10.1084/jem.184.6.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenough T C, Sullivan J L, Desrosiers R C. Declining CD4 T-cell counts in a person infected with nef-deleted HIV-1. N Engl J Med. 1999;340:236–237. doi: 10.1056/NEJM199901213400314. [DOI] [PubMed] [Google Scholar]
- 26.Hu J, Pope M, Brown C, O'Doherty U, Miller C J. Immunophenotypic characterization of simian immunodeficiency virus-infected dendritic cells in cervix, vagina, and draining lymph nodes of rhesus monkeys. Lab Investig. 1998;78:435–451. [PubMed] [Google Scholar]
- 27.Ignatius R, Isdell F, O'Doherty U, Pope M. Dendritic cells from skin and blood of macaques both promote SIV replication with T cells from different anatomical sites. J Med Primatol. 1998;27:121–128. doi: 10.1111/j.1600-0684.1998.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 28.Kestler H W, III, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 29.Kirchhoff F, Greenough T C, Brettler D B, Sullivan J L, Desrosiers R C. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 30.Kirchhoff F, Münch J, Carl S, Stolte N, Mätz-Rensing K, Fuchs D, Haaft P T, Heeney J L, Swigut T, Skowronski J, Stahl-Hennig C. The human immunodeficiency virus type 1 nef gene can to a large extent replace simian immunodeficiency virus nef in vivo. J Virol. 1999;73:8371–8383. doi: 10.1128/jvi.73.10.8371-8383.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knight S C. Bone-marrow-derived dendritic cells and the pathogenesis of AIDS. AIDS. 1996;10:807–817. doi: 10.1097/00002030-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Miller C J, Alexander N J, Sutjipto S, Lackner A A, Gettie A, Hendrickx A G, Lowenstine L J, Jennings M, Marx P A. Genital mucosal transmission of simian immunodeficiency virus: animal model for heterosexual transmission of human immunodeficiency virus. J Virol. 1989;63:4277–4284. doi: 10.1128/jvi.63.10.4277-4284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller C J, Alexander N J, Vogel P, Anderson J, Marx P A. Mechanism of genital transmission of SIV: a hypothesis based on transmission studies and the location of SIV in the genital tract of chronically infected female rhesus macaques. J Med Primatol. 1992;21:64–68. [PubMed] [Google Scholar]
- 34.Miller M D, Warmerdam M T, Gaston I, Greene W C, Feinberg M B. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med. 1994;179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morse M A, Zhou L J, Tedder T F, Lyerly H K, Smith C. Generation of dendritic cells in vitro from peripheral blood mononuclear cells with granulocyte-macrophage-colony-stimulating factor, interleukin-4, and tumor necrosis factor-alpha for use in cancer immunotherapy. Ann Surg. 1997;226:6–16. doi: 10.1097/00000658-199707000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nuovo G J, Forde A, MacConnell P, Fahrenwald R. In situ detection of PCR-amplified HIV-1 nucleic acids and tumor necrosis factor cDNA in cervical tissues. Am J Pathol. 1993;143:40–48. [PMC free article] [PubMed] [Google Scholar]
- 37.O'Doherty U, Ignatius R, Bhardwaj N, Pope M. Generation of monocyte-derived dendritic cells from precursors in rhesus macaque blood. J Immunol Methods. 1997;207:185–194. doi: 10.1016/s0022-1759(97)00119-1. [DOI] [PubMed] [Google Scholar]
- 38.O'Doherty U, Peng M, Gezelter S, Swiggard W J, Betjes M, Bhardwaj N, Steinman R M. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 1994;82:487–493. [PMC free article] [PubMed] [Google Scholar]
- 39.O'Doherty U, Steinman R M, Peng M, Cameron P U, Gezelter S, Kopeloff I, Swiggard W J, Pope M, Bhardwaj N. Dendritic cells freshly isolated from human blood express CD4 and mature into typical immunostimulatory dendritic cells after culture in monocyte-conditioned medium. J Exp Med. 1993;178:1067–1076. doi: 10.1084/jem.178.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinchuk L M, Polacino P S, Agy M B, Klaus S J, Clark E A. The role of CD40 and CD80 accessory cell molecules in dendritic cell-dependent HIV-1 infection. Immunity. 1994;1:317–325. doi: 10.1016/1074-7613(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 41.Pomerantz R J, de la Monte S M, Donegan S P, Rota T R, Vogt M W, Craven D E, Hirsch M S. Human immunodeficiency virus (HIV) infection of the uterine cervix. Ann Intern Med. 1988;108:321–327. doi: 10.7326/0003-4819-108-3-321. [DOI] [PubMed] [Google Scholar]
- 42.Pontzer C H, Irwin M J, Gascoigne N R, Johnson H M. T-cell antigen receptor binding sites for the microbial superantigen staphylococcal enterotoxin A. Proc Natl Acad Sci USA. 1992;89:7727–7731. doi: 10.1073/pnas.89.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pope M. Mucosal dendritic cells and immunodeficiency viruses. J Infect Dis. 1999;179(Suppl. 3):S427–S430. doi: 10.1086/314798. [DOI] [PubMed] [Google Scholar]
- 44.Pope M, Betjes M G, Romani N, Hirmand H, Cameron P U, Hoffman L, Gezelter S, Schuler G, Steinman R M. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994;78:389–398. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 45.Pope M, Elmore D, Ho D, Marx P. Dendritic cell-T cell mixtures, isolated from the skin and mucosae of macaques, support the replication of SIV. AIDS Res Hum Retrovir. 1997;13:819–827. doi: 10.1089/aid.1997.13.819. [DOI] [PubMed] [Google Scholar]
- 46.Pope M, Gezelter S, Gallo N, Hoffman L, Steinman R M. Low levels of HIV-1 infection in cutaneous dendritic cells promote extensive viral replication upon binding to memory CD4+ T cells. J Exp Med. 1995;182:2045–2056. doi: 10.1084/jem.182.6.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reece J C, Handley A J, Anstee E J, Morrison W A, Crowe S M, Cameron P U. HIV-1 selection by epidermal dendritic cells during transmission across human skin. J Exp Med. 1998;187:1623–1631. doi: 10.1084/jem.187.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay C R, Qin S, Lanzavecchia A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28:2760–2769. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 49.Scarlatti G, Tresoldi E, Bjorndal A, Fredriksson R, Colognesi C, Deng H K, Malnati M S, Plebani A, Siccardi A G, Littman D R, Fenyo E M, Lusso P. In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nat Med. 1997;3:1259–1265. doi: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]
- 50.Sinclair E, Barbosa P, Feinberg M B. The nef gene products of both simian and human immunodeficiency viruses enhance virus infectivity and are functionally interchangeable. J Virol. 1997;71:3641–3651. doi: 10.1128/jvi.71.5.3641-3651.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sodora D L, Sheridan K E, Marx P A, Connor R I. Immunization with a live, attenuated simian immunodeficiency virus vaccine leads to restriction of viral diversity in rhesus macaques not protected from pathogenic challenge. J Virol. 1999;73:4443–4446. doi: 10.1128/jvi.73.5.4443-4446.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sozzani S, Allavena P, Vecchi A, Mantovani A. The role of chemokines in the regulation of dendritic cell trafficking. J Leukoc Biol. 1999;66:1–9. doi: 10.1002/jlb.66.1.1. [DOI] [PubMed] [Google Scholar]
- 53.Spina C A, Kwoh T J, Chowers M Y, Guatelli J C, Richman D D. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J Exp Med. 1994;179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spira A I, Marx P A, Patterson B K, Mahoney J, Koup R A, Wolinsky S M, Ho D D. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stahl-Hennig C, Dittmer U, Nisslein T, Pekrun K, Petry H, Jurkiewicz E, Fuchs D, Wachter H, Rud E W, Hunsmann G. Attenuated SIV imparts immunity to challenge with pathogenic spleen-derived SIV but cannot prevent repair of the nef deletion. Immunol Lett. 1996;51:129–135. doi: 10.1016/0165-2478(96)02567-9. [DOI] [PubMed] [Google Scholar]
- 56.Stahl-Hennig C, Steinman R M, Tenner-Racz K, Pope M, Stolte N, Matz-Rensing K, Grobschupff G, Raschdorff B, Hunsmann G, Racz P. Rapid infection of oral mucosal-associated lymphoid tissue with simian immunodeficiency virus. Science. 1999;285:1261–1265. doi: 10.1126/science.285.5431.1261. [DOI] [PubMed] [Google Scholar]
- 57.Torres B A, Tanabe T, Johnson H M. Characterization of Nef-induced CD4 T cell proliferation. Biochem Biophys Res Commun. 1996;225:54–61. doi: 10.1006/bbrc.1996.1130. [DOI] [PubMed] [Google Scholar]
- 58.Torres B A, Tanabe T, Yamamoto J K, Johnson H M. HIV encodes for its own CD4 T-cell superantigen mitogen. Biochem Biophys Res Commun. 1996;225:672–678. doi: 10.1006/bbrc.1996.1228. [DOI] [PubMed] [Google Scholar]
- 59.Tsunetsugu-Yokota Y, Matsuda S, Maekawa M, Saito T, Takemori T, Takebe Y. Constitutive expression of the nef gene suppresses human immunodeficiency virus type 1 (HIV-1) replication in monocytic cell lines. Virology. 1992;191:960–963. doi: 10.1016/0042-6822(92)90272-q. [DOI] [PubMed] [Google Scholar]
- 60.van't Wout A B, Kootstra N A, Mulder-Kampinga G A, Albrecht-van Lent N, Scherpbier H J, Veenstra J, Boer K, Coutinho R A, Miedema F, Schuitemaker H. Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parenteral, and vertical transmission. J Clin Investig. 1994;94:2060–2067. doi: 10.1172/JCI117560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weissman D, Li Y, Ananworanich J, Zhou L J, Adelsberger J, Tedder T F, Baseler M, Fauci A S. Three populations of cells with dendritic morphology exist in peripheral blood, only one of which is infectable with human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1995;92:826–830. doi: 10.1073/pnas.92.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zaitseva M, Blauvelt A, Lee S, Lapham C K, Klaus-Kovtun V, Mostowski H, Manischewitz J, Golding H. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nat Med. 1997;3:1369–1375. doi: 10.1038/nm1297-1369. [DOI] [PubMed] [Google Scholar]
- 63.Zambruno G, Giannetti A, Bertazzoni U, Girolomoni G. Langerhans cells and HIV infection. Immunol Today. 1995;16:520–524. doi: 10.1016/0167-5699(95)80044-1. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus K A, Reimann K A, Reinhart T A, Rogan M, Cavert W, Miller C J, Veazey R S, Notermans D, Little S, Danner S A, Richman D D, Havlir D, Wong J, Jordan H L, Schacker T W, Racz P, Tenner-Racz K, Letvin N L, Wolinsky S, Haase A T. Sexual transmission and propagation of SIV and HIV in resting and activated CD4(+) T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 65.Zoeteweij J P, Golding H, Mostowski H, Blauvelt A. Cytokines regulate expression and function of the HIV coreceptor CXCR4 on human mature dendritic cells. J Immunol. 1998;161:3219–3223. [PubMed] [Google Scholar]