Abstract
Background:
Spatial orientation is a complex process involving vestibular sensory input and possibly cognitive ability. Previous research demonstrated that rotational spatial orientation was worse for individuals with profound bilateral vestibular dysfunction.
Objective:
Determine whether rotational and linear vestibular function were independently associated with large amplitude rotational spatial orientation perception in healthy aging.
Methods:
Tests of rotational spatial orientation accuracy and vestibular function [vestibulo-ocular reflex (VOR), ocular and cervical vestibular evoked myogenic potentials (VEMP)] were administered to 272 healthy community-dwelling adults participating in the Baltimore Longitudinal Study of Aging. Using a mixed model multiple linear regression we regressed spatial orientation errors on lateral semicircular canal function, utricular function (ocular VEMP), and saccular function (cervical VEMP) in a single model controlling for rotation size, age, and sex.
Results:
After adjusting for age, and sex, individuals with bilaterally low VOR gain (β = 20.9, p = 0.014) and those with bilaterally absent utricular function (β = 9.32, p = 0.017) made significantly larger spatial orientation errors relative to individuals with normal vestibular function.
Conclusions:
The current results demonstrate for the first time that either bilateral lateral semicircular canal dysfunction or bilateral utricular dysfunction are associated with worse rotational spatial orientation. We also demonstrated in a healthy aging cohort that increased age also contributes to spatial orientation ability.
Keywords: vestibular function, visuospatial ability, aging, spatial orientation
Introduction
Individuals with vestibular loss demonstrate greater performance deficits in spatial orientation, spatial memory, and spatial navigation than healthy adults [20,40,71]. Vestibular function declines with age [47,58]. Age related vestibular dysfunction is associated with impaired performance on spatial cognition tests [9], supporting the observation that older individuals have greater difficulty orienting and navigating through both real-world and virtual environments [1]. Animal and human models of vestibular loss demonstrate significant performance deficits in spatial navigation tasks, suggesting a vestibular mediated deficit [4,14,61,64,71]. This is consistent with the concept that alterations in vestibular signaling impact egocentric mental transformations of object versus self-rotation [22,24,45]. Spatial orientation includes both location of objects relative to the perceiver (egocentric) and location of objects relative to other objects independent of the position of the perceiver (allocentric) [42]. Both egocentric spatial orientation (location of objects “A” and “B” relative to the observer) and allocentric perspective taking (location of object “A” relative to object “B”) are implicated in the development of cognitive spatial maps [28]. Thus spatial orientation can be defined as awareness of and ability to regulate orientation of the body relative to space and/or the environment [60]. Thus spatial orientation ability likely contributes to planning and execution of serial motor tasks (such as the Perdue Pegboard or Tail-Making test Part B [9], or navigation to sequential locations [29]). Accurate spatial orientation in novel environments may depend on intact cognitive function to learn the novel spatial map, independent of egocentric self-motion cues. Sequentially linked objects may enhance learning novel maps in the context of reduced vestibular function, since individuals with profound vestibular loss demonstrate preserved sequence learning [39].
Spatial orientation and navigation are complex processes integrating sensory information from multiple modalities to update orientation in space relative to known external space locations [13,43]. Both egocentric and allocentric processes update the internal representation of position and orientation in space [19,31]. This internal representation of external coordinates depends on both linear and rotational vestibular input [56,69,72]. Individuals with either benign paroxysmal positional vertigo or acute vestibular neuritis demonstrate impairments in both egocentric and allocentric mental rotation [17]. In fact, peripheral vestibular disorders are associated with worse spatial memory and spatial navigation [8,71]. Previous work linked hippocampus function, spatial orientation, and vestibular function [14,41,65,73]. However, unlike vestibular reflexes, spatial orientation likely depends on both multisensory integration and cognitive processing to generate and update a spatial map. It is unlikely that internal spatial representation is solely based on vestibular signals since sensory integration begins at the first vestibular synapse in the vestibular nuclei [21]. Even when large directional discrepancies exist between visual and vestibular sensory cues there is strong bi-directional influence on heading perception [62]. Spatial perception has recently been shown to involve distributed cognitive processes [15,33,34,52,59,65]. Independent of self-motion sensory cues, cognitive processes may influence the ability to develop and store new spatial maps, necessary for spatial orientation. Recent evidence supports connections between the vestibular system and cognitive functions including visuospatial, attention, and memory abilities [9,33,63]. Therefore, accurate spatial orientation ability may depend on both intact vestibular signaling and intact cognitive function.
Here we investigate the relationship between rotational spatial orientation errors and vestibular function in a cross-sectional cohort of healthy adults. Since accuracy in this task implies use of a combination of egocentric motion signals and allocentric spatial maps based on the relative location of the labeled walls and corners to each other [44], only participants with normal global mental status as assessed by the Mini-Mental State Examination (MMSE) [27] were included. In this study, we provide a novel perspective on vestibular contributions to human spatial orientation by separately evaluating rotational and gravitational vestibular function [utricular, saccular, and lateral semicircular canal function]. We hypothesized that both rotational and linear vestibular function would be associated with spatial orientation errors [56,72].
Materials and Methods
The current study uses data from the Baltimore Longitudinal Study of Aging (BLSA) to evaluate the cross-sectional association between a spatial orientation updating task and tests of vestibular function (video head-impulse testing [vHIT], ocular vestibular evoked myogenic potentials [oVEMP], and cervical vestibular evoked myogenic potentials [cVEMP]). The spatial orientation task determined an individual’s ability to update his/her egocentric orientation to the environment after whole-body yaw-axis rotations.
Participants
The BLSA is an ongoing prospective continuous enrollment cohort study initiated in 1958 and currently conducted by the Intramural Research Program of the National Institute on Aging (IRP-NIA). Participants are community-dwelling men and women aged 20 and older who undergo a standardized array of tests over 3 days every 1-4 years at the Clinical Research Unit of the IRP-NIA in Baltimore, MD. This study includes a cross-sectional sample of all BLSA participants aged 60 and older seen between April 2016 and March 2018. During this time period, 272 healthy participants without known sensory or neurological impairments underwent vestibular and spatial orientation testing and were administered the Mini-Mental Status Exam (MMSE) by trained examiners. The MMSE is a brief test of mental status used to screen for cognitive impairment and dementia [27]. Participants with MMSE scores lower than 24 were excluded from the analyses due to possible cognitive impairment [23]. Years of education were recorded. All participants provided written informed consent. The BLSA study protocol was approved by the National Institute of Environmental Health Sciences Institutional Review Board.
Experimental Design
Vestibular Testing
Vestibulo-ocular reflex
Horizontal semicircular canal vestibulo-ocular reflex (VOR) gain measurements have been previously validated in older adults [47,48]. Briefly, seated participants wore a lightweight video-oculography system, with a camera recording right eye movements and an accelerometer recording head movement sampled at 250 Hz (GN Otometrics, Schaumberg, IL USA) and were instructed to look at a visual fixation target 1.25 meters away. A trained examiner tilted the participant’s head down to align Reid’s plane with the horizontal and then delivered 10 small amplitude (5°-15°) head impulses (peak velocity 150°-250°/second) to the right and left. Horizontal VOR gain was calculated as the ratio of the eye to head velocity [48]. Participants were excluded from head impulse testing if they could not see the target, reported restriction/pain during neck rotation, or had previous cervical spine surgery. VOR gain was used as a continuous variable and also categorized as: Normal (bilateral VOR gain > 0.62), unilateral hypofunction (either side VOR gain < 0.62), and bilateral hypofunction (bilateral VOR gain <0.62). This criteria was based on the sample mean minus two standard deviations (0.92 – 0.30) [11].
Vestibular Evoked Myogenic Potentials
Electromyographic (EMG) signals were recorded with pregelled, disposable, self-adhesive, Ag/AgCl electrodes (GN Otometrics, Schaumburg, IL, USA) using a commercial EMG system (Carefusion Synergy, version 14.1, Dublin, OH, USA). EMG signal processing included 2500x amplification and band-pass filtering between 3-500 Hz for ocular VEMPs and between 20–2000 Hz for cervical VEMPs [57].
Ocular VEMP
The ocular VEMP (oVEMP) evaluates mainly utricular function. Trained examiners delivered fifty midline head taps at Fz with an Aesculap model ACO12C reflex hammer with participants reclined at 30 degrees from horizontal and maintained a 20 degree upward gaze at a target on the ceiling. An inertial microswitch triggered the EMG recording. The oVEMP waveform is characterized by a negative peak (n10, approximately 10 ms after the head tap), followed by a positive peak (p16, approximately 16 ms after the head tap). An absent oVEMP response was defined as EMG recordings lacking definable n10 waves consistent with prior studies at the BLSA [46]. Participants unable to see the oVEMP fixation target due to visual impairments such as blindness, or who declined to participate were excluded.
Cervical VEMP
The cervical VEMP (cVEMP) evaluates mainly saccular function. While reclined at 30 degrees from horizontal, participants were instructed to lift and turn their heads to the right and left providing tonic background sternocleidomastoid activity. Air-conducted positive polarity tone bursts at 500 Hz and 125 dB SPL were delivered monaurally through an Audiocups noise-excluding headset (Amplivox, Eden Prairie, MN). The cVEMP waveform consists of a positive peak (p13, approximately 13 ms after each tone burst), followed by a negative peak (n23, approximately 23 ms after each tone burst). An absent cVEMP response was defined as EMG recordings lacking definable p13 waves consistent with prior studies at the BLSA [46]. Participants were excluded from the cVEMP test if they had conductive hearing loss or if they had pain when turning their head to the right or left.
For both cVEMP and oVEMP tests, normal vestibular function was defined as the presence of a vestibular evoked myogenic potential in both ears. Unilateral loss was defined as unilaterally absent function and bilateral loss was defined as bilaterally absent function.
Spatial Orientation Test
Each wall of the room was labeled alphabetically in a clockwise fashion (i.e., A, B, C, and D) and each label was positioned in the center of each wall (Figure 1). Subjects sat upright in a rotating chair with a footrest, placed in the center of the room initially facing wall A. The subject was then oriented to the labels of each wall (i.e., walls A, B, C, and D) and adjacent corners (i.e., corners AB, BC, CD, and DA) while being slowly rotated clockwise so that they could see each of the labeled locations. Participants then experienced one rotation each of 45, 90, and 135 degrees with their eyes open to demonstrate the velocity and distance of the turns prior to beginning the Spatial Orientation Test.
Figure 1.
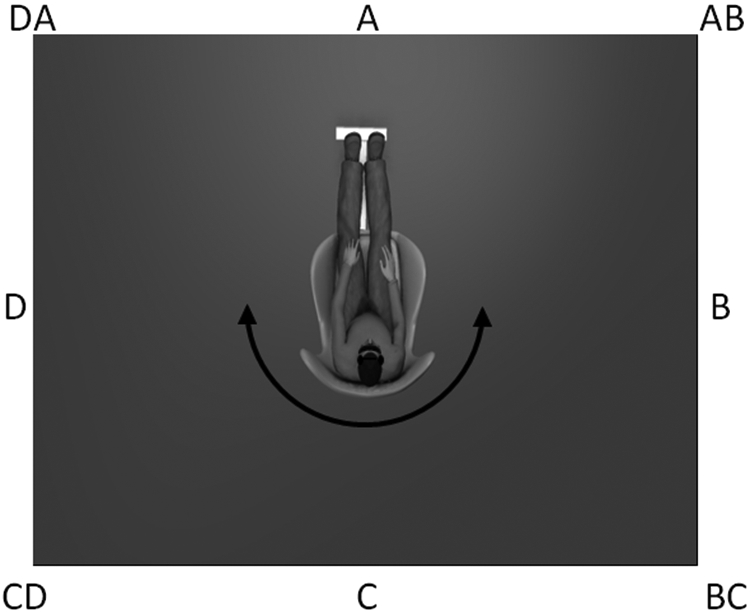
Illustration of experimental set up for spatial orientation test. Subjects were blindfolded and wore noise-attenuating headphones. Walls and corners were identified using the alpha-coordinate system shown based on 45 degree increments.
Participants wore a blindfold and noise-attenuating headphones (BOSE, Inc). The examiner applied 2 sets of 6 manually driven, whole-body rotations consisting of uniformly distributed and randomly ordered 45, 90, or 135 degree rotations alternating left and right [40]. The timing of each rotation was similar to Jáuregui-Renaud et al. (2008): approximately 1 second (45 degrees), 1.5 seconds (90 degrees), and 2 seconds (135 degrees) [40]. After each rotation, participants were asked to identify which way they were facing using the coordinates shown in Figure 1. The spatial orientation error was calculated by subtracting the reported rotation amplitude (based on starting and stopping locations) from the actual rotation amplitude. After their response was recorded, participants were informed of their actual orientation so that they were aware of the next starting position.
An interval of approximately 30 seconds between each rotation allowed any post-rotary sensation of motion to subside. After the 6th rotation, the chair was returned to wall A and the participant was instructed to remove his/her eye mask and rest for approximately one minute before beginning the second set of 6 rotations. Average spatial orientation errors were calculated for each rotation size.
Statistical Analysis
A single mixed multivariate linear model (Stata 14, StataCorp, LLC) regressed spatial orientation errors on categorized lateral semicircular canal function (normal, unilateral hypofunction, bilateral hypofunction), oVEMP function (bilaterally present, unilaterally absent, bilaterally absent), cVEMP function (bilaterally present, unilaterally absent, bilaterally absent) while controlling for rotation size (45, 90, and 135 degrees), age and sex. Post-hoc Wald comparisons for three level variables significant in the mixed model (VOR function, oVEMP function, and rotation size) were performed to compare differences across levels and are reported as chi-square statistics. Significance for the mixed effects model was tested at α = 0.05, and an adjusted α = 0.025 was used for each post-hoc comparisons. Raw data are accessible after application to the Baltimore Longitudinal Study of Aging.
Results
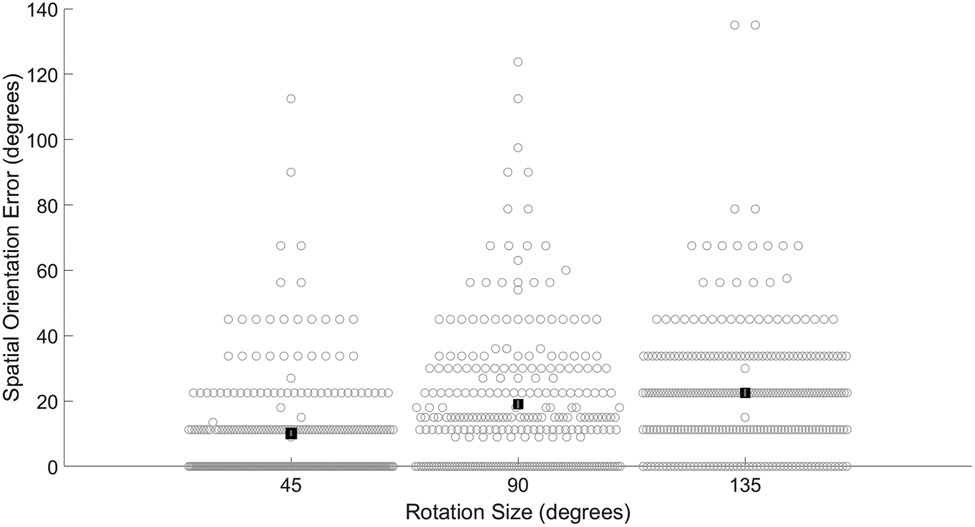
Participant demographics are presented in Table 1. The average VOR gain was 0.92 (SD 0.15). Based on VOR gain less than 0.62, 7 (2.6%) participants were classified with unilateral semicircular canal hypofunction and 3 (1.1%) with bilateral semicircular canal hypofunction. Based on absent oVEMP responses, 20 (7.4%) participants were classified with unilateral utricular dysfunction, and 17 (6.3%) participants were classified with bilateral utricular dysfunction. Based on absent cVEMP responses, 34 (12.5%) participants were classified with unilateral saccular dysfunction, and 38 (14.0%) participants were classified with bilateral saccular dysfunction. Average spatial orientation errors were 10.1 (SD 15.5) degrees for 45 degree rotations, 19.0 (SD 20.7) degrees for 90 degree rotations, and 22.5 (SD 19.8) degrees for 135 degree rotations, see Figure 2.
Table 1.
Participant demographics.
| Characteristic | Value |
|---|---|
| Age years (mean (SD), [range]) | 77.3 years (8.5), [60-95] |
| Sex (%) | |
| Male | 56.6% |
| Female | 43.4% |
| Race (%) | |
| White | 68.4% |
| Black | 26.5% |
| Other | 5.1% |
| Education, years (mean (SD)) | 16.9 (2.5), [7-24] |
| MMSE (mean (SD), [range]) | 28.6 (1.4), [24-30] |
| Mixed Vestibular Hypofunction (n) | |
| No Hypofunction | 180 |
| VOR only | 7 |
| cVEMP only | 47 |
| oVEMP only | 13 |
| VOR + cVEMP | 1 |
| VOR + oVEMP | 0 |
| cVEMP + oVEMP | 22 |
| VOR + cVEMP + oVEMP | 2 |
Figure 2.
Average spatial orientation errors significantly increase across rotation size. Filled black squares represent group average. Error bars indicate standard error of the mean. Light gray open circles represent all subject data. All pairwise comparisons were statistically significant.
In the multivariate linear regression model including semicircular canal, utricular, and saccular function, average spatial orientation error was significantly higher/worse with bilateral semicircular canal hypofunction (β = 20.9, p = 0.014), see Table 2. Individuals with bilateral semicircular canal hypofunction estimated rotation amplitudes 20 degrees larger compared to individuals with normal semicircular canal function. Individuals with unilateral semicircular canal hypofunction did not make significantly larger errors compared to individuals with normal semicircular canal function. In a post-hoc Wald comparison, individuals with bilateral semicircular canal hypofunction also made significantly larger errors than individuals with unilateral semicircular canal hypofunction (chi square (1) = 7.22, p = 0.0072). Average spatial orientation errors were also significantly larger with bilateral utricular dysfunction (β = 9.32, p = 0.017). This corresponds to a 9 degree larger rotational error with bilaterally absent oVEMP responses compared to bilaterally present oVEMP responses. In a post-hoc Wald comparison, individuals with bilaterally absent oVEMP responses also made significantly larger errors than individuals with unilaterally absent oVEMP responses (chi square (1) = 5.82, p = 0.016). Average spatial orientation error was significantly higher with older age (β = 0.39, p < 0.001). This corresponds to an almost 4 degree increase in spatial orientation errors for each decade. Average spatial orientation error was significantly larger for 90 degree rotations (β = 9.33, p < 0.001) and 135 degree rotations (β = 12.74, p < 0.001) compared to 45 degree rotations, see Figure 2. In post-hoc Wald comparisons, average spatial orientation errors were also significantly larger for 135 degree rotations compared to 90 degree rotations (chi square (1) = 10.0, p < 0.0016). Saccular function and sex were not significantly associated with spatial orientation errors.
Table 2.
Relationship between spatial orientation errors and vestibular function (semicircular canal, utricular, saccular function) controlling for education), age, and sex. * indicates significant relationships, S.E. is standard error, 95% CI is the 95% confidence interval, VIF is variance inflation factor.
| Variable | β | S.E. | 95% CI | VIF |
|---|---|---|---|---|
| Age | 0.39 * | 0.11 | [0.08, 0.61] | 1.07 |
| Sex | ||||
| Female | Ref | Ref | Ref | |
| Male | 0.71 | 1.80 | [−2.82, 4.26] | 1.01 |
| VOR gain | ||||
| Bilaterally Normal VOR gain | Ref | Ref | Ref | |
| Unilateral Reduced VOR gain | −6.28 | 5.64 | [−17.3, 4.78] | 1.04 |
| Bilateral Reduced VOR gain | 20.88 * | 8.47 | [4.27, 37.48] | 1.01 |
| Utricular Function | ||||
| Bilaterally Present oVEMP | Ref | Ref | Ref | |
| Unilaterally Absent oVEMP | −2.48 | 3.49 | [−9.31, 4.36] | 1.09 |
| Bilaterally Absent oVEMP | 9.32 * | 3.90 | [1.69, 16.96] | 1.12 |
| Sacular Function | ||||
| Bilaterally Present cVEMP | Ref | Ref | Ref | |
| Unilaterally Absent cVEMP | −1.48 | 2.82 | [−7.01, 4.05] | 1.14 |
| Bilaterally Absent cVEMP | −1.20 | 2.70 | [−6.50, 4.10] | 1.14 |
| Rotation size | ||||
| 45 Degree | Ref | Ref | Ref | |
| 90 Degree | 9.33 * | 1.08 | [7.23, 11.44] | 1.33 |
| 135 Degree | 12.73 * | 1.17 | [10.44, 15.04] | 1.33 |
| Intercept | −20.69 | 8.22 | [−36.8, −4.58 |
Discussion
New findings from this study demonstrate that in healthy older adults, age-related decline in lateral semicircular canal function, utricular function, and aging contribute to rotational spatial orientation accuracy. Individuals with bilateral hypofunction of the lateral semicircular canals made significantly larger spatial orientation errors than individuals with unilateral VOR hypofunction or bilaterally normal VOR gain. This is consistent with findings that projections from the medial vestibular nuclei contribute to head direction cells functioning as a compass [16]. Reduced or aberrant peripheral signaling regarding head rotation velocity may lead to reduced vestibular input projecting to head direction cells, leading to reduced spatial estimation ability [17]. The current study extends the known spatial orientation difficulties for individuals with profound bilateral vestibular loss [20,40], to include individuals with less severe and incomplete age related bilateral hypofunction. Consistent with previous reports, preserved unilateral vestibular function was sufficient to allow accurate spatial orientation after passive egocentric rotation [20,40]. Unilaterally reduced or asymmetric rotational vestibular function did not significantly degrade spatial orientation ability, at least for the motion parameters and timing used in the current study. Whether similar results would be obtained for individuals with unilateral hypofunction if rotational velocities exceeded inhibitory cut-off velocities should be investigated in future studies. The current results combined with previous studies [20,40], suggest a differential impact of unilateral vestibular hypofunction on spatial orientation following passive rotation and mental rotation. Individuals with bilaterally absent utricular function also demonstrated larger spatial orientation errors compared to individuals with any preserved utricular function. One possible explanation for the association with spatial errors and utricular function is that those participants had more complete vestibular loss (i.e. loss of both semicircular canal and otolith function). Several participants presented with a mixed loss of vestibular function, see Table 1. However, none of the participants in this study with bilateral VOR hypofunction also had bilaterally absent oVEMP or cVEMP responses. And the two individuals presenting with hypofunction based on VOR gain, oVEMP and cVEMP both had “normal” VOR gain on one side (VOR gain > 0.8) [2]. These results support the idea of an independent/complimentary utricular pathway for vestibular spatial orientation in humans. No relationship was identified between rotational spatial orientation errors and saccular function. This is expected since there was no vertical or tilting motion during the rotation in the yaw plane.
Unlike profound bilateral vestibular loss, age related vestibular loss can independently effect semicircular canals or otolith organs [3]. The participants with age related bilateral reduction in lateral semicircular canal or utricular function in this study, might be less capable of developing new egocentric spatial mapping due to reduced vestibular signaling [56]. Individuals with cognitive impairment have greater vestibular impairment [32,70]. Thus, compromised spatial orientation may be prevalent in the general aging population due to age related vestibular loss, consistent with elevated vestibular perceptual thresholds [6]. VOR gain does not improve as much after vestibular rehabilitation for individuals with mild cognitive impairment identified with the MMSE [54]. Reduced accuracy with spatial orientation may explain worse postural control seen in individuals with impaired cognition plus unilateral vestibular hypofunction [53], and future studies should examine this in detail. In this study, the MMSE score was an inclusion criterion, screening out participants with potential cognitive impairment since the task required individuals to integrate environmental anchors for a novel spatial map. Although the educational attainment of a few participants was less than high school, the average educational attainment included at least some college for this cohort. It is unclear whether individuals with cognitive impairment will experience worse spatial orientation perceptual performance, similar to worse performance on measures of postural sway or VOR gain [53,54]. Experiments designed to investigate interactions between vestibular input and spatial cognition related to self-motion perception are needed.
The spatial landmarks in this experiment were sequentially ordered (A, B, C, D including intermediate corners, see Figure 1); thus, a strategy of sequence memorization could allow for correct spatial orientation, independent of an allocentric spatial map. Sequence learning is a function of the cerebellum [67], and individuals with cerebellar disease have difficulty with learning sequence based navigation [68]. Rotational spatial orientation ability has been associated with gray matter volume of the cerebellum [19]. Although the current sequential design differs from other spatial orientation tasks [38,40,49], this design may be more robust to vestibular dysfunction. Individuals with bilateral vestibular loss demonstrate increased cerebellar activity during spatial navigation tasks, interpreted as a sequence related strategy for navigation [39]. Although possible, it is unlikely that the present results are based on sequence learning as similar results were reported using non-sequence oriented landmarks [40]. Future investigations should directly compare different methods for quantifying spatial orientation to determine whether accuracy depends on the methodology.
Detecting the start and stop of each rotation would provide rotation timing, facilitating spatial estimation when combined with a spatial sequence. Movement time, based on perceiving the start and stop of the rotations, could be employed to solve the question of how large each rotation was [30,35]. Vestibular stimulation has been shown to modify timekeeping [10]. These start and stop signals could originate in the vestibular or somatosensory system via vibration or shear forces at the chair [7,18]. Participants probably noticed that the different rotation sizes had slightly different time durations during the familiarization process. Even though time would probably be underestimated due to blindfolding [25] or more variable due to non-constant velocity rotations [10,35], the relative difference in perceived time across different amplitudes could be used to estimate rotation amplitude.
Independent of the vestibular contributions to rotational spatial orientation, we observed poorer accuracy with older age, corresponding to almost 4 degrees of error for every additional decade. In a similar study, age was not found to significantly impact spatial orientation errors [40]. The discrepancy between these studies is likely due to sample size as the current study was three times larger thereby increasing the power to detect the age association. The precise mechanism underlying this age association cannot be determined in the current study. However, self-motion perception has been attributed to a diffuse neural network [15]. Changes in white and grey matter with aging have been linked to alterations in diffuse cognitive networks [51,55]. Additional studies are needed to investigate whether the aging effect reported here reflects a change in the dynamics of neural networks used to process self-motion perception.
We observed a small but significant increase in spatial orientation errors with increasing rotation amplitude, independent of vestibular function or age. Previous studies did not separately evaluate the effect of rotation amplitude on spatial orientation accuracy, likely due to non-uniform distribution of rotation amplitudes [40]. It is unclear if the increasing error is an artifact of requiring verbal responses for spatial estimates rather than motor response (twisting a dial/pointer), as systematic increases in error do not appear to be present for smaller rotations when saccading or pointing to an initial target [36-38]. Proprioception has been shown to play a role in visuospatial mapping [36], potentially leading to greater accuracy with active responses (saccades or pointing) due to sensory feedback from the motor behavior. Since participants were provided with spatial feedback of the direction they were facing after each rotation (a spatial anchor within the sequence), early inaccuracies in spatial mapping could be overcome using feedback learning strategies [50]. Despite this potential for error reduction during the experiment, average spatial orientation errors reported here are similar to those reported in similar studies [12,20,26,40], suggesting limited within experiment change in accuracy. Future studies are needed to determine whether timing, sequential orientation training, or error based feedback serve to enhance spatial orientation ability as these may be beneficial compensatory strategies in the presence of age related reduced vestibular function.
Limitations
These cross-sectional data do not support causal inferences between vestibular function, and spatial orientation accuracy. However, these results highlight both semicircular canal and utricular function as potential mechanisms important for spatial orientation ability. There is often a discrepancy between caloric testing and video head impulse testing [5]. Had semicircular canal function been quantified with caloric responses the results may be different. This population consisted of healthy older adults without cognitive impairment (MMSE > 23) and the results presented here may differ for individuals with more severe cognitive or vestibular problems. Additionally, it is possible that participants with high educational attainment may have had mild cognitive impairment and passed the MMSE screening [23,66].
Conclusions
Accurate rotational spatial orientation was related to vestibular function, and age. As either semicircular canal function or utricular function decline, spatial orientation becomes less accurate.
Acknowledgments:
This work was supported in part by the National Institutes of Health [NIDCD K23 DC013056, NIDCD T32 DC000023].
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
REFERENCES
- [1].Adamo DE, Briceño EM, Sindone JA, Alexander NB, Moffat SD, Age differences in virtual environment and real world path integration., Front. Aging Neurosci 4 (2012) 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Agrawal Y, Van De Berg R, Wuyts F, Walther L, Magnusson M, Oh E, Sharpe M, Strupp M, Presbyvestibulopathy: Diagnostic criteria Consensus document of the classification committee of the Bárány Society, J. Vestib. Res. Equilib. Orientat 29 (2019) 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Agrawal Y, Zuniga MG, Davalos-Bichara M, Schubert MC, Walston JD, Hughes J, Carey JP, Decline in semicircular canal and otolith function with age., Otol. Neurotol 33 (2012) 832–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Anson ER, Ehrenburg MR, Wei EX, Bakar D, Simonsick E, Agrawal Y, Saccular function is associated with both angular and distance errors on the triangle completion test, Clin. Neurophysiol 130 (2019) 2137–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bell SL, Barker F, Heselton H, MacKenzie E, Dewhurst D, Sanderson A, A study of the relationship between the video head impulse test and air calorics, Eur. Arch. Oto-Rhino-Laryngology 272 (2015) 1287–1294. [DOI] [PubMed] [Google Scholar]
- [6].Bermúdez Rey MC, Clark TK, Wang W, Leeder T, Bian Y, Merfeld DM, Vestibular Perceptual Thresholds Increase above the Age of 40, Front. Neurol 7 (2016) 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Berthoz A, Israël I, Georges-François P, Grasso R, Tsuzuku T, Spatial memory of body linear displacement: What is being stored?, Science (80-. ). 269 (1995) 95–98. [DOI] [PubMed] [Google Scholar]
- [8].Bigelow RT, Agrawal Y, Vestibular involvement in cognition: Visuospatial ability, attention, executive function, and memory., J. Vestib. Res 25 (2015) 73–89. [DOI] [PubMed] [Google Scholar]
- [9].Bigelow RT, Semenov YR, Trevino C, Ferrucci L, Resnick SM, Simonsick EM, Xue Q-L, Agrawal Y, Association between visuospatial ability and vestibular function in the Baltimore Longitudinal Study of Aging., J. Am. Geriatr. Soc 63 (2015) 1837–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Binetti N, Siegler IA, Bueti D, Doricchi F, Time in motion: Effects of whole-body rotatory accelerations on timekeeping processes, Neuropsychologia. 48 (2010) 1842–1852. [DOI] [PubMed] [Google Scholar]
- [11].Blödow A, Pannasch S, Walther LE, Detection of isolated covert saccades with the video head impulse test in peripheral vestibular disorders., Auris. Nasus. Larynx 40 (2013) 348–51. [DOI] [PubMed] [Google Scholar]
- [12].Blouin J, Vercher JL, Gauthier GM, Paillard J, Bard C, Lamarre Y, Perception of passive whole-body rotations in the absence of neck and body proprioception, J. Neurophysiol 74 (1995) 2216–2219. [DOI] [PubMed] [Google Scholar]
- [13].Borel L, Lopez C, Péruch P, Lacour M, Vestibular syndrome: A change in internal spatial representation, Clin. Neurophysiol 38 (2008) 375–389. [DOI] [PubMed] [Google Scholar]
- [14].Brandt T, Schautzer F, Hamilton DA, Brüning R, Markowitsch HJ, Kalla R, Darlington C, Smith P, Strupp M, Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans, Brain. 128 (2005) 2732–2741. [DOI] [PubMed] [Google Scholar]
- [15].Britton Z, Arshad Q, Vestibular and Multi-Sensory Influences Upon Self-Motion Perception and the Consequences for Human Behavior, Front. Neurol 10 (2019) 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Butler WN, Smith KS, van der Meer MAA, Taube JS, The Head-Direction Signal Plays a Functional Role as a Neural Compass during Navigation, Curr. Biol 27 (2017) 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Candidi M, Micarelli A, Viziano A, Aglioti SM, Minio-Paluello I, Alessandrini M, Impaired mental rotation in benign paroxysmal positional vertigo and acute vestibular neuritis, Front. Hum. Neurosci 7 (2013) 783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Capelli A, Israël I, One second interval production task during post-rotatory sensation., J. Vestib. Res 17 (2007) 239–49. [PubMed] [Google Scholar]
- [19].Chrastil ER, Sherrill KR, Aselcioglu I, Hasselmo ME, Stern CE, Individual Differences in Human Path Integration Abilities Correlate with Gray Matter Volume in Retrosplenial Cortex, Hippocampus, and Medial Prefrontal Cortex, Eneuro. 4 (2017) ENEURO.0346–16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cohen BS, Provasi J, Leboucher P, Israël I, Effects of vestibular disorders on vestibular reflex and imagery, Exp. Brain Res 235 (2017) 2181–2188. [DOI] [PubMed] [Google Scholar]
- [21].Cullen KE, Taube JS, Our sense of direction: progress, controversies and challenges, Nat. Neurosci 20 (2017) 1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Deroualle D, Borel L, Devèze A, Lopez C, Changing perspective: The role of vestibular signals, Neuropsychologia. 79 (2015) 175–185. [DOI] [PubMed] [Google Scholar]
- [23].Dick JPR, Guiloff RJ, Stewart A, Mini-mental state examination in neurological patients, J. Neurol. Neurosurg. Psychiatry 47 (1984) 496–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dilda V, MacDougall HG, Curthoys IS, Moore ST, Effects of Galvanic vestibular stimulation on cognitive function, Exp. Brain Res 216 (2012) 275–285. [DOI] [PubMed] [Google Scholar]
- [25].Espinosa-Fernández L, Miró E, Cano M, Buela-Casal G, Age-related changes and gender differences in time estimation., Acta Psychol. (Amst) 112 (2003) 221–32. [DOI] [PubMed] [Google Scholar]
- [26].Fitzpatrick RC, Watson SRD, Passive motion reduces vestibular balance and perceptual responses, J. Physiol 593 (2015) 2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Folstein MF, Folstein SE, McHugh PR, “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician, J. Psychiatr. Res 12 (1975) 189–198. [DOI] [PubMed] [Google Scholar]
- [28].Gagnon SA, Brunyé TT, Gardony A, Noordzij ML, Mahoney CR, Taylor HA, Stepping Into a Map: Initial Heading Direction Influences Spatial Memory Flexibility, Cogn. Sci 38 (2014) 275–302. [DOI] [PubMed] [Google Scholar]
- [29].Gärling T, Böök A, Lindberg E, Spatial oriewntation and wayfinding in the designed environment: A conceptual analysis and some suggestions for postoccupancy evaluation, J. Archit. Plann. Res 3 (1986) 55–64. [Google Scholar]
- [30].Gibson JJ, Events are Perceivable But Time Is Not, in: Study Time II, Springer Berlin Heidelberg, Berlin, Heidelberg, 1975: pp. 295–301. [Google Scholar]
- [31].Glasauer S, Amorim M-A, Viaud-Delmon I, Berthoz A, Differential effects of labyrinthine dysfunction on distance and direction during blindfolded walking of a triangular path, Exp. Brain Res 145 (2002) 489–497. [DOI] [PubMed] [Google Scholar]
- [32].Harun A, Oh ES, Bigelow RT, Studenski S, Agrawal Y, Vestibular Impairment in Dementia, Otol. Neurotol 37 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hitier M, Besnard S, Smith PF, Vestibular pathways involved in cognition., Front. Integr. Neurosci 8 (2014) 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hitier M, Sato G, Zhang Y-F, Besnard S, Smith PF, Effects of electrical stimulation of the rat vestibular labyrinth on c-Fos expression in the hippocampus, Neurosci. Lett 677 (2018) 60–64. [DOI] [PubMed] [Google Scholar]
- [35].Israël I, Capelli A, Sablé D, Laurent C, Lecoq C, Bredin J, Multifactorial interactions involved in linear self-transport distance estimate: A place for time, Int. J. Psychophysiol 53 (2004) 21–28. [DOI] [PubMed] [Google Scholar]
- [36].Israël I, Ventre-Dominey J, Denise P, Vestibular information contributes to update retinotopic maps., Neuroreport. 10 (1999) 3479–83. [DOI] [PubMed] [Google Scholar]
- [37].Ivanenko Y, Viaud-Delmon I, Siegler I, Israël I, Berthoz A, The vestibulo-ocular reflex and angular displacement perception in darkness in humans: adaptation to a virtual environment, Neurosci. Lett 241 (1998) 168–170. [DOI] [PubMed] [Google Scholar]
- [38].Ivanenko Y, Grasso R, Israël I, Berthoz A, Spatial orientation in humans: perception of angular whole-body displacements in two-dimensional trajectories, Exp. Brain Res 117 (1997) 419–427. [DOI] [PubMed] [Google Scholar]
- [39].Jandl NM, Sprenger A, Wojak JF, Göttlich M, Münte TF, Krämer UM, Helmchen C, Dissociable cerebellar activity during spatial navigation and visual memory in bilateral vestibular failure, Neuroscience. 305 (2015) 257–267. [DOI] [PubMed] [Google Scholar]
- [40].Jáuregui-Renaud K, Sang FYP, Gresty MA, Green DA, Bronstein AM, Depersonalisation/derealisation symptoms and updating orientation in patients with vestibular disease, J. Neurol. Neurosurg. Psychiatry 79 (2008) 276–283. [DOI] [PubMed] [Google Scholar]
- [41].Kamil RJ, Jacob A, Ratnanather JT, Resnick SM, Agrawal Y, Vestibular Function and Hippocampal Volume in the Baltimore Longitudinal Study of Aging (BLSA), Otol. Neurotol 39 (2018) 765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Klatzky RL, Allocentric and Egocentric Spatial Representations: Definitions, Distinctions, and Interconnections, in: Freksa C, Habel C, Wender KF (Eds.), Spat. Cogn. An Interdiscip. Approach to Represent. Process. Spat. Knowl, Springer Berlin Heidelberg, Berlin, Heidelberg, 1998: pp. 1–17. [Google Scholar]
- [43].Koutakis P, Mukherjee M, Vallabhajosula S, Blanke DJ, Stergiou N, Path integration: Effect of curved path complexity and sensory system on blindfolded walking, Gait Posture. 37 (2013) 154–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Laurens J, Angelaki DE, The Brain Compass: A Perspective on How Self-Motion Updates the Head Direction Cell Attractor, Neuron. 97 (2018) 275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lenggenhager B, Lopez C, Blanke O, Influence of galvanic vestibular stimulation on egocentric and object-based mental transformations, Exp. Brain Res 184 (2007) 211–221. [DOI] [PubMed] [Google Scholar]
- [46].Li C, Layman AJ, Carey JP, Agrawal Y, Epidemiology of vestibular evoked myogenic potentials: data from the Baltimore Longitudinal Study of Aging., Clin. Neurophysiol 126 (2015) 2207–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Li C, Layman AJ, Geary R, Anson E, Carey JP, Ferrucci L, Agrawal Y, Epidemiology of vestibulo-ocular reflex function: data from the Baltimore Longitudinal Study of Aging, Otol. Neurotol 36 (2015) 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].MacDougall HG, Weber KP, McGarvie LA, The video head impulse test Diagnostic accuracy in peripheral vestibulopathy, Neurology. 73 (2009) 1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mackrous I, Simoneau M, Visuo-vestibular interaction: predicting the position of a visual target during passive body rotation, Neuroscience. 195 (2011) 45–53. [DOI] [PubMed] [Google Scholar]
- [50].Mackrous I, Simoneau M, Generalization of vestibular learning to earth-fixed targets is possible but limited when the polarity of afferent vestibular information is changed, Neuroscience. 260 (2014) 12–22. [DOI] [PubMed] [Google Scholar]
- [51].Marstaller L, Williams M, Rich A, Savage G, Burianová H, Aging and large-scale functional networks: White matter integrity, gray matter volume, and functional connectivity in the resting state, Neuroscience. 290 (2015) 369–378. [DOI] [PubMed] [Google Scholar]
- [52].Medendorp WP, Alberts BBGT, Verhagen WIM, Koppen M, Selen LPJ, Psychophysical Evaluation of Sensory Reweighting in Bilateral Vestibulopathy., Front. Neurol 9 (2018) 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Micarelli A, Viziano A, Della-Morte D, Augimeri I, Alessandrini M, Degree of functional impairment associated with vestibular hypofunction among older adults with cognitive decline, Otol. Neurotol 39 (2018) e392–e400. [DOI] [PubMed] [Google Scholar]
- [54].Micarelli A, Viziano A, Micarelli B, Augimeri I, Alessandrini M, Vestibular rehabilitation in older adults with and without mild cognitive impairment: Effects of virtual reality using a head-mounted display, Arch. Gerontol. Geriatr 83 (2019) 246–256. [DOI] [PubMed] [Google Scholar]
- [55].de Mooij SMM, Henson RNA, Waldorp LJ, Kievit RA, Age Differentiation within Gray Matter, White Matter, and between Memory and White Matter in an Adult Life Span Cohort, J. Neurosci 38 (2018) 5826–5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Muir GM, Brown JE, Carey JP, Hirvonen TP, Della Santina CC, Minor LB, Taube JS, Disruption of the head direction cell signal after occlusion of the semicircular canals in the freely moving chinchilla., J. Neurosci 29 (2009) 14521–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Nguyen KD, Welgampola MS, Carey JP, Test-Retest Reliability and Age-Related Characteristics of the Ocular and Cervical Vestibular Evoked Myogenic Potential Tests, Otol. Neurotol 31 (2010) 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Paige GD, Senescence of human visual-vestibular interactions. 1. Vestibulo-ocular reflex and adaptive plasticity with aging., J. Vestib. Res 2 (1992) 133–51. [PubMed] [Google Scholar]
- [59].Peer M, Salomon R, Goldberg I, Blanke O, Arzy S, Brain system for mental orientation in space, time, and person, Proc. Natl. Acad. Sci. U. S. A 112 (2015) 11072–11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Pietropaolo S, Crusio WE, Learning Spatial Orientation, in: Seel NM (Ed.), Encycl. Sci. Learn, Springer; US, 2012: pp. 1969–1971. [Google Scholar]
- [61].Popp P, Wulff M, Finke K, Rühl M, Brandt T, Dieterich M, Cognitive deficits in patients with a chronic vestibular failure, J. Neurol 264 (2017) 554–563. [DOI] [PubMed] [Google Scholar]
- [62].Rodriguez R, Crane BT, Effect of range of heading differences on human visual–inertial heading estimation, Exp. Brain Res 237 (2019) 1227–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Semenov YR, Bigelow RT, Xue Q-L, du Lac S, Agrawal Y, Association between vestibular and cognitive function in U.S. adults: data from the National Health and Nutrition Examination Survey., J. Gerontol. A. Biol. Sci. Med. Sci 71 (2016) 243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Smith PF, The vestibular system and cognition, Curr. Opin. Neurol 30 (2017). [DOI] [PubMed] [Google Scholar]
- [65].Smith PF, Darlington CL, Zheng Y, The Effects of Complete Vestibular Deafferentation on Spatial Memory and the Hippocampus in the Rat: The Dunedin Experience., Multisens. Res 28 (2015) 461–85. [DOI] [PubMed] [Google Scholar]
- [66].Spencer RJ, Wendell CR, Giggey PP, Katzel LI, Lefkowitz DM, Siegel EL, Waldstein SR, Psychometric limitations of the mini-mental state examination among nondemented older adults: An evaluation of neurocognitive and magnetic resonance imaging correlates, Exp. Aging Res 39 (2013) 382–397. [DOI] [PubMed] [Google Scholar]
- [67].Spencer RMC, Ivry RB, Sequence Learning is Preserved in Individuals with Cerebellar Degeneration when the Movements are Directly Cued, J. Cogn. Neurosci 21 (2009) 1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Tedesco AM, Bianchini F, Piccardi L, Clausi S, Berthoz A, Molinari M, Guariglia C, Leggio M, Does the cerebellum contribute to human navigation by processing sequential information?, Neuropsychology. 31 (2017) 564–574. [DOI] [PubMed] [Google Scholar]
- [69].Valerio S, Taube JS, Head Direction Cell Activity Is Absent in Mice without the Horizontal Semicircular Canals, J. Neurosci 36 (2016) 741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wei EX, Oh ES, Harun A, Ehrenburg M, Xue Q-L, Simonsick E, Agrawal Y, Increased Prevalence of Vestibular Loss in Mild Cognitive Impairment and Alzheimer’s Disease, Curr. Alzheimer Res 16 (2020) 1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Xie Y, Bigelow RT, Frankenthaler SF, Studenski SA, Moffat SD, Agrawal Y, Vestibular Loss in Older Adults Is Associated with Impaired Spatial Navigation: Data from the Triangle Completion Task, Front. Neurol 8 (2017) 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Yoder RM, Taube JS, Head Direction Cell Activity in Mice: Robust Directional Signal Depends on Intact Otolith Organs, J. Neurosci 29 (2009) 1061–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Yoder RM, Taube JS, The vestibular contribution to the head direction signal and navigation, Front. Integr. Neurosci 8 (2014) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]