Abstract
The cancer stem cell (SC) theory proposes that a population of SCs serves as the driving force behind fundamental tumor processes, including metastasis, recurrence, and resistance to therapy. The standard of care for patients with stage III and high-risk stage II colorectal cancer (CRC) includes surgery and adjuvant chemotherapy. Fluoropyrimidines and their combination with oxaliplatin increased the cure rates, being able to eradicate the occult metastatic SC in a fraction of patients. The treatment for unresectable metastatic CRC is based on chemotherapy, antibodies to VEGF and EGFR, and tyrosine-kinase inhibitors. Immunotherapy is used in MSI-H tumors. Currently used drugs target dividing cells and, while often effective at debulking tumor mass, these agents have largely failed to cure metastatic disease. SCs are generated either due to genetic and epigenetic alterations in stem/progenitor cells or to the dedifferentiation of somatic cells where diverse signaling pathways such as Wnt/β-catenin, Hedgehog, Notch, TGF-β/SMAD, PI3K/Akt/mTOR, NF-κB, JAK/STAT, DNA damage response, and Hippo-YAP play a key role. Anti-neoplastic treatments could be improved by elimination of SCs, becoming an attractive target for the design of novel agents. Here, we present a review of clinical trials assessing the efficacy of targeted treatment focusing on these pathways in CRC.
Keywords: cancer stem cell, colorectal cancer, molecular targeted therapies, clinical trial
1. Introduction
Colorectal cancer (CRC) is a significant global health concern, ranking as the third most common cancer worldwide. Despite progress in screening techniques and therapeutic strategies, CRC continues to be a leading cause of cancer-related deaths globally [1]. The heterogeneity of CRC presents a challenge for treatment and contributes to its variable prognosis. This complexity is attributed to diverse genetic, epigenetic, and environmental influences. Colorectal cancer stem cells (CRC-SCs) have been identified as a critical factor in the initiation, progression, metastasis, and recurrence of the disease. The theory of cancer stem cells (CSCs) proposes that a small population of cells, the CSCs, may be responsible for the fundamental processes of cancer. This model suggests that there is a hierarchical organization within tumors, with CSCs at the top due to their unique self-renewal and multipotent properties, which can give rise to a heterogeneous population through asymmetric division. CSCs were first described in acute myeloid leukemia by Dick and colleagues [2]. They made a significant discovery that not all leukemic cells propagate the disease when transplanted into immunodeficient mice [2,3]. Later, using similar experimental approaches, the presence of CSCs was demonstrated in solid cancers such as CRC [4,5]. Human CRC-SCs were first identified based on cell surface expression of CD133. CD133+ cells were consistently able to reproduce the original tumor in immunodeficient mice, while their CD133-counterparts were unable to give rise to xenografts [4,5]. CD133+ cells were largely resistant to oxaliplatin and/or 5-fluorouracil (5-FU) in vitro and in vivo, whereas CD133− cells showed a high sensitivity to the treatment [6]. Today, it is well accepted that CRC-SCs represent a phenotypically and functionally heterogeneous population. Accordingly, several SCs markers have been reported including CD44, EpCAM, CD166, leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5), aldehyde dehydrogenase 1 (ALDH1), EphrinB receptors, doublecortin-like kinase 1 (DCLK1), and others revised in [7].
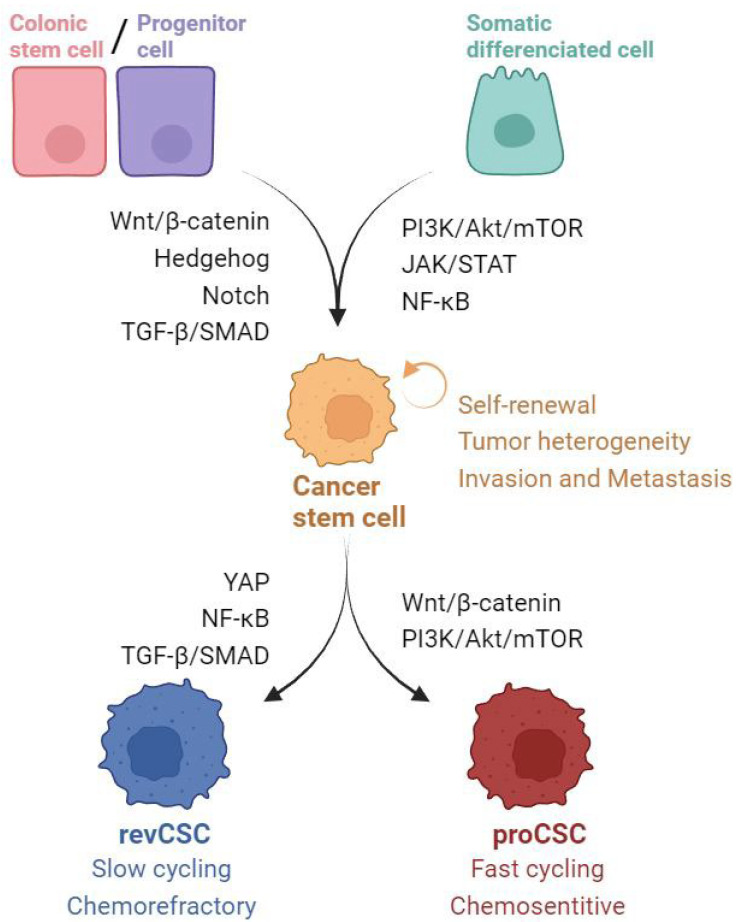
CRC-SCs have two main sources: SCs that have undergone malignant transformation through genetic or epigenetic alterations and differentiated epithelial non-SCs that acquire stemness through dedifferentiation [6,8,9,10,11]. The dual origin of SCs (Figure 1) underscores their complexity and plasticity, as they possess self-renewal capacity and potential to drive tumor growth, metastasis, and resistance to conventional therapies [9]. The transformation of normal colonic SCs into CSCs is believed to be influenced by deregulation in several key signaling pathways, including Wingless integrated-Beta-catenin (Wnt/β-catenin), Hedgehog, Notch, and TGF-β/SMAD (Figure 1). The dedifferentiation of non-stem epithelial cells into tumor-initiating cells depends mainly on the level of Wnt signaling and is also influenced by the tumor microenvironment (TME) through different signaling pathways, such as PI3K/Akt/mTOR, JAK/STAT, and NF-κB, which link inflammatory and immune signaling, angiogenesis, and tumor-initiating cells [11]. Recent advances have revealed a distinct SC type within CRC dynamics, termed revival colon SCs (revCSCs). In contrast to traditional proliferative CSCs, which prioritize proliferation, revCSCs emphasize survival over proliferation [12]. These SCs share transcriptional similarities with fetal intestinal SCs, suggesting the activation of early developmental programs in response to epithelial stress. Interestingly, revCSCs, which are slow-cycling cells, show a marked upregulation of Yes-associated protein (YAP) signaling. This pattern is complemented by increased signaling in pathways such as focal adhesion kinase (FAK), TNF-α, TGF-β, INF-γ, and NF-κB, which play a fundamental role not only in the initial formation of the primary tumor but also in metastatic dissemination and the emergence of chemoresistance [13].
Figure 1.
Signaling Pathways and Their Roles in Colorectal Cancer Stem cell (CRC-SC) hallmarks. These pathways collectively contribute to the intricate regulatory network that supports the malignancy and plasticity of CSCs and highlights potential therapeutic targets.
2. Targeted Drug Modulation of Stemness Pathways
Several signaling pathways are involved in both the development and maintenance of CRC-SCs. This review aims to focus on clinical trials investigating the potential of targeted drug modulation of stemness pathways, including Wnt/β-catenin, Hedgehog, Notch, TGF-β/SMAD, PI3K/Akt/mTOR, NF-κB, Janus kinase/signal transducer and activator of transcription (JAK/STAT), DNA damage response, and Hippo-YAP, in the treatment of CRC (Figure 1). The evaluation of immunotherapy strategies against CSCs is outside the scope of this review.
2.1. Wingless Integrated/Beta-catenin (Wnt/β-catenin) Pathway
The Wnt/β-catenin pathway is a crucial molecular pathway involved in cell development, proliferation, and differentiation and plays a key role in embryonic development and adult tissue homeostasis. Wnt/β-catenin signaling plays a key role in influencing cancer stemness [14,15]. Dysregulation of this pathway is common in cancers and more than 80% of CRC cases are associated with driver mutations in this pathway [16,17]. Sequence variations in the Wnt pathway components, such as loss of function mutations in adenomatous polyposis coli (APC) and activating mutations in β-catenin, significantly influence the development and progression of CRC [17].
Therapeutic strategies that aim to disrupt the Wnt signaling pathway can reduce the population of CSCs and inhibit tumor growth. Currently, there are drugs approved by the Food and Drug Administration (FDA) for other medical conditions that target the Wnt/β-catenin pathway, including niclosamide (a Frizzled receptor inhibitor), pimozide (a β-catenin inhibitor), and ethacrynic acid (a β-catenin-LEF1 inhibitor). Additionally, preclinical data support the use of these agents in various types of cancer, including CRC [17]. Niclosamide is currently undergoing evaluation in an ongoing phase 2 trial for metastatic CRC (clinicaltrials.gov identifier NCT02519582, accessed on 26 April 2024).
Regorafenib is a multitargeted kinase inhibitor approved for the treatment of patients with metastatic CRC (mCRC) who are refractory to standard chemotherapy [18]. Regorafenib treatment was shown to reduce the formation of tumor spheres and the side population in 5-FU-resistant CRC cell lines both in vitro and in immunocompromised mice. The stemness genes including Notch1, WNT1, CD44, and c-Myc were downregulated by regorafenib exposure via miR-34a induction [19]. A preclinical study has suggested that regorafenib may be useful in the adjuvant treatment of micrometastases in resected CRC [20]. However, a phase 3 trial that evaluated the efficacy and safety of regorafenib vs. placebo in patients with CRC following curative resection of liver metastases after completion of planned chemotherapy was prematurely terminated [21]. Only five patients were randomized. Serious adverse events (SAEs) were reported on 14.29% of the patients assigned to regorafenib [21]. A randomized phase 2 study is currently recruiting to evaluate the efficacy of durvalumab plus regorafenib compared to observation in patients with stage IV colon cancer who have achieved no evidence of disease following locoregional or systemic treatment. It is planned to enroll 182 mCRC patients. The primary endpoint is disease-free survival (NCT05382741).
Previous studies have shown that vitamin D can repress Wnt/β-catenin signaling in cancer cells [22]. In a phase 2 clinical trial involving 139 patients with mCRC, the addition of high-dose vitamin D3 to standard chemotherapy was compared to standard-dose vitamin D3. The study found a difference in median progression-free survival (PFS) between the two groups (13 vs. 11 months), although this difference was not statistically significant. No significant differences were found between high-dose and standard-dose vitamin D3 for objective response rate (ORR, 58% vs. 63%; p = 0.27) or overall survival (OS, median 24.3 months vs. 24.3 months, respectively; p = 0.43) [23].
Genistein is a glycogen synthase kinase-3 beta (GSK-3 β) inhibitor that blocks Wnt signaling by increasing the production of soluble Wnt inhibitory molecules. In a phase 1/2 pilot study, genistein was combined with FOLFOX or FOLFOX–bevacizumab for the first-line treatment of 13 mCRC patients. The study showed a favorable safety profile, with an ORR of 61.5% and a median PFS of 11.5 months (95% confidence interval, CI 4.9–21.7) [24]. Another selective GSK-3 β inhibitor, 9-ING-41, was studied as a single agent or with chemotherapy in a phase 1b/2 trial involving 63 patients with solid tumors. Only one of the thirteen patients with CRC who were treated achieved SD [25].
Various targeted therapies against porcupine, an enzyme that facilitates the acylation of Wnt proteins, have reached different phases of development in CRC. In a phase 1b trial, a small molecule inhibitor of porcupine O-acyltransferase (WNT974) did not show any clinical benefit when combined with encorafenib and cetuximab in BRAF V600E mutant patients. The trial observed dose-limiting toxicities (DLTs) in 4 out of 20 patients, and the overall response rate (ORR) was only 10%. As a result, a phase 2 trial was not started [26]. RXC004, another porcupine inhibitor, was evaluated in a clinical trial with 20 patients and was found to be safe at doses up to 2 mg. It is of note that RXC004 demonstrated disease stabilization in five patients with Wnt ligand-dependent tumors. It is currently being evaluated in a phase 2 trial for metastatic microsatellite stable CRC, either alone or in combination with nivolumab [27]. Tumors must have loss-of-function RNF43 mutation or R-spondin (RSPO) 2/3 fusion for enrollment.
Other target therapy approaches are under early stages of development for CRC treatment such us β-catenin inhibitors, RSPO antibodies, and later-generation β-catenin and frizzle receptor inhibitors [28].
2.2. Hedgehog Signaling Pathway
The Hedgehog signaling (Hh) pathway plays a crucial role in embryonic development and tissue regeneration. The Hh pathway has been linked to carcinogenesis in CRC and is also involved in maintaining stemness and drug resistance [29]. Smoothened (SMO) is a family of seven transmembrane proteins that are critical for Hh signal transduction on the cell membrane. Drugs targeting SMO have been shown to be the most effective way to modulate the activity of the Hh pathway.
Several SMO inhibitors (such as vismodegib or sonidegib) have been approved for treating basal cell carcinoma or hematological malignancies. Other novel SMO inhibitors are still in early development phases, revised in [30]. Hh pathway inhibitors have limited activity in unselected patients with solid tumors.
In the context of CRC, certain compounds have demonstrated efficacy in preclinical models, but their translation to the clinic has not been successful. In a phase 2a multiple basket study, patients with advanced refractory solid tumors and molecular alteration in the Hh pathway were treated with vismodegib, although no patients with CRC were found [31]. In another phase 2 trial, patients with previously untreated mCRC were randomized to receive either vismodegib or a placebo in combination with a standard chemotherapy regimen. The results showed that the vismodegib arm had an equivalent ORR and similar median PFS compared to the placebo arm, indicating a lack of efficacy [32].
While the Hh pathway is a promising target for cancer treatment, a better understanding of the mechanisms that cause aberrant Hh activation and its consequences in different tumor types is necessary for the development of new therapies.
2.3. Notch Pathway
The Notch signaling pathway is essential for cell fate decisions and contributes to the transformation process by inhibiting the differentiation of SCs and promoting their proliferation, thereby maintaining the CSC phenotype. Alterations in this pathway have been associated with CRC [33]. High Notch1 copy number gains have been described in over 22% of CRCs [34] and other downstream molecular alterations have also been described in CRC patients [35].
Notch1 activation can be inhibited targeting gamma-secretase (γ-secretase), an enzyme responsible for cleaving Notch receptors. A phase 2 study evaluated the efficacy of RO4929097, a γ-secretase inhibitor, in 37 mCRC patients who had previously received at least two lines of chemotherapy. There were no radiologic responses. The median PFS was 1.8 months (95% CI, 1.8–1.86) and median OS was 6.0 months (95% CI, 3.9–9.1) [36]. Other γ-secretase inhibitors have been tested in CRC in phase 1, but with limited efficacy. Two out of six patients with CRC showed SD with the combination of RO4929097 with cediranib in a phase 1 trial evaluating a total of twenty patients with solid tumors [37]. In a phase 1 study evaluating LY9000009, another gamma-secretase inhibitor, two out of five patients with CRC showed SD [38].
Monoclonal antibodies (moAbs) targeting Notch are also under development. Demcizumab, an anti-delta ligand-4 (DLL4), had a favorable safety profile in a phase 1 study and showed disease stabilization in 40% of 55 evaluable patients, including 10 patients with CRC [39]. A phase 2 clinical trial is currently evaluating demcizumab in combination with FOLFIRI in first- and second-line mCRC, but results have not yet been reported (NCT01189942).
Other approaches, including ADAM inhibitors and Hes-1 inhibitors, are currently in preclinical or early phase 1 clinical trials [35].
2.4. TGF-β/SMAD Pathway
TGF-β is a cytokine with multifunctional properties that can act as a tumor promoter or tumor suppressor in a cell- and context-dependent manner. The TGF-β/SMAD pathway acts as a tumor suppressor in early stages and later facilitates transformation through mechanisms such as the epithelial–mesenchymal transition (EMT), which endows cells with stem-like features [40]. In the context of CRC, some studies with colonic organoids and xenograft models have demonstrated that the TGF-β signaling pathway is specifically activated in the CSCs in human CRC [41,42]. Moreover, increased levels in the primary tumor or high plasma TGF-β levels in patients are associated with poor prognosis [43].
Preclinical data suggest that TGF-β could be a promising therapeutic target in mCRC. Strategies to block TGF-β signaling have been proposed and several drugs have been evaluated in early phase clinical trials with limited success. In CRC, these drugs include receptor kinase inhibitor, moAbs, and ligand traps, either as monotherapy or in combination with other anti-cancer drugs [40,41,42].
Vactosertib is an oral inhibitor of TGF-β type I receptor kinase. A phase 1b/2a study was conducted to evaluate vactosertib in combination with pembrolizumab in 105 previously treated, immunotherapy-naïve patients with microsatellite stable (MSS) mCRC (NCT03724851). The ORR was 13.3% (95% CI, 7.5–21.4). Median PFS and OS were 1.3 months (95% CI, 1.2–1.4) and 15.8 months (95% CI, 7.9-NR), respectively. The phase 2 part of the study is still ongoing [44].
In a single-arm phase 2 trial (NCT02688712), galunisertib, another TGF-β kinase inhibitor, was tested in 38 previously untreated patients with locally advanced rectal cancer in combination with neoadjuvant chemoradiotherapy, showing a complete response rate of 32% [45].
NIS793, a fully human anti-TGF-β moAb, has shown promising results in a phase 1b study combined with spartalizumab in MSS-CRC (n = 40) [46]. NIS793 has also been evaluated in a phase 2 trial in combination with FOLFOX/FOLFIRI + bevacizumab + tislelizumab vs. chemotherapy for second-line treatment (NCT04952753) in mCRC. Although no results are available, Novartis will discontinue the development of the anti-TGF-β moAb NIS793 for the treatment of cancer.
Also, the clinical results of ascrinvacumab (PF-03446962), a moAb against activin receptor-like kinase-1 (ALK1), a type I subclass of the TGF-β receptor with dose-dependent anti-angiogenic activity, have been disappointing in various tumor types [47,48]. In a study involving patients with chemorefractory mCRC (NCT02116894), the combination of PF-03446962 and regorafenib resulted in unacceptable toxicity without any evidence of anti-tumor activity, leading to the premature termination of the trial [49].
SAR439459, a next-generation anti-TGF-β antibody that inhibits all TGF-β isoforms, has been investigated against solid tumors. In 24 mCRC patients a disease control rate (DCR) of 25% and partial response (PR) of 4% was obtained. Nevertheless, the study was discontinued due to a lack of efficacy and a high risk of bleeding. No significant association between clinical response and plasma TGF-β level at baseline or modulation upon treatment was observed [50].
Bintrafusp alfa is a bifunctional fusion protein that targets PD-L1 and TGF-β. In CRC, bintrafusp alfa in monotherapy showed only modest anti-tumor activity. In an expansion cohort of a phase 1 trial (NCT02517398) evaluating bintrafusp alfa in monotherapy for heavily pretreated mCRC patients, the ORR was low (3.1%), as was the DCR (6.3%). Additionally, the median PFS was poor (1.3 months) and the median OS was 7.7 months [51]. Bintrafusp did not demonstrate significant anti-tumor activity in patients with high microsatellite instability (MSI-H) CRC who had previously received immunotherapy in a phase 2 trial with 15 patients. The median PFS was 1.8 months with no responses [52]. In a phase 2 trial, the combination with other immune-oncology agents (CV301—poxviral vaccine against CEA and MUC1, N-803—IL-15 superagonist, and M9241—tumor-targeted IL-12) showed preliminary clinical activity with a 12-month OS rate between 66.7% and 77.2% and a manageable safety profile in patients MSS CRC in the third or successive lines [53]. However, the phase 2 trial that evaluated the combination of bintrafusp with radiotherapy was terminated due to futility (NCT03436563).
SHR-1701 is a bifunctional anti-PD-L1/TGF-β agent. A phase 2 study combining SHR-1701 with standard therapy (XELOX and the anti-VEGF biosimilar BP102) in first-line treatment (NCT04856787) reported an ORR of 59.7% and a DCR of 83.9% in 62 mCRC patients. Median PFS was 10.3 months (95% CI, 8.3–13.7), with no mature OS data [54]. Grade ≥ 3 adverse events (AEs) were reported in 59.7% of patients with a 9.7% rate of discontinuation of SHR-1701, with anemia (8.1%) and decreased neutrophils (6.5%) being the most common toxicities.
Interference with EMT (directly or indirectly) and mesenchymal–epithelial transition (MET) has also been evaluated as a potential therapeutic strategy in preclinical models, with preliminary anti-tumor activity [55,56]. Nevertheless, further clinical research and additional studies are necessary before application in clinical practice.
2.5. PI3K/Akt/mTOR Pathway
The phosphoinositide 3-kinases (PI3Ks) are a family of activating enzymes that can phosphorylate the inositol ring in phosphatidylinositol membrane lipids. They are known to play a central role in cell growth, survival, metabolism, and cell mobility, which makes them a therapeutic target of great interest [57].
In models of colon cancer, a mutation in PIK3CA activates PI3K/Akt signaling and increases the expression of LGR5 and c-myc, promoting the survival and proliferation of stem cells. Tumor cells with a PIK3CA mutation and LGR5 expression exhibit poor sensitivity to first-line chemotherapy in vitro. In multivariate analysis, patients with a PIK3A mutation and LGR5 expression had poor outcomes for both DFS and OS [58]. PI3KCA mutations are present in 13% of mCRC cases and are associated with right-sided tumors, older patients, and poor prognosis [58,59,60].
Drugs that interfere with the PI3K pathway are currently undergoing clinical evaluation. These include PI3K inhibitors, dual inhibitors of PI3K and mTOR, AKT inhibitors, and mTOR inhibitors. PI3K inhibitors can be pan-inhibitors (such as buparlisib, pictilisib, copanlisib, pilaralisib, and sonolisib) or selective for the catalytic subunit p110 α (alpelisib, taselisib, inavolisib, and serabelisib).
In a randomized phase 2 trial involving patients with mCRC, the combination of sonolisib and cetuximab did not demonstrate superiority in terms of PFS or OS when compared to cetuximab alone. The median PFS was 59 days for cetuximab and sonolisib and 104 days for cetuximab (p = 0.77). Similarly, the OS for arm combination and cetuximab alone were 266 and 333 days, respectively (p = 0.83). The treatment-related toxicity was higher in the combination arm, especially in terms of all-grade nausea, vomiting, diarrhea, and rash [61].
In a phase 1 study with 52 patients with advanced solid tumors, the combination of pilaralisib and paclitaxel–carboplatin was evaluated. The best overall response was confirmed PR in seven patients (13.5%). It included one CRC patient (no response). No associations between PI3K pathway alterations and responses were observed [62]. Buparlisib has been evaluated in a phase 1 trial in combination with mFOLFOX6 [63]. A total of 17 patients received treatment with buparlisib, 13 of which were evaluate for dose-limiting toxicities (DLTs). The most common tumor type was CRC. DLT included hyperglycemia, transaminase elevation, neutropenia, and thrombocytopenia. One patient demonstrated an unconfirmed PR and three patients had stable disease (SD).
In a phase 1b trial for patients with advanced CRC and KRAS wildtype, buparlisib was combined with panitumumab [64]. The toxicities observed included fatigue, rash, and mucositis. Out of the seventeen patients, one (5.9%) who was PTEN and PIK3CA negative by immunohistochemistry had a PR, seven had SD, and nine had disease progression. The authors conclude that in this biomarker-unselected cohort, there was limited evidence to suggest activity.
The NCI-MATCH tumor-agnostic platform trial arm Z1F (NCT02465060) evaluated copanlisib in patients with PIK3CA mutations and KRAS wildtype. The study achieved its primary endpoint with an ORR of 16% (p = 0.0341). The most common toxicities observed were hyperglycemia, fatigue, diarrhea, hypertension, nausea, and rash. However, no clinical benefit (PR or SD) was observed in the four mCRC patients included [65].
Single-agent alpelisib has been evaluated in PIK3CA-altered advanced solid tumors (NCT01219699). Alpelisib in combination with fulvestrant is currently approved for the treatment of late-stage metastatic breast cancer in patients with a PIK3CA mutation. In the first dose-escalation and expansion study, 35 patients with mCRC (26.1%) were included. Two patients had PR. DCR and clinical benefit rate (CBR: complete response + PR + SD > 24 weeks) were 34.3% and 8.6%, respectively. Concomitant PIK3CA and KRAS mutations were detected in 13 of 17 (76.5%) colorectal tumors. In the complete cohort (n = 134) hyperglycemia, gastrointestinal (GI) toxicities, fatigue, and rash were the most frequent AEs [66].
In a randomized phase 2 study, the efficacy of adding alpelisib to encorafenib and cetuximab in previously treated BRAF-mutated mCRC patients was investigated [67]. The combination of cetuximab, encorafenib, and alpelisib resulted in a median PFS of 5.4 months and a confirmed ORR of 27% in 52 patients. The combination of encorafenib and cetuximab (n = 50) resulted in an mPFS of 4.2 months and an ORR of 22%. The triple combination showed favorable results for PFS, with an HR of 0.69 (0.43–1.11; p = 0.064).
There are other ongoing phase 1 trials in the treatment of CRC, evaluating alpelisib, capecitabine, and radiation in rectal cancer (NCT02550743) or alpelisib with capecitabine in mCRC with PIK3CA gene mutation (NCT04753203).
AKT proteins are crucial effectors of PI3K and are directly activated in response to PI3K. One of the key downstream target genes of AKT is the mammalian target of rapamycin (mTOR) complex, which is a conserved serine/threonine kinase. Dual inhibitors of PI3K and mTOR are in early phases of drug development, such as dactolisib (BEZ235) [68].
In colon cancer SC models in vitro, Chen et al. reported that dactolisib reduced colon SCs proliferation, decreased expression of the stem cell markers CD133 and Lgr5, and induced apoptosis [69]. Dactolisib had been evaluated in a phase 1 study in 33 patients with advanced solid tumors. The AEs included mucositis, fatigue, hyperglycemia, dehydration, and thrombocytopenia. Fifteen patients experienced SD as their best response, including four colorectal patients who had disease control for ≥16 weeks [70]. In addition, a phase 1b dose-escalation study of everolimus plus dactolisib showed no responses in 19 patients with advanced tumors, including 3 CRC patients [71].
Another PI3K/mTOR inhibitor, gedatolisib, has also been evaluated in combination with irinotecan in a dose-escalation study [72]. The most common treatment-related AEs were nausea, diarrhea, vomiting, and mucosal inflammation/stomatitis. The ORR was 4.7% (95% CI, 0.6–15.8). Clinical benefit was seen in seven patients (16.3%; 95% CI, 6.8–30.7%). The median PFS was 2.8 (95% CI, 1.7–3.7) months. There was no correlation between clinical benefit and the presence of activating PI3K mutations [73].
In preclinical models, it has been observed that the overexpression of ABCB1 and ABCG2 in CRC cells, as well as aberrant regulation of WNT/β-catenin and active glycogen synthase kinase 3-beta (GSK3β) signaling, may lead to resistance to gedatolisib [28,74]. While the initial results are promising, there are still many issues associated with the use of PI3K pathway inhibitors in CRC that need to be resolved. These include selecting the appropriate biomarkers of pathway activation, determining the best timing for drug use, identifying resistance mechanisms, and investigating whether combination therapies can improve treatment outcomes.
2.6. NF-κB Pathway
NF-κB transcription factors play a crucial role in various physiological processes, including innate and adaptive immune responses, cell proliferation, cell death, and inflammation. These factors can contribute to the transformation of normal colonic SCs into CSCs [75]. Moreover, NF-κB signaling in the tumor microenvironment can induce dedifferentiation of cancer cells, enhancing their stemness and resistance to therapies. A constitutive activation of the NF-κB pathway has been described in 60 to 80% of CRCs [76].
The NF-κB pathway is activated through ubiquitin-mediated protein degradation via the proteasome. Although preclinical studies have shown promise for the development of proteasome inhibitors in CRC [77], a phase 2 study found that single-agent bortezomib, an FDA-approved proteasome inhibitor, was ineffective in treating mCRC [78]. None of the 19 patients with CRC who received bortezomib showed an objective response, and the median time to progression was 5.1 weeks [78].
Curcumin is a natural compound that inhibits the NF-κB pathway as well as other mechanisms, such as the WNT/β-catenin pathway [79]. In a randomized phase 2a trial, curcumin was combined with FOLFOX and found to be safe and tolerable (primary outcome) in 28 patients with mCRC. The study found no statistically significant differences in PFS (HR 0.57; 95% CI, 0.24–1.36; p = 0.2). However, there was a significant difference in OS in the intention-to-treat population (HR 0.339; 95% CI, 0.141–0.815; p = 0.016) [80].
Other potential targeted therapies against the NF-κB pathway are still in the early phases of development. Several phase 1 trials are currently underway to investigate the potential use of protein arginine methyltransferase 5 (PRMT5) inhibitors in CRC. PRMT5 is an upstream protein that regulates the NF-κB pathway [81]. PMRT5 knockout murine models are unable to produce SCs and lead to lethality [82], demonstrating the role of PMRT5 in cell stemness. PRMT5 inhibitors have shown biological activity in cancer cell lines and animal models [83]. Strategies to develop new PMRT5 inhibitors and utilize existing FDA-approved drugs to target this molecular pathway are ongoing [84,85].
IκB kinase alpha, a key regulator of the NF-κB pathway, has been identified as a potential target in CRC. The selective IKβ inhibitor BIX02514 has demonstrated preclinical activity in CRC cell lines by modulating the NF-κB pathway [86].
Other FDA-approved therapies, such as glucocorticoids, dasatinib (a tyrosine kinase inhibitor), and guratimod (an anti-rheumatic drug), have also been suggested to inhibit the NF-κB axis in cancer and may have a potential role in future development for CRC patients, revised in [72].
2.7. JAK/STAT Pathway
Janus kinase (JAK) is a family of non-receptor cytoplasmic tyrosine kinases that includes four members: JAK1, JAK2, JAK3, and TYK2. Its clinical role in myeloproliferative diseases is well-established and various JAK/STAT inhibitors are approved for treatment [87].
The JAK/STAT pathway contributes to CRC by supporting the survival and proliferation of cancer SCs in the inflammatory tumor microenvironment and by inducing the EMT [88]. Therapies targeting JAK/STAT signaling can both inhibit the transformation of normal SCs to CSC and promote the dedifferentiation of cancer cells to a normal stem-like state.
JAK/STAT-targeted therapies have shown promise in preclinical studies on cell lines and tumor models [88] and their impact is under active clinical investigation. However, these trials have produced conflicting results with even early termination or no published findings.
On one hand, in a phase 3 clinical trial for pretreated mCRC patients [89], napabucasin, a STAT3 inhibitor, did not show any difference in OS compared to placebo in patients with refractory mCRC. Nevertheless, in patients with positive pSTAT3 expression, the OS was longer with napabucasin (5.1 vs. 3.0 months; HR 0.41; p = 0.0025). Recently, the combination of napabucasin and FOLFIRI in second-line therapy did not improve OS compared to FOLFIRI alone [90]. On the other hand, napabucasin can be safely combined with panitumumab in heavily pretreated RAS wildtype (WT) mCRC patients, showing encouraging anti-tumor activity in terms of DCR (52.1%), PFS (9.14 months), and OS (39.43 months), including 64.6% of patients who were previously treated with an anti-EGFR agent [91]. In a phase 1/2 trial the combination of napabucasin with pembrolizumab showed an ORR of 50% and 10% in mCRC with MSI-H and MSS, respectively. The DCR was 45% and the median response duration was 9 months in MSS mCRC [92].
Additionally, in a phase 2 trial for refractory mCRC patients, the addition of ruxolitinib (a selective JAK 1 and 2 inhibitor) to regorafenib did not result in any benefit in PFS or OS [93].
Finally, in a phase 1 first-in-human study with OPB-31121, a STAT3 inhibitor, Do Youn et al. observed tumor shrinkage in only two out of eighteen patients with solid tumors, both of whom had CRC [94].
Clinical trials are currently underway to determine the efficacy of JAK/STAT inhibitors in treating CRC and other solid tumors. Results are expected from inhibitors such as itacitinib (NCT02646748) and other STAT inhibitors (NCT03647839, NCT02983578).
2.8. DNA Damage Pathway
CSCs have a strong ability to repair DNA damage (DD) through multiple DNA damage response (DDR) pathways [95]. Poly ADP ribose polymerase-1 (PARP-1) mediates multiple DD repair mechanisms and it is highly expressed in CRC-SCs [96,97]. In CRC-SCs, PARP1 and other components of homologous recombination repair factors, such as RAD51 and MRE11, cooperate with the ATR-CHK1 axis to control replication stress and mitosis [98]. There are four PARP inhibitors (PARPis) that have received FDA approval for treating breast, ovary, pancreas, and prostate cancers, although in different clinical and biomarkers scenarios. PARPis are most effective in tumors associated with inability to repair DNA damage [99], including inherited or acquired mutations in BRCA1, BRCA2, or PALB2 and homologous recombination deficiency (HRD). In CRC, the mutation rates for genes involved in DDR, in HRD, and BRCA1/2 are 21%, 13–15%, and 1–8%, respectively [100]. Notably, at least one HRD gene mutation was present in over 75% of MSI-H patients and 9.5% of MSS patients [101].
In preclinical models, PARPis may enhance the cytotoxic effects of DNA-damaging chemotherapeutic agents [102]. The combination of 5-FU and veliparib (ABT-888) enhances CRC-SC death by increasing DNA damage accumulation and simultaneously inhibiting the mismatch repair (MMR) system in MMR-proficient cells [102]. Niraparib enhanced the cytotoxic activity of irinotecan in both MSI-H and MSS CRC, including in vitro and xenograft models. However, even the most sensitive CRC cell lines showed an EC50 of niraparib that was more than 10 times higher than that of BRCA-deficient cell lines [103]. Results from clinical trials of PARPis in mCRC included veliparib, olaparib, and neliparib.
Several clinical trials have evaluated the combination of veliparib with irinotecan. However, this treatment combination showed limited safety and poor tolerability in trials of escalating doses. The most common toxicities observed were diarrhea, GI toxicities, and neutropenia [104]. In a phase 2 randomized clinical trial [105], veliparib was compared to placebo, each with FOLFIRI +/− bevacizumab. There were no significant differences in any of the outcomes. The median PFS, which was the primary objective, was 12 months for veliparib and 11 months for placebo (HR, 0.94; 95% CI, 0.60–1.48). The median OS was 25 months for veliparib and 27 months for placebo (HR 1.26; 95% CI, 0.74–2.16). The response rate was 57% for veliparib and 62% for placebo. The veliparib group had significantly higher frequencies (p < 0.05) of anemia (39% vs. 19%, p = 0.019) and neutropenia (66% vs. 37%, p = 0.001).
Veliparib plus temozolomide was evaluated in a single-arm phase 2 study including 75 heavily pretreated mCRC patients. The primary endpoint was met with a DCR of 25%. In addition, 4% of patients achieved a PR with a median PFS of 1.8 months and a median OS of 6.6 months. Patient inclusion was not based on DDR status. There was a suggestion of worse outcomes for patients with MSI-H tumors [106].
A phase 1 study was conducted to evaluate the safety and tolerability of olaparib in combination with irinotecan [107]. Olaparib was administered continuously or intermittently. Continuous olaparib and irinotecan was associated with high toxicities. Intermittent olaparib was better tolerated, although at reduced doses. Common toxicities included fatigue, anorexia, diarrhea, nausea, vomiting, neutropenia, thrombocytopenia, and abdominal pain. Nine patients had SD as the best response, two from continuous olaparib and seven from an intermittent schedule (median duration: 7.4 months; range: 4 to 13 months). The authors considered this combination to be of low interest for further clinical development.
In a phase 2 study, olaparib in monotherapy was not effective in terms of response or PFS in patients with refractory mCRC (n = 33), regardless of MSS (n = 20) or MSI-H (n = 13) status. Only five patients had SD. Nausea, fatigue, and vomiting were the most commonly reported treatment-emergent adverse events [108].
The phase 3 LYNK-003 study was recently halted after enrolling 309 patients, short of its target enrollment of 525 patients [109]. The study aimed to compare olaparib alone or olaparib plus bevacizumab with 5-FU plus bevacizumab as standard maintenance therapy in patients with advanced CRC who have not progressed on first-line FOLFOX plus bevacizumab. At the prespecified interim analysis for PFS, the effectiveness of olaparib as a single therapy and in combination with bevacizumab compared to the control group did not meet the criteria for continuation as determined by the Data Monitoring Committee (DMC). Therefore, both experimental arms were discontinued (NCT04456699).
A phase 2 study assessed the safety and effectiveness of combining niraparib and panitumumab in previously treated patients with RAS wildtype (WT) mCRC [110]. The primary endpoint was the clinical benefit rate (CBR), defined as objective response and SD. Prior therapy with EGFR inhibitors (cetuximab or panitumumab) was not allowed. However, patients who had received first-line chemotherapy with oxaliplatin and had an objective response or SD for at least 4 months were eligible for the trial. The ORR was 25%, while the CBR was 83.3%. Anemia dermatitis, oral mucositis, hypertension, and neutropenia were the most common AEs (NCT03983993).
Although preclinical studies show PARPis activity in CRC models, clinical trials have failed to demonstrate efficacy in patients with mCRC cancer. To improve the effectiveness of inhibiting DNA repair mechanisms against mCRC, several approaches can be taken: (i) using new drug combinations, (ii) developing new drugs that target different repair mechanisms, and (iii) selectively targeting patients with tumors that have altered DNA repair mechanisms.
A recent study screened a drug library in a collection of primary CRC-SCs isolated from patients [111]. The study identified prexasertib (LY2606368) as an anti-CSC agent that acts in in vitro and in vivo models. Prexasertib (LY2606368) is a small ATP-competitive selective inhibitor of CHK1 and CHK2. The most sensitive CSCs to Chk1 inhibitor showed an ongoing DNA replication stress (RS) response and were p53 deficient and hyperdiploid. This response was independent of RAS mutational status. The safety and recommended phase 2 dose of the CHK1 inhibitor prexasertib have been defined in a phase 1 trial. Hematologic toxicity was the most frequent AE and was dose limiting. The ORR was 4.9% and 12.5% in combination with cetuximab and 5-fluorouracil, respectively [112].
There is a need for biomarkers to improve the prediction of response to therapy with inhibitors of DNA repair pathways in cohorts of patients with CRC. These biomarkers would include mutations in known DNA repair genes as well as genetic alterations and genomic rearrangements indicating a repair-defective phenotype. Currently, a phase 2, open-label, multicentric, single arm trial (PEMBROLA, NCT05201612) is evaluating the response to olaparib in combination with pembrolizumab specifically in patients with mCRC with HRD. HRD has been defined as the presence of a BRCA deleterious mutation and/or a low RAD51 score (cut-off <10%) [113].
2.9. Hippo-YAP Pathway
The Hippo pathway consists of two main domains: the core serine/threonine kinase domain, which is modulated by a variety of upstream signals, and the transcriptional module, which is responsible for downstream target gene expression [114,115]. The core kinase domain comprises MST1/2 (Hippo or Hpo), SAV1, and LATS1/2. The transcriptional module includes YAP and TAZ. The Hippo pathway is initiated by the activation of MST1/2 or MAP4K proteins. When the kinase cascade is inactive, nuclear YAP/TAZ binds to the transcriptional enhanced associate domain (TEAD) family of transcription factors (TEAD1–4), which activate the expression of pro-proliferative and survival-enhancing genes. The Hippo pathway regulates CSCs and contributes to tumor initiation and progression [116]. Depletion of TAZ significantly decreased tumor-seeding ability. In CRC cell models, constitutive YAP activation reinforced the expression of the stemness biomarkers and regulators ALDH1A3, LGR5, and OCT4. The four main components of the Hippo pathway, MST1, LATS1/2, YAP, and TAZ, have also been shown to play a role in the development of chemoresistance, such as to 5-fluoruracil and oxalipatin [117,118].
There is evidence for crosstalk between the Wnt/β-catenin and Hippo-YAP signaling pathways [119]. Hippo pathway signaling is deactivated epigenetically by YAP overexpression, deletion (MST2), or gene silencing (MST, LATS, and RASSF1). In addition, KRAS and YAP1 converge on the transcription factor FOS and activate a transcriptional program involved in regulating the EMT [120]. However, in some CRC models, YAP plays a suppressive role in tumor growth. Cheung et al. [115] demonstrated that the Hippo kinases LATS1/2 and MST1/2, which inhibit YAP activity, are necessary to maintain Wnt signaling and canonical stem cell function. Loss of LATS1/2 or overexpression of YAP was sufficient to suppress tumor growth in organoids, patient-derived xenografts, and mouse models of primary and mCRC.
Currently, the development of potential drugs targeting the Hippo pathway focuses mainly on the activity and expression of the Hippo core kinase, or the expression levels of downstream YAP/TAZ, and the interactions between YAP/TAZ and TEAD. However, there are still few clinical trials with Hippo/YAP pathway modulators in cancer, including CRC. Some of the first trials include TEAD inhibitor drugs. Patients with somatic NF2 mutations and other alterations of the Hippo-YAP1 pathway are included [121]. In CRC, there are limited data available, although it is estimated that sequence variations in NF2 may be less than 5%. Expression of NF2/Merlin was reduced in CRC cells as compared with adjacent non-cancerous cells [122].
Another first-in-human, phase 1a/1b open-label [123] study evaluated SW-682 in patients with advanced solid tumors with or without Hippo pathway alterations. This included solid tumors with NF2 mutations and other Hippo pathway sequence variations (FAT1, LATS1/2) or YAP fusions (NCT06251310T).
3. Challenges in Targeting Colorectal Cancer Stem Cells
Surgery followed by adjuvant chemotherapy (ACT) for patients with stage III and high-risk stage II colon cancer is the standard of care for patients with non-metastatic disease. ACT with fluoropyrimidines and combination with oxaliplatin increase cure rates. In a fraction of patients, they are able to eradicate occult disseminated CSCs [124]. The treatment for unresectable metastatic CRC is based on systemic therapy (cytotoxic chemotherapy, biologic therapy such as antibodies to VEGF and EGFR growth factors and their combinations, and tyrosine-kinase inhibitor). Immunotherapy is used in the subset of MSI-H tumors. Currently used drugs target rapidly dividing cells and, while often effective at debulking tumor mass, these agents have largely failed to cure disease in the metastatic setting. It has been suggested that CSCs possess characteristics of tumor dormancy and inherent resistance to cytotoxic drugs, which may result in disease relapse after initial therapy involving surgery and adjuvant chemotherapy and in metastases and chemoresistance. Tailored approaches against CSCs could potentially be useful at any of these moments, from the presence of minimally disseminated but quiescent tumor disease, metastases chemosensitive to initial treatments, and even the situation of clinical progression with refractory disease. Currently, there are several therapeutic strategies under preclinical and clinical evaluation that could specifically target and eliminate CSCs. Table 1 shows different randomized trials that evaluate the efficacy of different anti-target drugs with potential activity against SCs in CRC. It is important to note how the various clinical trials targeting stemness have been developed in different evolutionary stages and clinical scenarios of mCRC. Determining the appropriate drugs and timing of administration is a crucial factor for the potential effectiveness of this therapy against CRC-SCs.
Table 1.
Phase 2 and 3 randomized clinical trials evaluating targeted molecular pathways associated with colorectal cancer SCs.
| Trial | Pathway | Phase | CRC Patients | Intervention | Comparator | Significant Results | Reference |
|---|---|---|---|---|---|---|---|
| CORRECT | Wnt/β-Catenin | 3 | Pretreated | Regorafenib | Placebo | Regorafenib mOS, 6.4 months. Placebo 5 m. HR 0.77; one-sided p = 0.0052 | [18] |
| COAST | Wnt/β-Catenin | 3 | Following curative resection of liver metastases after completion of planned chemotherapy | Regorafenib | Placebo | Only 25 patients randomized. SAEs were observed in 14.29% with regorafenib | [24] |
| NCT01830621 | JAK/STAT | 3 | Pretreated | Napabucasin | Placebo | OS did not significantly differ. In pSTAT3-positive patients, napabucasin showed longer survival: median 5.1 months (95% CI 4.0–7.5; HR 0.41, 0.23–0.73, p = 0.0025) | [89] |
| LYNK-003 | DNA Damage Response | 3 | Advanced CRC patients who had not progressed on first-line FOLFOX plus bevacizumab | Olaparib/ Olaparib plus bevacizumab |
5-FU + bevacizumab | The study was terminated for futility after enrolling 309 patients | [109] |
| NCT00985192 | PI3K/Akt/mTOR | 2 | Pretreated | Sonolisib (PX-866) and cetuximab | Cetuximab | No PFS superiority. The treatment-related toxicity was higher in the combination arm | [61] |
| NCT02278133 | PI3K/Akt/mTOR | 2 | BRAF V600E-mutated mCRC | Alpelisib, encorafenib, and cetuximab | Encorafenib and cetuximab | The combination of cetuximab, encorafenib, and alpelisib resulted in a median PFS 5.4 months (HR 0.69; p = 0.064). ORR 27% in 52 patients | [67] |
| NCT01490996 | NF-κB | 2a | Pretreated | Curcumin + FOLFOX | FOLFOX | No statistically significant differences in PFS (HR 0.57; p = 0.2). However, there was a significant difference in OS in the ITT population (HR 0.339; p = 0.016) | [79] |
| NCT02119676 | JAK/STAT | 2 | Pretreated | Ruxolitinib + regorafenib | Regorafenib | The combination did not improve OS or PFS | [93] |
| NCT02305758 | DNA Damage Response | 2 | Pretreated | Veliparib + FOLFIRI | Placebo + FOLFIRI | No significant differences in PFS or OS. The veliparib group had significantly higher frequencies of anemia and neutropenia | [105] |
| NCT00636610 | Hedgehog | 2 | Pretreated | Vismodegib + FOLFOX/FOLFIRI + bevacizumab | Placebo + FOLFOX/ FOLFIRI + bevacizumab | The overall response rates for placebo-treated and vismodegib-treated patients were 51% (90% CI: 43–60) and 46% (90% CI: 37–55), respectively | [32] |
Notably, CSCs represent a small percentage (0.3% to 2.2%) of all cancer cells in CRC [125]. There is evidence that systemic spread can occur early in CRC [126]. Cells with functional and phenotypic properties of SCs have been isolated from primary colorectal tumors, metastases, and blood [127]. Although the expression of distinct stem cell markers in both tumor tissue and peripheral blood has been correlated with prognosis, we have no standardized and validated method in the clinic to enumerate or characterize CRC-SCs in real time [7]. The majority of studies have included patients with mCRC without selection based on molecular markers that could indicate the activity of the different signaling pathways. The identification of SC signatures specific to each patient could guide personalized treatment strategies.
CRC-SCs display a heterogeneous and dynamic phenotype that is influenced by mutations in epithelial cells and by extrinsic signals from the microenvironment [128]. Moreover, this plasticity allows SCs to adapt to the selective pressure that can be induced, for example, by anti-neoplastic treatment. The lack of efficacy of the different anti-target therapies in eradicating CRC-SC may be partly explained by these characteristics.
To address these challenges, the use of single-cell omics technologies offers a promising avenue to unravel the complex heterogeneity and plasticity of CSCs. By providing high-resolution insights into the molecular signatures of individual cancer cells, single-cell omics can significantly enhance our ability to identify specific biomarkers and therapeutic targets unique to CSCs. Moreover, single-cell analysis may facilitate the real-time monitoring of therapeutic responses and CSC dynamics, offering new approaches for managing cancer progression and resistance mechanisms [129].
In the clinical context, addressing the challenge of eradicating CSCs and preventing tumor recurrence while preserving normal SCs, which are crucial for tissue regeneration and homeostasis, requires a multifaceted approach. One such challenge is the inherent plasticity of CSCs, which allows them to alternate between stem-like and differentiated states. Furthermore, the identification of reliable biomarkers for CSCs remains a significant barrier to effective clinical management. Taking advantage of these cutting-edge technologies, researchers and clinicians can move closer to designing effective therapeutic strategies that address the unique challenges posed by CSCs in CRC. While the eradication of SCs has been hypothesized as a possible cure for solid tumors, including CRC [130], there is still considerable progress yet to be made.
Author Contributions
Conceptualization, methodology, writing—original draft preparation: J.M.-P., C.T., M.A.D.-C. and M.V.-A.; writing—review and editing, M.V.-A. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
No new data were created.
Conflicts of Interest
J.M.-P. has received payment or honoraria for lectures, presentations, manuscript writing, or educational events from MSD, Bristol-Myers, Merck, Servier. M.V.-A. has received grants or contracts from Roche and Amgen and consulting fees from Amgen, Sanofi, Servier, and Bayer and payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from MSD, Bristol-Myers, Merck, Servier, Amgen and participates on Advisory Boards of Amgen, Servier, and Merck. C.T. and M.A.D.-C. have no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA Cancer J. Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Lapidot T., Sirard C., Vormoor J., Murdoch B., Hoang T., Caceres-Cortes J., Minden M., Paterson B., Caligiuri M.A., Dick J.E. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 3.Bonnet D., Dick J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien C.A., Pollett A., Gallinger S., Dick J.E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 5.Ricci-Vitiani L., Lombardi D.G., Pilozzi E., Biffoni M., Todaro M., Peschle C., De Maria R. Identification and expansion of human colon-cancer-initiating cells. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 6.Todaro M., Alea M.P., Di Stefano A.B., Cammareri P., Vermeulen L., Iovino F., Tripodo C., Russo A., Gulotta G., Medema J.P., et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Abbasian M., Mousavi E., Arab-Bafrani Z., Sahebkar A. The most reliable surface marker for the identification of colorectal cancer stem-like cells: A systematic review and meta-analysis. J. Cell Physiol. 2019;234:8192–8202. doi: 10.1002/jcp.27619. [DOI] [PubMed] [Google Scholar]
- 8.Barker N., Ridgway R.A., van Es J.H., van de Wetering M., Begthel H., van den Born M., Danenberg E., Clarke A.R., Sansom O.J., Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 9.Schwitalla S., Fingerle A.A., Cammareri P., Nebelsiek T., Göktuna S.I., Ziegler P.K., Canli O., Heijmans J., Huels D.J., Moreaux G. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Clara J.A., Monge C., Yang Y., Takebe N. Targeting signalling pathways and the immune microenvironment of cancer stem cells—A clinical update. Nat. Rev. Clin. Oncol. 2020;17:204–232. doi: 10.1038/s41571-019-0293-2. [DOI] [PubMed] [Google Scholar]
- 11.Ebrahimi N., Afshinpour M., Fakhr S.S., Kalkhoran P.G., Shadman-Manesh V., Adelian S., Beiranvand S., Rezaei-Tazangi F., Khorram R., Hamblin M.R., et al. Cancer stem cells in colorectal cancer: Signaling pathways involved in stemness and therapy resistance. Crit. Rev. Oncol. Hematol. 2023;182:103920. doi: 10.1016/j.critrevonc.2023.103920. [DOI] [PubMed] [Google Scholar]
- 12.Malla S.B., Byrne R.M., Lafarge M.W., Corry S.M., Fisher N.C., Tsantoulis P.K., Mills M.L., Ridgway R.A., Lannagan T.R.M., Najumudeen A.K., et al. Pathway level subtyping identifies a slow-cycling biological phenotype associated with poor clinical outcomes in colorectal cancer. Nat. Genet. 2024;56:458–472. doi: 10.1038/s41588-024-01654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tape C.J. Plastic persisters: Revival stem cells in colorectal cancer. Trends Cancer. 2024;10:185–195. doi: 10.1016/j.trecan.2023.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Atlasi Y., Looijenga L., Fodde R. Cancer stem cells, pluripotency, and cellular heterogeneity: A WNTer perspective. Curr. Top. Dev. Biol. 2014;107:373–404. doi: 10.1016/B978-0-12-416022-4.00013-5. [DOI] [PubMed] [Google Scholar]
- 15.Zhao H., Ming T., Tang S., Ren S., Yang H., Liu M., Tao Q., Xu H. Wnt signaling in colorectal cancer: Pathogenic role and therapeutic target. Mol. Cancer. 2022;21:144. doi: 10.1186/s12943-022-01616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong Z., Virshup D.M. Wnt Signaling and Drug Resistance in Cancer. Mol. Pharmacol. 2020;97:72–89. doi: 10.1124/mol.119.117978. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y., Chen M., Deng K. Blocking the Wnt/beta-catenin signaling pathway to treat colorectal cancer: Strategies to improve current therapies (Review) Int. J. Oncol. 2023;62:24. doi: 10.3892/ijo.2022.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grothey A., Van Cutsem E., Sobrero A., Siena S., Falcone A., Ychou M., Humblet Y., Bouché O., Mineur L., Barone C., et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 19.Cai M.H., Xu X.G., Yan S.L., Sun Z., Ying Y., Wang B.K., Tu Y.X. Regorafenib suppresses colon tumorigenesis and the generation of drug resistant cancer stem-like cells via modulation of miR-34a associated signaling. J. Exp. Clin. Cancer Res. 2018;37:151. doi: 10.1186/s13046-018-0836-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu F.T., Man S., Xu P., Chow A., Paez-Ribes M., Lee C.R., Pirie-Shepherd S.R., Emmenegger U., Kerbel R.S. Efficacy of Cotargeting Angiopoietin-2 and the VEGF Pathway in the Adjuvant Postsurgical Setting for Early Breast, Colorectal, and Renal Cancers. Cancer Res. 2016;76:6988–7000. doi: 10.1158/0008-5472.CAN-16-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grothey A., Yoshino T., Xu R., Zalcberg D.J., Sargent M., Choti M., Rutstein M., Cupit L., Kuss I., Van Cutsem E. Adjuvant Regorafenib (Reg) in Stage Iv Colorectal Cancer (Crc) After Curative Treatment of Liver Metastases: A Phase III Randomized, Placebo (Pbo)-Controlled Trial (Coast) Ann. Oncol. 2014;25((Suppl. S4)):iv208. doi: 10.1093/annonc/mdu333.112. [DOI] [Google Scholar]
- 22.Larriba M.J., Gonzalez-Sancho J.M., Barbachano A., Niell N., Ferrer-Mayorga G., Munoz A. Vitamin D Is a Multilevel Repressor of Wnt/b-Catenin Signaling in Cancer Cells. Cancers. 2013;5:1242–1260. doi: 10.3390/cancers5041242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng K., Nimeiri H.S., McCleary N.J., Abrams T.A., Yurgelun M.B., Cleary J.M., Rubinson D.A., Schrag D., Miksad R., Bullock A.J., et al. Effect of High-Dose vs Standard-Dose Vitamin D3 Supplementation on Progression-Free Survival Among Patients with Advanced or Metastatic Colorectal Cancer: The SUNSHINE Randomized Clinical Trial. JAMA. 2019;321:1370–1379. doi: 10.1001/jama.2019.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pintova S., Dharmupari S., Moshier E., Zubizarreta N., Ang C., Holcombe R.F. Genistein combined with FOLFOX or FOLFOX-Bevacizumab for the treatment of metastatic colorectal cancer: Phase I/II pilot study. Cancer Chemother. Pharmacol. 2019;84:591–598. doi: 10.1007/s00280-019-03886-3. [DOI] [PubMed] [Google Scholar]
- 25.Safran H., Dhawan M.S., Cavalcante L., Souza A.L.D., Powell S.F., Billadeau D.D., Giles F.J., Munster P.N., Carneiro B.A. Glycogen synthase kinase-3 beta (GSK-3β) blockade with 9-ing-41 in gastrointestinal cancers: The 1801 phase Ib/II study. J. Clin. Oncol. 2020;38((Suppl. S4)):827. doi: 10.1200/JCO.2020.38.4_suppl.827. [DOI] [Google Scholar]
- 26.Tabernero J., Van Cutsem E., Garralda E., Tai D., De Braud F., Geva R., van Bussel M.T.J., Fiorella Dotti K., Elez E., de Miguel M.J., et al. A Phase Ib/II Study of WNT974 + Encorafenib + Cetuximab in Patients with BRAF V600E-Mutant KRAS Wild-Type Metastatic Colorectal Cancer. Oncologist. 2023;28:230–238. doi: 10.1093/oncolo/oyad007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopetz S., Morris V.K., O’Neil B., Bridgewater J.A., Graham J., Parkes E.E., Saunders M.P., Asken E., Goodwin L., Phillips C., et al. A multi-arm, phase 2, open-label study to assess the efficacy of RXC004 as monotherapy and in combination with nivolumab in patients with ring finger protein 43 (RNF43) or R-spondin (RSPO) aberrated, metastatic, microsatellite stable colorectal cancer following standard treatments. J. Clin. Oncol. 2022;40((Suppl. S16)):TPS3637. [Google Scholar]
- 28.Park W.J., Kim M.J. A New Wave of Targeting ‘Undruggable’ Wnt Signaling for Cancer Therapy: Challenges and Opportunities. Cells. 2023;12:1110. doi: 10.3390/cells12081110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu C., Zhu X., Liu W., Ruan T., Tao K. Hedgehog signaling pathway in colorectal cancer: Function, mechanism, and therapy. Onco Targets Ther. 2017;10:3249–3259. doi: 10.2147/OTT.S139639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quaglio D., Infante P., Di Marcotullio L., Botta B., Mori M. Hedgehog signaling pathway inhibitors: An updated patent review (2015–present) Expert Opin. Ther. Pat. 2020;30:235–250. doi: 10.1080/13543776.2020.1730327. [DOI] [PubMed] [Google Scholar]
- 31.Hainsworth J.D., Meric-Bernstam F., Swanton C., Hurwitz H., Spigel D.R., Sweeney C., Burris H., Bose R., Yoo B., Stein A., et al. Targeted Therapy for Advanced Solid Tumors on the Basis of Molecular Profiles: Results from MyPathway, an Open-Label, Phase IIa Multiple Basket Study. J. Clin. Oncol. 2018;36:536–542. doi: 10.1200/JCO.2017.75.3780. [DOI] [PubMed] [Google Scholar]
- 32.Berlin J., Bendell J.C., Hart L.L., Firdaus I., Gore I., Hermann R.C., Mulcahy M.F., Zalupski M.M., Mackey H.M., Yauch R.L., et al. A randomized phase II trial of vismodegib versus placebo with FOLFOX or FOLFIRI and bevacizumab in patients with previously untreated metastatic colorectal cancer. Clin. Cancer Res. 2013;19:258–267. doi: 10.1158/1078-0432.CCR-12-1800. [DOI] [PubMed] [Google Scholar]
- 33.Zhou B., Lin W., Long Y., Yang Y., Zhang H., Wu K., Chu Q. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduct. Target. Ther. 2022;7:95. doi: 10.1038/s41392-022-00934-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arcaroli J.J., Tai W.M., McWilliams R., Bagby S., Blatchford P.J., Varella-Garcia M., Purkey A., Quackenbush K.S., Song E.K., Pitts T.M., et al. A NOTCH1 gene copy number gain is a prognostic indicator of worse survival and a predictive biomarker to a Notch1 targeting antibody in colorectal cancer. Int. J. Cancer. 2016;138:195–205. doi: 10.1002/ijc.29676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tyagi A., Sharma A.K., Damodaran C. A Review on Notch Signaling and Colorectal Cancer. Cells. 2020;9:1549. doi: 10.3390/cells9061549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strosberg J.R., Yeatman T., Weber J., Coppola D., Schell M.J., Han G., Almhanna K., Kim R., Valone T., Jump H., et al. A phase II study of RO4929097 in metastatic colorectal cancer. Eur. J. Cancer. 2012;48:997–1003. doi: 10.1016/j.ejca.2012.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sahebjam S., Bedard P.L., Castonguay V., Chen Z., Reedijk M., Liu G., Cohen B., Zhang W.J., Clarke B., Zhang T., et al. A phase I study of the combination of RO4929097 and cediranib in patients with advanced solid tumours (PJC-004/NCI 8503) Br. J. Cancer. 2013;109:943–949. doi: 10.1038/bjc.2013.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pant S., Jones S.F., Kurkjian C.D., Infante J.R., Moore K.N., Burris H.A., McMeekin D.S., Benhadji K.A., Patel B.K.R., Frenzel M.J., et al. A first-in-human phase I study of the oral Notch inhibitor, LY900009, in patients with advanced cancer. Eur. J. Cancer. 2016;56:1–9. doi: 10.1016/j.ejca.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 39.Smith D.C., Eisenberg P.D., Manikhas G., Chugh R., Gubens M.A., Stagg R.J., Kapoun A.M., Xu L., Dupont J., Sikic B. A phase I dose escalation and expansion study of the anticancer stem cell agent demcizumab (anti-DLL4) in patients with previously treated solid tumors. Clin. Cancer Res. 2014;20:6295–6303. doi: 10.1158/1078-0432.CCR-14-1373. [DOI] [PubMed] [Google Scholar]
- 40.Massagué J., Sheppard D. TGF-β signaling in health and disease. Cell. 2023;186:4007–4037. doi: 10.1016/j.cell.2023.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qin X., Cardoso Rodriguez F., Sufi J., Vlckova P., Claus J., Tape C.J. An oncogenic phenoscape of colonic stem cell polarization. Cell. 2023;186:5554–5568.e18. doi: 10.1016/j.cell.2023.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Nakano M., Kikushige Y., Miyawaki K., Kunisaki Y., Mizuno S., Takenaka K., Tamura S., Okumura Y., Ito M., Ariyama H., et al. Dedifferentiation process driven by TGF-beta signaling enhances stem cell properties in human colorectal cancer. Oncogene. 2019;38:780–793. doi: 10.1038/s41388-018-0480-0. [DOI] [PubMed] [Google Scholar]
- 43.Lampropoulos P., Zizi-Sermpetzoglou A., Rizos S., Kostakis A., Nikiteas N., Papavassiliou A.G. TGF-beta signalling in colon carcinogenesis. Cancer Lett. 2012;314:1–7. doi: 10.1016/j.canlet.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 44.Kim T.W., Lee K.W., Ahn J.B., Hong Y.S., Kim S.Y., Kim J.W., Kim J.-W., Shin S.J., Beom S.H., Kim S.T., et al. 618P. Efficacy and safety of vactosertib and pembrolizumab combination in patients with previously treated microsatellite stable metastatic colorectal cancer. Ann. Oncol. 2023;34:S443. doi: 10.1016/j.annonc.2023.09.1809. [DOI] [Google Scholar]
- 45.Yamazaki T., Gunderson A.J., Gilchrist M., Whiteford M., Kiely M.X., Hayman A., O'Brien D., Ahmad R., Manchio J.V., Fox N., et al. Galunisertib plus neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer: A single-arm, phase 2 trial. Lancet Oncol. 2022;23:1189–1200. doi: 10.1016/S1470-2045(22)00446-6. [DOI] [PubMed] [Google Scholar]
- 46.Bauer T.M., Santoro A., Lin C.C., Garrido-Laguna I., Joerger M., Greil R., Spreafico A., Yau T., Goebeler M.E., Hütter-Krönke M.L., et al. Phase I/Ib, open-label, multicenter, dose-escalation study of the anti-TGF-beta monoclonal antibody, NIS793, in combination with spartalizumab in adult patients with advanced tumors. J. Immunother. Cancer. 2023;11:e007353. doi: 10.1136/jitc-2023-007353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simonelli M., Zucali P., Santoro A., Thomas M.B., de Braud F.G., Borghaei H., Berlin J., Denlinger C.S., Noberasco C., Rimassa L., et al. Phase I study of PF-03446962, a fully human monoclonal antibody against activin receptor-like kinase-1, in patients with hepatocellular carcinoma. Ann. Oncol. 2016;27:1782–1787. doi: 10.1093/annonc/mdw240. [DOI] [PubMed] [Google Scholar]
- 48.Wheatley-Price P., Chu Q., Bonomi M., Seely J., Gupta A., Goss G., Hilton J., Feld R., Lee C.W., Goffin J.R., et al. A Phase II Study of PF-03446962 in Patients with Advanced Malignant Pleural Mesothelioma. CCTG Trial IND.207. J. Thorac. Oncol. 2016;11:2018–2021. doi: 10.1016/j.jtho.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 49.Clarke J.M., Blobe G.C., Strickler J.H., Uronis H.E., Zafar S.Y., Morse M., Dropkin E., Howard L., O'Neill M., Rushing C.N., et al. A phase Ib study of the combination regorafenib with PF-03446962 in patients with refractory metastatic colorectal cancer (REGAL-1 trial) Cancer Chemother. Pharmacol. 2019;84:909–917. doi: 10.1007/s00280-019-03916-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robbrecht D., Doger B., Grob J.-J., Bechter O., Miguel M., Vieito M., Schadendorf D., Curigliano G., Borbath I., Butler M.O., et al. Safety and efficacy results from the expansion phase of the first-in-human study evaluating TGFβ inhibitor SAR439459 alone and combined with cemiplimab in adults with advanced solid tumors. J. Clin. Oncol. 2022;40:2524. doi: 10.1200/JCO.2022.40.16_suppl.2524. [DOI] [Google Scholar]
- 51.Spira A., Wertheim M.S., Kim E.J., Tan B., Lenz H.J., Nikolinakos P., Rich P.L., Jehl G., Machl A., Ito R., et al. Bintrafusp Alfa: A Bifunctional Fusion Protein Targeting PD-L1 and TGF-beta, in Patients with Pretreated Colorectal Cancer: Results from a Phase I Trial. Oncologist. 2023;28:e124–e127. doi: 10.1093/oncolo/oyac254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morris V., Lam M., Wang X., Overman M., Johnson B., Kee B., Wolff R.A., Dasari A., Zorrilla I.R., Tam A., et al. Phase II trial of bintrafusp alfa in patients with metastatic MSI-H cancers following progression on immunotherapy. J. Clin. Oncol. 2021;39:79. doi: 10.1200/JCO.2021.39.3_suppl.79. [DOI] [Google Scholar]
- 53.Pastor D.M., Redman J.M., Floudas C.S., Lamping E., Francis D.C., Cordes L.M., Marte J.L., Schlom J., Gulley J.L., Strauss J. Phase II evaluation of combination immunotherapy with CV301, N-803, bintrafusp alfa, and M9241 in patients with advanced small bowel and colorectal cancers. J. Clin. Oncol. 2023;41((Suppl. S4)):116. doi: 10.1200/JCO.2023.41.4_suppl.116. [DOI] [Google Scholar]
- 54.Xu R.H., Qiu M., Bai Y., Wang J., Gu K., Yang M., He Y., Yi C., Jin Y., Liu B., et al. SHR-1701 in combination with BP102 and XELOX as first-line (1L) treatment for patients (pts) with unresectable metastatic colorectal cancer (mCRC): Data from a phase II/III study. Ann. Oncol. 2023;34:S439. doi: 10.1016/j.annonc.2023.09.1802. [DOI] [Google Scholar]
- 55.Oh J., Barve M., Matthews C.M., Koon E.C., Heffernan T.P., Fine B., Grosen E., Bergman M.K., Fleming E.L., DeMars L.R., et al. Phase II study of Vigil(R) DNA engineered immunotherapy as maintenance in advanced stage ovarian cancer. Gynecol. Oncol. 2016;143:504–510. doi: 10.1016/j.ygyno.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 56.Hirano T., Satow R., Kato A., Tamura M., Murayama Y., Saya H., Kojima H., Nagano T., Okabe T., Fukami K. Identification of novel small compounds that restore E-cadherin expression and inhibit tumor cell motility and invasiveness. Biochem. Pharmacol. 2013;86:1419–1429. doi: 10.1016/j.bcp.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 57.He Y., Sun M.M., Zhang G.G., Yang J., Chen K.S., Xu W.W., Li B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021;6:425. doi: 10.1038/s41392-021-00828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Q., Shi Y.L., Zhou K., Wang L.L., Yan Z.X., Liu Y.L., Xu L.L., Zhao S.W., Chu H.L., Shi T.T., et al. PIK3CA mutations confer resistance to first-line chemotherapy in colorectal cancer. Cell Death Dis. 2018;9:739. doi: 10.1038/s41419-018-0776-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sastre J., Orden V., Martinez A., Bando I., Balbin M., Bellosillo B., Palanca S., Peligros-Gomez M.I., Mediero B., Llovet P., et al. Association Between Baseline Circulating Tumor Cells, Molecular Tumor Profiling, and Clinical Characteristics in a Large Cohort of Chemo-naive Metastatic Colorectal Cancer Patients Prospectively Collected. Clin. Colorectal Cancer. 2020;19:e110–e116. doi: 10.1016/j.clcc.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 60.Sastre J., Garcia-Alfonso P., Vieitez J.M., Cano M.T., Rivera F., Reina-Zoilo J.J., Salud-Salvia A., Quintero G., Robles-Díaz L., Safont M.J., et al. Influence of BRAF and PIK3CA mutations on the efficacy of FOLFIRI plus bevacizumab or cetuximab as first-line therapy in patients with RAS wild-type metastatic colorectal carcinoma and <3 baseline circulating tumour cells: The randomised phase II VISNU-2 study. ESMO Open. 2021;6:100062. doi: 10.1016/j.esmoop.2021.100062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bowles D.W., Kochenderfer M., Cohn A., Sideris L., Nguyen N., Cline-Burkhardt V., Schnadig I., Choi M., Nabell L., Chaudhry A., et al. A Randomized, Phase II Trial of Cetuximab with or without PX-866, an Irreversible Oral Phosphatidylinositol 3-Kinase Inhibitor, in Patients with Metastatic Colorectal Carcinoma. Clin. Colorectal Cancer. 2016;15:337–344.e2. doi: 10.1016/j.clcc.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 62.Wheler J., Mutch D., Lager J., Castell C., Liu L., Jiang J., Traynor A.M. Phase I Dose-Escalation Study of Pilaralisib (SAR245408, XL147) in Combination with Paclitaxel and Carboplatin in Patients with Solid Tumors. Oncologist. 2017;22:377-e37. doi: 10.1634/theoncologist.2016-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McRee A.J., Sanoff H.K., Carlson C., Ivanova A., O’Neil B.H. A phase I trial of mFOLFOX6 combined with the oral PI3K inhibitor BKM120 in patients with advanced refractory solid tumors. Investig. New Drugs. 2015;33:1225–1231. doi: 10.1007/s10637-015-0298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goodwin R., Jonker D., Chen E., Kennecke H., Cabanero M., Tsao M.S., Vickers M., Bohemier C., Lim H., Ritter H., et al. A phase Ib study of a PI3Kinase inhibitor BKM120 in combination with panitumumab in patients with KRAS wild-type advanced colorectal cancer. Investig. New Drugs. 2020;38:1077–1084. doi: 10.1007/s10637-019-00814-3. [DOI] [PubMed] [Google Scholar]
- 65.Damodaran S., Zhao F., Deming D.A., Mitchell E.P., Wright J.J., Gray R.J., Wang V., McShane L.M., Rubinstein L.V., Patton D.R., et al. Phase II Study of Copanlisib in Patients with Tumors with PIK3CAMutations: Results From the NCI-MATCHECOG-ACRINTrial (EAY131) Subprotocol, Z.1.F. J. Clin. Oncol. 2022;40:1552–1561. doi: 10.1200/JCO.21.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Juric D., Rodon J., Tabernero J., Janku F., Burris H.A., Schellens J.H.M., Middleton M.R., Berlin J., Schuler M., Gil-Martin M., et al. Phosphatidylinositol 3-Kinase α-Selective Inhibition with Alpelisib (BYL719) in PIK3CA-Altered Solid Tumors: Results from the First-in-Human Study. J. Clin. Oncol. 2018;36:1291–1299. doi: 10.1200/JCO.2017.72.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tabernero J.M., Van Geel R., Guren T.K., Yaeger R., Spreafico A., Faris J.E., Yoshino T., Yamada Y., Kim T.W., Bendell J.C., et al. Phase 2 results: Encorafenib (ENCO) and cetuximab (CETUX) with or without alpelisib (ALP) in patients with advanced BRAF-mutant colorectal cancer (BRAFm CRC) J. Clin. Oncol. 2016;34((Suppl. S15)):3544. [Google Scholar]
- 68.Maira S.M., Stauffer F., Brueggen J., Furet P., Schnell C., Fritsch C., Brachmann S., Chène P., De Pover A., Schoemaker K., et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol. Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 69.Chen J., Shao R., Li F., Monteiro M., Liu J.P., Xu Z.P., Gu W. PI3K/Akt/mTOR pathway dual inhibitor BEZ235 suppresses the stemness of colon cancer stem cells. Clin. Exp. Pharmacol. Physiol. 2015;42:1317–1326. doi: 10.1111/1440-1681.12493. [DOI] [PubMed] [Google Scholar]
- 70.Wise-Draper T.M., Moorthy G., Salkeni M.A., Karim N.A., Thomas H.E., Mercer C.A., Beg M.S., O'Gara S., Olowokure O., Fathallah H., et al. A Phase Ib Study of the Dual PI3K/mTOR Inhibitor Dactolisib (BEZ235) Combined with Everolimus in Patients with Advanced Solid Malignancies. Target. Oncol. 2017;12:323–332. doi: 10.1007/s11523-017-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bendell J.C., Kurkjian C., Infante J.R., Bauer T.M., Burris H.A., Greco F.A., Shih K.C., Thompson D.S., Lane C.M., Finney L.H., et al. A phase 1 study of the sachet formulation of the oral dual PI3K/mTOR inhibitor BEZ235 given twice daily (BID) in patients with advanced solid tumors. Investig. New Drugs. 2015;33:463–471. doi: 10.1007/s10637-015-0218-6. [DOI] [PubMed] [Google Scholar]
- 72.Zeligs K.P., Neuman M.K., Annunziata C.M. Molecular Pathways: The Balance between Cancer and the Immune System Challenges the Therapeutic Specificity of Targeting Nuclear Factor-κB Signaling for Cancer Treatment. Clin. Cancer Res. 2016;22:4302–4308. doi: 10.1158/1078-0432.CCR-15-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wainberg Z.A., Alsina M., Soares H.P., Brana I., Britten C.D., Del Conte G., Ezeh P., Houk B., Kern K.A., Leong S., et al. A Multi-Arm Phase I Study of the PI3K/mTOR Inhibitors PF-04691502 and Gedatolisib (PF-05212384) plus Irinotecan or the MEK Inhibitor PD-0325901 in Advanced Cancer. Target. Oncol. 2017;12:775–785. doi: 10.1007/s11523-017-0530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu C., Xing W., Yu H., Zhang W., Si T. ABCB1 and ABCG2 restricts the efficacy of gedatolisib (PF-05212384), a PI3K inhibitor in colorectal cancer cells. Cancer Cell Int. 2021;21:108. doi: 10.1186/s12935-021-01800-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu H., Lin L., Zhang Z., Zhang H., Hu H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020;5:209. doi: 10.1038/s41392-020-00312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soleimani A., Rahmani F., Ferns G.A., Ryzhikov M., Avan A., Hassanian S.M. Role of the NF-κB signaling pathway in the pathogenesis of colorectal cancer. Gene. 2020;726:144132. doi: 10.1016/j.gene.2019.144132. [DOI] [PubMed] [Google Scholar]
- 77.Hochwald S.N., Lind D.S., Malaty J., Copeland E.M., III, Moldawer L.L., MacKay S.L. Antineoplastic therapy in colorectal cancer through proteasome inhibition. Am. Surg. 2003;69:15–23. doi: 10.1177/000313480306900104. [DOI] [PubMed] [Google Scholar]
- 78.Mackay H., Hedley D., Major P., Townsley C., Mackenzie M., Vincent M., Degendorfer P., Tsao M.S., Nicklee T., Birle D., et al. A phase II trial with pharmacodynamic endpoints of the proteasome inhibitor bortezomib in patients with metastatic colorectal cancer. Clin. Cancer Res. 2005;11:5526–5533. doi: 10.1158/1078-0432.CCR-05-0081. [DOI] [PubMed] [Google Scholar]
- 79.Vallee A., Lecarpentier Y., Vallee J.N. Curcumin: A therapeutic strategy in cancers by inhibiting the canonical WNT/beta-catenin pathway. J. Exp. Clin. Cancer Res. 2019;38:323. doi: 10.1186/s13046-019-1320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Howells L.M., Iwuji C.O.O., Irving G.R.B., Barber S., Walter H., Sidat Z., Griffin-Teall N., Singh R., Foreman N., Patel S.R., et al. Curcumin Combined with FOLFOX Chemotherapy Is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. J. Nutr. 2019;149:1133–1139. doi: 10.1093/jn/nxz029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hartley A.V., Wang B., Jiang G., Wei H., Sun M., Prabhu L., Martin M., Safa A., Sun S., Liu Y., et al. Regulation of a PRMT5/NF-κB Axis by Phosphorylation of PRMT5 at Serine 15 in Colorectal Cancer. Int. J. Mol. Sci. 2020;21:3684. doi: 10.3390/ijms21103684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stopa N., Krebs J.E., Shechter D. The PRMT5 arginine methyltransferase: Many roles in development, cancer and beyond. Cell Mol. Life Sci. 2015;72:2041–2059. doi: 10.1007/s00018-015-1847-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yan Y., Zhao P., Wang Z., Liu Z., Wang Z., Zhang J., Ding Y., Hua X., Yu L. PRMT5 regulates colorectal cancer cell growth and EMT via EGFR/Akt/GSK3beta signaling cascades. Aging. 2021;13:4468–4481. doi: 10.18632/aging.202407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Feustel K., Falchook G.S. Protein Arginine Methyltransferase 5 (PRMT5) Inhibitors in Oncology Clinical Trials: A review. J. Immunother. Precis. Oncol. 2022;5:58–67. doi: 10.36401/JIPO-22-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prabhu L., Martin M., Chen L., Demir O., Jin J., Huang X., Motolani A., Sun M., Jiang G., Nakshatri H., et al. Inhibition of PRMT5 by market drugs as a novel cancer therapeutic avenue. Genes. Dis. 2023;10:267–283. doi: 10.1016/j.gendis.2022.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prescott J.A., Balmanno K., Mitchell J.P., Okkenhaug H., Cook S.J. IKK 1alpha plays a major role in canonical NF-κB signalling in colorectal cells. Biochem. J. 2022;479:305–325. doi: 10.1042/BCJ20210783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shawky A.M., Almalki F.A., Abdalla A.N., Abdelazeem A.H., Gouda A.M. A Comprehensive Overview of Globally Approved JAK Inhibitors. Pharmaceutics. 2022;14:1001. doi: 10.3390/pharmaceutics14051001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mengie Ayele T., Tilahun Muche Z., Behaile Teklemariam A., Bogale Kassie A., Chekol Abebe E. Role of JAK2/STAT3 Signaling Pathway in the Tumorigenesis, Chemotherapy Resistance, and Treatment of Solid Tumors: A Systemic Review. J. Inflamm. Res. 2022;15:1349–1364. doi: 10.2147/JIR.S353489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jonker D.J., Nott L., Yoshino T., Gill S., Shapiro J., Ohtsu A., Zalcberg J., Vickers M.M., Wei A.C., Gao Y., et al. Napabucasin versus placebo in refractory advanced colorectal cancer: A randomised phase 3 trial. Lancet Gastroenterol. Hepatol. 2018;3:263–270. doi: 10.1016/S2468-1253(18)30009-8. [DOI] [PubMed] [Google Scholar]
- 90.Shah M.A., Yoshino T., Tebbutt N.C., Grothey A., Tabernero J., Xu R.H., Cervantes A., Oh S.C., Yamaguchi K., Fakih M., et al. Napabucasin Plus FOLFIRI in Patients with Previously Treated Metastatic Colorectal Cancer: Results from the Open-Label, Randomized Phase III CanStem303C Study. Clin. Color. Cancer. 2023;22:100–110. doi: 10.1016/j.clcc.2022.11.002. [DOI] [PubMed] [Google Scholar]
- 91.Larson T., Ortuzar W.F., Bekaii-Saab T.S., Becerra C., Ciombor K.K., Hubbard J.M., Edenfield W.J., Shao S.H., Grothey A., Borodyansky L., et al. BBI608-224: A phase Ib/II study of cancer stemness inhibitor napabucasin (BBI-608) administered with panitumumab in KRAS wild-type patients with metastatic colorectal cancer. J. Clin. Oncol. 2017;35:677. doi: 10.1200/jco.2017.35.4_suppl.67. [DOI] [Google Scholar]
- 92.Kawazoe A., Kuboki Y., Shinozaki E., Hara H., Nishina T., Komatsu Y., Yuki S., Wakabayashi M., Nomura S., Sato A., et al. Multicenter Phase I/II Trial of Napabucasin and Pembrolizumab in Patients with Metastatic Colorectal Cancer (EPOC1503/SCOOP Trial) Clin. Cancer Res. 2020;26:5887–5894. doi: 10.1158/1078-0432.CCR-20-1803. [DOI] [PubMed] [Google Scholar]
- 93.Cubillo A., Garcia-Alfonso P., Miron M.L.L., Nemunaitis J., Flora D., Borg C., Mineur L., Vieitez J.M., Cohn A., Saylors G., et al. Randomized, double-blind, phase two study of ruxolitinib plus regorafenib in patients with relapsed/refractory metastatic colorectal cancer. Cancer Med. 2018;7:5382–5393. doi: 10.1002/cam4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oh D.Y., Lee S.H., Han S.W., Kim M.J., Kim T.M., Kim T.Y., Heo D.S., Yuasa M., Yanagihara Y., Bang Y.J. Phase I Study of OPB-31121, an Oral STAT3 Inhibitor, in Patients with Advanced Solid Tumors. Cancer Res. Treat. 2015;47:607–615. doi: 10.4143/crt.2014.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blanpain C., Mohrin M., Sotiropoulou P.A., Passegue E. DNA-damage response in tissue-specific and cancer stem cells. Cell Stem Cell. 2011;8:16–29. doi: 10.1016/j.stem.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 96.Ray Chaudhuri A., Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 2017;18:610–621. doi: 10.1038/nrm.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Quinonero F., Cepero A., Urbano D., Munoz-Gamez J.A., Martin-Guerrero S.M., Martin-Oliva D., Prados J., Melguizo C., Ortiz R. Identification of PARP-1 in cancer stem cells of gastrointestinal cancers: A preliminary study. J. Biosci. 2021;46:6. doi: 10.1007/s12038-020-00135-1. [DOI] [PubMed] [Google Scholar]
- 98.Manic G., Musella M., Corradi F., Sistigu A., Vitale S., Soliman Abdel Rehim S., Mattiello L., Malacaria E., Galassi C., Signore M., et al. Control of replication stress and mitosis in colorectal cancer stem cells through the interplay of PARP1, MRE11 and RAD51. Cell Death Differ. 2021;28:2060–2082. doi: 10.1038/s41418-020-00733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Groelly F.J., Fawkes M., Dagg R.A., Blackford A.N., Tarsounas M. Targeting DNA damage response pathways in cancer. Nat. Rev. Cancer. 2023;23:78–94. doi: 10.1038/s41568-022-00535-5. [DOI] [PubMed] [Google Scholar]
- 100.Knijnenburg T.A., Wang L., Zimmermann M.T., Chambwe N., Gao G.F., Cherniack A.D., Fan H., Shen H., Way G.P., Greene C.S., et al. Genomic and Molecular Landscape of DNA Damage Repair Deficiency across The Cancer Genome Atlas. Cell Rep. 2018;23:239–254.e6. doi: 10.1016/j.celrep.2018.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moretto R., Elliott A., Zhang J., Arai H., Germani M.M., Conca V., Xiu J., Stafford P., Oberley M., Abraham J., et al. Homologous Recombination Deficiency Alterations in Colorectal Cancer: Clinical, Molecular, and Prognostic Implications. J. Natl. Cancer Inst. 2022;114:271–279. doi: 10.1093/jnci/djab169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Paul S., Chatterjee S., Sinha S., Dash S.R., Pradhan R., Das B., Goutam K., Kundu C.N. Veliparib (ABT-888), a PARP inhibitor potentiates the cytotoxic activity of 5-fluorouracil by inhibiting MMR pathway through deregulation of MSH6 in colorectal cancer stem cells. Expert. Opin. Ther. Targets. 2023;27:999–1015. doi: 10.1080/14728222.2023.2266572. [DOI] [PubMed] [Google Scholar]
- 103.Genther Williams S.M., Kuznicki A.M., Andrade P., Dolinski B.M., Elbi C., O’Hagan R.C., Toniatti C. Treatment with the PARP inhibitor, niraparib, sensitizes colorectal cancer cell lines to irinotecan regardless of MSI/MSS status. Cancer Cell Int. 2015;15:14. doi: 10.1186/s12935-015-0162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cecchini M., Walther Z., Wei W., Hafez N., Pilat M.J., Boerner S.A., Durecki D.E., Eder J.P., Schalper K.A., Chen A.P., et al. NCI 7977: A Phase I Dose-Escalation Study of Intermittent Oral ABT-888 (Veliparib) plus Intravenous Irinotecan Administered in Patients with Advanced Solid Tumors. Cancer Res. Commun. 2023;3:1113–1117. doi: 10.1158/2767-9764.CRC-22-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gorbunova V., Beck J.T., Hofheinz R.D., Garcia-Alfonso P., Nechaeva M., Cubillo Gracian A., Mangel L., Elez Fernandez E., Deming D.A., Ramanathan R.K., et al. A phase 2 randomised study of veliparib plus FOLFIRI+/-bevacizumab versus placebo plus FOLFIRI+/-bevacizumab in metastatic colorectal cancer. Br. J. Cancer. 2019;120:183–189. doi: 10.1038/s41416-018-0343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pishvaian M.J., Slack R.S., Jiang W., He A.R., Hwang J.J., Hankin A., Dorsch-Vogel K., Kukadiya D., Weiner L.M., Marshall J.L., et al. A phase 2 study of the PARP inhibitor veliparib plus temozolomide in patients with heavily pretreated metastatic colorectal cancer. Cancer. 2018;124:2337–2346. doi: 10.1002/cncr.31309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen E.X., Jonker D.J., Siu L.L., McKeever K., Keller D., Wells J., Hagerman L., Seymour L. A Phase I study of olaparib and irinotecan in patients with colorectal cancer: Canadian Cancer Trials Group IND 187. Investig. New Drugs. 2016;34:450–457. doi: 10.1007/s10637-016-0351-x. [DOI] [PubMed] [Google Scholar]
- 108.Leichman L., Groshen S., O’Neil B.H., Messersmith W., Berlin J., Chan E., Leichman C.G., Cohen S.J., Cohen D., Lenz H.J., et al. Phase II Study of Olaparib (AZD-2281) after Standard Systemic Therapies for Disseminated Colorectal Cancer. Oncologist. 2016;21:172–177. doi: 10.1634/theoncologist.2015-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Takashima A., García-Alfonso P., Manneh R., Beşen A.A., Hong Y.S., Cuyle P.J., Yanez P., Burge M., Yoshino T., Kim T.W., et al. Olaparib with or without bevacizumab versus bevacizumab plus a fluoropyrimidine as maintenance therapy in advanced colorectal cancer: The randomized phase 3 LYNK-003 study. Eur. J. Cancer. 2024;205:114036. doi: 10.1016/j.ejca.2024.114036. [DOI] [PubMed] [Google Scholar]
- 110.Alese O.B., Diab M., Gbolahan O.B., McCook-Veal A., Shaib W.L., Wu C., Akce M., Liu Y., Lesinski G.B., El-Rayes B.F. Nipavect: Phase II study of niraparib and panitumumab in advanced RAS WT colorectal cancer. J. Clin. Oncol. 2023;41((Suppl. S16)):3579. doi: 10.1200/JCO.2023.41.16_suppl.3579. [DOI] [Google Scholar]
- 111.Manic G., Signore M., Sistigu A., Russo G., Corradi F., Siteni S., Musella M., Vitale S., De Angelis M.L., Pallocca M., et al. CHK1-targeted therapy to deplete DNA replication-stressed, p53-deficient, hyperdiploid colorectal cancer stem cells. Gut. 2018;67:903–917. doi: 10.1136/gutjnl-2016-312623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moore K.N., Hong D.S., Patel M.R., Pant S., Ulahannan S.V., Jones S., Meric-Bernstam F., Wang J.S., Aljumaily R., Hamilton E.P., et al. A Phase 1b Trial of Prexasertib in Combination with Standard-of-Care Agents in Advanced or Metastatic Cancer. Target. Oncol. 2021;16:569–589. doi: 10.1007/s11523-021-00835-0. [DOI] [PubMed] [Google Scholar]
- 113.Castroviejo-Bermejo M., Cruz C., Llop-Guevara A., Gutierrez-Enriquez S., Ducy M., Ibrahim Y.H., Gris-Oliver A., Pellegrino B., Bruna A., Guzmán M., et al. A RAD51 assay feasible in routine tumor samples calls PARP inhibitor response beyond BRCA mutation. EMBO Mol. Med. 2018;10:e9172. doi: 10.15252/emmm.201809172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Franklin J.M., Wu Z., Guan K.L. Insights into recent findings and clinical application of YAP and TAZ in cancer. Nat. Rev. Cancer. 2023;23:512–525. doi: 10.1038/s41568-023-00579-1. [DOI] [PubMed] [Google Scholar]
- 115.Cheung P., Xiol J., Dill M.T., Yuan W.C., Panero R., Roper J., Osorio F.G., Maglic D., Li Q., Gurung B., et al. Regenerative Reprogramming of the Intestinal Stem Cell State via Hippo Signaling Suppresses Metastatic Colorectal Cancer. Cell Stem Cell. 2020;27:590–604.e9. doi: 10.1016/j.stem.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bauzone M., Souidi M., Dessein A.F., Wisztorski M., Vincent A., Gimeno J.P., Monté D., Van Seuningen I., Gespach C., Huet G. Cross-talk between YAP and RAR-RXR Drives Expression of Stemness Genes to Promote 5-FU Resistance and Self-Renewal in Colorectal Cancer Cells. Mol. Cancer Res. 2021;19:612–622. doi: 10.1158/1541-7786.MCR-20-0462. [DOI] [PubMed] [Google Scholar]
- 117.Wang C.H., Baskaran R., Ng S.S., Wang T.F., Li C.C., Ho T.J., Hsieh D.J., Kuo C.H., Chen M.C., Huang C.Y. Platycodin D confers oxaliplatin Resistance in Colorectal Cancer by activating the LATS2/YAP1 axis of the hippo signaling pathway. J. Cancer. 2023;14:393–402. doi: 10.7150/jca.77322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li L., Li R., Wang Y. Identification of Small-molecule YAP-TEAD inhibitors by High-throughput docking for the Treatment of colorectal cancer. Bioorg. Chem. 2022;122:105707. doi: 10.1016/j.bioorg.2022.105707. [DOI] [PubMed] [Google Scholar]
- 119.Liu Y., Wang G., Yang Y. Increased TEAD4 expression and nuclear localization in colorectal cancer promote epithelial-mesenchymal transition and metastasis in a YAP-independent manner. Oncogene. 2016;35:2789–2800. doi: 10.1038/onc.2015.342. [DOI] [PubMed] [Google Scholar]
- 120.Shao D.D., Xue W., Krall E.B., Bhutkar A., Piccioni F., Wang X., Schinzel A.C., Sood S., Rosenbluh J., Kim J.W., et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014;158:171–184. doi: 10.1016/j.cell.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tolcher A.W., Lakhani N.J., McKean M., Lingaraj T., Victor L., Sanchez-Martin M., Kacena K., Malek K.S., Santillana S. A phase 1, first-in-human study of IK-930, an oral TEAD inhibitor targeting the Hippo pathway in subjects with advanced solid tumors. JCO. 2022;40:TPS3168. doi: 10.1200/JCO.2022.40.16_suppl.TPS3168. [DOI] [Google Scholar]
- 122.Arakawa H., Hayashi N., Nagase H., Ogawa M., Nakamura Y. Alternative splicing of the NF2 gene and its mutation analysis of breast and colorectal cancers. Hum. Mol. Genet. 1994;3:565–568. doi: 10.1093/hmg/3.4.565. [DOI] [PubMed] [Google Scholar]
- 123.Chen L., Milani de Marval P., Powell K., Johnson M., Falls G., Lawhorn B., Candi A., Kilonda A., Vanderhoydonck B., Marchand A., et al. SW-682: A novel TEAD inhibitor for the treatment of cancers bearing mutations in the Hippo signaling pathway; Proceedings of the American Association for Cancer Research Annual Meeting 2023; Orlando, FL, USA. 14–19 April 2023. [Google Scholar]
- 124.Oskarsson T., Batlle E., Massagué J. Metastatic stem cells: Sources, niches, and vital pathways. Cell Stem Cell. 2014;14:306–321. doi: 10.1016/j.stem.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dylla S.J., Beviglia L., Park I.K., Chartier C., Raval J., Ngan L., Pickell K., Aguilar J., Lazetic S., Smith-Berdan S., et al. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS ONE. 2008;3:e2428. doi: 10.1371/annotation/2aa6a20a-e63c-49b6-aeea-aae62435617f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hu Z., Ding J., Ma Z., Sun R., Seoane J.A., Scott Shaffer J., Suarez C.J., Berghoff A.S., Cremolini C., Falcone A., et al. Quantitative evidence for early metastatic seeding in colorectal cancer. Nat. Genet. 2019;51:1113–1122. doi: 10.1038/s41588-019-0423-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Grillet F., Bayet E., Villeronce O., Zappia L., Lagerqvist E.L., Lunke S., Charafe-Jauffret E., Pham K., Molck C., Rolland N., et al. Circulating tumour cells from patients with colorectal cancer have cancer stem cell hallmarks in ex vivo culture. Gut. 2017;66:1802–1810. doi: 10.1136/gutjnl-2016-311447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vasquez E.G., Nasreddin N., Valbuena G.N., Mulholland E.J., Belnoue-Davis H.L., Eggington H.R., Schenck R.O., Wouters V.M., Wirapati P., Gilroy K., et al. Dynamic and adaptive cancer stem cell population admixture in colorectal neoplasia. Cell Stem Cell. 2022;29:1213–1228.e8. doi: 10.1016/j.stem.2022.07.008. Erratum in Cell Stem Cell 2022, 29, 1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Frank M.H., Wilson B.J., Gold J.S., Frank N.Y. Clinical Implications of Colorectal Cancer Stem Cells in the Age of Single-Cell Omics and Targeted Therapies. Gastroenterology. 2021;160:1947–1960. doi: 10.1053/j.gastro.2020.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mai Y., Su J., Yang C., Xia C., Fu L. The strategies to cure cancer patients by eradicating cancer stem-like cells. Mol. Cancer. 2023;22:171. doi: 10.1186/s12943-023-01867-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created.