Abstract
The envelope protein encoded by the vaccinia virus A17L open reading frame is essential for virion assembly. Our mutagenesis studies indicated that cysteines 101 and 121 form an intramolecular disulfide bond and that cysteine 178 forms an intermolecular disulfide linking two A17L molecules. This arrangement of disulfide bonds has important implications for the topology of the A17L protein and supports a two-transmembrane model in which cysteines 101 and 121 are intraluminal and cysteine 178 is cytoplasmic. The structure of the A17L protein, however, was not dependent on these disulfide bonds, as a recombinant vaccinia virus with all three cysteine codons mutated to serines retained infectivity.
Crescent-shaped membranes, comprising the earliest defined vaccinia virus structures in the cytoplasm of infected cells, develop into spherical, immature virus particles and subsequently into dense, brick-shaped, infectious intracellular mature virions (IMV) (4). Some IMV are then wrapped by modified trans-Golgi membranes, transported to the periphery, and released from the cell (8, 19). Electron micrographs suggested that the crescent membranes, and their precursors which accumulate in the presence of the drug rifampin, are composed of single lipid bilayers unconnected to cellular organelles (3, 7, 9). Other studies, however, suggested that these viral structures are comprised of two closely apposed membranes that are derived from the cellular intermediate compartment connecting the endoplasmic reticulum to the Golgi network (11, 18, 21). Further biochemical, morphological, and genetic studies are needed to understand the biogenesis of IMV membranes and to resolve conflicting data. IMV contain at least 11 virus-encoded membrane proteins, of which A14L, A17L, and D13L are needed for assembly of the spicule-covered crescents (14, 17, 26, 27). Of these, the A17L protein is the best characterized. Disulfide-linked A17L protein dimers have been reported to form a complex with dimers of the A14L protein (16) and trimers of the A27L protein (15). The A17L protein is posttranslationally cleaved near the N and C termini (2, 15, 22) within AGX consensus motifs previously identified as cleavage sites for other vaccinia virus structural proteins (25). The C-terminal truncation of the A17L protein is dependent on the F10L kinase (2), which is also required for envelope formation (23, 24). The A17L (2, 5) and A14L (2) proteins are phosphorylated directly or indirectly by the F10L kinase, providing a role in morphogenesis for the latter enzyme.
The topology of the A17L protein determines the intracellular sites of protein interactions, cleavage, phosphorylation, and disulfide bond formation. Krijnse-Locker et al. (11) demonstrated that the N and C termini of the A17L protein are cytoplasmically oriented, consistent with an even number of membrane-spanning segments. Although the A17L protein has four hydrophobic domains, recent topological studies of Betakova et al. (1) supported a two-transmembrane model. In the present study, we mutagenized the three cysteines of the A17L protein and found evidence for a previously unrecognized intramolecular disulfide bond between cysteines 101 and 121 which is compatible with a two transmembrane model (1) but not a four transmembrane model (11). The formation of disulfide-bonded A17L dimers was dependent on cysteine 178, which is in the C-terminal cytoplasmic domain of the A17L protein. Nevertheless, the structure of the A17L protein was not dependent on these disulfide bonds, since a recombinant virus in which all three cysteine codons were mutated to serines retained infectivity.
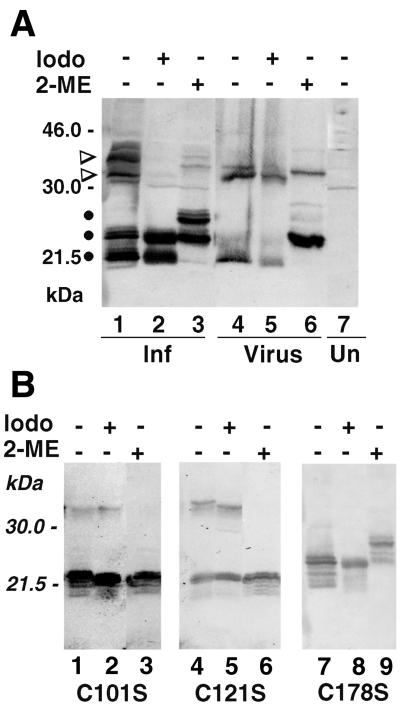
Evidence for intra- and intermolecular disulfide bonds.
Purified virions were treated with sodium dodecyl sulfate (SDS) and 2-mercaptoethanol (2-ME) and analyzed by SDS–12.5% polyacrylamide gel electrophoresis (PAGE) and Western blotting with an antibody to the mature N terminus of the A17L protein (2). As previously shown (2), the predominant band was a C-terminally truncated monomer of about 23 kDa (Fig. 1A, lane 6). In addition, a minor dimer band was detected, in agreement with observations of Krijnse-Locker and Griffiths (12), suggesting difficulty in completely reducing the intermolecular disulfide bond. When the reducing agent was omitted, there was relatively more dimer and the major dimer and monomer bands migrated more rapidly than the reduced forms, of which only trace amounts could be detected (Fig. 1A, lane 4), suggesting a more compact structure due to an intramolecular disulfide bond. Iodoacetamide or N-ethylmaleimide (NEM) is frequently used to alkylate free sulfhydryl groups and prevent artifactual postlysis disulfide bond formation. Addition of 50 mM iodoacetamide prior to virus disruption had no major effect on the relative amounts of the unreduced monomeric and dimeric forms of the A17L protein from IMV membranes (Fig. 1A, lane 5). In other experiments, cells were lysed in the presence of 50 mM iodoacetamide and 20 mM NEM and the alkylating agents were maintained during virus purification through a sucrose cushion. Under these conditions, the dimer and monomer bands were also similar in intensity (data not shown). Although the effect was barely evident, iodoacetamide caused the monomer species to migrate slightly faster than the form that received neither iodoacetamide nor 2-ME (Fig. 1A, compare lanes 5 and 4), presumably due to alkylation of the same cysteine that can form an intermolecular disulfide bond.
FIG. 1.
Western blots of unreduced and reduced forms of the A17L protein. (A) BSC-1 cells in a six-well plate were infected with 10 PFU of vaccinia virus strain WR per cell. After 18 h, the cells were harvested in the absence or presence of 50 mM iodoacetamide. Washed cell pellets were resuspended in 30 μl of extraction buffer (1% NP-40, 150 mM NaCl, 1 mM EDTA, 20 mM Tris-HCl [pH 7.5], 1 mM phenylmethylsulfonyl fluoride) with or without 50 mM iodoacetamide. The samples were incubated for 10 min at room temperature and clarified by centrifugation, and 15 μl of the supernatant was treated with SDS with or without 2-ME and analyzed by SDS-PAGE and Western blotting with A17L-specific antibody. Sucrose gradient-purified vaccinia virions were resuspended in extraction buffer with or without iodoacetamide and analyzed as described above. Abbreviations: Iodo, iodoacetamide; Inf, lysate of infected cells; Un, lysate of uninfected cells; Virus, purified virions. The positions of marker proteins and their masses in kilodaltons are indicated on the left. A17L monomers and dimers are indicated by dots and wedges, respectively. (B) BSC-1 cells in six-well plates were transfected with 2 μg of plasmid in DOTAP (N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate; Boehringer Mannheim) and infected 4 h later with vA17LΔ5 in the absence of IPTG. After 48 h, the cells were harvested and the A17L protein was analyzed as for panel A. C101S, C121S, and C178S refer to plasmids containing codon 101, 121, or 178 mutated from cysteine to serine. Other abbreviations are as in panel A.
Lysates of vaccinia virus-infected BSC-1 cells were analyzed in a manner similar to that used for purified virions. The polypeptide pattern, however, was more complex due to mixtures of N- and C-terminally cleaved and uncleaved forms. Under favorable electrophoretic conditions, the C-terminally truncated form of the A17L monomer was resolved from its uncleaved precursor (2), as shown in Fig. 1A, lane 3. The incompletely reduced disulfide-bonded dimer was also resolved as faint C-terminally truncated and precursor species (Fig. 1A, lane 3). Without 2-ME, the dimer bands were more prominent and both the dimer and monomer bands migrated more rapidly than the reduced forms, consistent with the presence of an intramolecular disulfide bond (Fig. 1A, lane 1). Iodoacetamide had only a slight effect on the mobility of the unreduced monomers (Fig. 1A, lane 2), suggesting that the intramolecular disulfide bond had been formed before cell lysis. In contrast, there was much less of the dimeric forms of A17L in the presence of iodoacetamide (Fig. 1A, lane 2) than in its absence (Fig. 1A, lane 1), consistent with postlysis dimer formation. In some experiments, however, iodoacetamide had little effect on the amount of dimer, corresponding to data obtained by Krijnse-Locker and Griffiths (12) using NEM as the alkylating agent. One explanation is that some of the A17L protein exists in a noncovalently linked dimeric form with closely apposed cysteines so that disulfide bond formation can compete with alkylation.
Effects of cysteine-to-serine mutations on disulfide bond formation.
The A17L open reading frame (ORF) contains cysteines at positions 101, 121, and 178. To determine which ones are involved in disulfide bond formation, we needed a way of expressing mutated proteins during a virus infection. The A17L ORF with its late promoter was inserted into a plasmid, and the cysteines were individually mutated to serines using the QuickChange Site-Directed Mutagenesis Kit (Stratagene) and appropriate primers. For expression, the plasmids were transfected into cells that were subsequently infected with a conditionally lethal vaccinia virus mutant, vA17LΔ5, with a stringently repressed A17L gene (26). In the absence of isopropyl-β-d-thiogalactopyranoside (IPTG), the only detectable A17L protein was derived from the transfected plasmids but all other viral proteins were expressed from the viral genome. The 2-ME-induced decrease in mobility of the monomeric species detected with the wild-type A17L protein (Fig. 1A) was not observed when either cysteine 101 or 121 was changed to serine, although a dimeric species was still formed (Fig. 1B, lanes 1 to 6). However, no dimeric form was detected when cysteine 178 was changed to serine but the 2-ME-induced shift of the monomeric species occurred (Fig. 1B, lanes 7 to 9). These data suggested that the intramolecular disulfide bond of the wild-type A17L protein forms between cysteines 101 and 121 and that cysteine 178 is required for intermolecular disulfide bond formation.
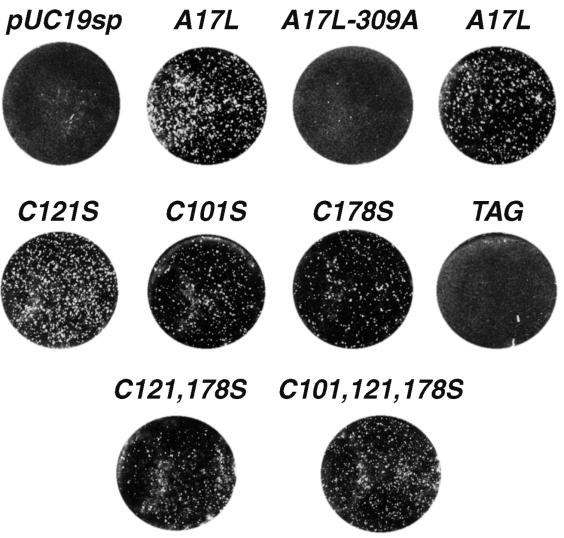
Complementation of plaque formation by transfected A17L genes.
We used the transfection-infection protocol described above to determine whether the mutated A17L genes are functional for virus assembly and infectivity. Repression of A17L gene expression results in a severe reduction of vA17LΔ5 plaque size and number (26). Plaque formation was enhanced in the absence of IPTG, however, by transfection of a plasmid containing the A17L gene regulated by its own late promoter (Fig. 2A, A17L). Remarkably, plaque formation was also enhanced by transfection of the A17L gene with mutations of cysteine 101 (Fig. 2A, C101S), 121 (Fig. 2A, C121S), or 178 (Fig. 2A, C178S). Furthermore, the A17L gene with mutations of two cysteines (Fig. 2A, C121, 178S) or three cysteines (Fig. 2A, C101, 121, 178S) also rescued plaques. By contrast, enhanced plaque formation did not occur when a stop codon was formed by introducing an A residue at position 309 to give a C-terminal truncation (Fig. 2A, A17L-309A) or when eight amino acids between the second and third hydrophobic domains of A17L were replaced with a similar-length influenza virus hemagglutinin epitope tag (1) (Fig. 2A, TAG). These results indicated that neither the intramolecular nor the intermolecular disulfide bonds of the A17L protein are needed for virus assembly and spread, whereas certain other mutations produce nonfunctional A17L proteins.
FIG. 2.
Complementation with mutated A17L proteins. BSC-1 cells were transfected with a plasmid and infected with vA17LΔ5 in the absence of IPTG as described in the legend to Fig. 1B. After 48 h, the plates were stained with crystal violet. Plasmid abbreviations: pUC19sp, vector without the A17L ORF; A17L, unmutated A17L ORF; A17L-309A, stop codon at nucleotide 309; C101S, codon 101 changed from cysteine to serine; C121S, codon 121 changed from cysteine to serine; C178S, codon 178 changed from cysteine to serine; C121, 178S, codons 121 and 178 changed from cysteines to serines; C101, 121, 178S, all three cysteine codons changed to serines; TAG, equivalent number of codons between the second and third hydrophobic domains of the A17L ORF replaced with a hemagglutinin tag sequence. Two wells were transfected with a plasmid containing the unmutated A17L ORF.
Isolation and characterization of mutant vaccinia viruses with cysteine-to-serine mutations.
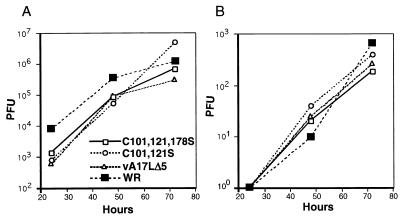
Following the protocol in the previous section, BSC-1 cells were transfected with the plasmid containing all three cysteine mutations and infected with vA17LΔ5. Viruses in plaques that formed in the absence of IPTG were subjected to repeated rounds of plaque purification, and 30 were amplified and screened by SDS-PAGE under reducing conditions and Western blotting. The majority of isolated viruses made the wild-type A17L protein. Three viruses that made A17L proteins with or without IPTG that migrated faster than the wild type in the presence of 2-ME were further characterized. One of these formed disulfide-linked dimers, and two did not. PCR analysis indicated that each virus had a single copy of the A17L gene that had replaced the inducible copy, and sequencing indicated that one (vA17LC101,121S) had serine substitutions of the first two cysteines but retained the cysteine at position 178, whereas in the other two (vA17LC101,121,178S), which did not form A17L protein dimers, serines replaced all three cysteines. The yields of the A17L cysteine mutants in both the cell lysates and medium were similar to those of WR and vA17LΔ5 at 37.5°C in the presence of IPTG (Fig. 3). Thus, the three cysteines of the A17L ORF were not required for replication of vaccinia virus.
FIG. 3.
Virus yields. BSC-1 cells were infected with 1 PFU of vaccinia virus WR, vA17LΔ5, vA17LC101,121S, or vA17LC101,121,178S per cell. Infection with vA17LΔ5 was in the presence of IPTG. At 24, 48, and 72 h after infection, the medium was removed and clarified and the cells were harvested. C101, 121S and C101, 121, 178S are abbreviations of vA17LC101,121S and vA17LC101,121,178S, respectively. Virus titers of the cell lysates (A) and medium (B) were determined by plaque assay.
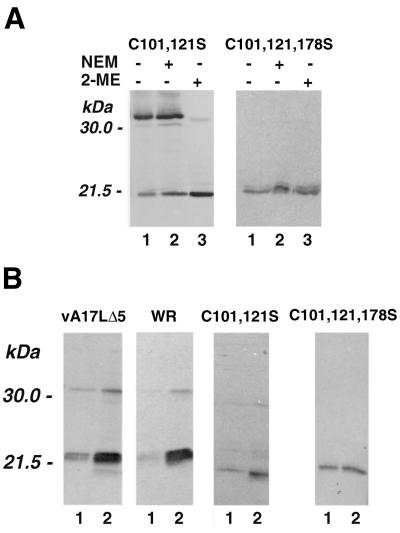
When the A17L protein of purified vA17LC101,121S virions was examined, the disulfide-linked dimer was present but the 2-ME-induced mobility change of the monomer was not observed (Fig. 4A), consistent with the roles of cysteines 101 and 121 in intramolecular disulfide bond formation. As predicted, the A17L protein of purified vA17LC101,121,178S virions lacked both inter- and intramolecular disulfide bonds (Fig. 4A). The absence of the intermolecular disulfide bond correlated with the ability to efficiently extract the A17L protein from purified vA17LC101,121,178S virions in the absence of a reducing agent (Fig. 4B). In contrast, the extraction of the A17L protein from vA17LΔ5 (made in the presence of IPTG), WR, or vA17LC101,121S virions was enhanced by a reducing agent (Fig. 4B). The extraction of other membrane proteins in the absence of a reducing agent, detected by polyclonal antiserum to vaccinia virus, was not enhanced by the mutations of the A17L protein (data not shown), suggesting that the general integrity of the membrane had not been radically altered. In addition, preincubation of purified viruses at elevated temperatures prior to plaque formation did not reveal enhanced thermal sensitivity of the mutants (data not shown).
FIG. 4.
Western blot analysis of A17L proteins of purified vA17LC101,121S and vA17LC101,121,178S. Infected BSC-1 cells were harvested after 48 h, and virions were purified by sedimentation through a sucrose cushion and CsCl gradient centrifugation. (A) Proteins were extracted in the presence or absence of NEM and treated with SDS in the presence or absence of 2-ME. (B) Purified virions were extracted with 1% Triton X-114 in phosphate-buffered saline for 10 min at 32°C without (lanes 1) or with (lanes 2) 100 mM dithiothreitol. The supernatants were recovered after centrifugation in a microcentrifuge. Dithiothreitol (100 mM) was added to the extracts without a reducing agent, and the samples were denatured by boiling in 2% SDS and analyzed by Western blotting.
Conclusions.
The present study confirmed previous reports regarding the existence of disulfide-bonded dimers of the A17L protein, provided evidence for an intramolecular disulfide bridge, and demonstrated that cysteine 178 is required for the former and cysteines 101 and 121 are required for the latter. Disulfide bonds usually form within the endoplasmic reticulum of mammalian cells or the periplasm of prokaryotic cells, which provide a favorable redox potential, as well as molecular chaperones that assist in folding (6, 10, 13). It seems likely, therefore, that the disulfide bonds of the A17L protein would form in the endoplasmic reticulum. In this regard, a recent topological analysis of the A17L protein provided evidence for two membrane-spanning domains and placed cysteines 101 and 121 in an intraluminal location (1), consistent with stoichiometric disulfide bond formation. In contrast, a suggested model with four membrane-spanning domains (11) would place cysteines 101 and 121 in different compartments and is incompatible with the data presented here. Cysteine 178 is located in the cytoplasmic tail of the A17L protein, accounting for the variable amounts of disulfide-bonded molecules reported previously (12). It seems likely, however, that cysteine 178 residues of noncovalently linked A17L dimers are closely apposed, allowing some cytoplasmic disulfide bond formation and further disulfide bond formation when virions are released by cell lysis.
The A17L protein is required for assembly of vaccinia virions and is conserved in other vertebrate poxviruses. Of the three cysteines, the two that form the intramolecular disulfide bond are more highly conserved, as they are present in the orthologous protein of molluscum contagiosum virus, a distantly related member of the poxvirus family (20). Based on the conservation of the two cysteines, we anticipated that they might be required for proper folding or function of the A17L protein. To test this hypothesis, a recombinant vaccinia virus with all three cysteines of the A17L protein mutated to serines was constructed. The infectivity of this mutant in cell culture established that the disulfide bonds of the A17L protein are not needed for virion assembly or spread. Although the disulfide bonds help to retain the A17L protein in the viral membrane, as shown by the need for reducing agents to extract the wild type but not the mutated polypeptide, the mutant virions were not noticeably less heat stable than wild-type virions. Our inability to demonstrate an appreciable in vitro effect of mutating cysteines, however, does not preclude advantages of disulfide bond formation with regard to the assembly, structure, or stability of vaccinia virions in vivo.
Acknowledgments
We thank Elizabeth J. Wolffe and Douglas M. Moore for unpublished information first suggesting an intramolecular disulfide bond in the A17L protein and Andrea S. Weisberg for help preparing the figures.
REFERENCES
- 1.Betakova T, Wolffe E J, Moss B. Membrane topology of the vaccinia virus A17L envelope protein. Virology. 1999;261:347–356. doi: 10.1006/viro.1999.9870. [DOI] [PubMed] [Google Scholar]
- 2.Betakova T, Wolffe E J, Moss B. Regulation of vaccinia virus morphogenesis: phosphorylation of the A14L and A17L membrane proteins and C-terminal truncation of the A17L protein are dependent on the F10L protein kinase. J Virol. 1999;73:3534–3543. doi: 10.1128/jvi.73.5.3534-3543.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dales S, Mosbach E H. Vaccinia as a model for membrane biogenesis. Virology. 1968;35:564–583. doi: 10.1016/0042-6822(68)90286-9. [DOI] [PubMed] [Google Scholar]
- 4.Dales S, Siminovitch L. The development of vaccinia virus in Earle's L strain cells as examined by electron microscopy. J Biophys Biochem Cytol. 1961;10:475–503. doi: 10.1083/jcb.10.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Derrien M, Punjabi A, Khanna R, Grubisha O, Traktman P. Tyrosine phosphorylation of A17 during vaccinia virus infection: involvement of the H1 phosphatase and the F10 kinase. J Virol. 1999;73:7287–7296. doi: 10.1128/jvi.73.9.7287-7296.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freedman R B. The formation of protein disulphide bonds. Curr Opin Struct Biol. 1995;5:85–91. doi: 10.1016/0959-440x(95)80013-q. [DOI] [PubMed] [Google Scholar]
- 7.Grimley P M, Rosenblum E N, Mims S J, Moss B. Interruption by rifampin of an early stage in vaccinia virus morphogenesis: accumulation of membranes which are precursors of virus envelopes. J Virol. 1970;6:519–533. doi: 10.1128/jvi.6.4.519-533.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiller G, Weber K. Golgi-derived membranes that contain an acylated viral polypeptide are used for vaccinia virus envelopment. J Virol. 1985;55:651–659. doi: 10.1128/jvi.55.3.651-659.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollinshead M, Vanderplasschen A, Smith G L, Vaux D J. Vaccinia virus intracellular mature virions contain only one lipid membrane. J Virol. 1999;73:1503–1517. doi: 10.1128/jvi.73.2.1503-1517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang C, Sinskey A J, Lodish H F. Oxidized redox state of glutathionine in the endoplasmic reticulum. Science. 1992;257:1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- 11.Krijnse-Locker J, Schleich S, Rodriguez D, Goud B, Snijder E J, Griffiths G. The role of a 21-kDa viral membrane protein in the assembly of vaccinia virus from the intermediate compartment. J Biol Chem. 1996;271:14950–14958. doi: 10.1074/jbc.271.25.14950. [DOI] [PubMed] [Google Scholar]
- 12.Krijnse Locker J, Griffiths G. An unconventional role for cytoplasmic disulfide bonds in vaccinia virus proteins. J Cell Biol. 1999;144:267–279. doi: 10.1083/jcb.144.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rietsch A, Beckwith J. The genetics of disulfide bond metabolism. Annu Rev Genet. 1998;32:163–184. doi: 10.1146/annurev.genet.32.1.163. [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez D, Esteban M, Rodríguez J R. Vaccinia virus A17L gene product is essential for an early step in virion morphogenesis. J Virol. 1995;69:4640–4648. doi: 10.1128/jvi.69.8.4640-4648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez D, Rodriguez J-R, Esteban M. The vaccinia virus 14-kilodalton fusion protein forms a stable complex with the processed protein encoded by the vaccinia virus A17L gene. J Virol. 1993;67:3435–3440. doi: 10.1128/jvi.67.6.3435-3440.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodríguez J R, Risco C, Carrascosa J L, Esteban M, Rodríguez D. Characterization of early stages in vaccinia virus membrane biogenesis: implications of the 21-kilodalton protein and a newly identified 15-kilodalton envelope protein. J Virol. 1997;71:1821–1833. doi: 10.1128/jvi.71.3.1821-1833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodríguez J R, Risco C, Carrascosa J L, Esteban M, Rodríguez D. Vaccinia virus 15-kilodalton (A14L) protein is essential for assembly and attachment of viral crescents to virosomes. J Virol. 1998;72:1287–1296. doi: 10.1128/jvi.72.2.1287-1296.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salmons T, Kuhn A, Wylie F, Schleich S, Rodriguez J R, Rodriguez D, Esteban M, Griffiths G, Krijnse Locker J. Vaccinia virus membrane proteins p8 and p16 are cotranslationally inserted into the rough endoplasmic reticulum and retained in the intermediate compartment. J Virol. 1997;71:7404–7420. doi: 10.1128/jvi.71.10.7404-7420.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmelz M, Sodeik B, Ericsson M, Wolffe E J, Shida H, Hiller G, Griffiths G. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans Golgi network. J Virol. 1994;68:130–147. doi: 10.1128/jvi.68.1.130-147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Senkevich T G, Koonin E V, Bugert J J, Darai G, Moss B. The genome of molluscum contagiosum virus: analysis and comparison with other poxviruses. Virology. 1997;233:19–42. doi: 10.1006/viro.1997.8607. [DOI] [PubMed] [Google Scholar]
- 21.Sodeik B, Doms R W, Ericsson M, Hiller G, Machamer C E, van't Hof W, van Meer G, Moss B, Griffiths G. Assembly of vaccinia virus: role of the intermediate compartment between the endoplasmic reticulum and the Golgi stacks. J Cell Biol. 1993;121:521–541. doi: 10.1083/jcb.121.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi T, Oie M, Ichihashi Y. N-terminal amino acid sequences of vaccinia virus structural proteins. Virology. 1994;202:844–852. doi: 10.1006/viro.1994.1406. [DOI] [PubMed] [Google Scholar]
- 23.Traktman P, Caligiuri A, Jesty S A, Sankar U. Temperature-sensitive mutants with lesions in the vaccinia virus F10 kinase undergo arrest at the earliest stage of morphogenesis. J Virol. 1995;69:6581–6587. doi: 10.1128/jvi.69.10.6581-6587.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S, Shuman S. Vaccinia virus morphogenesis is blocked by temperature-sensitive mutations in the F10 gene, which encodes protein kinase 2. J Virol. 1995;69:6376–6388. doi: 10.1128/jvi.69.10.6376-6388.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitehead W S, Hruby D E. Differential utilization of a conserved motif for the proteolytic maturation of vaccinia virus proteins. Virology. 1994;200:154–161. doi: 10.1006/viro.1994.1174. [DOI] [PubMed] [Google Scholar]
- 26.Wolffe E J, Moore D M, Peters P J, Moss B. Vaccinia virus A17L open reading frame encodes an essential component of nascent viral membranes that is required to initiate morphogenesis. J Virol. 1996;70:2797–2808. doi: 10.1128/jvi.70.5.2797-2808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Moss B. Immature viral envelope formation is interrupted at the same stage by lac operator-mediated repression of the vaccinia virus D13L gene and by the drug rifampicin. Virology. 1992;187:643–653. doi: 10.1016/0042-6822(92)90467-4. [DOI] [PubMed] [Google Scholar]