Abstract
We investigated the effect of anti-macrophage inflammatory protein 2 immunoglobulin G (aMIP-2 IgG) on the progression of influenza virus-induced pneumonia in mice. When mice were infected with a mouse lung-adapted strain of influenza A/PR/8/34 virus by intranasal inoculation, neutrophil counts in the bronchoalveolar lavage fluid (BALF) increased in parallel with the kinetics of MIP-2 production, which peaked 2 days after infection. After intracutaneous injection of a dose of 10 or 100 μg of aMIP-2 IgG once a day on days 0 and 1, neutrophil counts in BALF on day 2 were reduced to 49 or 37%, respectively, of the value in the control infected mice administered anti-protein A IgG. The antibody administration also improved lung pathology without affecting virus replication. Furthermore, by prolonged administration with a higher or lower dose for up to 5 days, body weight loss became slower and finally 40% of mice in both treatment groups survived potentially lethal pneumonia. These findings suggest that MIP-2-mediated neutrophil infiltration during the early phase of infection might play an important role in lung pathology. Thus, MIP-2 was considered to be a novel target for intervention therapy in potentially lethal influenza virus pneumonia in mice.
In influenza virus infection in mice via the intranasal route, a typical pathological feature is the presence of areas of lung surface consolidation, which is one kind of lung injury accompanied by extensive inflammatory infiltration and hemorrhage (20). It has been suggested that hyperreaction of the host defense system is involved in the pathogenesis of consolidation and that morbidity and mortality are immunopathological consequences (12, 22). Toms et al. (26) reported that the inflammatory response in the upper respiratory tract after intranasal infection of ferrets with influenza A virus consisted of 90% neutrophils 1 day after infection. Thus, neutrophil infiltration during the early phase of infection is considered to be a characteristic feature of influenza virus infection (23). Several studies (5, 18) revealed that influenza virus infection has the potential to induce the production of chemokines, many of which have been shown to possess chemotactic activity for inflammatory and immune effector cells and which may contribute to the pathogenesis of inflammatory diseases (7, 11, 13). Since the initial discovery of interleukin-8, a chemokine prototype (29, 30), this cytokine is now classified into two groups, α-chemokines (CXC family) and β-chemokines (CC family) by a few structural and functional dissimilarities; α-Chemokines especially show chemotactic activity for neutrophils (8). We now know that chemokines and their receptors are expressed by a wide variety of cells under positive or negative regulation of certain cytokines, whose expression is also regulated by chemokines in specific cells, and chemokine function extends far beyond chemotactic activity to various processes such as lymphocyte recruitment, angiogenesis, human immunodeficiency virus replication, and anti-tumor activity (for reviews, see references 2 and 21).
We have previously reported (10) that influenza virus infection could induce the production of macrophage inflammatory protein 2 (MIP-2), a mouse counterpart of α-chemokines (27), in a mouse infection model in vitro and in vivo. In addition to killing the invading microbes, neutrophils can also cause tissue injuries such as lung damage in adult respiratory distress syndrome and other inflammatory diseases by producing superoxides or certain enzymes (3, 25). Although Cook et al. (6) demonstrated that MIP-1α, a member of β-chemokines, is an important mediator of inflammatory responses to certain viral infections such as coxsackievirus-induced myocarditis, the pathological role of MIP-2 in vivo has not yet been studied. In light of these facts, we studied the effect of anti-MIP-2 immunoglobulin G (aMIP-2 IgG) on the progression of lethal influenza virus pneumonia in mice.
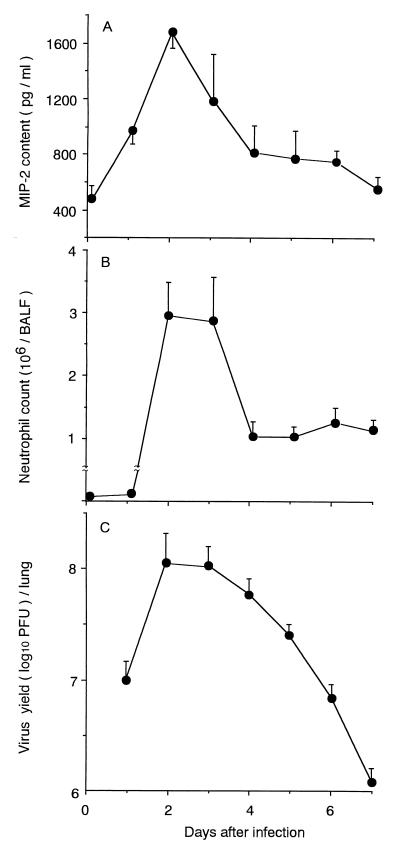
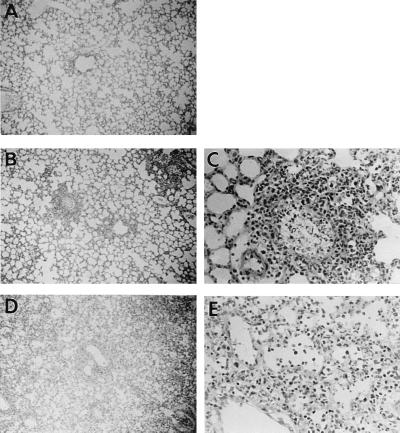
In this study, an outbred specific-pathogen-free strain of ICR female mice 4 weeks old (body weight, approximately 17 g) obtained from SLC Co. Ltd. (Hamamatsu, Japan) was used for infection by intranasal inoculation of a virus solution containing 4,000 PFU/25 μl (four 50% lethal doses of virus) of a mouse lung-adapted strain of influenza A/PR/8/34 (PR8) virus (H1N1 subtype). We initially examined the kinetics of the MIP-2 concentration and virus yields in lung homogenates and counted the neutrophils in bronchoalveolar lavage fluid (BALF). The MIP-2 concentration was assayed by antibody sandwich enzyme-linked immunosorbent assay in which rabbit unlabeled and biotinylated aMIP-2 IgG antibodies were used as the capture and secondary antibodies, respectively, followed by the addition of peroxidase-coupled streptavidin and substrate for color development, as described previously (19). For standardization of MIP-2 concentration, MIP-2 was purified from the conditioned medium of lipopolysaccharide-stimulated RAW264.7 cells (LPS-CM) by aMIP-2 IgG-coupled Sepharose column (19). To obtain hyperimmune aMIP-2 IgG, a fusion construct of MIP-2 to protein A was used as an antigen to enable the generation of a sufficiently large antibody response because of the low molecular weight of MIP-2 itself (19). Western blot analysis of lyophilized LPS-CM confirmed that this antibody gave a single band with a molecular weight of 6,000, which was identical to that of purified MIP-2 but different from those of any influenza virus-coded proteins (15). The number of neutrophils in BALF was calculated by the formula T × R/100, where T is the total number of cells in BALF determined in a hemocytometer and R is the rate (%) of neutrophils in Giemsa-staining BALF cells, as described previously (16). Virus yield was determined by a plaque method (17). As shown in Fig. 1, a low level of MIP-2 (500 pg/ml) was detected immediately after infection (on day 0) as the background level. However, the MIP-2 level significantly increased to a peak on day 2 and then sharply decreased, although slightly elevated levels were maintained until day 5. On day 0, only a low level of macrophages was detected in BALF (<103 cells/BALF). In correlation with the increase in MIP-2 concentration, neutrophil counts increased sharply 2 days postinfection (p.i.) and remained almost the same on the next day. In such an early phase of infection, neutrophils comprised 70 to 90% of total cells in BALF and the remaining cells were mostly macrophages. In the next phase of infection, the lymphocyte population gradually increased from 20 to 50% over time. In contrast to the kinetics of MIP-2, neutrophil counts remained at the elevated levels, suggesting that additional chemotactic factors such as leukotriene B4 might contribute to this phenomenon as if compensating for the low level of MIP-2 as reported previously (9). As for virus replication in the lung, the virus titer demonstrated 7 log10 PFU on day 1, reaching the maximal level (almost 8 log10 PFU) on day 2 and then gradually decreasing thereafter. Thus, the kinetics of the MIP-2 concentration and neutrophil count could be considered similar to those of virus replication. As shown in Fig. 2, histopathological studies of the infected lung obtained on day 2 indicated marked neutrophil infiltration into the intra- and peribronchial spaces. On day 6 when mice began to die, lung pathology became more severe, accompanied by massive lymphocyte infiltration and hemorrhage.
FIG. 1.
Time-related changes in MIP-2 content, neutrophil count, and virus yield in the lung after intranasal infection of mice with PR8 virus. MIP-2 content (A) and virus yield (C) in lung tissue homogenate and neutrophil count in BALF (B) were monitored immediately after infection (day 0) to 7 days after infection. Each point shows the mean ± standard deviation (SD) for five mice (SDs indicated by the error bars).
FIG. 2.
Histopathological study of lungs from mice infected with PR8 virus. Lungs were obtained from uninfected mice (A) and from infected mice on day 2 (B and C) or day 6 after infection (D and E) and then processed for histopathological staining. Magnifications, ×87 for panels A, B, and D and ×348 for panels C and E.
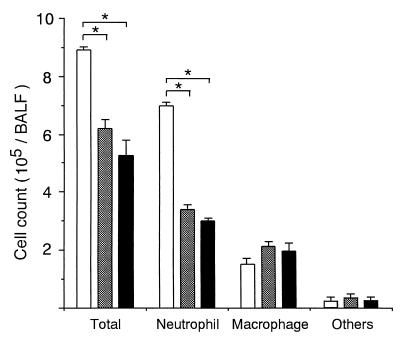
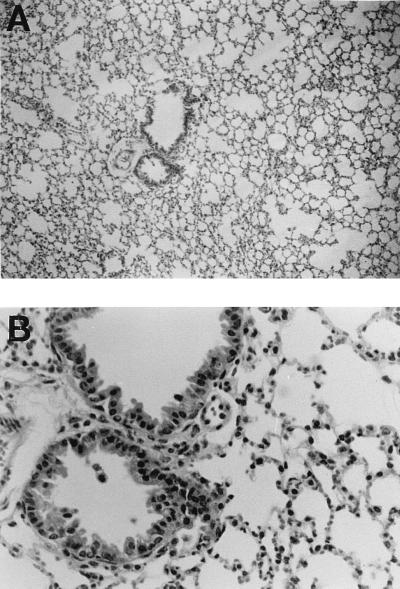
Based on the kinetic studies described above, the cell composition and virus yield in BALF were compared for the aMIP-2 IgG-treated and control groups on day 2. Because MIP-2–protein A fusion protein was used as an antigen to prepare aMIP-2 IgG, rabbit anti-protein A IgG (Sigma, St. Louis, Mo.) was used for the control group. Ten to 15 mice were used for each experimental group. When infected mice were treated twice with intracutaneous injections on days 0 and 1 at a dose of 10 or 100 μg of aMIP-2 IgG or 100 μg of anti-protein A IgG/day/mouse, neutrophil counts in treated groups decreased significantly in a dose-dependent manner to 49 and 37% of the control group at doses of 10 and 100 μg/day, respectively (Fig. 3). There were no significant differences in macrophage and other cell counts, including lymphocytes and epitheliar cells, among the groups. Consequently, total cell counts in the treatment groups decreased to 70 and 58% of that of the control group at doses of 10 and 100 μg/day, respectively. These findings were verified histopathologically, as shown in Fig. 4. Compared with the lungs of control mice on day 2 shown in Fig. 2, the lungs of treated mice (100 μg/day) on the same day showed less neutrophil infiltration around the bronchia and intrabronchial spaces. The administration of aMIP-2 IgG did not affect virus yields (Table 1). These findings suggest that administration of aMIP-2 IgG specifically reduces neutrophil exudation into BALF during the early phase of infection without affecting virus replication. Previous reports have demonstrated that interleukin-8, which belongs to the same chemokine family as MIP-2, enhances the cytopathic effect and virus replication in infections with positive-strand RNA viruses such as encephalomyocarditis virus, but not in infections with negative-strand RNA viruses such as vesicular stomatitis virus (14). Conversely, MIP-1, which belongs to another chemokine family, enhances the growth of negative-strand RNA viruses such as influenza virus but not that of positive-strand RNA viruses such as coxsackievirus (6). Thus, our data were considered consistent with these reports.
FIG. 3.
Effect of anti-MIP-2 IgG administration on the total cell count and cell composition in BALF obtained from mice infected with PR8 virus. Infected mice received intracutaneous injections a total of two times (on days 0 and 1) with either 100 μg of anti-protein A IgG (white bar), 10 μg of anti-MIP-2 IgG (checkered bar), or 100 μg of anti-MIP-2 IgG (black bar) per day per mouse. On day 2 after infection, the total cell counts and cell compositions of BALF were examined. Each point shows the mean ± SD for five mice (SDs indicated by the error bars). An asterisk indicates that the value was significantly different from the control value (P < 0.01 by unpaired t test).
FIG. 4.
Effect of anti-MIP-2 IgG administration on lung histopathology. Infected mice received intracutaneous injections a total of two times (on days 0 and 1) with 100 μg of anti-MIP-2 IgG/day/mouse. On the second day after infection, the lung was collected and processed for histopathological staining. Magnifications, ×88 for panel A and ×352 for panel B.
TABLE 1.
Effect of anti-MIP-2 IgG administration on virus growth in the lungs of mice infected with a mouse lung-adapted PR8 virus
| Treatment group (dose [μg/ml]) | Virus yielda in lung by the following day after infection:
|
||||
|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 7 | |
| Anti-protein A (100) | 8.04 ± 0.25 | 8.00 ± 0.04 | 7.86 ± 0.10 | 7.44 ± 0.08 | 6.09 ± 0.09 |
| Anti-MIP-2 (10) | 8.00 ± 0.23 | 7.95 ± 0.43 | 7.73 ± 0.38 | 7.50 ± 0.13 | 6.35 ± 0.35 |
| Anti-MIP-2 (100) | 7.85 ± 0.35 | 7.72 ± 0.33 | 7.62 ± 0.11 | 7.42 ± 0.16 | 6.88 ± 0.13 |
Virus yield is expressed as mean ± SD (log10 PFU/lung) for the five mice in each treatment group.
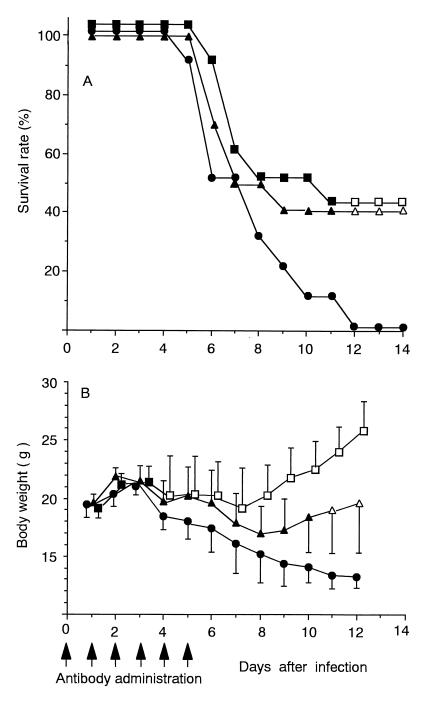
We further studied whether administration of aMIP-2 IgG improved the survival rate and the body weight loss, which is a sensitive indicator of the progression of viral pneumonia in mice (24). In this case, duration of antibody administration was prolonged to 5 days p.i. based on the kinetics of the MIP-2 concentration (Fig. 1). As shown in Fig. 5A, control mice began to die on day 5. Thereafter, mortality increased, and all control mice had died by day 12. In contrast, mice in the treated group receiving high (100-μg/day) or low (10-μg/day) doses began to die on day 6, 1 day later than the control group, and 40% of mice in both treatment groups continued to survive until day 14. Survival rates of both treatment groups were significantly higher than that of control group on day 12 and thereafter. However, there was a tendency toward a slower decrease in survival rates in the high-dose group compared to that in the low-dose group between 6 and 10 days p.i. When a dose of 300 μg/day was used, the survival rates were similar to that of 100 μg/day (data not shown). The beneficial effect of aMIP-2 IgG administration was also shown in body weight loss with a clearer dose dependency than that shown by survival rates. In the control group, body weight successively decreased from 4 days p.i. By administering a higher dose, body weight loss ceased between 4 and 6 days p.i. and recovered somewhat in the subsequent period, demonstrating significantly higher values than those of control group after as early as 4 days p.i., whereas recovery was smaller in the low-dose group and significant differences were obtained only after 10 days p.i. in that group.
FIG. 5.
Effect of anti-MIP-2 IgG administration on the survival rate and body weight loss. Infected mice received a total of six intracutaneous injections once daily for six days (indicated by the six arrows below the abscissa) with either 100 μg of anti-protein A IgG (closed circles) (control), 10 μg of anti-MIP-2 IgG (closed triangles), or 100 μg of anti-MIP-2 IgG (closed squares) per day per mouse, and the survival rate (A) and body weight (B) of the mice were monitored. Each point shows the mean ± SD for 10 to 15 mice in each experimental group (SDs indicated by the error bars). An open symbol indicates that the value was significantly different from the control value, with P values of 0.04 (squares) and 0.01 (triangles). The data in panel A were assessed by Fisher's exact probability test, and the data in panel B were assessed by unpaired t test.
It has been demonstrated that both nitric oxide (NO) and active oxygen radicals are involved in the pathogenesis of influenza virus-induced pneumonia in mice (1, 20). It is well-known that these tissue-toxic molecules are produced by neutrophils, especially chemokine-attracted neutrophils (3, 8, 16, 25). Although the correlation between pyrexia and neutrophil response was shown in influenza A virus infections of animal models (26), the pathological role of MIP-2-mediated neutrophil exudation has not yet been clarified. Several studies demonstrated that the use of antibody against chemokines leads to prevention or attenuation of certain acute inflammatory diseases in animal models due to reduction of neutrophil infiltration (4, 8, 28). It has been shown that the ability of neutrophils to produce active oxygen radicals is significantly reduced after the chemokine is blocked by the antibody (4). Leukotriene B4 is known to enhance NO synthesis in neutrophils (16) and reported to be induced actually in a later phase of influenza virus infection in mice (9). Indeed, when NO contents in BALF from infected mice were measured by an automated NO detector–high-pressure liquid chromatograph system, these values did not significantly differ between the untreated control group and groups treated with aMIP-2 IgG (unpublished data). Taking these facts together with our findings, it is suggested that administration of aMIP-2 IgG might partially eliminate the tissue-toxic activity of these radical molecules by the reduction of neutrophil exudation, resulting in attenuation, but not complete prevention, of lung tissue injury. However, it is evident that MIP-2 plays an important pathological role and thereby is considered a novel target for intervention therapy in influenza virus-induced pneumonia.
Acknowledgments
This study was supported in part by a grant for research into traditional medicine from the Tokyo Metropolitan Government.
REFERENCES
- 1.Akaike T, Noguchi Y, Ijiri S, Setoguchi K, Suga M, Zheng Y M, Dietzschold B, Maeda H. Pathogenesis of influenza virus-induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc Natl Acad Sci USA. 1996;93:2448–2455. doi: 10.1073/pnas.93.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baggioloni M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 3.Blake D R, Hall N D, Bacon P A, Dieppe P A, Halliwell B, Gutteridge J M. Effect of a specific iron chelating agent on animal models of inflammation. Ann Rheumat Dis. 1983;42:89–93. doi: 10.1136/ard.42.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carvalho G L, Wakabayashi G, Shimazu M, Karahashi T, Yoshida M, Yamamoto S, Matsushima K, Mukaida N, Clark B D, Takabayashi T, Brandt C T, Kitajima M. Anti-interleukin-8 monoclonal antibody reduces free radical production and improves hemodynamics and survival rate in endotoxic shock in rabbits. Surgery. 1997;122:60–68. doi: 10.1016/s0039-6060(97)90265-8. [DOI] [PubMed] [Google Scholar]
- 5.Choi A M K, Jacoby D B. Influenza virus A induces interleukin-8 gene expression in human airway epithelial cells. FEBS Lett. 1992;309:327–329. doi: 10.1016/0014-5793(92)80799-m. [DOI] [PubMed] [Google Scholar]
- 6.Cook D N, Beck M A, Coffman T M, Kirby S L, Sheridan J F, Pragnell I B, Smithies O. Requirement of MIP-1 α for an inflammatory response to viral infection. Science. 1995;269:1583–1585. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 7.Driscoll K E. Macrophage inflammatory proteins: biology and role in pulmonary inflammation. Exp Lung Res. 1994;20:473–490. doi: 10.3109/01902149409031733. [DOI] [PubMed] [Google Scholar]
- 8.Harada A, Mukaida N, Matsushima K. Interleukin 8 as a novel target for intervention therapy in acute inflammatory diseases. Mol Med Today. 1996;11:482–489. doi: 10.1016/1357-4310(96)10042-3. [DOI] [PubMed] [Google Scholar]
- 9.Hennet T, Zeilterner H J, Frei K, Peterhans E. A kinetic study of immune mediators in the lungs of mice infected with influenza A virus. J Immunol. 1992;149:932–939. [PubMed] [Google Scholar]
- 10.Hirabayashi T, Ochiai H, Sakai S, Nakajima K, Terasawa K. Inhibitory effect of ferulic acid and isoferulic acid on murine interleukin-8 production in response to influenza virus infections in vitro and in vivo. Planta Med. 1995;61:221–226. doi: 10.1055/s-2006-958060. [DOI] [PubMed] [Google Scholar]
- 11.Huang S, Paulauskis J D, Godleski J J, Kobzik L. Expression of macrophage inflammatory protein-2 and KC mRNA in pulmonary inflammation. Am J Pathol. 1992;141:981–988. [PMC free article] [PubMed] [Google Scholar]
- 12.Hurd J, Hearth R E. Effect of cyclophosphamide on infections in mice caused by virulent and avirulent strains of influenza virus. Infect Immun. 1975;11:886–889. doi: 10.1128/iai.11.5.886-889.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iida N, Grotendorst G R. Cloning and sequencing of a new gro transcript from activated human monocytes: expression in leukocytes and wound tissue. Mol Cell Biol. 1990;10:5596–5599. doi: 10.1128/mcb.10.10.5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khabar K S A, Al-Zoghaibi F, Murayama T, Matsushima K, Mukaida N, Siddiqui Y, Dhalla M, Al-Ahdal M N. Interleukin-8 selectively enhances cytopathic effect (CPE) induced by positive-strand RNA viruses in the human WISH cell line. Biochim Biophys Res Commun. 1997;235:774–778. doi: 10.1006/bbrc.1997.6872. [DOI] [PubMed] [Google Scholar]
- 15.Lamb R A. Genes and proteins of the influenza viruses. In: Krug R M, editor. The influenza viruses. New York, N.Y: Plenum Press; 1989. pp. 1–87. [Google Scholar]
- 16.Moncada S, Palmer R M J, Higgs E A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 17.Ochiai H, Kurokawa M, Hayashi K, Niwayama S. Antibody-mediated growth of influenza A NWS virus in macrophagelike cell line P388D1. J Virol. 1988;62:20–26. doi: 10.1128/jvi.62.1.20-26.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ochiai H, Ikesue A, Kurokawa M, Nakajima K, Nakagawa H. Enhanced production of rat interleukin-8 by in vitro and in vivo infections with influenza A NWS virus. J Virol. 1993;67:6811–6814. doi: 10.1128/jvi.67.11.6811-6814.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ochiai H, Sakai S, Kogure T, Hirabayashi T, Nakajima K, Terasawa K. Development and some applications of enzyme-linked immunosorbent assay system for murine macrophage inflammatory protein-2 (MIP-2) Mediat Inflamm. 1996;5:206–209. doi: 10.1155/S0962935196000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oda T, Akaie T, Hamamoto T, Suzuki F, Hirano T, Maeda H. Oxygen radicals in influenza-induced pathogenesis and treatment with pyran polymer-conjugated SOD. Science. 1989;244:974–976. doi: 10.1126/science.2543070. [DOI] [PubMed] [Google Scholar]
- 21.Rollins B J. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 22.Singer S H, Noguchi P, Kirschstein R L. Respiratory diseases in cyclophosphamide-treated mice. Infect Immun. 1972;5:957–960. doi: 10.1128/iai.5.6.957-960.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sweet C, Smith H. Pathogenesis of influenza virus. Microbiol Rev. 1980;44:303–330. doi: 10.1128/mr.44.2.303-330.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tashiro M, Homma M. Pneumotropism of Sendai virus in relation to protease-mediated activation in mouse lungs. Infect Immun. 1983;39:879–888. doi: 10.1128/iai.39.2.879-888.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tate R M, Repine J E. Neutrophils and the adult respiratory distress syndrome. Am Rev Respir Dis. 1983;128:552–559. doi: 10.1164/arrd.1983.128.3.552. [DOI] [PubMed] [Google Scholar]
- 26.Toms G L, Davies J A, Woodward C G, Sweet C, Smith H. The relation of pyrexia and nasal inflammatory responses to virus levels in nasal washings of ferrets infected with influenza viruses of differing virulence. Br J Exp Pathol. 1977;58:444–458. [PMC free article] [PubMed] [Google Scholar]
- 27.Wolpe S D, Shery B, Juers D, Davateils G, Yurt R W, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci USA. 1989;86:612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yokoi K, Mukaida N, Harada A, Watanabe Y, Matsushima K. Prevention of endotoxemia-induced acute respiratory distress syndrome-like lung injury in rabbits by a monoclonal antibody to IL-8. Lab Invest. 1997;76:375–384. [PubMed] [Google Scholar]
- 29.Yoshimura T, Matsushima K, Oppenheim J J, Leonard E J. Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)-stimulated human blood mononuclear leukocytes: partial characterization and separation from interleukin 1 (IL-1) J Immunol. 1987;139:788–793. [PubMed] [Google Scholar]
- 30.Yoshimura T, Matsushima K, Oppenheim J J, Leonard E J. Purification of a human monocyte-derived neutrophil chemotactic factor that shares sequence homology with other host defense cytokines. Proc Natl Acad Sci USA. 1987;84:9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]