Abstract
We demonstrate here that Sendai virus (SeV) blocks alpha interferon (IFN-α) signaling to signal transducers and activators of transcription (STATs) in HeLa cells. IFN-α-stimulated tyrosine phosphorylation of STATs and subsequent formation of the IFN-stimulated gene factor 3 transcription complex were inhibited in SeV-infected cells, resulting in inefficient induction of IFN-stimulated gene products. None of the components of the signaling pathway—type I IFN receptor subunits Jak1, Tyk2, Stat1, Stat2, and p48—was degraded. Moreover, tyrosine phosphorylation of Jak1 in response to IFN-α was unaffected at the early phase of infection, suggesting that oligomerization of the receptor subunits proceeded normally. In contrast to Jak1, IFN-α-stimulated tyrosine phosphorylation of Tyk2 was partially inhibited. Therefore, this partial inhibition of activation of Tyk2 probably contributes to the subsequent failure in the activation of STATs.
In response to virus infection, cells secrete interferons (IFNs), which play important roles at an early phase of host defense mechanisms. IFN-α/β establishes an antiviral state in cells by induction of IFN-stimulated gene (ISG) products, including antiviral proteins such as double-stranded-RNA-dependent protein kinase (PKR) and 2′, 5′-oligoadenylate synthetase (6, 22, 27). Binding of IFN-α/β to the type I IFN receptor causes oligomerization of receptor subunits, IFN-αR1 and IFN-αR2, and cross-activation of receptor-associated tyrosine kinases (JAK family), Jak1 and Tyk2, by phosphorylation of particular tyrosine residues in activation loops (8, 13). These activated JAKs tyrosine phosphorylate signal transducers and activators of transcription (STATs), Stat1 (Stat1α and Stat1β) and Stat2, which are recruited to the receptor complex (12, 20, 24). Upon phosphorylation, Stat1 and Stat2 form a heterodimer, combine with p48, and migrate to the nucleus to function as active ISG factor 3 (ISGF3), which binds to IFN-stimulated response elements (ISREs) and activates transcription of ISGs (26).
To counteract the antiviral action of IFN-α/β, several viruses have evolved strategies in which induction of ISG products is suppressed by blocking IFN-α/β signaling. Adenovirus produces E1A protein, which inhibits formation of ISGF3 by reduction of p48 (16, 17). Human cytomegalovirus blocks IFN-α/β signaling by decreasing Jak1 and p48 (18, 19). In cells persistently infected with mumps virus, a decreased level of Stat1α results in poor induction of ISG products (31). It has also been demonstrated that human herpes virus 8 encodes an IFN regulatory factor, which inhibits responses to IFN-α/β and IFN-γ (4, 32). On the other hand, poxviruses, including vaccinia virus, have evolved distinct strategies in which viruses produce IFN receptor homologues to block binding of IFN to the intact IFN receptors of hosts (1, 28).
Sendai virus (SeV), a prototype paramyxovirus, is also capable of suppressing the antiviral action of IFN-α/β (30). Our previous study showed that this suppression was unique in that even UV-inactivated SeV retained the anti-IFN ability. Neither viral replication nor the secondary transcription was required for the suppression (30). Here we demonstrate that SeV blocks IFN-α signaling to STATs without degradation of any components of the IFN-α signaling pathway and further reveal the partial inhibition of IFN-stimulated activation of Tyk2, one of the upstream molecules of STATs, in SeV-infected cells. Thus, this study represents a novel viral mechanism by which IFN-α signaling is blocked (17, 19, 28, 32).
Initially we determined conditions under which SeV could effectively inhibit the antiviral action of IFN-α. The antiviral action was estimated by inhibition of replication of the vesicular stomatitis virus New Jersey strain, as described previously (30). Significant inhibition was observed only when SeV infection preceded but did not follow IFN-α treatment (data not shown). From this result, we speculated that SeV targets a component or components of the IFN-α/β signaling pathway rather than antiviral products such as PKR, since the antiviral action would be suppressed even in IFN-α-pretreated cells if SeV targets antiviral proteins like vaccinia virus does (2, 3). To examine whether SeV actually affects IFN-α-stimulated formation of ISGF3, nuclear extracts of SeV-infected cells were analyzed by electrophoretic mobility shift assays (EMSAs) using a 32P-labeled probe containing the ISRE sequence as described in reference 10. Cells were infected with SeV at the indicated time points and harvested at 2 h after replacement with medium containing IFN-α. IFN-α treatment prior to SeV infection resulted in formation of ISGF3 (Fig. 1, lanes 6 and 7), while no ISGF3 complex was detected in cells infected with SeV at 12 h before IFN treatment (Fig. 1, lane 3). SeV infection at 1 or 4 h before IFN treatment also resulted in a substantial inhibition of formation of ISGF3 (Fig. 1, lanes 4 and 5), indicating that the anti-IFN state was almost established at the early phase of infection.
FIG. 1.
ISGF3 complex formation in SeV-infected cells. HeLa cells were mock-infected (m) (lanes 1 and 2) or infected with SeV at the indicated times and harvested at 2 h after replacement with fresh medium containing 103 IU of IFN-α per ml (recombinant human IFN-α2a from Takeda Chemical Industries Ltd., Osaka, Japan) (lanes 2 to 7) or no IFN-α (lane 1). Nuclear extracts were prepared according to the “mini-extracts” method (23, 25) and analyzed by EMSA with a 32P-labeled ISG15 ISRE probe as described previously (10). The arrow indicates the position of ISGF3. SeVpB (30), a temperature-sensitive mutant isolated from a carrier culture of BHK cells persistently infected with SeV strain Nagoya 1-60 (11), was used for all of the experiments in this study. HeLa cells were grown in Eagle's minimum essential medium (MEM) supplemented with 5% bovine serum and 3% tryptose phosphate broth.
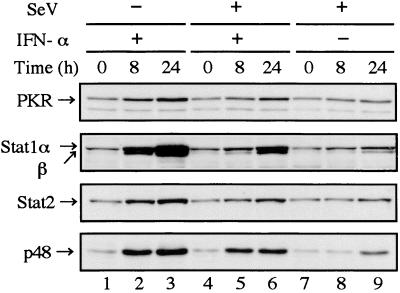
To examine whether induction of ISG products was consequently suppressed in infected cells, the levels of Stat1, Stat2, and p48 in addition to PKR were estimated by Western blot analysis. This would simultaneously provide information regarding components of the signaling pathway, since Stat1, Stat2, and p48 are not only ISG products (15) but also components of ISGF3. As shown in Fig. 2, induction of the ISG products was significantly suppressed in infected cells (Fig. 2, lanes 5 and 6). Weak induction of the ISG products was observed in infected cells in the absence of exogenously added IFN-α (Fig. 2, lane 9). This may be due to the action of autocrine IFN-α/β secreted by infected cells, since HeLa cells are a good IFN-α/β-producing cell line (29). Levels of expression of Stat1, Stat2, and p48 at 0 h in infected cells (Fig. 2, lane 4 or 7) were almost the same as those in uninfected cells (Fig. 2, lane 1), suggesting there was no degradation of components of ISGF3 at 2 h postinfection (hpi).
FIG. 2.
Poor induction of ISG products in SeV-infected cells. HeLa cells were mock infected (lanes 1 to 3) or infected with SeV (lanes 4 to 9), and then the media were replaced at 2 hpi with fresh medium containing 103 IU of IFN-α per ml (lanes 2, 3, 5, and 6) or no IFN-α (lanes 1, 4, 7, 8, and 9). The cells were harvested at 0 (lanes 1, 4, and 7), 8 (lanes 2, 5, and 8), and 24 (lanes 3, 6, and 9) h after IFN-α treatment. Total-cell extracts (20 μg of protein) prepared according to the method of Lee et al. (14) were subjected to Western blot analysis as described previously (9). Anti-PKR rabbit polyclonal (sc-707) (A), anti-Stat1 mouse monoclonal (sc-464) (B), anti-Stat2 rabbit polyclonal (sc-476) (C), and anti-p48 rabbit polyclonal (sc-496) (D) antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.) were used as the first antibody. The same blotting membrane was stripped and reprobed. The protein concentration was determined as described previously (9).
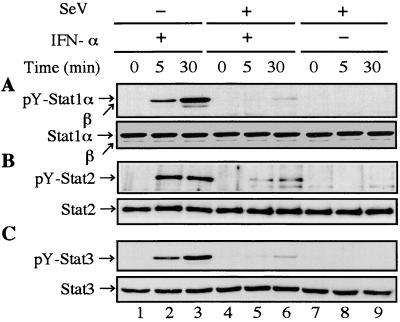
Since none of the components of ISGF3 was degraded, we next examined whether SeV infection affected IFN-α-stimulated tyrosine phosphorylation of Stat1, Stat2, and Stat3. Stat3 is one of the downstream molecules of the IFN-α signal transduction, although it is not a component of ISGF3. To exclude effects of autocrine IFN-α/β secreted by HeLa cells on signal transduction, further analyses were restricted to the early phase of infection (2 hpi). Infected cells were harvested at the indicated times after IFN-α treatment. Total-cell extracts were subjected to Western blot analysis with an antiphosphotyrosine (701) Stat1 or antiphosphotyrosine (705) Stat3 antibody. For detection of tyrosine-phosphorylated Stat2, total-cell extracts were immunoprecipitated with an anti-Stat2 antibody before Western blot analysis with an antiphosphotyrosine antibody. As shown in Fig. 3, apparent inhibition of tyrosine phosphorylation of STATs was observed in infected cells (Fig. 3, lanes 5 and 6). SeV infection did not affect expression levels of the STAT proteins (Fig. 3). These results suggested that the failure of formation of ISGF3 is attributable to the inhibition of IFN-α-stimulated tyrosine phosphorylation of Stat1 and Stat2.
FIG. 3.
Inhibition of IFN-α-stimulated tyrosine phosphorylation of Stat1, Stat2, and Stat3 in SeV-infected cells. HeLa cells were mock infected (lanes 1 to 3) or infected (lanes 4 to 9) with SeV at 2 h prior to replacement with fresh medium containing 103 IU of IFN-α per ml (lanes 2, 3, 5, and 6) or no IFN-α (lanes 1, 4, 7, 8, and 9). The cells were harvested at 0 (lanes 1, 4, and 7), 5 (lanes 2, 5, and 8), or 30 (lanes 3, 6, and 9) min after IFN-α treatment. Total-cell extracts (50 μg of protein) were subjected to Western blot analysis with anti-phospho-(Tyr 701)-Stat1 (no. 9171) (A) and anti-phospho-(Tyr 705)-Stat3 (no. 9131) (C) rabbit polyclonal antibodies (New England Biolabs, Inc.). To detect tyrosine-phosphorylated Stat2, total-cell extracts (500 μg) were immunoprecipitated with an anti-Stat2 antibody (sc-476) (B) before Western blot analysis with antiphosphotyrosine mouse monoclonal antibody (sc-7020) (Santa Cruz Biotechnology, Inc.). Each blotting membrane was stripped and reprobed with anti-Stat1 (sc-464) (A) mouse monoclonal antibody or anti-Stat2 (sc-476) (B) or anti-Stat3 (sc-7179) (C) rabbit polyclonal antibody (Santa Cruz Biotechnology, Inc.).
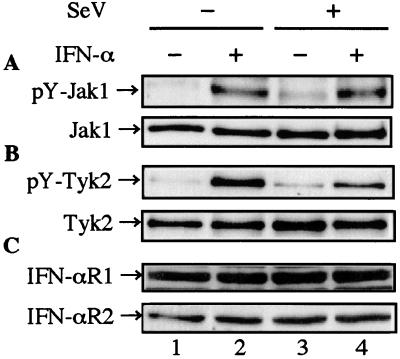
The receptor-associated kinases Jak1 and Tyk2 are responsible for the activation of STATs. To examine whether the JAKs in infected cells were activated by IFN-α stimulation, levels of tyrosine-phosphorylated JAKs in response to IFN-α were estimated (Fig. 4A and B). Infected cells were harvested at the indicated time after IFN-α treatment. Total-cell extracts were immunoprecipitated by an anti-Jak1 or an anti-Tyk2 antibody before Western blot analysis with an antiphosphotyrosine antibody. As shown in Fig. 4A, no inhibition of tyrosine phosphorylation of Jak1 was observed (Fig. 4A, lanes 2 and 4), suggesting that binding of IFN-α to the type I IFN receptor and subsequent oligomerization of the receptor subunits, IFN-αR1 and IFN-αR2, proceeded normally. In contrast, IFN- α-stimulated tyrosine phosphorylation of Tyk2 was partially inhibited (Fig. 4B, lanes 2 and 4). This finding was also confirmed by using a specific anti-phospho-Tyk2 (Tyr 1054/1055) rabbit polyclonal antibody (no. 9321) (New England Biolabs, Inc., Beverly, Mass.) against phosphotyrosine residues (1054/1055) in the activation loop of Tyk2 (data not shown). The expression levels of both Jak1 and Tyk2, as well as those of both IFN-αR1 and IFN-αR2, were stable irrespective of IFN-α treatment and SeV infection (Fig. 4). These results, together with those shown in Fig. 2 and 3, revealed that SeV infection did not degrade any components of the signaling pathway.
FIG. 4.
Effects of SeV infection on IFN-α-stimulated tyrosine phosphorylation of Jak1 and Tyk2 and on expression levels of the type I IFN receptor subunits IFN-αR1 and IFN-αR2. HeLa cells were mock infected (lanes 1 and 2) or infected with SeV (lanes 3 and 4) at 2 h prior to replacement with fresh medium containing 103 IU of IFN-α per ml (lanes 2 and 4) or no IFN-α (lanes 1 and 3). The cells were harvested at 15 min after IFN-α treatment. The total-cell extracts (1 mg) were immunoprecipitated with anti-Jak1 (no. 06-272) (A) and anti-Tyk2 (no. 06-638) (B) rabbit polyclonal antibodies (Upstate Biotechnology, Inc., Lake Placid, N.Y.) before Western blot analysis with antiphosphotyrosine mouse monoclonal antibody (sc-7020). The same blotting membrane was stripped and reprobed with anti-Jak1 (J24320) (A) and anti-Tyk2 (T20220) (B) mouse monoclonal antibodies (Transduction Laboratories, Lexington, Ky.). To detect the type I IFN receptor subunits, total-cell extracts (50 μg) were subjected to Western blot analysis with anti-IFN-αR (IFN-αR1) (sc-845) and anti-IFN-α/βR (IFN-αR2) (sc-704) rabbit polyclonal antibodies (Santa Cruz Biotechnology, Inc.) (C).
Didcock et al. have recently demonstrated unresponsiveness of SeV-infected cells to IFN-α/β mainly by means of transfection experiments with an IFN-α/β-responsive plasmid (5). Our results presented here are essentially consistent with their results and further demonstrate that SeV blocks IFN-α signaling to STATs without degradation of any components of the IFN-α signaling pathway (Fig. 3 and 4). The normal IFN-stimulated tyrosine phosphorylation of Jak1 (Fig. 4A) suggests that neither binding of IFN-α to the receptor nor the subsequent oligomerization of the receptor subunits is inhibited. These findings imply that the signal of IFN-α passes through the cell membrane but hardly reaches STATs in SeV-infected cells.
The mechanism by which IFN-α signaling to the STATs is blocked remains to be elucidated. We have found that the activation of Tyk2 was partially inhibited, in contrast to normal tyrosine phosphorylation of Jak1 (Fig. 4B). Accordingly, it is reasonable to speculate that the partial inhibition of tyrosine phosphorylation of Tyk2 contributes to the subsequent failure in tyrosine phosphorylation of the STATs. However, it is unclear at present whether only this partial inhibition is responsible for the prevention of signaling to the STATs. Nevertheless, this finding is of significance and suggests that the other mechanism behind this partial inhibition, if present, targets a signaling process very close to this Tyk2 activation. Phorbol ester specifically inhibits IFN-α-stimulated tyrosine phosphorylation of Tyk2 by inducing a specific tyrosine phosphatase activity against Tyk2, but not Jak1 (21). The tyrosine phosphatase inhibitor vanadate could reverse this inhibitory effect. Although the inhibitory pattern of phorbol ester is similar to that in SeV-infected cells, our preliminary experiments showed that vanadate treatment never reversed the inhibitory effects of SeV on IFN-α-stimulated tyrosine phosphorylation of Stat1 and Tyk2 (unpublished), suggesting that no phosphatase is involved in the blocking process. It has recently been found that the SeV C proteins (C′, C, Y1, and Y2) are responsible for the suppression mechanisms (7, 9). Therefore, analyses of molecular interactions between C proteins and cellular factors (e.g., components of the IFN-α signaling pathway) will greatly contribute to elucidation of the mechanisms by which activation of Tyk2 is inhibited.
Acknowledgments
We thank S. Kubo for excellent technical assistance and Y. Kimura for giving a boost to our research. We also thank Y. Ohnishi, A. Kato, and Y. Nagai for constant encouragement of our research.
This work was supported in part by a research fund from Y. Kokami and by a grant from the Ministry of Education, Science, Sports and Culture, Japan.
REFERENCES
- 1.Alcami A, Smith G L. Receptors for gamma-interferon encoded by poxviruses: implications for the unknown origin of vaccinia virus. Trends Microbiol. 1996;4:321–326. doi: 10.1016/0966-842x(96)10051-2. [DOI] [PubMed] [Google Scholar]
- 2.Beattie E, Denzler K L, Tartaglia J, Perkus M E, Paoletti E, Jacobs B L. Reversal of the interferon-sensitive phenotype of a vaccinia virus lacking E3L by expression of the reovirus S4 gene. J Virol. 1995;69:499–505. doi: 10.1128/jvi.69.1.499-505.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beattie E, Tartaglia J, Paoletti E. Vaccinia virus-encoded eIF-2 alpha homolog abrogates the antiviral effect of interferon. Virology. 1991;183:419–422. doi: 10.1016/0042-6822(91)90158-8. [DOI] [PubMed] [Google Scholar]
- 4.Burysek L, Yeow W S, Pitha P M. Unique properties of a second human herpesvirus 8-encoded interferon regulatory factor (vIRF-2) J Hum Virol. 1999;2:19–32. [PubMed] [Google Scholar]
- 5.Didcock L, Young D F, Goodbourn S, Randall R E. Sendai virus and simian virus 5 block activation of interferon-responsive genes: importance for virus pathogenesis. J Virol. 1999;73:3125–3133. doi: 10.1128/jvi.73.4.3125-3133.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gale M, Jr, Katze M G. Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon-induced protein kinase. Pharmacol Ther. 1998;78:29–46. doi: 10.1016/s0163-7258(97)00165-4. [DOI] [PubMed] [Google Scholar]
- 7.Garcin D, Latorre P, Kolakofsky D. Sendai virus C proteins counteract the interferon-mediated induction of an antiviral state. J Virol. 1999;73:6559–6565. doi: 10.1128/jvi.73.8.6559-6565.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gauzzi M C, Velazquez L, McKendry R, Mogensen K E, Fellous M, Pellegrini S. Interferon-alpha-dependent activation of Tyk2 requires phosphorylation of positive regulatory tyrosines by another kinase. J Biol Chem. 1996;271:20494–20500. doi: 10.1074/jbc.271.34.20494. [DOI] [PubMed] [Google Scholar]
- 9.Gotoh B, Takeuchi K, Komatsu T, Yokoo J, Kimura Y, Kurotani A, Kato A, Nagai Y. Knockout of the Sendai virus C gene eliminates the viral ability to prevent the interferon-α/β-mediated responses. FEBS Lett. 1999;459:205–210. doi: 10.1016/s0014-5793(99)01241-7. [DOI] [PubMed] [Google Scholar]
- 10.Gutch M J, Reich N C. Repression of the interferon signal transduction pathway by the adenovirus E1A oncogene. Proc Natl Acad Sci USA. 1991;88:7913–7917. doi: 10.1073/pnas.88.18.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimura Y, Ito Y, Shimokata K, Nishiyama Y, Nagata I, Kitoh J. Temperature-sensitive virus derived from BHK cells persistently infected with HVJ (Sendai virus) J Virol. 1975;15:55–63. doi: 10.1128/jvi.15.1.55-63.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotenko S V, Izotova L S, Mirochnitchenko O V, Lee C, Pestka S. The intracellular domain of interferon-alpha receptor 2c (IFN-alphaR2c) chain is responsible for Stat activation. Proc Natl Acad Sci USA. 1999;96:5007–5012. doi: 10.1073/pnas.96.9.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnan K, Pine R, Krolewski J J. Kinase-deficient forms of Jak1 and Tyk2 inhibit interferon alpha signaling in a dominant manner. Eur J Biochem. 1997;247:298–305. doi: 10.1111/j.1432-1033.1997.00298.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee C K, Bluyssen H A, Levy D E. Regulation of interferon-alpha responsiveness by the duration of Janus kinase activity. J Biol Chem. 1997;272:21872–21877. doi: 10.1074/jbc.272.35.21872. [DOI] [PubMed] [Google Scholar]
- 15.Lehtonen A, Matikainen S, Julkunen I. Interferons up-regulate STAT1, STAT2, and IRF family transcription factor gene expression in human peripheral blood mononuclear cells and macrophages. J Immunol. 1997;159:794–803. [PubMed] [Google Scholar]
- 16.Leonard G T, Sen G C. Effects of adenovirus E1A protein on interferon-signaling. Virology. 1996;224:25–33. doi: 10.1006/viro.1996.0503. [DOI] [PubMed] [Google Scholar]
- 17.Leonard G T, Sen G C. Restoration of interferon responses of adenovirus E1A-expressing HT1080 cell lines by overexpression of p48 protein. J Virol. 1997;71:5095–5101. doi: 10.1128/jvi.71.7.5095-5101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller D M, Rahill B M, Boss J M, Lairmore M D, Durbin J E, Waldman J W, Sedmak D D. Human cytomegalovirus inhibits major histocompatibility complex class II expression by disruption of the Jak/Stat pathway. J Exp Med. 1998;187:675–683. doi: 10.1084/jem.187.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller D M, Zhang Y, Rahill B M, Waldman W J, Sedmak D D. Human cytomegalovirus inhibits IFN-alpha-stimulated antiviral and immunoregulatory responses by blocking multiple levels of IFN-alpha signal transduction. J Immunol. 1999;162:6107–6113. [PubMed] [Google Scholar]
- 20.Pestka S, Kotenko S V, Muthukumaran G, Izotova L S, Cook J R, Garotta G. The interferon gamma (IFN-gamma) receptor: a paradigm for the multichain cytokine receptor. Cytokine Growth Factor Rev. 1997;8:189–206. doi: 10.1016/s1359-6101(97)00009-9. [DOI] [PubMed] [Google Scholar]
- 21.Petricoin E, III, David M, Igarashi K, Benjamin C, Ling L, Goelz S, Finbloom D S, Larner A C. Inhibition of alpha interferon but not gamma interferon signal transduction by phorbol esters is mediated by a tyrosine phosphatase. Mol Cell Biol. 1996;16:1419–1424. doi: 10.1128/mcb.16.4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Player M R, Torrence P F. The 2-5A system: modulation of viral and cellular processes through acceleration of RNA degradation. Pharmacol Ther. 1998;78:55–113. doi: 10.1016/S0163-7258(97)00167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sadowski H B, Gilman M Z. Cell-free activation of a DNA-binding protein by epidermal growth factor. Nature. 1993;362:79–83. doi: 10.1038/362079a0. [DOI] [PubMed] [Google Scholar]
- 24.Schindler C, Darnell J E., Jr Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem. 1995;64:621–651. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- 25.Schreiber E, Matthias P, Muller M M, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stark G R, Kerr I M. Interferon-dependent signaling pathways: DNA elements, transcription factors, mutations, and effects of viral proteins. J Interferon Res. 1992;12:147–151. doi: 10.1089/jir.1992.12.147. [DOI] [PubMed] [Google Scholar]
- 27.Stark G R, Kerr I M, Williams B R, Silverman R H, Schreiber R D. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 28.Symons J A, Alcami A, Smith G L. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell. 1995;81:551–560. doi: 10.1016/0092-8674(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 29.Wathelet M G, Berr P M, Huez G A. Regulation of gene expression by cytokines and virus in human cells lacking the type-I interferon locus. Eur J Biochem. 1992;206:901–910. doi: 10.1111/j.1432-1033.1992.tb16999.x. [DOI] [PubMed] [Google Scholar]
- 30.Yokoo J, Gotoh B, Komatsu T, Takeuchi K, Miyadai T. Replication-incompetent Sendai virus can suppress the antiviral action of type I interferon. Arch Virol. 1999;144:1043–1055. doi: 10.1007/s007050050568. [DOI] [PubMed] [Google Scholar]
- 31.Yokosawa N, Kubota T, Fujii N. Poor induction of interferon-induced 2′,5′-oligoadenylate synthetase (2-5 AS) in cells persistently infected with mumps virus is caused by decrease of STAT-1 alpha. Arch Virol. 1998;143:1985–1992. doi: 10.1007/s007050050434. [DOI] [PubMed] [Google Scholar]
- 32.Zimring J C, Goodbourn S, Offermann M K. Human herpesvirus 8 encodes an interferon regulatory factor (IRF) homolog that represses IRF-1-mediated transcription. J Virol. 1998;72:701–707. doi: 10.1128/jvi.72.1.701-707.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]