Abstract
Binding of human immunodeficiency virus type 1 (HIV-1) to CD4 receptors induces multiple cellular signaling pathways, including the MEK/ERK cascade. While the interaction of X4 HIV-1 with CXCR4 does not seem to activate this pathway, viruses using CCR5 for entry efficiently activate MEK/ERK kinases (W. Popik, J. E. Hesselgesser, and P. M. Pitha, J. Virol. 72:6406–6413, 1998; W. Popik and P. M. Pitha, Virology 252:210–217, 1998). Since the importance of MEK/ERK in the initial steps of viral replication is poorly understood, we have examined the role of MEK/ERK signaling in the CD3- and CD28 (CD3/CD28)-mediated activation of HIV-1 replication in resting peripheral blood CD4+ T lymphocytes infected with X4 or R5 HIV-1. We have found that the MEK/ERK inhibitor U0126 selectively inhibited CD3/CD28-stimulated replication of X4 HIV-1, while it did not affect the replication of R5 HIV-1. Inhibition of the CD3/CD28-stimulated MEK/ERK pathway did not affect the formation of the early proviral transcripts in cells infected with either X4 or R5 HIV-1, indicating that virus reverse transcription is not affected in the absence of MEK/ERK signaling. In contrast, the levels of nuclear provirus in cells infected with X4 HIV-1, detected by the formation of circular proviral DNA, was significantly lower in cells stimulated in the presence of MEK/ERK inhibitor than in the absence of the inhibitor. However, in cells infected with R5 HIV-1, the inhibition of the MEK/ERK pathway did not affect nuclear localization of the proviral DNA. These data suggest that the nuclear import of X4, but not R5, HIV-1 is dependent on a CD3/CD28-stimulated MEK/ERK pathway.
CCR5-specific (R5) strains of human immunodeficiency virus type 1 (HIV-1) have been implicated in the transmission of virus infection and are predominantly found during the asymptomatic stages of HIV infection (31, 42). In contrast, X4 strains that use CXCR4 coreceptors for entry are generally associated with disease progression, decline in peripheral CD4+ T-lymphocyte levels, and the onset of clinical symptoms of AIDS (9).
Cellular tropism of HIV-1 is primarily determined by utilization of chemokine receptors. Changes in HIV-1 coreceptor utilization usually correlate with changes in the V3 loop of the viral envelope glycoprotein (9, 29). In addition to the role that chemokine receptors play as HIV-1 entry cofactors, these receptors are able to activate different signaling pathways upon interaction with HIV-1 envelope during entry. However, the role of HIV-1-induced signaling pathways in viral pathogenesis is not clear.
While chemokine receptor signaling in established cell lines is not necessary for viral entry (1, 12, 14), signaling events seem to play a role in postentry events (6), aberrant expression of inflammatory genes (25), CD4+ T-cell depletion (24), and deregulated cell adhesion and chemotaxis during HIV infection (8). It was shown that binding of HIV-1 envelope glycoproteins from X4 or R5 viruses to chemokine receptors rapidly induced phosphorylation of the tyrosine kinase Pyk2 (10, 23). In addition, macrophage-tropic HIV-1 and simian immunodeficiency virus (SIV) induced calcium signaling through the CCR5 receptor (38). Recently, R5 HIV-1 envelope was shown to induce tyrosine phosphorylation of focal adhesion kinase (FAK) and its association with the CCR5 receptor (8). However, due to the structural complexity of the chemokine receptors, signaling events induced by the interaction with specific ligands may not be mimicked entirely by binding of HIV-1. Specifically, binding of SDF-1, a natural ligand for CXCR4, stimulates the mitogen-activated protein kinase (MAPK) ERK pathway; however, interaction of X4 HIV-1 with CXCR4 did not activate this pathway (23, 25). In contrast, viruses using CCR5 for entry efficiently activated MEK/ERK, as well as JNK and p38 MAPKs (26).
The role of MAPK ERK in the HIV-1 life cycle is not completely understood. Thus, it has been suggested that ERK pathway plays a role in HIV-1 replication by enhancing the infectivity of virions through Vif-dependent (39) and Vif-independent mechanisms (18, 40), possibly by the establishment of a functional reverse transcription complex. In this regard, ERK was shown to phosphorylate HIV-1 Gag matrix protein p17 (4), which then, together with Vpr, promotes nuclear translocation of a preintegration complex and, consequently, stimulates virus infectivity.
Activation of CD4+ T cells is critical for efficient replication of HIV-1 in these cells. In quiescent T cells, HIV-1 entry occurs efficiently; however, the extent of postentry events in quiescent cells is not clear (33, 35, 41). Optimal T-cell activation through T-cell receptor (TCR)/CD28 was shown to be required for efficient reverse transcription and productive HIV-1 infection (21, 35). However, the possibility that activation of signaling cascades upon engagement of CD4 or chemokine coreceptors by HIV-1 may bypass a requirement for a full T-cell activation for virus replication has not been considered.
Based on the differential ability of R5 and X4 HIV-1 to induce the MEK/ERK pathway, which constitutes a part of the TCR/CD28-mediated signaling involved in T-cell activation, we hypothesize that R5 viruses may be able to replicate and spread in suboptimally activated CD4+ T cells. Interestingly, recent findings showed a significant replicative advantage of R5 over X4 HIV-1 in suboptimally activated T lymphocytes (37). Similarly, recent results obtained in a hu-PBL-SCID mice model showed that X4 HIV-1 strains were highly virulent when injected at the time the transferred human T cells were highly activated and were less infectious and poorly cytopathic when the majority of T cells at the moment of viral infection were quiescent or memory cells. In contrast, R5 HIV-1 was virulent independently of the state of target cell activation in the SCID mouse environment (13).
In the present study, we have investigated the role of MEK/ERK signaling in CD3 and CD28 (CD3/CD28)-stimulated HIV-1 replication in resting peripheral blood CD4+ T lymphocytes infected with X4 or R5 HIV-1. We have shown that in cells stimulated by cross-linking of CD3 and CD28 receptors, R5, but not X4, HIV-1 replicated efficiently in the presence of a MEK/ERK inhibitor. Our results further suggest that in the absence of MEK/ERK signaling, restricted replication of X4 HIV-1 resulted from inefficient completion of late preintegration steps and/or nuclear import of preintegration complexes and consequent inhibition of X4 HIV-1 provirus integration.
MATERIALS AND METHODS
Reagents.
Phosphospecific antibodies detecting phosphorylated and total forms of ERK1/2 were purchased from New England Biolabs. MEK/ERK pathway inhibitor U0126 was purchased from Promega. RNase-free DNase was obtained from Gibco BRL. Monoclonal anti-CD3 (clone UCHT1) and anti-CD28 (clone CD28.2) antibodies, as well as control immunoglobulin G1 (IgG1), were obtained from Sigma. Anti-HLA-DR (clone G46-6) antibody was purchased from Pharmingen.
Isolation and purification of primary CD4+ T lymphocytes.
Highly enriched preparations of CD4+ T lymphocytes were isolated from peripheral blood of healthy, HIV-seronegative donors. Lymphocytes obtained by using LSM lymphocyte separation medium (Organon Teknika) were depleted of monocytes/macrophages by 2 h of adherence to plastic in the presence of 2% of human heat-inactivated AB serum (Sigma). Depletion of monocytes was repeated two more times. CD4+ T lymphocytes were isolated by a negative selection with a human T-cell CD4 subset column kit (R&D Systems) and subsequently incubated with monoclonal antibody against activation marker HLA-DR (Pharmingen). Positively selected cells expressing HLA-DR were removed by using antimouse antibody-conjugated magnetic beads (Dynal). The isolated resting CD4+ T cells were cultured overnight at 37°C in RPMI 1640 medium with 2 mM l-glutamine (Life Technologies) and supplemented with gentamicin (50 μg/ml) and heat-inactivated 5% human AB serum.
Virus preparation and infection.
Virus stocks of the infectious X4 NL4-3 and R5 Ba-L and 49-5 clones of HIV-1 were prepared as described previously (25, 26). The culture supernatants containing viruses were collected and clarified by centrifugation at 1,500 × g for 15 min, filtered through a 0.45-μm-pore-diameter filter, and frozen at −80°C. Before infection, virus preparations were treated with RNase-free DNase (200 U/ml) for 1 h at room temperature to eliminate potential contamination with viral DNA. The virus titer was monitored by the reverse transcriptase (RT) activity assay (25, 26). CD4+ T lymphocytes were infected by incubation with NL4-3, Ba-L, or 49-5 HIV-1 (20 RT cpm/cell) for 3 h at 37°C, followed by extensive washing to remove unbound virus. After the infection, the resting cells were cultured for 3 days in RPMI medium with 5% human serum without any stimulation. Before stimulation with anti-CD3/CD28 antibodies, the cells were washed, centrifuged, and resuspended in a fresh culture medium at 0.5 × 106 cells/ml.
Stimulation of HIV-1 replication in infected resting CD4+ T cells.
The infected CD4+ T cells were stimulated by cross-linking of CD3 and CD28 receptors by incubation in six-well plates precoated with monoclonal anti-CD3 (clone UCHT-1; Sigma) and anti-CD28 (clone CD28.2; Sigma) antibodies prebound to plastic-immobilized goat antimouse Ig (Sigma) as described previously (32). In unstimulated controls, cells were exposed to isotype-matched control IgG1. To study the effect of MEK/ERK inhibition, a specific inhibitor, U0126 (10 μM in 0.1% dimethyl sulfoxide), was preincubated with cells at 37°C for 15 min before being added to plates and stimulated with anti-CD3/CD28 antibodies. Cells stimulated without the inhibitor were exposed to 0.1% dimethyl sulfoxide.
Western blot analysis.
Cells were solubilized in ice-cold 1% Triton X-100 lysis buffer supplemented with protease and phosphatase inhibitors as described previously (25). After 30 min on ice, the lysates were clarified by centrifugation, and the protein concentration was determined with the Pierce bicinchoninic acid protein assay reagent. Proteins (20 μg) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10% acrylamide), transferred to nitrocellulose membranes, and probed with specific antibodies (diluted 1:1,000), followed by incubation with secondary horseradish peroxidase-conjugated antibody (1:100,000). Bound antibodies were detected with the SuperSignal ULTRA chemiluminescent substrate (Pierce).
RT-PCR analysis of CD4 and chemokine receptor expression.
Total RNA was isolated from cells with the RNeasy RNA purification system (Qiagen), and 1 μg of DNase-treated RNA was used for cDNA synthesis with Superscript II RNase H− RT and oligo(dT)12–18 primers (Gibco BRL). One-tenth of this reaction was used as a template for PCR amplification with Taq polymerase (SuperMix; Gibco BRL). The primers for CXCR4, CCR5, CD4, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were described previously (26). Amplifications were performed for 30 cycles (94°C for 45 s, 50°C for 1 min, and 72°C for 1.5 min), and PCR products were resolved by electrophoresis in a 2% agarose gel and visualized by ethidium bromide staining.
Flow cytometry.
Expression of CD4 and CXCR4 on purified CD4+ T lymphocytes was determined by fluorescence-activated cell sorter (FACS) as described previously (32). The following antibodies were used: fluorescein isothiocyanate (FITC)-conjugated antihuman CD4 monoclonal antibody (Becton Dickinson), phycoerythrin (PE)-conjugated antihuman CXCR4 antibody 12G5 (Pharmingen), and FITC- and PE-conjugated mouse IgG isotype controls. After being washed twice, the cells were examined with an Epics Elite FACS (Coulter).
PCR analysis of HIV-1 DNA.
Cells (1 × 106 to 1.5 × 106) were lysed with 200 μl of PCR buffer consisting of 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 2.5 mM MgCl2, 0.1 mg of gelatin per ml, 0.45% Nonidet P-40, 0.45% Tween 20, and 100 μg of proteinase K per ml. After protein digestion for 2 h at 56°C and inactivation for 10 min at 95°C, different amounts of cell lysate (0.3 to 5μl) were subjected to 25 to 30 cycles of PCR with Taq polymerase in a total volume of 50 μl containing 0.2 μM oligonucleotide primers in PCR SuperMix (Gibco BRL). The PCR conditions and HIV-1-specific oligonucleotide primers detecting early R/U5, late long terminal repeat (LTR)/gag, and 2LTR DNA were described previously (30). Assays for integrated HIV-1 DNA were performed as described previously (7). Briefly, the first PCR was carried out by using nested primers—Alu-LTR 5′, specific for conserved sequences of human Alu; and Alu-LTR 3′, from conserved HIV-1 LTR sequences. Amplifications with serially diluted cells were performed by using Elongase polymerase (Gibco BRL) for 22 cycles. For the second PCRs, aliquots of 1, 2, or 5 μl of the 10-fold-diluted first PCR product were amplified with Taq polymerase for 30 cycles with primers NI-2 5′ and NI-2 3′, detecting part of the HIV-1 LTR as described previously (7). PCR products were analyzed by electrophoresis in 2% agarose gel and visualized by ethidium bromide staining.
RESULTS
Establishment of X4 and R5 HIV-1 infection in resting CD4+ T lymphocytes.
We have earlier shown that X4 and R5 HIV-1 differ in their ability to activate the MEK/ERK signaling pathway during interaction with specific chemokine coreceptors (25). These observations prompted us to investigate the significance of MEK/ERK pathway activation in the induction of X4 and R5 virus replication in resting CD4+ T cells. For this purpose, we have established a resting CD4+ T-cell model of HIV-1 infection.
Negatively selected CD4+ T lymphocytes were purified from the peripheral blood of HIV-seronegative donors. Purified T cells were then cultured for 24 h in the absence of any activation. Subsequently, the cells were either left uninfected or were incubated for 3 h with X4 NL4-3 or R5 Ba-L HIV-1 and then washed extensively to remove unbound virus. After infection, the cells were cultured for 3 days without any stimulation. No virus production, as measured by the RT assay, could be detected in culture medium during this time.
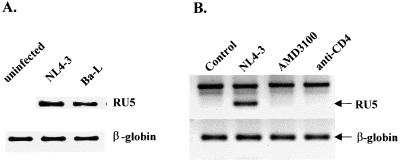
To measure the efficiency of HIV-1 entry into resting CD4+ T cells, we first examined by RT-PCR analysis the expression of CD4 and CXCR4 and CCR5, which serve as major HIV-1 entry coreceptors used by NL4-3 and Ba-L, respectively. We have found that the expression of CD4 as well as CXCR4 and CCR5 mRNA did not change noticeably in unstimulated cells 3 days after infection with X4 and R5 HIV-1 (data not shown). Synthesis of strong-stop DNA, the early product of HIV-1 reverse transcription (LTR R/U5 region) synthesized shortly after viral entry, was detected only in infected cells (Fig. 1A), and the levels of R/U5 products synthesized after entry of X4 NL4-3 and R5 Ba-L were similar. This suggests that both viruses entered the cells with comparable efficiency. Specificity of the R/U5 signal was confirmed by showing the persistence of the signal in unstimulated cells for at least 3 days after infection (see Fig. 5). In addition, we did not detect any R/U5 signal in cells infected with X4 NL4-3 in the presence of AMD3100, an antagonist of CXCR4 known to inhibit X4 HIV-1 entry (11), or in cells pretreated with anti-CD4 antibody Q4120, which blocks CD4-mediated HIV-1 entry (Fig. 1B).
FIG. 1.
HIV-1 entry into resting peripheral blood CD4+ T lymphocytes. (A) HIV-1 entry into unstimulated CD4+ T lymphocytes. The cells were infected for 3 h with NL4-3 or Ba-L (20 RT cpm/cell) or were left uninfected. PCR amplification of total-cell lysates was performed with R/U5 primers specific for strong-stop DNA as described in Materials and Methods. Amplification of the β-globin gene was used to control the amount of DNA in each sample. PCR products were resolved by electrophoresis in 2.5% agarose gels and stained with ethidium bromide. (B) Inhibition of X4 HIV-1 entry into CD4+ T cells by CD4 and CXCR4 antagonists. The cells were preincubated for 30 min with anti-CD4 antibody (5 μg/ml) or AMD3100 (500 ng/ml), followed by infection with NL4-3 as described for panel A. PCR amplification and analysis of R/U5 product were performed as described for panel A and in Materials and Methods.
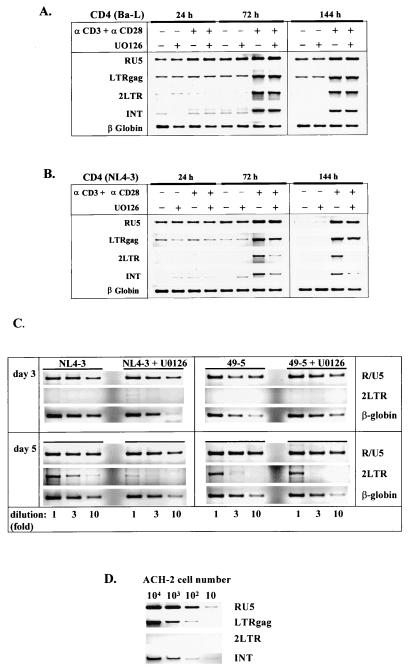
FIG. 5.
Differential effect of the MEK/ERK inhibitor U0126 on nuclear import and/or integration of X4 and R5 HIV-1 proviral DNA. (A and B) Analysis of the sequential steps of reverse transcription and subsequent nuclear import and integration of the proviral DNA was performed with specific oligonucleotide primer sets and under the conditions described in Materials and Methods. The peripheral blood resting CD4+ T lymphocytes infected with Ba-L (A), NL4-3 (B), or with the isogenic pair (49-5 and NL4-3) of HIV-1 strains (C) were left untreated, were treated with MEK/ERK inhibitor U0126 (10 μM) alone, or were stimulated for different periods of time with immobilized anti-CD3/CD28 antibodies (αCD3 and αCD28) in the absence or presence of U0126. Subsequently, the cells were harvested and analyzed by PCR for RT products that were early (RU5), late (LTRgag), nuclear (2LTR circles), and integrated (INT) into the host genome, respectively. The amount of total DNA was controlled by amplification of the cellular β-globin gene. (D) As controls, serial dilutions of the HIV-1-infected ACH-2 cells, containing one HIV-1 provirus per cell, were amplified with the same set of primers. Note that, in these cells, 2LTR circles were not present.
Restricted replication of X4, but not R5, HIV-1 in resting CD4+ T cells in the absence of MEK/ERK signaling.
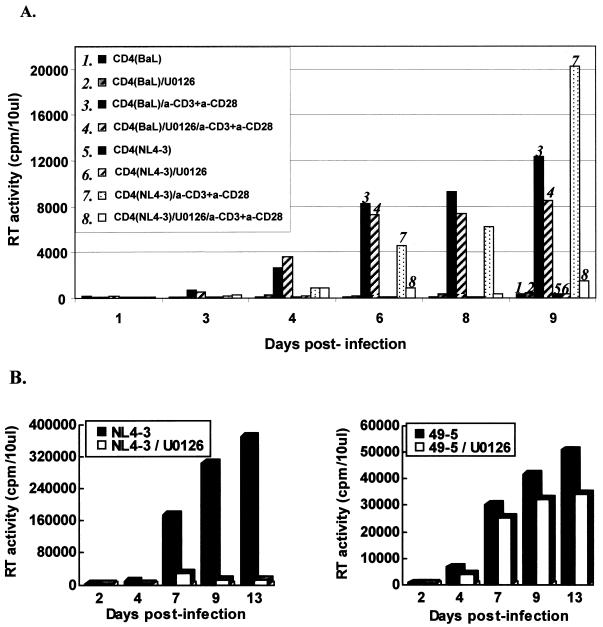
We next studied the ability of the anti-CD3/anti-CD28 antibodies to stimulate productive infection in infected resting CD4+ T cells in which the MEK/ERK pathway was inhibited. The cells, infected with X4 NL4-3 or R5 Ba-L or with the isogenic pair of HIV-1 strains, NL4-3 and 49-5, were stimulated with immobilized monoclonal anti-CD3 and anti-CD28 antibodies in the presence or absence of the specific MEK/ERK pathway inhibitor U0126. Productive replication and the amount of the virus released into the medium were determined by analysis of RT activity in culture supernatants. Figure 2A shows that costimulation with anti-CD3/CD28 antibodies rendered cells competent for productive infection. At days 3 and 4 after stimulation, replication of Ba-L and NL4-3 was clearly detected, although the levels of Ba-L HIV-1 replication were initially two- to threefold higher than those of NL4-3 replication. The presence of U0126 did not significantly affect the replication of R5 HIV-1 Ba-L. In contrast, replication of X4 NL4-3 was severely inhibited in the presence of MAPK inhibitor. The inhibitory effect of U0126 on the X4 HIV-1 replication did not result from a general toxicity of the drug, since replication of Ba-L was not significantly affected over a period of 9 days.
FIG. 2.
Restricted replication of X4 HIV-1 in infected resting CD4+ T lymphocytes in the absence of MEK/ERK pathway signaling. Purified peripheral blood CD4+ T lymphocytes were infected with NL4-3 or Ba-L (A) or with a pair of isogenic viruses, 49-5 and NL4-3 (B), as described in Materials and Methods. Subsequently, the cells were left untreated or were stimulated with immobilized anti-CD3/CD28 antibodies in the absence or presence of U0126 inhibitor (10 μM). Virus production was monitored by the RT activity released into culture medium.
To provide more definitive evidence for the role of R5 and X4 HIV-1 envelopes in the observed effect and to exclude possible effects of other viral determinants, we used a pair of isogenic viruses, NL4-3 and 49-5, which differ exclusively in the envelope region. A recombinant clone, 49-5 (24), is based on X4 NL4-3 and contains the envelope V3 loop sequence derived from R5 Ba-L HIV-1. The results presented in Fig. 2B show that, in contrast to X4 NL4-3, replication of R5 49-5 HIV-1, which uses CCR5 for entry (24), was not significantly inhibited in the presence of the MEK/ERK inhibitor. Together, these results suggest that the MEK/ERK activity stimulated by CD3/CD28 is required to support viral replication and productive infection of X4 HIV-1.
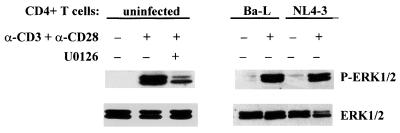
To ensure that U0126 inhibits the MEK/ERK pathway in CD4+ T cells and to exclude the possibility that ERK activity may be differentially altered upon infection, we examined the phosphorylation status of ERK1/2 in uninfected cells as well as in cells infected with Ba-L and NL4-3. The cells were preincubated for 30 min without or with U0126 at 10 μM and activated with immobilized anti-CD3/CD28 antibodies for 24 h, and then ERK1/2 phosphorylation was assessed in cell lysates by Western blot analysis with phosphospecific antibodies. Figure 3 shows that U0126 significantly inhibited phosphorylation and activation of ERK1/2. In addition, we did not observe any significant changes in the activation of ERK by anti-CD3/CD28 antibodies in infected cells.
FIG. 3.
U0126 inhibits ERK1/2 phosphorylation induced by CD3 and CD28 ligation in CD4+ T lymphocytes. Purified peripheral blood CD4+ T cells (uninfected) were preincubated for 30 min at 37°C in the absence or presence of MEK/ERK inhibitor U0126 (10 μM). Cells infected with Ba-L or NL4-3, as well as the uninfected cells preincubated in the absence of U0126, were subsequently activated with plastic-immobilized monoclonal anti-CD3/CD28 antibodies or were left unactivated. Cells preincubated with U0126 were activated with immobilized anti-CD3/CD28 antibodies for 24 h in the presence of the inhibitor before lysis. Triton X-100 cell lysates were prepared, and proteins (20 μg/lane) were resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and probed with antibodies specific for phosphorylated forms of ERK1/2 (P-ERK1/2) and total ERK1/2.
Restricted replication of X4 NL4-3 does not result from downregulation of CD4 or CXCR4 expression.
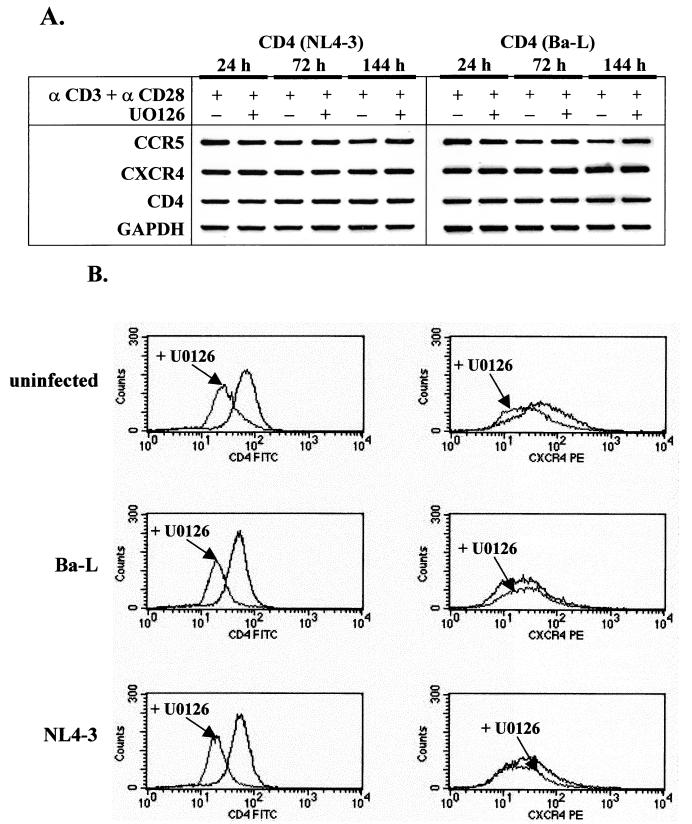
To examine whether the observed restriction of NL4-3 replication can be ascribed to the downregulation of CD4 and/or CXCR4 receptors by the inhibitor, we first analyzed the expression of HIV-1 receptor and coreceptor mRNAs by RT-PCR in cells infected with NL4-3. For comparison, we also analyzed the expression of HIV-1 receptor mRNAs in cells infected with Ba-L HIV-1. Figure 4A shows that the presence of U0126 did not affect significantly the expression of CD4 or CXCR4 mRNA after stimulation of the cells with anti-CD3/CD28 antibodies over a period of 6 days. In addition, no major differences in the expression of CCR5 coreceptor were detected. Since the expression of HIV-1 receptors could be altered by posttranscriptional mechanisms, we analyzed the cell surface expression of CD4 and CXCR4 receptors by FACS.
FIG. 4.
Analysis of the chemokine and CD4 receptor expression in HIV-1-infected CD4+ T lymphocytes stimulated with anti-CD3/CD28 antibodies in the presence or absence of MEK/ERK signaling. (A) Total RNA was isolated from purified CD4+ T cells infected with NL4-3 or Ba-L and stimulated for the indicated period of time with immobilized anti-CD3/CD28 antibodies in the absence or presence of MEK/ERK inhibitor U0126 (10 μM). PCR analysis was performed with synthesized cDNA under conditions described in Materials and Methods. PCR products were resolved by electrophoresis in 2.5% agarose gels and stained with ethidium bromide. GAPDH mRNA served as an internal control. (B) FACS analysis of surface expression of CD4 and CXCR4 in resting CD4+ T lymphocytes. Uninfected resting CD4+ T cells and cells infected with Ba-L or NL4-3 and stimulated for 6 days with immobilized anti-CD3/CD28 antibodies in the absence or presence of U0126 (10 μM) were stained with PE- or FITC-conjugated monoclonal antibodies and subsequently analyzed as described in Materials and Methods.
We have found that in the presence of U0126 inhibitor, surface expression of CD4 receptors was downregulated by about 51% in both uninfected and HIV-1-infected CD4+ T lymphocytes (Fig. 4B) and thus seems unlikely to result in specific inhibition of NL4-3, but not Ba-L or 49-5, HIV-1 replication. The surface expression of CXCR4 receptors was not significantly changed by the inhibitor or infection. In the presence of the inhibitor, CXCR4 was expressed at levels of 74 to 117%, compared with those in controls in the absence of the inhibitor. We therefore conclude that restricted replication of X4 NL4-3 in the presence of MEK/ERK inhibitor does not result from a specific downregulation of the expression of CD4 or CXCR4 receptors. This conclusion is also underscored by the fact that, in our model, a significant portion of the resting cells were infected, and therefore the initial replication of HIV-1 should be independent of the level of HIV-1 receptor or coreceptor expression.
In addition, the levels of the earliest reverse transcription products (R/U5 signal) formed after X4 and R5 HIV-1 entry were similar in the absence or presence of U0126 (Fig. 5). This suggests that some changes in the surface expression of the HIV-1 receptors do not play a major role in the observed restricted replication of NL4-3 in the presence of MEK/ERK inhibitor.
Activation of the MEK/ERK signaling pathway is not required for the early preintegration steps of HIV-1 replication.
To define MAPK-dependent steps in HIV-1 replication that control establishment of productive infection in resting CD4+ T lymphocytes, we used PCR to analyze proviral reverse transcription products. The set of oligonucleotide primers that can specifically identify different forms of proviral DNA transcripts was used (7, 30). Infected CD4+ T cells were collected at different times after stimulation with anti-CD3/CD28 antibodies, and the first products of reverse transcription synthesized after viral entry were identified by amplification of total DNA with the LTR R/U5 primers. It can be seen that these early proviral transcripts were present in both unstimulated and stimulated cells infected with either R5 Ba-L (Fig. 5A) or X4 NL4-3 (Fig. 5B) HIV-1. Inhibition of the MAPK pathway by treatment of the cells with U0126 inhibitor did not affect the levels of the amplified R/U5 DNA, indicating that the early step of reverse transcription is independent of the MAPK pathway and CD3/CD28 stimulation. In unstimulated cells infected with NL4-3, the R/U5 signals significantly diminished on day 6 (144 h), suggesting that the unintegrated proviral DNA disappears faster in X4 NL4-3-infected cells than in cells infected with R5 Ba-L HIV-1.
The LTR/gag fragments representing DNA formed after the second template switch were present at 24 h poststimulation in the CD3/CD28-stimulated cells and the unstimulated controls. The relative levels of LTR/gag DNA were not significantly affected by the presence of U0126 inhibitor. However, the levels of LTR/gag DNA increased at 72 h after the CD3/CD28 stimulation in both cells infected with NL4-3 and those infected with Ba-L and remained increased over a period of 6 days after stimulation. This contrasts with the accumulation of the transcripts in unstimulated cells that could be detected on day 6 only in cells infected with Ba-L, but not those infected with NL4-3 HIV-1. These results indicate that in cells infected with X4 or R5 HIV-1, virus transcription proceeds almost to completion, even in the absence of the MAPK signaling pathway. However, in unstimulated cells, the levels and/or stability of preintegration forms of HIV-1 DNA were significantly higher in cells infected with R5 than in those infected with X4 HIV-1.
Activation of ERK is required for successful completion of reverse transcription or nuclear import of X4 NL4-3 proviral DNA in infected CD4+ T cells.
We further investigated whether activation of the MEK/ERK pathway induced by engagement of TCR/CD28 is required for completion of reverse transcription or nuclear import of X4 HIV-1 DNA. To this end, we monitored the levels of PCR-amplified circular proviral DNA (2LTR signal) as a marker for full completion of reverse transcription and translocation of the preintegration complexes into the nucleus. Since 2LTR viral DNA is formed exclusively in the nucleus (after synthesis of full-length viral DNA), it is commonly used as a marker for completion of preintegration steps and nuclear import of HIV-1 DNA (43). The amount of total DNA was controlled by amplifying the cellular β-globin gene. In addition, as a control, cellular lysates from HIV-1-infected ACH-2 cells, containing one integrated HIV-1 provirus per cell, were prepared and amplified with the same set of primers (Fig. 5D). Figure 5 shows that, in the absence of CD3/CD28 stimulation, the levels of 2LTR DNA circles were undetectable in cells infected with NL4-3. However, very faint bands, corresponding to 2LTR circles, could be detected at 24 h poststimulation in cells infected with Ba-L.
Formation of 2LTR circles was detected in infected cells stimulated for at least 72 h with anti-CD3/CD28 antibodies. However, in CD4+ T cells infected with X4 NL4-3 and stimulated through TCR/CD28 in the presence of MEK/ERK inhibitor, the relative levels of 2LTR circle signals were significantly lower (about 10-fold [Fig. 5C]) than those in infected cells stimulated in the absence of the inhibitor. In contrast, in cells infected with Ba-L (Fig. 5A) or R5 49-5 (Fig. 5C) HIV-1, containing the envelope V3 loop from Ba-L in the background of NL4-3, the presence of MEK/ERK inhibitor did not significantly affect formation of 2LTR DNA circles. These results suggest that full completion of preintegration steps and/or nuclear import of X4 HIV-1 DNA, but not R5 HIV-1 provirus, is significantly impaired in the presence of MEK/ERK pathway inhibition.
The levels of integrated HIV-1 DNA were determined by using nested PCR amplification with Alu-LTR primers in the initial PCR and a pair of NI-2 primers, which allows amplification of a portion of the HIV-1 LTR, for the second PCR (7). Integrated DNA was detected only in infected cells stimulated for 72 h with anti-CD3/CD28 antibodies. No integration signal was present in infected unstimulated cells. However, a significant difference in integration of Ba-L and NL4-3 provirus was observed in the cells treated with MEK/ERK inhibitor. While the integration of HIV-1 Ba-L DNA was unaffected in the presence of U0126 inhibitor, integration of NL4-3 DNA was greatly inhibited. Since we have shown that the completion of the preintegration steps of NL4-3 HIV-1 provirus is significantly inhibited by the MEK/ERK inhibitor, we assume that, as a consequence, integration of the provirus is inhibited as well. However, it is not clear whether the MEK/ERK pathway directly regulates the process of integration of proviral DNA into the host genome.
Together, our results suggest that activation of the MAPK ERK pathway during CD3/CD28 stimulation is necessary for the full completion of the preintegration steps and/or nuclear import of X4 HIV-1 DNA and replication of the virus in CD4+ T lymphocytes. In contrast, CD3/CD28-induced MEK/ERK signaling is not required for efficient replication of R5 Ba-L or 49-5 HIV-1 in CD4+ T cells. Studies are in progress to delineate the mechanism(s) responsible for the observed differential requirements for MEK/ERK signaling for the replication of X4 and R5 HIV-1.
DISCUSSION
We have shown in this study that replication of R5 HIV-1 in infected resting peripheral blood CD4+ T lymphocytes stimulated with anti-CD3/CD28 antibodies is independent of the stimulation of the MEK/ERK pathway. In contrast, replication of X4 HIV-1 was dependent on the presence of a functional MEK/ERK pathway. We have further shown that this restricted replication of X4, but not R5, HIV-1 was a result of inefficient completion of late preintegration steps and/or nuclear import of proviral DNA.
To mimic the activation of T cells that takes place during antigen presentation, we have used an in vitro model of resting CD4+ T cells infected with X4 or R5 HIV-1 and subsequently stimulated by cross-linking of CD3 and CD28 receptors with plastic-immobilized monoclonal antibodies (32). Importantly, this stimulation allows for productive replication of both X4 and R5 HIV-1 in the cells. Since it was shown that CD4+ T cells downregulated CCR5, but not CXCR4, expression in response to CD28 costimulation (27), we used cells that were infected with HIV-1 before stimulation with anti-CD3/CD28 antibodies. Our results showed that both X4 and R5 HIV-1 entered the cells with comparable efficiency in the absence of stimulation, as determined by analysis of the levels of R/U5 DNA, the product of HIV-1 reverse transcription synthesized shortly after viral entry.
It is well established that the MEK/ERK pathway constitutes a part of TCR/CD28-mediated signaling involved in T-cell activation (5) and can be specifically inhibited by the MEK/ERK inhibitor U0126 (15). Accordingly, we have shown that stimulation of the resting CD4+ T cells with the anti-CD3/CD28 antibodies in the presence of U0126 results in a significant inhibition of ERK phosphorylation and activation (Fig. 3). In addition, we did not observe any significant changes in the activation of ERK by the anti-CD3/CD28 antibodies in uninfected and infected cells. Thus, differential replication of X4 and R5 HIV-1 in cells stimulated in the presence of U0126 suggests that X4 and R5 viruses may differ in their requirements for the MEK/ERK stimulation for viral replication. By using a pair of isogenic viruses, X4 NL4-3 and R5 49-5, that differ only in the V3 region of the envelope, we showed that the observed restricted replication of X4 HIV-1 in the absence of MEK/ERK signaling can be overcome by changing the HIV-1 coreceptor specificity.
We speculate that the observed differences may be related to differential signaling induced upon entry of R5 and X4 viruses. In this regard, we have shown that the interaction of SIVs with CCR5 stimulated the MEK/ERK pathway (26). In contrast, engagement of CXCR4 by X4 HIV-1 did not induce this pathway (25). However, both X4 and R5 viruses stimulated the MEK/ERK pathway through CD4 receptor, independently of signaling through chemokine receptors (3, 25, 26). Interestingly, engagement of CD4 and CCR5 receptors by SIV significantly enhanced the MEK/ERK as well as JNK and p38 MAPK signaling, compared to binding to CCR5-negative cells. These results suggest that CD4 receptor-induced signaling can be significantly intensified by the engagement of CCR5, but not CXCR4, coreceptors. Consequently, R5 HIV-1 replication in “preactivated” cells may require suboptimal activation delivered by the engagement of CD3 and CD28 receptors in the absence of MEK/ERK activity. In contrast, X4 HIV-1 will require MEK/ERK activity induced by CD3/CD28 receptors for optimal replication. Whether these quantitative differences in expression of the MEK/ERK cascade or possible synergistic interaction with other HIV-1 binding-induced pathways lead to the observed different outcome for X4 and R5 HIV-1 replication requires further investigation.
Since we performed our experiments with unselected primary CD4+ T lymphocytes, we cannot exclude the possibility that the observed effects may be at least partially due to infection of different CD4+ T-cell subpopulations by X4 and R5 HIV-1. Two major subsets of CD4+ T cells, naive and memory cells, differ functionally from each other and show different patterns of intracellular signaling upon CD3 stimulation (17). However, previous studies showed that surface expression of the HIV-1 coreceptors on CD4+ T cells was differentially expressed on naive versus memory T cells, with CCR5 mostly restricted to the CD26 high subset of memory CD45RO+ cells and CXCR4 expressed on both memory and naive CD45RA+ CD4+ T cells (2, 22). In addition, it was shown that X4 HIV-1 replicates preferentially in memory cells (32) and does not replicate productively in naive cells stimulated with anti-CD3/CD28 antibodies (28). It therefore seems conceivable that X4 and R5 HIV-1 replicated preferentially in a population of memory cells in our in vitro system. However, whether the memory cells are the only infected subset of CD4+ T cells in our system is not clear.
In accordance with our results, it was recently shown that R5 HIV-1 has a significant replicative advantage over X4 HIV-1 in suboptimally activated T lymphocytes (37). However, the nature of this envelope-dependent restriction was not clarified. Similarly, recent results suggest that R5 and X4 HIV-1 induce different pathogenic effects in hu-PBL-SCID mice, depending on the state of activation of T cells (13). While X4 strains were highly virulent only when injected at a time when the transferred human T cells were highly activated, the R5 HIV-1 strains caused CD4+ T-cell depletion and immune dysfunction independent of the state of activation of the target cells.
To define MEK/ERK-dependent steps in the viral life cycle that restrict replication of X4 HIV-1 in CD4+ T cells, we have used PCR analysis. In our model, a possible downregulation of HIV-1 receptors should not have a critical effect early in HIV-1 infection, since CD4+ T cells were “latently” infected, and thus HIV-1 early replication events did not involve engagement of HIV-1 receptors. Although we did not find any evidence of a significant modulation of CD4, CXCR4, or CCR5 at the transcriptional levels, surface expression of the CD4 receptor was decreased by 51% in the presence of MEK/ERK inhibitor. Interestingly, a similar reduction was observed in both uninfected and infected cells. Together with the observation that CXCR4 coreceptor was not significantly modulated by U0126, changes in the expression of HIV-1 receptors cannot satisfactorily explain the observed restriction of the X4 HIV-1 replication in resting CD4+ T cells.
PCR analysis of the preintegration stages of HIV-1 replication performed with LTR/gag-specific primers suggested that reverse transcription proceeded significantly to completion in CD3/CD28-stimulated infected cells and was independent of MEK/ERK activation. Using LTR/gag primers, we could not conclude whether the reverse transcription proceeded to full completion, resulting in double-stranded and blunt-ended DNAs that serve as the substrate for HIV-1 integrase. However, the 2LTR circular forms of viral DNA that are formed exclusively in the nucleus (36) are used as a marker for full completion of reverse transcription and translocation of the preintegration complex into the nucleus (43). Using primers detecting 2LTR circle forms of HIV-1 DNA, we have found that a full completion of preintegration steps and/or nuclear import of X4 HIV-1 DNA was significantly impaired in the presence of MEK/ERK inhibitor. In contrast, nuclear import of R5 HIV-1 DNA was not significantly affected by the inhibitor. Similarly, integration of HIV-1 DNA into the host genome followed the same pattern. However, it is not clear whether the MEK/ERK pathway directly regulates some aspects of integration or merely overcomes a primary block related to full completion of reverse transcription and/or nuclear import of preintegration complexes.
The nature of the specific MEK/ERK targets that may be involved in nuclear import of preintegration complexes is not clear. It has been suggested that phosphorylation of HIV-1 Gag MA protein localized in the viral reverse transcription complexes is required for nuclear transport of viral DNA (4). However, whether HIV-1 Gag represents the only viral target for MEK/ERK is unknown. It seems possible that cellular factors that serve as MEK/ERK substrates may also be required for efficient completion of reverse transcription and nuclear import of preintegration complexes. In this regard, a nuclear factor of activated T cells, NFATc, was shown to facilitate completion of HIV-1 reverse transcription (20). Interestingly, regulation of NFATc in T cells requires the activity of multiple effector pathways, including ERK (16). In addition, it was suggested that c-Myc, which is regulated by the MEK/ERK pathway (19), controls HIV-1 DNA nuclear import without an effect on viral full-length synthesis (34). Further experiments will be required in order to understand the role of MEK/ERK signaling in X4 and R5 HIV-1 replication and pathogenesis.
ACKNOWLEDGMENTS
This study was supported by NIH grants AI42557 (W.P.) and AI40838 (P.M.P.).
We thank B. Chesebro for the HIV-1 49-5 plasmid and D. Schols for the gift of AMD3100, T. Pierson for help with FACS analysis, and T. Alce for critical reading of the manuscript.
REFERENCES
- 1.Atchison R E, Gosling J, Monteclaro F S, Franci C, Digilio L, Charo I F, Goldsmith M A. Multiple extracellular elements of CCR5 and HIV-1 entry: dissociation from response to chemokines. Science. 1996;274:1924–1926. doi: 10.1126/science.274.5294.1924. [DOI] [PubMed] [Google Scholar]
- 2.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briant L, Robert-Hebmann V, Sivan V, Brunet A, Pouyssegur J, Devaux C. Involvement of extracellular signal-regulated kinase module in HIV-mediated CD4 signals controlling activation of nuclear factor-κB and AP-1 transcription factors. J Immunol. 1998;160:1875–1885. [PubMed] [Google Scholar]
- 4.Bukrinskaya A G, Ghorpade A, Heinzinger N K, Smithgall T E, Lewis R E, Stevenson M. Phosphorylation-dependent human immunodeficiency virus type 1 infection and nuclear targeting of viral DNA. Proc Natl Acad Sci USA. 1996;93:367–371. doi: 10.1073/pnas.93.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantrell D. T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 6.Chackerian B, Long E M, Luciw P A, Overbaugh J. Human immunodeficiency virus type 1 coreceptors participate in postentry stages in the virus replication cycle and function in simian immunodeficiency virus infection. J Virol. 1997;71:3932–3939. doi: 10.1128/jvi.71.5.3932-3939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chun T-W, Stuyver L, Mizell S B, Ehler A L, Mican J A, Baseler M, Lloyd A L, Nowak M A, Fauci A S. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cicala C, Arthos J, Ruiz M, Vaccarezza M, Rubbert A, Riva A, Wildt K, Cohen O, Fauci A S. Induction of phosphorylation and intracellular association of CC chemokine receptor 5 and focal adhesion kinase in primary human CD4+ T cells by macrophage-tropic HIV envelope. J Immunol. 1999;163:420–426. [PubMed] [Google Scholar]
- 9.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis C B, Dikic I, Unutmaz D, Hill C M, Arthos J, Siani M A, Thompson D A, Schlessinger J, Littman D R. Signal transduction due to HIV-1 envelope interactions with chemokine receptors CXCR4 or CCR5. J Exp Med. 1997;186:1793–1798. doi: 10.1084/jem.186.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donzella G A, Schols D, Lin S W, Este J A, Nagashima K A, Maddon P J, Allaway G P, Sakmar T P, Henson G, De Clercq E, Moore J P. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4:72–77. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- 12.Doranz B J, Orsini M J, Turner J D, Hoffman T L, Berson J F, Hoxie J A, Peiper S C, Brass L F, Doms R W. Identification of CXCR4 domains that support coreceptor and chemokine receptor functions. J Virol. 1999;73:2752–2761. doi: 10.1128/jvi.73.4.2752-2761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fais S, Lapenta C, Santini S M, Spada M, Parlato S, Logozzi M, Rizza P, Belardelli F. Human immunodeficiency virus type 1 strains R5 and X4 induce different pathogenic effects in hu-PBL-SCID mice, depending on the state of activation/differentiation of human target cells at the time of primary infection. J Virol. 1999;73:6453–6459. doi: 10.1128/jvi.73.8.6453-6459.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farzan M, Choe H, Martin K A, Sun Y, Sidelko M, Mackay C R, Gerard N P, Sodroski J, Gerard C. HIV-1 entry and macrophage inflammatory protein-1-β-mediated signaling are independent functions of the chemokine receptor CCR5. J Biol Chem. 1997;272:6854–6857. doi: 10.1074/jbc.272.11.6854. [DOI] [PubMed] [Google Scholar]
- 15.Favata M F, Horiuchi K Y, Manos E J, Daulerio A J, Stradley D A, Feeser W S, Van Dyk D E, Pitts W J, Earl R A, Hobbs F, Copeland R A, Magolda R L, Scherle P A, Trzaskos J M. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 16.Genot E, Cleverley S, Henning S, Cantrell D. Multiple p21ras effector pathways regulate nuclear factor of activated T cells. EMBO J. 1996;15:3923–3933. [PMC free article] [PubMed] [Google Scholar]
- 17.Hall S R, Heffernan B M, Thompson N T, Rowan W C. CD4+ CD45RA+ and CD4+ CD45RO+ T cells differ in their TCR-associated signaling responses. Eur J Immunol. 1999;29:2098–2106. doi: 10.1002/(SICI)1521-4141(199907)29:07<2098::AID-IMMU2098>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 18.Jacque J-M, Mann A, Enslen H, Sharova N, Brichacek B, Davis R J, Stevenson M. Modulation of HIV-1 infectivity by MAPK, a virion-associated kinase. EMBO J. 1998;17:2607–2618. doi: 10.1093/emboj/17.9.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerkhoff E, Houben R, Loffler S, Troppmair J, Lee J E, Rapp U R. Regulation of c-myc expression by Ras/Raf signalling. Oncogene. 1998;16:211–216. doi: 10.1038/sj.onc.1201520. [DOI] [PubMed] [Google Scholar]
- 20.Kinoshita S, Chen B K, Kaneshima H, Nolan G P. Host control of HIV-1 parasitism in T cells by the nuclear factor of activated T cells. Cell. 1998;95:595–604. doi: 10.1016/s0092-8674(00)81630-x. [DOI] [PubMed] [Google Scholar]
- 21.Korin Y D, Zack J A. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J Virol. 1998;72:3161–3168. doi: 10.1128/jvi.72.4.3161-3168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee B, Sharron M, Montaner L J, Weissman D, Doms R W. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci USA. 1999;96:5215–5220. doi: 10.1073/pnas.96.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Misse D, Cerutti M, Noraz N, Jourdan P, Favero J, Devauchelle G, Yssel H, Taylor N, Veas F. A CD4-independent interaction of human immunodeficiency virus-1 gp120 with CXCR4 induces their cointernalization, cell signaling, and T-cell chemotaxis. Blood. 1999;93:2454–2462. [PubMed] [Google Scholar]
- 24.Penn M L, Grivel J-C, Schramm B, Goldsmith M A, Margolis L. CXCR4 utilization is sufficient to trigger CD4+ T cell depletion in HIV-1-infected human lymphoid tissue. Proc Natl Acad Sci USA. 1999;96:663–668. doi: 10.1073/pnas.96.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Popik W, Hesselgesser J E, Pitha P M. Binding of human immunodeficiency virus type 1 to CD4 and CXCR4 receptors differentially regulates expression of inflammatory genes and activates the MEK/ERK signaling pathway. J Virol. 1998;72:6406–6413. doi: 10.1128/jvi.72.8.6406-6413.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Popik W, Pitha P M. Early activation of mitogen-activated protein kinase kinase, extracellular signal-regulated kinase, p38 mitogen-activated protein kinase, and c-Jun N-terminal kinase in response to binding of simian immunodeficiency virus to Jurkat T cells expressing CCR5 receptor. Virology. 1998;252:210–217. doi: 10.1006/viro.1998.9466. [DOI] [PubMed] [Google Scholar]
- 27.Riley J L, Levine B L, Craighead N, Francomano T, Kim D, Carroll R G, June C H. Naïve and memory CD4 T cells differ in their susceptibilities to human immunodeficiency virus type 1 infection following CD28 costimulation: implications for transmission and pathogenesis. J Virol. 1998;72:8273–8280. doi: 10.1128/jvi.72.10.8273-8280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roederer M, Raju P A, Mitra D K, Herzenberg L A, Herzenberg L A. HIV does not replicate in naive CD4 T cells stimulated with CD3/CD28. J Clin Investig. 1997;99:1555–1564. doi: 10.1172/JCI119318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scarlatti G, Tresoldi E, Bjorndal A, Fredriksson R, Colognesi C, Deng H K, Malnati M S, Plebani A, Siccardi A G, Littman D R, Fenyo E M, Lusso P. In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nat Med. 1997;3:1259–1265. doi: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]
- 30.Schmidtmayerova H, Alfano M, Nuovo G, Bukrinsky M. Human immunodeficiency virus type 1 T-lymphotropic strains enter macrophages via a CD4- and CXCR4-mediated pathway: replication is restricted at a postentry level. J Virol. 1998;72:4633–4642. doi: 10.1128/jvi.72.6.4633-4642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuitemaker H, Koot M, Kootstra N A, Dercksen M W, de Goede R E Y, van Steenwijk R P, Lange J M A, Eeftink Schattenkerk J K M, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spina C A, Prince H E, Richman D D. Preferential replication of HIV-1 in the CD45RO memory cell subset of primary CD4 lymphocytes in vitro. J Clin Investeg. 1997;99:1774–1785. doi: 10.1172/JCI119342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spina C A, Guatelli J C, Richman D D. Establishment of a stable, inducible form of human immunodeficiency virus type 1 DNA in quiescent CD4 lymphocytes in vitro. J Virol. 1995;69:2977–2988. doi: 10.1128/jvi.69.5.2977-2988.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun Y, Clark E A. Expression of the c-myc proto-oncogene is essential for HIV-1 infection in activated T cells. J Exp Med. 1999;189:1391–1397. doi: 10.1084/jem.189.9.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Y, Pinchuk L M, Agy M B, Clark E A. Nuclear import of HIV-1 DNA in resting CD4+ T cells requires a cyclosporin A-sensitive pathway. J Immunol. 1997;158:512–517. [PubMed] [Google Scholar]
- 36.Varmus H, Swanstrom R. Replication of retroviruses. In: Weiss R, Teich N, Varmus H, Coffin J, editors. RNA tumor viruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1985. p. 369. [Google Scholar]
- 37.Vicenzi E, Bordignon P P, Biswas P, Brambilla A, Bovolenta C, Cota M, Sinigaglia F, Poli G. Envelope-dependent restriction of human immunodeficiency virus type 1 spreading in CD4+ T lymphocytes: R5 but not X4 viruses replicate in the absence of T-cell receptor restimulation. J Virol. 1999;73:7515–7523. doi: 10.1128/jvi.73.9.7515-7523.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weissman D, Rabin R L, Arthos J, Rubbert A, Dybul M, Swofford R, Venkatesan S, Farber J M, Fauci A S. Macrophage-tropic HIV and SIV envelope proteins induce a signal through the CCR5 chemokine receptor. Nature. 1997;389:981–985. doi: 10.1038/40173. [DOI] [PubMed] [Google Scholar]
- 39.Yang X, Gabuzda D. Mitogen-activated protein kinase phosphorylates and regulates the HIV-1 Vif protein. J Biol Chem. 1998;273:29879–29887. doi: 10.1074/jbc.273.45.29879. [DOI] [PubMed] [Google Scholar]
- 40.Yang X, Gabuzda D. Regulation of human immunodeficiency virus type 1 infectivity by the ERK mitogen-activated protein kinase signaling pathway. J Virol. 1999;73:3460–3466. doi: 10.1128/jvi.73.4.3460-3466.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zack J, Haislip A M, Krogstad P, Chen I S Y. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol. 1992;66:1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Genotypic and phenotypic characterization of HIV-1 in patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 43.Zybarth G, Reiling N, Schmidtmayerova H, Sherry B, Bukrinsky M. Activation-induced resistance of human macrophages to HIV-1 infection in vitro. J Immunol. 1999;162:400–406. [PubMed] [Google Scholar]