Abstract
Virus infections are devastating to neonates, and the induction of active antiviral immunity in this age group is an important goal. Here, we show that a single neonatal DNA vaccination induces cellular and humoral immune responses which are maintained for a significant part of the animal's life span. We employ a sensitive technique which permits the first demonstration and quantitation, directly ex vivo, of virus-specific CD8+ T cells induced by DNA immunization. One year postvaccination, antigen-specific CD8+ T cells were readily detectable and constituted 0.5 to 1% of all CD8+ T cells. By several criteria—including cytokine production, perforin content, development of lytic ability, and protective capacity—DNA vaccine-induced CD8+ memory T cells were indistinguishable from memory cells induced by immunization with a conventional (live-virus) vaccine. Analyses of long-term humoral immune responses revealed that, in contrast to the strong immunoglobulin G2a (IgG2a) skewing of the humoral response seen after conventional vaccination, IgG1 and IgG2a levels were similar in DNA-vaccinated neonatal and adult animals, indicating a balanced T helper response. Collectively, these results show that a single DNA vaccination within hours or days of birth can induce long-lasting CD8+ T- and B-cell responses; there is no need for secondary immunization (boosting). Furthermore, the observed immune responses induced in neonates and in adults are indistinguishable by several criteria, including protection against virus challenge.
Conventional vaccines provide the safest and most effective prophylactic measures against viral diseases. A focused vaccination program has led to the eradication of smallpox and, by the early years of the next millenium, global vaccination should have eliminated poliomyelitis. Despite these successes, infectious diseases remain major contributors to human morbidity and mortality. The World Health Organization mortality figures for 1998 place infections (acute lower-respiratory-tract infections and human immunodeficiency virus) as the third and fourth most frequent causes of adult death worldwide, and infectious diseases kill 2 to 5 million children (<5 years of age) annually. For several reasons, neonates and infants are at heightened risk of viral infection and disease, but vaccination is often delayed in this age group, in part because maternal antibodies can inactivate conventional vaccines, preventing the induction of active immunity in the young. The lack of a widely applicable vaccination strategy which could safely and effectively confer lifelong protective immunity after a single administration early in life has led us to examine the efficacy of DNA vaccination in neonates. We (13) and others (6, 24, 26, 27, 39) have previously shown that DNA vaccination in the first few hours of life can prime both cytotoxic T lymphocyte (CTL) and antibody responses. Furthermore, protective immunity can be induced against some pathogens even when the vaccine recipient carries maternal antibodies (13, 21), validating one proposed advantage of DNA vaccination. However, the longevity, quantity, and quality of DNA-induced neonatal responses have not been characterized.
In our studies, we have used Lymphocytic choriomeningitis virus (LCMV), the prototype of the Arenaviridae family, which includes human pathogens such as Lassa virus. LCMV is itself a human pathogen (causing aseptic meningitis) and teratogen (causing hydrocephalus) (19, 32, 44). LCMV infection of its natural host, the mouse, is well characterized. In this model, viral clearance and protective immunity are effected primarily by major histocompatibility complex (MHC) class I restricted CD8+ T cells (7, 17, 30). In BALB/c mice (H-2d) the overwhelming majority of antiviral CTLs are specific for a single immunodominant, nonameric CTL epitope peptide contained within the viral nucleoprotein (NP) (42), and a recombinant viral vaccine encoding this sequence can confer solid protection against subsequent LCMV challenge (17). Protective immunity also can be conferred by DNA vaccines expressing either the full-length NP gene (23, 47, 48) or a minigene encoding a ubiquitinated form of the dominant epitope (29).
The overall goal of the present study was to establish whether DNA-induced protective immunity could be maintained for a significant fraction of an animal's life span in the absence of revaccination. We have (i) demonstrated, for the first time, DNA-induced CD8+ T-cell responses directly ex vivo (in the absence of significant secondary stimulation); (ii) quantitated these responses and compared them to those induced by a conventional (live-virus) vaccine; (iii) evaluated perforin and gamma interferon (IFN-γ) levels in CD8+ memory T cells induced by DNA immunization and compared them to those in memory cells induced by conventional immunization; (iv) demonstrated CTL memory up to a year after a single DNA vaccination of both neonates and adults; (v) evaluated the serum antibody levels and immunoglobulin G (IgG) isotypes 6 months postvaccination; and (vi) determined the protective efficacy of the immune responses at these late time points.
MATERIALS AND METHODS
Mice.
All mice were BALB/c (H-2d haplotype). Adult animals were purchased from the Scripps Research Institute breeding facility and were bred (by D.E.H.) to generate mice for neonatal DNA immunization.
DNA immunizations.
The plasmid pCMVNP encodes the full length LCMV NP (Armstrong strain); pCMV, the vector control, contains no LCMV sequences. The construction of both of these plasmids has been previously described (47). Plasmids were propagated in Escherichia coli by standard techniques and purified with an Endofree plasmid purification kit (Qiagen, Chatsworth, Calif.) according to the manufacturer's instructions. Adult mice (6 to 16 weeks of age) received a single 50-μg dose of plasmid DNA dissolved in 50 μl of saline in the tibialis anterior muscle. Neonatal mice (3 days old) were injected in the upper left thigh with 50 μg of plasmid dissolved in 25 μl of saline.
Intracellular cytokine staining (ICCS) and perforin staining.
One year after DNA vaccination, splenocytes were harvested and single cell suspensions of splenocytes were prepared. Cells (2 × 106) were incubated for 5 h in 200 μl of RPMI 1640 medium containing 50 μM 2-mercaptoethanol, 150 U of recombinant human interleukin-2 per ml, and 2 μg of brefeldin A per ml in the presence or absence of 10−7 M peptide corresponding to the immunodominant H-2d-restricted LCMV NP CD8+ T-cell epitope (NP118–126; sequence RPQASGVYM). After stimulation, the cells were washed with phosphate-buffered saline (PBS) and 5% fetal bovine serum (FBS), stained for 30 min with an anti-mouse CD8 cychrome-conjugated antibody (PharMingen, San Diego, Calif.), washed with PBS and 5% FBS, fixed in cold PBS and 2% formaldehyde, and permeabilized with Cytofix-Cytoperm (PharMingen). Intracellular cytokines were stained with fluorescein isothiocyanate-labeled antibody specific for mouse IFN-γ (PharMingen). To detect intracellular perforin, cells were fixed with 2% formaldehyde in PBS for 20 min on ice, washed, and permeabilized with 0.1% saponin (Sigma, St. Louis, Mo.) in PBS containing 1% FBS. Perforin antibody (clone P1-8, Kamiya Biomedical Co.) was diluted 1:400 in 0.3% saponin-PBS and added to the cells. Following a 30-min incubation on ice, the cells were washed with 0.1% saponin and incubated with polyclonal goat anti-rat Ig-phycoerythrin (PharMingen) for 30 min. After washing, cells were stained for 30 min with anti-CD8-cychrome and anti-IFN-γ–fluorescein isothiocyanate. The cells were washed with 0.1% saponin and then with PBS and 5% FBS and were stored at 4°C in PBS containing 2% formaldehyde until analysis. In all cases, stained cells were acquired on a FACScan flow cytometer (100,000 to 800,000 events per sample) and analyzed with CELLQUEST software (Becton Dickinson, San Jose, Calif.).
Identification of CTL priming by DNA vaccination.
Directly ex vivo, CD8+ memory T cells have little or no detectable lytic activity and therefore cannot be identified in a standard in vitro cytotoxicity assay. However, the CD8+ memory cells present in a successfully vaccinated mouse can rapidly proliferate in response to virus infection, yielding a lytic response that is detectable by 4 days postinfection (p.i.). In contrast, naïve mice must generate a primary cytotoxic T-cell response from a limited number of naïve T cells of the appropriate specificity, a process that takes about 5 to 6 days to become detectable in a standard in vitro lytic assay. Therefore, virus-specific CTL activity detectable at 4 days p.i. indicates that the mouse was previously successfully vaccinated. Neonatal or adult mice were inoculated with DNA as described above, and 6 months or 1 year later each animal was infected with LCMV (2 × 105 PFU; Armstrong strain) by intraperitoneal injection. Spleens were removed 4 days p.i. One half was reserved for later virus titration (see below), and the remainder was assayed for in vitro cytolytic activity by a standard chromium release assay, described elsewhere (41).
Virus titrations.
Spleen samples were snap frozen by immersion in liquid nitrogen. Each sample was weighed and then homogenized in 1 ml of complete 199 medium (199 medium [Gibco BRL] plus 10% FBS plus penicillin, streptomycin, and l-glutamine). Aliquots of serial dilutions were plated on subconfluent monolayers of Vero cells. After a 1-h incubation period, the virus was removed and the monolayers were overlaid with complete 199 medium containing 0.5% agarose and incubated at 37°C. Three to four days later, the cells were formalin fixed, the agarose plugs were removed, and the monolayers were stained with crystal violet. Plaques were counted, and the data were used to calculate the number of PFU per gram of spleen. The lower limit of detection was approximately 200 PFU per g.
Evaluation of plasmid DNA-induced serum IgG responses.
Serum was prepared from coagulated whole blood from mice at 6 months postimmunization. Ninety-six-well plates (Falcon 3912 Microtest III flexible assay plates; Becton Dickinson) were coated with 100 μl of target antigen, comprising purified LCMV in PBS (200 ng of total protein/well). Following overnight incubation at room temperature, the unbound antigen was removed and the wells were blocked with blocking buffer [3% bovine serum albumin (BSA fraction V; Sigma), 0.2% Tween 20 in 1× PBS] and washed with wash buffer (0.1% Tween 20, 1× PBS). The serum samples were serially diluted in blocking buffer and added to the target plate (100 μl/well). Following a 1-h incubation at room temperature, the liquid was aspirated and the wells were washed three times with wash buffer. Total bound IgG, IgG1, or IgG2a was detected with appropriate secondary antibodies conjugated to horseradish peroxidase (1:4,000 dilution, 100 μl per well; Southern Biotechnology, Birmingham, Ala.). After a 1-h incubation at room temperature, the second antibody was removed and the wells were washed three times with wash buffer. Horseradish peroxidase activity was measured by incubation for 30 min with 100 μl of the substrate o-phenylenediamine dihydrochloride in urea hydrogen peroxide (Sigmafast OPD tablets; Sigma), followed by the addition of 1 N HCl (100 μl per well). Absorbance at 492 nm was measured with a Titertek Multiscan Plus (Flow Laboratories, McLean, Va.). For each mouse, the endpoint titer was defined as the first serum dilution at which the mean optical density of triplicate samples did not lie at least 3 standard deviations above the background optical density.
Statistics.
Statistical relatedness was calculated with the Mann-Whitney rank sum test (SigmaStat; SPSS, Chicago, Ill.).
RESULTS
Antigen-specific CD8+ T-cell responses are detectable directly ex vivo 1 year after neonatal or adult DNA vaccination.
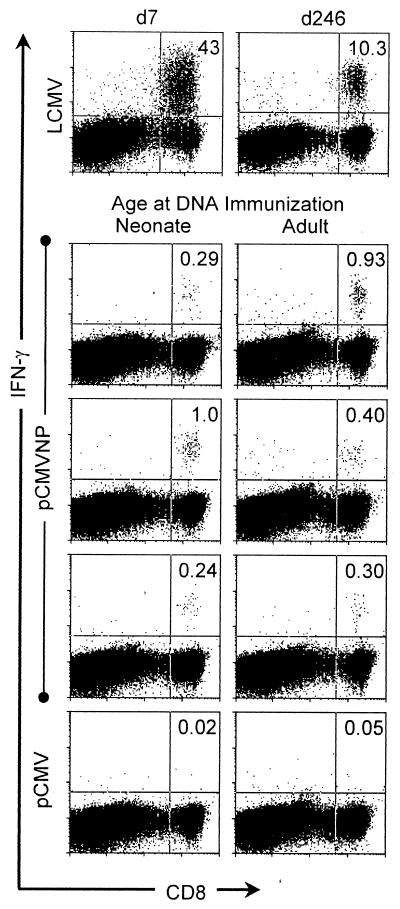
The exquisite specificity of the T-cell receptor for its cognate peptide/MHC complex, coupled with the ability to detect intracellular cytokines in responding cells, has recently made it possible to detect extremely small numbers of primary or memory antigen-specific T cells within a large and heterogeneous population of nonspecific cells. Primed antigen-specific T cells initiate cytokine production within minutes of T-cell receptor engagement (37), and in the presence of inhibitors of secretion, these cytokines accumulate within the cell where they can be detected by staining with fluorescently labeled anti-cytokine antibodies, followed by flow cytometry (11). By this ICCS technique, we have analyzed neonatal and adult DNA vaccinees for the presence of LCMV NP-specific CD8+ T cells 1 year after vaccination. Neonatal (3-day-old) or adult (at least 6-week-old) mice were vaccinated either with pCMV (as a negative control) or with pCMVNP. One year later, spleen cells were harvested and virus-specific cells were measured directly ex vivo by ICCS (see Materials and Methods). As positive controls, similar analyses were carried out using cells from an acutely infected mouse 7 days p.i. and from a mouse immunized by conventional means (live virus) 246 days previously (Fig. 1). As shown in Fig. 1, virus-specific cells were abundant in day 7 mice, representing approximately 43% of all CD8+ T cells, a number similar to that seen previously in our laboratory (37) and by others using MHC class I tetramers (25); LCMV-specific memory cells constituted >10% of all splenic CD8+ T cells at 246 days p.i. Most importantly, antigen-specific CD8+ cells were readily detectable in DNA-immunized mice at 1 year postvaccination, forming discrete populations of CD8+ IFN-γ+ cells (Fig. 1) which were not present in negative control (pCMV) vaccinees. This is the first identification, directly ex vivo, of CD8+ T-cell responses induced by DNA immunization. Between 0.24 and 1% of CD8+ T cells in neonatal and adult vaccinees were epitope-specific memory cells. There was no significant difference in the percentage of memory cells present in adult compared to neonatal vaccinees. Although the numbers of memory cells are 10- to 40-fold lower than those induced by live-virus immunization, it is remarkable that a year after a single inoculation of DNA within days of birth up to 1 in 100 CD8+ cells remains specific for the encoded antigen. Mice immunized with pCMV showed no discrete CD8+ IFN-γ+ signal after peptide stimulation (Fig. 1), and similar background levels were observed in pCMVNP vaccinees in the absence of peptide stimulation (data not shown).
FIG. 1.
Virus-specific CD8+ T cells are detectable directly ex vivo 1 year after DNA immunization of neonates or adults. Neonatal or adult mice were immunized with pCMVNP or pCMV. One year later, splenocytes were assayed, directly ex vivo, by flow cytometry for expression of CD8 (x axis) and IFN-γ (y axis) after 5 h of peptide stimulation as described in Materials and Methods. As positive controls, splenocytes were included from LCMV-infected mice at either 7 (d7) or 246 (d246) days p.i. The number in the top right-hand corner of each dot plot indicates the percentage of CD8+ cells which were IFN-γ+ (i.e., specific for the CTL epitope peptide NP118–126). The data shown are representative of three independent experiments.
DNA-induced and virus-induced CD8+ memory T cells produce similar levels of IFN-γ upon antigen contact.
Virus-induced and DNA-induced epitope-specific CD8+ T cells produced IFN-γ in response to a 5-h peptide stimulation in vitro (Fig. 1). We estimated the extent of IFN-γ production in both cell populations by determining the mean fluorescence intensity of cells stained for IFN-γ. IFN-γ staining was similar in all antigen-specific cells analyzed (data not shown), suggesting that the mode of immunization (live virus or DNA) has little effect on expression of this effector function.
DNA-induced and virus-induced CD8+ memory T cells contain similar low levels of perforin.
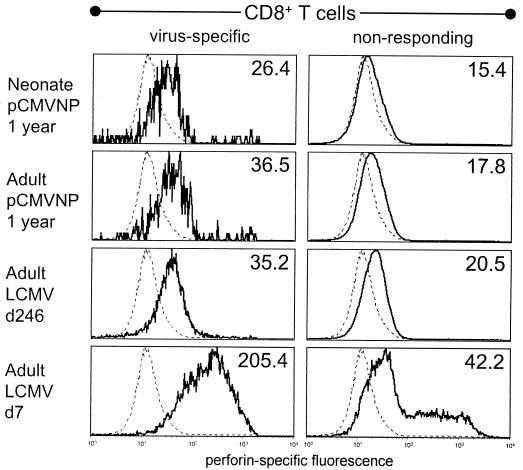
As shown above and elsewhere (3, 18, 37), CD8+ memory T cells can quickly initiate cytokine production upon antigen contact. However, to eradicate certain virus infections—including LCMV infection—CD8+ T cells must lyse infected cells in a perforin-dependent manner (16, 38). It is therefore of interest to determine if this protein is present in CD8+ memory T cells. The perforin-mediated lytic capacity of CD8+ memory T cells is somewhat controversial; although several studies indicate that the acquisition of strong lytic activity requires several hours of antigen stimulation (3, 10), low-level lytic activity has been reported (31). We therefore quantitated perforin levels directly ex vivo in CD8+ memory T cells present 1 year after DNA vaccination of adults and neonates and compared them to the levels in virus-induced memory cells and virus-specific CD8+ T cells at the peak of the antiviral immune response. In individual mice, perforin levels were evaluated in IFN-γ+ (antigen-specific) CD8+ cells and in nonresponding (IFN-γ−) CD8+ cells. As shown in Fig. 2, perforin was detectable in antigen-specific CD8+ T cells following both DNA immunization and virus infection. The highest median level of perforin (205.4) was found during acute virus infection. In all three long-term immune groups (DNA-immunized neonates and adults and virus-immune subjects), the median level of perforin was consistently higher in virus-specific CD8+ T cells than in nonresponding CD8+ T cells from the same mouse. We have not yet determined whether this reflects persistently low levels of perforin in the virus-specific cells or the initiation of perforin synthesis during the 5-h peptide stimulation. Collectively, these data indicate that the age at which animals were vaccinated had no measurable impact on CD8+ T-cell effector functions. Furthermore, the mode of immunization has minimal impact on the effector status of individual CD8+ memory T cells, since virus-induced memory cells contained levels of IFN-γ and perforin similar to those found in their DNA vaccine-induced counterparts.
FIG. 2.
Perforin is detectable in CD8+ memory T cells induced by DNA vaccination or virus infection. CD8+ T cells from the same splenocytes used in the experiment shown in Fig. 1 were stained for perforin and IFN-γ and analyzed by flow cytometry. For all mice, perforin-specific fluorescence was measured in virus-specific CD8+ T cells (i.e., those which produced IFN-γ in response to peptide) and also in nonresponding (IFN-γ−) CD8+ T cells. A representative mouse from each group is shown (solid line), and the median fluorescence is indicated in the upper right corner of each histogram. Splenocytes from perforin-deficient mice were included as a negative staining control and are shown as a dotted line in each histogram; the median fluorescence of perforin-deficient CD8+ T cells was 12.9. d7, day 7; d246, day 246.
Accelerated antiviral CTL responses after virus challenge.
We have demonstrated that, for at least 1 year postvaccination and even in the absence of boosting, both neonatal and adult vaccinees maintained a population of antigen-specific CD8+ T cells. Following antigen contact, these memory cells produced IFN-γ (Fig. 1) and contained low levels of perforin (Fig. 2). We considered it important to demonstrate that DNA-induced memory cells or their progeny could lyse target cells, since lysis is required for the clearance of LCMV infection (16, 38). Neonates or adults received a single injection of pCMVNP or the vector control plasmid pCMV, and 6 months or 1 year later were challenged with LCMV (Fig. 3). Four days postchallenge, splenocytes from each individual animal were tested in vitro for lytic activity against MHC-matched target cells coated with the immunodominant LCMV NP epitope peptide. As explained in Materials and Methods, the presence of detectable cytolytic T-cell activity 4 days p.i. is indicative of preexisting virus-specific immunity. Six months after DNA immunization, 3 of 4 adult vaccinees and 5 of 5 neonatal vaccinees showed accelerated lytic activity (Fig. 3A and B). CTL activity also was detectable 1 year postvaccination in 4 of 4 adult vaccinees and in 4 of 5 neonatal vaccinees (Fig. 3C and D). In each experiment, CTL activity at 4 days p.i. was also evaluated using splenocytes from several nonimmune mice; the maximum background level of lysis seen in any of these mice is shown (Fig. 3). Thus, DNA vaccination of neonatal or adult mice with no subsequent boosting induces CD8+ memory T cells that are detectable up to 1 year postimmunization. These cells respond to antigen contact by secreting IFN-γ (Fig. 1), contain low levels of perforin (Fig. 2), and mount rapid cytolytic responses upon virus challenge (Fig. 3). This is the first study to demonstrate the lifelong maintenance of CTL following a single neonatal administration of plasmid DNA.
FIG. 3.
Accelerated antigen-specific lytic CD8+ T cell responses at 6 months and 1 year after DNA vaccination of neonatal or adult mice. Adult (▾, panels A and C) or neonatal (●, panels B and D) mice received a single 50-μg injection of pCMVNP. Six months (panels A and B) or 1 year (panels B and D) later, mice were infected with LCMV, and 4 days thereafter were evaluated for the presence of lytic CTL activity. Each line represents the percent specific 51Cr release for an individual mouse, determined at the indicated effector-to-target cell (E:T) ratios. For each experiment, the nonimmune mouse (either pCMV inoculated or given no DNA) showing the highest level of background lysis at day 4 p.i. is shown (open squares).
Similar long-lived IgG responses in DNA-immunized neonates and adults.
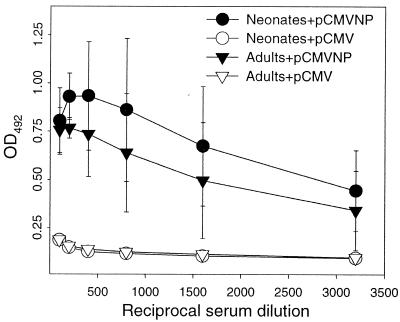
Many vaccines exert their protective effect at least in part by eliciting humoral immune responses. Although long-term antiviral antibody responses have been described after adult and neonatal DNA vaccination (14, 26, 28, 39, 45), there have been few comparative studies of adult and neonatal vaccinees. To address this issue, LCMV-specific IgG serum antibodies were measured by enzyme-linked immunosorbent assay in individual animals 6 months after DNA vaccination (Fig. 4). For clarity, only the means and standard deviations for each group are shown. All animals that received pCMVNP as either neonates or adults were LCMV seropositive 6 months later (Fig. 4), with endpoint titers of >1:3,200. The difference in antibody titers between neonatal and adult vaccine groups was not statistically significant (P = 0.17). As expected, none of the adult or neonatal pCMV vaccinees showed evidence of LCMV-specific antibodies (Fig. 4). We conclude from these data that a single vaccination with pCMVNP within 3 days of birth leads to the generation of long-lived humoral responses that are similar to those seen in adult vaccinees.
FIG. 4.
DNA vaccination of neonates or adults induces long-lived IgG responses. Neonatal mice (circles) or adult mice (triangles) were injected with pCMV (open symbols) or pCMVNP (solid symbols). The representative experiment shown used four mice per vaccine group. Six months later, serum from each mouse was tested by enzyme-linked immunosorbent assay for the presence of LCMV-specific antibodies as described in Materials and Methods. At each serum dilution, the mean and standard error for each vaccine group are shown.
Similar long-term antibody isotypes in adult and neonatal vaccinees.
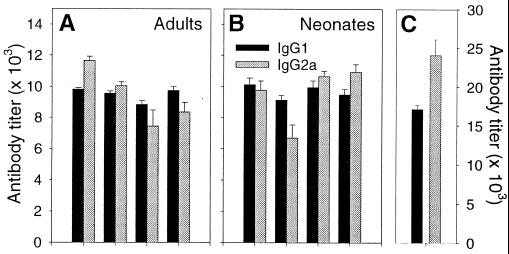
Immunization regimens that lead to high levels of IgG2a in adults often result in IgG1-dominated responses in neonates (4, 34). The characteristic IgG1 or IgG2a skewing of the humoral response is established shortly after initial priming and remains unaltered by boosting regimens which lead to alternate skewing among naïve animals (22). To determine the isotype profiles several months after neonatal and adult pCMVNP vaccination, sera were tested for the presence of LCMV-specific antibodies with detection reagents specific for IgG1 or IgG2a. Endpoint titers for each mouse were determined in triplicate, and the geometric mean titers for each isotype are shown in Fig. 5. Infection with LCMV leads to an IgG2a-dominated response among BALB/c mice (Fig. 5C), as previously reported (35). In contrast, neonatal immunization with pCMVNP induces similar titers of IgG1 and IgG2a, although at levels well below that seen after virus infection (Fig. 5B). Thus, there was no polarization of the long-term antibody response in neonates vaccinated with DNA. Similar isotype profiles were seen in adult vaccinees (Fig. 5A). Together, these data show that DNA immunization within the first 3 days of life can, in the absence of boosting, successfully prime long-lived antibody responses with a titer and isotype profile very similar to those responses primed in adulthood (Fig. 4 and 5). Others have reported that, 6 months after DNA immunization, similar IgG levels are present in adult and neonatal vaccinees (39), but one recent study noted an age-dependent difference in isotype profile (26). We find similar IgG levels (Fig. 4) and isotype patterns (Fig. 5), regardless of the age at immunization.
FIG. 5.
Similar isotype responses in mice immunized as neonates or adults. The isotype profile of the LCMV-specific antibodies induced by pCMVNP immunization of adult (A) or neonatal (B) mice are shown. Black bars, IgG1; gray bars, IgG2a. Each paired set of bars shows the average titers for a single animal, and error bars representing the standard deviation are included. For comparative purposes, the isotype profile present 6 weeks after virus infection is shown (C). Note that the left-hand y axis scale refers to panels A and B, and the right-hand y axis scale refers to panel C.
Long-term DNA-induced immunity protects against virus challenge.
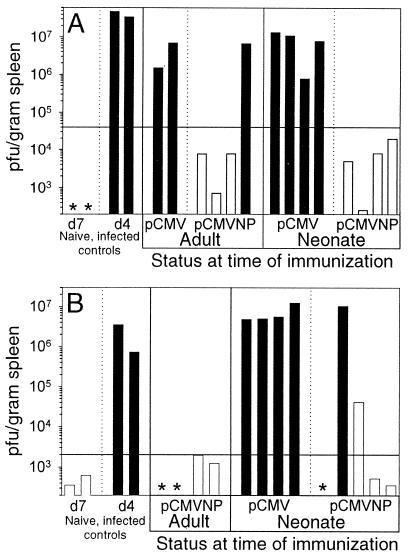
Viral clearance and protective immunity against LCMV are conferred by CD8+ CTLs. We have demonstrated the long-term maintenance of LCMV-specific CD8+ T cells (Fig. 1) and CTLs (Fig. 3) in mice immunized with pCMVNP as neonates and adults. To establish whether these long-lived responses were biologically relevant, we next evaluated their ability to limit LCMV multiplication at 6 months and 1 year postvaccination (Fig. 6). The mice were infected with LCMV and 4 days p.i., their splenic titers were determined; these are shown for individual mice (log10 scale). To provide a baseline virus titer against which vaccinees could be compared, nonimmune mice were infected with LCMV, and their splenic titers were determined 4 days p.i. In each panel, a horizontal line signifies a 99.9% (1,000-fold) reduction from the average titer in these nonimmune day 4 p.i. control mice. To demonstrate the strong correlation between protective immunity and the presence of CTLs, the bars in Fig. 6 are color coded; white bars represent animals in which lytic CTL activity was detectable (Fig. 3), while black bars denote animals devoid of detectable CTLs. Virus which was below the level of detection in five mice is indicated by an asterisk in Fig. 6; all of these mice were positive for CTLs (Fig. 3) and therefore had been infected and had cleared the virus.
FIG. 6.
Neonatal and adult DNA immunization provides long-term protection against viral challenge. Neonatal and adult mice were immunized as indicated. Six months (A) or 1 year (B) postimmunization, the mice were challenged with LCMV, and 4 days later their spleens were harvested for virus titration. Each bar represents the amount of infectious virus present per gram of spleen in a single animal at 4 days p.i. (d4) (or 7 days p.i. for the d7 control mice). To display the correlation between protective immunity and the presence of CTL, the bars are color coded; white bars designate animals in which lytic CTL activity was detected in the experiment shown in Fig. 3, while black bars designate animals devoid of CTL activity. The two virus control groups shown in Fig. 3 were also analyzed. The virus titers in nonimmune mice infected with LCMV 4 days prior to titration (d4) were used as a baseline against which the DNA vaccines were compared. The horizontal bars indicate a 99.9% (1,000-fold) decrease from the average titers of these d4 mice. The lower limit of detection is 200 PFU per gram; mice in which plaques were undetectable (but which displayed CTL activity and thus had been successfully infected) are indicated by asterisks.
Six months after DNA immunization (Fig. 6A), 7 of 8 pCMVNP vaccinees (3 of 4 adult and 4 of 4 neonatal subjects) showed >1,000-fold reductions in LCMV titer 4 days after LCMV challenge. A similar reduction was seen in 8 of 9 pCMVNP vaccinees (4 of 4 adult and 4 of 5 neonatal subjects) 1 year postimmunization (Fig. 6B). None of the mice immunized with pCMV showed a reduction in titer approaching these levels. There is an excellent correlation (26 of 27 DNA-immunized mice) between CTL activity and protective immunity. Only one mouse with detectable CTLs did not meet the stringent criterion of a 1,000-fold reduction in titer (Fig. 6B); however, infectious virus was markedly (∼95%) reduced, even in this animal.
Therefore, DNA vaccination within the first 3 days of life primes antigen-specific effector T cells (Fig. 1) and CTLs (Fig. 3) and is as effective as immunization in adulthood for priming long-lived protective antiviral immunity (Fig. 6).
DISCUSSION
Neonates are profoundly sensitive to a variety of viral infections, and early antiviral immunization is vital to reduce the incidence and severity of viral diseases in this age group. However, early postnatal immunization is not entirely without risk. Live viral vaccines at doses administered to adults might overwhelm the neonatal immune system and cause inadvertent pathological consequences. Killed vaccines, although safer, often do not stimulate the strong responses necessary to provide long-lived protective immunity, and they are rather ineffective in stimulating the CD8+ T-cell responses which are critical for protection against a wide variety of intracellular pathogens. In addition, numerous examples indicate that neonates and adults may respond differently to immunological challenges (33). For these reasons, few vaccines are given in the neonatal period; most are withheld until infancy, and repeated booster immunizations are administered throughout infancy and early childhood to ensure adequate levels of protection. A preferable situation would be the single administration of a safe vaccine immediately after birth, capable of providing a high level of protective immunity that could be maintained throughout life in the absence of boosting. DNA vaccines are an attractive alternative to the immunization of neonates, and in our previous study we documented the acquisition of short-term humoral and cell-mediated immune responses against LCMV after a single administration of pCMVNP within hours of birth. These responses were capable of successfully controlling a challenge infection until at least 6 weeks after birth (13). Protective immunity after neonatal DNA vaccination has also been observed with rodent models of influenza virus (5, 6), herpesvirus (21) and rabies virus (39, 40) infections and in a primate model of hepatitis B virus (27). In contrast, plasmid vaccination against the circumsporozoite antigen of malaria induces tolerance when administered to neonates and protective immunity when given to adults (15, 24).
The present study had several goals: first, to determine if humoral and cellular immunity primed by neonatal DNA vaccination could be maintained for an extended period of time in the absence of revaccination; second, to quantitate, directly ex vivo, CD8+ T-cell responses at 1 year postvaccination; third, to compare the effector status of long-term DNA-induced memory cells with the status of memory cells induced by conventional (live-virus) immunization; and fourth, to evaluate the protective efficacy of these memory T cells. In all cases, we carried out parallel analyses in neonatal and adult vaccinees to determine if the age at DNA immunization was an important variable for any of the responses being measured. Our data (Fig. 1) show that antigen-specific CD8+ T cells can be maintained in vivo without revaccination for at least 1 year (roughly half of the lifespan of a mouse). The relative proportions of antigen-specific CD8+ effector T cells were similar in neonatal and adult vaccinees. In both cases, ∼0.5% to 1% of CD8+ T cells were NP specific 1 year after DNA vaccination, a number only ∼2-fold lower than that observed 1 month after vaccination with a recombinant vaccinia virus expressing LCMV NP (1 to 2%, our unpublished data). NP-specific CD8+ memory cells constituted ∼10% of CD8+ T cells at 246 days after live virus immunization, a figure consistent with that described with peptide tetramer staining (25). The memory cells induced by DNA immunization appeared qualitatively similar to those induced by conventional immunization, having comparable levels of IFN-γ and perforin. Furthermore, DNA vaccine-induced cells permitted the generation of an accelerated CTL response detected by target cell lysis (Fig. 3), and most importantly, the immunity induced by neonatal DNA vaccination could confer protection against virus challenge 1 year later (Fig. 6). Thus, the mode of immunization affected the quantity, but not the quality, of CD8+ memory T cells.
Analysis of the anti-NP humoral response 6 months postimmunization revealed that neonatal vacciness had levels of serum IgG that were indistinguishable from those observed in immunized adults (Fig. 4), confirming that early postnatal exposure to a plasmid-expressed antigen can prime a B-cell response as efficiently as can exposure in adulthood. Since the half-life of antibodies in murine serum is approximately 10 to 14 days (36), high antibody titers at 6 months postvaccination are indicative of ongoing antibody synthesis, presumably by long-lived plasma cells (36). It has been previously reported that neonates show a bias towards an IgG1 antibody response after protein immunization, which is overcome by DNA immunization (22). Our results confirm the presence of a balanced antibody response after neonatal DNA immunization similar to that seen in vaccinated adults and show that this is maintained well into adulthood.
While the above data show conclusively that DNA immunization of neonates and adults can induce long-term immunity, the underlying mechanisms remain unclear. For example, might the longevity of our DNA-induced memory cells depend on the continued production of NP antigen from persistent plasmid DNA? Plasmid DNA inoculation induces a local antigen-specific inflammatory response (12, 46) which leads to destruction of the transfected muscle fibers (9) and therefore to the eradication of many of the antigen-expressing cells. It is probable that a similar fate would befall transfected cells of all types, including antigen-presenting cells (8). Thus it seems unlikely that significant quantities of NP would persist in the vaccinee. Furthermore, several studies—mainly in the LCMV model—suggest that antigen is not required for the maintenance of memory cells (1, 2, 20). However, while we doubt the importance of persistent NP in driving the DNA-induced memory cell response demonstrated here, we cannot altogether exclude the possibility; one report describes the in vivo detection of plasmid DNA and expression of the encoded luciferase protein a remarkable 19 months after DNA inoculation (43).
The data presented here indicate that long-lived protective immunity can be induced by neonatal vaccination with plasmid DNA. By all of the immunological criteria examined, these long-lived responses appear to be efficiently maintained in the absence of boosting and are quantitatively and qualitatively indistinguishable from the responses induced in adults. Furthermore, the DNA vaccine-induced responses are relatively abundant and are qualitatively similar to those induced by conventional (live-virus) immunization. Thus, DNA vaccines may offer a safe and attractive solution to the problems associated with neonatal vaccination.
ACKNOWLEDGMENTS
We are grateful to Annette Lord for excellent secretarial support.
This work was supported by NIH grant AI-37186.
Footnotes
Manuscript 12423 of the Scripps Research Institute.
REFERENCES
- 1.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 2.Asano M S, Ahmed R. CD8 T cell memory in B cell-deficient mice. J Exp Med. 1996;183:2165–2174. doi: 10.1084/jem.183.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmann M F, Barner M, Viola A, Kopf M. Distinct kinetics of cytokine production and cytolysis in effector and memory T cells after viral infection. Eur J Immunol. 1999;29:291–299. doi: 10.1002/(SICI)1521-4141(199901)29:01<291::AID-IMMU291>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 4.Barrios C, Brawand P, Berney M, Brandt C, Lambert P H, Siegrist C A. Neonatal and early life immune responses to various forms of vaccine antigens qualitatively differ from adult responses: predominance of a Th2-biased pattern which persists after adult boosting. Eur J Immunol. 1996;26:1489–1496. doi: 10.1002/eji.1830260713. [DOI] [PubMed] [Google Scholar]
- 5.Bot A, Bot S, Bona C. Enhanced protection against influenza virus of mice immunized as newborns with a mixture of plasmids expressing hemagglutinin and nucleoprotein. Vaccine. 1998;16:1675–1682. doi: 10.1016/s0264-410x(98)00054-1. [DOI] [PubMed] [Google Scholar]
- 6.Bot A, Bot S, Garcia-Sastre A, Bona C. DNA immunization of newborn mice with a plasmid-expressing nucleoprotein of influenza virus. Viral Immunol. 1996;9:207–210. doi: 10.1089/vim.1996.9.207. [DOI] [PubMed] [Google Scholar]
- 7.Byrne J A, Oldstone M B A. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus: clearance of virus in vivo. J Virol. 1984;51:682–686. doi: 10.1128/jvi.51.3.682-686.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Condon C, Watkins S C, Celluzzi C M, Thompson K, Falo J L D. DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 9.Davis H L, Millan C L, Watkins S C. Immune-mediated destruction of transfected muscle fibers after direct gene transfer with antigen-expressing plasmid DNA. Gene Ther. 1997;4:181–188. doi: 10.1038/sj.gt.3300380. [DOI] [PubMed] [Google Scholar]
- 10.Ehl S, Klenerman P, Aichele P, Hengartner H, Zinkernagel R M. A functional and kinetic comparison of antiviral effector and memory cytotoxic T lymphocyte populations in vivo and in vitro. Eur J Immunol. 1997;27:3404–3413. doi: 10.1002/eji.1830271240. [DOI] [PubMed] [Google Scholar]
- 11.Ferrick D A, Schrenzel M D, Mulvania T, Hsieh B, Ferlin W G, Lepper H. Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma delta T cells in vivo. Nature. 1995;373:255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 12.Hassett D E, Whitton J L. DNA immunization. Trends Microbiol. 1996;4:307–312. doi: 10.1016/0966-842x(96)10048-2. [DOI] [PubMed] [Google Scholar]
- 13.Hassett D E, Zhang J, Whitton J L. Neonatal DNA immunization with an internal viral protein is effective in the presence of maternal antibodies and protects against subsequent viral challenge. J Virol. 1997;71:7881–7888. doi: 10.1128/jvi.71.10.7881-7888.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho T Y, Hsiang C Y, Hsiang C H, Chang T J. DNA vaccination induces a long-term antibody response and protective immunity against pseudorabies virus in mice. Arch Virol. 1998;143:115–125. doi: 10.1007/s007050050272. [DOI] [PubMed] [Google Scholar]
- 15.Ichino M, Mor G, Conover J, Weiss W R, Takeno M, Ishii K J, Klinman D M. Factors associated with the development of neonatal tolerance after the administration of a plasmid DNA vaccine. J Immunol. 1999;162:3814–3818. [PubMed] [Google Scholar]
- 16.Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen K J, Podack E R, Zinkernagel R M, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 17.Klavinskis L S, Whitton J L, Oldstone M B A. Molecularly engineered vaccine which expresses an immunodominant T-cell epitope induces cytotoxic T lymphocytes that confer protection from lethal virus infection. J Virol. 1989;63:4311–4316. doi: 10.1128/jvi.63.10.4311-4316.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lalvani A, Brookes R, Hambleton S, Britton W J, Hill A V, McMichael A J. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsen P D, Chartrand S A, Tomashek K M, Hauser L G, Ksiazek T G. Hydrocephalus complicating lymphocytic choriomeningitis virus infection. Pediatr Infect Dis J. 1993;12:528–531. doi: 10.1097/00006454-199306000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Lau L L, Jamieson B D, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–652. [PubMed] [Google Scholar]
- 21.Manickan E, Yu Z, Rouse B T. DNA immunization of neonates induces immunity despite the presence of maternal antibody. J Clin Investig. 1997;100:2371–2375. doi: 10.1172/JCI119777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez X, Brandt C, Saddallah F, Tougne C, Barrios C, Wild F, Dougan G, Lambert P H, Siegrist C A. DNA immunization circumvents deficient induction of T helper type 1 and cytotoxic T lymphocyte responses in neonates and during early life. Proc Natl Acad Sci USA. 1997;94:8726–8731. doi: 10.1073/pnas.94.16.8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martins L P, Lau L L, Asano M S, Ahmed R. DNA vaccination against persistent viral infection. J Virol. 1995;69:2574–2582. doi: 10.1128/jvi.69.4.2574-2582.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mor G, Yamshchikov G, Sedegah M, Takeno M, Wang R, Houghten R A, Hoffman S, Klinman D M. Induction of neonatal tolerance by plasmid DNA vaccination of mice. J Clin Investig. 1996;98:2700–2705. doi: 10.1172/JCI119094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 26.Pertmer T M, Robinson H L. Studies on antibody responses following neonatal immunization with influenza hemagglutinin DNA or protein. Virology. 1999;257:406–414. doi: 10.1006/viro.1999.9666. [DOI] [PubMed] [Google Scholar]
- 27.Prince A M, Whalen R, Brotman B. Successful nucleic acid based immunization of newborn chimpanzees against hepatitis B virus. Vaccine. 1997;15:916–919. doi: 10.1016/s0264-410x(96)00248-4. [DOI] [PubMed] [Google Scholar]
- 28.Rhodes G H, Abai A M, Margalith M, Kuwahara-Rundell A, Morrow J, Parker S E, Dwarki V J. Characterization of humoral immunity after DNA injection. Dev Biol Stand. 1994;82:229–236. [PubMed] [Google Scholar]
- 29.Rodriguez F, An L L, Harkins S, Zhang J, Yokoyama M, Widera G, Fuller J T, Kincaid C, Campbell I L, Whitton J L. DNA immunization with minigenes: low frequency of memory CTL and inefficient antiviral protection are rectified by ubiquitination. J Virol. 1998;72:5174–5181. doi: 10.1128/jvi.72.6.5174-5181.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulz M, Aichele P, Vollenweider M, Bobe F W, Cardinaux F, Hengartner H, Zinkernagel R M. Major histocompatibility complex-dependent T cell epitopes of lymphocytic choriomeningitis virus nucleoprotein and their protective capacity against viral disease. Eur J Immunol. 1989;19:1657–1668. doi: 10.1002/eji.1830190921. [DOI] [PubMed] [Google Scholar]
- 31.Selin L K, Welsh R M. Cytolytically active memory CTL present in lymphocytic choriomeningitis virus-immune mice after clearance of virus infection. J Immunol. 1997;158:5366–5373. [PubMed] [Google Scholar]
- 32.Sheinbergas M M. Hydrocephalus due to prenatal infection with the lymphocytic choriomeningitis virus. Infection. 1976;4:185–191. doi: 10.1007/BF01638922. [DOI] [PubMed] [Google Scholar]
- 33.Siegrist C A, Lambert P H. Immunization with DNA vaccines in early life: advantages and limitations as compared to conventional vaccines. Springer Semin Immunopathol. 1997;19:233–243. doi: 10.1007/BF00870271. [DOI] [PubMed] [Google Scholar]
- 34.Siegrist C A, Saddallah F, Tougne C, Martinez X, Kovarik J, Lambert P H. Induction of neonatal TH1 and CTL responses by live viral vaccines: a role for replication patterns within antigen presenting cells? Vaccine. 1998;16:1473–1478. doi: 10.1016/s0264-410x(98)00111-x. [DOI] [PubMed] [Google Scholar]
- 35.Slifka M K, Ahmed R. Long-term antibody production is sustained by antibody-secreting cells in the bone marrow following acute viral infection. Ann N Y Acad Sci. 1996;797:166–176. doi: 10.1111/j.1749-6632.1996.tb52958.x. [DOI] [PubMed] [Google Scholar]
- 36.Slifka M K, Antia R, Whitmire J K, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 37.Slifka M K, Rodriguez F, Whitton J L. Rapid on/off cycling of cytokine production by virus-specific CD8+ T cells. Nature. 1999;401:76–79. doi: 10.1038/43454. [DOI] [PubMed] [Google Scholar]
- 38.Walsh C M, Matloubian M, Liu C C, Ueda R, Kurahara C G, Christensen J L, Huang M T, Young J D, Ahmed R, Clark W R. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci USA. 1994;91:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Xiang Z, Pasquini S, Ertl H C. Immune response to neonatal genetic immunization. Virology. 1997;228:278–284. doi: 10.1006/viro.1996.8384. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Xiang Z, Pasquini S, Ertl H C. Effect of passive immunization or maternally transferred immunity on the antibody response to a genetic vaccine to rabies virus. J Virol. 1998;72:1790–1796. doi: 10.1128/jvi.72.3.1790-1796.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitton J L, Tishon A. Use of CTL clones in vitro to map CTL epitopes. In: Oldstone M B A, editor. Animal virus pathogenesis: a practical approach. Oxford, England: Oxford University Press; 1990. pp. 104–115. [Google Scholar]
- 42.Whitton J L, Tishon A, Lewicki H, Gebhard J R, Cook T, Salvato M S, Joly E, Oldstone M B A. Molecular analyses of a five-amino-acid cytotoxic T-lymphocyte (CTL) epitope: an immunodominant region which induces nonreciprocal CTL cross-reactivity. J Virol. 1989;63:4303–4310. doi: 10.1128/jvi.63.10.4303-4310.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolff J A, Ludtke J J, Acsadi G, Williams P, Jani A. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum Mol Genet. 1992;1:363–369. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]
- 44.Wright R, Johnson D, Neumann M, Ksiazek T G, Rollin P, Keech R V, Bonthius D J, Hitchon P, Grose C F, Bell W E, Bale J F., Jr Congenital lymphocytic choriomeningitis virus syndrome: a disease that mimics congenital toxoplasmosis or Cytomegalovirus infection. Pediatrics. 1997;100:E9. doi: 10.1542/peds.100.1.e9. [DOI] [PubMed] [Google Scholar]
- 45.Yankauckas M A, Morrow J E, Parker S E, Abai A, Rhodes G H, Dwarki V J, Gromkowski S H. Long-term anti-nucleoprotein cellular and humoral immunity is induced by intramuscular injection of plasmid DNA containing NP gene. DNA Cell Biol. 1993;12:771–776. doi: 10.1089/dna.1993.12.771. [DOI] [PubMed] [Google Scholar]
- 46.Yokoyama M, Hassett D E, Zhang J, Whitton J L. DNA immunization can stimulate florid local inflammation, and the antiviral immunity induced varies depending on injection site. Vaccine. 1997;15:553–560. doi: 10.1016/s0264-410x(97)00213-2. [DOI] [PubMed] [Google Scholar]
- 47.Yokoyama M, Zhang J, Whitton J L. DNA immunization confers protection against lethal lymphocytic choriomeningitis virus infection. J Virol. 1995;69:2684–2688. doi: 10.1128/jvi.69.4.2684-2688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zarozinski C C, Fynan E F, Selin L K, Robinson H L, Welsh R M. Protective CTL-dependent immunity and enhanced immunopathology in mice immunized by particle bombardment with DNA encoding an internal virion protein. J Immunol. 1995;154:4010–4017. [PubMed] [Google Scholar]