Abstract
Accessory protein Vpr of human immunodeficiency virus type 1 (HIV-1) arrests cell cycling at G2/M phase in human and simian cells. Recently, it has been shown that Vpr also causes cell cycle arrest in the fission yeast Schizosaccharomyces pombe, which shares the cell cycle regulatory mechanisms with higher eukaryotes including humans. In this study, in order to identify host cellular factors involved in Vpr-induced cell cycle arrest, the ability of Vpr to cause elongated cellular morphology (cdc phenotype) typical of G2/M cell cycle arrest in wild-type and various mutant strains of S. pombe was examined. Our results indicated that Vpr caused the cdc phenotype in wild-type S. pombe as well as in strains carrying mutations, such as the cdc2-3w, Δcdc25, rad1-1, Δchk1, Δmik1, and Δppa1 strains. However, other mutants, such as the cdc2-1w, Δwee1, Δppa2, and Δrad24 strains, failed to show a distinct cdc phenotype in response to Vpr expression. Results of these genetic studies suggested that Wee1, Ppa2, and Rad24 might be required for induction of cell cycle arrest by HIV-1 Vpr. Cell proliferation was inhibited by Vpr expression in all of the strains examined including the ones that did not show the cdc phenotype. The results supported the previously suggested possibility that Vpr affects the cell cycle and cell proliferation through different pathways.
Human immunodeficiency virus type 1 (HIV-1) is a causative agent of AIDS. In addition to the viral genes, such as gag, pro, pol, and env, common to all of the replication-competent retroviruses, the HIV-1 genome has genes for accessory proteins that are thought to play important roles in viral replication and pathogenesis. One of the HIV-1 accessory proteins, Vpr, is a virion-associated protein of 14 kDa. Despite its small size, Vpr has been shown to have multiple functions including nuclear translocation of the preintegration complex (20, 53, 63), regulation of apoptosis (3, 61), inhibition of cell proliferation (30, 50), induction of cell differentiation (30), and host cell cycle arrest at G2/M phase (22, 55). The G2/M cell cycle arrest by Vpr is conserved among primate lentiviruses including HIV-2 and simian immunodeficiency viruses (14, 24, 51, 62), suggesting that it may play an important role in viral replication. It has been shown that the transcriptional activity of the HIV-1 long terminal repeat is elevated in the cells at G2 phase, leading to efficient virus production, and that the virus with intact Vpr can be selected for in vivo (17). It has also been suggested from a recent study that Vpr might enhance the fidelity of DNA repair through its ability to arrest the cell cycle at G2 phase and might protect unintegrated HIV provirus from intracellular defenses against exogenous DNA (21). Therefore, the ability of Vpr to cause G2/M arrest appears to be instrumental to HIV-1 propagation.
To elucidate the molecular mechanism for Vpr-induced cell cycle arrest, a number of attempts to identify the host proteins which physically interact with Vpr have been made. The results of those studies revealed that Vpr could bind various proteins, including uracil DNA glycosylase (UNG) (7), HHR23A (18, 65), and a human homologue of mov34 (33), which have been implicated in cell cycle control. However, the functional significance of the interaction between Vpr and these cellular proteins is still unclear. In fact, it was shown that the ability of Vpr to bind UNG did not correlate with its ability to induce cell cycle arrest (60). Therefore, a different approach for identifying host factors functionally involved in Vpr-induced cell cycle arrest appeared necessary.
Previous studies have shown that Vpr-induced cell cycle arrest is associated with inactivation of p34cdc2 kinase, a key regulator of the G2/M transition (19, 54). The kinase activity of p34cdc2 is mainly regulated by proteins Wee1 and Cdc25 (28, 46). Specifically, p34cdc2 is inhibited by Wee1 via phosphorylation of its tyrosine residue at position 15 (Y15) when a cell is not ready for mitosis (37, 58). When a cell is prepared for mitosis, p34cdc2 is activated by Cdc25-mediated dephosphorylation of Y15, leading to G2/M transition (39). When DNA replication or repair of damaged DNA is incomplete, a checkpoint control mechanism is induced, which results in inhibition of p34cdc2 probably through activation of Wee1 or inactivation of Cdc25, causing cell cycle arrest at the G2/M boundary (28, 46). Therefore, it is possible that Vpr causes cell cycle arrest by affecting Wee1, Cdc25, or other checkpoint control molecules.
For elucidation of cell cycle regulatory mechanisms, the fission yeast Schizosaccharomyces pombe has been used as a good model system for a number of years, because (i) it shares the cell cycle regulatory mechanisms with higher eukaryotes including humans, (ii) a variety of well-defined mutant strains are available, facilitating genetic studies, and (iii) G2/M cell cycle arrest is manifested as an easily noticeable elongated morphology called the cdc phenotype (28, 40, 44). It has been demonstrated that Vpr also causes cell cycle arrest in S. pombe, suggesting that this organism may be a useful model for studying the molecular mechanism of Vpr-induced G2/M arrest (67, 68). Indeed, S. pombe has successfully been used for studying the antagonism of pentoxifylline against the effects of Vpr (69) and the structure-function relationship of Vpr (10). In this study, to identify host cellular factors involved in Vpr-induced cell cycle arrest, HIV-1NL4-3 Vpr was expressed in wild-type and various mutant strains of S. pombe and its effects on cellular morphology and proliferation were examined. The results demonstrated that among the genes involved in cell cycle regulation, wee1+, rad24+, and ppa2+ were necessary for induction of the cdc phenotype by Vpr, suggesting that the products of these genes, Wee1, Rad24, and Ppa2, may play important roles in Vpr-induced cell cycle arrest.
MATERIALS AND METHODS
Molecular cloning of the HIV-1 vpr gene into an S. pombe expression vector.
The vpr gene fragment was prepared by PCR using an infectious DNA clone of HIV-1NL4-3 (1) given by Akio Adachi (Tokushima University, Tokushima, Japan) as a template and a pair of oligonucleotide primers (5′-CGGGATCCCGAGGACAGATGGAACAAGCCC-3′ and 5′-CAATAGCAATTGGTACAAGCAGTTTTAGGC-3′). The amplified product was digested with BamHI and MfeI and subcloned between the BamHI and EcoRI sites of pBluescript SK II(+) (Stratagene), and its nucleotide sequence was verified. Then, the BamHI-EcoRV fragment containing the Vpr-coding region was prepared from the plasmid and inserted between the BamHI and SmaI sites of the pREP-1 vector (36) downstream of the thiamine-repressible nmt1 promoter. The constructed vector was named pREP1-vpr. With pREP1-vpr as a template, a mutant vpr gene fragment carrying C-to-T and T-to-C substitutions at the first and second nucleotides, respectively, of codon 67 was generated by primer-directed PCR mutagenesis as described previously (35). Introduction of the mutation was verified by nucleotide sequencing so that the mutant gene encodes Vpr whose Leu67 is replaced by Ser. The fragment was cloned in the pREP-1 vector to construct pREP1-L67S.
Culture and transformation of S. pombe.
S. pombe strains used in this study are listed in Table 1. Mutant strains were originally obtained from Paul Nurse (Imperial Cancer Research Fund, London, United Kingdom), Antony M. Carr (University of Sussex, Brighton, United Kingdom), and Mitsuhiro Yanagida (Kyoto University, Kyoto, Japan). Strains designated as “our stock” in Table 1 were generated in our laboratory by modifying their nutrition requirement properties through mating and tetrad analysis. Fission yeast cells were grown at 30°C in minimal medium (MM) supplemented or not supplemented with leucine (250 μg/ml), using standard culture techniques (2). As for temperature-sensitive (ts) mutants, different conditions were used as specified below. For repression of the nmt1 promoter, 10 μM thiamine was added to the medium. To induce transcription from the nmt1 promoter, cells were washed twice with MM without thiamine and then reinoculated into MM lacking thiamine. Transformation of S. pombe with plasmid DNA was carried out by the lithium acetate method as described previously (47).
TABLE 1.
S. pombe strains used in this study
| Brief genotype | Genotypea | Description | Source |
|---|---|---|---|
| Wild type | Wild type | Normal cell cycle; normal DNA damage and replication checkpoints | Our stock |
| cdc2-1w | cdc2-1w | Constitutively active Cdc2 refractory to Wee1-mediated Y15 phosphorylation | Our stock |
| cdc2-3w | cdc2-3w | Cdc2 activated by Cdc25-independent dephosphorylation of Y15 | Our stock |
| Δwee1 | ura4-D18 wee1::ura4+ | No expression of Wee1, a negative regulator of Cdc2 | Our stock |
| wee1-50 | wee1-50 | Wee1 ts mutant functional at 23°C, but not at 32.5°C | Our stock |
| Δnim1 | ura4-D18 nim1::ura4+ | No expression of Nim1, a negative regulator of Wee1 | Our stock |
| wee1-50 Δmik1 | ura4-294 wee1-50 mik1::ura4+ | No expression of Mik1, the twin kinase of Wee1, with the wee1-50 mutation | Our stock |
| cdc2-3w Δcdc25 | cdc2-3w ura4-D18 cdc25::ura4+ | No expression of Cdc25, a positive regulator of Cdc2, with the cdc2-3w mutation | P. Nurse |
| rad1-1 | rad1-1 | Nonfunctional Rad1, a transducer for DNA damage and replication checkpoints | Our stock |
| Δchk1 | ura4-D18 chk1::ura4+ | No expression of Chk1, a transducer for DNA damage and replication checkpoints | Our stock |
| Δrad24 | ura4-D18 rad24::ura4+ | No expression of Rad24 involved in cell cycle control and DNA damage checkpoint | Our stock |
| Δcds1 | ura4-D18 cds1::ura4+ | No expression of Cds1, a transducer for DNA replication checkpoint | Our stock |
| Δppa1 | ura4-D18 ppa1::ura4+ | No expression of Ppa1, a catalytic subunit of PP2A | M. Yanagida |
| Δppa2 | ura4-D18 ppa2::ura4+ | No expression of Ppa2, a catalytic subunit of another isozyme of PP2A | M. Yanagida |
The genetic background common to all of the strains (h− leu1-32) is omitted.
Examination of proliferation and morphology of yeast cells.
Yeast cells were grown in MM supplemented or not supplemented with thiamine, and an aliquot was taken at various time points. The number of cells in the sample was measured by using a particle counter (Z1; Beckman Coulter, Inc.). Morphology of the cells was observed under a phase-contrast microscope (Nikon Corp.) without fixation, and representative pictures were taken by using a charge-coupled device camera (KV-26B; Hitachi Denshi, Ltd.) and printed by a video copy processor (SCT-P67; Mitsubishi Electric Corp.). Staining of the nucleus with 4′,6-diamidino-2-phenylindole (DAPI) was carried out by the standard method (2).
Protein analysis.
S. pombe cells in the mid-log growth phase were seeded in MM supplemented or not supplemented with thiamine at a density of 3 × 105 per ml and grown at 30°C for 15 h with vigorous shaking. Then, cell extracts were prepared in HB buffer as described previously (41), electrophoresed on a sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS–10% PAGE) gel, and blotted to a polyvinylidene difluoride (PVDF) membrane. HIV-1 Vpr was detected by anti-HIV-1NL4-3 Vpr rabbit serum (NIH AIDS Research and Reference Reagent Program) and a peroxidase-conjugated anti-rabbit immunoglobulin G (IgG) mouse antibody (Amersham Pharmacia Biotech). The binding of the antibodies was visualized by using a BM chemiluminescence blotting kit (Roche Diagnostics).
Cell cycle analysis.
S. pombe cells in the mid-log growth phase were seeded in nitrogen-limited MM supplemented or not supplemented with thiamine at a density of 2 × 105 per ml and grown at 30°C for 36 h with vigorous shaking. Then, the cells were fixed with 70% ethanol, treated with RNase A (0.1 mg/ml) in 50 mM sodium citrate (pH 7.0) for 2 h, and stained with propidium iodide (10 μg/ml) overnight. Next day, DNA contents of the cells were measured by a flow cytometer (EPICS XL; Beckman Coulter, Inc.).
RESULTS
Wild-type Vpr, but not the L67S mutant, induced G2/M cell cycle arrest in S. pombe associated with the cdc phenotype.
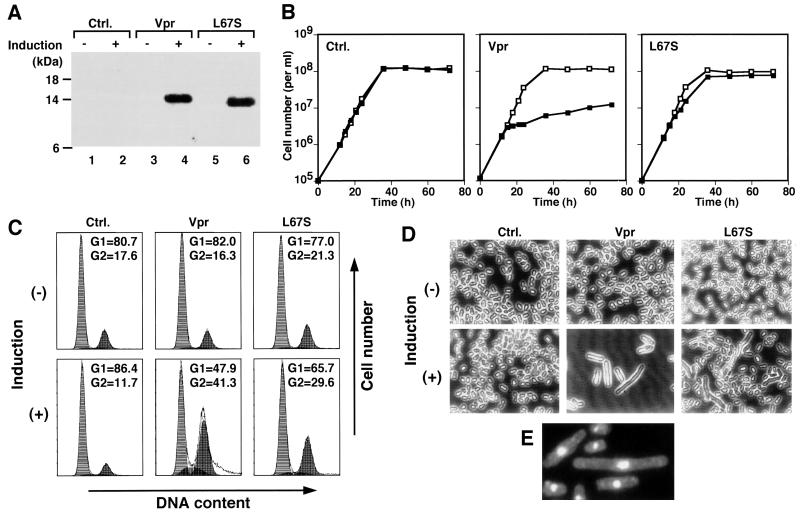
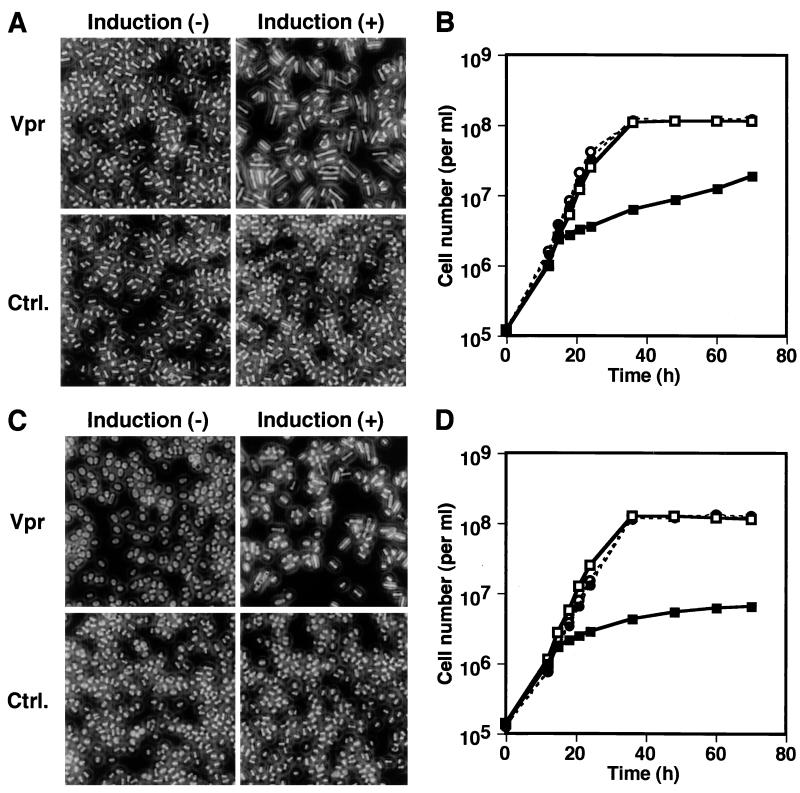
It has previously been shown that expression of HIV-1 Vpr in S. pombe causes cell cycle arrest (67, 68). To confirm the observation, the vpr gene was cloned in thiamine-repressible expression vector pREP-1 and introduced into wild-type S. pombe cells. When the cells were grown in the absence of thiamine, Vpr expression was induced (Fig. 1A, lane 4) and cell proliferation was markedly inhibited (Fig. 1B). Normally, nitrogen-starved S. pombe cells are arrested in G1 phase of the cell cycle, as shown by flow cytometric analysis of the control cells carrying the pREP-1 vector (Fig. 1C). Although S. pombe cells carrying pREP1-vpr showed a similar cell cycle profile under Vpr-repressing conditions, a large proportion of Vpr-expressing cells were arrested at G2/M phase (Fig. 1C). Microscopic observation demonstrated that the Vpr-expressing cells manifested an elongated morphology typical of the cdc phenotype representing G2/M cell cycle arrest (Fig. 1D). In addition, DAPI staining of the Vpr-expressing cells revealed that the elongated cells carried a single nucleus (Fig. 1E), further confirming that the cells were arrested at G2/M phase. The Leu67-to-Ser (L67S) substitution in Vpr has been shown to decrease the ability of the protein to induce G2/M cell cycle arrest in human cells (34). When the Vpr with the L67S substitution (VprL67S) was expressed in S. pombe (Fig. 1A, lane 6), cell proliferation was affected only slightly and the level of G2/M arrest was reduced compared with the arrest induced by wild-type Vpr (Fig. 1B and C). Most of the VprL67S-expressing cells failed to manifest the cdc phenotype, consistent with the reduced effects on the cell cycle (Fig. 1D). These results indicated that the effects of wild-type and mutant Vpr on the cell cycle of S. pombe cells were similar to those on the human cell cycle, suggesting that a common mechanism is involved in Vpr-induced cell cycle arrest in these different species. It was also shown that microscopic detection of the elongated morphology (cdc phenotype) was useful for evaluating the level of Vpr-induced G2/M arrest.
FIG. 1.
Effects of wild-type Vpr and VprL67S expression on S. pombe cell growth properties. Wild-type S. pombe cells carrying pREP-1 (Ctrl.), pREP1-vpr (Vpr), and pREP1-L67S (L67S) were compared. (A) Immunoblot analysis of Vpr expression. Cells were grown in the presence (−) or absence (+) of thiamine for 15 h, and cell extracts were prepared, fractionated by SDS–10% PAGE, and transferred to a PVDF membrane. Vpr was detected by a rabbit antiserum to HIV-1NL4-3 Vpr (NIH AIDS Research and Reference Reagent Program) and a peroxidase-conjugated mouse anti-rabbit Ig antibody. Binding of the secondary antibody was visualized by using a BM chemiluminescence blotting kit (Roche Diagnostics). (B) Cells were grown under Vpr-inducing (■) or noninducing (□) conditions and were counted at the indicated time points. (C) Cells were grown in the low-nitrogen medium supplemented (−) or not supplemented (+) with thiamine for 36 h, fixed with ethanol, treated with RNase A, and stained with propidium iodide. Then, cellular DNA content was measured by flow cytometry. The numbers in each graph indicate the percentages of the cells in G1 and G2 phases. (D) Photomicrographs of cells grown under Vpr-inducing (+) and noninducing (−) conditions for 36 h. Original magnification, ×400. (E) DAPI staining of wild-type S. pombe manifesting the Vpr-induced cdc phenotype. Original magnification, ×630.
wee1+, but not cdc25+, was required for induction of the cdc phenotype by Vpr.
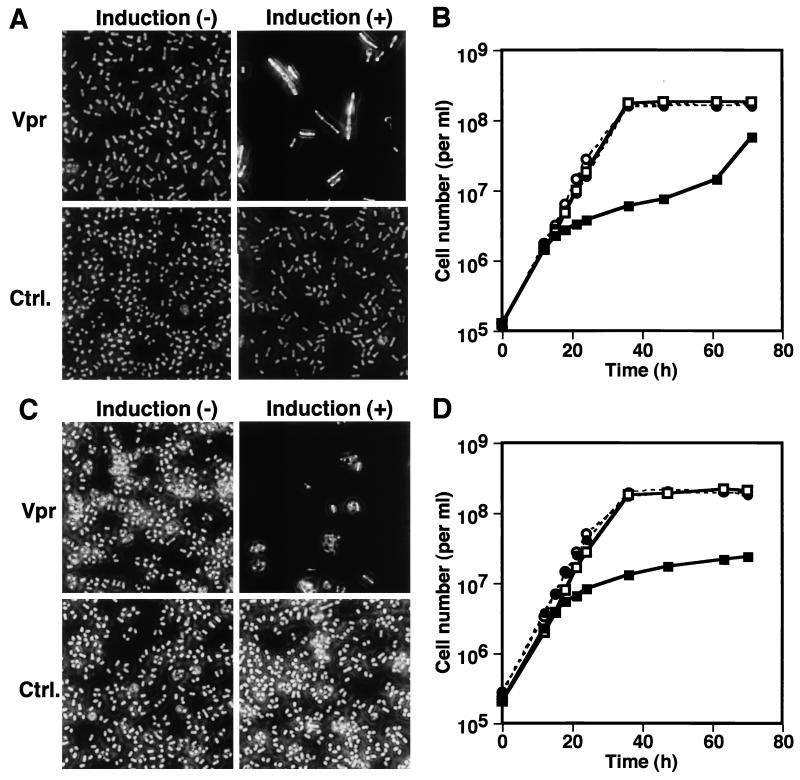
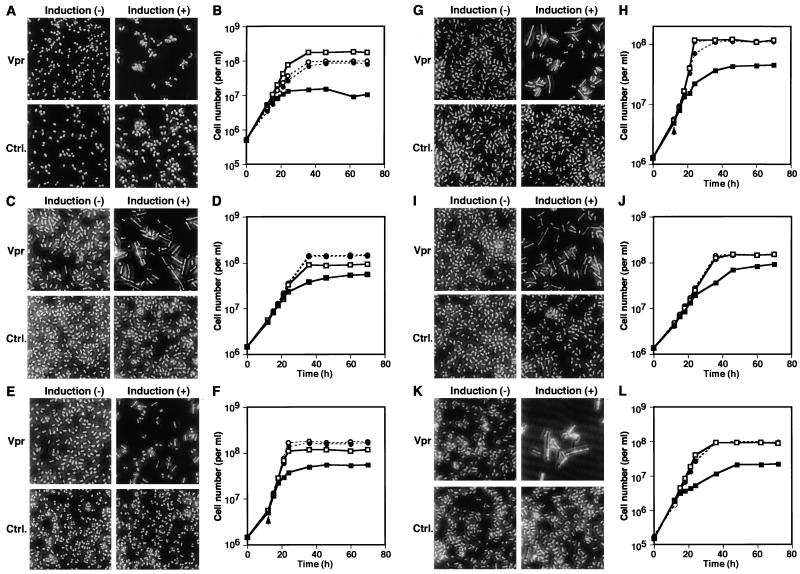
In order to examine whether Vpr affects the cell cycle through the Wee1 or the Cdc25 pathway, Vpr expression was induced in wild-type S. pombe and the cdc2 mutant cdc2-3w and cdc2-1w strains (Table 1). cdc2-3w encodes p34cdc2, whose Y15 is dephosphorylated in a Cdc25-independent manner. On the other hand, cdc2-1w encodes p34cdc2, which is refractory to Wee1-mediated Y15 phosphorylation. Although both of them are defined as constitutively active mutants, cdc2-3w responds to the negative regulation by overexpression of Wee1, whereas cdc2-1w does not (58). When Vpr was expressed in the cdc2-3w strain, the cdc phenotype was clearly observed and cell proliferation was inhibited (Fig. 2A and B). On the other hand, Vpr expression in the cdc2-1w strain failed to show the cdc phenotype, and only inhibition of cell proliferation was observed (Fig. 2C and D). These results suggested that Wee1-mediated phosphorylation of p34cdc2 might be necessary for Vpr-induced cell cycle arrest. To further examine this possibility, Vpr was expressed in a Δwee1 mutant (Table 1). As shown in Fig. 3A and B, the cdc phenotype was not manifested by Vpr in the Δwee1 strain, whereas cell proliferation was inhibited. The level of Vpr expression in the Δwee1 strain in the absence of thiamine was comparable to that in wild-type S. pombe (Fig. 4, lanes 2 and 4). When the effects of Vpr expression on the viability of wild-type and Δwee1 strains of S. pombe were compared, no significant difference was observed (data not shown). Therefore, failure of the Δwee1 strain to manifest a Vpr-induced cdc phenotype was not due to lack of Vpr expression or a higher susceptibility to Vpr-mediated cell killing. The requirement of Wee1 activity for the Vpr-induced cdc phenotype was also confirmed with ts mutant wee1-50 strain (Table 1). The wee1-50 cells grown at a permissive temperature (23°C) clearly showed the cdc phenotype in response to Vpr expression (Fig. 3C). In contrast, manifestation of the distinct cdc phenotype was no longer observed when the temperature was shifted to 32.5°C (Fig. 3E), demonstrating that Wee1 activity was required for Vpr-induced cell cycle arrest. Proliferation of the wee1-50 strain was inhibited by Vpr at both temperatures (Fig. 3D and F). A temperature shift from 23 to 32.5°C did not affect the susceptibility of wild-type S. pombe to the effects of Vpr (Fig. 3G and H). As a “twin kinase” of Wee1, Mik1 plays a supplementary role in regulating p34cdc2 through phosphorylation of Y15 (29, 31). Vpr expression in a double mutant wee1-50 Δmik1 strain (Table 1) at 23°C caused both the cdc phenotype and inhibition of proliferation (Fig. 3I and J), indicating that Mik1 was not required for Vpr-induced cell cycle arrest. At 32.5°C, the wee1-50 Δmik1 strain manifested a lethal phenotype both in the presence and absence of induction of Vpr expression (data not shown). The effects of Vpr in the absence of Cdc25 on a Δcdc25 cdc2-3w double mutant were examined (Table 1) since S. pombe carrying the cdc25 null mutation alone is not viable (57). Induction of Vpr expression in this strain caused the cdc phenotype as well as inhibition of proliferation (Fig. 3K and L), indicating that Cdc25 was dispensable for Vpr-induced cell cycle arrest. In a mutant Δnim1 strain (Table 1) deficient in the kinase which negatively regulates Wee1 (11, 48, 66), Vpr expression induced the cdc phenotype as well as inhibition of proliferation (data not shown).
FIG. 2.
Effects of Vpr expression on morphology and proliferation of the cdc2-3w and cdc2-1w strains of S. pombe. S. pombe cells bearing the cdc2-3w (A and B) or cdc2-1w (C and D) mutation transformed with pREP1-vpr (Vpr; squares) or pREP-1 (Ctrl.; circles) were grown in the presence (−; open symbols) or absence (+; solid symbols) of thiamine. Photomicrographs (A and C) show representative morphology of the cells at 36 h. Graphs (B and D) indicate the numbers of the cells counted at the indicated time points.
FIG. 3.
Requirement of wee1 for manifestation of the Vpr-induced cdc phenotype. Δwee1 (A and B), wee1-50 (C, D, E, and F), wild-type (G and H), wee1-50 Δmik1 (I and J), and Δcdc25 cdc2-3w (K and L) S. pombe strains transformed with pREP1-vpr (Vpr; squares) or pREP-1 (Ctrl.; circles) were grown in the presence (−; open symbols) or absence (+; solid symbols) of thiamine at 30 (A, B, K, and L) or 23°C (C, D, I, and J) throughout the experiment or at 23°C until 12 h (arrowhead) and 32.5°C thereafter (E, F, G and H). Photomicrographs (A, C, E, G, I, and K) show representative morphology of the cells at 36 h. Graphs (B, D, F, H, J, and L) indicate the numbers of the cells counted at the indicated time points.
FIG. 4.
Immunoblot analysis of Vpr expression. Wild-type (WT), Δwee1, Δppa2, and Δrad24 S. pombe cells carrying the pREP1-vpr vector were grown under noninducing (−) or inducing (+) conditions for 15 h, and cell extracts were prepared, fractionated by SDS–10% PAGE, and transferred to a PVDF membrane. Vpr was detected by an rabbit antiserum to HIV-1NL4-3 Vpr (NIH AIDS Research and Reference Reagent Program) and peroxidase-conjugated mouse anti-rabbit Ig antibody. Binding of the secondary antibody was visualized by using a BM chemiluminescence blotting kit (Roche Diagnostics).
Requirement of rad24+ for the Vpr-induced cdc phenotype.
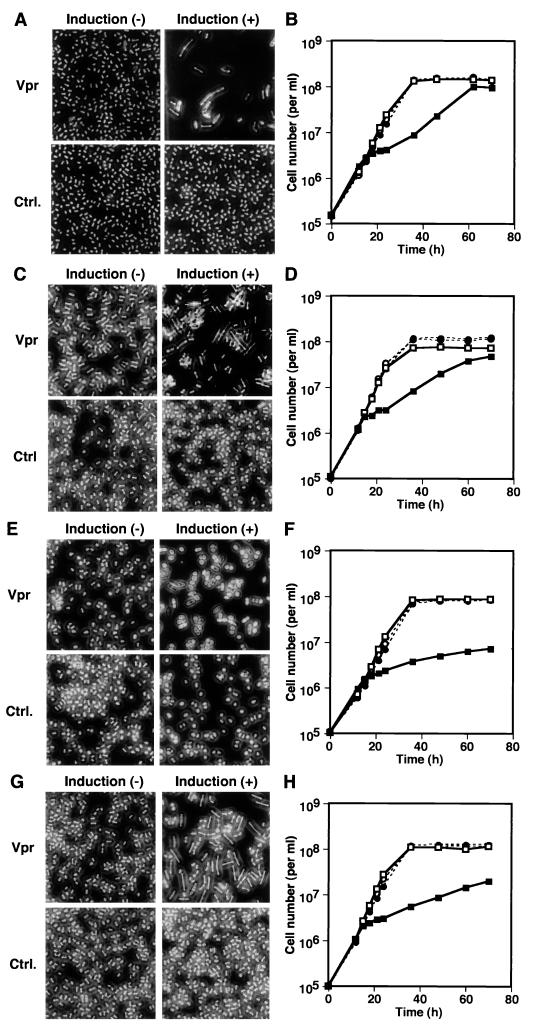
It has previously been suggested that Vpr may cause cell cycle arrest through a pathway similar to the DNA damage checkpoint pathway (52). To examine this possibility, the effects of Vpr on various cell cycle checkpoint mutants were examined. Rad1 and Chk1 are signal transducers required for both DNA damage and replication checkpoints (46). Strains carrying both rad1-1, which encodes nonfunctional Rad1, and Δchk1, which expresses no Chk1, manifested the cdc phenotype in response to Vpr expression (Table 1; Fig. 5A and C). DAPI staining of the Vpr-expressing rad1-1 and Δchk1 cells revealed that most of the elongated cells carried one nucleus (data not shown). Although proliferation of the rad1-1 and Δchk1 strains was clearly inhibited under Vpr-inducing conditions at earlier time points up to 36 h postinduction, their growth curves appeared to catch up with that of the controls at later time points (Fig. 5B and D). These results indicated that both of rad1+ and chk1+ were dispensable for Vpr-induced cell cycle arrest, while they may play some role in sustaining the arrest. rad24+ was identified as a multicopy suppressor of a radiation-sensitive mutation of S. pombe and is thought to be involved in regulation of the cell cycle timing and DNA damage checkpoint control through negative effects on Cdc25 (12, 15, 16, 49, 59). Unlike rad1-1 and Δchk1 mutants, the Δrad24 strain (Table 1) failed to reveal the cdc phenotype in response to Vpr expression, although its proliferation was markedly inhibited (Fig. 5E and F). The level of Vpr expression in the Δrad24 strain was comparable to that in wild-type S. pombe (Fig. 4, lane 8). Another mutant, the Δcds1 strain, deficient in the DNA replication checkpoint (Table 1) was susceptible to both induction of the cdc phenotype and inhibition of proliferation by Vpr (Fig. 5G and H).
FIG. 5.
Influence of cell cycle checkpoint molecules on the susceptibility to the effects of Vpr. rad1-1 (A and B), Δchk1 (C and D), Δrad24 (E and F), and Δcds1 (G and H) S. pombe cells transformed with pREP1-vpr (Vpr; squares) or pREP-1 (Ctrl.; circles) were grown in the presence (−; open symbols) or absence (+; solid symbols) of thiamine. Photomicrographs (A, C, E, and G) show representative morphology of the cells at 36 h. Graphs (B, D, F, and H) indicate the numbers of the cells counted at the indicated time points.
Vpr failed to induce the cdc phenotype in the Δppa2 strain.
It has been shown that okadaic acid, a potent inhibitor of protein phosphatase 2A (PP2A), can abrogate Vpr-induced cell cycle arrest in mammalian and fission yeast cells, suggesting that PP2A may be required for manifestation of the effects of Vpr (54, 68). Fission yeast PP2A consists of a catalytic subunit, either Ppa1 or Ppa2, and two regulatory subunits, Paa1 and Pab2 (25, 27). To investigate the possibility that PP2A might be involved in Vpr-induced cell cycle arrest, the effects of Vpr expression on cellular morphology and proliferation in mutant Δppa1 and Δppa2 strains were examined (Table 1). Vpr expression in the Δppa1 strain caused clearly elongated morphology and inhibition of cell proliferation (Fig. 6A and B). On the other hand, Vpr affected the morphology of the Δppa2 strain only marginally (Fig. 6C). Vpr was expressed in the Δppa2 strain as efficiently as in wild-type S. pombe (Fig. 4, lane 6) and inhibited cell proliferation (Fig. 6D). These results suggested that ppa2+, but not ppa1+, was necessary for the inhibitory effects of Vpr on the cell cycle.
FIG. 6.
Requirement of ppa2, which encodes a catalytic subunit of PP2A, for the Vpr-induced cdc phenotype. Δppa1 (A and B) and Δppa2 (C and D) S. pombe cells transformed with pREP1-vpr (Vpr; squares) or pREP-1 (Ctrl.; circles) were grown in the presence (−; open symbols) or absence (+; solid symbols) of thiamine. Photomicrographs (A and C) show representative morphology of the cells at 36 h. Graphs (B and D) indicate the numbers of the cells counted at the indicated time points.
Susceptibility of several mutants to Vpr-induced cell cycle arrest was discordant with their responsiveness to DNA damage.
In order to compare the mechanisms of Vpr-induced cell cycle arrest and the DNA damage checkpoint, several mutant strains of S. pombe carrying pREP1-vpr were grown under Vpr-repressing conditions and treated with bleomycin, which induces DNA double-strand breaks. As expected from previous studies (16), wild-type S. pombe and the Δwee1 wee1-50 Δmik1 strains incubated at the permissive temperature responded to bleomycin-induced DNA damage, revealing the cdc phenotype, whereas the cdc2-3w Δcdc25 strain did not (Table 2). The size of cdc2-3w cells was somewhat increased by bleomycin treatment (Table 2), probably because the mutant remains responsive to DNA damage-induced Cdc25 inhibition (16). However, the change in the cell size of the cdc2-3w strain was marginal compared with the cdc phenotype induced by Vpr. Other radiation-sensitive mutants, such as the rad1-1 and rad24 strains, failed to respond to bleomycin as expected (15, 56). The sensitivity of PP2A mutants to DNA damage has not previously been described, and our data indicated that both Δppa1 and Δppa2 strains manifested the cdc phenotype in response to bleomycin treatment (Table 2). These results demonstrated that in S. pombe susceptibility to Vpr-induced cell cycle arrest was not necessarily correlated with responsiveness to DNA damage.
TABLE 2.
Effects of genetic background of S. pombe on induction of cell cycle arrest in response to Vpr and DNA damage
| Brief genotype |
cdc phenotype induction in response to:
|
|
|---|---|---|
| Vpra | DNA damageb | |
| Wild type | + | + |
| cdc2-3w | + | +/−c |
| cdc2-3w Δcdc25 | + | − |
| Δwee1 | − | + |
| wee1-50 Δmik1d | + | + |
| rad1-1 | + | − |
| Δrad24 | − | − |
| Δppa1 | + | + |
| Δppa2 | − | + |
Cells in the mid-log growth phase under Vpr-repressing conditions were treated with bleomycin (50 mU/ml) for 6 h and were examined for the presence or absence of the cdc phenotype with a microscope.
Cell size was marginally increased, but not to the level of an unequivocal cdc phenotype.
At 23°C.
DISCUSSION
In this study, we exploited the fission yeast S. pombe for identifying the cellular factors involved in Vpr-induced cell cycle arrest. The fission yeast S. pombe, serving as a useful model organism for elucidating the mechanism for cell cycle regulation, has been shown to be susceptible to Vpr-induced cell cycle arrest (67, 68). Although it has previously been suggested that the effects of Vpr on the cell cycle were species or cell type specific (43, 62), a recent study demonstrated that the effects of the mutations in Vpr on its functions in S. pombe and human cells were similar (10). Our data on VprL67S, which was not examined in S. pombe in the previous study (10), also indicated that the effects of the Vpr mutation in S. pombe were similar to those in human cells (34). These observations suggest that Vpr may induce cell cycle arrest in S. pombe and human cells through a common mechanism despite the large phylogenetic distance between these species.
It has previously been shown that G2/M cell cycle arrest by HIV-1 Vpr is associated with inactivation of p34cdc2 kinase, a key regulator of the G2/M transition (19, 54). The kinase activity of p34cdc2 is mainly regulated by the relative activities of Wee1 and Cdc25, although other mechanisms also appear to contribute to the regulation (28, 46). Our data demonstrated that Wee1, but not Cdc25, was required for induction of the cdc phenotype by Vpr in S. pombe, suggesting that Vpr may affect the host cell cycle by increasing the Wee1 activity rather than inhibiting Cdc25. Like Wee1, Mik1 has been shown to negatively regulate p34cdc2 through Y15 phosphorylation. However, our data indicated that Mik1 was dispensable for the Vpr-induced cdc phenotype.
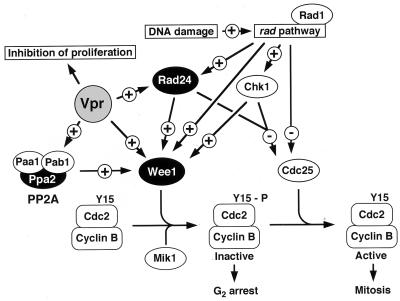
What might be the mechanism by which Vpr affects the Wee1 activity? Although Vpr may activate Wee1 directly (Fig. 7), Vpr has no known domain or activity that mediates immediate interaction with Wee1. Therefore, it is possible that Vpr affects Wee1 through a mechanism which involves additional cellular factors. In S. pombe, kinase Nim1 negatively affects Wee1 activity (11, 48, 66). However, Vpr caused the cdc phenotype in a Δnim1 mutant, making it unlikely that Nim1 or the pathway upstream of Nim1 is involved in Vpr-induced cell cycle arrest. A Nim1-related kinase, Cdr2, which may also negatively regulate Wee1, has recently been identified (8, 23). Additional experiments using a cdr2 mutant may reveal whether Cdr2 plays any role in the effects of Vpr on the cell cycle. Alternatively, it is possible that Vpr blocks the G2/M transition by inhibiting Wee1 degradation, since a recent study suggested that degradation of Wee1 might be a prerequisite for entry into mitosis (38). These possibilities are currently being investigated in our laboratories.
FIG. 7.
A putative mechanism of Vpr-induced cell cycle arrest. A model for relevant pathways of cell cycle regulation and DNA damage checkpoint has been summarized from previous studies (9, 46). Positive and negative regulations are indicated by arrows labeled + and −, respectively. P, Y15 phosphorylation.
PP2A is also thought to play a role in cell cycle regulation through its positive or negative effects on Wee1 or Cdc25, respectively (26). S. pombe PP2A consists of two regulatory subunits, Paa1 and Pab2 (25), and a catalytic subunit, Ppa1 or Ppa2; Ppa2 contributes to the majority of the phosphatase activity and is a target of the inhibitory effect of okadaic acid (27). Our data demonstrated that Ppa2, but not Ppa1, was required for manifestation of the Vpr-induced cdc phenotype. This observation is compatible with the previous studies showing that okadaic acid rescues mammalian and S. pombe cells from Vpr-induced cell cycle arrest (54, 68).
It is possible that Vpr mimics cell cycle checkpoint control, which delays the G2/M transition, in response to DNA damage and incomplete DNA replication. Our data showed that Vpr caused the cdc phenotype in the cdc2-3w strain, which appears to be specifically defective in the DNA replication checkpoint (13). We also showed that cds1+, which is involved in the DNA replication checkpoint (5, 42), was dispensable for the Vpr-induced cdc phenotype. Therefore, Vpr does not seem to utilize the DNA replication checkpoint pathway for inducing G2/M arrest. Involvement of DNA damage checkpoint control in Vpr-induced cell cycle arrest has previously been suggested (52). However, another study reached the opposite conclusion that Vpr induced cell cycle arrest by a mechanism which differs from DNA damage checkpoint control (4). A currently proposed model for DNA damage checkpoint control (9, 46) speculates that DNA damage is recognized by an as yet unidentified sensor molecule and that the signal is transduced through the rad checkpoint pathway, which involves multiple proteins, including Rad1 (56), and effector molecules, such as Wee1 (Fig. 7). There is some evidence that Chk1, which can phosphorylate Wee1, mediates the DNA damage signal from the rad pathway (45, 64) (Fig. 7). However, our data indicated that neither Rad1 nor Chk1 was required for induction of the cdc phenotype by Vpr. These results suggest that Vpr does not utilize Rad1, Chk1, or the DNA damage checkpoint control pathway upstream of these molecules for inducing cell cycle arrest. It should be mentioned that the rad1-1 and Δchk1 strains appeared to recover from the effects of Vpr after prolonged induction of Vpr expression (Fig. 5B and D), suggesting that Rad1 and Chk1 might play some role in the maintenance, if not the induction, of cell cycle arrest by Vpr. Unlike rad1+ and chk1+, rad24+ was shown to be necessary for manifestation of the Vpr-induced cdc phenotype. Rad24, an S. pombe homolog of the mammalian 14-3-3 protein, was identified as a DNA damage checkpoint molecule which determines the timing of mitosis (15). Recent studies have suggested that 14-3-3 binds to Cdc25 phosphorylated by Chk1, inhibits its nuclear translocation, and thereby prevents it from dephosphorylating p34cdc2 (12, 16, 49, 59). Our data indicated that Cdc25 does not play a major role in induction of the cdc phenotype by Vpr. Therefore, it is possible that Rad24 negatively regulates p34cdc2 through an alternative pathway, which may involve Wee1 (Fig. 7). Taken together, these findings suggest that Vpr does not mimic the entire DNA damage checkpoint control, but rather may partially utilize it for inducing G2/M arrest. Also supporting this possibility are the data in this study indicating that the susceptibility of S. pombe mutants to Vpr-induced cell cycle arrest is not always correlated with their responsiveness to DNA damage (Table 2).
Vpr inhibited proliferation of all of the strains used in this study including the ones that did not show the cdc phenotype in response to Vpr expression. It has been suggested in a previous study (67) that Vpr-induced cell cycle arrest may be Wee1 independent, based on an observation that Vpr inhibited the proliferation of the cdc2-1w strain. However, the morphology of cdc2-1w S. pombe cells expressing Vpr was not described in the study. Our present study reveals that Vpr could inhibit proliferation of cdc2-1w cells without causing the cdc phenotype, indicating that Wee1 is required for the Vpr-induced cdc phenotype but not inhibition of cell proliferation. Therefore, Vpr appears to affect the cell cycle and proliferation through distinct pathways (Fig. 7) as suggested by previous studies (43, 69). Since the toxicity of Vpr has been documented, not only for mammalian cells but also for other systems including bacteria (6) and the budding yeast Saccharomyces cerevisiae (32), Vpr may affect the viability of S. pombe by deteriorating a basic biological function common to various species.
Although it is still unknown whether the human homologs of Wee1, Ppa2, and Rad24 are involved in Vpr-induced G2/M arrest, our preliminary data suggested that expression of human WEE1 in the Δwee1 strain of S. pombe restored susceptibility to the Vpr-induced cdc phenotype (Y. Nagai and M. Masuda, unpublished data). Further studies using S. pombe on the mechanism by which Vpr affects human cellular functions may provide useful insights into the molecular basis for AIDS pathogenesis and development of a novel therapeutic intervention.
ACKNOWLEDGMENTS
We thank A. Adachi for the molecular clone of HIV-1NL4-3 and P. Nurse, A. M. Carr, and M. Yanagida for the mutant strains of S. pombe.
Y.N. and N.O. are students of the Faculty of Medicine, University of Tokyo, participating in the Free Quarter internship program.
This study was supported in part by research grants from the Ministry of Human Health and Welfare and the Ministry of Education, Science, Sports and Culture of Japan.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfa C, Fantes P, Hyams J, McLeod M, Warbick E. Experiments with fission yeast: a laboratory course manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1993. [Google Scholar]
- 3.Ayyavoo V, Mahboubi A, Mahalingam S, Ramalingam R, Kudchodkar S, Williams W V, Green D R, Weiner D B. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor kappaB. Nat Med. 1997;3:1117–1123. doi: 10.1038/nm1097-1117. [DOI] [PubMed] [Google Scholar]
- 4.Bartz S R, Rogel M E, Emerman M. Human immunodeficiency virus type 1 cell cycle control: Vpr is cytostatic and mediates G2 accumulation by a mechanism which differs from DNA damage checkpoint control. J Virol. 1996;70:2324–2331. doi: 10.1128/jvi.70.4.2324-2331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boddy M N, Furnari B, Mondesert O, Russell P. Replication checkpoint enforced by kinases Cds1 and Chk1. Science. 1998;280:909–912. doi: 10.1126/science.280.5365.909. [DOI] [PubMed] [Google Scholar]
- 6.Bodeus M, Margottin F, Durand H, Rouer E, Benarous R. Inhibition of prokaryotic cell growth by HIV1 Vpr. Res Virol. 1997;148:207–213. doi: 10.1016/s0923-2516(97)83990-8. [DOI] [PubMed] [Google Scholar]
- 7.Bouhamdan M, Benichou S, Rey F, Navarro J-M, Agostini I, Spire B, Camonis J, Slupphaug G, Vigne R, Benarous R, Sire J. Human immunodeficiency virus type 1 Vpr protein binds to the uracil DNA glycosylase DNA repair enzyme. J Virol. 1996;70:697–704. doi: 10.1128/jvi.70.2.697-704.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breeding C S, Hudson J, Balasubramanian M K, Hemmingsen S M, Young P G, Gould K L. The cdr2+ gene encodes a regulator of G2/M progression and cytokinesis in Schizosaccharomyces pombe. Mol Biol Cell. 1998;9:3399–3415. doi: 10.1091/mbc.9.12.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caspari T, Carr A M. DNA structure checkpoint pathways in Schizosaccharomyces pombe. Biochimie. 1999;81:173–181. doi: 10.1016/s0300-9084(99)80050-9. [DOI] [PubMed] [Google Scholar]
- 10.Chen M, Elder R T, Yu M, O'Gorman M G, Selig L, Benarous R, Yamamoto A, Zhao Y. Mutational analysis of Vpr-induced G2 arrest, nuclear localization, and cell death in fission yeast. J Virol. 1999;73:3236–3245. doi: 10.1128/jvi.73.4.3236-3245.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman T R, Tang Z, Dunphy W G. Negative regulation of the Wee1 protein kinase by direct action of the Nim1/Cdr1 mitotic inducer. Cell. 1993;72:919–929. doi: 10.1016/0092-8674(93)90580-j. [DOI] [PubMed] [Google Scholar]
- 12.Conklin D S, Galaktionov K, Beach D. 14-3-3 proteins associate with cdc25 phosphatases. Proc Natl Acad Sci USA. 1995;92:7892–7896. doi: 10.1073/pnas.92.17.7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enoch T, Nurse P. Mutation of fission yeast cell cycle control genes abolishes dependence of mitosis on DNA replication. Cell. 1990;60:665–673. doi: 10.1016/0092-8674(90)90669-6. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher, T. M., III, B. Brichacek, N. Sharova, M. A. Newman, G. Stivahtis, P. M. Sharp, M. Emerman, B. H. Hahn, and M. Stevenson. 1996. Nuclear import and cell cycle arrest functions of the HIV-1 Vpr protein are encoded by two separate genes in HIV-2/SIVSM. EMBO J. 15:6155–6165. [PMC free article] [PubMed]
- 15.Ford J C, Al-Khodairy F, Fotou E, Sheldrick K S, Griffiths D J F, Carr A M. 14-3-3 protein homologs required for the DNA damage checkpoint in fission yeast. Science. 1994;265:533–535. doi: 10.1126/science.8036497. [DOI] [PubMed] [Google Scholar]
- 16.Furnari B, Rhind N, Russell P. Cdc25 mitotic inducer targeted by Chk1 DNA damage checkpoint kinase. Science. 1997;277:1495–1497. doi: 10.1126/science.277.5331.1495. [DOI] [PubMed] [Google Scholar]
- 17.Goh W C, Rogel M E, Kinsey C M, Michael S F, Fultz P N, Nowak M A, Hahn B H, Emerman M. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat Med. 1998;4:65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]
- 18.Gragerov A, Kino T, Ilyina-Gragerova G, Chrousos G P, Pavlakis G N. HHR23A, the human homologue of the yeast repair protein RAD23, interacts specifically with Vpr protein and prevents cell cycle arrest but not the transcriptional effects of Vpr. Virology. 1998;245:323–330. doi: 10.1006/viro.1998.9138. [DOI] [PubMed] [Google Scholar]
- 19.He J, Choe S, Walker R, Di Marzio P, Morgan D O, Landau N R. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinzinger N K, Bukinsky M I, Haggerty S A, Ragland A M, Kewalramani V, Lee M-A, Gendelman H E, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jowett J B, Xie Y-M, Chen I S Y. The presence of human immunodeficiency virus type 1 Vpr correlates with a decrease in the frequency of mutations in a plasmid shuttle vector. J Virol. 1999;73:7132–7137. doi: 10.1128/jvi.73.9.7132-7137.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jowett J B M, Planelles V, Poon B, Shah N P, Chen M-L, Chen I S Y. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J Virol. 1995;69:6304–6313. doi: 10.1128/jvi.69.10.6304-6313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanoh J, Russell P. The protein kinase Cdr2, related to Nim1/Cdr1 mitotic inducer, regulates the onset of mitosis in fission yeast. Mol Biol Cell. 1998;9:3321–3334. doi: 10.1091/mbc.9.12.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kewalramani V N, Park C S, Gallombardo P A, Emerman M. Protein stability influences human immunodeficiency virus type 2 Vpr virion incorporation and cell cycle effect. Virology. 1996;218:326–334. doi: 10.1006/viro.1996.0201. [DOI] [PubMed] [Google Scholar]
- 25.Kinoshita K, Nemoto T, Nabeshima K, Kondoh H, Niwa H, Yanagida M. The regulatory subunits of fission yeast protein phosphatase 2A (PP2A) affect cell morphogenesis, cell wall synthesis and cytokinesis. Genes Cells. 1996;1:29–45. doi: 10.1046/j.1365-2443.1996.02002.x. [DOI] [PubMed] [Google Scholar]
- 26.Kinoshita N, Ohkura H, Yanagida M. Distinct, essential roles of type 1 and 2A protein phosphatases in the control of the fission yeast cell division cycle. Cell. 1990;63:405–415. doi: 10.1016/0092-8674(90)90173-c. [DOI] [PubMed] [Google Scholar]
- 27.Kinoshita N, Yamano H, Niwa H, Yoshida T, Yanagida M. Negative regulation of mitosis by the fission yeast protein phosphatase ppa2. Genes Dev. 1993;7:1059–1071. doi: 10.1101/gad.7.6.1059. [DOI] [PubMed] [Google Scholar]
- 28.Lee M, Nurse P. Cell cycle control genes in fission yeast and mammalian cells. Trends Genet. 1988;4:287–290. doi: 10.1016/0168-9525(88)90171-0. [DOI] [PubMed] [Google Scholar]
- 29.Lee M S, Enoch T, Piwnica-Worms H. mik1+ encodes a tyrosine kinase that phosphorylates p34cdc2 on tyrosine 15. J Biol Chem. 1994;269:30530–30537. [PubMed] [Google Scholar]
- 30.Levy D N, Fernandes L S, Williams W V, Weiner D B. Induction of cell differentiation by human immunodeficiency virus 1 vpr. Cell. 1993;72:541–550. doi: 10.1016/0092-8674(93)90073-y. [DOI] [PubMed] [Google Scholar]
- 31.Lundgren K, Walworth N, Booher R, Dembski M, Kirschner M, Beach D. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell. 1991;64:1111–1122. doi: 10.1016/0092-8674(91)90266-2. [DOI] [PubMed] [Google Scholar]
- 32.Macreadie I G, Thorburn D R, Kirby D M, Castelli L A, de Rozario N L, Azad A A. HIV-1 protein Vpr causes gross mitochondrial dysfunction in the yeast Saccharomyces cerevisiae. FEBS Lett. 1997;410:145–149. doi: 10.1016/s0014-5793(97)00542-5. [DOI] [PubMed] [Google Scholar]
- 33.Mahalingam S, Ayyavoo V, Patel M, Kieber-Emmons T, Kao G D, Muschel R J, Weiner D B. HIV-1 Vpr interacts with a human 34-kDa mov34 homologue, a cellular factor linked to the G2/M phase transition of the mammalian cell cycle. Proc Natl Acad Sci USA. 1998;95:3419–3424. doi: 10.1073/pnas.95.7.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahalingam S, Ayyavoo V, Patel M, Kieber-Emmons T, Weiner D B. Nuclear import, virion incorporation, and cell cycle arrest/differentiation are mediated by distinct functional domains of human immunodeficiency virus type 1 Vpr. J Virol. 1997;71:6339–6347. doi: 10.1128/jvi.71.9.6339-6347.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masuda M, Hanson C A, Alvord W G, Hoffman P M, Ruscetti S K, Masuda M. Effects of subtle changes in the SU protein of ecotropic murine leukemia virus on its brain capillary endothelial cell tropism and interference properties. Virology. 1996;215:142–151. doi: 10.1006/viro.1996.0017. [DOI] [PubMed] [Google Scholar]
- 36.Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993;123:127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- 37.McGowan C H, Russell P. Human Wee1 kinase inhibits cell division by phosphorylating p34cdc2 exclusively on Tyr15. EMBO J. 1993;12:75–85. doi: 10.1002/j.1460-2075.1993.tb05633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michael W M, Newport J. Coupling of mitosis to the completion of S phase through Cdc34-mediated degradation of Wee1. Science. 1998;282:1886–1889. doi: 10.1126/science.282.5395.1886. [DOI] [PubMed] [Google Scholar]
- 39.Millar J B A, McGowan C H, Lenaers G, Jones R, Russell P. p80cdc25 mitotic inducer is the tyrosine phosphatase that activates p34cdc2 kinase in fission yeast. EMBO J. 1991;10:4301–4309. doi: 10.1002/j.1460-2075.1991.tb05008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchison J M. The fission yeast, Schizosaccharomyces pombe. Bioessays. 1990;12:189–191. doi: 10.1002/bies.950120409. [DOI] [PubMed] [Google Scholar]
- 41.Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- 42.Murakami H, Okayama H. A kinase from fission yeast responsible for blocking mitosis in S phase. Nature. 1995;374:817–819. doi: 10.1038/374817a0. [DOI] [PubMed] [Google Scholar]
- 43.Nishino Y, Myojin T, Kamata M, Aida Y. Human immunodeficiency virus type 1 vpr gene product prevents cell proliferation on mouse NIH3T3 cells without the G2 arrest of the cell cycle. Biochem Biophys Res Commun. 1997;232:550–554. doi: 10.1006/bbrc.1997.6186. [DOI] [PubMed] [Google Scholar]
- 44.Nurse P, Thuriaux P, Nasmyth K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1976;146:167–178. doi: 10.1007/BF00268085. [DOI] [PubMed] [Google Scholar]
- 45.O'Connell M J, Raleigh J M, Verkade H M, Nurse P. Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. EMBO J. 1997;16:545–554. doi: 10.1093/emboj/16.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okayama H, Nagata A, Jinno S, Murakami H, Tanaka K, Nakashima N. Cell cycle control in fission yeast and mammals: identification of new regulatory mechanisms. Adv Cancer Res. 1996;69:17–62. doi: 10.1016/s0065-230x(08)60859-3. [DOI] [PubMed] [Google Scholar]
- 47.Okazaki K, Okazaki N, Kume K, Jinno S, Tanaka K, Okayama H. High-frequency transformation method and library transducing vectors for cloning mammalian cDNAs by trans-complementation of Schizosaccharomyces pombe. Nucleic Acids Res. 1990;18:6485–6489. doi: 10.1093/nar/18.22.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parker L L, Walter S A, Young P G, Piwnica-Worms H. Phosphorylation and inactivation of the mitotic inhibitor Wee1 by the nim1/cdr1 kinase. Nature. 1993;363:736–738. doi: 10.1038/363736a0. [DOI] [PubMed] [Google Scholar]
- 49.Peng C-Y, Graves P R, Thoma R S, Wu Z, Shaw A S, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- 50.Planelles V, Bachelerie F, Jowett J B M, Haislip A, Xie Y, Banooni P, Masuda T, Chen I S Y. Fate of the human immunodeficiency virus type 1 provirus in infected cells: a role for vpr. J Virol. 1995;69:5883–5889. doi: 10.1128/jvi.69.9.5883-5889.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Planelles V, Jowett J B M, Li Q-X, Xie Y, Hahn B, Chen I S Y. Vpr-induced cell cycle arrest is conserved among primate lentiviruses. J Virol. 1996;70:2516–2524. doi: 10.1128/jvi.70.4.2516-2524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poon B, Jowett J B M, Stewart S A, Armstrong R W, Rishton G M, Chen I S Y. Human immunodeficiency virus type 1 vpr gene induces phenotypic effects similar to those of the DNA alkylating agent, nitrogen mustard. J Virol. 1997;71:3961–3971. doi: 10.1128/jvi.71.5.3961-3971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Popov S, Rexach M, Zybarth G, Reiling N, Lee M-A, Ratner L, Lane C M, Moore M S, Blobel G, Bukrinsky M. Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. EMBO J. 1998;17:909–917. doi: 10.1093/emboj/17.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Re F, Braaten D, Franke E K, Luban J. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J Virol. 1995;69:6859–6864. doi: 10.1128/jvi.69.11.6859-6864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rogel M E, Wu L I, Emerman M. The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J Virol. 1995;69:882–888. doi: 10.1128/jvi.69.2.882-888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rowley R, Subramani S, Young P G. Checkpoint controls in Schizosaccharomyces pombe: rad1. EMBO J. 1992;11:1335–1342. doi: 10.1002/j.1460-2075.1992.tb05178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Russell P, Nurse P. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell. 1986;45:145–153. doi: 10.1016/0092-8674(86)90546-5. [DOI] [PubMed] [Google Scholar]
- 58.Russell P, Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987;49:559–567. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- 59.Sanchez Y, Wong C, Thoma R S, Richman R, Wu Z, Piwnica-Worms H, Elledge S J. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- 60.Selig L, Benichou S, Rogel M E, Wu L I, Vodicka M A, Sire J, Benarous R, Emerman M. Uracil DNA glycosylase specifically interacts with Vpr of both human immunodeficiency virus type 1 and simian immunodeficiency virus of sooty mangabeys, but binding does not correlate with cell cycle arrest. J Virol. 1997;71:4842–4846. doi: 10.1128/jvi.71.6.4842-4846.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stewart S A, Poon B, Jowett J B M, Chen I S Y. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stivahtis G L, Soares M A, Vodicka M A, Hahn B H, Emerman M. Conservation and host specificity of Vpr-mediated cell cycle arrest suggest a fundamental role in primate lentivirus evolution and biology. J Virol. 1997;71:4331–4338. doi: 10.1128/jvi.71.6.4331-4338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vodicka M A, Koepp D M, Silver P A, Emerman M. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 1998;12:175–185. doi: 10.1101/gad.12.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walworth N, Davey S, Beach D. Fission yeast chk1 protein kinase links the rad checkpoint pathway to cdc2. Nature. 1993;363:368–371. doi: 10.1038/363368a0. [DOI] [PubMed] [Google Scholar]
- 65.Withers-Ward E S, Jowett J B M, Stewart S A, Xie Y-M, Garfinkel A, Shibagaki Y, Chow S A, Shah N, Hanaoka F, Sawitz D G, Armstrong R W, Souza L M, Chen I S Y. Human immunodeficiency virus type 1 Vpr interacts with HHR23A, a cellular protein implicated in nucleotide excision DNA repair. J Virol. 1997;71:9732–9742. doi: 10.1128/jvi.71.12.9732-9742.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu L, Russell P. Nim1 kinase promotes mitosis by inactivating Wee1 tyrosine kinase. Nature. 1993;363:738–741. doi: 10.1038/363738a0. [DOI] [PubMed] [Google Scholar]
- 67.Zhang C, Rasmussen C, Chang L-J. Cell cycle inhibitory effects of HIV and SIV Vpr and Vpx in the yeast Schizosaccharomyces pombe. Virology. 1997;230:103–112. doi: 10.1006/viro.1997.8459. [DOI] [PubMed] [Google Scholar]
- 68.Zhao Y, Cao J, O'Gorman M R G, Yu M, Yogev R. Effect of human immunodeficiency virus type 1 protein R (vpr) gene expression on basic cellular function of fission yeast Schizosaccharomyces pombe. J Virol. 1996;70:5821–5626. doi: 10.1128/jvi.70.9.5821-5826.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao Y, Yu M, Chen M, Elder R T, Yamamoto A, Cao J. Pleiotropic effects of HIV-1 protein R (Vpr) on morphogenesis and cell survival in fission yeast and antagonism by pentoxifylline. Virology. 1998;246:266–276. doi: 10.1006/viro.1998.9208. [DOI] [PubMed] [Google Scholar]