Abstract
Human T-cell leukemia virus (HTLV) Tax protein has been implicated in the HTLV oncogenic process, primarily due to its pleiotropic effects on cellular genes involved in growth regulation and cell cycle control. To date, several approaches attempting to correlate Tax activation of the CREB/activating transcription factor (ATF) or NFκB/Rel transcriptional activation pathway to cellular transformation have yielded conflicting results. In this study, we use a unique HTLV-2 provirus (HTLVc-enh) that replicates by a Tax-independent mechanism to directly assess the role of Tax transactivation in HTLV-mediated T-lymphocyte transformation. A panel of well-characterized tax-2 mutations is utilized to correlate the respective roles of the CREB/ATF or NFκB/Rel signaling pathway. Our results demonstrate that viruses expressing tax-2 mutations that selectively abrogate NFκB/Rel or CREB/ATF activation display distinct phenotypes but ultimately fail to transform primary human T lymphocytes. One conclusion consistent with our results is that the activation of NFκB/Rel provides a critical proliferative signal early in the cellular transformation process, whereas CREB/ATF activation is required to promote the fully transformed state. However, complete understanding will require correlation of Tax domains important in cellular transformation to those Tax domains important in the modulation of gene transcription, cell cycle control, induction of DNA damage, and other undefined activities.
The human T-cell leukemia virus types 1 and 2 (HTLV-1 and HTLV-2) are oncogenic retroviruses associated with human T-cell malignancies and degenerative neurological disorders (reviewed in reference 18). HTLV-mediated cellular transformation and disease is a multistep process facilitated by the pleiotropic effects of the viral Tax protein. Tax is a transcriptional activator of the HTLV long terminal repeat (LTR) as well as many cellular promoters. Tax interacts with transcription factors and/or their regulatory components to activate or modulate several major transcription factor pathways, including the cyclic-AMP response element and activating transcription factor (ATF) binding (CREB/ATF) proteins (1, 6–9, 22, 54, 59, 62), NFκB/Rel (10, 24–26, 28, 29, 31, 33, 34, 61), and serum response factor (15, 53). Tax activation of the CREB/ATF pathway is critical for efficient viral gene expression and replication (2, 4, 6, 56, 60, 64).
Tax displays oncogenic potential in several experimental systems and recently has been shown to be essential for HTLV-2-mediated cellular transformation of human T lymphocytes (46). The precise mechanism by which Tax initiates the malignant process is unclear but likely involves several points of transcriptional and posttranscriptional disregulation in the infected T lymphocyte. To this end, Tax has been shown to activate a number of cellular genes involved in the regulation of cell proliferation, including interleukin-2 (IL-2), IL-3, IL-2 receptor, proliferating cell nuclear antigen, c-fos, c-sis, G-CSF, and GM-CSF (5, 13, 14, 21, 27, 39, 40, 42, 55, 57). Furthermore, Tax interferes with cell cycle control by altering the activity of p53, the mitotic checkpoint regulator MAD1, cyclin D, cyclin-dependent kinase 4 (cdk4) and cdk6, and the cdk inhibitor p16INK4A (23, 30, 36, 38, 41, 47, 52). Complete understanding of the mechanism of T-lymphocyte transformation will require correlation of Tax domains important in modulating gene transcription, cell cycle control, and induction of DNA damage with those required for cellular transformation.
A number of mutants in both HTLV-1 Tax (Tax-1) and HTLV-2 Tax (Tax-2) have been described that selectively abrogate the ability of Tax to activate transcription through the CREB/ATF or NFκB/Rel signaling pathway (45, 48, 49, 58). The major functional regions or domains of Tax important for transactivation by the CREB/ATF or NFκB/Rel signaling pathway are similar but not identical in Tax-1 and Tax-2 (45). Several approaches have been used to evaluate the domains of Tax-1 used to immortalize or transform rodent cell lines or primary human T lymphocytes. These analyses have yielded conflicting results as to whether Tax-1 activation of the CREB/ATF or NFκB/Rel pathway correlates with cellular transformation (3, 20, 43, 44, 51). The transforming domains of Tax-2 have yet to be defined, and their elucidation may provide insight into the differential pathogenesis exhibited by HTLV-1 and HTLV-2.
We recently reported the generation and characterization of a chimeric HTLV-2 that replicates by a Tax-independent mechanism (46). The Tax response element (TRE) in the U3 region was replaced with the enhancer (c-enh) from the cytomegalovirus (CMV) immediate-early promoter. Transcription of the chimeric HTLV-2 (HTLVc-enh) was efficiently directed by this heterologous promoter-enhancer. Also, this unique virus transformed primary human T lymphocytes with an efficiency similar to that of wild-type HTLV-2 (wtHTLV-2) (46). The functional HTLVc-enh provides the first opportunity to perform a tax mutational analysis in an infectious virus without compromising the ability of the mutant viruses to efficiently replicate. In this study, we examined the effects of select Tax-2 mutants on the ability of HTLV-2 to transform human T lymphocytes. In our study, transformation is defined as continuous growth in culture in the absence of exogenous IL-2. Our results indicate that viruses expressing tax mutants that selectively abrogate NFκB/Rel or CREB/ATF fail to induce IL-2-independent T-lymphocyte transformation. Our results suggest that activation of NFκB/Rel provides a critical proliferative signal early in the cellular transformation process, whereas CREB/ATF activation promotes sustained cell growth and IL-2-independent cellular transformation.
MATERIALS AND METHODS
Cells.
The 729-6 B-cell line (hereafter called 729) and the human leukemic T-cell line JM4 (55) were maintained in Iscove's medium (Mediatech Inc.) supplemented with 10% fetal calf serum (FCS), penicillin (100 U/ml), streptomycin (100 μg/ml), and 2 mM glutamine. BJAB cells, a Burkitt's lymphoma human B-cell line, were maintained in RPMI 1640 medium containing the same supplements. Peripheral blood lymphocytes (PBL) were isolated from the blood of healthy donors by centrifugation over Ficoll-Paque (Pharmacia). Cells were maintained in culture in RPMI 1640 medium supplemented with 20% FCS and antibiotics.
Plasmids.
The wt LTR-2-chloramphenicol acetyltransferase (CAT) (wtLTR-CAT) reporter construct and the control vector SV2neo have been described elsewhere (12, 19). The HTLVc-enh proviral clone contains the enhancer (c-enh) from the CMV immediate-early promoter in place of the TRE in both the 5′ and 3′ LTRs and has been previously described (46). Previously characterized tax-2 mutations (45) are designated by the wt amino acid single-letter code, the position in the Tax protein, followed by the mutated amino acid single-letter code or Term (termination or stop). These mutations were cloned into the HTLVc-enh proviral clone to generate HTLVc-enhF4Term (ΔTax), HTLVc-enhH3N, HTLVc-enhC29S, HTLVc-enhT113A, HTLVc-enhS130A/L131F, HTLVc-enhY290Term, and HTLVc-enhI319R/L320S.
Transfections and CAT assay.
Plasmid DNA was introduced into cells by electroporation as previously described (11). Briefly, cells were washed with phosphate-buffered saline and resuspended (2 × 107 cells/ml) in RPMI 1640 medium supplemented with 20% FCS, penicillin (100 U/ml), streptomycin (100 μg/ml), and 2 mM glutamine. A total of 5 × 106 cells were electroporated with 25 μg of DNA (900-mF charge; 250-V potential). Cells were transferred to 3 ml of medium and grown at 37°C for 48 h. Permanently transfected cells (stable transfectants) containing the wt or CMV enhancer-containing proviral clones were isolated following incubation in 24-well culture dishes (5 × 105 cell/ml) in medium supplemented with 10% FCS, penicillin (100 U/ml), streptomycin (100 μg/ml), 2 mM glutamine, and Geneticin (1.0 mg/ml). Following a 4- to 5-week selection period, viable cells were expanded in culture for further analysis. Permanently transfected cells are designated “729,” followed by the clone with which they were transfected.
Transfections for CAT assays included 5 μg of wtLTR-CAT, 15 μg of the proviral DNA clone, and 5 μg of pCMVβGal expression vector. Forty-eight hours posttransfection, cells were harvested and enumerated, and 106 cells were subjected to a β-galactosidase (β-Gal) colorimetric assay to normalize for transfection efficiency. Briefly, cells were lysed by sonication in 60 μl of 0.25 mM Tris (pH 7.8) and centrifuged for 15 min at 4°C; 30 μl of extract was incubated for 1 to 5 h at room temperature in 1 mM MgCl2, 50 mM β-mercaptoethanol, 66 mM NaHPO4-Na2PO4, and 0.9 mg of o-nitrophenyl-β-d-galactopyranoside per ml. The reaction was terminated by the addition of Na2CO3, and the absorbance was quantitated at 410 nm. Extracts were made from the remainder of the cells and lysates, were normalized for transfection efficiency, and were subjected to CAT assays as described elsewhere (17). Percentages of 14[C]chloramphenicol acetylation were quantified by the Molecular Dynamics Imaging System.
Metabolic labeling and immunoprecipitation.
Permanently transfected 729 cell lines were metabolically labeled with [35S]methionine cysteine (Trans-35S-label, 100 mCi/ml; ICN Biochemicals, Inc.) in methionine-cysteine-free RPMI 1640 medium supplemented with 10% dialyzed FCS. Cells were lysed in radioimmunoprecipitation assay buffer (0.05 M Tris-HCl [pH 8.0], 0.1% sodium dodecyl sulfate [SDS], 1.0% Triton X-100, 0.15 M NaCl, 2.0 mM phenylmethylsulfonyl fluoride), and lysates were clarified by centrifugation at 100,000 × g (1 h at 4°C). Clarified extracts were immunoprecipitated with HTLV-2 patient antiserum containing antibody against p24 Gag in the presence of protein A-Sepharose (Pharmacia). Immunoreactive proteins were fractionated by SDS-polyacrylamide gel electrophoresis and visualized by autoradiography.
Syncytium and transformation assays.
Syncytium and transformation assays were performed as previously described (19). Briefly, 729 producer cells (5 × 105) were irradiated with 10,000 rads and then cocultivated either with 105 BJAB cells or 2 × 106 PBL (isolated from the blood of healthy donors by centrifugation over Ficoll-Paque) in 24-well culture plates. Syncytia were scored in BJAB cocultures 5 to 7 days postplating. Transformed T cells, defined as continuous growth in the absence of IL-2, grew out of PBL cocultures at 7 to 8 weeks postplating. In both cases, the presence of HTLV-2 expression was confirmed by detection of structural Gag protein in the culture supernatant by p19 Gag enzyme-linked immunosorbent assay (ELISA) (Cellular Products, Buffalo, N.Y.) with a detection sensitivity of 25 pg/ml.
RESULTS
Establishment of HTLVc-enh provirus-expressing cells with distinct Tax transactivation phenotypes.
The chimeric proviral clone, termed HTLVc-enh, contains the CMV enhancer in place of the TRE in both LTRs. Following introduction into human lymphoid cells, this clone produces infectious virus particles that replicate by a Tax-independent mechanism and are capable of infecting and transforming primary human T lymphocytes with an efficiency similar to wt HTLV-2 (46). This unique reagent allowed us to directly determine that Tax is essential for HTLV-2-mediated transformation of primary human T cells (46). We have recently generated and characterized a panel of Tax-2 mutants identifying regions within the 331-amino-acid protein important for activation of promoters through CREB/ATF or NFκB/Rel signaling (45). One of the next critical steps in understanding HTLV pathogenesis as well as providing insight into the origin of other T-cell leukemias and lymphomas is to correlate Tax activation of the CREB/ATF and NFκB/Rel signaling pathways with HTLV-mediated cellular transformation. Therefore, our tax-2 point mutants, which display distinct transactivation phenotypes, were inserted into the functional HTLVc-enh proviral clone. The Tax transactivation phenotypes of the panel of Tax mutant proviral clones are expected to comprise four distinct groups. These include (i) activation of both CREB/ATF and NFκB/Rel (wt Tax and mutant H3N), (ii) activation of NFκB/Rel only (mutant C29S in the putative zinc binding domain, truncation mutant Y290Term, and mutant I319R/L320S, a point mutant similar to Tax-1 M47 [49]), (iii) activation of CREB/ATF only (mutant S130A/L131F, a point mutant similar to Tax-1 M22 [49]), and (iv) activation of neither CREB/ATF nor NFκB/Rel (truncation mutant F4Term and mutant T113A). We first confirmed the expected transactivation profiles of the tax-2 mutants with respect to CREB/ATF and NFκB/Rel activation when expressed from the HTLVc-enh proviral clone. The DNA proviral clones were cotransfected with the CREB/ATF-dependent reporter plasmid, LTR-2-CAT, or the NFκB/Rel-dependent reporter plasmid, HIV-κB-CAT, into human JM4 T cells, and CAT activity was quantified. The results summarized in Table 1 confirm that the Tax mutants have the expected transactivation activity (45) when expressed from the chimeric HTLVc-enh proviral clone.
TABLE 1.
Phenotypic analysis of HTLVc-enh tax mutant clonesa
| Proviral plasmid | % Chloroamphenicol acetylation by reporter plasmid
|
||
|---|---|---|---|
| pU3R-1-CAT (HTLV-1 LTR) | LTR-2-CAT (HTLV-2 LTR) | HIV-κB-CAT (HIV-κB-TATA) | |
| Vector | <5 | <5 | <5 |
| HTLVc-enh(wtTax) | 100 | 100 | 100 |
| HTLVc-enhH3N | 92 | 75 | 105 |
| HTLVc-enhC29S | <5 | <5 | 73 |
| HTLVc-enhT113A | <5 | <5 | <5 |
| HTLVc-enhS130A/L131F | 59 | 79 | <5 |
| HTLVc-enhF4Term(ΔTax) | <5 | <5 | <5 |
| HTLVc-enhY290Term | <5 | <5 | 57 |
| HTLVc-enhI319R/L320S | <5 | <5 | 121 |
JM4 T cells were cotransfected by electroporation with 5 μg of the indicated CAT reporter plasmid (pU3R-1-CAT, LTR-2-CAT, or HIV-κB-CAT), 5 μg pCMVβGal, and 15 μg of the indicated HTLVc-enh proviral plasmid encoding wt or mutant forms of Tax-2 or SV2neo vector control. After 48 h of growth, cells were harvested, normalized for transfection efficiency (β-Gal activity), and assayed for CAT activity. The numbers represent percent chloramphenicol acetylation values averaged over three independent experiments and normalized to the value for HTLVc-enh(wtTax) (100%).
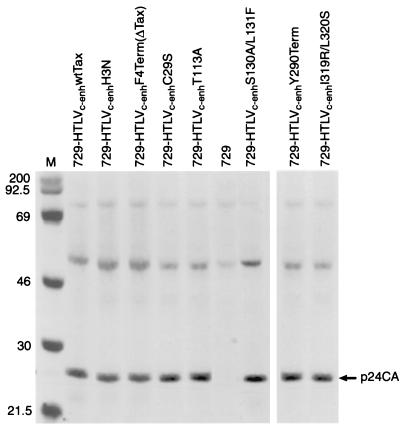
Our next goal was to determine the capacity of HTLVc-enh proviral clones expressing Tax mutants to synthesize viral proteins and direct viral replication. To this end, permanent 729 B-cell transfectants expressing wt Tax and mutant Tax HTLVc-enh proviral clones were isolated and further characterized. To monitor the production of viral proteins in these transfectants, cells were metabolically labeled, and lysates were subjected to immunoprecipitation (anti-p24Gag) and SDS-polyacrylamide gel electrophoresis analysis. Each of the transfectants chosen for this study produced similar levels of p24 Gag capsid protein (Fig. 1). Although each of the tax gene mutations was designed to maintain the integrity of the overlapping rex gene reading frame, the efficient expression of Gag protein confirms that Rex is fully functional. In addition, each transfectant was shown to contain full-length proviral genomes as assessed by PCR and Southern blotting (data not shown).
FIG. 1.
Immunoprecipitation of [35S]methionine-cysteine-labeled 729-HTLVc-enh producer cells. 729 cells (uninfected B-cell line) and 729 viral producer cell lines expressing viruses with various Tax mutants, as indicated, were metabolically labeled and cell lysates were prepared. Transfectant cell lysates were normalized by scintillation counting of trichloroacetic acid precipitates, and equivalent amounts were immunoprecipitated with human antiserum directed against the HTLV-2 p24 Gag capsid protein (CA). The sizes (in kilodaltons, indicated on the left) were determined by comparison to protein markers (Amersham) (lane M). Regardless of the Tax transactivation phenotype, each producer cell line expresses similar levels of p24 Gag capsid.
To assess whether the various transfectants continued to express Tax with the expected transactivation phenotype, cell clones were transfected with LTR-2-CAT or HIV-κB-CAT, and CAT activity was measured. The results, summarized in Table 2, indicate that the proviruses continue to display the expected Tax mutant transactivation phenotypes.
TABLE 2.
Phenotype of virus expressed from permanent transfectants
| Cell line | % Chloroamphenicol acetylationa
|
Syncytium induction in BJAB cellsc | |
|---|---|---|---|
| LTR-2-CAT (HTLV-2 LTR) | HIV-κB-CAT (HIV-κB-TATA) | ||
| 729 (uninfected) | <5 | <5 | − |
| 729-wtHTLV | NDb | NDb | +(100) |
| 729-HTLVc-enh(wtTax) | 100 | 100 | +(100) |
| 729-HTLVc-enhH3N | 80 | 102 | +(100) |
| 729-HTLVc-enhF4Term(ΔTax) | <5 | <5 | +(100) |
| 729-HTLVc-enhC29S | <5 | 79 | +(100) |
| 729-HTLVc-enhT113A | <5 | <5 | +(100) |
| 729-HTLVc-enhS130A/L131F | 81 | <5 | +(100) |
| 729-HTLVc-enhY290Term | <5 | 56 | +(100) |
| 729-HTLVc-enhI319R/L320S | <5 | 125 | +(100) |
Permanently transfected 729 cells were cotransfected by electroporation with 5 μg of the indicated CAT reporter plasmid (LTR-2-CAT or HIV-κB-CAT) and 5 μg of pCMVβGal. After 48 h of growth, cells were harvested, lysates were made and normalized for transfection efficiency (β-Gal activity), and cells were assayed for CAT activity. The numbers represent percent chloramphenicol acetylation values averaged over three independent experiments and normalized to the value for 729-HTLVc-enh(wtTax) (100%).
ND, not determined.
Permanently transfected 729 cells were irradiated with 10,000 rads, and serial 10-fold dilutions of irradiated cells were incubated with 5 × 105 BJAB cells in 24-well culture plates. Cells were fed twice a week with RPMI-1640 supplemented with 20% FCS and antibiotics. Cultures were scored positive (+) or negative (−) for syncytia at 3 to 7 days postplating. Numbers in parentheses indicate the minimum number of 729 producer cells required for syncytium induction following coculture with 5 × 105 BJAB cells.
Infection and replication by HTLVc-enh-expressing Tax mutants.
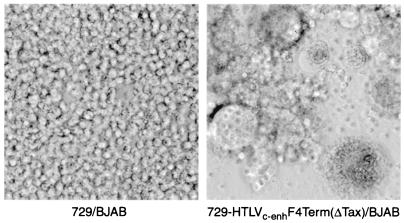
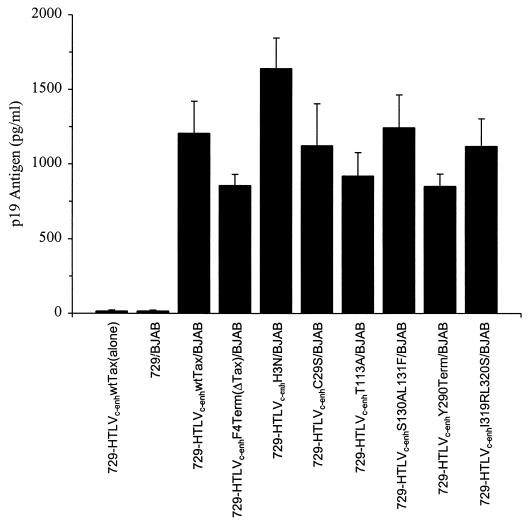
To evaluate the capacity of the chimeric mutant viruses to produce infectious progeny virions, the transfectants were irradiated and cocultured with the human B-cell line BJAB. Productive infection of BJAB cells by HTLV-2 results in rapid induction of syncytia with cytopathicity (19, 46). Figure 2 shows the results of a representative assay comparing syncytium formation between irradiated uninfected 729 cells or irradiated 729-HTLVc-enhF4Term(ΔTax) cells upon coculture with BJAB cells. Cocultivation of all 729-HTLVc-enh tax mutant cell clones with BJAB cells resulted in syncytium formation (Table 2). To address the efficiency at which the viruses replicate and induce syncytia, 10-fold serial dilutions of irradiated producer cells were cocultured with BJAB cells. Syncytia were induced with as few as 100 irradiated producer cells (Table 2), and there was no apparent difference in the time course of syncytium induction by either the tax wild-type or tax mutant HTLVc-enh (data not shown). BJAB cells cocultured with 100 irradiated producer cells expressing the HTLVc-enh Tax mutants exhibit similar levels of Gag protein in the culture supernatant, as assessed by p19 Gag ELISA at 7 days postcoculture (Fig. 3). Taken together, these results demonstrate that HTLVc-enh replicates and spreads with similar efficiency regardless of tax or mutant tax genes.
FIG. 2.
A representative HTLVc-enh syncytium induction assay in BJAB cells. A total of 5 × 105 uninfected 729 cells or a representative stable transfectant, 729-HTLVc-enhF4Term(ΔTax), were irradiated with 10,000 rads and cocultured with 105 BJAB cells. Syncytia were scored in BJAB cell cocultures microscopically 3 to 5 days postplating. Cells were photographed 3 days postplating.
FIG. 3.
p19 Gag expression in BJAB cells. Permanently transfected 729 cells or mock 729 cells were irradiated with 10,000 rads, and 100 cells were cocultivated with 5 × 105 BJAB cells in 24-well culture plates. Syncytia were microscopically visible in BJAB cocultures 5 days postplating. At day 7, viral particle production was estimated in the culture supernatants by p19 Gag antigen ELISA. The error bars indicate the standard deviation from three replicate wells. 729-HTLVc-enhwtTax(alone), day 7 supernatant from 100 irradiated producer cells alone or without BJAB cells (the amount of p19 Gag detected in this sample was <25 pg/ml).
Transformation of human T cells by HTLVc-enh-expressing Tax mutants.
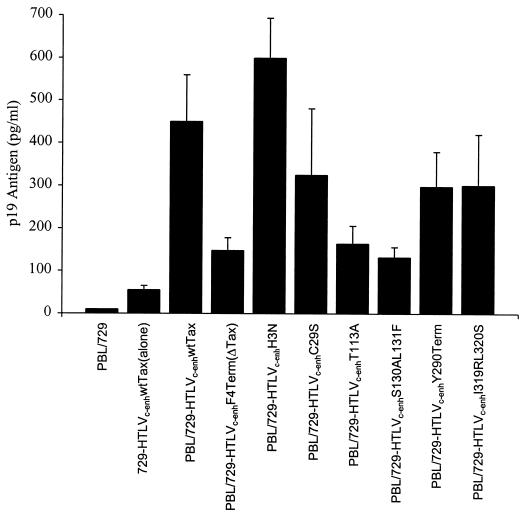
Experiments were next performed to determine which Tax mutant phenotypes are required for HTLV-2-mediated transformation of primary human T lymphocytes. A typical transformation assay included irradiated 729 producer cells harboring either wtHTLV-2, HTLVc-enhwtTax, or HTLVc-enhmutantTax-2 proviral clones and freshly isolated human PBL in 24-well plates. In an attempt to provide an environment similar to that which occurs in vivo, the PBL were not previously activated with phytohemagglutinin and IL-2, nor did the media contain exogenous IL-2. Cell number and viability were monitored at approximately weekly intervals in order to follow cellular proliferation as a result of viral infection. Viral replication was confirmed by p19 Gag ELISA of culture supernatant at 3 weeks postcocultivation, a time point at which HTLV productively infected PBL would be expected to produce viral particles (as measured by p19 Gag), whereas particle production from residual irradiated viral producer cells would be expected to be low (Fig. 4). These results indicate that each virus, regardless of the Tax transactivation phenotype, is capable of productively infecting PBL. Therefore, Tax is not necessary for HTLVc-enh infection of PBL.
FIG. 4.
p19 Gag expression in infected PBL. Cell supernatants were obtained at day 21, as described in the legend to Fig. 5. Viral particle production was estimated in the culture supernatant by p19 Gag antigen ELISA. The error bars indicate the standard deviation from three replicate wells. To control for particle production from 729 producer cells (background), culture supernatant from 106 irradiated cells, designated 729-HTLVc-enhwtTax cells(alone), was measured after 3 weeks in culture.
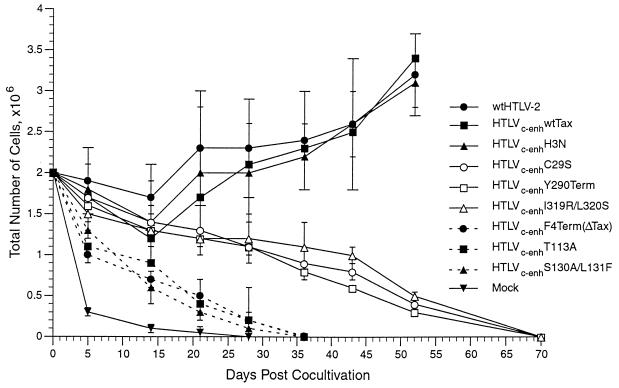
Multiple transformation assays were performed and established four distinct growth patterns, and a representative assay is presented in Fig. 5. Pattern 1 is represented by the negative control and presents the growth of PBL cocultured with irradiated uninfected 729 cells (Mock). A rapid decrease in viable cells was observed, and no viable cultures are produced. Pattern 2 is represented by PBL/729-wtHTLV-2, PBL/729-HTLVc-enhwtTax, and PBL/729-HTLVc-enhH3N cocultures, in which the characteristic transformation process is observed. Initially, there was a slight decrease in cell number, followed by a rapid expansion of cells for the duration of the assay. Flow cytometric analysis determined that these cells were primarily CD8+ T cells (46 and data not shown). The expression of HTLV-2 was confirmed by the detection of p24Gag capsid protein in HTLVc-enhwtTax-transformed cells (46) and HTLVc-enhH3N mutant-transformed cells (data not shown). IL-2 was not added to the culture media, indicating that viability and growth of transformed cells is not dependent on exogenous IL-2. However, transformed cells did respond to exogenous IL-2 and the capacity to efficiently establish viable IL-2-independent T-cell lines could be enhanced by providing IL-2 at 5 to 6 weeks following coculture (data not shown).
FIG. 5.
Growth curve of HTLVc-enh T-lymphocyte transformation assay. Human PBL were isolated by Ficoll-Paque and cocultivated with irradiated (10,000 rads) 729 virus producer cells (described in Table 2) or 729 uninfected control cells. PBL (2 × 106) were cocultured with irradiated donor cells (106) in 24-well plates. Cells were fed once per week with RPMI 1640 supplemented with 20% FCS. Cell viability was determined by trypan blue exclusion staining at 0, 5, 14, 21, 28, 36, 43, 53, and 70 days postcocultivation. Four distinct growth patterns were observed. The mean and standard deviation was determined from three independent samples of each coculture: pattern 1 contains PBL/729 negative control (Mock) coculture; pattern 2 contains PBL/729-HTLVc-enhF4Term(ΔTax), PBL/729-HTLVc-enhT113A, and PBL/729-HTLVc-enhS130A/L131F cocultures showing the critical importance of NFκB/Rel activation; pattern 3 contains PBL/729-HTLVc-enhC29S, PBL/729-HTLVc-enhY290Term, and PBL/729-HTLVc-enhI320R/L320S cocultures showing the requirement for activation of CREB/ATF in addition to NFκB/Rel; and pattern 4 contains PBL/729-wtHTLV-2, PBL/729-HTLVc-enhwtTax, and PBL/729-HTLVc-enhH3N or fully transformed cells.
Pattern 3 is distinct and slightly shifted from the negative control and is represented by PBL/729-HTLVc-enhF4Term(ΔTax), PBL/729-HTLVc-enhS130A/L131F, and PBL/729-HTLVc-enhT113A cocultures. A progressive loss of viable cells over time was seen, and by 36 days postcoculture, no viable cells remained. The slight positive shift of this curve compared to the negative control is attributed to the presence of replicating virus (Fig. 4); HTLV particles have been shown to be mitogenic for primary T cells (16, 63). The observation that HTLVc-enhS130A/L131F fails to transform cells and displays a growth-inducing phenotype similar to HTLVc-enhF4Term(ΔTax), which makes no Tax, suggests that Tax activation of the NFκB/Rel signaling pathway correlates with an initial phase of the cellular transformation process.
Pattern 4 is represented by PBL/729-HTLVc-enhC29S, PBL/729-HTLVc-enhY290Term, and PBL/729-HTLVc-enhI319R/L320S cocultures; each virus encodes a distinct Tax mutant that maintains NFκB/Rel activation but is abrogated in CREB/ATF activation. Within the first 2 weeks postcoculture, a decline in cell number is observed that is similar to that in wtHTLV-2, HTLVc-enhwtTax, or HTLVc-enhH3N infection. Although these cells remain viable for up to 2 months postcoculture, they never enter an expansion phase, and at approximately 70 days postcoculture no viable cells remain. Consistent with the phenotype of wtHTLV-2-infected cells, flow cytometric analysis at 50 days postcoculture determined that these cells were primarily CD8+ T-cells (data not shown). The simplest interpretation is that sustained viability of the cells is attributed to Tax activation of NFκB/Rel and is consistent with the reciprocal failure of HTLVc-enhS130A/L131F to induce cell proliferation. Addition of IL-2 to the cultures at day 50 failed to result in immortalization or sustain cell proliferation (data not shown). These in vitro transformation assays of the wt and seven tax mutant HTLVc-enh viruses are consistent with the conclusion that Tax transactivation of NFκB/Rel correlates with cell proliferation and the initiation of the transformation process and that CREB/ATF signaling pathway promotes sustained cell growth and IL-2-independent cellular transformation.
DISCUSSION
In this study, seven tax mutants were analyzed in the context of a replication-competent HTLV provirus. The ultimate goal was to directly correlate the role of Tax activation of the CREB/ATF or NFκB/Rel signaling pathway with HTLV-mediated T-lymphocyte transformation. This is the first investigation into the role of Tax-2 activation of NFκB/Rel and CREB/ATF pathways in the transformation process. Our approach makes use of a well-characterized HTLV-2 (HTLVc-enh) provirus that replicates by a Tax-independent mechanism. This unique virus eliminates the variation that independent tax mutations may have on viral transcription and replication efficiency. Our results indicate that Tax-2 mutant viruses that selectively abrogate NFκB/Rel or CREB/ATF fail to induce long-term growth of IL-2-independent T lymphocytes. Our results with HTLVc-enhS130A/L131F are consistent with the hypothesis that Tax activation of NFκB/Rel provides an early proliferative signal to HTLV-infected cells that is absolutely critical for the initiation of cell growth and the transformation process. One can equate this early step with maintaining cell growth and viability in culture by activating cellular growth genes, including IL-2 and IL-2 receptor genes. This hypothesis is supported further by the growth of primary cells infected with HTLVc-enhC29S, HTLVc-enhY290Term, and HTLVc-enhI319R/L320S. These viruses, which encode a mutant Tax protein that activates NFκB/Rel but not CREB/ATF, facilitate cell viability for up to 2 months. However, the capacity of Tax to activate CREB/ATF is also critical to the transformation process, since PBL infected with these HTLVc-enh Tax mutants fail to exhibit the transformation profile observed with wt Tax-expressing virus. Although activation of both the NFκB/Rel and CREB/ATF pathways are implicated in the in vitro transformation process, we cannot rule out the possible contribution of other mechanisms critical for Tax transformation.
The role of CREB/ATF or NFκB/Rel pathways in Tax-1-mediated transformation has been previously studied using several different experimental systems, and the results have been controversial. One study indicated that Tax-1 activation of NFκB/Rel was dispensable but that activation of the CREB/ATF pathway was critical for the morphological transformation of Rat-2 cells (50). Two similar studies using Rat-1 cells implicated the NFκB/Rel pathway in the morphological transformation process (32, 58). Studies using various delivery systems investigated the ability of Tax-1 mutants to immortalize primary human peripheral blood mononuclear cells (PBMCs), again with inconclusive results. One study using retroviral vectors indicated that Tax-1 activation of NFκB/Rel is sufficient to promote the growth response of PBL to IL-2. However, clonal expansion of CD4+ T cells required activation of the CREB/ATF pathway (3). Another study indicated that a Tax-1 variant incapable of activating NFκB/Rel when expressed from a herpesvirus saimiri vector retained its immortalizing potential for primary human T cells (44). A more recent study has examined the role of Tax-1 in immortalization or transformation of PBMCs in the context of a full-length HTLV-1 (43). Their results indicated that the activation of the NFκB/Rel pathway by Tax-1 correlates with IL-2-dependent immortalization of PBMCs and that CREB/ATF activation was dispensable. It is likely that these conflicting observations may be due to substantial differences in experimental systems used or the Tax-1 mutations characterized.
Our study, distinct in approach and use of HTLV-2, more closely resembles the HTLV-1 immortalization report by Robek and Ratner (43) because both utilize full-length proviruses and primary human cells as transformation targets. Both attempt to mimic natural infection in vivo, but there are several major differences. First, we use irradiated virus producer cells, not transfection, to efficiently infect PBL. Second, the PBL are not previously activated with phytohemagglutinin and IL-2, nor is the culture media supplemented with exogenous IL-2. Therefore, the endpoint for our assay is IL-2-independent cell growth, not IL-2-dependent immortalization. Lastly, the functional HTLVc-enh replicates by a Tax-independent mechanism. Thus, tax mutations do not have a compromising effect on viral gene transcription and the ability of the mutant viruses to efficiently replicate. Our results are in agreement with Robek and Ratner in that NFκB/Rel activation directly correlates with cellular transformation. However, the importance of Tax activation of the CREB/ATF pathway in the transformation process cannot be directly compared because of differences between our systems. Our results indicate that CREB/ATF activation correlates with the emergence of fully transformed cells that proliferate independently of IL-2. This possibility was not addressed in previous IL-2-dependent immortalization assays.
HTLV-1 and HTLV-2 have distinct pathogenic properties but transform primary human T lymphocytes with similar efficiencies. These distinct pathogenic properties may be attributable to differences in Tax-1- and Tax-2-mediated transformation. The major functional regions or domains of Tax that are important for transactivation by the CREB/ATF or NFκB/Rel signaling pathway are similar but not identical in Tax-1 and Tax-2 (45). In addition, HTLV-1-transformed cells are associated with the constitutive activation of the Jak/STAT pathway, whereas in HTLV-2-transformed cells Jak/STAT is not activated, suggesting that the mechanism of in vitro transformation is different (35, 37).
A complete understanding of the mechanism of T-lymphocyte transformation will require correlation of Tax domains important in cellular transformation with those domains important in modulating gene transcription, cell cycle control, induction of DNA damage, and other undefined modes of action (7, 23, 30, 36, 38, 41, 47, 52). The HTLV-2 experimental system described in this study as well as the similar HTLV-1 system in development are useful for the dissection of Tax domains in the transformation process. Future comparisons between HTLV-1 and HTLV-2 will be critical for our understanding of the pathogenesis of HTLV infection.
ACKNOWLEDGMENTS
We thank Kathleen Boris-Lawrie and Michael Lairmore for critical comments.
This work is supported by grants from the National Institutes of Health (CA77556) and the Leukemia Society of America. P.L.G. is a scholar of the Leukemia Society of America.
REFERENCES
- 1.Adya N, Giam C-Z. Distinct regions in human T-lymphotropic virus type 1 Tax mediate interactions with activator protein CREB and basal transcription factors. J Virol. 1995;69:1834–1841. doi: 10.1128/jvi.69.3.1834-1841.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adya N, Zhao L-J, Huang W, Boros I, Giam C-Z. Expansion of CREB's DNA recognition specificity by Tax results from interaction with Ala-Ala-Arg at positions 282–284 near the conserved DNA-binding domain of CREB. Proc Natl Acad Sci USA. 1994;91:5642–5646. doi: 10.1073/pnas.91.12.5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akagi T, Ono H, Nyunoya H, Shimotohno K. Characterization of peripheral blood T-lymphocytes transduced with HTLV-I Tax mutants with different trans-activating phenotypes. Oncogene. 1997;14:2071–2078. doi: 10.1038/sj.onc.1201045. [DOI] [PubMed] [Google Scholar]
- 4.Anderson M G, Dynan W S. Quantitative studies of the effect of HTLV-1 Tax protein on CREB protein-DNA binding. Nucleic Acids Res. 1994;22:3194–3201. doi: 10.1093/nar/22.15.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballard D W, Bohnlein E, Lowenthal J W, Wano Y, Franza B R, Greene W C. HTLV-I tax induces cellular proteins that activate the κB element in the IL-2 receptor α gene. Science. 1988;241:1652–1655. doi: 10.1126/science.241.4873.1652. [DOI] [PubMed] [Google Scholar]
- 6.Bantignies F, Rousset R, Desbois C, Jalinot P. Genetic characterization of transactivation of the human T-cell leukemia virus type 1 promoter: binding of Tax to Tax-responsive element 1 is mediated by the cyclic AMP-responsive members of the CREB/ATF family of transcription factors. Mol Cell Biol. 1996;16:2174–2182. doi: 10.1128/mcb.16.5.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bex F, Yin M J, Burny A, Gaynor R B. Differential transcriptional activation by human T-cell leukemia virus type 1 Tax mutants is mediated by distinct interactions with CREB binding protein and p300. Cell. 1998;18:2392–2405. doi: 10.1128/mcb.18.4.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodor J, Walker W, Flemington E, Spetz A L, Habener J F. Modulation of Tax and PKA-mediated expression of HTLV-1 promoter via cAMP response element binding and modulator proteins CREB and CREM. FEBS Lett. 1995;377:413–418. doi: 10.1016/0014-5793(95)01299-0. [DOI] [PubMed] [Google Scholar]
- 9.Brauweiler A, Garl P, Franklin A A, Giebler H A, Nyborg J K. A molecular mechanism for human T-cell leukemia virus latency and Tax transactivation. J Biol Chem. 1995;270:12814–12822. doi: 10.1074/jbc.270.21.12814. [DOI] [PubMed] [Google Scholar]
- 10.Brockman J A, Scherer D C, McKinsey T A, Hall S M, Qi X, Lee W, Ballard D W. Coupling of a signal response domain in IκBα to multiple pathways for NF-κB activation. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cann A J, Koyanagi Y, Chen I S Y. High efficiency transfection of primary human lymphocytes and studies of gene expression. Oncogene. 1988;3:123–128. [Google Scholar]
- 12.Chen I Y, McLaughlin J, Gasson J C, Clark S C, Golde D W. Molecular characterization of genome of a novel human T-cell leukaemia virus. Nature. 1983;305:502–505. doi: 10.1038/305502a0. [DOI] [PubMed] [Google Scholar]
- 13.Cross S L, Feinberg M B, Wolf J B, Holbrook N J, Wong-Staal F, Leonard W J. Regulation of the human interleukin-2 receptor alpha promoter: activation of a nonfunctional promoter by the transactivator gene of HTLV-I. Cell. 1987;49:47–56. doi: 10.1016/0092-8674(87)90754-9. [DOI] [PubMed] [Google Scholar]
- 14.Fujii M, Sassone-Corsi I, Verma I M. c-fos promoter trans-activation by the tax 1 protein of human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1988;85:8526–8530. doi: 10.1073/pnas.85.22.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujii M, Tsuchiya H, Chuhjo T, Akizawa T, Seiki M. Interaction of HTLV-1 Tax1 with p67SRF causes the aberrant induction of cellular immediate early genes through CArG boxes. Genes Dev. 1992;6:2006–2076. doi: 10.1101/gad.6.11.2066. [DOI] [PubMed] [Google Scholar]
- 16.Gazzolo L, Duc Dodon M. Direct activation of resting T lymphocytes by human T-lymphotropic virus type I. Nature. 1987;326:714–717. doi: 10.1038/326714a0. [DOI] [PubMed] [Google Scholar]
- 17.Gorman C M, Moffat L F, Howard B H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982;2:1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green P L, Chen I S Y. Molecular features of the human T-cell leukemia virus: mechanisms of transformation and leukemogenicity. In: Levy J A, editor. The Retroviridae. Vol. 3. New York, N.Y: Plenum Press; 1994. pp. 227–311. [Google Scholar]
- 19.Green P L, Ross T M, Chen I S Y, Pettiford S. Human T-cell leukemia virus type II nucleotide sequences between env and the last exon of tax/rex are not required for viral replication or cellular transformation. J Virol. 1995;69:387–394. doi: 10.1128/jvi.69.1.387-394.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwanaga Y, Tsukahara T, Ohashi T, Tanaka Y, Arai M, Nakamura M, Ohtani K, Koya Y, Kannagi M, Yamamoto N, Fujii M. Human T-cell leukemia virus type 1 Tax protein abrogates interleukin-2 dependence in a mouse T-cell line. J Virol. 1999;73:1271–1277. doi: 10.1128/jvi.73.2.1271-1277.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeang K-T, Chiu R, Santos E, Kim S-J. Induction of the HTLV-I LTR by Jun occurs through the Tax-responsive 21-bp elements. Virology. 1991;181:218–227. doi: 10.1016/0042-6822(91)90487-v. [DOI] [PubMed] [Google Scholar]
- 22.Jeang K-T, Boros I, Brady J, Radonovich M, Khoury G. Characterization of cellular factors that interact with the human T-cell leukemia virus type I p40x-responsive 21-base-pair sequence. J Virol. 1988;62:4499–4509. doi: 10.1128/jvi.62.12.4499-4509.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin D Y, Spencer F, Jeang K T. Human T-cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell. 1998;93:1–20. doi: 10.1016/s0092-8674(00)81148-4. [DOI] [PubMed] [Google Scholar]
- 24.Jin D-Y, Giordano V, Kilber K V, Nakano H, Jeang K-T. Role of adapter function in oncoprotein-mediated activation of NFκB: HTLV-1 Tax interacts directly with IκB kinase γ. J Biol Chem. 1999;274:17402–17405. doi: 10.1074/jbc.274.25.17402. [DOI] [PubMed] [Google Scholar]
- 25.Kanno T, Borwn K, Franzoso G, Siebenlist U. Kinetic analysis of human T-cell leukemia virus type I Tax-mediated activation of NF-κB. Mol Cell Biol. 1994;14:6443–6451. doi: 10.1128/mcb.14.10.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacoste J, Cohen L, Hiscott J. NF-κB activity in T cells stably expressing the Tax protein of human T cell lymphotropic virus type I. Virology. 1991;184:553–562. doi: 10.1016/0042-6822(91)90425-b. [DOI] [PubMed] [Google Scholar]
- 27.Leung K, Nabel G J. HTLV-I transactivator induces interleukin-2 receptor expression through an NFκB-like factor. Nature. 1988;333:776–778. doi: 10.1038/333776a0. [DOI] [PubMed] [Google Scholar]
- 28.Li C-C H, Ruscetti F W, Rice N, Chen E, Yang N-S, Mikovits J, Longo D L. Differential expression of Rel family members in human T-cell leukemia virus type I-infected cells: transcriptional activation of c-rel by Tax protein. J Virol. 1993;67:4205–4213. doi: 10.1128/jvi.67.7.4205-4213.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindholm P, Marriott S, Gitlin S, Bohan C, Brady J. Induction of nuclear factor NF-κB DNA binding activity after exposure of lymphoid cells to soluble Tax1 protein. New Biol. 1991;2:1034–1043. [PubMed] [Google Scholar]
- 30.Low K G, Dorner L F, Fernando D B, Grossman J, Jeang K T, Comb M J. Human T-cell leukemia virus type I Tax releases cell cycle arrest induced by p16INK4a. J Virol. 1997;71:1956–1962. doi: 10.1128/jvi.71.3.1956-1962.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maggirwar S B, Harhaj E, Sun S-C. Activation of NF-κB/Rel by Tax involves degradation of IκBα and is blocked by a proteasome inhibitor. Oncogene. 1995;11:993–998. [PubMed] [Google Scholar]
- 32.Matsumoto K, Shibata H, Fujisawa J-I, Inoue H, Hakura A, Tsukahara T, Fujji M. Human T-cell leukemia virus type 1 Tax protein transforms rat fibroblasts via two distinct pathways. J Virol. 1997;71:4445–4451. doi: 10.1128/jvi.71.6.4445-4451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKinsey T A, Brockman J A, Scherer D C, Al-Murrani S W, Green P L, Ballard D W. Inactivation of IκBβ by human T-cell leukemia virus type 1 Tax: a potential mechanism for constitutive induction of NF-κB. Mol Cell Biol. 1996;16:2083–2090. doi: 10.1128/mcb.16.5.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennett B L, Li J, Young D B, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NFκB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 35.Migione T-S, Lin J-X, Cereseto A, Mulloy J C, O'Shea J J, Franchini G, Leonard W J. Constitutively activated Jak-STAT pathway in T-cells transformed with HTLV-1. Science. 1995;269:79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- 36.Mulloy J C, Kislyakova T, Cereseto A, Casareto L, LoMonico A, Fullen J, Lorenzi M V, Cara A, Nicot C, Giam C-Z, Franchini G. Human T-lymphotropic/leukemia virus type 1 Tax abrogates p53-induced cell cycle arrest and apoptosis through its CREB/ATF functional domain. J Virol. 1998;72:8852–8860. doi: 10.1128/jvi.72.11.8852-8860.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulloy J C, Migione T-S, Ross T M, Ton N, Green P L, Leonard W J, Franchini G. Human and simian T-cell leukemia viruses type 2 (HTLV-2 and STLV-2pan-p) transform T-cells independently of Jak/STAT activation. J Virol. 1998;72:4408–4412. doi: 10.1128/jvi.72.5.4408-4412.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neuveut C, Low K G, Maldarelli F, Schmitt I, Majone F, Grassmann R, Jeang K T. Human T-cell leukemia virus type 1 Tax and cell cycle progression: role of cyclin D-cdk and p110Rb. Mol Cell Biol. 1998;18:3620–3632. doi: 10.1128/mcb.18.6.3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nimer S D. Tax responsiveness of the GM-CSF promoter is mediated by mitogen-inducible sequences other than kappa B. New Biol. 1991;3:997–1004. [PubMed] [Google Scholar]
- 40.Nimer S D, Gasson J C, Hu K, Smallberg I, Chen I S Y, Rosenblatt J D. Activation of the GM-CSF promoter by HTLV-I and HTLV-II tax proteins. Oncogene. 1989;4:671–676. [PubMed] [Google Scholar]
- 41.Pise-Masison C A, Choi K S, Radonovich M, Dittmer J, Kim S J, Brady J N. Inhibition of p53 transactivation function by the human T-cell lymphotropic virus type 1 Tax protein. J Virol. 1998;72:1165–1170. doi: 10.1128/jvi.72.2.1165-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ressler S, Morris G F, Marriott S J. Human T-cell leukemia virus type 1 Tax transactivates the human proliferating cell nuclear antigen promoter. J Virol. 1997;71:1181–1190. doi: 10.1128/jvi.71.2.1181-1190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robek M D, Ratner L. Immortalization of CD4+ and CD8+ T-lymphocytes by human T-cell leukemia virus type 1 Tax mutants expressed in a functional molecular clone. J Virol. 1999;73:4856–4865. doi: 10.1128/jvi.73.6.4856-4865.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosin R, Koch C, Schmitt I, Semmes O J, Jeang K-T, Grassmann R. A human T-cell leukemia virus Tax variant incapable of activating NFκB retains its immortalizing potential for primary T-lymphocytes. J Biol Chem. 1998;273:6698–6703. doi: 10.1074/jbc.273.12.6698. [DOI] [PubMed] [Google Scholar]
- 45.Ross T M, Minella A C, Fang Z Y, Pettiford S M, Green P L. Mutational analysis of the human T-cell leukemia virus type 2 Tax. J Virol. 1997;71:8912–8917. doi: 10.1128/jvi.71.11.8912-8917.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ross T M, Pettiford S M, Green P L. The tax gene of human T-cell leukemia virus type 2 is essential for transformation of human T lymphocytes. J Virol. 1996;70:5194–5202. doi: 10.1128/jvi.70.8.5194-5202.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmitt I, Rosin O, Rohwer P, Gossen M, Grassmann R. Stimulation of cyclin-dependent kinase activity and G1- to S-phase transition in human lymphocytes by the human T-cell leukemia/lymphotropic virus type 1 tax protein. J Virol. 1998;72:633–640. doi: 10.1128/jvi.72.1.633-640.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Semmes O J, Jeang K-T. Mutational analysis of human T-cell leukemia virus type I Tax: region necessary for function determined with 47 mutant proteins. J Virol. 1992;66:7183–7192. doi: 10.1128/jvi.66.12.7183-7192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith M R, Greene W C. Identification of HTLV-I tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990;4:1875–1885. doi: 10.1101/gad.4.11.1875. [DOI] [PubMed] [Google Scholar]
- 50.Smith M R, Greene W C. Molecular biology of the type I human T-cell leukemia virus (HTLV-I) and adult T-cell leukemia. J Clin Investig. 1991;87:761–766. doi: 10.1172/JCI115078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith M R, Greene W C. Type I human T-cell leukemia virus Tax protein transforms rat fibroblasts through the cyclic adenosine monophosphate response element binding protein/activating transcription factor pathway. J Clin Investig. 1991;88:1038–1042. doi: 10.1172/JCI115364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki T, Kitao S, Matsushima H, Yoshida M. HTLV-1 Tax protein interacts with cyclin-dependent kinase inhibitor p16INK4A and counteracts its inhibitory activity towards CDK4. EMBO J. 1996;15:1807–1814. [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki T, Hirai H, Fujisawa J, Fujita T, Yoshida M. A transactivator Tax of human T-cell leukemia virus type 1 binds to NF-κB p50 and serum response factor (SRF) and associates with enhancer DNAs of the NFκB site and CArG box. Oncogene. 1993;8:2391–2397. [PubMed] [Google Scholar]
- 54.Tie F, Adya N, Greene W C, Giam C-Z. Interaction of the human T-lymphotropic virus type 1 Tax dimer with CREB and the viral 21-base-pair repeat. J Virol. 1996;70:8368–8374. doi: 10.1128/jvi.70.12.8368-8374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trejo S R, Fah W E, Ratner L. c-sis/PDGF-B promoter transactivator by the Tax protein of the human T-cell leukemia virus type 1. J Biol Chem. 1996;271:14584–14590. doi: 10.1074/jbc.271.24.14584. [DOI] [PubMed] [Google Scholar]
- 56.Wagner S, Green M R. HTLV-I Tax protein stimulation of DNA binding of bZIP proteins by enhancing dimerization. Science. 1993;262:395–399. doi: 10.1126/science.8211160. [DOI] [PubMed] [Google Scholar]
- 57.Wano Y, Feinberg M, Hosking J B, Bogerd H, Greene W C. Stable expression of the tax gene of type I human T-cell leukemia virus in human T cells activates specific cellular genes involved in growth. Proc Natl Acad Sci USA. 1988;85:9733–9737. doi: 10.1073/pnas.85.24.9733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamaoka S, Inoue H, Sakurai M, Sugiyama T, Hazama M, Yamada T, Hatanaka M. Constitutive activation of NF-κB is essential for transformation of rat fibroblasts by the human T-cell leukemia virus type 1 Tax protein. EMBO J. 1996;15:873–887. [PMC free article] [PubMed] [Google Scholar]
- 59.Yin M J, Gaynor R B. Complex formation between CREB and Tax enhances the binding affinity of CREB for the human T-cell leukemia virus type 1 21-base-pair repeats. Mol Cell Biol. 1996;16:3156–3168. doi: 10.1128/mcb.16.6.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yin M J, Paulssen E, Seeler J, Gaynor R B. Chimeric proteins composed of Jun and CREB define domains required for interaction with the human T-cell leukemia virus type 1 Tax protein. J Virol. 1995;69:6209–6218. doi: 10.1128/jvi.69.10.6209-6218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yin M-J, Christerson L B, Yamamoto Y, Kwak Y-T, Xu S, Mercurio F, Barbosa M, Cobb M H, Gaynor R B. HTLV-1 Tax protein binds to MEKK1 to stimulate IκB kinase activity and NFκB activation. Cell. 1998;93:875–884. doi: 10.1016/s0092-8674(00)81447-6. [DOI] [PubMed] [Google Scholar]
- 62.Yin M-J, Paulssen E J, Seeler J S, Gaynor R B. Protein domains involved in both in vivo and in vitro interactions between human T-cell leukemia virus type I Tax and CREB. J Virol. 1995;69:3420–3432. doi: 10.1128/jvi.69.6.3420-3432.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zack J A, Cann A J, Lugo J P, Chen I S Y. AIDS virus production from infected peripheral blood T cells following HTLV-I-induced mitogenic stimulation. Science. 1988;240:1026–1029. doi: 10.1126/science.2835813. [DOI] [PubMed] [Google Scholar]
- 64.Zhao L-J, Giam C-Z. Human T-cell lymphotrophic virus type I (HTLV-I) transcriptional activator, Tax, enhances CREB binding to the HTLV-I 21 base-pair repeats by protein-protein interactions. Proc Natl Acad Sci USA. 1992;89:7070–7074. doi: 10.1073/pnas.89.15.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]