Abstract
Background
The first dengue outbreak in Sao Tome and Principe was reported in 2022. Entomological investigations were undertaken to establish the typology of Aedes larval habitats, the distribution of Ae. aegypti and Ae. albopictus, the related entomological risk and the susceptibility profile of Ae. aegypti to insecticides, to provide evidence to inform the outbreak response.
Methodology/Principal findings
Entomological surveys were performed in all seven health districts of Sao Tome and Principe during the dry and rainy seasons in 2022. WHO tube and synergist assays using piperonyl butoxide (PBO) and diethyl maleate (DEM) were carried out, together with genotyping of F1534C/V1016I/V410L mutations in Ae. aegypti. Aedes aegypti and Ae. albopictus were found in all seven health districts of the country with high abundance of Ae. aegypti in the most urbanised district, Agua Grande. Both Aedes species bred mainly in used tyres, discarded tanks and water storage containers. In both survey periods, the Breteau (BI > 50), house (HI > 35%) and container (CI > 20%) indices were higher than the thresholds established by WHO to indicate high potential risk of dengue transmission. The Ae. aegypti sampled were susceptible to all insecticides tested except dichlorodiphenyltrichloroethane (DDT) (9.2% mortality, resistant), bendiocarb (61.4% mortality, resistant) and alpha-cypermethrin (97% mortality, probable resistant). A full recovery was observed in Ae. aegypti resistant to bendiocarb after pre-exposure to synergist PBO. Only one Ae. aegypti specimen was found carrying F1534C mutation.
Conclusions/Significance
These findings revealed a high potential risk for dengue transmission throughout the year, with the bulk of larval breeding occurring in used tyres, water storage and discarded containers. Most of the insecticides tested remain effective to control Aedes vectors in Sao Tome, except DDT and bendiocarb. These data underline the importance of raising community awareness and implementing routine dengue vector control strategies to prevent further outbreaks in Sao Tome and Principe, and elsewhere in the subregion.
Author summary
During the first dengue outbreak in Sao Tome and Principe reported in 2022, entomological investigations were undertaken to establish the typology of Aedes larval habitats, the distribution of Ae. aegypti and Ae. albopictus, the related entomological risk and the susceptibility profile of Ae. aegypti to insecticides to inform the outbreak response. Surveys revealed the presence of Ae. aegypti and Ae. albopictus in all seven health districts of the country with high abundance of Ae. aegypti in the most urbanised district, Agua Grande. Both Aedes species bred mainly in used tyres, discarded tanks and water storage containers suggesting good waste management and improved water supply system could help to reduce Aedes densities and the risk of dengue transmission. Analyses also revealed that most of the insecticides tested remain effective to control Aedes vectors in Sao Tome, except dichlorodiphenyltrichloroethane and bendiocarb. These findings revealed a high potential risk for dengue transmission throughout the year and underline the importance of raising community awareness and implementing routine dengue vector control strategies to prevent further outbreaks in Sao Tome and Principe, and elsewhere in the subregion.
Background
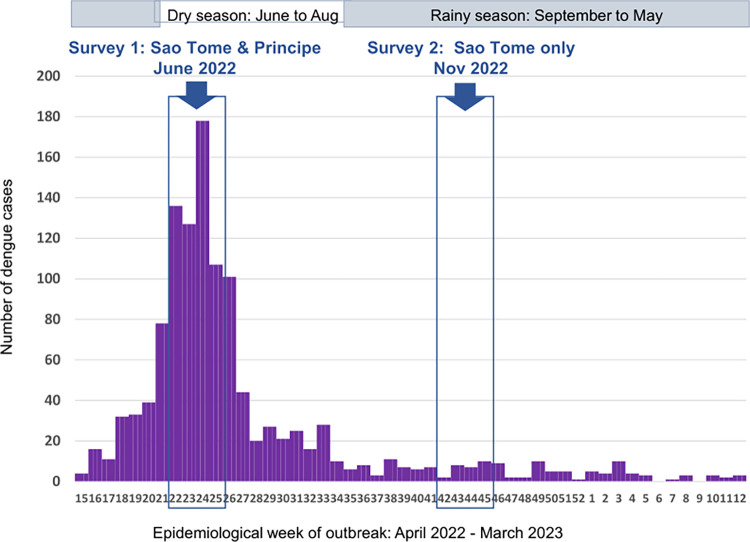
Dengue is the most important mosquito-borne viral disease in the world in terms of morbidity and mortality. Indeed, one modelling estimate indicates 390 million dengue virus infections per year, of which 96 million (67–136 million) manifest clinically [1]. Dengue is caused by the dengue virus (DENV) belonging to the Flavivirus genus and Flaviviridae family. This virus is transmitted to vertebrates including humans by the bite of infected female Aedes species mosquitoes. Formerly, dengue was considered scarce in Africa probably due to the similarity of symptoms with other infectious diseases like malaria. In countries with endemic malaria, it was therefore often misdiagnosed [2]. However, during the past two decades, increasing numbers of dengue cases have been reported across the continent with major outbreaks in some countries, including Gabon [3], Burkina Faso [4], and Angola [5]. In addition, a study undertaken in Douala Cameroon in 2020 demonstrated that ~13% of acute febrile patients presenting in health facilities in the city are due to dengue [6], suggesting that the dengue prevalence in Africa may be higher than expected. The first ever-recorded case of dengue in Sao Tome and Principe, an island state in Central Africa, was reported in April 2022, reaching a cumulative total of 1,200 cases by March 2023, with the peak of the outbreak in June 2022 (Fig 1). Most cases (68%) were reported in the district of Agua Grande, the most urbanized zone in the island. Since there is no specific treatment nor efficient vaccine against dengue, the control of dengue outbreaks remains reliant primarily on vector control, through the destruction of Aedes larval habitats and the application of insecticides to either treat larval habitats or control adults [7]. The implementation of this control strategy requires a good knowledge of the vectors involved in the outbreak.
Fig 1. Timing of entomological surveys in relation to cases reported during dengue outbreak in Sao Tome and Principe, 2022–2023.
Both of the vectors most frequently associated with dengue epidemics, Aedes aegypti and Ae. albopictus, are found in Central Africa, but these species have different origins. Aedes aegypti originated from African forests [8] whereas Ae. albopictus originated from South-East Asia forest [9], but has since spread globally and invaded all the continents over the last four decades. This invasive species was first reported in Central Africa in early 2000s in Cameroon [10] and has rapidly spread to almost all central African countries including Sao Tome and Principe where it was first reported in 2016 [11]. Studies performed in Central Africa on larval ecology of both species showed that they colonise the same types of larval habitats composed mainly of used tyres, discarded tanks and water storage containers [12]. However, in the cities where both species are present, Ae. aegypti has been found to prefer larval habitats located in downtown neighbourhoods with high building density while Ae. albopictus was mostly found in peri-urban or rural locations surrounded by vegetation [13–15]. The emergence of insecticide resistance in Aedes mosquitoes can seriously compromise vector control using insecticides as demonstrated in some countries [16,17]. Data generated in numerous Central African countries reveals significant variation of insecticide resistance according to Aedes species tested, the origin of mosquito and the type of insecticide [18–23]. Though to our knowledge, use of insecticide in central Africa is mainly for control of malaria vectors through impregnated mosquito net and indoor spraying, and in agriculture for the protection of crops. Both major mechanisms, metabolic resistance and target site resistance, generally found involved in insecticide resistance in Aedes mosquitoes, were suspected as the main causes of resistance in Central Africa. Indeed, knock down resistance (kdr) mutations, F1534C, V410L and V1016G were detected in Ae. aegypti with high frequency for 1534C [20]. Some cytochrome P450 genes were found over expressed in Ae. aegypti and Ae. albopictus from Central Africa [20].
In Sao Tome and Principe data on Aedes are very scarce. To fill this knowledge gap, this entomological investigation aimed to assess the typology of larval habitats, the entomological risk using Stegomyian indices, the current distribution of Ae. aegypti and Ae. albopictus as well as the resistance profile of Ae. aegypti to insecticides.
Methods
Study area
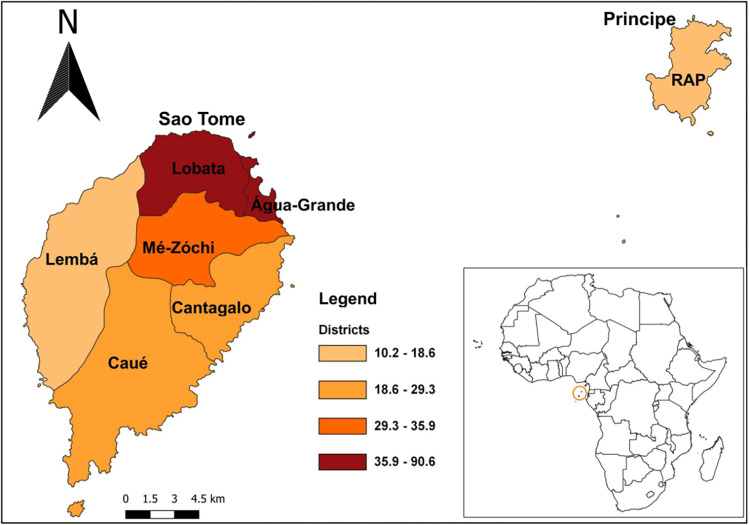
Surveys were carried out in Sao Tome and Principe, an island state of the western equatorial coast of Central Africa. The country consists of two islands, Sao Tome, the bigger island subdivided into six health districts: Agua Grande, Lobata, Me-zochi, Caue, Cantagalo and Lemba, and Principe, the smaller island (RAP: Região Autonóma do Príncipe) comprising one health district (Fig 2). The population of both islands is estimated as 200,000 inhabitants [24]. The climate is humid tropical characterised by two seasons: a long rainy season of nine months duration from September to May and a shorter dry season from June to August; mean annual rainfall is 1,382 mm. Mean temperatures vary a few degrees throughout the year, ranging between 22°C and 26°C. The lowest average temperatures occur during the dry season, while the rainy season experiences higher temperatures(https://climateknowledgeportal.worldbank.org/country/sao-tome-and-principe/climate-data-historical). Agriculture and fishing are the predominant economic activities.
Fig 2. Map of Sao Tome and Principe indicating the seven health districts.
Colors indicate population density by health district. The map was created in QGIS version 3.22.12 with the base layer shapefiles downloaded in the publicly available repository GADM version 4.1 (https://gadm.org/download_country.html).
Sampling and entomological surveys
Entomological investigations were carried out in June and November 2022, corresponding to the dry and rainy seasons respectively (Fig 1). Nationwide surveys were undertaken in clusters of randomly selected dwellings in each of seven health districts of the country. In each district, a minimum of three clusters were randomly selected; each cluster consisted of 15 dwellings per neighbourhood. During the surveys, each selected dwelling and its surroundings was inspected to record all natural and/or artificial containers with water (potential larval habitats), and number containing at least one larvae or pupae (positive larval habitats). On basis of the nature of the container, source and use of the water, potential larval habitats were classified into three categories: domestic, peri-domestic, and natural. Domestic containers were defined as human-filled receptacles (e.g. storage tanks), while peri-domestic (e.g. discarded tanks, used tyres), and natural receptacles (e.g. rock and tree holes, leaf axils) were those filled by rain [14]. Larvae and pupae found per container were collected and transported to the insectary, isolated from predators such as Lutzia tigripes, and reared to adults. Emerged adults were morphologically identified alive using a suitable taxonomic key [25,26]. Adult mosquitoes identified as Ae. aegypti or Ae. albopictus from different districts were pooled and reared until obtaining the 2–5 day-old adults of G1 generation used to perform adult bioassays to insecticide. The number of immature stages of each species was estimated from the proportion of emerging adults of each species. During the field investigation all the discarded tanks identified were destroyed and water storage containers were treated with larvicide (Bacillus thuringiensis israelensis) (Fig 3). Local entomologists from each health district in Sao Tome and Principe were trained on the identification of Aedes larval habitats and their destruction or treatment. Advice was also provided to the population on how to avoid and eliminate Aedes larval habitats in their environment.
Fig 3. Pictures indicating some examples of action taken on the field during the dengue outbreak to control Aedes larvae.
A, Destroying plastic saucer of flower pot by perforation with holes; B, Destroying of tree hole (natural larval habitat) by creating drainage channel; C, Destroying of discarded tanks by perforation; D, Destroying used tyres by cutting; E, Water storage container (cement tanks) treated with Bacillus thuringiensis israelensis (Bti).
Entomological indices
The level of infestation was estimated using traditional Stegomyia indices including Breteau index (BI, the number of positive containers per 100 prospected houses), house index (HI, the percentage of houses infested), and container index (CI, percentage of positive containers). Estimated reference thresholds of HI, BI, and CI established by WHO for dengue and yellow fever transmission risk were applied: whenever HI > 35%, BI > 50, and CI > 20%, the location is considered as high risk of urban transmission of yellow fever virus, whereas HI < 4%, BI < 5 and CI < 3%, is considered to indicate a low risk of disease transmission [27]. Similarly, the categories of low HI < 0.1%, medium HI 0.1%-5% and high HI > 5% were established for classifying risk of dengue transmission [28].
Insecticide resistance evaluation in Ae. aegypti
For this study the Ae. aegypti Benin strain was used as the reference full-susceptible laboratory strain. Bioassays were performed according to WHO protocol using 2–5 days old G1 generation from the field. Four replicates of 20–25 females per tube were exposed to 0.03% deltamethrin, 0.40% permethrin, 0.25% pirimiphos-methyl, 0.05% alphacypermethrin, 0.1% bendiocarb, 1% fenitrothion and 4% dichlorodiphenyltrichloroethane (DDT) for 1 hour. Mortality was recorded 24 hours later and mosquitoes alive or dead after exposure were stored in RNA later or silica gel, respectively. The resistance status was defined as follows: susceptible (mortality rate between 98–100%), probable resistance (mortality rate between 90–97%), and resistant (mortality rate < 90%) [29].
Adult synergist assay with PBO and DEM
To evaluate the potential role of oxidases and glutathione S-transferases (GSTs) in metabolic resistance mechanisms, synergist assays with 4% piperonyl butoxide (PBO) and 8% diethyl maleate (DEM) were performed. Two-to- five-days-old G1 adults from the field were pre-exposed for one hour to PBO or DEM impregnated papers and after that immediately exposed to the selected insecticide. Mortality was scored 24 hrs later and compared to the results obtained with each insecticide without synergist, according to WHO standards [29]. The comparison of mortality rates after pre-exposure of mosquitoes to synergist and without pre-exposure to synergist was performed using Chi-square test.
Knockdown resistance (kdr) genotyping in Ae. aegypti
Thirty specimens of Ae. aegypti from G0 selected randomly were genotyped for three different kdr mutations: V1016I, V410L and F1534C, chosen because these mutations have been described as involved in pyrethroid resistance of Ae. aegypti mosquito [30,31]. These mutations have also been previously reported in Central Africa [20]. Based on Moyes et al., review, F1534C and V410L are associated with insecticide resistance and V1016I is associated with insecticide resistance when combined to other kdr mutations [31]. Genomic DNA was extracted using the Livak protocol [32], and genotyping of the V1016I, V410L and F1534C mutations was performed by real-time melting curve quantitative PCR [33]. Each PCR reaction was performed in a 21.5 μL volume PCR tube containing 2 μL of DNA sample, 10 μL of SYBR Green (SuperMix), and 1.25 μL of each primer. The amplification conditions were set as follow: 95°C for 3 min, followed by 40 cycles of (95°C for 20 s, 60°C for 1 min and 72°C for 30 s) and then final steps of 72°C for 5 min, 95°C for 1 min, 55°C for 30 s and 95°C for 30 s.
Data analysis
Categorical variables were summarised by percentages and confidence interval, and numeric variables by means and standard deviations; and compared using the Chi squared and Kruskal Wallis tests, respectively. All statistical analyses were performed using R v4.2.1 and RStudio v2023.03.0 (R Core Team, 2018), and a p-value < 0.05 was considered statistically significant.
Results
Pre-imaginal infestation
In total we investigated 173 and 241 houses in 22 neighbourhood clusters across six health districts of Sao Tome, during the dry and rainy seasons respectively. In addition, 42 houses in four neighbourhoods were surveyed in Principe (RAP) during the dry season only.
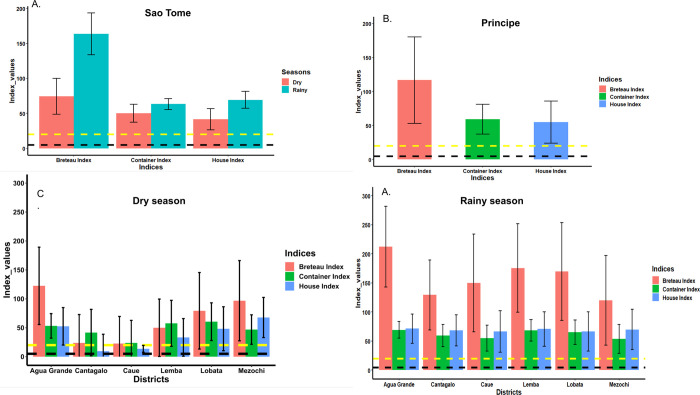
In Sao Tome, out of 253 potential larval habitats for Aedes inspected during the dry season 123 (50.2%) were found positive for Ae. aegypti and/or Ae. albopictus, while 385 of 624 potential containers (61.7%) were positive in the rainy season (Table 1). Aedes aegypti and Ae. albopictus spp. larvae were sometimes found together with Eretmapodites chrysogaster, Lutzia tigripes and Culex sp. larvae in the same larval habitats. The Stegomyia indices in the dry season were significantly lower than those calculated in the rainy season: house index (HI 41.5 vs 69.3, p<0.0001), Breteau index (BI 74.3 vs 163.5, p<0.0001), and container index (CI 50.2 vs 63.2, p = 0.0005) respectively (Fig 4A and S1 Table). However, in both seasons, all the Stegomyia indices calculated were high and above the thresholds established by WHO to indicate potential high risk for dengue transmission (Fig 4A). When analyses were performed according to location, the house index varied significantly between districts during the dry season (χ2 = 28.117, df = 5, p < 0.00001) with highest index (HI 67.7) in Mezochi and lowest index (HI 9.5) in Cantagalo (Fig 4C and S2 Table). A similar pattern was observed for the Breteau Index with the highest index (BI 122.5) in Agua Grande and lowest (BI 22.7) in Caue (χ2 = 26.064, df = 5, p <0.00001). However, no significant difference was found for the container index. When similar analyses were performed for data collected during the rainy season, there was no significant variation in the three indexes between the districts (Fig 4D and S3 Table).
Table 1. Container type infested with larvae and or pupae of Aedes spp. in rainy and dry seasons in Sao Tome, 2022.
| Dry season (June 2022) | Rainy season (November 2022) | |||||||
|---|---|---|---|---|---|---|---|---|
| Container | N inspected | N positive | % positive | Relative contribution: % of positive containers | N inspected | N positive | % positive | Relative contribution: % of positive containers |
| Domestic | 74 | 35 | 47.3 | 27.6 | 164 | 79 | 48.2 | 20.5 |
| Water storage | 48 | 22 | 45.8 | 17.3 | 130 | 61 | 46.9 | 15.8 |
| Flower pot | 13 | 9 | 69.2 | 7.1 | 23 | 11 | 47.8 | 2.9 |
| Animal drinking bowl | 13 | 4 | 30.8 | 3.1 | 11 | 7 | 63.6 | 1.8 |
| Peridomestic | 175 | 91 | 52.0 | 71.6 | 430 | 283 | 65.8 | 73.5 |
| Used tyres | 67 | 35 | 52.2 | 27.6 | 163 | 111 | 68.1 | 28.8 |
| Discarded tanks | 70 | 34 | 48.6 | 26.8 | 150 | 96 | 64 | 24.9 |
| Plastic covers | 0 | 0 | - | 0 | 29 | 20 | 69 | 5.2 |
| Tin cans | 14 | 5 | 35.7 | 3.9 | 23 | 12 | 52.2 | 3.1 |
| Miscellaneous | 24 | 17 | 70.8 | 13.4 | 65 | 44 | 67.7 | 11.4 |
| Natural | 4 | 1 | 25 | 0.8 | 30 | 23 | 76.7 | 6.0 |
| Bamboo | 0 | 0 | - | 0 | 11 | 11 | 100 | 2.8 |
| Coconut shells | 0 | 0 | - | 0 | 7 | 4 | 57.1 | 1.0 |
| Snail shells | 0 | 0 | - | 0 | 7 | 3 | 42.9 | 0.9 |
| Tree holes | 1 | 1 | 100 | 0.8 | 2 | 2 | 100 | 0.5 |
| Rock holes | 0 | 0 | - | 0 | 3 | 3 | 100 | 0.8 |
| Axil of plant | 3 | 0 | 0 | 0 | 0 | 0 | - | 0 |
| Overall | 253 | 127 | 50.2 | 100 (n = 127) | 624 | 385 | 61.7 | 100 (n = 385) |
N inspected, Number of containers with water inspected; N positive, Number of containers found with immature stages of Aedes spp.; % positive, Percentage of each type of container found with immature stages of Aedes spp.; Relative contribution: Percentage of all positive containers found with immature stages of Aedes spp.
Fig 4. Stegomyia indices calculated in Sao Tome and Principe.
A, Indices calculated in Sao Tome according to season. B, Indices calculated in Principe during the dry season. C, Indices calculated during the dry season in Sao Tome per health district; D, Indices calculated during the rainy season in Sao tome per health district.
In Principe, all three indexes calculated during the dry season (HI = 54.8, BI = 116.7 and CI = 59.0) were high and superior to the threshold established by WHO for potential high transmission risk of dengue (Fig 4B).
Typology and prevalence of larval habitat
During the entomological investigations in Sao Tome and Principe, all three categories of Aedes larval habitats were found: human-filled domestic (water storage container, flower pot and animal drinking bowl, rain-filled peri-domestic (used tyres, tin cans, car wrecks and miscellaneous) and natural (tree holes, rock holes, snail shells, coconut shells, leaf axil of plants). The number of each type of potential container and proportion of containers infested with immature stages of Aedes for each island is presented in Table 1 (Sao Tome) and Table 2 (Principe).
Table 2. Container type infested with larvae and/or pupae of Aedes spp. in dry season (June 2022) in Principe.
| Container | N inspected |
N positive |
% positive |
Relative contribution: % of positive containers |
|---|---|---|---|---|
| Domestic | 65 | 37 | 56.9 | 67.3 |
| Water storage | 61 | 34 | 55.7 | 61.8 |
| Flower pot | 2 | 1 | 50.0 | 1.8 |
| Animal drinking bowl | 2 | 2 | 100 | 3.7 |
| Peridomestic | 25 | 18 | 72.0 | 32.7 |
| Used tyres | 12 | 9 | 75.0 | 16.4 |
| Discarded tanks | 10 | 8 | 80.0 | 14.5 |
| Miscellaneous | 3 | 1 | 33.3 | 1.8 |
| Natural | 0 | - | - | 0.0 |
| Overall | 90 | 55 | 61.1 | 100 (n = 55) |
N inspected, Number of containers with water inspected; N positive, Number of containers found with immature stages of Aedes spp.; % positive, Percentage of each type of containers found with immature stages of Aedes spp.; Relative contribution: Percentage of all positive containers found with immature stages of Aedes spp.
In Sao Tome the most numerous potential larval habitats for Aedes spp. inspected were used tyres followed by discarded tanks and water storage containers, independent of the season. These containers were also found to be the most infested with larvae and /or pupae of Aedes spp. (Table 1). High infestation rates were observed in all domestic and peridomestic containers inspected on Sao Tome, however the greater number of discarded containers in the peridomestic environment results in these contributing most to Aedes vector breeding in the locations surveyed, in both seasons. An increased contribution of natural larval habitats was also noted during the rainy season, with 5.98% of natural containers found to be infested with Aedes spp. in the wet season compared to just 0.79% in the dry season. In Principe where inspections were only carried out during the dry season, domestic water storage containers were the most numerous potential larval habitats, and also the main receptacles found infested (61.82%) with immature stages of Ae. aegypti and/or Ae. albopictus. This was followed by used tyres and discarded tanks (Table 2).
Distribution of Aedes aegypti and Ae. albopictus in Sao Tome and Principe
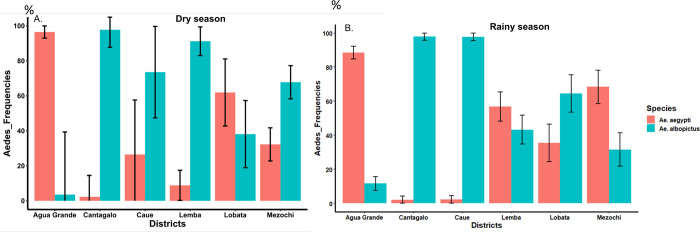
In Sao Tome, 1,224 and 2,995 specimens of Aedes spp. were morphologically identified during the dry and rainy seasons respectively. These specimens were comprised of 55.47% Ae. aegypti versus 44.53% Ae. albopictus (χ2 = 2.16, df = 1, p > 0.05) in the dry season, and 53.46% Ae. aegypti versus 46.54% Ae. albopictus (χ2 = 0.89, df = 1, p > 0.05) in the rainy season. Thus overall, Ae. aegypti was the most prevalent Aedes species in both seasons in Sao Tome. However, when analysed according to the district, Ae. albopictus was found most prevalent in Caue and Cantagalo irrespective to the season, while Ae. aegypti was the predominant species in Agua Grande (Fig 5). Nevertheless, in Lemba, Lobata and Mezochi the relative prevalence of the two Aedes species did vary according to the season, with a significantly higher prevalence of Ae. albopictus seen in Lemba and Mezochi during dry season (Fig 5).
Fig 5. Distribution and prevalence of Ae. aegypti and Ae. albopictus per health district.
A, dry season; B, rainy season.
In Principe, where investigations were only carried out during the dry season, a total of 126 individuals of Aedes spp. were identified comprising 48% Ae. aegypti and 52% Ae. albopictus.
Insecticide resistance profile in Ae. aegypti
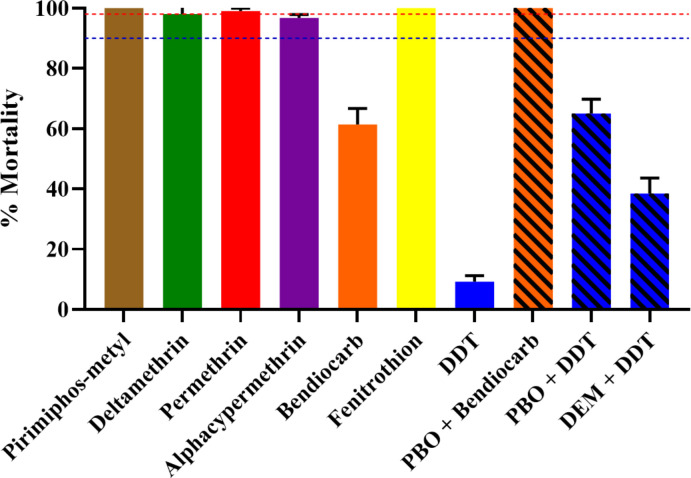
Unfortunately, not enough Ae. albopictus larvae survived rearing to G1 adults for bioassays. In total 1000 Ae. aegypti adults from Sao Tome were tested with seven insecticides. The results revealed that this sample was resistant to DDT (9.2% mortality) and bendiocab (61.4% mortality), and probable resistance to 0.005% alphacypermethrin (97.1% mortality), but was susceptible to 0.25% pirimiphos-methyl, 0.03% deltamethrin, 0.40% permethrin, and fenitrothion (with 100%, 98%, 99% and 100% mortality respectively) (Fig 6).
Fig 6. Mortality rates of adult Aedes aegypti from Sao Tome when exposed to insecticides alone or with 1 h preexposure to synergist.
Error bars represent standard error of the mean. PBO Piperonyl butoxide. DEM, diethyl maleate.
Synergist assay
Results from synergist assays showed a full recovery of susceptibility to bendiocarb after PBO pre-exposure (61.4±5.3 mortality without PBO pre-exposure vs 100.0 ± 0.0% mortality after PBO pre-exposure, p<0.0001). However for DDT, only a strong but not full recovery to DDT susceptibility was observed after pre-exposure to PBO and DEM (9.2 ±2.0 mortality without PBO pre-exposure vs 65.0 ±4.7 mortality after PBO pre-exposure, p<0.0001; Fig 4).
F1534C, V1016I and V410L kdr genotyping
Among the 28 specimens of Ae. aegypti G0 from Sao Tome that were genotyped, no individual was detected with V1016 or V410 resistance mutations, while one heterozygote was found with F1534C resistance mutation.
Discussion
This was the first study in Sao Tome and Principe to assess the distribution of Ae. aegypti and Ae. albopictus, the typology of larval habitats, and the susceptibility profile of Ae. aegypti to insecticides. The investigations were performed during the first dengue outbreak reported in the country in 2022 [24]. Epidemiological data collected during the outbreak revealed the presence of positive cases of dengue across all the seven health districts of Sao Tome and Principe, however, more than 68% of cases were resident in the health district of Agua Grande [24] which is the most urbanized and most populated area in Sao Tome and Principe.
Entomological risk and distribution of Ae. aegypti and Ae. albopictus
The Stegomyia indices calculated in Sao Tome were considerably higher than the thresholds established by WHO for potential dengue and yellow fever transmission [27], in both seasons. Nonetheless, the values of the Breteau index (BI) obtained during the rainy season were two-times higher compared to the dry season, indicating that the potential risk for dengue transmission is more pronounced during the rainy season. In general BI was highest in Agua Grande compared to other health districts indicative of the high transmission in this location, which has a higher building density. This observation is supported by the epidemiological data across the country [Água Grande (818 cases), Mézôchi (181), Lobata (97), Lemba (20), Caué (23), Cantagalo (47) and Principe (14)] [24]. The entomological data collected during this dengue outbreak show that both Ae. aegypti and Ae. albopictus are found throughout the country, though geographical and seasonal differences in their distribution were noted. Ae. albopictus was found to be the most abundant in four health districts, whereas Ae. aegypti was the predominant species in Agua Grande. This observation is in accordance with prior data from other locations where both species are sympatric[13–15]. Indeed, studies conducted along an urbanization gradient have demonstrated that Ae. aegypti prefers urban locations with high building density while Ae. albopictus is more abundant in rural or peri-urban areas with high vegetation index [34]. This matches with the observations made in different health districts in Sao Tome and Principe. Furthermore, banana trees were found in almost all habitations in the country and could serve as natural larval habitat for Ae. albopictus. This could explain why, even if Ae. albopictus was introduced recently as suggested by Reis et al [11], this invasive species has become the most predominant species in 4/7 health districts in Sao Tome and Principe. Previous studies in Central Africa have shown that Ae. albopictus tends to replace resident species Ae. aegypti, in several locations where both are found sympatric [35–37].
Typology of larval habitats
The entomological investigation revealed that used tyres, discarded tanks and domestic water storage containers were both the most abundant and the most infested larval habitats for Aedes spp. in Sao Tome, irrespective of the season; whereas water storage containers were found to be the principal breeding site in Principe. This difference between the two islands can be explained by deficiencies in the water supply system in Principe, which was more pronounced than in Sao Tome, and led residents to store water in containers for a long period. In fact, some household owners reported they often store water for up to a month in drums, bucket and jerrycans. Socio-anthropological studies could help to better elucidate this phenomenon. The high presence of used tyres and discarded tanks positive for immature stages of Ae. aegypti and Ae. albopictus is in accordance with previous data from other Central African cities such as Bangui in the Central African Republic [14], Brazzaville in the Republic of the Congo [38], Yaoundé and Douala in Cameroon [13,15,21,36]. On the other hand, the situation in Principe is closer to what is generally found in Southeast Asia where domestic water storage containers are the most prevalent and productive larval habitats for Ae. aegypti [39]. In general, these observations suggest that a good waste management system, recycling of used tyres, and communication to support behaviour change could help to reduce breeding sites and the density of Aedes in the country. Indeed, during our field surveys advice was given to the population on how to avoid or eliminate Aedes larval habitats, all discarded containers that were found were destroyed through perforation, saucers of flower pots were perforated, and large water storage containers (cement tanks) were treated using Bacillus thuringiensis israelensis (Bti) (Fig 3). This larvicide was chosen because it’s highly specific to Diptera, might be considered a biological control agent and its prior reported effectiveness to control dengue vectors [40–42]. Furthermore, Aedes in central Africa have consistently been found susceptible to Bti [18,22,43]. Bti was also readily available on the islands as it was already being used for Anopheles larval control by the malaria elimination programme.
Insecticide susceptibility profile in Ae. aegypti
This first study in Sao Tome revealed that Ae. aegypti is resistant to bendiocab and DDT, but remains susceptible to permethrin, alphacypermethrin, deltamethrin, pirimiphos-methyl and fenitrothion. These findings suggest that all the insecticides tested, except for the two resistant compounds, could be recommended to control Ae. aegypti in this country. A decreasing susceptibility of Ae. aegypti population from Central Africa towards DDT, notably in Brazzaville and Yaoundé, was already described in 1970s [44], which may reflect a continuing selection pressure on Aedes populations as suggested previously [43]. Indeed, during the last decade DDT resistance has repeatedly been reported in Ae. aegypti in multiple locations [15,20,21,43,45,46]. A loss of susceptibility was also observed to bendiocarb with moderate level of resistance. Similar results were recently found in Cameroon [23] and Burkina Faso [47,48] in Africa, and several countries outside Africa such as Malaysia [46], Colombia [49], and Mexico [50]. The source of selection driving the observed resistance to DDT and bendiocarb in Ae. aegypti populations remains unclear, as the programmatic use of insecticides against Aedes is limited in the African region [19]. As suggested previously [15,43], domestic use of insecticides through indoor spraying and impregnated bed nets, and agricultural use could be the main sources of resistance selection in Aedes vectors in Central Africa, whilst the use of pesticides in agriculture for the protection of crops could promote the emergence of resistance in mosquitoes through contamination of breeding sites and resting places of mosquitoes [15]. During the current outbreak the insecticide used for fumigation was pirimiphos-methyl, an organophosphate, but staff from the National Malaria Elimination Program reported that bendiocarb has been used for indoor residual spraying in Sao Tome in the past. Even though loss of susceptibility in Aedes vectors has been reported in other locations in central Africa [19–21], Ae. aegypti collected from Sao Tome showed a good level of susceptibility to both type I and II pyrethroids tested.
A full recovery of susceptibility to bendiocarb was observed in Ae. aegypti from Sao Tome after pre-exposure to PBO synergist suggesting that the cytochrome P450 monooxygenases are playing the main role in the observed resistance. On the other hand, only partial recovery of susceptibility to DDT was seen, both after pre-exposure to PBO or DEM synergists. This observation suggests a possible implication of both cytochrome P450 monooxygenases and glutathione S-transferases in DDT-resistant Ae. aegypti as suspected previously [51]. Among the three kdr mutations 1534, 410 and 1016 genotyped only one specimen was found to possesses the 1534C allele, confirming the susceptibility to pyrethroids in Ae. aegypti in Sao Tome although these results should be treated with caution since the sample size was small and thus sensitivity for detecting resistance mutations is low. These mutations are known to be widely distributed in Ae. aegypti [31] and have previously been detected in other samples from Central Africa [20,21]. A further limitation of this study was our inability to assess insecticide susceptibility in Ae. albopictus, due to the low number of specimens successfully reared to G1 adults from larvae collected. Although these data were useful for informing vector control in urban areas, such as Agua Grande where most cases were reported and Ae. aegypti was the predominant vector, it could be useful to extend this study to obtain a complete picture, since Ae. albopictus was found to be most prevalent species in four of the other six health districts in Sao Tome and Principe, as well as to continue to monitor insecticide resistance in both species across the country.
Conclusions
This study has provided for the first time the typology of Aedes larval habitats and the distribution of Ae. aegypti and Ae. albopictus across Sao Tome and Principe. The results revealed that these species bred mainly in used tyres, discarded tanks and water storage containers. A pattern of susceptibility to insecticides in Ae. aegypti was established enabling this country to quickly implement insecticide-based control interventions in case of future outbreaks. Indeed, organophosphates notably pirimiphos-methyl was used to control Aedes adults in Sao Tome and Principe during this dengue outbreak. Findings generated in this study helped to give advice to the population on practical actions to limit the proliferation of Aedes and the Ministry of Health to implement an efficient strategy to control dengue vectors in Sao Tome and Principe. Continued future engagement with political leaders and local communities will also be key to improve systems of water supply and waste management, reducing the number of potential breeding sites in the domestic and peri-domestic environment to reduce the risk of future outbreaks. As there is currently a lack of control programs against arbovirus in many countries in Africa, we recommend using an integrated vector control strategic approach to build upon the successes of well-established vector control program against malaria, to reduce the risk of mosquito-borne disease transmission in general, and to achieve more efficiencies in the fight against mosquito-borne diseases.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(XLSX)
Acknowledgments
We would like to thank the populations living in the different survey sites for their cooperation during mosquito sampling.
Data Availability
All the relevant data generated during this study are included in the manuscript and its supporting information.
Funding Statement
This study was supported by the World Health Organisation to BK and by UK International Development from the UK Government [Health Research Programme Consortia (RPC): RAFT (Resilience Against Future Threats through Vector Control), PO8615] to SEC. The funders had no role in study design, data collection and analysis, preparation of the manuscript, or decision to publish.
References
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. : The global distribution and burden of dengue. Nature 2013, 496(7446):504–507. doi: 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amarasinghe A, Kuritsk JN, Letson GW, Margolis HS: Dengue virus infection in Africa. Emerg Infect Dis 2011, 17(8):1349–1354. doi: 10.3201/eid1708.101515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leroy EM, Nkoghe D, Ollomo B, Nze-Nkogue C, Becquart P, Grard G, et al. : Concurrent chikungunya and dengue virus infections during simultaneous outbreaks, Gabon, 2007. Emerg Infect Dis 2009, 15(4):591. doi: 10.3201/eid1504.080664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarnagda Z, Cissé A, Bicaba BW, Diagbouga S, Sagna T, Ilboudo AK, et al. : Dengue fever in Burkina Faso, 2016. Emerg Infect Dis 2018, 24(1):170. doi: 10.3201/eid2401.170973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz E, Meltzer E, Mendelson M, Tooke A, Steiner F, Gautret P, et al. : Detection on four continents of dengue fever cases related to an ongoing outbreak in Luanda, Angola, March to May 2013. Euro Surveill 2013, 18(21):20488. [PubMed] [Google Scholar]
- 6.Simo Tchetgna H, Sado Yousseu F, Kamgang B, Tedjou A, McCall PJ, Wondji CS: Concurrent circulation of dengue serotype 1, 2 and 3 among acute febrile patients in Cameroon. PLoS Negl Trop Dis 2021, 15(10):e0009860. doi: 10.1371/journal.pntd.0009860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO: Pesticides and their application: for the control of vectors and pests of public health importance. 2006. [Google Scholar]
- 8.Christophers S: Aedes aegypti (L.) the yellow fever mosquito: its life history, bionomics and structure. Cambridge University Press, New York, 1960. xii+ 739 pp. [Google Scholar]
- 9.Smith C: The history of dengue in tropical Asia and its probable relationship to the mosquito Aedes aegypti. J Trop Med Hyg 1956, 59(10):243–251. [PubMed] [Google Scholar]
- 10.Fontenille D, Toto JC: Aedes (Stegomyia) albopictus (Skuse), a potential new Dengue vector in southern Cameroon. Emerg Infect Dis 2001, 7(6):1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reis S, Cornel AJ, Melo M, Pereira H, Loiseau C: First record of Aedes albopictus (Skuse 1894) on São tomé island. Acta Trop 2017, 171:86–89. [DOI] [PubMed] [Google Scholar]
- 12.Ngoagouni C, Kamgang B, Nakouné E, Paupy C, Kazanji M: Invasion of Aedes albopictus (Diptera: Culicidae) into central Africa: what consequences for emerging diseases? Parasit Vectors 2015, 8(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamgang B, Happi JY, Boisier P, Njiokou F, Hervé JP, Simard F, et al. : Geographic and ecological distribution of the dengue and chikungunya virus vectors Aedes aegypti and Aedes albopictus in three major Cameroonian towns. Med Vet Entomol 2010, 24(2):132–141. [DOI] [PubMed] [Google Scholar]
- 14.Kamgang B, Ngoagouni C, Manirakiza A, Nakouné E, Paupy C, Kazanji M: Temporal patterns of abundance of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) and mitochondrial DNA analysis of Ae. albopictus in the Central African Republic. PLoS Negl Trop Dis 2013, 7(12):e2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamgang B, Yougang AP, Tchoupo M, Riveron JM, Wondji C: Temporal distribution and insecticide resistance profile of two major arbovirus vectors Aedes aegypti and Aedes albopictus in Yaounde, the capital city of Cameroon. Parasit Vectors 2017, 10(1):469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcombe S, Darriet F, Tolosa M, Agnew P, Duchon S, Etienne M, et al. : Pyrethroid resistance reduces the efficacy of space sprays for dengue control on the island of Martinique (Caribbean). PLoS Negl Top Dis 2011, 5(6):e1202. doi: 10.1371/journal.pntd.0001202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Amin HM, Gyawali N, Graham M, Alam MS, Lenhart A, Hugo LE, et al. : Insecticide resistance compromises the control of Aedes aegypti in Bangladesh. Pest Manag Sci 2023. [DOI] [PubMed] [Google Scholar]
- 18.Kamgang B, Marcombe S, Chandre F, Nchoutpouen E, Nwane P, Etang J, et al. : Insecticide susceptibility of Aedes aegypti and Aedes albopictus in Central Africa. Parasit Vectors 2011, 4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamgang B, Wilson-Bahun TA, Yougang AP, Lenga A, Wondji CS: Contrasting resistance patterns to type I and II pyrethroids in two major arbovirus vectors Aedes aegypti and Aedes albopictus in the Republic of the Congo, Central Africa. Infect Dis Poverty 2020, 9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montgomery M, Harwood JF, Yougang AP, Wilson-Bahun TA, Tedjou AN, Keumeni CR, et al. : Spatial distribution of insecticide resistant populations of Aedes aegypti and Ae. albopictus and first detection of V410L mutation in Ae. aegypti from Cameroon. Infect Dis Poverty 2022, 11(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yougang AP, Keumeni CR, Wilson-Bahun TA, Tedjou AN, Njiokou F, Wondji C, et al. : Spatial distribution and insecticide resistance profile of Aedes aegypti and Aedes albopictus in Douala, the most important city of Cameroon. Plos One 2022, 17(12):e0278779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yougang AP, Kamgang B, Tedjou AN, Wilson-Bahun TA, Njiokou F, Wondji CS: Nationwide profiling of insecticide resistance in Aedes albopictus (Diptera: Culicidae) in Cameroon. Plos One 2020, 15(6):e0234572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yougang AP, Kamgang B, Bahun TAW, Tedjou AN, Nguiffo-Nguete D, Njiokou F, et al. : First detection of F1534C knockdown resistance mutation in Aedes aegypti (Diptera: Culicidae) from Cameroon. Infect Dis Poverty 2020, 9(06):51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards FW: Mosquitoes of the Ethiopian Region. III.-Culicine adults and pupae. British Museum, 1941. 499 pp. [Google Scholar]
- 25.Jupp PG: Mosquitoes of Southern Africa: culicinae and toxorhynchitinae: Ekogilde Publishers; 1996. [Google Scholar]
- 26.WHO: Technical guide for a system of yellow fever surveillance. In.: World Health Organisation; 1971. [Google Scholar]
- 27.PAHO: Dengue and dengue hemorrhagic fever in the Americas: guidelines for prevention and control. In. Washington DC: Pan American Health Organisation; 1994. [Google Scholar]
- 28.WHO: Entomological surveillance for Aedes spp. in the context of Zika virus: interim guidance for entomologists. 2016. [Google Scholar]
- 29.Livak KJ: Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics 1984, 107(4):611–634. doi: 10.1093/genetics/107.4.611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris AF, Rajatileka S, Ranson H: Pyrethroid resistance in Aedes aegypti from Grand Cayman. Am J Trop Med Hyg 2010, 83(2):277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moyes CL, Vontas J, Martins AJ, Ng LC, Koou SY, Dusfour I, et al.: Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl Trop Dis 2017, 11(7):e0005625. doi: 10.1371/journal.pntd.0005625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saavedra-Rodriguez K, Urdaneta-Marquez L, Rajatileka S, Moulton M, Flores A, et al. : A mutation in the voltage-gated sodium channel gene associated with pyrethroid resistance in Latin American Aedes aegypti. Insect Mol Biol 2007, 16(6):785–798. [DOI] [PubMed] [Google Scholar]
- 33.MS/STP: Rapport de situation de la dengue. SitRep No 42, 27 mars 2023 2023. [Google Scholar]
- 34.Wilson-Bahun TA, Kamgang B, Lenga A, Wondji CS: Larval ecology and infestation indices of two major arbovirus vectors, Aedes aegypti and Aedes albopictus (Diptera: Culicidae), in Brazzaville, the capital city of the Republic of the Congo. Parasit Vectors 2020, 13(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tedjou AN, Kamgang B, Yougang AP, Wilson-Bahun TA, Njiokou F, Wondji CS: Patterns of ecological adaptation of Aedes aegypti and Aedes albopictus and Stegomyia indices highlight the potential risk of arbovirus transmission in Yaoundé, the Capital City of Cameroon. Pathogens 2020, 9(6):491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vannavong N, Seidu R, Stenström TA, Dada N, Overgaard HJ: Effects of socio-demographic characteristics and household water management on Aedes aegypti production in suburban and rural villages in Laos and Thailand. Parasit Vectors 2017, 10(1):170. doi: 10.1186/s13071-017-2107-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Araújo AP, Araujo Diniz DF, Helvecio E, De Barros RA, De Oliveira CMF, Ayres CFJ, et al. : The susceptibility of Aedes aegypti populations displaying temephos resistance to Bacillus thuringiensis israelensis: a basis for management. Parasit Vectors 2013, 6(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmad Zaki Z, Che Dom N, Ahmed Alhothily I: Efficacy of Bacillus thuringiensis treatment on Aedes population using different applications at high-rise buildings. Trop Med Infect Dis 2020, 5(2):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tissera H, Samaraweera P, Jayamanne B, Janaki M, U Chulasiri M, Rodrigo C, et al. : Use of Bacillus thuringiensis israelensis in integrated vector control of Aedes sp. in Sri Lanka: a prospective controlled effectiveness study. Trop Med Int Health 2018, 23(2):229–235. [DOI] [PubMed] [Google Scholar]
- 40.Ngoagouni C, Kamgang B, Brengues C, Yahouedo G, Paupy C, Nakoune E, et al. : Susceptibility profile and metabolic mechanisms involved in Aedes aegypti and Aedes albopictus resistant to DDT and deltamethrin in the Central African Republic. Parasit Vectors 2016, 9(1):599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cox J, Grillet ME, Ramos OM, Amador M, Barrera R: Habitat segregation of dengue vectors along an urban environmental gradient. Am J Trop Med Hyg 2007, 76(5):820–826. [PubMed] [Google Scholar]
- 42.Tedjou AN, Kamgang B, Yougang AP, Njiokou F, Wondji CS: Update on the geographical distribution and prevalence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae), two major arbovirus vectors in Cameroon. Plos Negl Trop Dis 2019, 13(3):e0007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamgang B, Wilson-Bahun TA, Irving H, Kusimo MO, Lenga A, Wondji CS: Geographical distribution of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) and genetic diversity of invading population of Ae. albopictus in the Republic of the Congo. Wellcome Open Res 2018, 3:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mouchet J, Cordellier R, Germain M, Carnevale P, Barathe J, Sannier C: Résistance aux insecticides d’Aedes aegypti et Culex pipiens fatigans en Afrique Centrale. WHO/VBC/72/381, 12P 1972. [Google Scholar]
- 45.Ayorinde A, Oboh B, Oduola A, Otubanjo O: The insecticide susceptibility status of Aedes aegypti (Diptera: Culicidae) in farm and nonfarm sites of Lagos State, Nigeria. J Insect Sci 2015, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishak IH, Jaal Z, Ranson H, Wondji CS: Contrasting patterns of insecticide resistance and knockdown resistance (kdr) in the dengue vectors Aedes aegypti and Aedes albopictus from Malaysia. Parasit Vectors 2015, 8:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toé HK, Zongo S, Guelbeogo MW, Kamgang B, Viana M, Tapsoba M, et al. : Multiple insecticide resistance and first evidence of V410L kdr mutation in Aedes (Stegomyia) aegypti (Linnaeus) from Burkina Faso. Medical and veterinary entomology 2022, 36(3):309–319. [DOI] [PubMed] [Google Scholar]
- 48.Sombié A, Saiki E, Yaméogo F, Sakurai T, Shirozu T, Fukumoto S, et al. : High frequencies of F1534C and V1016I kdr mutations and association with pyrethroid resistance in Aedes aegypti from Somgandé (Ouagadougou), Burkina Faso. Trop Med Health 2019, 47:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deming R, Manrique-Saide P, Medina Barreiro A, Cardeña EUK, Che-Mendoza A, Jones B, et al. : Spatial variation of insecticide resistance in the dengue vector Aedes aegypti presents unique vector control challenges. Parasit Vectors 2016, 9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Solis-Santoyo F, Rodriguez AD, Penilla-Navarro RP, Sanchez D, Castillo-Vera A, Lopez-Solis AD, et al. : Insecticide resistance in Aedes aegypti from Tapachula, Mexico: Spatial variation and response to historical insecticide use. PLoS Negl Trop Dis 2021, 15(9):e0009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ishak IH, Kamgang B, Ibrahim SS, Riveron JM, Irving H, Wondji CS: Pyrethroid Resistance in Malaysian populations of dengue vector Aedes aegypti is mediated by CYP9 family of Cytochrome P450 genes. PLoS Negl Trop Dis 2017, 11(1):e0005302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(XLSX)
Data Availability Statement
All the relevant data generated during this study are included in the manuscript and its supporting information.