SUMMARY:
Ex vivo lungs are useful for a variety of experiments to collect physiological data while excluding confounding variables of in vivo experiments. Commercial setups are often expensive and limited in what types of data they can collect. We describe a method for building a fully modular setup, adaptable to various study designs.
Ex vivo lung preparations are a useful model that can be translated to many different fields of research, complementing corresponding in-vivo and in-vitro models. Laboratories wishing to use isolated lungs need to be aware of important steps and inherent challenges to establish a setup that is affordable, reliable, and that can be easily adapted to fit the topic of interest. This paper describes a do-it-yourself model for ex vivo rat lung ventilation and perfusion to study drug- and gas-effects on pulmonary vascular tone, independent of changes in cardiac output. Creating this model includes a) the design and construction of the apparatus, and b) the lung isolation procedure. This model results in a setup that is more cost-effective than commercial alternatives and yet modular enough to adapt to changes in specific research questions. Various obstacles had to be resolved to ensure a consistent model that is capable of being used for a variety of different research topics. Once established, this model has proven to be highly adaptable to different questions and is easily altered for different fields of study.
INTRODUCTION:
Ex vivo lung perfusion (EVLP) techniques1 have seen a rise in usage in the past decade as a means of studying lung transplantations2, ischemia/reperfusion3, lung metabolism4, and immune responses5. Isolated, but intact, ventilated and perfused lungs offer the critically important ability to directly assess the response of the lungs, including the pulmonary vasculature, to potential interventions and/or therapeutics without potential confounders such as neuronal and hormonal input or changing hemodynamics in vivo, while maintaining the physiological interplay of ventilation and perfusion in contrast to in vitro conditions. A proposal looking at immune responses in lungs5, for example, will need the same quality of data as a study focused on increasing the donor pool size6 for lung transplantations. EVLP can be used across a variety of species, including mice3, rats7–12, pigs13, and humans2. Therefore, it is necessary to establish a model that can produce reliable data from a variety of different experimental parameters. Clinical relevance will be generated in subsequent studies using the EVLP model as a tool.
While commercial setups are available for purchase for most species, they can often be cost-prohibitive and confine researchers to a specific brand of equipment and proprietary software. Any deviation from the out-of-the-box setup (e.g., going from one species to another) requires foresight and working around the provided setup that may prove to be difficult or impossible. In the following, a do-it-yourself (DIY) setup for rat isolated lungs that is both modular and cost-effective, as well as the surgical procedure for isolating the lungs, are described.
PROTOCOL:
The in vivo portion of the experiments (from general anesthesia to euthanasia) will require prior approval by the respective Institutional Animal Care and Use Committee (IACUC). All procedures described herein were approved (protocol number M1700168) by the IACUC at Vanderbilt University Medical Center, Nashville, Tennessee, and were performed in compliance with the ARRIVE guidelines14. Prior to the experimentation, all rats were housed in the institute’s animal care facility, with free access to water and food. Including different studies outside the purview of this manuscript, we have used a total of 148 male Sprague Dawley rats (Charles River, Wilmington, MA), 7–10 weeks old, with a weight between 250–400 g so far.
1. Apparatus construction
NOTE: All parts, including respective manufacturers, are listed in the Table of Materials.
Table of Materials
| Name of Material/Equipment | Company | Catalog Number |
|---|---|---|
| 1,000 mL Glass Beaker | Pyrex, Chicago, IL | |
| 1,500 mL Glass Beaker | Pyrex, Chicago, IL | |
| Air Trap Compliance Chamber | Radnoti | 130149 |
| Bioamplifiers | CWE Inc | BPM-832 |
| Clamps | Fisher Scientific | S02626 |
| DAQ (Data Acquisition) | National Instruments, Austin, TX | NI USB-6343 |
| Gas Mixer | CWE Inc, Ardmore, PA | GSM-4 |
| Heating Coil | Radnoti, Covina, CA | 158822 |
| Heating Plate | Thermo Fisher Scientific, Waltham, MA | 11–100-49SH |
| Heparin | Pfizer | W63422 |
| LabVIEW Full Development System 2014 | National Instruments | |
| Pentobarbital | Diamondback Drugs | G2270–0235-50 |
| pH700 Probe | OAKTON, Vernon Hills, IL | EW-35419–10 |
| Polystat Water Bath | Cole-Parmer | EW-12121–02 |
| Rodent Ventilator | Harvard Apparatus, Holliston, MA | Model 683 |
| Roller Pump | Cole-Parmer, Wertheim, Germany | Ismatec REGLO Digital MS 2/8 |
| Sprague Dawley Rat | Charles River, Wilmington, MA | Strain code 001 |
| VetScan i-STAT | Abraxis, Chicago, IL | i-STAT 1 |
1.1. To hold each piece of equipment in place, construct a custom-built lattice to allow for easy configuration and incorporation of new devices (Figure 1). Attach aluminum poles (1 to 2 ft in length, 1 cm in diameter, available at any hardware store) to each other using cross clamps to create a 3D lattice placed on a plastic tray (30 in x 21 in) to prevent spillage of fluids.
Figure 1: A custom-built lattice to allow for easy configuration and incorporation of new devices.
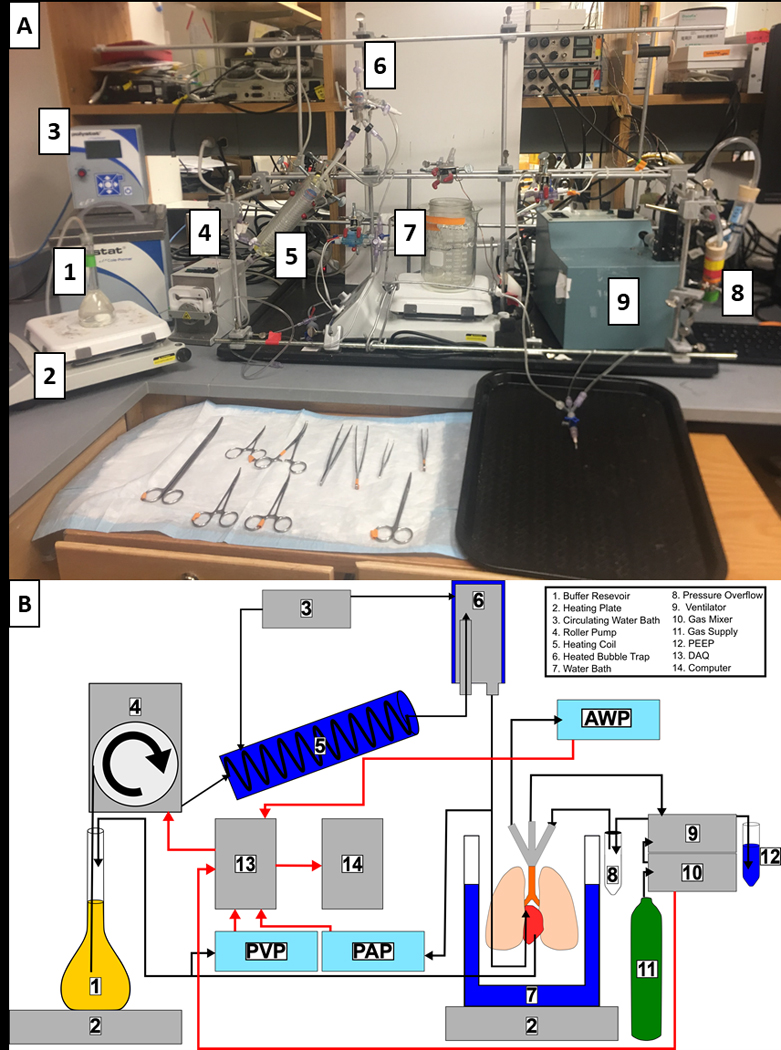
(A) Photograph of the custom-made lattice used to house all the equipment and devices. Not shown are the pressure overflow, gas mixer, gas supply, DAQ, or computer. (B) Schematic of the closed circuit used to perfuse the lung and each device involved in the setup: 1) The buffer is stored in a volumetric flask to reduce volume and surface area. 2) Heating plates are used to heat both the buffer and the lung chamber. A digital thermometer is used to monitor to temperature inside the lung chamber. 3) Circulating water bath heats the heating coil and air trap to maintain a constant temperature. 4) In the constant-flow setup described here, the roller pump is used to control lung buffer perfusion. 5) Buffer is passed through a heating coil to maintain its temperature. 6) The air trap ensures no air bubbles reach the lung and helps maintain the temperature. 7) A custom double boiler is used to create a humid environment for the lung and maintain its temperature. 8) A pressure overflow is placed between the ventilator and the lung to prevent over-ventilating the lung. 9) A volume-controlled ventilator is used for reliable tidal volumes. 10) The gas mixer can be used to create different gas compositions. 11) Various gas tanks such as O2, CO2, and N2 are needed for ventilating different compositions of gas. 12) A simple water column is connected to the ventilator to adjust the positive end expiratory pressure on the lung. 13) The DAQ system is responsible for collecting all the data and sending it to the computer. 14) Data are collected and visualized live on the computer. Abbreviations: DAQ = data acquisition system; PEEP = positive end expiratory pressure; AWP = Airway Pressure.
1.2. Mount pressure transducers at an equal height to the lungs and connect them to the lines for he pulmonary artery (PA), pulmonary vein (PV), and to the ventilation tube.
NOTE: These transducers transmit data to their respective bioamplifiers connected to the data acquisition system and its software.
1.3. Rather than using commercially available cannulas, craft custom cannulas (Figure 2) from segments of 2 mm wide, hard plastic tubing that are flared at the ending using an open flame to allow for secure tying-off of sutures. Bend them into a U-shape to reduce stress on the lungs during hanging and to fit in the lung chamber.
Figure 2: Custom-made cannulas.
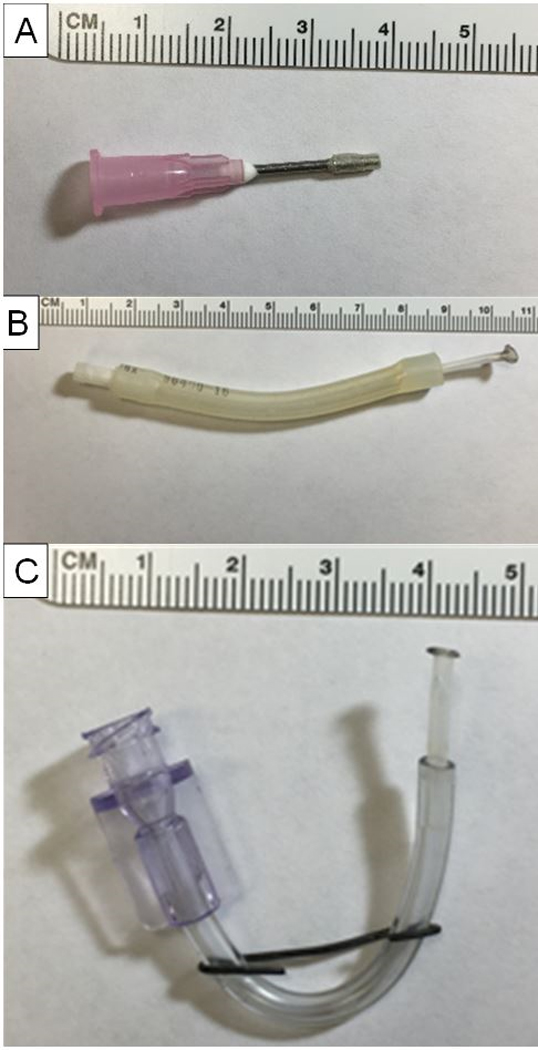
(A) Tracheal cannula (made from an 18 G needle). (B) Pulmonary artery cannula. (C) Pulmonary vein cannula.
1.4. To make the chamber the lungs are to be ventilated and perfused in, place a 1,000 mL beaker inside a 1,500 mL beaker with a water bath between the two and inside the 1,000 mL beaker creating a double boiler. Place these beakers on a heating plate set to 48 °C, creating a chamber for the lungs that is both humid and resistant to fluctuations in temperature.
1.5. Keep the buffer for the experiment in a 150 mL volumetric flask placed on a heating plate set to 37 °C. Use a magnetic stirring bar to circulate the buffer inside the beaker. Set the meniscus of the buffer at a height such that it is 4 cm above the lung to create an innate 4 cmH2O of pressure on the PV. During the surgery, ensure that the animal’s lungs are at the same height as the buffer to reduce hydrostatic edema formation.
NOTE: Using a flask minimizes the amount of surface area in contact with room air, which minimizes gas diffusion.
1.6. Use a roller pump to move the buffer through a circuit consisting of a heating coil and an air trap prior to perfusing the lung. Recycle the effluent from the PV back into the volumetric flask. Connect both the heating coil and air trap to a circulating water bath also set to 37 °C. Adjust the temperature of the water bath according to the pump speed, so the perfusate has a constant temperature of 37 °C.
2. Procedure
2.1. Prior to the start of the experiment, prepare the setup.
2.1.1. Ensure that the software is running (see below) and properly collecting data.
2.1.2. Calibrate all pressure transducers daily to ensure they are not drifting.
2.1.3. Prepare the buffer (see Table 1 for ingredients for a physiological saline solution with 4% bovine serum albumin [BSA]) and ensure that the pH is at 7.4 using HCl to adjust accordingly. Set up the perfusion system with warmed buffer (Table 1) circulating throughout, ensuring that no air bubbles are present. Add BSA at least 30 min prior to the start of the experiment to give it sufficient time to dissolve. In the absence of an oxygenator, bubble gas into the perfusion buffer prior to the addition of BSA with a gas composition of 65% N2, 30% O2, and 5% CO2 to mimic the CO2 levels of in vivo lungs; this will prevent the solution from foaming once BSA is added.
Table 1:
Krebs buffer composition.
| NaCl | 119.0 mM |
| NaHCO3 | 24.0 mM |
| Glucose | 5.5 mM |
| CaCl2 | 1.6 mM |
| KCl | 4.7 mM |
| MgSO4 | 1.17 mM |
| NaH2PO4 | 1.18 mM |
| Bovine serum albumin (98%) | 4% solution (4.0816 g / 100 mL) |
2.1.4. Prepare the operating area with all necessary surgical tools, sutures, and tape. Incline the operating board at a 15° angle so that the anesthetized rat can be positioned with its head elevated above the rest of the body and the trachea and heart-lung block can be manipulated easily.
2.1.5. Wearing proper personal protective equipment, weigh the rat and give an intraperitoneal injection of pentobarbital (65 mg kg−1). After 10 min, use a toe pinch to ensure that the surgical plane of general anesthesia has been reached; administer more anesthetic if necessary.
2.2. Transfer the rat to the operating area and fixate it to the operating board by taping its front legs separately followed by the hind legs together, making sure that the front legs are taped loose enough to allow a pain reflex to be visualized should anesthesia not be deep enough (Figure 3A).
Figure 3: Isolation process of the rat lungs.
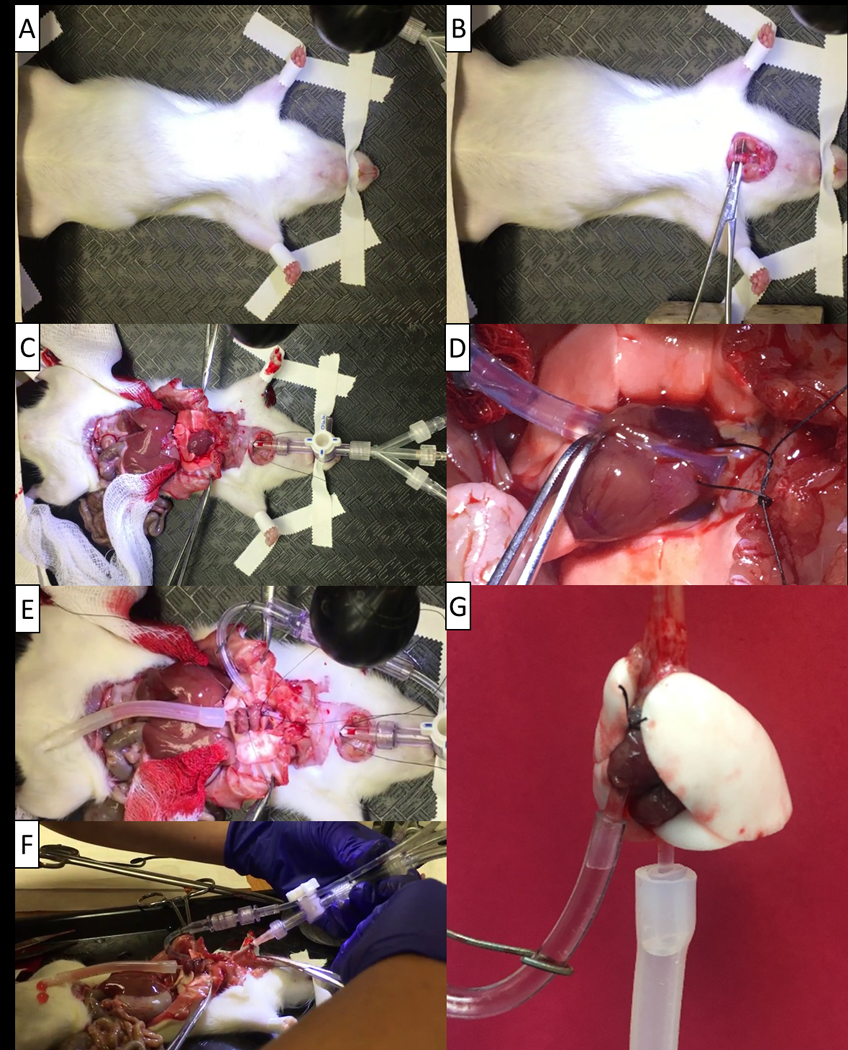
(A) The rat is securely taped down after anesthesia with the mouth taped as well to ensure minimal movement. (B) Proper isolation and placement of the forceps for performing the tracheostomy. (C) Spreading the rib cage to open the surgical area and minimize the risk of a broken rib puncturing the lungs. (D) Placement of the pulmonary artery cannula. (E) Placement of the pulmonary vein cannula following it being secured to the heart. (F) Removal of the trachea and heart-lung block from the rat. (G) The rat isolated lungs hanging prior to placement in the chamber.
2.3. Secure the head of the rat by taping the mouth with a long, thin strip behind the front teeth without restricting the tongue or airflow for the still spontaneously breathing rat (Figure 3B).
2.4. Perform a tracheostomy by pinching the skin above the trachea using forceps and cutting with surgical scissors. Using curved forceps, bluntly dissect the muscle and tissue to reach the trachea. Ensure that there is no bleeding during this step.
2.4.1. Pass curved forceps underneath the trachea and open them to allow room to pass 3–0 sutures underneath that can then be pretied into a box knot (Figure 3B). Make a small incision between the cartilage rings of the trachea and insert the tracheal cannula (modified 18 G needle with 1 mm diameter tubing glued around the tip to create a notch). Tie off the suture, ensuring that no air can escape from the tracheal incision and that the trachea does not have any strain put on it by the cannula.
2.5. Set the ventilator to run with a gas mixture of 30% O2, 5% CO2, and 65% N2 with a tidal volume (VT) of 10 mL kg−1, a positive end expiratory pressure (PEEP) of 0 cmH2O, and a rate of 60 breaths min-1.
2.6. Once the tracheal cannula is secured with the suture, begin ventilating the rat with the above settings.
NOTE: The surgical method used was adapted from that by Nelson et al.9 and is described step-by-step.
2.7. Remove the fur from the abdomen of the rat using large surgical scissors and forceps.
NOTE: Hair removal ointments can also be used prior to the start of the experiment.
2.8. While holding the xiphoid process with forceps, make a small horizontal incision below the ribs, ensuring that the diaphragm stays intact. Once the internal organs can be safely visualized, widen the horizontal cut to expose the entire diaphragm.
2.9. Taking extreme care to avoid puncturing the lungs, inject heparin (3,000 U kg−1) into the inferior vena cava (IVC) with a 22 G syringe.
2.10. Again, hold the xiphoid process with a forceps and cut cranially along the sternum while constantly visualizing the lungs to prevent cutting them.
2.11. To properly see the heart-lung block, spread the rib cage apart using two large forceps and be sure to avoid breaking a rib, which can result in a sharp bone fragment puncturing the lung (Figure 3C).
NOTE: Once the rib cage has been opened, use a PEEP of 2–3 cmH2O, set by a water lock attached to the expiratory limb of the ventilator, to avoid atelectasis.
2.12. At this time, cut the IVC to euthanize the rat by exsanguination. Pay attention that a least one minute has passed since the injection of heparin (2.9.) to give it enough time to circulate and prevent microclots in the lungs.
2.13. When placing the perfusion cannulas, constantly check the pressures to make sure that no sudden changes occur.
NOTE: A sudden spike in pressure while the pump is running at a constant speed indicates blockage formation, while a sudden decrease can indicate a leakage.
2.14. Trim any excess thymus to allow for easier visualization of the pulmonary vasculature. Locate the PA, pass a small, curved forceps underneath, and again pass a 3–0 suture underneath and pretie into a box knot.
2.15. Make a small incision into the right ventricle of the heart and insert the PA cannula (made from 2 mm diameter plastic tubing flared at the end using an open flame to allow for sutures to be tied around), being gentle to avoid rupturing the adjacent artery. Secure the cannula using the suture and perfuse starting at 1.5 mL min−1 (Figure 3D).
2.16. Immediately excise the apex of the heart to avoid a pressure buildup in the lungs. Using small, curved forceps, rupture the mitral valve and visually ensure that the forceps are able to enter the left atrium unrestricted.
2.17. Place a pretied 3–0 suture around the heart below the atrium (Figure 3E).
2.18. Insert the PV cannula (similar construction to PA cannula) into the left atrium and ensure that buffer can flow out of it prior to tying off the suture.
NOTE: It is critical that the PV cannula is placed past the mitral valve to ensure that it can be properly tied off while not being placed too deep as to injure the PV or encumber flow (Figure 3E). Take extra care with tying the suture as tying it too loose results in lost flow, while tying it too tight can damage the heart, weakening the placement of the cannula, and can cause leakages.
2.19. Trim excess tissue between the thoracic cavity and the tracheostomy incision using blunt-tipped scissors to avoid damaging the trachea. Ensure that the entire trachea below the tracheal cannula and the entire heart-lung block are visible.
2.20. Remove the heart-lung block and trachea by holding the tracheal cannula and excising connective tissue behind the trachea with a pair of curved, blunt-tipped scissors, leaving the esophagus attached for extra structural support (Figure 3F).
2.21. Gradually increase the flow rate to a maximum flow of 40 mL kg−1 min-1. Allow the first 50 mL of buffer to flow out of the system to remove any inflammatory cytokines that may be released (Figure 3G).
2.22. Gently move the isolated lungs to the double boiler chamber created with the two beakers. Make sure that the PA, PV, or tracheal cannula do not become twisted at any point while moving the lungs or hanging them.
NOTE: Prevention of atelectasis during lung retrieval and connection to the setup is important, especially when EVLP is used for already damaged lungs or when interventions prior to EVLP are planned. This can be achieved by, for example, a small clip on the trachea after inspiration before transfer to the ventilator15.
3. Data acquisition
For data collection, any commercially available analog-to-digital converter and data acquisition (DAQ) system will suffice. Pay attention that the sampling frequency is sufficient for the given purpose (we chose 200 Hz) and that all relevant dependent parameters from bioamplifiers and other input devices are collected concurrently (we chose airway pressure, PA and PV pressure)16. Independent parameters such as, e.g., perfusion speed, ventilation rate, ventilated gas composition, buffer electrolyte composition, and lung weight should be recorded as well.
REPRESENTATIVE RESULTS
Following 10 min of stabilization and baseline readings, we randomized a first set of 10 male Sprague Dawyley rats into five small groups: global no-flow ischemia for 5, 7.5, 8, 9 or 10 min (n = 2 per group) followed by reperfusion; these limited preliminary dose-finding experiments were conducted to identify the longest possible ischemia time to still allow sufficient ventilation and reperfusion before the eventual development of a precipitous and irreversible increase in airway pressure and edema formation. Importantly, during ischemia, we chose not to stop the ventilation but to rather reduce its rate from 60 to 20 breaths min−1 to prevent deflation and irreversible atelectasis of the ex-situ lungs. Applying a constant PEEP likely achieves the same goal, in analogy to the tracheas of to-be-transplanted lungs being clamped after inspiration in clinical practice17. Figure 4 suggests that under these conditions, the longest ischemia time to still allow 150 min reperfusion was 8 min.
Figure 4: Representative results on lung viability after ischemia and reperfusion.
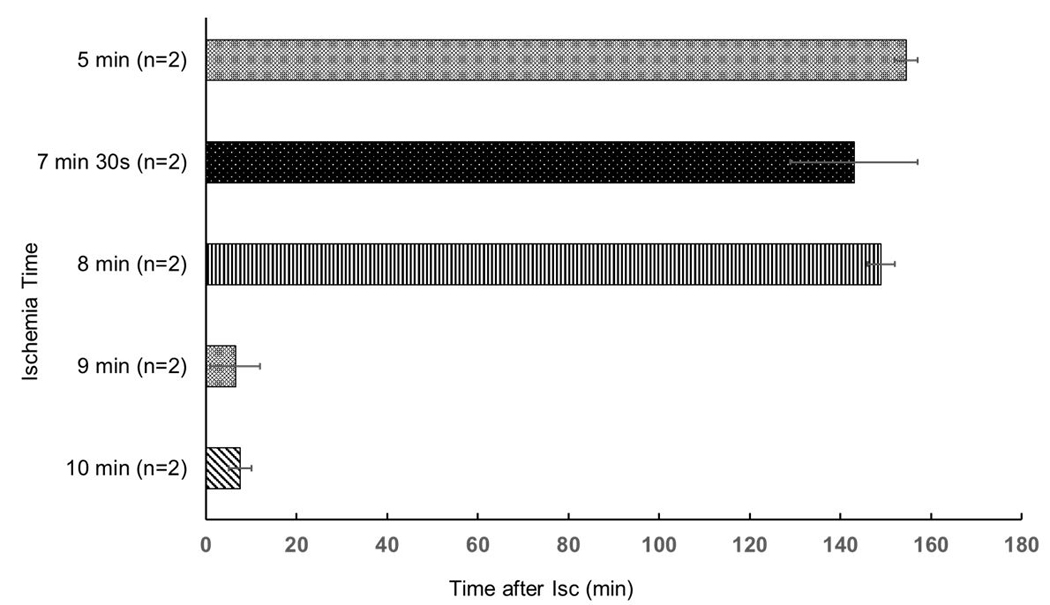
Lung viability was defined as survival time until the lungs exhibited a fulminant increase in airway pressure and became fully edematous. Data of limited experiments per group are displayed as mean ± standard error of the mean. Statistical tests were not conducted because of the low number of experiments per group.
DISCUSSION:
More than 100 experiments have been successfully performed in our lab using this setup. The modular design of this customized setup gave great flexibility to potential changes in experimental requirements. While other setups utilize a deoxygenator18 to mimic constant oxygen consumption and CO2 production by end organs, this simplified model did not employ this feature due to the focus on studying the effects of different gas compositions on pulmonary vascular tone. This approach, in which CO2 is controlled only by the inspired gas concentration, also allowed for differential regulation of CO2 independent from minute ventilation.
A closed circuit (Figure 1) was used to recirculate the modified Krebs buffer (Table 1) to conserve waste of BSA, an important cost-limiting step. Perfusion with constant flow mimicking a constant cardiac output was utilized to allow for the study of different doses of pharmacological agents on pulmonary vascular tone independent of concomitant cardiac output changes in-vivo. This can easily be modified to a constant-pressure perfusion by integrating an adjustable height pressure column in the circuit and adjusting the pump speed accordingly; for that, an additional flow probe is required, though, since flow, instead of pressure, now becomes the dependent variable.
The method of ventilation was with positive rather than more physiological negative pressure, mimicking an unconscious or anesthetized, intubated, and ventilated rather than a spontaneously breathing subject. Changing this to negative pressure ventilation would require the use of a sealed chamber for hanging the lungs and making corresponding changes to setting up the ventilator11 but is still possible to incorporate into the apparatus with minimal effort.
The buffer was maintained at a constant temperature by a heating plate and passed through a heating coil and heated air trap to ensure no dissolved gasses escaped solution causing the lung to be perfused with air emboli. To perfuse the lungs, a peristaltic pump was used. The roller pump we used had the great advantage of having both manual controls and voltage inputs and outputs for easy connection to the DAQ. This made it possible to not only record the pump speed but also to control the pump electronically. One important aspect of in vivo lungs that is absent in the isolated model is pulsatile flow from the heart. By using sinusoidal, square, or triangle waves generated by the software as voltage outputs to the pump, the setup is able to mimic the pulsatile blood flow coming from an in vivo heart. This effect was slightly dampened by the Windkessel effect from tubing between the buffer and the lungs but was still seen in both the PA and PV pressure recordings. Non-compliant tubing was used to minimize the Windkessel effect. A three-way stopcock placed in the circuit coming from the PV allowed for collection of effluent for analysis of injury markers (e.g., lactate dehydrogenase).
Of concern was maintaining a consistent metabolic environment—glucose concentration—in the isolated lungs similar to what would be seen in vivo. The compositions of the buffer as well as ventilated gasses play an important role. Valenza et al. showed in a porcine EVLP model that glucose consumption in lungs correlated with lung function4. As a means to keep the glucose concentration constant in the recirculated buffer despite ongoing glucose metabolism, the glucose level of the solution was checked hourly and glucose added back into the buffer to keep the composition as close to the target of 5.5 mM as possible. A modified Krebs buffer (Table 1) with an electrolyte composition that mimics blood was used for the perfusion. However, this presented problems when trying to measure the glucose concentration. A standard blood glucose measurement device (ACCU-CHEK, Aviva) was unable to read the glucose levels of the buffer, so other options needed to be explored. Glucose test strips did not prove accurate enough to measure the glucose of the solution when compared to a more clinically relevant device (VetScan i-STAT 1)19, which worked reliably.
While other EVLP setups utilize additives such as Perfadex and Celsior20, this setup used BSA due to prior success with previous experiments and its relative affordability if recirculated. This addition was necessary to maintain a physiological oncotic pressure in the lungs as experiments performed without BSA deteriorated much more rapidly, typically within 30 min of extraction, due to pulmonary edema. Others have also reported success when using higher concentrations of BSA and whole blood. This has the added benefit of allowing the blood to be fractionated to suit the experiment. Lungs that were extracted by a skilled technician in a timely manner were able to survive up to 6 h under control conditions before edema formation became a problem. The latter was apparent when observing changes in the lungs, such as gradual changes in color from white to pink, an increase in size due to edema formation, and sharp pressure increases in the pulmonary vasculature During the procedure, it is important to measure the electrolyte composition of the buffer after the addition of BSA; measured Ca2+, for example, decreased in the buffer following the addition of BSA, implying that BSA is chelating Ca2+, reducing the amount that is available for the lungs21.
In summary, several commercial isolated lung kits are available for purchase that require significantly less time invested into starting up. However, these can be limited in scope to what they can do and might not offer the ability to adapt to different experimental parameters. Taking the DIY approach offers a system that is on par with the commercial setups, while also allowing for flexible adaption to a variety of research topics. Once the initial barrier of entry is overcome, the system can be run with minimal oversight and teaching required.
ACKNOWLEDGMENTS:
Support provided, in part, by a Merit Review Award (101 BX003482) from the U.S. Department of Veteran Affairs Biomedical Laboratory R&D Service, a NIH grant (5R01 HL123227), a Transformative Project Award (962204) from the American Heart Association, and by institutional funds awarded to Dr. Riess. Dr. Balzer received unrelated funding from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project number BA 6287/1–1. The authors would like to thank Matthew D. Olsen, Chun Zhou, Zhu Li and Rebecca C. Riess for their valuable contributions to the study.
Footnotes
A complete version of this article that includes the video component is available at http://dx.doi.org/10.3791/64740.
DISCLOSURES:
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES:
- 1.Uhlig S & Taylor AE. Methods in Pulmonary Research. (Birkhäuser, 1998). [Google Scholar]
- 2.Ghaidan H et al. Ten year follow-up of lung transplantations using initially rejected donor lungs after reconditioning using ex vivo lung perfusion. J Cardiothorac Surg. 14 (1), 125, doi: 10.1186/s13019-019-0948-1, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stone ML et al. Ex Vivo Perfusion With Adenosine A2A Receptor Agonist Enhances Rehabilitation of Murine Donor Lungs After Circulatory Death. Transplantation. 99 (12), 2494–2503, doi: 10.1097/TP.0000000000000830, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valenza F et al. The consumption of glucose during ex vivo lung perfusion correlates with lung edema. Transplant Proc. 43 (4), 993–996, doi: 10.1016/j.transproceed.2011.01.122, (2011). [DOI] [PubMed] [Google Scholar]
- 5.Sayner SL et al. Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circ Res. 95 (2), 196–203, doi: 10.1161/01.RES.0000134922.25721.d9, (2004). [DOI] [PubMed] [Google Scholar]
- 6.McAuley DF et al. Clinical grade allogeneic human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am J Physiol Lung Cell Mol Physiol. 306 (9), L809–815, doi: 10.1152/ajplung.00358.2013, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pego-Fernandes PM et al. Experimental model of isolated lung perfusion in rats: first Brazilian experience using the IL-2 isolated perfused rat or guinea pig lung system. Transplant Proc. 42 (2), 444–447, doi: 10.1016/j.transproceed.2010.01.016, (2010). [DOI] [PubMed] [Google Scholar]
- 8.Noda K et al. Successful prolonged ex vivo lung perfusion for graft preservation in rats. Eur J Cardiothorac Surg. 45 (3), e54–60, doi: 10.1093/ejcts/ezt598, (2014). [DOI] [PubMed] [Google Scholar]
- 9.Nelson K et al. Method of isolated ex vivo lung perfusion in a rat model: lessons learned from developing a rat EVLP program. J Vis Exp. (96), doi: 10.3791/52309, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bassani GA et al. Ex Vivo Lung Perfusion in the Rat: Detailed Procedure and Videos. PLoS One. 11 (12), e0167898, doi: 10.1371/journal.pone.0167898, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watson KE, Segal GS & Conhaim RL Negative pressure ventilation enhances acinar perfusion in isolated rat lungs. Pulm Circ. 8 (1), 2045893217753596, doi: 10.1177/2045893217753596, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohsumi A et al. A method for translational rat ex vivo lung perfusion experimentation. Am J Physiol Lung Cell Mol Physiol. 319 (1), L61–L70, doi: 10.1152/ajplung.00256.2019, (2020). [DOI] [PubMed] [Google Scholar]
- 13.Hozain AE et al. Multiday maintenance of extracorporeal lungs using cross-circulation with conscious swine. J Thorac Cardiovasc Surg. 159 (4), 1640–1653 e1618, doi: 10.1016/j.jtcvs.2019.09.121, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilkenny C, Browne WJ, Cuthill IC, Emerson M & Altman DG Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 8 (6), e1000412, doi: 10.1371/journal.pbio.1000412, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Zanden JE, Leuvenink HGD, Verschuuren EAM, Erasmus ME & Hottenrott MC A translational rat model for ex vivo lung perfusion of pre-injured lungs after brain death. PLoS One. 16 (12), e0260705, doi: 10.1371/journal.pone.0260705, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cleveland WJ et al. Implementation of LabVIEW as a Virtual Instrument in a Cost-Effective Isolated Lung Setup. FASEB J. 33 (1), 846.849, doi: 10.1096/fasebj.2019.33.1_supplement.846.9, (2019). [DOI] [Google Scholar]
- 17.Jamieson SW, Stinson EB, Oyer PE, Baldwin JC & Shumway NE Operative technique for heart-lung transplantation. J Thorac Cardiovasc Surg. 87 (6), 930–935 (1984). [PubMed] [Google Scholar]
- 18.Liu M et al. Alterations of nitric oxide synthase expression and activity during rat lung transplantation. Am J Physiol Lung Cell Mol Physiol. 278 (5), L1071–1081, doi: 10.1152/ajplung.2000.278.5.L1071, (2000). [DOI] [PubMed] [Google Scholar]
- 19.Riess ML et al. Glucose Measurements in Blood-Free balanced Salt Solutions with Three Devices (i-STAT®, Glucose Test Strips and ACCU-CHEK® Aviva). Anesth Analg. 128 (5), S433 (2019). [Google Scholar]
- 20.Menezes AQ et al. Comparison of Celsior and Perfadex lung preservation solutions in rat lungs subjected to 6 and 12 hours of ischemia using an ex-vivo lung perfusion system. Clinics (Sao Paulo). 67 (11), 1309–1314, doi: 10.6061/clinics/2012(11)15, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riess ML et al. Electrolyte Measurements in Blood-Free Balanced Salt Solutions – Comparison of i-STAT® with ABL80. Anesth Analg 130 (5 ), 984 (2020.). [Google Scholar]


