Abstract
Myelin plays a pivotal role in the efficient transmission of nerve impulses. Disruptions in myelin integrity are associated with numerous neurological disorders, including multiple sclerosis. In the central nervous system (CNS), myelin is formed by oligodendrocytes. Remyelination refers to the re-formation of the damaged myelin sheath by newly formed oligodendrocytes. Steroids have gained attention for their potential modulatory effects on myelin in both health and disease. Steroids are traditionally associated with endocrine functions, but their local synthesis within the nervous system has generated significant interest. The term “neuroactive steroids” refers to steroids that can act on cells of the nervous system. In the healthy state, neuroactive steroids promote myelin formation, maintenance, and repair by enhancing oligodendrocyte differentiation and maturation. In pathological conditions, such as demyelination injury, multiple neuroactive steroids have shown promise in promoting remyelination. Understanding the effects of neuroactive steroids on myelin could lead to novel therapeutic approaches for demyelinating diseases and neurodegenerative disorders. This review highlights the potential therapeutic significance of neuroactive steroids in myelin-related health and diseases. We review the synthesis of steroids by neurons and glial cells and discuss the roles of neuroactive steroids on myelin structure and function in health and disease. We emphasize the potential promyelinating effects of the varying levels of neuroactive steroids during different female physiological states such as the menstrual cycle, pregnancy, lactation, and postmenopause.
Keywords: Neurosteroids, Oligodendrocytes, Steroid hormone synthesis, Myelin, Multiple sclerosis
Highlights of the Study
Alterations of myelin are linked to various neurological disorders, including multiple sclerosis.
Neuroactive steroids can be synthesized within the CNS and modulate myelin formation in both health and disease.
Variations in neuroactive steroids during different female physiological states profoundly affect myelination in diseases such as multiple sclerosis.
Introduction
The pathological loss of the myelin sheath around axons because of injury or disease is known as demyelination [1]. It results in an inefficient transmission of action potentials along the axon and eventually loss of sensory, motor, and cognitive functions [2]. Remyelination is one of the few regenerative processes that takes place in the adult CNS [3], and is triggered spontaneously following demyelination injury [3]. Remyelination involves the repair of the myelin sheath to restore saltatory conduction of nerve impulses and resolve functional deficits resulting from demyelination [4]. Demyelination occurs in several neurological diseases including multiple sclerosis (MS), stroke, and spinal cord injury [5]. MS is a neurodegenerative, inflammatory, archetypal demyelination disease that affects about 2.5 million people worldwide [6, 7]. The incidence of MS has progressively increased during the last 2 decades in the Middle East and worldwide [8–10].
Demyelinating diseases such as MS are prevalent in women with a ratio of 3:1 [11]. The severity and the course of MS seem to be changing depending on the physiological states such as stages of the estrus cycle, pregnancy, postpartum, and menopause. Each of these physiological states has unique hormonal profiles that are thought to influence the course of de-/remyelination. The most important hormones such as estrogens, progestogens, androgens, and glucocorticoids are lipophilic steroid hormones. Other nonsteroidal hormones associated with postpartum such as prolactin and oxytocin might also affect the process of de-/remyelination. This review will focus mainly on steroidal hormones, and the roles of nonsteroidal hormones on de-/remyelination have been reviewed elsewhere [12]. We will also discuss the mechanisms of synthesis and the de-/remyelinating action of the four major steroid hormones. This review will be limited to de-/remyelination in females because of the prevalence of MS in women, and the unique hormonal changes throughout their reproductive experience.
Neuroactive Steroids: Synthesis and Mechanism of Action
Neuronal and nonneuronal cells within the central nervous system possess the enzymatic tools to locally synthesize steroid hormones, called neurosteroids. This “in-house” production of steroid hormones does not preclude the action of peripherally synthesized hormones in the CNS. Thus, the term “neuroactive steroids” was coined for steroids produced in the nervous system (neurosteroids), as well as steroids produced by the peripheral glands [13, 14]. All neuroactive steroids share a common precursor, cholesterol [15]. As the blood-brain barrier, as well as the blood-nerve barrier, does not allow the passage of cholesterol from the periphery into the nervous system, all the cholesterol used in steroidogenesis is locally synthesized within the nervous system [16]. The first step in steroidogenesis involves the translocation of cholesterol from the cytoplasm to the mitochondrial membranes by the action of the steroidogenic acute regulatory protein (StAR) and the 18-kDa translocator protein (TSPO) (formerly known as peripheral benzodiazepine receptor) [17], where the enzyme P450 side-chain cleavage (P450scc), the rate-limiting enzyme for the conversion of cholesterol to pregnanolone, is present [18]. This enzyme is localized in the inner mitochondrial membrane of steroidogenic endocrine cells, astrocytes, oligodendrocytes, and neurons [15, 19, 20]. Thus, these cells convert cholesterol into pregnenolone, which can be further metabolized into any one of the neuroactive steroids as will be discussed below [18]. Both TSPO and StAR are significantly upregulated in neurodegenerative diseases including MS and brain injury models induced by neurotoxins. This TSPO/StAR upregulation promotes a series of steroidogenic processes within the CNS, which might be one of the neuroprotective mechanisms of the brain after injury [18, 21, 22]. It is worth mentioning that the role of TSPO in steroidogenesis in rodents has recently been questioned [23], and therefore, the players involved in cholesterol trafficking and de novo steroidogenesis may differ between species and steroidogenic cells.
Steroid hormones act classically by binding to intracellular receptors [24]. These receptors subsequently change their conformation and translocate into the nucleus where they bind to specific response elements and modulate the expression of several genes (Fig. 1A) [24]. Steroid receptors include progesterone receptors, estrogen receptors, androgen receptors (AR), and glucocorticoid receptors (GR) [25]. In addition to this classical transcriptional effect, steroids can rapidly act by interacting with membrane-associated classical steroid receptors (Fig. 1B), membrane-bound receptors such as the gamma-aminobutyric acid A (GABAA) receptor (Fig. 1C), and ion channels located on the plasma membrane such as chloride channels (Fig. 1D) [18, 25–27]. The seven-transmembrane G-protein-coupled receptors have also been described as membrane targets of neuroactive steroids (Fig. 1E). For example, the cannabinoid type 1 (CB1) receptor can be negatively modulated by pregnenolone, which binds to a specific allosteric site [28, 29]. Neuroactive steroids signal through various mechanisms including changing the levels of intracellular calcium or activation of kinases in different signaling pathways [30, 31], regulating the inflammatory responses of neural cells [32]. We will now proceed to discuss the synthesis and remyelinating effects of the four major neuroactive steroids (progestagens, estrogens, glucocorticoids, and androgens) within the CNS.
Fig. 1.
Mechanism of action of neuroactive steroids. Neuroactive steroids bind to their steroid receptors (SR), which then form dimers that bind to steroid receptor response element (SRE) and regulate gene transcription(A). Neuroactive steroids can also bind to classical steroid receptors located in the plasma membrane (B), putative steroid receptors in the plasma membrane such as the GABAA receptor (C), ion channels related to neurotransmitter receptors such as chloride channels (D), and transmembrane G-protein-coupled receptors such as cannabinoid type 1 receptor (E). Through these receptors, steroids activate many of the signaling pathways such as increasing intracellular levels of calcium and inositol 1,45-trisphosphate (IP3), activation of G-proteins, mitogen-activated protein kinase (MAPK), and phosphoinositide-3 kinase (PI3K)/Akt pathways [26].
Progesterone
Progesterone is the naturally occurring progestagen. It is widely known for its role during pregnancy. In addition, progesterone exerts several neuroprotective and promyelinating effects on the nervous system as shown in several experimental models of MS [16, 33–37]. The synthesis of progesterone in both rodents and human females takes place mainly in the ovaries [16, 38]. During pregnancy, however, the placenta takes over the major role in producing progesterone in humans, but not in rodents, in which the ovaries continue to be the main source for the synthesis of progesterone [16]. The adrenal glands also produce progesterone and are considered the primary source of progesterone in males [16, 39]. Progesterone is also synthesized de novo within the central nervous system [16, 40].
The first step of progesterone formation is the conversion of cholesterol into pregnenolone by the enzymatic action of P450scc [16, 41]. The second step involves the oxidation of pregnenolone to progesterone, a reaction that takes place in the endoplasmic reticulum and is catalyzed by the 3β-hydroxysteroid-dehydrogenase (3β-HSD) enzyme (Fig. 2) [15, 41]. The 3β-HSD enzyme is expressed in astrocytes, oligodendrocytes, and neurons under both normal and pathological conditions [15, 20, 25, 30, 42, 43]. Astrocytes are more active in the production of progesterone than oligodendrocytes, owing to the higher activity of the 3β-HSD enzyme [20]. Microglia express the enzyme P450scc and therefore can produce pregnenolone de novo [44] but not progesterone because they lack the enzyme 3β-HSD [15].
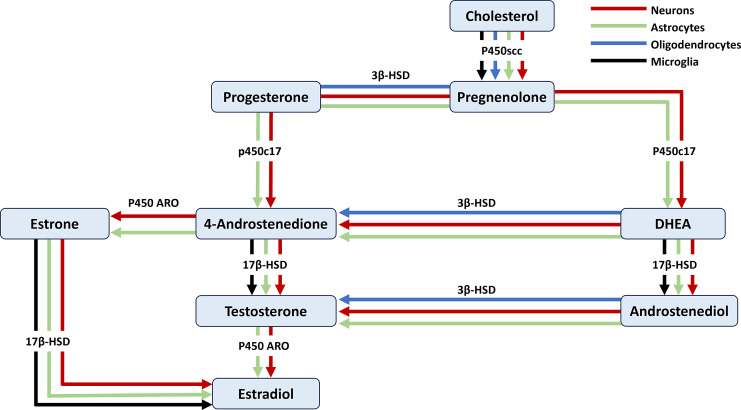
Fig. 2.
Synthesis of progesterone and ALLO. The synthesis of progesterone begins with the conversion of cholesterol to pregnenolone by the enzyme P450scc followed by its conversion to progesterone by the enzyme 3β-HSD. Allopregnanolone is the result of progesterone metabolism into 5α-dihydroprogesterone by the enzyme 5α-R and further metabolism by the enzyme 3α-HSD. Arrows represent cellular expression of steroid-converting enzymes in neurons (red), astrocytes (green), oligodendrocytes (blue), and microglia (black).
Progesterone has been shown to play a role in the process of remyelination [16]; the formation of myelin during both development and remyelination after a demyelinating injury is enhanced by progesterone [45]. The first evidence of such promyelinating potential for progesterone was shown in a model of sciatic nerve cryo-lesion injury in male mice [46]. In this model, remyelination failed when the local synthesis of progesterone was blocked or when the receptor mediating the action of progesterone was antagonized [46]. On the other hand, administration of progesterone, or its precursor, pregnenolone, had a promyelinating effect observed as an increase in the thickness of the myelin sheath during remyelination [45, 46]. Adding progesterone to cultures of dorsal root ganglia was also shown to increase axonal myelination confirming the important role of progesterone in myelin formation and repair [45, 46]. At the molecular levels, progesterone was shown to increase the expression of both myelin basic protein (MBP) and 2′,3′-cyclic-nucleotide 3′-phosphodiesterase (CNPase) in primary cultures of rat oligodendrocytes [47, 48]. In addition, it increased the branching of oligodendrocytes in primary cultures of oligodendrocyte progenitor cells [49]. Furthermore, the addition of progesterone to the culture medium of cerebellar slices made from 7-day-old rats and mice increased the expression of MBP and stimulated the proliferation and maturation of OPCs [50].
Progesterone enhanced remyelination in vivo in ethidium bromide (EB)-induced lesions in cerebellar peduncles of 9-month-old male rats [51]. It also restored the levels of MBP to the preinjury levels 3 days after the complete transection of the spinal cord [52], likely by enhancing the proliferation and the maturation of OPCs into myelinating oligodendrocytes [53].
The effects of progesterone in the EAE model are somewhat controversial. While some studies have shown that progesterone attenuates the severity of the disease [33, 34, 54, 55], others have shown that it worsens the symptoms of EAE [56]. In the cuprizone model, progesterone provided only moderate prevention from demyelination, while when combined with estrogen, it was shown to significantly decrease the resulting demyelination [57]. Progesterone also enhanced remyelination, increased the density of oligodendrocytes, and promoted the expression of myelin proteins including MBP and proteolipid protein [58] following CPZ-induced demyelination. Another study has shown that progesterone promoted remyelination and increased the expression of MBP following cuprizone-induced demyelination in a PR-dependent manner [59] likely by inducing a switch of microglial phenotype from proinflammatory (M1) to regulatory (M2) phenotype [60]. Similarly, the synthetic progestin Nestorone promoted myelination following CPZ-induced demyelination in mice by increasing the expression of the myelin synthesis markers Olig2, Myt1, and Sox17 [61]. Using lysolecithin-demyelinated brain slices, both progesterone and nestorone increased the proliferation and migration of OPCs to the area of demyelination [35]. This effect was absent in slices that were prepared from PR knockout mice [35]. Table 1 illustrates the promyelinating effects of progesterone.
Table 1.
Promyelinating effects of progesterone
| Experimental models | Effects | References |
|---|---|---|
| Primary cultures of oligodendrocytes | ↑ MBP | [47] |
| ↑ CNPase | ||
| Primary cultures of OPCs | ↑ Branching of OPCs | [49] |
| Cerebellar slices | ↑ MBP | [50, 62] |
| ↑ Proliferation of OPC | ||
| ↑ Maturation of OPC | ||
| Lysolecithin-demyelinated cerebellar slices | ↑ Proliferation of OPC | [35] |
| ↑ Migration of OPC | ||
| EB demyelination in CCP | ↑ Remyelination of oligodendrocytes | [51] |
| Spinal cord transection | ↑ MBP | [52, 53] |
| ↑ Proliferation of OPC | ||
| ↑ Maturation of OPC | ||
| EAE | ↓ Demyelination | [33, 34, 54, 55] |
| ↓ Severity of symptoms | ||
| EAE | ↑ Severity of symptoms | [56] |
| CPZ | Moderate prevention | [57] |
| CPZ | ↑ Proliferation of OPC | [59] |
| ↑ Maturation of OPC | ||
| ↑ MBP and PLP | ||
| CPZ | ↑ Remyelination | [58] |
| ↑ MBP and PLP | ||
| ↑ Maturation of oligodendrocytes | ||
| CPZ | ↑ MBP | [60] |
| ↑ Remyelination | ||
| Spinal cord injury | ↑ Proliferation of OPC | [53] |
| CPZ | ↑ Remyelination of oligodendrocytes | [61] |
MBP, myelin basic protein; CNPase, 2′,3′-cyclic-nucleotide 3′-phosphodiesterase; OPC, oligodendrocyte progenitor cell; EB, ethidium bromide; CCP, caudal cerebellar peduncle; EAE, experimental autoimmune encephalomyelitis; CPZ, cuprizone; PLP, proteolipid protein.
Aside from its direct effect on PR, progesterone can exert a promyelinating effect indirectly through its metabolite allopregnanolone (ALLO) [62–64]. ALLO is produced by the conjoint actions of 5α-reductase (5α-R) and 3α-hydroxysteroid-dehydrogenase enzymes on progesterone [15]. Neurons and all glial cells express the 5α-R enzyme and thus can metabolize progesterone into 5α-dihydroprogesterone. Microglia, on the other hand, do not express the 3α-hydroxysteroid-dehydrogenase enzyme and thus cannot produce ALLO (Fig. 2) [15]. ALLO exerts its effects by potentiating the effect of the endogenous neurotransmitter GABA on the GABAA receptor [16].
Allopregnanolone
ALLO has been shown to enhance the proliferation of OPCs through the GABAA receptor in an autocrine/paracrine manner in vitro [65]. This effect was completely abolished when the synthesis of ALLO was blocked using the 5α-R inhibitor L685-273 [65]. ALLO also increases the basal expression of MBP in cerebellar slice cultures, an effect that was blocked when either ALLO-synthesizing enzyme 5α-R was inhibited or when the GABAA receptor was pharmacologically antagonized by bicuculline [62]. The administration of ALLO delays the onset of demyelination in mouse models of Niemann-Pick C disease, an irreversible neurodegenerative disease with no current treatment [63]. Furthermore, the administration of ALLO following the induction of EAE in mice reduced myelin and axonal injury and reduced the behavioral deficits associated with EAE [66]. ALLO can also modulate inflammation by reducing levels of inflammatory cytokines, including IL-1β and TNF-α, produced by peripheral macrophages [66, 67]. In addition, pretreatment of oligodendrocytes with ALLO protects against TNF-α-induced toxicity in vitro. Moreover, dysregulation of ALLO synthesis has been shown in the brains of MS patients; indeed, levels of ALLO were significantly reduced in the myelin of the brains of postmortem MS patients. This reduction in levels of ALLO was associated with diminished levels of the enzyme 5α-R [66]. It is thus plausible that the deficiency in ALLO production contributes to the pathogenesis of MS.
The promyelinating effects of ALLO were also shown experimentally; ALLO contributed to enhanced remyelination of the lysolecithin-induced demyelination in the corpus callosum of pregnant rats in a GABAA receptor-dependent manner [68]. These findings were further confirmed using the synthetic analog of ALLO, ganaxolone. Treatment of ovariectomized rats with ganaxolone resulted in enhanced remyelination, upregulated expression of myelin proteins (MBP, MAG), and enhanced microglial clearance of damaged myelin [69]. Table 2 summarizes the effects of ALLO on myelination/remyelination.
Table 2.
Promyelinating effects of ALLO
| Experimental models | Effects | References |
|---|---|---|
| Cerebellar slices | ↑ MBP | [62] |
| Primary culture of oligodendrocytes | ↓ TNF-α-induced cytotoxicity | [66] |
| Niemann-Pick C | Delayed onset of demyelination | [63] |
| EAE | ↓ Severity of disease | [66] |
| ↓ Inflammation | ||
| Lysolecithin-demyelination of the CC | ↑ Remyelination | [68, 69] |
| ↑ Proliferation of OPC | ||
| ↑ Maturation of OPC | ||
| ↑ MBP and MAG | ||
| ↑ Clearance of myelin debris |
OPC, oligodendrocyte progenitor cell; MBP, myelin basic protein; TNF-α, tumor necrosis factor-α; EAE, experimental autoimmune encephalomyelitis; CC, corpus callosum; MAG, myelin-associated glycoprotein.
Androgens
Androgens play an essential role in the regulation of several physiological processes including male sexual development, muscle and bone growth, in addition to a role in behavior and cognition [70, 71].
Testosterone
The most studied androgen with neuroprotective effects is testosterone [35]. Testosterone can be synthesized de novo within the CNS and exerts neuroprotective effects on the CNS [72, 73].
The gonads and the adrenal glands are the two main sources of testosterone [20, 74]. Dehydroepiandrosterone (DHEA), a precursor for estrogens and androgens, is synthesized by metabolizing pregnenolone by the enzyme P450c17 in the endoplasmic reticulum [18]. In the CNS, only astrocytes and neurons possess this enzyme and thus can produce DHEA de novo from cholesterol [75]. Testosterone is produced either by the metabolism of progesterone or DHEA into androstenedione and further metabolism into testosterone by the enzyme 17β-HSD. Testosterone can also be produced by the metabolism of 5-androsten-3β,17β-diol (androstenediol: ADIOL) by the enzyme 3β-HSD, which is present in neurons, astrocytes, and oligodendrocytes [15] (Fig. 3).
Fig. 3.
Pathways involved in the synthesis of testosterone and estradiol. Arrows represent cellular expression in neurons (red), astrocytes (green), oligodendrocytes (blue), and microglia (black).
The effects of testosterone on myelin are both beneficial and detrimental. The administration of testosterone delays myelinogenesis as observed by MRI of developing rat brains [76]; on the other hand, testosterone ameliorates the symptoms of EAE [72, 77]. The effects of testosterone are mediated through AR [78]. Testosterone enhances the proliferation and maturation of OPCs following cuprizone-induced demyelination [79], an action that is blocked by the AR antagonist flutamide [80]. Treatment with flutamide also prevents the amelioration of EAE symptoms by treatment with testosterone, supporting the role of AR in mediating the neuroprotective effects of androgens [81]. Testosterone promotes the proliferation and differentiation of OPCs and restores the expression of MBP following lysolecithin-induced demyelination in the spinal cord of male mice [82]. Similarly, testosterone increases the density of OPCs, mature oligodendrocytes, and the expression of MBP 7 days after lysolecithin-induced demyelination in the corpus callosum [83]. The conversion of testosterone into estradiol by the action of aromatase (P450 ARO) might also be responsible for the observed effects of testosterone treatment [73, 84]. In addition, testosterone can be metabolized to 5α-dihydrotestosterone (5α-DHT), which can bind to AR [85]. The effects of 5α-DHT, however, are mainly on the development of the male reproductive system [80]. Using 5α-DHT (a nonaromatizable androgen) as well as AR antagonists are important in studying whether the neuroprotective effects of androgens are mediated through AR or the conversion of testosterone into estradiol [73, 86, 87]. A summary of the myelinating/remyelinating effects of testosterone is shown in Table 3.
Table 3.
Promyelinating effects of testosterone and its metabolites
| Experimental models | Effects | References |
|---|---|---|
| EAE | ↓ EAE severity | [72] |
| CPZ | ↑ Proliferation of OPC | [79, 80] |
| ↑ Maturation of OPC | ||
| Lysolecithin-induced demyelination in the spinal cord | ↑ Proliferation of OPC | [82] |
| ↑ Maturation of OPC | ||
| ↑ MBP | ||
| Lysolecithin-induced demyelination in the CC | ↑ Proliferation of OPC | [83] |
| ↑ Maturation of OPC | ||
| ↑ MBP | ||
| EB demyelination of the CC | ↑ Proliferation of OPC | [96] |
| ↑ Maturation of OPC | ||
| ↑ Remyelination |
EAE, experimental autoimmune encephalomyelitis; CPZ, cuprizone; OPC, oligodendrocyte progenitor cell; MBP, myelin basic protein; EB, ethidium bromide; CC, corpus callosum.
DHEA
Another androgen that has an impact on the remyelination process is DHEA, a precursor of testosterone that has weak androgenic effects with neuroprotective properties and potent anti-inflammatory effects [88–90]. DHEA prevents the development of EAE in mice [88]. In humans, DHEA is one of the most abundant steroids in the circulation [88], but its levels are lower in MS patients, and MS patients reported an improvement in their symptoms after receiving DHEA replacement therapy [88].
The mechanism by which DHEA exerts its effect is still not fully understood; it could mediate its effect through AR, ER, or modulation of membrane receptors such as GABAA, N-methyl-d-aspartate receptors, and sigma 1 receptors [91–93]. In addition, these effects might be attributed to its conversion to estradiol or testosterone [75, 93]. The observation that DHEA does not have a specific receptor led researchers to believe that it can be further metabolized into an active hormone that mediates the observed neuroprotective effects [75, 94, 95]. One of the metabolites of DHEA that was shown to have neuroprotective effects is ADIOL [96, 97].
ADIOL
The importance of ADIOL as a neuroactive steroid became evident after the observation that the conversion of DHEA to ADIOL is decreased in neurodegenerative conditions such as Alzheimer’s disease, suggesting a role for this hormone in these pathologies [98]. The neuroprotective potentials of ADIOL have not been fully explored.
Pregnenolone is metabolized into DHEA by the enzyme P450c17. The enzyme 17β-hydroxysteroid dehydrogenase (17β-HSD) mediates the conversion of DHEA into ADIOL [99]. The 17β-HSD family contains at least 14 different members that can convert DHEA to ADIOL [99] (Fig. 3).
Both activated microglia, which are usually found concentrated in areas of injury within the CNS, and resting microglia, can convert DHEA into ADIOL [75, 100]. Peripheral macrophages that might infiltrate into the CNS after brain injury can also convert DHEA into ADIOL [75]. Indeed, both microglia and peripheral macrophages express the enzyme 17β-HSD [101]. Depletion or inhibition of these ADIOL-producing cells impairs remyelination [102]. ADIOL possesses both estrogenic and androgenic properties in addition to being a precursor of androgens and estrogens [97].
Following a pathological insult to the CNS, immune activation causes an increase in the number of microglia that have a high capacity to convert DHEA into ADIOL, suggesting a neuroprotective role of this hormone in such conditions [75]. The effects of ADIOL may be manifested through ERβ [97, 103]; indeed, ADIOL competes with estradiol for ERβ [104]. Knockout of the 17β-HSD enzyme responsible for converting DHEA to ADIOL or ERβ was shown to cause an exaggerated inflammatory response in both microglia and astrocytes, suggesting that ADIOL might be playing a role in maintaining the homeostasis of the CNS [97]. ADIOL increases the density of OPCs and their maturation following EB-induced demyelination in the CC of male rats. These findings were associated with better remyelination [96, 97].
Estrogens
Estrogens have three main biological forms, estrone (E1), estradiol (E2), and estriol (E3). Estradiol has two naturally occurring optical isomers, 17α-estradiol and 17β-estradiol, with the latter being the most potent of all estrogens [73, 105] and which is the focus of this section [73, 105]. In addition to its functions in reproduction, 17β-estradiol (referred to hereafter as estradiol) has several neuroprotective actions in animal models and human diseases such as Parkinson’s disease, stroke, and MS [106].
As with progesterone, the ovaries are considered the main site for estradiol synthesis in both human and rodent females; however, only in humans does the placenta participate in the production of estradiol during pregnancy. Estradiol is also produced in the male gonads [107, 108]. Interestingly, estradiol can be produced locally within the nervous tissue [15]. Synthesis of estradiol can occur via several different pathways (Fig. 3). First, the enzyme 17-hydroxylase-c17,20-lyase (P450c17) converts progesterone into androstenedione. Next, androstenedione is metabolized into testosterone by the 17β-HSD and further aromatized into estrogen by the P450 ARO. Estradiol is also produced by the aromatization of androstenedione into estrone and further metabolism by the enzyme 17β-HSD [15]. In addition, when pregnenolone is metabolized into DHEA, it can be converted into either androstenedione or androstenediol, both of which can be further converted to testosterone and eventually estradiol (Fig. 3) [15].
In the CNS, only neurons and astrocytes have the complete machinery necessary to produce estradiol de novo [15, 18]. Oligodendrocytes and microglia lack both the P450c17 and P450 ARO enzymes and therefore are unable to produce estradiol [15, 109]. Microglia, however, express the 17β-HSD enzyme, enabling them to produce estradiol from estrone but not de novo [15].
Estradiol was first reported to affect myelin in 1966 when the administration of estradiol to neonatal rat brains was shown to increase the process of myelination [110]. Estradiol speeds up myelinogenesis in the brains of developing rats [76], enhances the proliferation of OPCs, and promotes the formation of the membrane sheath of oligodendrocytes in vitro [49]. Treatment with estradiol following oxygen-induced apoptosis (subjecting cells to 80% oxygen) prevented the death of oligodendrocytes and attenuated the loss of MBP [111]. It also increased the survival of developing oligodendrocytes in vitro in response to deprivation of oxygen-glucose [112]. Moreover, estradiol prevents the loss of myelin in a neonatal hypoxic-ischemic injury model, in which carotid ligation was performed on 6-day-old pups [112].
Estradiol significantly decreased demyelination of the corpus callosum following 3 weeks of cuprizone administration compared to controls, an effect that continued after even 5 weeks of cuprizone diet [113]. Furthermore, estradiol prevented the loss of mature myelinating oligodendrocytes in the corpus callosum of mice subjected to 3 and 5 weeks of cuprizone diet [113]. Although estradiol has been shown to increase the proliferation of OPCs in vitro [49], it did not affect the number of OPCs in vivo following cuprizone-induced demyelination [113]. A recent study showed that estradiol decreased demyelination following 5 weeks of cuprizone administration possibly by promoting the regulatory M2 microglial polarization in mice [114]. Estradiol can also exert a promyelinating effect indirectly by activating the release of insulin-like growth factor I (IGF-1) from astrocytes, and IGF-1 enhances the maturation of OPCs into myelinating oligodendrocytes [57, 115].
Two estradiol receptors, estrogen receptor α (ERα) and β (ERβ) [116], are expressed by OPCs and mature oligodendrocytes [117]. ERβ is the most widely expressed isoform of ER in the brain; however, most experimental models have shown that effects mediated through ERα are more protective [79]. While ERα ligands have been shown to mediate the anti-inflammatory effects of estradiol, ERβ ligands promote remyelination independent of inflammation [115].
In the EAE model of demyelination, ERα ligand (propyl pyrazole triol) was found to be protective during both the acute and chronic phases of the disease [116]. Treatment with ERα ligand also reduced the levels of the proinflammatory cytokines TNFα and interleukin-6. On the other hand, ERβ ligand (diaryl propionitrile) was protective only during the chronic stage of EAE and did not have a significant effect on any of the proinflammatory or anti-inflammatory cytokines [116, 118], suggesting that the neuroprotective effects of estrogen-mediated through ERβ are dissociated from the anti-inflammatory effects of estradiol [116]. Table 4 summarizes the effects of estradiol on myelination/remyelination processes.
Table 4.
Promyelinating effects of estradiol
| Experimental models | Effects | References |
|---|---|---|
| Primary cultures of OPCs | ↑ Proliferation of OPC | [49] |
| ↑ Formation of oligodendrocyte membranes | ||
| Oxidative injury in primary cultures oligodendrocyte progenitors | ↑ Survival of developing oligodendrocytes | [112] |
| Primary cultures of mature oligodendrocytes | ↓ Apoptosis of oligodendrocytes | [111] |
| ↓ Loss of MBP | ||
| Carotid ligation in neonates | ↓ Loss of MBP | [112] |
| CPZ | ↑ Myelination scores | [113] |
| ↑ Survival of oligodendrocytes | ||
| CPZ | ↑ IGF-1 | [57] |
| ↑ MBP | ||
| ↑ PLP | ||
| CPZ | ↓ Demyelination | [114] |
| ↑ MBP |
OPC, oligodendrocyte progenitor cell; MBP, myelin basic protein; CPZ, cuprizone; IGF-1, insulin-like growth factor 1; PLP, myelin proteolipid protein.
Glucocorticoids
Glucocorticoids (cortisol in human, corticosterone in rodents) were named based on their role in increasing blood glucose concentration [119]. They are synthesized primarily in the adrenal cortex of the adrenal gland of both males and females [119]. The increase in the endogenous levels of glucocorticoids seen in MS patients with Cushing syndrome and during the third trimester of pregnancy was associated with remission of MS symptoms, which suggests a protective role for glucocorticoids [120, 121]. Glucocorticoids have been used for over 50 years in treating diseases with an inflammatory component such as rheumatoid arthritis, allergic reactions, and MS due to their ability to suppress inflammatory responses [122–124]. However, the effects of glucocorticoids are both beneficial and detrimental to CNS-related diseases [119, 125, 126]. Despite their effectiveness in the early stages of MS, prolonged treatment with glucocorticoids leads to the development of resistance after which these agents have no beneficial effect on the course of the disease [121]. This prolonged treatment also leads to the development of conditions such as diabetes and osteoporosis [121].
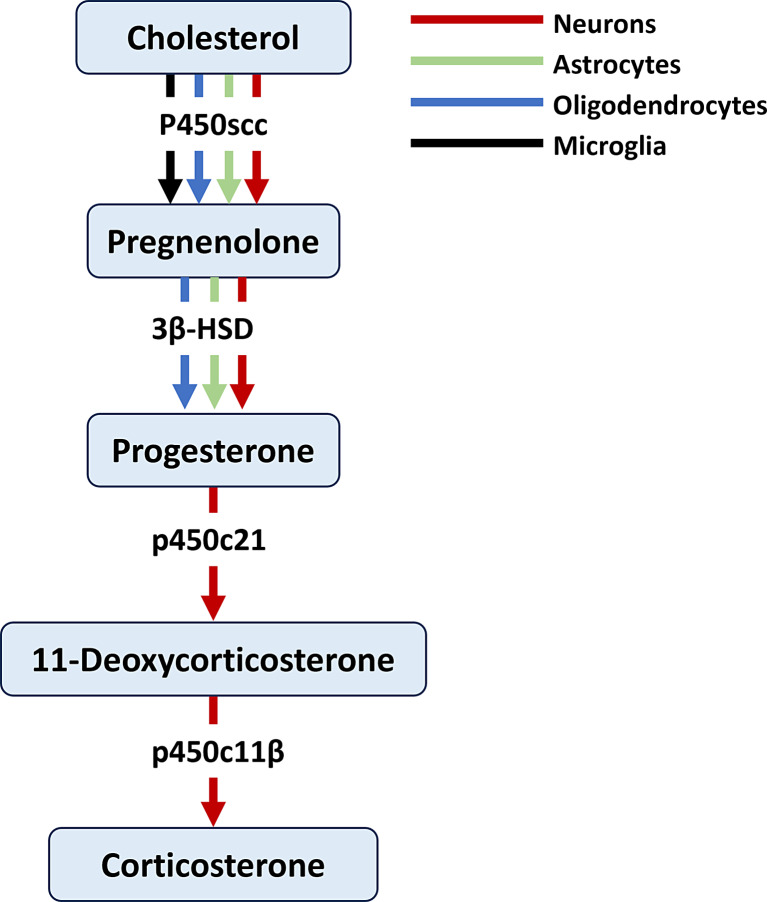
The adrenal cortex was thought to be the exclusive site of the synthesis of corticosterone. Corticosterone disappears from the circulation after adrenalectomy in rats [127, 128]. However, accumulated evidence has shown that the CNS has the required machinery for synthesizing glucocorticoids from cholesterol including the enzymes P450c21 and P450c11β and that the synthesis of corticosterone in rodents can take place within the CNS [129–133]. The metabolism of progesterone into 11-deoxycorticosterone and subsequently into corticosterone was shown to take place in neurons (Fig. 4) [127]. Whether glial cells contribute substantially to corticosterone production is not yet known.
Fig. 4.
Pathways involved in the synthesis of glucocorticoids. Only neurons metabolize progesterone into 11-deoxycorticosterone and subsequently to corticosterone. Arrows represent cellular expression of steroid-converting enzymes in neurons (red), astrocytes (green), and oligodendrocytes (blue).
Even though glucocorticoids are widely used in the treatment of diseases such as MS, their effects on the remyelination process are still controversial and might be dependent on the concentration and the time of exposure [134]. Glucocorticoids promote the differentiation of oligodendrocytes and enhance the process of myelinogenesis in vitro [135–137]. Moreover, the addition of dexamethasone, a potent synthetic glucocorticoid, to cultured neurons accelerates the process of axonal myelination, an effect that is blocked by the GR antagonist RU486 [138]. On the other hand, the administration of corticosterone to adrenalectomized rats inhibits the proliferation of OPCs in both the grey matter and the myelin of the brain [125]. Dexamethasone was also found to have detrimental effects on primary cultures of OPCS [134]. It reduces the expression of IGF-1 in astrocytes and depresses the expression of IGF-1 receptors in OPCs, which are essential for the differentiation of these cells into mature oligodendrocytes. Moreover, the negative effect of dexamethasone was shown to be associated with a downregulation of the differentiation marker CNPase [134].
The synthetic glucocorticoid methylprednisolone delays remyelination by oligodendrocytes in EB-induced demyelination of rat spinal cord. This delay in remyelination was attributed to a delay in the differentiation, but not the recruitment, of OPCs to the site of injury [139]. However, there is experimental evidence to suggest that methylprednisolone protects against the death of mature oligodendrocytes and increases their numbers following spinal cord injury [140].
The effect of glucocorticoids on myelination in the EAE model appears to contradict the effects seen in gliotoxin-induced demyelination models. Levels of corticosterone were demonstrated to increase throughout the course of EAE-induced demyelination damage possibly to counteract the deleterious effects of EAE [141]. It has been noted that the administration of exogenous glucocorticoids prevents the manifestation of EAE pathology [142] by acting through GR [121]. The apparent opposing effects of glucocorticoids on demyelination induced by focal administration of gliotoxin to myelin and that seen in the EAE model are probably due to the anti-inflammatory effects of these glucocorticoids on the systemic immune response associated with EAE [143]. The effects of glucocorticoids on myelination are summarized in Table 5.
Table 5.
Effects of glucocorticoids on myelination
| Experimental models | Effects | References |
|---|---|---|
| Primary cultures of oligodendrocytes | ↑ Myelinogenesis | [135] |
| Primary cultures of OPCs | ↑ Differentiation of OPC | [136] |
| Primary cultures of OPCs | ↑ Differentiation of OPC | [138] |
| Primary cultures of OPCs | ↓ CNPase | [134] |
| ↓ IGF-1 receptors | ||
| EB demyelination in spinal cord | ↓ Differentiation of OPC | [139] |
| Adrenalectomy | ↓ Proliferation of OPC | [125] |
| Spinal cord injury | ↑ Mature oligodendrocytes | [140] |
| ↓ Death of oligodendrocytes | ||
| EAE | ↓ EAE pathology | [142] |
OPC, oligodendrocyte progenitor cell; CNPase, 2′,3′-cyclic-nucleotide 3′-phosphodiesterase; IGF-1, insulin-like growth factor 1; EB, ethidium bromide.
De-/Remyelination during Different Physiological States
Overall, neuroactive steroids exert promyelinating effects. Owing to the prevalence of MS in females, the course and severity of MS symptoms were monitored during different physiological states characterized by large variations in the levels of circulating neuroactive steroids. In the following paragraphs, we summarize the course of demyelination symptoms in different physiological states such as the estrus cycle, pregnancy, lactation, and menopause.
De-/Remyelination during the Menstrual Cycle
Menstruation is suggested to affect the course of demyelinating conditions. For example, 40% of women with relapsing-remitting MS reported worsening of MS symptoms before menstruation [144, 145]. This exacerbation in disease symptoms has been linked to the sharp drop in the levels of ovarian hormones, mainly progesterone and estradiol, just before menstruation [144].
De-/Remyelination during Pregnancy
The most potent effect of steroid hormones on the course of demyelination is seen during pregnancy. A decrease in both symptomatic relapses and the number of active lesions in the brain of MS patients is seen during pregnancy, especially during the third trimester [146, 147]. This reduction is followed by a rebound of the disease in the first 3 months postpartum [145, 147]. The European multicenter study Pregnancy in Multiple Sclerosis (PRIMS) explored the effect of pregnancy and the postpartum state on the course of MS disease. This study included 227 MS patients with first pregnancies and 14 MS patients with second pregnancies; patients were followed 1 year before pregnancy, during pregnancy, and 1 year postpartum [148]. This study showed that there was a marked reduction in the rate of relapses during pregnancy, especially during the third trimester, compared to prepregnancy year. In addition, the rate of relapses was significantly higher during the first 3 months postpartum compared to the prepregnancy level [148]. MS patients show an 80% decrease in relapse rate during the third trimester of pregnancy compared to prepregnancy [149]. This improvement is higher than what current MS treatments offer to date (30–60% reduction in the relapse rate) [150–152]. On the other hand, the postpartum period is characterized by an increased relapse rate and number of lesions observed with magnetic resonance imaging [153]. The observed reduction in the rate of relapses during the third trimester coincides with the significant increase in the levels of pregnancy-related hormones such as progesterone, estradiol, estriol, prolactin, and cortisol. The increased number of relapses postpartum is attributed to the abrupt decrease in the level of these hormones during the postpartum period [149].
Animal models have been used to explore de-/remyelination processes during the peripartum period. Pregnancy in rodents takes 21–22 days followed by 3 weeks of lactation [154]. Experimental models of demyelination used to explore de-/remyelination include EAE and gliotoxin-induced demyelination models. Pregnant mice have been shown to be less susceptible to developing EAE, especially during middle and late pregnancy compared to virgin controls; moreover, the timing of EAE onset following immunization was delayed in pregnant animals compared to virgin controls [155]. Interestingly, this attenuation of EAE during pregnancy was lost during the postpartum period [156]. Furthermore, the proliferation and maturation of OPCs were enhanced following lysolecithin-induced demyelination in the corpus callosum and in the dorsal column of the spinal cord in early pregnancy (gestational day 7) when compared to virgins [157]. These effects were accompanied by enhanced axonal remyelination [157]. In addition, remyelination is enhanced in response to gliotoxin-induced demyelination in the rat corpus callosum during late pregnancy relative to virgin and postpartum rats. This pregnancy-associated promyelinating effect is likely mediated by the progesterone metabolite ALLO, which is elevated during this period of pregnancy [68]. Taken together, these studies indicate that remyelination is enhanced during pregnancy (especially during late pregnancy), an effect that is likely lost during postpartum.
De-/Remyelination and Lactation
Clinical evidence indicates that the course of demyelinating disease is improved during breastfeeding. Breastfeeding for at least 2 months resulted in significantly lowered relapses during the first 6 months postpartum compared to nonbreastfeeding in MS patients [158]. Experimental evidence shows that postpartum lactation promotes the remyelination process. At the cellular level, lactation enhances the maturation of oligodendrocytes and axonal myelination in the uninjured corpus callosum of lactating mice at postpartum day 14 [157]. Lactation has been shown to have promyelinating effects in the lysolecithin-demyelinated corpus callosum of postpartum rats. Indeed, lactation increases the cell density of OPCs and the expression of the myelin markers, including CNPase and MAG, involved in the initial stage of myelin recovery, while reducing the demyelination injury [159]. The mechanism underlying these promyelinating effects of lactation is not yet known and needs further exploration.
De-/Remyelination and Postmenopause
Menopause is associated with a decline in the levels of neuroactive steroids. The decline in progesterone levels is first observed during the 30s, while estrogen levels show reduction by the late 40s [160]. Natural menopause is observed in women around the age of 50 years, and this age is not affected by the diagnosis of MS. Because MS is usually diagnosed in young adulthood, most women living with MS will undergo menopause after MS diagnosis. However, recent evidence shows an increase in late-onset MS diagnosis as well [161]. Menopause affects the course of demyelinating conditions like MS. A large-scale study has shown that women with MS have more inflammatory disease activity in terms of relapses compared to men up to the age of menopause. This however changes after the age of 50 years after which the difference disappears and the course of the disease becomes similar between men and women [162].
Conclusion
Neuroactive steroids have been studied for their potential effects on remyelination in various neurological conditions, particularly in the context of demyelinating diseases such as MS. The potential involvement of neuroactive steroids in remyelination is elucidated by the upregulated production of these hormones in the nervous system, the enhanced expression of multiple steroid receptors by neural cells following a demyelination injury, and the remission of MS in patients during such physiological states as pregnancy. Understanding the impact of each of these agents on myelinating cells requires attention to their specific mechanisms of action. It is plausible that different steroids are more effective at certain stages of the de-/remyelination processes. Therefore, research on the impact of single or combinations of neuroactive steroids (e.g., estradiol and progesterone) on different stages of de-/remyelination might be fruitful.
Statement of Ethics
An ethics statement is not applicable because this study is based exclusively on the published literature.
Conflict of Interest Statement
The authors declare no conflicts of interest.
Funding Sources
The research program on neuroactive steroids and demyelination performed in Abdeslam Mouihate’s laboratory was supported by Kuwait University Research Grants Nos. YM11/11, YM 02/16, and MY01/22.
Author Contributions
Samah Kalakh performed the bibliographic search and drafted the manuscript. Abdeslam Mouihate participated in the drafting of the paper and supervised the paper editing. Both authors approved the final manuscript.
Funding Statement
The research program on neuroactive steroids and demyelination performed in Abdeslam Mouihate’s laboratory was supported by Kuwait University Research Grants Nos. YM11/11, YM 02/16, and MY01/22.
Data Availability Statement
All data presented are included in this review.
References
- 1. Franklin RJ, Gallo V. The translational biology of remyelination: past, present, and future. Glia. 2014;62(11):1905–15. [DOI] [PubMed] [Google Scholar]
- 2. Chong SY, Chan JR. Tapping into the glial reservoir: cells committed to remaining uncommitted. J Cell Biol. 2010;188(3):305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9(11):839–55. [DOI] [PubMed] [Google Scholar]
- 4. Franklin RJ, Kotter MR. The biology of CNS remyelination: the key to therapeutic advances. J Neurol. 2008;255(Suppl 1):19–25. [DOI] [PubMed] [Google Scholar]
- 5. Domingues HS, Portugal CC, Socodato R, Relvas JB. Corrigendum: oligodendrocyte, astrocyte and microglia crosstalk in myelin development, damage, and repair. Front Cell Dev Biol. 2016;4:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Procaccini C, De Rosa V, Pucino V, Formisano L, Matarese G. Animal models of multiple sclerosis. Eur J Pharmacol. 2015;759:182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14(2):183–93. [DOI] [PubMed] [Google Scholar]
- 8. Alroughani RA, Aref HM, Bohlega SA, Dahdaleh MP, Feki I, Al Jumah MA, et al. Natalizumab treatment for multiple sclerosis: Middle East and North Africa regional recommendations for patient selection and monitoring. BMC Neurol. 2014;14:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simpson S Jr., Taylor BV. The Scandinavian paradox revisited: editorial comment on Berg-Hansen et al. ‘High prevalence and no latitude gradient of multiple sclerosis in Norway. Mult Scler. 2014;20(13):1675–7. [DOI] [PubMed] [Google Scholar]
- 10. Akhtar S, Al-Hashel JY, Alroughani R. Impact of the first Gulf war on multiple sclerosis risk in Kuwait: a quasi-experimental study. BMC Neurol. 2023;23(1):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coyle PK. What can we learn from sex differences in MS? J Pers Med. 2021;11(10):1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Breton JM, Long KLP, Barraza MK, Perloff OS, Kaufer D. Hormonal regulation of oligodendrogenesis II: implications for myelin repair. Biomolecules. 2021;11(2):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diotel N, Charlier TD, Lefebvre d’Hellencourt C, Couret D, Trudeau VL, Nicolau JC, et al. Steroid transport, local synthesis, and signaling within the brain: roles in neurogenesis, neuroprotection, and sexual behaviors. Front Neurosci. 2018;12:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Panzica GC, Melcangi RC. The endocrine nervous system: source and target for neuroactive steroids. Brain Res Rev. 2008;57(2):271–6. [DOI] [PubMed] [Google Scholar]
- 15. Giatti S, Boraso M, Melcangi RC, Viviani B. Neuroactive steroids, their metabolites, and neuroinflammation. J Mol Endocrinol. 2012;49(3):R125–34. [DOI] [PubMed] [Google Scholar]
- 16. Schumacher M, Hussain R, Gago N, Oudinet JP, Mattern C, Ghoumari AM. Progesterone synthesis in the nervous system: implications for myelination and myelin repair. Front Neurosci. 2012;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Papadopoulos V, Fan J, Zirkin B. Translocator protein (18 kDa): an update on its function in steroidogenesis. J Neuroendocrinol. 2018;30(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Melcangi RC, Garcia-Segura LM, Mensah-Nyagan AG. Neuroactive steroids: state of the art and new perspectives. Cell Mol Life Sci. 2008;65(5):777–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Robel P, Baulieu EE. Neurosteroids biosynthesis and function. Trends Endocrinol Metab. 1994;5(1):1–8. [DOI] [PubMed] [Google Scholar]
- 20. Zwain IH, Yen SS. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinology. 1999;140(8):3843–52. [DOI] [PubMed] [Google Scholar]
- 21. Sierra A, Lavaque E, Perez-Martin M, Azcoitia I, Hales DB, Garcia-Segura LM. Steroidogenic acute regulatory protein in the rat brain: cellular distribution, developmental regulation and overexpression after injury. Eur J Neurosci. 2003;18(6):1458–67. [DOI] [PubMed] [Google Scholar]
- 22. Garcia-Ovejero D, Azcoitia I, Doncarlos LL, Melcangi RC, Garcia-Segura LM. Glia-neuron crosstalk in the neuroprotective mechanisms of sex steroid hormones. Brain Res Brain Res Rev. 2005;48(2):273–86. [DOI] [PubMed] [Google Scholar]
- 23. Liere P, Liu GJ, Pianos A, Middleton RJ, Banati RB, Akwa Y. The comprehensive steroidome in complete TSPO/PBR knockout mice under basal conditions. Int J Mol Sci. 2023;24(3):2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Panzica GC, Balthazart J, Frye CA, Garcia-Segura LM, Herbison AE, Mensah-Nyagan AG, et al. Milestones on steroids and the nervous system: 10 years of basic and translational research. J Neuroendocrinol. 2012;24(1):1–15. [DOI] [PubMed] [Google Scholar]
- 25. Caruso D, Pesaresi M, Abbiati F, Calabrese D, Giatti S, Garcia-Segura LM, et al. Comparison of plasma and cerebrospinal fluid levels of neuroactive steroids with their brain, spinal cord and peripheral nerve levels in male and female rats. Psychoneuroendocrinology. 2013;38(10):2278–90. [DOI] [PubMed] [Google Scholar]
- 26. Rupprecht R. Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology. 2003;28(2):139–68. [DOI] [PubMed] [Google Scholar]
- 27. Borowicz KK, Piskorska B, Banach M, Czuczwar SJ. Neuroprotective actions of neurosteroids. Front Endocrinol. 2011;2:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vallee M, Vitiello S, Bellocchio L, Hebert-Chatelain E, Monlezun S, Martin-Garcia E, et al. Pregnenolone can protect the brain from cannabis intoxication. Science. 2014;343(6166):94–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Raux PL, Drutel G, Revest JM, Vallee M. New perspectives on the role of the neurosteroid pregnenolone as an endogenous regulator of type-1 cannabinoid receptor (CB1R) activity and function. J Neuroendocrinol. 2022;34(2):e13034. [DOI] [PubMed] [Google Scholar]
- 30. Garcia-Segura LM, Melcangi RC. Steroids and glial cell function. Glia. 2006;54(6):485–98. [DOI] [PubMed] [Google Scholar]
- 31. Faroni A, Magnaghi V. The neurosteroid allopregnanolone modulates specific functions in central and peripheral glial cells. Front Endocrinol. 2011;2:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yilmaz C, Karali K, Fodelianaki G, Gravanis A, Chavakis T, Charalampopoulos I, et al. Neurosteroids as regulators of neuroinflammation. Front Neuroendocrinol. 2019;55:100788. [DOI] [PubMed] [Google Scholar]
- 33. Garay L, Gonzalez Deniselle MC, Meyer M, Costa JJ, Lima A, Roig P, et al. Protective effects of progesterone administration on axonal pathology in mice with experimental autoimmune encephalomyelitis. Brain Res. 2009;1283:177–85. [DOI] [PubMed] [Google Scholar]
- 34. Yu HJ, Fei J, Chen XS, Cai QY, Liu HL, Liu GD, et al. Progesterone attenuates neurological behavioral deficits of experimental autoimmune encephalomyelitis through remyelination with nucleus-sublocalized Olig1 protein. Neurosci Lett. 2010;476(1):42–5. [DOI] [PubMed] [Google Scholar]
- 35. Hussain R, El-Etr M, Gaci O, Rakotomamonjy J, Macklin WB, Kumar N, et al. Progesterone and Nestorone facilitate axon remyelination: a role for progesterone receptors. Endocrinology. 2011;152(10):3820–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Luoma JI, Kelley BG, Mermelstein PG. Progesterone inhibition of voltage-gated calcium channels is a potential neuroprotective mechanism against excitotoxicity. Steroids. 2011;76(9):845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Singh M, Su C. Progesterone and neuroprotection. Horm Behav. 2013;63(2):284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tuckey RC. Progesterone synthesis by the human placenta. Placenta. 2005;26(4):273–81. [DOI] [PubMed] [Google Scholar]
- 39. Gutai JP, Meyer WJ 3rd, Kowarski AA, Migeon CJ. Twenty-four hour integrated concentrations of progesterone, 17-hydroxyprogesterone and cortisol in normal male subjects. J Clin Endocrinol Metab. 1977;44(1):116–20. [DOI] [PubMed] [Google Scholar]
- 40. Singh M, Su C. Progesterone, brain-derived neurotrophic factor and neuroprotection. Neuroscience. 2013;239:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21(1):1–56. [DOI] [PubMed] [Google Scholar]
- 42. Akwa Y, Sananes N, Gouezou M, Robel P, Baulieu EE, Le Goascogne C. Astrocytes and neurosteroids: metabolism of pregnenolone and dehydroepiandrosterone. Regulation by cell density. J Cell Biol. 1993;121(1):135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ciriza I, Carrero P, Frye CA, Garcia-Segura LM. Reduced metabolites mediate neuroprotective effects of progesterone in the adult rat hippocampus. The synthetic progestin medroxyprogesterone acetate (Provera) is not neuroprotective. J Neurobiol. 2006;66(9):916–28. [DOI] [PubMed] [Google Scholar]
- 44. Germelli L, Da Pozzo E, Giacomelli C, Tremolanti C, Marchetti L, Wetzel CH, et al. De novo neurosteroidogenesis in human microglia: involvement of the 18 kDa translocator protein. Int J Mol Sci. 2021;22(6):3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schumacher M, Guennoun R, Ghoumari A, Massaad C, Robert F, El-Etr M, et al. Novel perspectives for progesterone in hormone replacement therapy, with special reference to the nervous system. Endocr Rev. 2007;28(4):387–439. [DOI] [PubMed] [Google Scholar]
- 46. Koenig HL, Schumacher M, Ferzaz B, Thi AN, Ressouches A, Guennoun R, et al. Progesterone synthesis and myelin formation by Schwann cells. Science. 1995;268(5216):1500–3. [DOI] [PubMed] [Google Scholar]
- 47. Jung-Testas I, Schumacher M, Robel P, Baulieu EE. The neurosteroid progesterone increases the expression of myelin proteins (MBP and CNPase) in rat oligodendrocytes in primary culture. Cell Mol Neurobiol. 1996;16(3):439–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jung-Testas I, Baulieu EE. Steroid hormone receptors and steroid action in rat glial cells of the central and peripheral nervous system. J Steroid Biochem Mol Biol. 1998;65(1–6):243–51. [DOI] [PubMed] [Google Scholar]
- 49. Marin-Husstege M, Muggironi M, Raban D, Skoff RP, Casaccia-Bonnefil P. Oligodendrocyte progenitor proliferation and maturation is differentially regulated by male and female sex steroid hormones. Dev Neurosci. 2004;26(2–4):245–54. [DOI] [PubMed] [Google Scholar]
- 50. Ghoumari AM, Baulieu EE, Schumacher M. Progesterone increases oligodendroglial cell proliferation in rat cerebellar slice cultures. Neuroscience. 2005;135(1):47–58. [DOI] [PubMed] [Google Scholar]
- 51. Ibanez C, Shields SA, El-Etr M, Baulieu EE, Schumacher M, Franklin RJ. Systemic progesterone administration results in a partial reversal of the age-associated decline in CNS remyelination following toxin-induced demyelination in male rats. Neuropathol Appl Neurobiol. 2004;30(1):80–9. [DOI] [PubMed] [Google Scholar]
- 52. Labombarda F, Gonzalez S, Gonzalez Deniselle MC, Garay L, Guennoun R, Schumacher M, et al. Progesterone increases the expression of myelin basic protein and the number of cells showing NG2 immunostaining in the lesioned spinal cord. J Neurotrauma. 2006;23(2):181–92. [DOI] [PubMed] [Google Scholar]
- 53. Labombarda F, Gonzalez SL, Lima A, Roig P, Guennoun R, Schumacher M, et al. Effects of progesterone on oligodendrocyte progenitors, oligodendrocyte transcription factors, and myelin proteins following spinal cord injury. Glia. 2009;57(8):884–97. [DOI] [PubMed] [Google Scholar]
- 54. Garay L, Gonzalez Deniselle MC, Lima A, Roig P, De Nicola AF. Effects of progesterone in the spinal cord of a mouse model of multiple sclerosis. J Steroid Biochem Mol Biol. 2007;107(3–5):228–37. [DOI] [PubMed] [Google Scholar]
- 55. Yates MA, Li Y, Chlebeck P, Proctor T, Vandenbark AA, Offner H. Progesterone treatment reduces disease severity and increases IL-10 in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2010;220(1–2):136–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hoffman GE, Le WW, Murphy AZ, Koski CL. Divergent effects of ovarian steroids on neuronal survival during experimental allergic encephalitis in Lewis rats. Exp Neurol. 2001;171(2):272–84. [DOI] [PubMed] [Google Scholar]
- 57. Acs P, Kipp M, Norkute A, Johann S, Clarner T, Braun A, et al. 17beta-estradiol and progesterone prevent cuprizone provoked demyelination of corpus callosum in male mice. Glia. 2009;57(8):807–14. [DOI] [PubMed] [Google Scholar]
- 58. Kashani IRP, Hedayatpour AP, Pasbakhsh PP, Kafami LP, Khallaghi BM, Malek FM. Progesterone enhanced remyelination in the mouse corpus callosum after cuprizone induced demyelination. Iran J Med Sci. 2015;40(6):507–14. [PMC free article] [PubMed] [Google Scholar]
- 59. El-Etr M, Rame M, Boucher C, Ghoumari AM, Kumar N, Liere P, et al. Progesterone and nestorone promote myelin regeneration in chronic demyelinating lesions of corpus callosum and cerebral cortex. Glia. 2015;63(1):104–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Aryanpour R, Pasbakhsh P, Zibara K, Namjoo Z, Beigi Boroujeni F, Shahbeigi S, et al. Progesterone therapy induces an M1 to M2 switch in microglia phenotype and suppresses NLRP3 inflammasome in a cuprizone-induced demyelination mouse model. Int Immunopharmacol. 2017;51:131–9. [DOI] [PubMed] [Google Scholar]
- 61. El-Etr M, Akwa Y, Rame M, Schumacher M, Sitruk-Ware R. Nestorone®, a 19nor-progesterone derivative boosts remyelination in an animal model of demyelination. CNS Neurosci Ther. 2021;27(4):464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ghoumari AM, Ibanez C, El-Etr M, Leclerc P, Eychenne B, O’Malley BW, et al. Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. J Neurochem. 2003;86(4):848–59. [DOI] [PubMed] [Google Scholar]
- 63. Ahmad I, Lope-Piedrafita S, Bi X, Hicks C, Yao Y, Yu C, et al. Allopregnanolone treatment, both as a single injection or repetitively, delays demyelination and enhances survival of Niemann-Pick C mice. J Neurosci Res. 2005;82(6):811–21. [DOI] [PubMed] [Google Scholar]
- 64. Brinton RD. Neurosteroids as regenerative agents in the brain: therapeutic implications. Nat Rev Endocrinol. 2013;9(4):241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gago N, El-Etr M, Sananes N, Cadepond F, Samuel D, Avellana-Adalid V, et al. 3alpha,5alpha-Tetrahydroprogesterone (allopregnanolone) and gamma-aminobutyric acid: autocrine/paracrine interactions in the control of neonatal PSA-NCAM+ progenitor proliferation. J Neurosci Res. 2004;78(6):770–83. [DOI] [PubMed] [Google Scholar]
- 66. Noorbakhsh F, Ellestad KK, Maingat F, Warren KG, Han MH, Steinman L, et al. Impaired neurosteroid synthesis in multiple sclerosis. Brain. 2011;134(Pt 9):2703–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Reyes-Garcia MG, Hernandez-Hernandez F, Hernandez-Tellez B, Garcia-Tamayo F. GABA (A) receptor subunits RNA expression in mice peritoneal macrophages modulate their IL-6/IL-12 production. J Neuroimmunol. 2007;188(1–2):64–8. [DOI] [PubMed] [Google Scholar]
- 68. Kalakh S, Mouihate A. Enhanced remyelination during late pregnancy: involvement of the GABAergic system. Sci Rep. 2019;9(1):7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mouihate A, Kalakh S. Ganaxolone enhances microglial clearance activity and promotes remyelination in focal demyelination in the corpus callosum of ovariectomized rats. CNS Neurosci Ther. 2020;26(2):240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ikeda Y, Aihara K, Yoshida S, Akaike M, Matsumoto T. Effects of androgens on cardiovascular remodeling. J Endocrinol. 2012;214(1):1–10. [DOI] [PubMed] [Google Scholar]
- 71. Otto-Duessel M, He M, Jones JO. Tissue-selective regulation of androgen-responsive genes. Endocr Res. 2012;37(4):203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Palaszynski KM, Loo KK, Ashouri JF, Liu HB, Voskuhl RR. Androgens are protective in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J Neuroimmunol. 2004;146(1–2):144–52. [DOI] [PubMed] [Google Scholar]
- 73. Spence RD, Voskuhl RR. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front Neuroendocrinol. 2012;33(1):105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23(8):963–87. [DOI] [PubMed] [Google Scholar]
- 75. Jellinck PH, Kaufmann M, Gottfried-Blackmore A, McEwen BS, Jones G, Bulloch K. Selective conversion by microglia of dehydroepiandrosterone to 5-androstenediol-A steroid with inherent estrogenic properties. J Steroid Biochem Mol Biol. 2007;107(3–5):156–62. [DOI] [PubMed] [Google Scholar]
- 76. Prayer D, Roberts T, Barkovich AJ, Prayer L, Kucharczyk J, Moseley M, et al. Diffusion-weighted MRI of myelination in the rat brain following treatment with gonadal hormones. Neuroradiology. 1997;39(5):320–5. [DOI] [PubMed] [Google Scholar]
- 77. Fargo KN, Foster AM, Sengelaub DR. Neuroprotective effect of testosterone treatment on motoneuron recruitment following the death of nearby motoneurons. Dev Neurobiol. 2009;69(12):825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev. 2007;28(7):778–808. [DOI] [PubMed] [Google Scholar]
- 79. Patel R, Moore S, Crawford DK, Hannsun G, Sasidhar MV, Tan K, et al. Attenuation of corpus callosum axon myelination and remyelination in the absence of circulating sex hormones. Brain Pathol. 2013;23(4):462–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hussain R, Ghoumari AM, Bielecki B, Steibel J, Boehm N, Liere P, et al. The neural androgen receptor: a therapeutic target for myelin repair in chronic demyelination. Brain. 2013;136(Pt 1):132–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Matejuk A, Hopke C, Vandenbark AA, Hurn PD, Offner H. Middle-age male mice have increased severity of experimental autoimmune encephalomyelitis and are unresponsive to testosterone therapy. J Immunol. 2005;174(4):2387–95. [DOI] [PubMed] [Google Scholar]
- 82. Bielecki B, Mattern C, Ghoumari AM, Javaid S, Smietanka K, Abi Ghanem C, et al. Unexpected central role of the androgen receptor in the spontaneous regeneration of myelin. Proc Natl Acad Sci USA. 2016;113(51):14829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zahaf A, Kassoussi A, Hutteau-Hamel T, Mellouk A, Marie C, Zoupi L, et al. Androgens show sex-dependent differences in myelination in immune and non-immune murine models of CNS demyelination. Nat Commun. 2023;14(1):1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Garcia-Segura LM, Veiga S, Sierra A, Melcangi RC, Azcoitia I. Aromatase: a neuroprotective enzyme. Prog Neurobiol. 2003;71(1):31–41. [DOI] [PubMed] [Google Scholar]
- 85. Askew EB, Gampe RT Jr., Stanley TB, Faggart JL, Wilson EM. Modulation of androgen receptor activation function 2 by testosterone and dihydrotestosterone. J Biol Chem. 2007;282(35):25801–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kemppainen JA, Langley E, Wong CI, Bobseine K, Kelce WR, Wilson EM. Distinguishing androgen receptor agonists and antagonists: distinct mechanisms of activation by medroxyprogesterone acetate and dihydrotestosterone. Mol Endocrinol. 1999;13(3):440–54. [DOI] [PubMed] [Google Scholar]
- 87. Ghayee HK, Auchus RJ. Basic concepts and recent developments in human steroid hormone biosynthesis. Rev Endocr Metab Disord. 2007;8(4):289–300. [DOI] [PubMed] [Google Scholar]
- 88. Du C, Guan Q, Khalil MW, Sriram S. Stimulation of Th2 response by high doses of dehydroepiandrosterone in KLH-primed splenocytes. Exp Biol Med. 2001;226(11):1051–60. [DOI] [PubMed] [Google Scholar]
- 89. Gudemez E, Ozer K, Cunningham B, Siemionow K, Browne E, Siemionow M. Dehydroepiandrosterone as an enhancer of functional recovery following crush injury to rat sciatic nerve. Microsurgery. 2002;22(6):234–41. [DOI] [PubMed] [Google Scholar]
- 90. Tan XD, Dou YC, Shi CW, Duan RS, Sun RP. Administration of dehydroepiandrosterone ameliorates experimental autoimmune neuritis in Lewis rats. J Neuroimmunol. 2009;207(1–2):39–44. [DOI] [PubMed] [Google Scholar]
- 91. Labrie F, Belanger A, Luu-The V, Labrie C, Simard J, Cusan L, et al. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids. 1998;63(5–6):322–8. [DOI] [PubMed] [Google Scholar]
- 92. Iruthayanathan M, Zhou YH, Childs GV. Dehydroepiandrosterone restoration of growth hormone gene expression in aging female rats, in vivo and in vitro: evidence for actions via estrogen receptors. Endocrinology. 2005;146(12):5176–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pesaresi M, Maschi O, Giatti S, Garcia-Segura LM, Caruso D, Melcangi RC. Sex differences in neuroactive steroid levels in the nervous system of diabetic and non-diabetic rats. Horm Behav. 2010;57(1):46–55. [DOI] [PubMed] [Google Scholar]
- 94. Regelson W, Kalimi M. Dehydroepiandrosterone (DHEA): the multifunctional steroid. II. Effects on the CNS, cell proliferation, metabolic and vascular, clinical and other effects. Mechanism of action? Ann N Y Acad Sci. 1994;719:564–75. [DOI] [PubMed] [Google Scholar]
- 95. Schmidt M, Kreutz M, Loffler G, Scholmerich J, Straub RH. Conversion of dehydroepiandrosterone to downstream steroid hormones in macrophages. J Endocrinol. 2000;164(2):161–9. [DOI] [PubMed] [Google Scholar]
- 96. Kalakh S, Mouihate A. The promyelinating properties of androstenediol in gliotoxin-induced demyelination in rat corpus callosum. Neuropathol Appl Neurobiol. 2015;41(7):964–82. [DOI] [PubMed] [Google Scholar]
- 97. Saijo K, Collier JG, Li AC, Katzenellenbogen JA, Glass CK. An ADIOL-ERβ-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell. 2011;145(4):584–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Weill-Engerer S, David JP, Sazdovitch V, Liere P, Schumacher M, Delacourte A, et al. In vitro metabolism of dehydroepiandrosterone (DHEA) to 7alpha-hydroxy-DHEA and Delta5-androstene-3beta,17beta-diol in specific regions of the aging brain from Alzheimer’s and non-demented patients. Brain Res. 2003;969(1–2):117–25. [DOI] [PubMed] [Google Scholar]
- 99. Moeller G, Adamski J. Integrated view on 17beta-hydroxysteroid dehydrogenases. Mol Cell Endocrinol. 2009;301(1–2):7–19. [DOI] [PubMed] [Google Scholar]
- 100. Tsutsui K, Matsunaga M, Miyabara H, Ukena K. Neurosteroid biosynthesis in the quail brain: a review. J Exp Zool Comp Exp Biol. 2006;305(9):733–42. [DOI] [PubMed] [Google Scholar]
- 101. Gottfried-Blackmore A, Sierra A, Jellinck PH, McEwen BS, Bulloch K. Brain microglia express steroid-converting enzymes in the mouse. J Steroid Biochem Mol Biol. 2008;109(1–2):96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Crawford AH, Chambers C, Franklin RJ. Remyelination: the true regeneration of the central nervous system. J Comp Pathol. 2013;149(2–3):242–54. [DOI] [PubMed] [Google Scholar]
- 103. Hackenberg R, Turgetto I, Filmer A, Schulz KD. Estrogen and androgen receptor mediated stimulation and inhibition of proliferation by androst-5-ene-3 beta,17 beta-diol in human mammary cancer cells. J Steroid Biochem Mol Biol. 1993;46(5):597–603. [DOI] [PubMed] [Google Scholar]
- 104. Kim JK, Levin ER. Estrogen signaling in the cardiovascular system. Nucl Recept Signal. 2006;4:e013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Barha CK, Galea LA. Influence of different estrogens on neuroplasticity and cognition in the hippocampus. Biochim Biophys Acta. 2010;1800(10):1056–67. [DOI] [PubMed] [Google Scholar]
- 106. Azcoitia I, Yague JG, Garcia-Segura LM. Estradiol synthesis within the human brain. Neuroscience. 2011;191:139–47. [DOI] [PubMed] [Google Scholar]
- 107. Mellon SH, Griffin LD, Compagnone NA. Biosynthesis and action of neurosteroids. Brain Res Brain Res Rev. 2001;37(1–3):3–12. [DOI] [PubMed] [Google Scholar]
- 108. Hess RA. Estrogen in the adult male reproductive tract: a review. Reprod Biol Endocrinol. 2003;1:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gago N, Akwa Y, Sananes N, Guennoun R, Baulieu EE, El-Etr M, et al. Progesterone and the oligodendroglial lineage: stage-dependent biosynthesis and metabolism. Glia. 2001;36(3):295–308. [DOI] [PubMed] [Google Scholar]
- 110. Curry JJ 3rd, Heim LM. Brain myelination after neonatal administration of oestradiol. Nature. 1966;209(5026):915–6. [DOI] [PubMed] [Google Scholar]
- 111. Gerstner B, Sifringer M, Dzietko M, Schuller A, Lee J, Simons S, et al. Estradiol attenuates hyperoxia-induced cell death in the developing white matter. Ann Neurol. 2007;61(6):562–73. [DOI] [PubMed] [Google Scholar]
- 112. Gerstner B, Lee J, DeSilva TM, Jensen FE, Volpe JJ, Rosenberg PA. 17beta-estradiol protects against hypoxic/ischemic white matter damage in the neonatal rat brain. J Neurosci Res. 2009;87(9):2078–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Taylor LC, Gilmore W, Ting JP, Matsushima GK. Cuprizone induces similar demyelination in male and female C57BL/6 mice and results in disruption of the estrous cycle. J Neurosci Res. 2010;88(2):391–402. [DOI] [PubMed] [Google Scholar]
- 114. Aryanpour R, Zibara K, Pasbakhsh P, Jame’ei SB, Namjoo Z, Ghanbari A, et al. 17β-Estradiol reduces demyelination in cuprizone-fed mice by promoting M2 microglia polarity and regulating NLRP3 inflammasome. Neuroscience. 2021;463:116–27. [DOI] [PubMed] [Google Scholar]
- 115. Arevalo MA, Santos-Galindo M, Bellini MJ, Azcoitia I, Garcia-Segura LM. Actions of estrogens on glial cells: implications for neuroprotection. Biochim Biophys Acta. 2010;1800(10):1106–12. [DOI] [PubMed] [Google Scholar]
- 116. Tiwari-Woodruff S, Morales LB, Lee R, Voskuhl RR. Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER)alpha and ERbeta ligand treatment. Proc Natl Acad Sci U S A. 2007;104(37):14813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Takao T, Flint N, Lee L, Ying X, Merrill J, Chandross KJ. 17beta-estradiol protects oligodendrocytes from cytotoxicity induced cell death. J Neurochem. 2004;89(3):660–73. [DOI] [PubMed] [Google Scholar]
- 118. Crawford DK, Mangiardi M, Song B, Patel R, Du S, Sofroniew MV, et al. Oestrogen receptor beta ligand: a novel treatment to enhance endogenous functional remyelination. Brain. 2010;133(10):2999–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Hardy RS, Raza K, Cooper MS. Endogenous glucocorticoids in inflammation: contributions of systemic and local responses. Swiss Med Wkly. 2012;142:w13650. [DOI] [PubMed] [Google Scholar]
- 120. Elenkov IJ, Webster EL, Torpy DJ, Chrousos GP. Stress, corticotropin-releasing hormone, glucocorticoids, and the immune/inflammatory response: acute and chronic effects. Ann N Y Acad Sci. 1999;876:1–13; discussion -3. [DOI] [PubMed] [Google Scholar]
- 121. Tischner D, Reichardt HM. Glucocorticoids in the control of neuroinflammation. Mol Cell Endocrinol. 2007;275(1–2):62–70. [DOI] [PubMed] [Google Scholar]
- 122. Rosen J, Miner JN. The search for safer glucocorticoid receptor ligands. Endocr Rev. 2005;26(3):452–64. [DOI] [PubMed] [Google Scholar]
- 123. Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, Bulloch K. Steroid hormone receptor expression and function in microglia. Glia. 2008;56(6):659–74. [DOI] [PubMed] [Google Scholar]
- 124. Clarner T, Parabucki A, Beyer C, Kipp M. Corticosteroids impair remyelination in the corpus callosum of cuprizone-treated mice. J Neuroendocrinol. 2011;23(7):601–11. [DOI] [PubMed] [Google Scholar]
- 125. Alonso G. Prolonged corticosterone treatment of adult rats inhibits the proliferation of oligodendrocyte progenitors present throughout white and gray matter regions of the brain. Glia. 2000;31(3):219–31. [DOI] [PubMed] [Google Scholar]
- 126. Wyrwoll CS, Holmes MC, Seckl JR. 11β-hydroxysteroid dehydrogenases and the brain: from zero to hero, a decade of progress. Front Neuroendocrinol. 2011;32(3):265–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Higo S, Hojo Y, Ishii H, Komatsuzaki Y, Ooishi Y, Murakami G, et al. Endogenous synthesis of corticosteroids in the hippocampus. PLoS One. 2011;6(7):e21631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Gomez-Sanchez EP, Ahmad N, Romero DG, Gomez-Sanchez CE. Is aldosterone synthesized within the rat brain? Am J Physiol Endocrinol Metab. 2005;288(2):E342–6. [DOI] [PubMed] [Google Scholar]
- 129. Gomez-Sanchez EP, Zhou M, Gomez-Sanchez CE. Mineralocorticoids, salt and high blood pressure. Steroids. 1996;61(4):184–8. [DOI] [PubMed] [Google Scholar]
- 130. Gomez-Sanchez EP, Gomez-Sanchez CE. First there was one, then two.why more 11beta-hydroxysteroid dehydrogenases? Endocrinology. 1997;138(12):5087–8. [DOI] [PubMed] [Google Scholar]
- 131. Mellon SH. Neurosteroid regulation of central nervous system development. Pharmacol Ther. 2007;116(1):107–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ibanez C, Shields SA, El-Etr M, Leonelli E, Magnaghi V, Li WW, et al. Steroids and the reversal of age-associated changes in myelination and remyelination. Prog Neurobiol. 2003;71(1):49–56. [DOI] [PubMed] [Google Scholar]
- 133. Taves MD, Ma C, Heimovics SA, Saldanha CJ, Soma KK. Measurement of steroid concentrations in brain tissue: methodological considerations. Front Endocrinol. 2011;2:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chesik D, De Keyser J. Progesterone and dexamethasone differentially regulate the IGF-system in glial cells. Neurosci Lett. 2010;468(3):178–82. [DOI] [PubMed] [Google Scholar]
- 135. Kumar S, Cole R, Chiappelli F, de Vellis J. Differential regulation of oligodendrocyte markers by glucocorticoids: post-transcriptional regulation of both proteolipid protein and myelin basic protein and transcriptional regulation of glycerol phosphate dehydrogenase. Proc Natl Acad Sci U S A. 1989;86(17):6807–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Barres BA, Lazar MA, Raff MC. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994;120(5):1097–108. [DOI] [PubMed] [Google Scholar]
- 137. Garcia-Segura LM, Chowen JA, Naftolin F. Endocrine glia: roles of glial cells in the brain actions of steroid and thyroid hormones and in the regulation of hormone secretion. Front Neuroendocrinol. 1996;17(2):180–211. [DOI] [PubMed] [Google Scholar]
- 138. Chan JR, Phillips LJ 2nd, Glaser M. Glucocorticoids and progestins signal the initiation and enhance the rate of myelin formation. Proc Natl Acad Sci U S A. 1998;95(18):10459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Chari DM, Zhao C, Kotter MR, Blakemore WF, Franklin RJ. Corticosteroids delay remyelination of experimental demyelination in the rodent central nervous system. J Neurosci Res. 2006;83(4):594–605. [DOI] [PubMed] [Google Scholar]
- 140. Lee JM, Yan P, Xiao Q, Chen S, Lee KY, Hsu CY, et al. Methylprednisolone protects oligodendrocytes but not neurons after spinal cord injury. J Neurosci. 2008;28(12):3141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. MacPhee IA, Antoni FA, Mason DW. Spontaneous recovery of rats from experimental allergic encephalomyelitis is dependent on regulation of the immune system by endogenous adrenal corticosteroids. J Exp Med. 1989;169(2):431–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Schweingruber N, Reichardt SD, Luhder F, Reichardt HM. Mechanisms of glucocorticoids in the control of neuroinflammation. J Neuroendocrinol. 2012;24(1):174–82. [DOI] [PubMed] [Google Scholar]
- 143. Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335(1):2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Zorgdrager A, De Keyser J. The premenstrual period and exacerbations in multiple sclerosis. Eur Neurol. 2002;48(4):204–6. [DOI] [PubMed] [Google Scholar]
- 145. Holmqvist P, Wallberg M, Hammar M, Landtblom AM, Brynhildsen J. Symptoms of multiple sclerosis in women in relation to sex steroid exposure. Maturitas. 2006;54(2):149–53. [DOI] [PubMed] [Google Scholar]
- 146. Haddady S, Low HP, Billings-Gagliardi S, Riskind PN, Schwartz WJ. Pregnancy modulates precursor cell proliferation in a murine model of focal demyelination. Neuroscience. 2010;167(3):656–64. [DOI] [PubMed] [Google Scholar]
- 147. Bove R, Musallam A, Healy BC, Raghavan K, Glanz BI, Bakshi R, et al. Low testosterone is associated with disability in men with multiple sclerosis. Mult Scler. 2014;20(12):1584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med. 1998;339(5):285–91. [DOI] [PubMed] [Google Scholar]
- 149. Patas K, Engler JB, Friese MA, Gold SM. Pregnancy and multiple sclerosis: feto-maternal immune cross talk and its implications for disease activity. J Reprod Immunol. 2013;97(1):140–6. [DOI] [PubMed] [Google Scholar]
- 150. Paty DW, Li DK. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group. Neurology. 1993;43(4):662–7. [DOI] [PubMed] [Google Scholar]
- 151. Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995;45(7):1268–76. [DOI] [PubMed] [Google Scholar]
- 152. Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol. 1996;39(3):285–94. [DOI] [PubMed] [Google Scholar]
- 153. Airas L, Kaaja R. Pregnancy and multiple sclerosis. Obstet Med. 2012;5(3):94–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Westwood FR. The female rat reproductive cycle: a practical histological guide to staging. Toxicol Pathol. 2008;36(3):375–84. [DOI] [PubMed] [Google Scholar]
- 155. Langer-Gould A, Garren H, Slansky A, Ruiz PJ, Steinman L. Late pregnancy suppresses relapses in experimental autoimmune encephalomyelitis: evidence for a suppressive pregnancy-related serum factor. J Immunol. 2002;169(2):1084–91. [DOI] [PubMed] [Google Scholar]
- 156. McClain RM, Wolz E, Davidovich A, Edwards J, Bausch J. Reproductive safety studies with genistein in rats. Food Chem Toxicol. 2007;45(8):1319–32. [DOI] [PubMed] [Google Scholar]
- 157. Gregg C, Shikar V, Larsen P, Mak G, Chojnacki A, Yong VW, et al. White matter plasticity and enhanced remyelination in the maternal CNS. J Neurosci. 2007;27(8):1812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Hellwig K, Rockhoff M, Herbstritt S, Borisow N, Haghikia A, Elias-Hamp B, et al. Exclusive breastfeeding and the effect on postpartum multiple sclerosis relapses. JAMA Neurol. 2015;72(10):1132–8. [DOI] [PubMed] [Google Scholar]
- 159. Mouihate A, Kalakh S. Breastfeeding promotes oligodendrocyte precursor cells division and myelination in the demyelinated corpus callosum. Brain Res. 2023;1821:148584. [DOI] [PubMed] [Google Scholar]
- 160. Ferrell RJ, O'Connor KA, Rodriguez G, Gorrindo T, Holman DJ, Brindle E, et al. Monitoring reproductive aging in a 5-year prospective study: aggregate and individual changes in steroid hormones and menstrual cycle lengths with age. Menopause. 2005;12(5):567–77. [DOI] [PubMed] [Google Scholar]
- 161. Koch-Henriksen N, Thygesen LC, Stenager E, Laursen B, Magyari M. Incidence of MS has increased markedly over six decades in Denmark particularly with late onset and in women. Neurology. 2018;90(22):e1954–63. [DOI] [PubMed] [Google Scholar]
- 162. Magyari M, Koch-Henriksen N. Quantitative effect of sex on disease activity and disability accumulation in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2022;93(7):716–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data presented are included in this review.