Abstract
Background and Objectives
Serum neurofilament light chain (sNfL) levels correlate with multiple sclerosis (MS) disease activity, but the dynamics of this correlation are unknown. We evaluated the relationship between sNfL levels and radiologic MS disease activity through monthly assessments during the 24-week natalizumab treatment interruption period in RESTORE (NCT01071083).
Methods
In the RESTORE trial, participants with relapsing forms of MS who had received natalizumab for ≥12 months were randomized to either continue or stop natalizumab and followed with MRI and blood draws every 4 weeks to week 28 and again at week 52 The sNfL was measured, and its dynamics were correlated with the development of gadolinium-enhancing (Gd+) lesions. Log-linear trend in sNfL levels were modeled longitudinally using generalized estimating equations with robust variance estimator from baseline to week 28.
Results
Of 175 patients enrolled in RESTORE, 166 had serum samples for analysis. Participants with Gd+ lesions were younger (37.7 vs 43.1, p = 0.001) and had lower Expanded Disability Status Scale scores at baseline (2.7 vs 3.4, p = 0.017) than participants without Gd+ lesions. sNfL levels increased in participants with Gd+ lesions (n = 65) compared with those without (n = 101, mean change from baseline to maximum sNfL value, 12.1 vs 3.2 pg/mL, respectively; p = 0.003). As the number of Gd+ lesions increased, peak median sNfL change also increased by 1.4, 3.0, 4.3, and 19.6 pg/mL in the Gd+ lesion groups of 1 (n = 12), 2–3 (n = 18), 4–9 (n = 21), and ≥10 (n = 14) lesions, respectively. However, 46 of 65 (71%) participants with Gd+ lesions did not increase above the 95th percentile threshold of the group without Gd+ lesions. The initial increase of sNfL typically trailed the first observation of Gd+ lesions, and the peak increase in sNfL was a median [interquartile range] of 8 [0, 12] weeks after the first appearance of the Gd+ lesion.
Discussion
Although sNfL correlated with the presence of Gd+ lesions, most participants with Gd+ lesions did not have elevations in sNfL levels. These observations have implications for the use and interpretation of sNfL as a biomarker for monitoring MS disease activity in controlled trials and clinical practice.
Introduction
MRI is a sensitive measure of focal CNS inflammation that has been validated in international consensus guidelines as a useful prognostic tool in multiple sclerosis (MS).1,2 However, MRI is expensive and cumbersome, reducing its typical use to intervals of 6 months or longer.3 Hence, a blood-based biomarker that identifies patients with inflammatory activity could help monitor disease activity more frequently and assess the need for a change in treatment or other management.
Neurofilaments are a structural protein specific to neurons that comprise 85% of the CNS cytoskeleton proteins.4 When released by injured neurons, neurofilaments pass into the CSF and eventually the serum.5 Neurofilaments are very stable, which makes them an attractive fluid-based biomarker to measure injury in many neurologic conditions.5 In patients with MS, increased serum neurofilament light chain (sNfL) levels correlate with MS disease activity,6-9 may predict worse long-term clinical and MRI outcomes,9 and are reduced by disease-modifying treatments.7,8,10 However, the temporal dynamics of sNfL, particularly their relationship with fluctuations in disease activity measured on MRI, remain unknown.
The RESTORE trial (NCT01071083)11 was an exploratory, randomized, partially placebo-controlled study with the objective of exploring the course of MS disease activity and the effects of pharmacokinetic and pharmacodynamic parameters in participants undergoing interruption of natalizumab therapy for up to 24 weeks compared with those remaining on natalizumab.11 The unique study design of RESTORE involved monthly serum sampling and MRI and afforded the opportunity to correlate MRI disease activity during the planned 24-week natalizumab dosage interruption and blood-based biomarkers such as sNfL.12-15
Understanding the temporal relationship between sNfL levels and gadolinium-enhancing (Gd+) lesions provides insight into the interaction between inflammation and neuroaxonal damage, as measured by MRI and sNfL, and the relative time course of new lesions on MRI and changes in sNfL levels. Therefore, this analysis will inform how sNfL might be used as a biomarker for monitoring disease activity in patients with MS.
Methods
Study Design and Participants
We conducted a post hoc analysis on a subset of participants with MS enrolled in RESTORE11 (NCT01071083) who had serum samples available for sNfL measurements at 4-week time intervals corresponding to MRI acquisition. The RESTORE study design and results were reported previously.11 Briefly, RESTORE enrolled patients aged 18–60 years with relapsing forms of MS who had been treated with natalizumab for ≥12 months before randomization and without relapses during those 12 months. Participants were excluded if, during screening, the presence of Gd+ lesions and antinatalizumab antibodies were detected, if they had a history of significant infectious illness or of significant disease other than MS, and if they were unable to undergo monthly MRI scans for 6 months.
Enrolled participants had received natalizumab (Tysabri) for ≥1 year and had no relapses in the previous year. Participants were randomized (1:1:2) to continue natalizumab, switch to placebo, or switch to other treatments (intramuscular interferon-β-1a, glatiramer acetate), or intravenous methylprednisolone [MP] 1 g every 4 weeks starting 12 weeks after randomization) for 24 weeks and then switch back to natalizumab for the follow-up period (weeks 28–52). In the other-therapies group, patients and their neurologist selected the immunomodulatory therapy on an individual basis. When deemed beneficial, MP was administered once every 4 weeks starting at week 12 of the randomized treatment period. Participants could receive a 3–5-day course of high-dose corticosteroids per local standard of care and/or restart natalizumab before week 28 if they had a protocol-defined clinical relapse or MRI disease activity per the rescue criteria and thus would immediately enter the follow-up period.
MRI was performed every 4 weeks during the randomized treatment period starting at week 0, at the time of suspected relapse, and at final visit. Some samples had missing data, but most participants had sNfL data for at least 7 of the 9 time points. MRIs were acquired using standardized protocol T2 and T1 sequences with and without Gd, as described previously.11 The number and volume of Gd+ lesions were assessed at each time point by the Central MRI Reading Center (NeuroRx Research, Montreal, Canada).
Standard Protocol Approvals, Registrations, and Patient Consents
Each site's institutional review board reviewed and approved the study protocol and amendments, and all participants provided written informed consent. The study was performed in accordance with the Declaration of Helsinki and International Conference on Harmonization Guideline on Good Clinical Practice and is registered with ClinicalTrials.gov, NCT01071083.
sNfL Assay
A sensitive single-molecule array assay (SIMOA NF-light Advantage Kit; Quanterix, Lexington, MA) was used to measure sNfL levels in sera. Detailed methodology was previously described.7 The assay was analytically validated for the fit-for-purpose sNfL evaluation. The measurements were performed in one round of experiments using a single batch of reagents. At baseline and every 4 weeks to week 28 (or at the time of rescue) and week 52, sNfL levels were measured.
Data Analysis
Descriptive statistics of sNfL levels and percentage change in sNfL from baseline were presented. Median percentage change in sNfL levels, stratified by Gd+ lesion number, was compared using the Wilcoxon rank-sum test. To evaluate the magnitude of sNfL elevations, participants who did not develop Gd+ lesions were used to calculate a 95th percentile threshold for sNfL levels and percentage change from baseline. This 95th percentile threshold was used to derive sensitivity and specificity for elevations in sNfL to predict the presence of Gd+ lesions.
As a sensitivity analysis, age adjustment was conducted as previously described (Harp et al. 2022).
Log-linear trends in sNfL levels were modeled longitudinally using generalized estimating equations (GEEs) with robust variance estimator from baseline to week 28 with the goal of identifying which predictors were most influential on sNfL. The following covariates were included: age, sex, race, body mass index, cumulative Gd+ lesion count, baseline Expanded Disability Status Scale (EDSS) score, years since diagnosis, Gd+ lesion volume, baseline creatinine clearance, relapse occurrence during the study, DMT, and 4-week visit intervals. Interactions with time were assessed but ultimately were left out of the analysis because the added complexity did not affect the overall findings. The standardized beta coefficients were also evaluated to assess the impact of each variable in terms of SD on the dependent variable. The estimate from the standardized betas translates to the impact on sNfL for every 1 SD increase for the predictor. Of the patients who experience GD+ lesions during their randomized treatment period, missing sNfL measurements comprised 9.2%, 5.4%, and 12.5% of the data during the scheduled visit immediately preceding, during, and immediately following the identification of the incident GD+ lesion, respectively, and missingness was assumed to be missing at random. GraphPad Prism 7 software (GraphPad Software, Inc.) was used for graphical representation and basic statistical results.
Data Availability
Anonymized participant data collected during the trial will be shared with qualified scientific researchers who provide a methodologically sound proposal. Proposals should be submitted through Vivli (vivli.org). To gain access, data requestors will need to sign a data sharing agreement. Data are made available for 1 year on a secure platform.
Results
Participants
Of the 175 participants randomized into the RESTORE trial, 166 had longitudinal sNfL measurements that included a baseline value and were included in this analysis. sNfL data were available for 166 participants at baseline, 130 at week 4, 125 at week 8, 153 at week 12, 141 at week 16, 104 at week 20, 100 at week 24, 152 at week 28, and 151 at week 52.
Baseline demographic and clinical characteristics of participants are presented in Table 1. Participants who did not (n = 101) and did (n = 65) develop Gd+ lesions during the study had a mean age of 43.1 ± 9.8 and 37.7 ± 9.2 years (p = 0.001) and disease duration of 9.8 ± 6.3 or 8.5 ± 5.4 years (p = 0.187), respectively. Before beginning natalizumab, 41.6% of those who did not and 41.5 who did develop Gd+ lesions during the study were considered to have high disease activity. Participants in both groups were moderately disabled at study baseline, as evidenced by a median [interquartile range] EDSS score of 3.5 [2.0, 4.0] in the group who did not and 2.8 [1.5, 3.5] in those who did develop Gd+ lesions (p = 0.024).
Table 1.
Patient Demographic and Clinical Characteristics
|
Characteristic, mean (SD) unless otherwise stated |
Stratified by Gd+ lesion during follow-up | |||
| Gd+ Neg. | Gd+ Pos. | Total | ||
| n = 101 | n = 65 | p Value | N = 166 | |
| Female (%) | 81 (80.2) | 47 (72.3) | 0.321 | 128 (77.1) |
| Age, y, mean ± SD | 43.1 ± 9.8 | 37.7 ± 9.2 | 0.001 | 41.0 ± 9.9 |
| Race, White, % | 92 (91.1) | 59 (90.8) | 0.861 | 151 (91.0) |
| EDSS score at baseline | ||||
| Mean ± SD | 3.4 ± 1.8 | 2.7 ± 1.5 | 0.017 | 3.1 ± 1.7 |
| Median [IQR] | 3.5 [2.0, 4.0] | 2.8 [1.5, 3.5] | 0.024 | 3.0 [2.0, 4.0] |
| Disease duration, y, mean ± SD | 9.8 ± 6.3 | 8.5 ± 5.4 | 0.187 | 9.3 ± 5.96 |
| No. of cumulative Gd+ lesions during follow-up, mean ± SD | 0.0a | 6.86 ± 9.4 | <0.001 | 2.69 ± 6.8 |
| Weeks until NfL peak after Gd+ lesion | ||||
| Mean ± SD | — | 8.9 ± 14.3 | — | — |
| Median [IQR] | — | 8 [0, 12] | — | — |
| Baseline creatinine clearance, µmol/L, mean ± SD | 69.0 ± 11.9 | 69.8 ± 12.3 | — | 69.3 ± 12.0 |
Abbreviations: EDSS = Expanded Disability Status Scale; Gd+ = gadolinium-enhancing; IQR = interquartile range.
Exclusion criteria of the RESTORE trial included any evidence of Gd-enhancement at screening.
Relationship Between sNfL Levels and Gd+ Lesion Development
The development of Gd+ lesions varied between assigned treatment cohorts. For those who discontinued natalizumab (n = 121), 56 (46.3%) remained free of Gd+ lesions and 65 (53.7%) developed 1 or more Gd+ lesions. None of the 45 participants who continued natalizumab developed Gd+ lesions. Therefore, in the current analysis, the group without Gd+ lesions consisted of 45 participants who continued on natalizumab and 56 who discontinued natalizumab (n = 101).
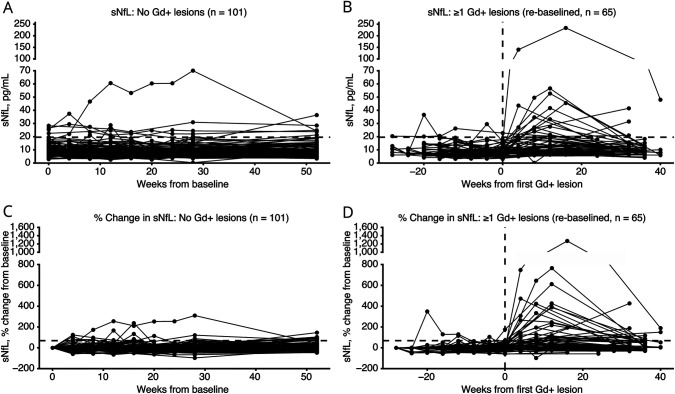
Longitudinal data for sNfL levels were examined for their relation to Gd+ lesions, irrespective of randomized treatment assignment. In the participants who developed Gd+ lesions (n = 65), the mean ± SD sNfL levels were 8.9 ± 3.9 pg/mL at baseline and increased to 20.0 ± 30.5 pg/mL at week 28 (mean difference with 95% confidence interval [CI]: 10.7 (2.77–18.58), Student t-test p = 0.009; Figure 1B and eTable 1). For participants who did not develop Gd+ lesions (n = 101), sNfL levels remained low, with a mean ± SD sNfL level at baseline of 9.7 ± 5.0 pg/mL (p = 0.267) and at 28 weeks of 10.2 ± 7.9 pg/mL (p = 0.004; Figure 1A and eTable 1). Compared with those without Gd+ lesions, participants with Gd+ lesions had significantly higher sNfL mean levels at 28 weeks, which correlated to ∼9 weeks after the first Gd+ lesion (20.0 ± 30.5 vs 10.2 ± 7.9 pg/mL; p = 0.004; eTable 1 and Figure 1, A and B). Figure 1, B and D plot sNfL from the time of first Gd+ lesion development for each participant. The mean ± SD percentage increase in sNfL from baseline to week 28 in participants who developed Gd+ lesions was 126.6% ± 232.2% vs 8.9% ± 46.2% (p < 0.001) in the participants who did not develop Gd+ lesions (Figure 1, C and D, eTable 1).
Figure 1. Timecourse of sNfL Levels According to Gd+ Lesions Status.
Individual subject sNfL levels over time among subjects without Gd+ lesions (absolute values A, percentage changes C) and with Gd+ lesions (absolute values B, percentage changes D). Horizontal dotted line in A and B represents 19.6 pg/mL change in sNfL from baseline. Horizontal dotted line in C and D represents the 68.8% change in sNfL from baseline. Vertical dotted line in B and D represents time of first detected Gd+ lesion (week 0). Gd+ = gadolinium-enhancing; sNfL = serum neurofilament light chain.
Among participants who did not develop Gd+ lesions, the 95th percentile threshold for sNfL levels and percentage change from baseline were 19.6 pg/mL and 68.8% increase from baseline. There were 5 participants who never developed Gd+ lesions and who had sNfL levels above the 95th percentile. Most were among the oldest participants in the study (ages 28, 48, 51, 58, and 59 years) and had high EDSS scores at baseline (4.0, 5.0, 6.0, 7.0, and 7.0); 4 of the 5 had disability progression without relapses during the study and 1 had a clinical relapse and spinal stenosis. None other of these 5 participants had adverse events that would be expected to increase sNfL levels (i.e., stroke, traumatic brain injury, seizure). Using these cutoffs, peak sNfL >19.6 pg/mL yielded 27.9% sensitivity and 91.8% specificity; peak sNfL 68.9% above baseline sNfL yielded 42.6% sensitivity and 82.7% specificity (Table 2).
Table 2.
Sensitivity and Specificity of sNfL Elevations Above the 95th Percentile Thresholds
| Gd+ lesion occurrence | |||
| Gd+ | No Gd+ | ||
| Peak sNfL | |||
| Above 19.6 pg/mL | 17 | 8 | PPV: 67.5% |
| Below 19.6 pg/mL | 44 | 90 | NPV: 67.7% |
| Sensitivity: 27.9% | Specificity: 91.8% | ||
| Peak sNfL % change | |||
| Above 68.8% | 26 | 17 | PPV: 59.9% |
| Below 68.8% | 35 | 81 | NPV: 70.4% |
| Sensitivity: 42.6% | Specificity: 82.7% | ||
Sensitivity: the proportion of people with Gd+ who were above the specified sNfL threshold compared with the total number of people who developed Gd+.
Specificity: the proportion of people with no Gd+ who were below the specified threshold of sNfL compared with the total number of people with no Gd+.
PPV: positive predictive value or probability that an elevation in sNfL above the identified threshold will have Gd+ lesions.
NPV: negative predictive value or the probability that the absence of an elevation in sNfL above the identified threshold will have Gd+ lesions.
Gd+ = gadolinium-enhancing; sNfL = serum neurofilament light.
Changes in sNfL Levels and Association With the Number of Gd+ Lesions
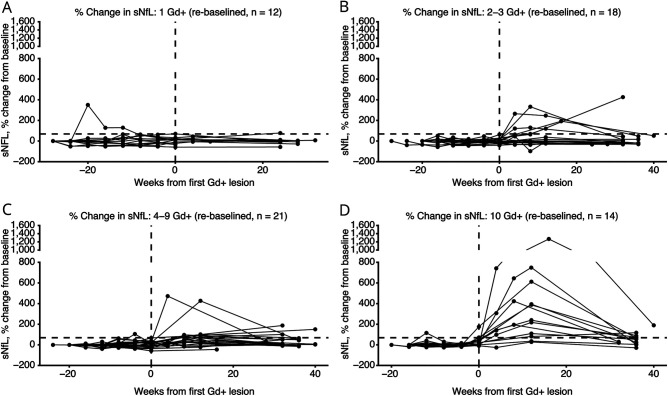
The change between baseline and peak sNfL increased among participants who developed a greater number of Gd+ lesions (Table 3). The mean sNfL percentage change increased by 48.4% (1 new Gd+ lesion, Figure 2A); 92.2% (2–3 new Gd+ lesions, Figure 2B); 103.8% (4–9 new Gd+ lesions, Figure 2C); and 371.1% (≥10 new Gd+ lesions, Figure 2D). Any increases in sNfL levels followed the initial appearance of Gd+ lesions in 80% of patients; 13 patients (20%) reached peak sNfL before the first Gd+ lesion was identified. When looking at the 95th percentile threshold, only 13.8% (n = 4/65) participants crossed the 19.6 pg/mL sNfL threshold before the presence of their first Gd+ lesion and 23.1% (n = 15/65) breached the 19.6 pg/mL sNfL threshold after their first Gd+ lesion. Within the participants who developed Gd+ lesions, 70.8% (n = 46/65) never breached the 19.6 pg/mL sNfL threshold and 10.7% (n = 7/65) did not have sNfL greater than their baseline levels. Peak sNfL was delayed by an average of 8.7 ± 14.4 weeks after first Gd+ lesion identification (Figure 1, B and D). In 9.2% (6/65) of participants with Gd+ lesions, sNfL levels were not available at week 52.
Table 3.
Maximum sNfL Increase in Participants by Maximum Number of Gd+ Lesions
| Maximum sNfL change for each group | Maximum No. of Gd+ lesions | |||||
| Gd+ lesions | 0 | >0 | 1 | 2–3 | 4–9 | ≥10 |
| n = 101 | n = 65 | n = 12 | n = 18 | n = 21 | n = 14 | |
| Mean sNfL ±SD | 3.2 ± 6.6 | 12.1 ± 28.7 | 3.9 ± 8.4 | 5.6 ± 7.8 | 5.6 ± 7.8 | 36.0 ± 56.5 |
| p-valuea | ─ | 0.003 | 0.89 | 0.58 | 0.32 | <0.001 |
| Median sNfL [IQR] | 2.1 [0.5, 4.1] | 4.10 [1.6, 12.2] | 1.4 [0.4, 4.9] | 3.0 [0.9, 8.6] | 4.3 [2.7, 7.2] | 19.5 [8.3, 35.8] |
| p-valueb | ─ | <0.001 | 0.94 | 0.31 | 0.003 | <0.001 |
| Participants with a peak sNfL ≥95th percentile of 19.6 pg/mL, n (%) | 11 (10.9) | 19 (29.2) | 2 (16.7) | 4 (22.2) | 3 (14.3) | 10 (71.4) |
| Participants with no sNfL peak > baseline, n (%) |
18 (17.8) | 7 (10.7) | 3 (25.0) | 3 (16.7) | 1 (5.0) | 0 (0.0) |
| Maximum % sNfL change for each group | Maximum No. of Gd+ lesions | |||||
| Gd+ lesions | 0 | >0 | 1 | 2–3 | 4–9 | ≥10 |
| n = 101 | n = 65 | n = 15 | n = 25 | n = 16 | n = 9 | |
| Mean ± SD | 38.2 ± 54.6 | 145.1 ± 223.1 | 48.4 ± 100.5 | 92.2 ± 130.3 | 103.8 ± 127.7 | 371.1 ± 355.2 |
| p-valuea | ─ | <0.001 | 0.79 | 0.10 | 0.04 | <0.001 |
| Median [IQR] | 22.8 [5.8, 66.9] | 63.9 [18.2, 190.0] | 20.0 [5.3, 64.6] | 36.7 [7.6, 127.7] | 68.2 [31.2, 100.7] | 239.6 [117.5, 423.6] |
| p-valueb | ─ | <0.001 | 0.73 | 0.28 | 0.003 | <0.001 |
| Participants with % change in sNfL ≥95th percentile of +68.8%, n (%) | 25 (24.8) | 29 (44.6) | 2 (16.7) | 6 (33.3) | 10 (47.6) | 11 (78.6) |
Abbreviations: Gd+ = gadolinium-enhancing; IQR = interquartile range; sNfL = serum neurofilament light chain.
t-test p-value; p-value indicates comparison with Gd+ = 0 group.
Wilcoxon rank sum test, p-value indicates comparison with Gd+ = 0 group.
Figure 2. Timecourse of Changes in sNfL Levels According to Number of Gd+ Lesions.
Proportional change in individual subject sNfL levels over time according to the number of Gd+ lesions observed in each subject. Vertical dotted line represents time of first detected Gd+ lesion; horizontal dotted line represents the 68.8% change in sNfL from baseline. Gd+ = gadolinium-enhancing; sNfL = serum neurofilament light chain.
Sensitivity analysis after adjusting for age did not significantly alter the findings (eFigure 1). Age-adjusted sNfL >10.6 pg/mL yielded 36.1% sensitivity and 91.8% specificity (eTable 2). Similarly, the age-adjusted maximum sNfL increase in participants by maximum number of Gd+ lesions changed little (eTable 3). Since age-adjustment changes the 95th percentile threshold and individual values equally, age adjustment did not change the percent elevations shown in Table 2 and Figure 1.
To explore the relationship between sNfL, baseline demographics, disease characteristics, and MRI findings, sNfL was longitudinally modeled using GEE. Models were generated for the overall and Gd+ populations with consistent findings. For participants who developed Gd+ lesions (n = 65), the number of cumulative Gd+ lesions and baseline EDSS were the only significant predictors for sNfL (eTable 4). This translated into an average of 4.2 and 6.3 percent increase in sNfL for each additional Gd+ lesion and level of EDSS worsening, respectively (p < 0.001). GEE modeling did not find DMT treatment to affect the relationship.
Discussion
There is a need for standardized fluid biomarkers that can inform the clinical practice of neurology. Serum concentrations of NfL have emerged as a promising biomarker for acute axonal injury and neurologic disease activity. As a potential measure of disease activity, sNfL could lend insight into the presence and evolution of neuroinflammatory diseases when clinical presentations of disease activity are less sensitive to tissue injury. In this study, we explored the association between sNfL levels and development of Gd+ lesions on brain MRI to better understand how sNfL might reflect the dynamics of MS focal inflammatory disease activity. As expected, average sNfL levels increased among participants who developed Gd+ lesions compared with those who did not. In general, a high number of Gd+ lesions were associated with high sNfL levels. However, increased sNfL was not consistently observed with Gd+ lesions, and indeed was particularly uncommon in patients with only a few Gd+ lesions. Even with very high Gd+ lesion levels, many participants showed no increase in sNfL that was greater than their baseline level, which may explain why Gd+ lesion volume was not a significant factor when modeling increased sNfL. In addition, large variability in sNfL increases were observed among participants, even those with similar numbers of Gd+ lesions, suggesting that there may be differing levels of tissue injury associated with each Gd+ lesion.
In general, we did not see the first increase in sNfL until after initial development of Gd+ lesions and observed a peak increase in sNfL an average of 9 weeks after the first Gd+ lesion was seen. It appears to take at least several weeks for NfL to start being released from CNS tissue with inflammatory injury and become measurable in the peripheral blood. This delay is important for clinicians to recognize when interpreting sNfL measures in clinical practice. sNfL levels remained elevated after resolution of Gd+ lesions in many participants. The time course of sNfL resolution is difficult to assess in this study because most participants had a gap in sNfL sampling between 12–16 weeks after Gd+ lesion development and the final study visit. sNfL levels remained low and stable in participants who continued on natalizumab, as well as in participants who discontinued natalizumab and did not develop Gd+ lesions. This finding is in agreement with results from a large clinical trial in secondary progressive MS, where natalizumab was shown to reduce sNfL concentrations compared with placebo, with or without evidence of acute inflammatory disease activity.16 A more recent study,17 using data from the same clinical trial, concluded that sNfL was not associated with disability progression in the absence of relapses and MRI evidence of inflammatory disease activity. Taken together, these observations suggest that sNfL could be used as a biomarker for inflammatory disease activity, but the relation to disability progression independent of relapses or new lesion formation is still controversial.
The large majority (80%) of sNfL elevations followed the appearance of the first Gd+ lesions, which led us to find sNfL a relatively specific biomarker of MRI disease activity in MS (82%–91.9% specificity), but somewhat low sensitivity (27.9%–42.6% sensitive). Analyses of age-adjusted sNfL levels yielded similar results. Through closer disease activity monitoring, sNfL might still contribute to improving outcomes in MS. Serial imaging for clinically stable patients is costly and often impractical; sNfL could be a marker of subclinical disease activity both in clinical trials that may require frequent imaging, such as phase 2 studies in relapsing MS or in routine clinical practice. However, our study highlights the limitations of sNfL for monitoring disease activity: Its elevation was delayed weeks to several months after Gd+ development and was, in this 28-week study, an insensitive marker of the acute focal inflammation observed on MRI. Another caveat is that elevated levels of sNfL could be attributed to non-MS–related complications.18
This study has limitations. As a post hoc analysis, these results should be interpreted as hypothesis-generating rather than definitive. Multiple Gd+ lesions in the same patient, sometimes developing sequentially over time and each with a potentially different contribution of sNfL, were combined within the same patient to a single sNfL value at each time point. Thus, the contribution of each Gd+ lesion to sNfL levels could not be determined. Furthermore, some Gd+ lesions may have been short-lived and not detected within 4-week MRI intervals. Since Gd+ lesions did not appear until week 12 or later of the 28-week treatment interruption period, there was limited time to explore subsequent sNfL pharmacokinetics beyond 12–16 weeks after Gd+ lesion development. The sNfL peak and subsequent resolution might have occurred at time points later than this study's follow-up or at times with missing samples. While missingness was assumed to be random, we do not have evidence for this. Since our analysis focused on the peak sNfL observed, missing sNfL measurements would, at worst, render our study conservative by underrepresenting of the true peak in sNfL.
In conclusion, approximately two-thirds of participants with the onset of new Gd+ lesions (46/65) did not have sNfL levels that crossed the 95th percentile threshold, and most sNfL elevations were after the initial appearance of Gd+ lesions. Although monthly paired MRI and serum sampling demonstrated an association between sNfL and MRI as indicators of disease activity, sNfL did not correlate closely with onset of new Gd+ lesions. These attributes need to be recognized when considering implementation of sNfL as a biomarker for MS disease activity and individual management of patients with MS.
Acknowledgment
We thank Suzanne Szak and Tatiana Plavina for their assistance in preliminary data gathering for this manuscript. Editorial support for the preparation of this manuscript was provided by Excel Scientific Solutions (Fairfield, CT, USA) and funded by Biogen. Coauthor Douglas Jeffrey, MD, died April 20, 2023.
Glossary
- EDSS
Expanded Disability Status Scale
- Gd+
gadolinium-enhancing
- GEE
generalized estimating equations
- IQR
interquartile range
- MP
methylprednisolone
- MS
multiple sclerosis
- [s]NfL
[serum] neurofilament light chain
Appendix. Authors
| Name | Location | Contribution |
| Robert J. Fox, MD | Mellen Center for Multiple Sclerosis, Neurological Institute, Cleveland Clinic, OH | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Bruce A.C. Cree, MD, PhD, MAS | Weill Institute for Neurosciences, Department of Neurology, University of California San Francisco | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Jérôme de Sèze, MD | Department of Neurology, Hôpital Civil, Strasbourg, France | Drafting/revision of the manuscript for content, including medical writing for content |
| Ralf Gold, MD | Department of Neurology, St. Josef Hospital, Ruhr University, Bochum, Germany | Drafting/revision of the manuscript for content, including medical writing for content |
| Hans-Peter Hartung, MD | Department of Neurology, Heinrich-Heine-University, Düsseldorf, Germany; Brain and Mind Center, University of Sydney, Australia; Department of Neurology, Palacky University Olomouc, Czech Republic | Drafting/revision of the manuscript for content, including medical writing for content |
| Douglas Jeffery, MD, PhD | Piedmont HealthCare, Mooresville, NC | Drafting/revision of the manuscript for content, including medical writing for content |
| Ludwig Kappos, MD | Research Center for Clinical Neuroimmunology and Neuroscience and MS Center; Departments of Head, Spine and Neuromedicine, Clinical Research and Biomedical Engineering, University Hospital and University of Basel, Switzerland; | Drafting/revision of the manuscript for content, including medical writing for content |
| Xavier Montalban, MD, PhD | Vall d’Hebron University Hospital, Barcelona, Spain | Drafting/revision of the manuscript for content, including medical writing for content |
| Bianca Weinstock-Guttman, MD | Jacobs Multiple Sclerosis Center and Pediatric Multiple Sclerosis Center of Excellence, Jacobs School of Medicine and Biomedical Sciences, Buffalo, NY | Drafting/revision of the manuscript for content, including medical writing for content |
| Carol M. Singh, BS | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content |
| Arman Altincatal, MS | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Nicholas Belviso, PharmD, PhD | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Robin L. Avila, PhD | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Pei-Ran Ho, MD | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content |
| Ray Su, PhD | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content |
| Robert Engle, PhD | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content |
| Dipen Sangurdekar, PhD | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content |
| Carl de Moor, PhD | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content |
| Elizabeth Fisher, PhD | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content |
| Bernd C. Kieseier, MD | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content |
| Richard A. Rudick, MD | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content |
Footnotes
Editorial, page e209456
Study Funding
The study was sponsored and funded by Biogen (Cambridge, MA).
Disclosure
R.J. Fox receives consulting fees from AB Science, Biogen, Bristol Myers Squibb, EMD Serono, Genentech, Genzyme, Greenwich Biosciences, Immunic, INmune Bio, Janssen, Lily, Novartis, Sanofi, Siemens, and TG Therapeutics; advisory boards for AB Science, Biogen, Immunic, Janssen, Novartis, Sanofi; and clinical trial contract and research grant funding from Biogen, Novartis, and Sanofi. B.A.C. Cree receives consulting fees from Alexion, Atara, Autobahn, Avotres, Biogen, EMD Serono, Gossamer Bio, Horizon, Neuron23, Novartis, Sanofi, TG Therapeutics; and Therini Bio; and research support from Genentech. J. de Sèze is an investigator for clinical trials for Alexion, Biogen, Chugai, Genzyme, MedDay, Merck, Novartis, Roche, and Teva; receives consultant fees from Alexion, Allergan, Almirall, Biogen, Chugai, Genzyme, Merck, Novartis, Roche, and Teva; and receives travel costs for being a congress presenter for Allergan, Alexion, Biogen, Chugai, Genzyme, Merck, Novartis, Roche, and Teva. R. Gold receives honoraria and/or research support from Bayer Healthcare, Biogen, Merck Serono, Novartis, and Teva Neuroscience. H.-P. Hartung receives consulting fees and/or steering/data monitoring committees fees from Bayer Healthcare, Biogen, BMS, Celgene, GeNeuro, Horizon Therapeutics, Merck, Novartis, Octapharma, Receptos, Roche, Sanofi-Genzyme, and TG Therapeutics. D. Jeffery is deceased. The relevant disclosures are: received research funding from Biogen and Genentech; speaker/consulting fees from Acorda, Bayer Healthcare, Biogen, Genentech, GlaxoSmithKline, Novartis, Questcor, Serono, and Teva. L. Kappos received payments to his institution (University Hospital Basel/Foundation Clinical Neuroimmunology and Neuroscience Basel) for steering committee and advisory board services for Actelion, Bayer, BMS, df-mp Molnia & Pohlmann, Celgene, Eli Lilly, EMD Serono, Genentech, Glaxo Smith Kline, Janssen, Japan Tobacco, Merck, MH Consulting, Minoryx, Novartis, F. Hofffmann-La Roche Ltd, Senda Biosciences Inc., Sanofi, Santhera, Shionogi BV, TG Therapeutics,and Wellmera; and license fees for Neurostatus UHB products; and grants from Novartis, Innosuisse, and Roche. X. Montalbán has received speaker fees and/or travel expenses for scientific meetings or steering committees and/or advisory boards for clinical trials from Actelion, Almirall, Bayer Healthcare, Biogen, Celgene, Genzyme, Merck, Novartis, Oryzon Genomics, Roche, Sanofi-Genzyme, and Teva. B. Weinstock-Guttman receives advisory boards and/or educational program fees from Biogen, Celgene, EMD Serono, Genentech, Genzyme, Novartis, Sanofi-Aventis, and Teva; and research support from Biogen, EMD Serono, Novartis, and Teva Neuroscience. C.M. Singh, N. Belviso, and R.L. Avila are employees of, and hold stock/stock options in, Biogen. A. Altincatal, P.-R. Ho, R. Su, R. Engle, D. Sangurdekar, B.C. Kieseier, C. DeMoor, E. Fisher, and R.A. Rudick are former employees of, and hold stock/stock options in, Biogen. Go to Neurology.org/N for full disclosures.
References
- 1.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi: 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 2.Wattjes MP, Ciccarelli O, Reich DS, et al. Magnetic Resonance Imaging in Multiple Sclerosis study group, Consortium of Multiple Sclerosis Centres, North American Imaging in Multiple Sclerosis Cooperative MRI guidelines working group. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021;20(8):653-670. doi: 10.1016/S1474-4422(21)00095-8 [DOI] [PubMed] [Google Scholar]
- 3.Tomassini V, Sinclair A, Sawlani V, et al. Diagnosis and management of multiple sclerosis: MRI in clinical practice. J Neurol. 2020;267(10):2917-2925. doi: 10.1007/s00415-020-09930-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuan A, Rao MV, Veeranna, Nixon RA. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol. 2017;9(4):a018309. doi: 10.1101/cshperspect.a018309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreira-Atuesta C, Reyes S, Giovanonni G, Gnanapavan S. The evolution of neurofilament light chain in multiple sclerosis. Front Neurosci. 2021;15:642384. doi: 10.3389/fnins.2021.642384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuhle J, Barro C, Disanto G, et al. Serum neurofilament light chain in early relapsing remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Mult Scler. 2016;22(12):1550-1559. doi: 10.1177/1352458515623365 [DOI] [PubMed] [Google Scholar]
- 7.Disanto G, Barro C, Benkert P, et al. Serum Neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81(6):857-870. doi: 10.1002/ana.24954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novakova L, Zetterberg H, Sundstrom P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology. 2017;89(22):2230-2237. doi: 10.1212/WNL.0000000000004683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhle J, Plavina T, Barro C, et al. Neurofilament light levels are associated with long-term outcomes in multiple sclerosis. Mult Scler. 2020;26(13):1691-1699. doi: 10.1177/1352458519885613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantó E, Barro C, Zhao C, et al. Association between serum neurofilament light chain levels and long-term disease course among patients with multiple sclerosis followed up for 12 years. JAMA Neurol. 2019;76(11):1359-1366. doi: 10.1001/jamaneurol.2019.2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox RJ, Cree BA, De Seze J, et al. MS disease activity in RESTORE: a randomized 24-week natalizumab treatment interruption study. Neurology. 2014;82(17):1491-1498. doi: 10.1212/WNL.0000000000000355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryerson LZ, Foley J, Chang I, et al. Risk of natalizumab-associated PML in patients with MS is reduced with extended interval dosing. Neurology. 2019;93(15):e1452-e1462. doi: 10.1212/WNL.0000000000008243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chisari CG, Grimaldi LM, Salemi G, et al.; Italian MS Register Study Group. Clinical effectiveness of different natalizumab interval dosing schedules in a large Italian population of patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2020;91(12):1297-1303. doi: 10.1136/jnnp-2020-323472 [DOI] [PubMed] [Google Scholar]
- 14.Butzkueven H, Kappos L, Spelman T, et al. No evidence for loss of natalizumab effectiveness with every-6-week dosing: a propensity score-matched comparison with every-4-week dosing in patients enrolled in the Tysabri Observational Program (TOP). Ther Adv Neurol Disord. 2021;14:17562864211042458. doi: 10.1177/17562864211042458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryerson LZ, Naismith RT, Krupp LB, et al. No difference in radiologic outcomes for natalizumab patients treated with extended interval dosing compared with standard interval dosing: real-world evidence from MS PATHS. Mult Scler Relat Disord. 2022;58:103480. doi: 10.1016/j.msard.2021.103480 [DOI] [PubMed] [Google Scholar]
- 16.Kapoor R, Sellebjerg F, Hartung H, et al. Natalizumab reduces serum concentrations of neurofilament light chain in secondary progressive multiple sclerosis patients from the phase 3 ASCEND study (S12.008). Neurology. 2019;92(15_supplement):S12-S008. doi: 10.1212/wnl.92.15_supplement.s12.008 [DOI] [Google Scholar]
- 17.Gafson AR, Jiang X, Shen C, et al. Serum neurofilament light and multiple sclerosis progression independent of acute inflammation. JAMA Netw Open. 2022;5(2):e2147588. doi: 10.1001/jamanetworkopen.2021.47588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sotirchos ES, Fitzgerald KC, Singh CM, et al. Associations of sNfL with clinico-radiological measures in a large MS population. Ann Clin Transl Neurol. 2023;10(1):84-97. doi: 10.1002/acn3.51704 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized participant data collected during the trial will be shared with qualified scientific researchers who provide a methodologically sound proposal. Proposals should be submitted through Vivli (vivli.org). To gain access, data requestors will need to sign a data sharing agreement. Data are made available for 1 year on a secure platform.