Abstract
Tissue-invasive cytomegalovirus (CMV) disease represents a well-recognized complication after kidney transplantation. However, direct involvement of the urogenital tract and CMV-ureteritis occur less frequently. Nephrogenic adenomas are benign lesions of the urinary tract preferentially reported in kidney transplant recipients. We herein report a second case of a 33-year-old male kidney transplant recipient with acute post-renal allograft dysfunction due to CMV-positive ureteral nephrogenic adenoma. A causal connection might be suspected but remains to be proven.
Keywords: case report, nephrogenic adenoma, cytomegalovirus, kidney transplantation, allograft dysfunction, post-renal, infection, urologic complication
Introduction
Transplantation is the treatment of choice for patients with end-stage kidney disease (1). Given current highly effective immunosuppressive regimens, infectious complications represent a main cause for morbidity and mortality in patients after solid organ transplantation (2). Infections with cytomegalovirus (CMV) are among the most common opportunistic infections occurring in kidney transplant recipients and negatively affect transplant outcome (3). CMV-induced asymptomatic viremia and systemic disease are well recognized, as is tissue-invasive disease with classical involvement of the upper and lower gastrointestinal tract, the lungs or the liver (4). However, direct involvement of the kidney graft or the urogenital tract is rare.
Nephrogenic adenoma is a particular, uncommon benign lesion of the urinary tract with a wide range of histopathological characteristics mimicking malignant neoplasms (5). Nephrogenic adenomas mainly arise in the bladder while other locations in the urinary tract are less frequent (6). In kidney transplant recipients, the occurrence of nephrogenic adenoma in the bladder has been reported with incidences ranging from 0.53 to 4.3 per 100 transplants (7, 8). Despite various hypotheses, the underlying pathogenesis for the development of nephrogenic adenoma has not been completely elucidated to date.
Herein, we report a rare case of a kidney transplant recipient with acute post-renal allograft dysfunction due to CMV-positive ureteral nephrogenic adenoma and discuss a potential link between both conditions.
Case description
A 33-year-old male of West-African descent with end-stage kidney disease due to hypertensive nephropathy received a kidney transplant from a deceased donor 6 years after initiating hemodialysis treatment. His past medical history was remarkable for hypertensive cardiopathy, chronic hepatitis B, and latent tuberculosis, for which treatment had been completed 2 years before transplantation.
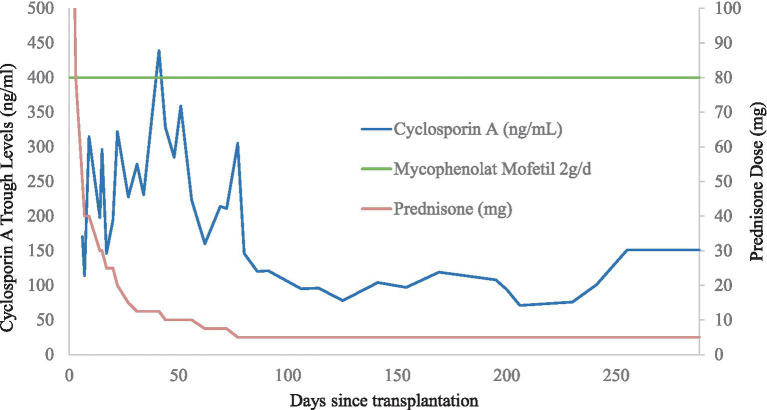
Transplant allocation parameters included a kidney donor profile index of 4%, 0/8 HLA matches, and intermediate CMV and EBV-risk-constellations (donor+/recipient+). Transplant surgery was performed (left donor kidney into right iliac fossa) with a cold ischemia time of 11 h and a warm ischemia time of 27 min. The immunosuppressive regimen included basiliximab as induction therapy as well as cyclosporin A, mycophenolate mofetil (MMF) and prednisolone as maintenance therapy. According to local practice, a preemptive approach was followed using regular monitoring of CMV viremia without prophylactic antiviral therapy.
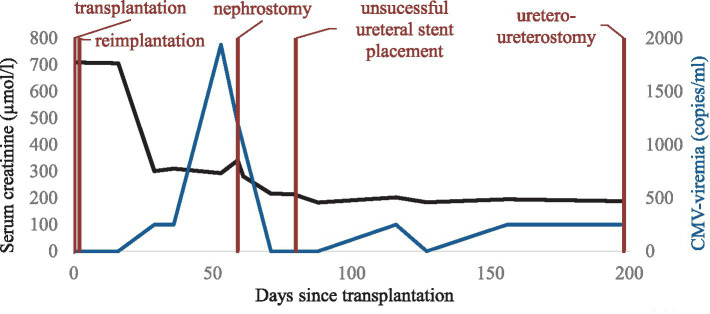
The immediate postoperative course was complicated by delayed graft function requiring continued hemodialysis treatment. On postoperative day 2, magnetic resonance imaging (MRI) of the kidney graft showed subtotal stenosis of the transplant artery at the outflow of the right common iliac artery due to dissection of the right common iliac artery and kidney graft infarcts. Therefore, explantation, thromboendarterectomy of the right common iliac artery, ventral reconstruction of the common iliac artery using a pericard patch and re-implantation of the allograft was performed on the same day. Postoperative duplex ultrasound showed restored graft perfusion. Subsequently, graft function slowly recovered allowing discontinuation of hemodialysis therapy on postoperative day 13 and stabilization of graft function at a serum creatinine level of 270 µmol/l corresponding to an estimated glomerular filtration rate (eGFR) of 26 mL/min/1.73 m2 according to chronic kidney disease epidemiology (CKD-EPI) formula (Figure 1).
Figure 1.
Timeline after kidney transplantation. CMV, cytomegalovirus.
Two months post-transplant during a regular visit to the transplant outpatient clinic, increasing CMV viremia of 1940 copies/ml was detected after low grade viremia at <500 copies/ml had been weekly monitored since a month post-transplant. At this time, valganciclovir at therapeutic dosage was started. Only 6 days later, a rise in serum creatinine to 341 μmoL/L was noted as well as new-onset microhematuria that had been retrospectively present since the preceding week. Duplex ultrasound of the kidney graft newly revealed hydronephrosis grade III. Consequently, urgent percutaneous nephrostomy was placed leading to a prompt fall in serum creatinine. Three weeks after treatment start, CMV viremia was undetectable and valganciclovir was stopped. Due to this satisfying response to valganciclovir, MMF was maintained at the same dose of 2 g per day (Figure 1). BK viremia was undetectable throughout follow-up.
Three months after nephrostomy placement, further urologic work-up by antegrade pyelography was performed revealing a stenotic ureteral lesion in proximity to the bladder. However, an attempt to insert a ureteral stent during the same session was unsuccessful. Therefore, definitive surgical treatment was undertaken consisting of secondary uretero-ureterostomy with the ipsilateral native ureter. Subsequently, the nephrostomy could be removed, and the serum creatinine remained stable at 193 μmoL/L (Figure 2).
Figure 2.
Immunosuppressive regimen and cyclosporin A trough levels after transplantation.
The histological examination of the resected transplant ureter showed presence of a PAX8-positive cell proliferation with surrounding fibrosis (Figure 3) consistent with nephrogenic adenoma. There were no signs of malignancy. Surprisingly, several cells showed cytopathic changes characteristic for CMV (Figure 4) and immunohistochemistry was positive for cytomegalovirus (CH2- and DDG-antibodies, Dako, dilution 1:400, pre-treatment H2 30 95; Figure 5). In the simultaneously taken kidney graft sample, signs of acute tubular injury without further anomalies were seen; however, tissue sampling was limited. CMV immunohistochemistry and SV40 staining, as BK-virus marker, were negative.
Figure 3.
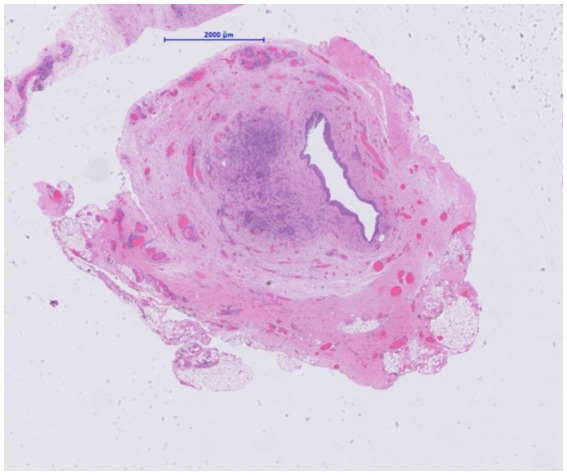
Resected ureter segment. Narrowed lumen in transplant ureter due to the presence of a cellular proliferation (H&E, x 10).
Figure 4.
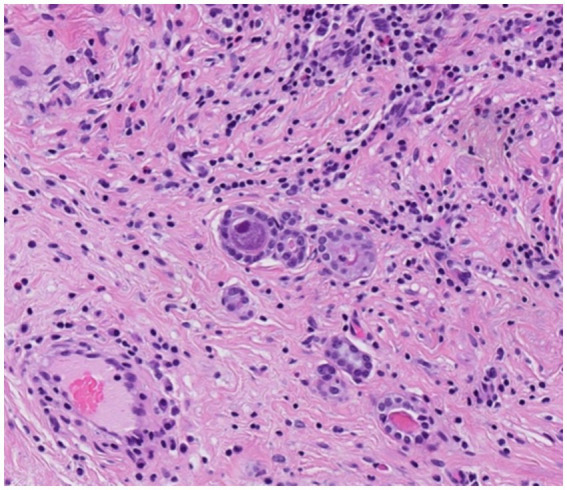
Resected ureter segment. Microglandular proliferation of a nephrogenic adenoma with typical cytopathic appearance of a CMV-infected cell (H&E, x 200).
Figure 5.
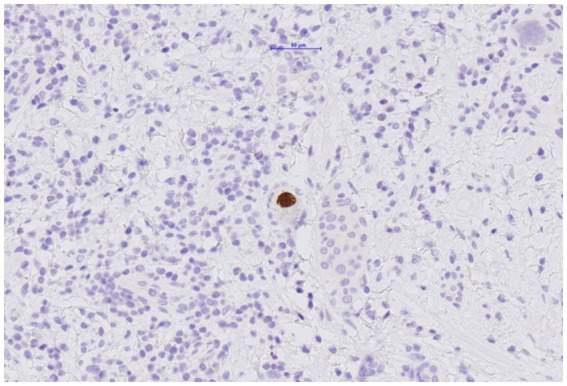
Resected ureter segment. Cells within nephrogenic adenoma with positive immunohistochemic staining for CMV.
In the presence of CMV-positive nephrogenic adenoma, another course of valganciclovir at therapeutic dosage was introduced for 6 weeks. Immunosuppressive therapy was maintained unchanged (Figure 1). The following clinical course was unremarkable with a serum creatinine value measured at 136 µmol/l at last follow-up and disappearance of hematuria after the last urologic intervention.
Discussion
We present the case of a kidney transplant recipient with acute post-renal kidney graft dysfunction due to CMV-positive nephrogenic adenoma of the ureter. To the best of our knowledge, this is the second described case of ureteral nephrogenic adenoma with CMV superinfection to date.
Acute kidney graft dysfunction has been estimated to occur with an incidence of 21% in the first 6 months after kidney transplantation and adversely affects patient and transplant outcomes (9, 10). Causes for acute kidney graft dysfunction include transplant-specific etiologies such as acute rejection, BK virus nephropathy and calcineurin inhibitor toxicity in addition to classical causes of acute kidney injury. In a single center cohort of 326 kidney transplant recipients, urinary tract obstruction accounted for 10% of cases of acute kidney injury in the early post-transplant period (9). Both CMV ureteritis and nephrogenic adenoma represent possible, albeit infrequent causes for post-renal acute kidney graft dysfunction.
CMV ureteritis is a rare manifestation of CMV-related tissue-invasive disease that has been increasingly recognized in kidney transplant recipients during the last decades and been linked to the progressive use of mycophenolate in the transplant setting (11–15). Its main manifestations include mild fever, urinary obstruction and kidney impairment. Risk factors for the development of CMV ureteritis are acute allograft rejection, the use of depleting immunosuppression or MMF as well as the absence of prophylactic antiviral therapy (11, 12). In our patient, use of an MMF-based immunosuppression and lack of antiviral prophylaxis following the preemptive therapy approach might have favored the occurrence of CMV-associated tissue-invasive disease.
Nephrogenic adenoma of the urinary tract may present with various symptoms. According to a single center retrospective analysis of 32 cases of nephrogenic adenoma, symptoms were present in 72% of patients including hematuria, urinary symptoms or incontinence, flank pain and hydronephrosis (6). In our patient, new-onset microhematuria was retrospectively noted 1 week before acute worsening of graft function together with the finding of hydronephrosis. There were no urinary symptoms nor painful graft site.
Until now, the pathogenesis of the development of nephrogenic adenoma remains incompletely understood. Several hypotheses have been put forward including the development from remnant mesonephric tissue, the development as metaplastic response to local trauma, irritation, inflammation or immunosuppression as well as the development from shed, secondarily implanted renal tubular cells (16). Indeed, in a landmark study in 24 kidney transplant recipients, bladder nephrogenic adenoma has been shown to derive from the kidney graft (i.e., donor) using fluorescence in situ hybridization studies of sex chromosomes (17). However, controversial data exist outside the transplant setting (16).
In our patient, nephrogenic adenoma of the ureter was found to be CMV-positive. We are aware of four previously reported cases of CMV-positive nephrogenic adenomas in kidney transplant recipients (18–21); while three of them affected the bladder, only one case involving the transplant ureter has been described so far (Table 1). Most of the cases were diagnosed within 1 year post-transplant, all of them by histological analysis. In the majority of general cases of nephrogenic adenoma in kidney transplant recipients published so far, CMV testing has not been reported (Table 2) (7, 8, 17, 22–36). On one hand it may be speculated whether CMV-induced local inflammation may have predisposed to the development of nephrogenic adenoma. CMV encodes several proteins inhibiting the assembly and trafficking of cellular proteins, which participate in immune recognition (e.g., major histocompatibility complex 1 and major histocompatibility complex 2). Consequently, CMV hides infected cells from adaptive immunity (37). This immune evasive capability not only helps CMV to persist within its host cells, but may further may predispose to the formation of metaplasia such as nephrogenic adenoma. In addition, sequential surgical procedures may have played a causative role in our case. Thus, the explantation and reimplantation of the allograft due to the artery dissection may have led to substantial shedding of tubular epithelial cells into the ureter and bladder of our patient finally leading to the formation of nephrogenic adenoma (17). On the other hand, nephrogenic adenoma per se may have favored CMV reactivation. Indeed, CMV reactivation secondary to inflammatory stimuli has been suggested previously (38). Interestingly, nephrogenic adenoma of the bladder positive for BK polyomavirus has similarly been reported in a kidney transplant recipient (34). According to the histological findings, the authors suggested BK virus contributed to cell atypia, but was not a causative factor for the development of nephrogenic adenoma.
Table 1.
Reported cases of nephrogenic adenoma with CMV-infection after kidney transplantation.
| Author | Time after kidney transplantation | Symptoms | Location | Detection of CMV | Therapy |
|---|---|---|---|---|---|
| Beaudry 1983 (18) | 5.33 years | Gross hematuria | Over 50% of bladder surface including the site of the reimplanted ureter | Biopsy and serum | Withdrawal of azathioprine |
| Buzelin 1988 (19) | 2.4 years | Gross hematuria, dysuria | Bladder | Biopsy | Resection |
| Redman 2000 (20) | 1 year | Vesical calculi | Bladder next to the ureteroneo-cystostomy | Biopsy | Resection |
| Hung 2001 (21) | 3 months | Ureteral obstruction, gross hematuria | Ureter | Biopsy | Resection, Valganciclovir, withdrawal of azathioprine |
| Our case | 2 months | Ureteral obstruction, hematuria | Ureter | Biopsy and serum | Valganciclovir and resection |
Table 2.
Previous cases of nephrogenic adenoma after kidney transplantation.
| Year | Author | Number of Cases | Supposed precipitating factors | CMV testing | Total |
|---|---|---|---|---|---|
| 1975 | Gordon et al. (22) | 1 | Impaired immunologic surveillance | Not reported | 1 |
| 1982 | Behesti et al. (23) | 1 | Renal transplantation, UTI | Not reported | 2 |
| 1988 | Gonzalez et al. (24) | 8 | Renal transplantation | Not reported | 10 |
| 1992 | Zeidan et al. (25) | 2 | Renal transplantation, transurethral resection of the prostate | Not reported | 12 |
| 1995 | Colombo et al. (26) | 5 | Mechanical trauma and recurrent UTI | 3 with postoperative systemic CMV infection, 2–4 years before nephrogenic adenoma | 17 |
| 1996 | Fournier et al. (8) | 9 | Ureterovesical anastomosis, chronic prostatitis, vesicorenal reflux, cyclophosphamide, condyloma | No viroid inclusions in biopsy | 26 |
| 1997 | Tse et al. (27) | 7 | Immunosuppression, ureterovesical anastomosis, recurrent UTI, changes of JJ stents | Not reported | 33 |
| 1998 | Banyai-Falger et al. (7) | 7 | Recurrent UTI, surgical procedure of renal transplantation | 2 with CMV disease, unrelated to nephrogenic adenoma | 40 |
| 1998 | Pycha et al. (28) | 12 | Renal transplantation, inflammation | Not reported | 52 |
| 2002 | Whang et al. (29) | 1 | Kidney-pancreas transplantation with drainage of the pancreas into the bladder | Not reported | 53 |
| 2002 | Mazal et al. (17) | 29 | Shedding of renal tubular cells due to trauma, infection and/or immunosuppression | Not reported | 82 |
| 2008 | Kim et al. (30) | 1 | UTI and bladder stones | Not reported | 83 |
| 2009 | Ladenheim et al. (31) | 1 | Renal transplantation | Not reported | 84 |
| 2013 | Voss et al. (32) | 1 | Renal transplantation | Not reported | 85 |
| 2014 | Kuzaka et al. (33) | 2 | Renal transplantation, immunosuppression, reoperation | Not reported | 87 |
| 2015 | Alexiev et al. (34) | 1 | BK Virus infection | Not reported | 88 |
| 2017 | North et al. (35) | 1 | Recurrent UTI | Not reported | 89 |
| 2020 | Kahn et al. (36) | 1 | Surgery | Not reported | 90 |
CMV, cytomegalovirus; UTI, urinary tract infection; JJ, double J.
Currently supported preventive strategies for CMV in kidney transplant recipients include prophylactic and preemptive therapy approaches (39). Preemptive CMV therapy includes regular monitoring of CMV viremia and start of antiviral therapy in case of viral replication at pre-specified levels. In patients with high-risk constellation (donor +/ recipient -) and after induction with depleting agents, a prophylactic approach may be chosen (40). However, the diagnosis of CMV-related tissue-invasive disease requires detection of CMV in the tissue by histology (cytopathic changes) or immunohistochemistry (39). In addition, CMV-related tissue-invasive disease may occur in the absence of CMV viremia as has also been reported for CMV ureteritis (14). Fortunately, in our patient, local symptoms were accompanied by a simultaneous rise in CMV viremia leading to prompt start of antiviral treatment, although being stopped after viremia was undetectable. However, the clinical course of our patient might advocate for a lower viremia threshold for instauration of antiviral therapy. Indeed, during the month preceding the acute rise in CMV viremia, low-grade viremia (< 500 copies/ml) had been present.
Management of the patient with ureteral nephrogenic adenoma reported by Hung et al. included resection of the lesion and pyeloplasty, intravenous ganciclovir treatment for 2 weeks and withdrawal of the antimetabolite azathioprin (21). The optimal duration of antiviral treatment for CMV-related tissue-invasive disease is not known. However, longer therapy courses are generally admitted in these cases (39). Therapy of nephrogenic adenoma usually involves endoscopic resection of the lesion with variable reported recurrence rates (6, 41). In our patient, valganciclovir treatment was re-started after diagnosis of CMV-related tissue-invasive disease for a total of 6 weeks without concomitant change in immunosuppression. Given the shortness of the lesion-free transplant ureter, surgical reconstruction after ureter resection involved proximal uretero-ureterostomie between transplanted and patient ureter.
In conclusion, CMV-ureteritis and nephrogenic adenomas are rare causes for acute post-renal kidney graft dysfunction. Diagnosis of tissue-invasive CMV disease requires histological evidence of CMV at the affected site. To the best of our knowledge, this is the second reported case of ureteral nephrogenic adenoma with CMV superinfection in a kidney transplant recipient. A causal link might be suspected but remains to be proven (42, 43).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
NH: Investigation, Visualization, Writing – original draft, Writing – review & editing. MM: Resources, Writing – review & editing. L-YM: Supervision, Investigation, Writing – original draft, Writing – review & editing.
Acknowledgments
We thank the patient who consented to the publication of this case report. This work has been presented as oral communication at the 55th Annual Meeting of the Swiss Society of Nephrology.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Department of Nephrology and Hypertension, Inselspital, Bern University Hospital, Bern, Switzerland.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. (1999) 341:1725–30. doi: 10.1056/NEJM199912023412303, PMID: [DOI] [PubMed] [Google Scholar]
- 2.Awan AA, Niu J, Pan JS, Erickson KF, Mandayam S, Winkelmayer WC, et al. Trends in the causes of death among kidney transplant recipients in the United States (1996-2014). Am J Nephrol. (2018) 48:472–81. doi: 10.1159/000495081, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartmann A, Sagedal S, Hjelmesaeth J. The natural course of cytomegalovirus infection and disease in renal transplant recipients. Transplantation. (2006) 82:S15–7. doi: 10.1097/01.tp.0000230460.42558.b0, PMID: [DOI] [PubMed] [Google Scholar]
- 4.Razonable RR, Humar A. Practice ASTIDCo. cytomegalovirus in solid organ transplantation. American J Transplantation: Official J American Society of Transplantation and the American society of transplant surgeons. (2013) 13:93–106. doi: 10.1111/ajt.12103 [DOI] [PubMed] [Google Scholar]
- 5.Amin W, Parwani AV. Nephrogenic adenoma. Pathol Res Pract. (2010) 206:659–62. doi: 10.1016/j.prp.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 6.Gordetsky J, Gennaro KH, Selph JP, Rais-Bahrami S. Nephrogenic adenoma: clinical features, management, and diagnostic pitfalls. Urology. (2016) 95:29–33. doi: 10.1016/j.urology.2016.04.032, PMID: [DOI] [PubMed] [Google Scholar]
- 7.Banyai-Falger S, Maier U, Susani M, Wiener H, Watschinger B, H??rl WH, et al. High incidence of nephrogenic adenoma of the bladder after renal transplantation. Transplantation. (1998) 65:511–4. doi: 10.1097/00007890-199802270-00010, PMID: [DOI] [PubMed] [Google Scholar]
- 8.Fournier G, Menut P, Moal MC, Hardy E, Volant A, Mangin P. Nephrogenic adenoma of the bladder in renal transplant recipients: a report of 9 cases with assessment of deoxyribonucleic acid ploidy and long-term followup. J Urol. (1996) 156:41–4. doi: 10.1016/S0022-5347(01)65932-9, PMID: [DOI] [PubMed] [Google Scholar]
- 9.Panek R, Tennankore KK, Kiberd BA. Incidence, etiology, and significance of acute kidney injury in the early post-kidney transplant period. Clin Transpl. (2016) 30:66–70. doi: 10.1111/ctr.12660, PMID: [DOI] [PubMed] [Google Scholar]
- 10.Mehrotra A, Rose C, Pannu N, Gill J, Tonelli M, Gill JS. Incidence and consequences of acute kidney injury in kidney transplant recipients. American J Kidney Dis: Official J National Kidney Foundation. (2012) 59:558–65. doi: 10.1053/j.ajkd.2011.11.034 [DOI] [PubMed] [Google Scholar]
- 11.Thomas M, Russ G, Mathew T, Rao Mohan M, Cooper J, Walker R. Four cases of CMV ureteritis: emergence of a new pattern of disease? Clin Transpl. (2001) 15:354–8. doi: 10.1034/j.1399-0012.2001.150509.x, PMID: [DOI] [PubMed] [Google Scholar]
- 12.Ersan S, Yorukoglu K, Sert M, Atila K, Celik A, Gulcu A, et al. Unusual case of severe late-onset cytomegalovirus-induced hemorrhagic cystitis and ureteritis in a renal transplant patient. Ren Fail. (2012) 34:247–50. doi: 10.3109/0886022X.2011.647209, PMID: [DOI] [PubMed] [Google Scholar]
- 13.Rico JE, Cardona X, Rodelo J, Reino A, Arias LF, Arbeláez M. Ureterostomy cytomegalovirus infection presenting as stoma ulceration in a kidney allograft receptor: a case report. Actas Urol Esp. (2008) 32:649–52. doi: 10.1016/s0210-4806(08)73903-2 [DOI] [PubMed] [Google Scholar]
- 14.Vaessen C, Kamar N, Mehrenberger M, Mazerolles C, Mengelle C, Rischmann P, et al. Severe cytomegalovirus ureteritis in a renal allograft recipient with negative CMV monitoring. Nephrol Dial Transplant. (2005) 20:227–30. doi: 10.1093/ndt/gfh531 [DOI] [PubMed] [Google Scholar]
- 15.Fusaro F, Murer L, Busolo F, Rigamonti W, Zanon GF, Zacchello G. CMV and BKV ureteritis: which prognosis for the renal graft? J Nephrol. (2003) 16:591–4. PMID: [PubMed] [Google Scholar]
- 16.Alexiev BA, LeVea CM. Nephrogenic adenoma of the urinary tract:a review. Int J Surg Pathol. (2012) 20:121–9. doi: 10.1177/1066896912439095 [DOI] [PubMed] [Google Scholar]
- 17.Mazal PR, Schaufler R, Altenhuber-Müller R, Haitel A, Watschinger B, Kratzik C, et al. Derivation of nephrogenic adenomas from renal tubular cells in kidney-transplant recipients. N Engl J Med. (2002) 347:653–9. doi: 10.1056/NEJMoa013413, PMID: [DOI] [PubMed] [Google Scholar]
- 18.Beaudry C, Bertrand PE, Laplante L, Houde M, Lamoureux C, Laverdiere M, et al. Nephrogenic adenoma of the bladder after kidney transplantation: spontaneous improvement with azathioprine removal; surgical trauma and cytomegalovirus infection as possible etiologic factors. J Urol. (1983) 130:1183–5. doi: 10.1016/S0022-5347(17)51746-2, PMID: [DOI] [PubMed] [Google Scholar]
- 19.Buzelin F, Hourmant MY, Audoin AF, Karam G, Philippot D. Nephrogenic metaplasia of the bladder in renal transplant patients. J Urol (Paris). (1988) 94:323–7. [PubMed] [Google Scholar]
- 20.Redman JF, Parham DM. Calculus-producing and cytomegalovirus-infected nephrogenic adenoma of the bladder in a prepubertal renal transplant recipient. Urology. (2000) 56:508. doi: 10.1016/S0090-4295(00)00661-0, PMID: [DOI] [PubMed] [Google Scholar]
- 21.Hung SY, Tseng HH, Chung HM. Nephrogenic adenoma associated with cytomegalovirus infection of the ureter in a renal transplant patient: presentation as ureteral obstruction. Transplant Int: Official J European Society for Organ Transplantation. (2001) 14:111–4. doi: 10.1111/j.1432-2277.2001.tb00024.x, PMID: [DOI] [PubMed] [Google Scholar]
- 22.Gordon HL, Kerr SG. Nephrogenic adenoma of bladder in immunosuppressed renal transplantation. Urology. (1975) 5:275–7. doi: 10.1016/0090-4295(75)90031-X [DOI] [PubMed] [Google Scholar]
- 23.Behesti M, Morales A. Nephrogenic adenoma of bladder developing after renal transplantation. Urology. (1982) 20:298–9. doi: 10.1016/0090-4295(82)90645-8, PMID: [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez JA, Watts JC, Alderson TP. Nephrogenic adenoma of the bladder: report of 10 cases. J Urol. (1988) 139:45–7. doi: 10.1016/S0022-5347(17)42285-3, PMID: [DOI] [PubMed] [Google Scholar]
- 25.Zeidan BS, Shabtai M, Waltzer WC, Miller F, Rapaport FT. Nephrogenic adenoma in renal transplant recipients. Transplant Proc. (1992) 24:752–4. [PubMed] [Google Scholar]
- 26.Colombo T, Altziebler S, Primus G, Kroll W, Onetto F, Ratschek M, et al. Nephrogenic adenoma after kidney transplantation. Urologe A. (1995) 34:489–93. [PubMed] [Google Scholar]
- 27.Tse V, Khadra M, Eisinger D, Mitterdorfer A, Boulas J, Rogers J. Nephrogenic adenoma of the bladder in renal transplant and non-renal transplant patients: a review of 22 cases. Urology. (1997) 50:690–6. doi: 10.1016/S0090-4295(97)00334-8, PMID: [DOI] [PubMed] [Google Scholar]
- 28.Pycha A, Mian C, Reiter WJ, Brössner C, Haitel A, Wiener H, et al. Nephrogenic adenoma in renal transplant recipients: a truly benign lesion? Urology. (1998) 52:756–61. doi: 10.1016/S0090-4295(98)00371-9, PMID: [DOI] [PubMed] [Google Scholar]
- 29.Whang M, Katz L, Ongcapin E, Geffner S, Friedman G, Mulgaonkar S, et al. Nephrogenic adenomas occurring in a patient with simultaneous kidney-pancreas transplant. Urology. (2000) 55:949. doi: 10.1016/S0090-4295(00)00465-9, PMID: [DOI] [PubMed] [Google Scholar]
- 30.Kim MS, Kim SK, Kim SI, Kim YS, Jeong HJ. Ureteral nephrogenic adenoma in a renal allograft patient. Int J Urol. (2008) 15:178–9. doi: 10.1111/j.1442-2042.2007.01958.x, PMID: [DOI] [PubMed] [Google Scholar]
- 31.Ladenheim H, Frable W. Cytology of a recurrent nephrogenic adenoma in a renal transplant patient. Diagn Cytopathol. (2009) 37:468–70. doi: 10.1002/dc.21056, PMID: [DOI] [PubMed] [Google Scholar]
- 32.Voss K, Peppas D. Recurrent nephrogenic adenoma: a case report of resolution after treatment with antibiotics and nonsteroidal anti-inflammatory medication. Urology. (2013) 82:1156–7. doi: 10.1016/j.urology.2013.04.024, PMID: [DOI] [PubMed] [Google Scholar]
- 33.Kuzaka B, Pudełko P, Powała A, Górnicka B, Radziszewski P. Nephrogenic adenoma of the urinary bladder: a report of three cases and a review of the literature. Ann Transplant. (2014) 19:153–6. doi: 10.12659/AOT.889441, PMID: [DOI] [PubMed] [Google Scholar]
- 34.Alexiev BA, Papadimitriou JC, Drachenberg CB. BK polyomavirus-infected nephrogenic adenoma of the urinary bladder in a renal transplant recipient: a case report. Pathol Res Pract. (2015) 211:697–701. doi: 10.1016/j.prp.2015.06.006, PMID: [DOI] [PubMed] [Google Scholar]
- 35.North D, Jaw J, Hill P, Bateman S, Barraclough N, Langham R. Nephrogenic adenoma complicating renal transplantation: a case report and discussion. Transplant Proc. (2017) 49:2381–3. doi: 10.1016/j.transproceed.2017.09.003, PMID: [DOI] [PubMed] [Google Scholar]
- 36.Khan I, Obeid M, Hasan N, Jaradat F, Sengupta B, Tabbal M, et al. Nephrogenic adenoma of the urinary bladder after kidney transplantation: long-term follow-up. Case Rep Transplant. (2020) 2020:1–3. doi: 10.1155/2020/8831966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lucin P, Mahmutefendic H, Blagojevic Zagorac G, Ilic TM. Cytomegalovirus immune evasion by perturbation of endosomal trafficking. Cell Mol Immunol. (2015) 12:154–69. doi: 10.1038/cmi.2014.85, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kline JN, Hunninghake GM, He B, Monick MM, Hunninghake GW. Synergistic activation of the human cytomegalovirus major immediate early promoter by prostaglandin E2 and cytokines. Exp Lung Res. (1998) 24:3–14. doi: 10.3109/01902149809046050, PMID: [DOI] [PubMed] [Google Scholar]
- 39.Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, et al. The third international consensus guidelines on the Management of Cytomegalovirus in solid-organ transplantation. Transplantation. (2018) 102:900–31. doi: 10.1097/TP.0000000000002191, PMID: [DOI] [PubMed] [Google Scholar]
- 40.Mella A, Mariano F, Dolla C, Gallo E, Manzione AM, Di Vico MC, et al. Bacterial and viral infection and Sepsis in kidney transplanted patients. Biomedicine. (2022) 10. doi: 10.3390/biomedicines10030701, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zougkas K, Kalafatis M, Kalafatis P. Nephrogenic adenoma of the urinary bladder. Int Urol Nephrol. (2004) 36:513–7. doi: 10.1007/s11255-004-0848-7 [DOI] [PubMed] [Google Scholar]
- 42.World Medical Association . World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 310:2191–4. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 43.Steering Committee of the Istanbul Summit . Organ trafficking and transplant tourism and commercialism: the declaration of Istanbul. Lancet. (2008) 372:5–6. doi: 10.1016/S0140-6736(08)60967-8, PMID: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.