Abstract
Background:
The flow-through INSTI™ HIV-1/HIV-2 Rapid Antibody (INSTI) test is a 60 s FDA-approved test for HIV-1 and HIV-2 antibody testing using whole blood and plasma.
Objective:
We evaluated the performance of INSTI using plasma and simulated whole blood specimens.
Study design:
INSTI’s performance in plasma specimens from commercial seroconversion panels was assessed by estimating the relative sensitivity using a 50% cumulative frequency analysis and by comparing its performance with other FDA-approved rapid tests (RTs). INSTI was further evaluated using 320 HIV-1 plasma specimens collected during a cross-sectional study and with 107 HIV-1 and 24 HIV-2 simulated whole blood specimens. Sensitivity and specificity were calculated using 615 known HIV-1 group M/O and 80 HIV-2 (Western blot (WB)-positive), and 497 HIV-negative plasma specimens, respectively.
Results:
In HIV-1 seroconversion panels, INSTI became reactive 9 days before a positive WB. When compared to FDA-approved antibody-based lateral flow RTs, INSTI detected significantly more early infections. Among HIV-1-infected cross-sectional plasma samples, INSTI detected 23 (27%) of 85 Architect-positive/Multispot-negative or indeterminate specimens. For plasma specimens, the sensitivity was 99.84% for HIV-1 and 100% for HIV-2, and the specificity was 99.80%. Using simulated whole blood from seroconverters, INSTI performed similarly to plasma.
Conclusions:
INSTI performed significantly better than antibody-based lateral flow RTs during early stages of seroconversion. Sensitivity and specificity were within the manufacturer’s reported ranges. Considering the observed test performance and the almost immediate results, INSTI is an accurate option to detect HIV-1/HIV-2 antibodies in point-of-care settings where lab testing is not feasible.
Keywords: HIV-1/2 rapid test, Whole blood testing, CLIA-waived test, Early HIV-1 infections
1. Background
HIV rapid tests (RTs) are widely used to expand access to HIV testing to non-clinical sites and provide opportunities for faster linkage to care and treatment [1–3]. FDA-approved RTs have high sensitivity for HIV detection in persons with established HIV infection (IgG-positive) and high antibody titers, but vary in their ability to detect low antibody titers, as found during early stages of seroconversion [4–6].
The INSTI™ HIV-1/HIV-2 Rapid Antibody test (INSTI, bioLytical Laboratories Inc; Canada) received approval by the Food and Drug Administration (FDA) for detection of HIV-1 antibodies in 2010 and HIV-2 antibodies in 2015, and Clinical Laboratory Improvement Amendment (CLIA) waiver status in 2012. The INSTI point-of-care (POC) RT provides a result, within 60 s, from fingerstick whole blood in CLIA-waived settings, and from venous/fingerstick whole blood or plasma in settings with moderate complexity [7]. The feasibility of its use in POC settings was shown in an evaluation where untrained operators obtained accurate results when following the manufacturer’s written instructions for fingerstick whole blood [8]. INSTI has been successfully evaluated in several studies outside the United States (U.S.) [9–13]. The reported sensitivities were ≥ 99% using fingerstick whole blood and plasma from individuals with HIV-1 (group M and O) and HIV-2 infections [9].
The lower sensitivity of some RTs during early stages of HIV infection increases the risk of misdiagnosing persons with active viral replication and high risk of transmission [6,14–17]. Because RT evaluation in health care settings using specimens in the early stages of seroconversion can be challenging [18], the number of studies evaluating the performance of INSTI in early HIV infections is limited. One study using residual serum showed that INSTI performed well compared to other POC tests for the detection of early HIV infections [19]. Due to the technical difficulties and expense associated with obtaining whole blood from seroconverting individuals, plasma and simulated whole blood prepared from commercial seroconversion panels were used to evaluate INSTI’s seroconversion sensitivity in our study. Specimens previously collected in several studies were also used to evaluate assay performance, including a prospective study to detect acute infections and well-characterized seroconversion panels.
2. Objective
The objective of this study was to evaluate the laboratory performance of INSTI using plasma and simulated whole blood specimens from early and established HIV infections, and plasma specimens from HIV-uninfected persons.
3. Study design
3.1. Sample sets
3.1.1. Plasma specimens from commercial HIV-1 seroconversion panels
To assess assay performance for detecting early infections, well-characterized frozen HIV-1 seroconversion panels from U.S. donors were obtained from Zeptometrix, Inc. (n = 12; Buffalo, NY, USA) and BBI-SeraCare Diagnostics (n = 14; Milford, MA, USA) [4,20,21]. Each panel included at least one WB-indeterminate specimen (9 seroconverters, 64 total specimens) or WB-positive (17 seroconverters, 166 total specimens) and all panels had at least one HIV-1 RNA-positive specimen.
3.1.2. Plasma specimens from HIV-1-positive persons at different infection stages (STOP study)
The Screening Targeted Populations to Interrupt On-going Chains of HIV Transmission with Enhanced Partner Notification (STOP) study was a multi-site, prospective study designed to evaluate methods to detect acute HIV-1 infections [22–24]. For this analysis, 85 HIV-1 specimens were classified as early infections for testing positive on the antigen/antibody (Ag/Ab) combo screening assay (Abbott Architect HIV Ag/Ab combo (ARC) immunoassay; Chicago, IL, USA), negative or indeterminate for the supplemental assay Bio-Rad Multispot HIV-1/HIV-2 rapid tests (Multispot), and positive for HIV-1 nucleic acid (Abbott m2000 HIV-1 RNA (m2000VL) viral load; Chicago, IL, USA). Two-hundred three specimens were classified as established infections and were ARC- and Multispot-positive, and 32 specimens were repeatedly ARC-reactive, but HIV-1 RNA negative and classified as ARC false-reactive [25]. In the STOP study, Multispot, a flow-through rapid tests, was used as a supplemental test following the CDC/APHL diagnostic algorithm, but was discontinued in 2016. Remnant plasma specimens were stored frozen at −30 °C until shipped to CDC.
3.1.3. Plasma specimens from established HIV-1 and HIV-2 infections and uninfected persons
Subsets from two previous CDC studies of characterized plasma sets were used to assess INSTI sensitivity and specificity [5,26]. For sensitivity, a total of 695 plasma specimens from persons with established HIV infection (HIV Western blot (WB)-positive) were tested with INSTI. Of these, 501 specimens were from the U.S. and presumed to be infected with HIV-1 subtype B [5,26]. The remaining 114 specimens were from Cameroon, and p17 (gag) and gp41 (env) sequences were available; 111 were HIV-1 group M subtypes A, G, F1, F2, D, CRF_01, CRF_02, CRF_11, CRF_09, and CRF_13 and three were HIV-1 group O [20]. The HIV-2 subset included 80 HIV-2 WB-positive plasma specimens from Ivory Coast (Boca Biolistics, Inc., Coconut Creek, FL) [20]. HIV-2 specimens were tested with Multispot HIV-1/HIV-2 rapid test (Bio-Rad Laboratories, Redmond, WA) in the field, and further characterized at CDC with serologic and molecular testing. Previous integrase region sequence analysis showed that both HIV-2 groups A and B were included in this sample set. To evaluate assay specificity, 499 HIV-uninfected plasma specimens found to be negative for antibody and nucleic acid testing at both a blood donation center and CDC were included [5,26].
3.1.4. Plasma specimens from HIV-1-positive persons receiving antiretroviral therapy
EDTA-whole blood from 35 HIV-1-positive persons from the U.S. receiving antiretroviral therapy were purchased from SeraCare Diagnostics. The blood was shipped overnight at ambient temperature, processed to obtain plasma, and stored at −80 °C until testing.
3.1.5. Simulated whole blood specimens from HIV-1 and HIV-2 plasma specimens
A subset of 107 plasma specimens from the previously characterized commercial HIV-1 seroconversion panels [4,20] and 24 HIV-2 plasma specimens from Ivory Coast [20] was used to prepare simulated HIV-infected whole blood. Plasma specimens were thawed and mixed to achieve a hematocrit of 40% using washed red blood cells (blood group O) to simulate HIV-infected whole blood.
3.1.6. Human subjects approvals
The STOP study was approved by the Institutional Review Boards for the University of California at San Francisco, the University of North Carolina at Chapel Hill, and the New York City Department of Health & Mental Hygiene, and by a research determination at CDC (HSR 6193). The remaining specimens were of commercial source and unlinked from personal identifiers.
3.2. HIV testing
INSTI is a manual, visually read, immunofiltration flow-through rapid test with a spill-free membrane that incorporates an IgG capture procedural control. It detects HIV-1 and/or HIV-2 antibodies in 50 μl of human blood, whole blood, serum and plasma in as little as 60 s. Each test includes a lancet, pipette, and alcohol swab for fingerstick testing. INSTI was performed as indicated in the package insert for plasma and whole blood specimens [7]. All testing was performed by two trained laboratory technicians at ambient temperature in singlet and repeated only if invalid results were obtained. Plasma specimens from 35 HIV-1-positive individuals receiving antiretroviral therapy were also tested with the m2000VL assay using the 0.8 ml sample volume protocol.
3.3. Analysis
Serial plasma specimens from 17 seroconverters (n = 166) were used to estimate the relative sensitivity of INSTI during seroconversion. Test reactivity was compared to historical data obtained from other tests with this same panel using a previously described 50% cumulative frequency [4,5,20,27].
Paired comparisons of INSTI results and all other FDA-approved RTs were done using McNemar’s statistical test with one degree of freedom and continuity correction (95% confidence interval) to analyze the differences in reactivity among seroconverters.
Results from plasma testing were compared to previous results from different FDA-approved diagnostic assays and to results obtained with INSTI using simulated whole blood specimens.
4. Results
4.1. INSTI assay performance using plasma specimens from commercial HIV-1 seroconversion panels
The relative sensitivity of INSTI using 166 seroconverter plasma specimens was estimated by calculating the 50% cumulative frequency [4,5,20,27]. The sequence of test reactivity, expressed as the number of days before the first positive HIV-1 WB, is shown in Fig. 1. INSTI was estimated to become reactive nine days before WB positivity. Compared with historical data of other antibody-based RTs with the same specimen set, INSTI reactivity was observed three days before another flow-through test, Reveal G2 and G3 Rapid HIV-1 antibody (Reveal G2/G3; MedMira Laboratories, Inc.; Halifax, Nova Scotia, Canada), four days before the lateral flow tests Clearview HIV-1/2 STAT-PAK (Statpak; Inverness Medical, Princeton, NJ) and Clearview HIV-1/2 Complete (Complete; Inverness Medical, Princeton, NJ), and seven and eight days before the lateral flow tests Uni-Gold Recombigen HIV (Unigold; Trinity Biotech USA, St. Louis, MO) and OraQuick ADVANCE Rapid HIV-1/2 antibody test (OraQuick; OraSure Technologies, Inc.; Bethlehem, PA), respectively.
Fig. 1.
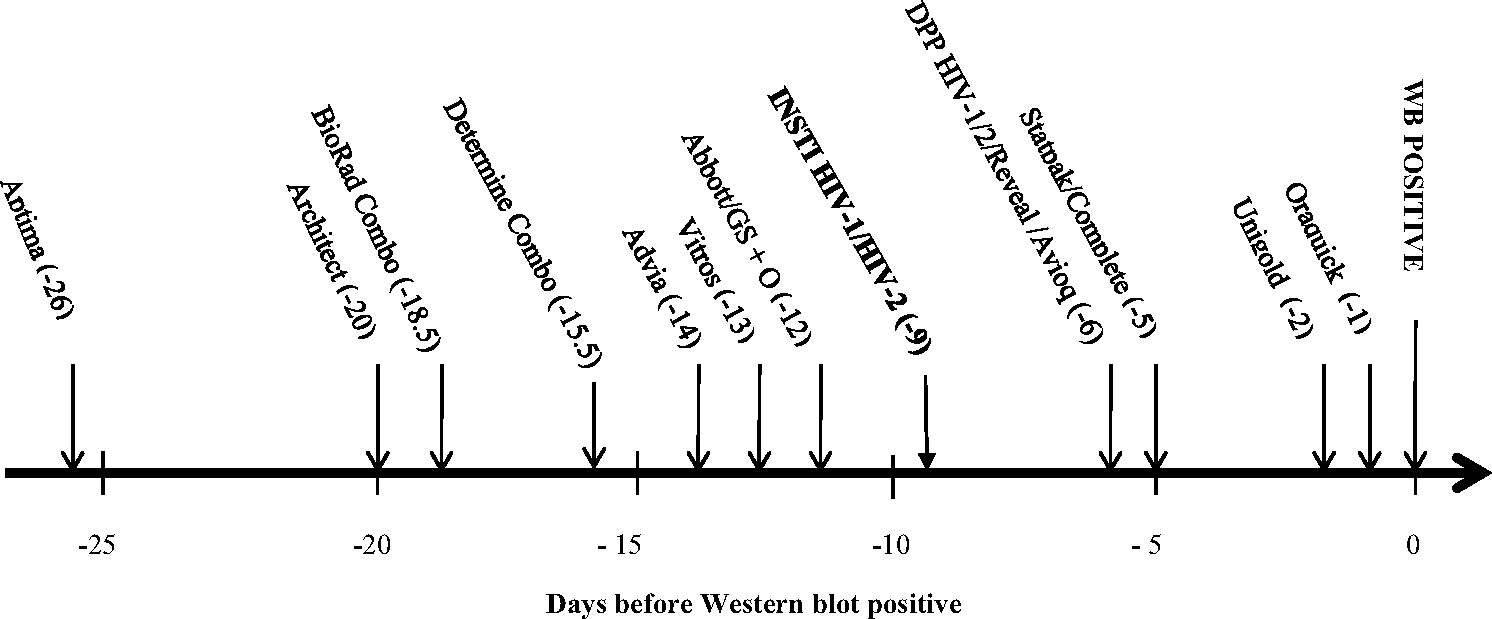
Sensitivity of assay reactivity for 17 plasma seroconverters during early HIV-1 infections as number of days before first positive Western blot (WB) when 50% of specimens tested with each test became positive.
The names, abbreviations, and sources, of the HIV assays previously evaluated11,12 are as follows: APTIMA HIV-1 Quantitative assay (Aptima, Gen-Probe, Inc., San Diego, CA); ARCHITECT® HIV Ag/Ab Combo assay (Architect; Abbott Diagnostics, Wiesbaden, Germany; CE marked version was used as the US version was not available when testing was conducted); GS HIV Combo Ag/Ab (BioRad Combo; Bio-Rad Laboratories, Redmond, WA); Determine™ HIV-1/2 Ag/Ab Combo (Determine Combo (rapid test); Alere Medical Co., Ltd. Scarborough, ME); GS HIV-1/HIV-2 PLUS O EIA (GS + O; Bio-Rad Laboratories, Redmond, WA); VITROS anti-HIV 1 + 2 assay (Vitros; Ortho-Clinical Diagnostics, Buckinghamshire, UK); ADVIA Centaur HIV 1/O/2 enhanced assay (Advia; Bayer, Tarrytown, NY); Abbott HIVAB HIV-1/2 (rDNA) EIA (Abbott; Abbott Laboratories, Abbott Park, IL); Avioq HIV-1 Microelisa system (Avioq; Avioq, Inc, Rockville, MD); INSTI™ HIV-1/HIV-2 Rapid Antibody test (INSTI (rapid test), BioLytical Laboratories Inc., Canada); Clearview HIV-1/2 STAT-PAK (Statpak (rapid test); Inverness Medical, Princeton, NJ); Clearview COMPLETE HIV-1/2 (Complete (rapid test); Inverness Medical, Princeton, NJ); DPP HIV-1/2 (DPP® HIV-1/2 Assay (rapid test); CHEMBIO Diagnostics Systems, Inc., Medford, NY); Reveal G2 and G3 Rapid HIV-1 antibody tests (Reveal G2 or G3 (rapid test); MedMira Laboratories, Inc.; Halifax, Nova Scotia, Canada); OraQuick ADVANCE Rapid HIV-1/2 antibody test (Oraquick (rapid test); OraSure Technologies, Inc., Bethlehem, PA); Uni-Gold Recombigen HIV (Unigold (rapid test); Trinity Biotech USA, St. Louis, MO). These assays have manufacturer reported point estimates for sensitivity ranging from 99.60% to 100.00% and point estimates for specificity ranging from 98.60% to 99.90%. The Genetic Systems HIV-1 Western blot (WB; Bio-Rad Laboratories, Redmond, WA) and Cambridge Biotech HIV-1 Western blot (WB; Maxim Biomedical Inc., Rockville, MD) have been shown to give concordant interpretations in studies conducted to qualify use in our clinical laboratory and were used interchangeably.
The paired comparison of antibody reactivity using 230 plasma specimens from 26 individuals in early stages of HIV-1 infection is shown in Table 1. In early HIV-1 infections, the comparison showed that INSTI detected significantly more infections than lateral flow RTs DPP HIV-1/2, Statpak, Complete, Unigold, and OraQuick. INSTI also detected significantly more positive samples than the flow-through RT, Reveal G2/G3. Determine™ HIV-1/2 Ag/Ab Combo rapid test (Determine Combo; Orgenic Ltd., Yavne, Israel) detected significantly more early HIV-1 infections than INSTI.
Table 1.
Paired comparison analysis of INSTI and other FDA-approved rapid tests (RT) using plasma specimens.
| Rapid test | Results of a 2 × 2 table comparing a RT and INSTI | ||||
|---|---|---|---|---|---|
|
|
|||||
| NR/NR | NR/R | R/NR | R/R | p value | |
|
| |||||
| Determine Comboa | 102 | 2 | 34 | 92 | <0.0001 * |
| Reveal G2/G3b | 134 | 14 | 1 | 80 | 0.0019 * |
| DPP HIV-1/2a | 134 | 15 | 2 | 79 | 0.0036 * |
| Statpaka | 135 | 20 | 1 | 74 | <0.0001 * |
| Completea | 135 | 22 | 1 | 72 | <0.0001 * |
| Unigoldb | 134 | 23 | 1 | 71 | <0.0001 * |
| Oraquickb | 133 | 34 | 2 | 60 | <0.0001 * |
McNemar’s test was used to compare the performance of INSTI and other FDA-approved RTs using early HIV-1 infection from 26 seroconverters. Results from INSTI testing were compared to historical data using the same specimens tested with Determine HIV-1/2 Ag/Ab Combo rapid test (Determine Combo), DPP HIV1/2 assay (DPP HIV-1/2), Clearview HIV-1/2 STAT-PAK (Statpak) and Clearview HIV-12/Complete (Complete) (an = 230) and with test OraQuick ADVANCE (Oraquick), Uni-Gold Recombigen HIV (Unigold), and Reveal G2 and G3 Rapid HIV-1 antibody test (Reveal) (bn = 229). NR: non-reactive; R: reactive
statistically significant.
4.2. INSTI performance using HIV-1 plasma specimens from the STOP study
Among 85 early HIV-1 samples confirmed with nucleic acid testing (74 were ARC-reactive/Multispot-negative and 11 were ARC-reactive/Multispot-indeterminate), INSTI was reactive in 15 (20.3%) Multispot-negative and 8 (72.7%) Multispot-indeterminate samples (Table 2). INSTI detected all 203 established HIV-1 infections. None of thirty-two samples false-reactive with ARC (HIV-1 RNA negative) were reactive with INSTI.
Table 2.
Summary of INSTI reactivity using HIV-1 plasma specimens from the STOP study.
| INSTI results |
|||
|---|---|---|---|
| Results of previous testing of ARC-positive plasma specimens | Total | positive negative | |
|
| |||
| MS-PL negative/SP-FS negative | 51 | 7 | 44 |
| MS-PL negative/SP-FS positive | 2 | 0 | 2 |
| MS-PL negative/SP-FS not available | 21 | 8 | 13 |
| Total | 74 | 15 | 59 |
| MS-PL indeterminate/SP-FS negative | 9 | 6 | 3 |
| MS-PL indeterminate/SP-FS positive | 1 | 1 | 0 |
| MS-PL indeterminate/SP-FS not available | 1 | 1 | 0 |
| Total | 11 | 8 | 3 |
| MS-PL positive/SP-FS negative | 9 | 9 | 0 |
| MS-PL positive/SP-FS positive | 174 | 174 | 0 |
| MS-PL positive/SP-FS not available | 20 | 20 | 0 |
| Total | 203 | 203 | 0 |
ARC: Abbott Architect; MS: Bio-Rad Multispot; SP: Clearview Stat-Pak rapid test; PL: plasma; FS: fingerstick whole blood; MS-PL-negative and –indeterminate specimens all tested positive with NAT.
From data obtained during field testing, the Statpak POC screening test using fingerstick whole blood detected two and one of the 53 ARC-reactive/Multispot-negative and 10 ARC-reactive/Multispot-indeterminate samples, respectively. Statpak using fingerstick whole blood missed nine established HIV-1 infections that were detected by INSTI with plasma (Table 2).
4.3. Sensitivity and specificity of INSTI
The sensitivity and 95% confidence intervals (CI) among 615 established HIV-1 infections (WB-positive) and 80 HIV-2 infections are shown in Table 3. The sensitivity of INSTI for HIV-1 infections was 99.8%, while the sensitivity for HIV-2 infections was 100%. INSTI detected all infections within HIV-1 groups M (non-B subtypes) and O, and HIV-2 groups A and B, but missed one HIV-1 group M subtype B infection. The specificity of INSTI was 99.8% (CI: 98.87–99.96%) among samples from 499 HIV-uninfected individuals.
Table 3.
Sensitivitya and specificity of INSTI HIV-1/HIV-2 antibody test.
| INSTI results |
||||||
|---|---|---|---|---|---|---|
| Total | Reactive | Non-reactive | Repeatdly Invalid | Sensitivity (%) | 95% Confidence Intervals | |
|
| ||||||
| HIV-1 Group M | ||||||
| HIV-1 B subtype | 501 | 500 | 1 | 0 | 99.8 | 98.88–99.96 |
| HIV-1 non-B subtype | 111 | 110 | 0 | 1 | 100 | 96.63–100 |
| HIV-1 Group O | 3 | 3 | 0 | 0 | 100 | 43.85–100 |
| HIV-2 (Groups A and B) | 80 | 80 | 0 | 0 | 100 | 95.42–100 |
| HIV-uninfected | 499 | 1 | 498 | 0 | 99.8 | 98.87–99.96 |
Overall sensitivity 99.84% [95% Confidence Interval: 99.08– 99.97%].
4.4. INSTI performance using specimens from patients on antiretroviral therapy
Plasma viral load was measured in 35 HIV-1-positive persons receiving antiretroviral therapy. Twenty-one persons on antiretroviral therapy had undetectable viral loads (target not detected), five had detectable HIV-1 RNA but below the measurable level of detection (<40 copies/ml), and nine had quantifiable viral loads with a mean of 163 copies/ml (range: 48–391 copies/ml). All 35 plasma specimens were reactive with INSTI.
4.5. INSTI performance using whole blood specimens from persons with HIV-1 and HIV-2 infections
To further assess INSTI performance in early HIV-1 infections, 40 sequential samples (three HIV-1 RNA-negative and 37 HIV-1 RNA-positive plasmas) from eight seroconverters were selected to prepare simulated whole blood. INSTI results with plasma and whole blood were compared to plasma results generated with two FDA-approved Ag/Ab (IgG/IgM) combo assays (one laboratory-based and one RT) and one FDA-approved IgG/IgM antibody-based immunoassay (IgG/IgM-IA), to evaluate how soon after infection INSTI detects antibodies in plasma and whole blood. Of 37 plasma samples with detectable HIV-1 RNA, 32 (86.5%) were positive using the Ag/Ab combo laboratory-based assay, 28 (75.7%) were positive with the Ag/Ab RT, 21 (56.8%) were positive using the IgG/IgM-IA, and 20 (54.1%) and 24 (64.9%) were detected by INSTI using plasma and whole blood, respectively. Of 28 Ag/Ab RT-positive plasma samples, 13 (46.4%) were Ag-positive and six (21.4%) were Ag/Ab-positive. INSTI was nonreactive in seven of 13 Ag-only positive samples and in five of six Ag/Ab-positive samples early in seroconversion. INSTI using plasma performed similarly to the IgG/IgM-IA; however, INSTI with simulated whole blood showed greater reactivity than both INSTI and the IgG/IgM-IA using plasma. To address possible false reactivity, Table 4 shows the reactivity of INSTI in four seroconverters that had five discrepant results between plasma and whole blood when compared to results from two Ag/Ab Combo assays and one IgG/IgM-IA. For three samples collected prior to seroconversion (HIV-1 NAT-negative samples), INSTI was not positive using plasma but gave false-positive results for one simulated whole blood sample. The remaining four discrepant simulated whole blood results were reactive early during infection when only NAT and Ag/Ab combo tests were reactive. Antibody reactivity was not detected for these times points in the IgG/IgM-IA or the Ag/Ab RT.
Table 4.
Comparison of INSTI performance with discrepant results in simulated whole blood and plasma from four HIV-1 seroconverters with other assays during positive and negative time points
| Sample | Days between bleeds | NAT | IA | IA | RT | INSTI | ||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Ag/Ab | IgG/IgM | Ag | Ab | plasma | whole blood | |||
|
| ||||||||
| 924-04 | nonreactive | − | − | − | − | − | + | |
| 924-05 | 16 | reactive | + | − | + | − | − | − |
| 924-06 | 7 | reactive | + | + | + | + | + | + |
| 924-07 | 2 | reactive | + | + | − | + | + | + |
| 924-08 | 5 | reactive | + | + | − | + | + | + |
|
| ||||||||
| 931-05 | reactive | + | − | − | − | − | + | |
| 931-06 | 13 | reactive | + | + | + | + | + | + |
| 931-07 | 5 | reactive | + | + | − | + | + | + |
| 931-08 | 2 | reactive | + | + | − | + | + | + |
| 931-09 | 7 | reactive | + | + | − | + | + | + |
|
| ||||||||
| 940-01 | reactive | + | − | − | − | − | − | |
| 940-02 | 7 | reactive | + | − | + | − | − | + |
| 940-03 | 4 | reactive | + | + | + | + | + | + |
| 940-04 | 4 | reactive | + | + | − | + | + | + |
| 940-05 | 3 | reactive | + | + | − | + | + | + |
|
| ||||||||
| 944-01 | reactive | − | − | − | − | − | − | |
| 944-02 | 2 | reactive | − | − | − | − | − | − |
| 944-03 | 5 | reactive | + | − | + | − | − | + |
| 944-04 | 2 | reactive | + | − | + | − | − | + |
| 944-05 | 5 | nonreactive | + | + | − | + | + | + |
| 944-06 | 2 | reactive | + | + | − | + | + | + |
NAT: FDA-approved diagnostic nucleic acid test; IA Ag/Ab: FDA-approved laboratory-based antigen/antibody immunoassay; IA IgG/IgM: FDA-approved laboratory-based IgG/IgM immunoassay; RT Ag/Ab: FDA-approved antigen/antibody rapid test
INSTI was reactive using all 24 HIV-2 simulated whole blood specimens. INSTI was non-reactive for all 15 simulated HIV-negative whole blood specimens (data not shown).
5. Discussion
This is the first study to describe the performance of the CLIA-waived antibody-based INSTI RT using longitudinal plasma specimens from U.S. seroconverters likely infected with subtype B. Our findings show that the relative reactivity of INSTI in early HIV-1 infection places it among the most sensitive RTs. INSTI reacts six days after DC, which also detects p24 antigen. Paired comparison analysis using plasma specimen results revealed that INSTI performs significantly better than other FDA-approved CLIA-waived antibody-based RTs that require longer incubation times to obtain results. INSTI is the only RT available in the U.S. that gives results in approximately 60 s [12], and has been shown to perform similarly to plasma when fingerstick whole blood was used by untrained personnel [8], which makes it an optimal choice for certain POC settings.
The overall sensitivity of INSTI for detecting divergent HIV-1 (including non-B group M subtypes and group O infections) and HIV-2 and its specificity using plasma specimens fell within the confidence intervals reported by the manufacturer [7]. INSTI also performed well using plasma specimens from several studies. Samples from the STOP study were characterized using Multispot as a supplemental test, prior to the test being discontinued. In this sample set, INSTI was reactive in more specimens than Multispot during early stages of HIV-1 infection. In plasma and simulated whole blood from commercial seroconverters, INSTI also detected most samples that were positive with an IgG/IgM-IA but primarily missed samples that were only p24 antigen reactive. Furthermore, the performance of INSTI was not affected by using plasma or whole blood specimens from persons on antiretroviral treatment. Overall, these results indicate that the INSTI RT can detect antibodies when titers are increasing during seroconversion and supports recent findings that suggest INSTI can detect IgM, the first antibody rising during seroconversion [28].
Though our results show that INSTI performed well using simulated whole blood, the use of simulated rather than whole blood is a limitation of our study. INSTI showed false reactivity with one HIV-1 NAT-negative sample. Four samples that were INSTI-reactive using whole blood were non-reactive with INSTI, an IgG/IgM immunoassay and the differentiation Ag/Ab RT using plasma. INSTI reactivity using simulated whole blood occurred mostly with samples in the early stages of HIV-1 infection, which could reflect false reactivity or some other reason INSTI did not detect those time points using plasma. Using INSTI and IgG/IgM-IA results generated with plasma as our reference, reactivity between plasma and whole blood specimens was similar in seroconversion samples. Assuming that the use of fingerstick whole blood with INSTI in POC settings may yield similar results to plasma, then many infections missed using other RTs, such as occurred with using Statpak in the STOP study [24], could likely be detected with INSTI.
The benefits of a faster CLIA-waived RT like INSTI, that also has a high sensitivity for antibody detection, could improve testing workflows in POC settings. The ease of use of INSTI and almost instant results makes this test an accurate option for settings where recommended laboratory testing is not feasible, or for sites testing persons who might not return to receive their test results.
Acknowledgments
We want to acknowledge William M. Switzer, lead of the HIV Diagnostics and Incidence team, for kindly reviewing this manuscript.
Funding
CDC intramural funding.
Footnotes
Competing interest
No financial disclosures were reported by the authors of this paper.
Ethical approval
The study was approved by CDC through a research determination in accordance with federal human participants’ protection regulations and CDC policies and procedures.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention, or any of the authors’ affiliated institutions. Use of brand names is for identification purposes and does not imply endorsement by the US Department of Health and Human Services, the Public Health Service, or the Centers for Disease Control and Prevention.
References
- [1].Cherutich P, Bunnell R, Mermin J, HIV testing: current practice and future directions, Curr. HIV/AIDS Rep. 10 (2) (2013) 134–141. [DOI] [PubMed] [Google Scholar]
- [2].Martin EG, et al. , Use of a rapid HIV testing algorithm to improve linkage to care, J. Clin. Virol. 52 (Suppl. 1) (2011) S11–S15. [DOI] [PubMed] [Google Scholar]
- [3].Patel P, et al HIV Rapid, screening: missed opportunities for HIV diagnosis and prevention, J. Clin. Virol. 54 (1) (2012) 42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Masciotra S, et al. , Evaluation of an alternative HIV diagnostic algorithm using specimens from seroconversion panels and persons with established HIV infections, J. Clin. Virol. 52 (Suppl. 1) (2011) S17–S22. [DOI] [PubMed] [Google Scholar]
- [5].Owen SM, et al. , Alternative algorithms for human immunodeficiency virus infection diagnosis using tests that are licensed in the United States, J. Clin. Microbiol. 46 (5) (2008) 1588–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Delaney KP, et al. , Evaluation of the performance characteristics of 6 rapid HIV antibody tests, Clin. Infect. Dis. 52 (2) (2011) 257–263. [DOI] [PubMed] [Google Scholar]
- [7].bioLytical Laboratories Inc., INSTITM HIV-1/HIV-2 Antibody test kit [package insert]. Vancouver, 2015. Canada. [Google Scholar]
- [8].Galli RA, et al. , Evaluation of the accuracy and ease of use of a rapid HIV-1 Antibody Test performed by untrained operators at the point of care, J. Clin. Virol. 58 (Suppl 1) (2013) e65–e69. [DOI] [PubMed] [Google Scholar]
- [9].Pavie J, et al. , Sensitivity of five rapid HIV tests on oral fluid or finger-stick whole blood: a real-time comparison in a healthcare setting, PLoS One 5 (7) (2010) e11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Casalino E, et al. , Twelve months of routine HIV screening in 6 emergency departments in the Paris area: results from the ANRS URDEP study, PLoS One 7 (10) (2012) e46437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sherman GG, Lilian RR, Coovadia AH, The performance of 5 rapid HIV tests using whole blood in infants and children: selecting a test to achieve the clinical objective, Pediatr. Infect. Dis. J. 31 (3) (2012) 267–272. [DOI] [PubMed] [Google Scholar]
- [12].Lee BE, et al HIV, et al. , Rapid HIV tests in acute care settings in an area of low HIV prevalence in Canada, J. Virol. Methods 172 (1–2) (2011) 66–71. [DOI] [PubMed] [Google Scholar]
- [13].Gennotte AF, et al. , Feasibility and acceptability of HIV screening through the use of rapid tests by general practitioners in a Brussels area with a substantial African community, HIV Med. 14 (Suppl. 3) (2013) 57–60. [DOI] [PubMed] [Google Scholar]
- [14].Stekler JD, Golden MR, Learning from the missed opportunities for HIV testing, Sex. Transm. Infect. 85 (1) (2009) 2–3. [DOI] [PubMed] [Google Scholar]
- [15].Stekler JD, et al. , Relative accuracy of serum, whole blood, and oral fluid HIV tests among Seattle men who have sex with men, J. Clin. Virol. 58 (Suppl. 1) (2013) e119–e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stekler JD, et al. , HIV testing in a high-incidence population: is antibody testing alone good enough? Clin. Infect. Dis. 49 (3) (2009) 444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bayer R, Oppenheimer GM, Routine HIV testing, public health, and the USPSTF-an end to the debate, N. Engl. J. Med. 368 (10) (2013) 881–884. [DOI] [PubMed] [Google Scholar]
- [18].Laforgerie E, et al. , Sensitivity of 8 CE (European Community)-approved rapid disposable tests for anti-HIV antibody detection during and after seroconversion, J. Virol. Methods 165 (1) (2010) 105–107. [DOI] [PubMed] [Google Scholar]
- [19].Cook D, Detection of Early Sero-Conversion HIV infection using the INSTI HIV-1 antibody point-of-care test, Open AIDS J. 4 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Masciotra S, et al. , Performance of the alere determine HIV-1/2 Ag/Ab combo rapid test with specimens from HIV-1 seroconverters from the US and HIV-2 infected individuals from ivory coast, J. Clin. Virol. 58 (Suppl. 1) (2013) e54–e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nasrullah M, et al. , Performance of a fourth-generation HIV screening assay and an alternative HIV diagnostic testing algorithm, AIDS 27 (5) (2013) 731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Geren KME, Tomlinson C, Hobohm D, et al. , Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm-United States, 2011–2013, MMWR 62 (24) (2013). [PMC free article] [PubMed] [Google Scholar]
- [23].Pandori MW, et al. , The Multispot rapid HIV-1/HIV-2 differentiation assay is comparable with the Western blot and an immunofluorescence assay at confirming HIV infection in a prospective study in three regions of the United States, J. Clin. Virol. 58 (Suppl. 1) (2013) e92–e96. [DOI] [PubMed] [Google Scholar]
- [24].Peters PJ, et al. , Screening yield of HIV antigen/antibody combination and pooled HIV RNA testing for acute HIV infection in a high-prevalence population, JAMA 315 (7) (2016) 682–690. [DOI] [PubMed] [Google Scholar]
- [25].Centers for Disease Control and Prevention and Association of Public Health Laboratories, Laboratory testing for the diagnosis of HIV infection: updated recommendations. 2014. [Google Scholar]
- [26].Wesolowski LG, et al. , Performance of an alternative laboratory-based algorithm for diagnosis of HIV infection utilizing a third generation immunoassay, a rapid HIV-1/HIV-2 differentiation test and a DNA or RNA-based nucleic acid amplification test in persons with established HIV-1 infection and blood donors, J. Clin. Virol. 52 (Suppl. 1) (2011) S45–S49. [DOI] [PubMed] [Google Scholar]
- [27].Masciotra S, et al. , Performance evaluation of the CHEMBIO DPP® (dual path platform) HIV-1/2 assay in early and established infections, J. Clin. Virol. 70 (2015) 97–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Moshgabadi N, et al. , Sensitivity of a rapid point of care assay for early HIV antibody detection is enhanced by its ability to detect HIV gp41 IgM antibodies, J. Clin. Virol. (2015). [DOI] [PubMed] [Google Scholar]


