Abstract
Background
Data on the epidemiology of sexually transmitted infections (STIs) among transgender women (TGW) with and without human immunodeficiency virus (HIV) are limited.
Methods
We analyzed baseline data collected from a cohort of adult TGW across 6 eastern and southern US cities between March 2018 and August 2020 (n = 1018). Participants completed oral HIV screening, provided self-collected rectal and urogenital specimens for chlamydia and gonorrhea testing, and provided sera specimens for syphilis testing. We assessed associations with ≥1 prevalent bacterial STI using modified Poisson regression.
Results
Bacterial STI prevalence was high and differed by HIV status: 32% among TGW with HIV and 11% among those without HIV (demographic-adjusted prevalence ratio = 1.91; 95% confidence interval = 1.39–2.62). Among TGW without HIV, bacterial STI prevalence differed by geographic region, race and ethnicity, and gender identity, and was positively associated with reporting >1 sexual partner, hazardous alcohol use, homelessness, having safety concerns regarding transit to health care, and no prior receipt of gender-affirming health services. Among TGW with HIV, older age was inversely associated with bacterial STI.
Conclusions
TGW had a high prevalence of bacterial STIs. The prevalence and correlates of bacterial STI differed by HIV status, highlighting the unique needs and risks of TGW with and without HIV. Tailored interventions may reduce sexual health-related inequities.
Keywords: transgender women, sexually transmitted infections, health disparities, HIV, situated vulnerabilities
In a multicity, community-based sample of adult transgender women in the United States, the prevalence and correlates of bacterial sexually transmitted infections differed substantially by HIV status, highlighting the unique needs and risks of transgender women with and without HIV.
(See the Editorial Commentary by Rushmore on pages 1603–5.)
Sexually transmitted infections (STIs) are increasing in the United States [1], and are associated with a higher risk of human immunodeficiency virus (HIV) transmission and acquisition and reduced quality of life [2, 3]. Transgender women (TGW) are disproportionately affected by HIV and other STIs. HIV prevalence among TGW in the United States is estimated to be 14%—more than 42 times the national HIV prevalence (0.3%) [4]. Data on bacterial STIs among TGW in the United States are sparse, but available data suggest a similarly high burden [5]. Most epidemiological studies of STIs among TGW, however, have been limited to clinic-based samples; there remains limited community-based studies characterizing the bacterial STI burden among TGW in the United States [6, 7].
The high HIV and STI burden among TGW is driven by a complex array of biological, behavioral, social, and structural-level factors that often co-occur to enhance risks [8]. In addition to low condom use during anal intercourse with cisgender male sexual partners, small, dense sexual networks may facilitate HIV and STI transmission among TGW and partners [4, 9–11]. Sexual health disparities are also situated alongside other social and health conditions disproportionally affecting transgender people (eg, social and economic marginalization, substance use, violence, and health care barriers) [8, 12]. These multilevel, situated vulnerabilities are recognized as drivers of HIV risk, but the degree to which they impact bacterial STI risk among TGW remains incompletely understood [12–16].
Intersectional stigma and discrimination associated with living with HIV among TGW exacerbate situated vulnerabilities [17, 18], which may place TGW with HIV at heightened STI risk. Conversely, once diagnosed, TGW with HIV may have increased access to health care that could lead to differences in access to STI testing and treatment. Other STI studies among TGW have not typically characterized the unique epidemiology and contexts associated with STIs that may differ between those with and without HIV [6, 19]. We hypothesize that a person's lived experience with HIV could modify whether sociodemographic factors, sexual behaviors, and situated vulnerabilities are associated with bacterial STI outcomes. Elucidating the distribution and drivers of bacterial STIs unique to TGW with and without HIV may facilitate tailored prevention and treatment strategies optimally suited for each population.
This cross-sectional study aimed to characterize the prevalence and correlates of bacterial STIs by HIV status among a community-based sample of adult TGW in 6 cities in the eastern and southern United States.
METHODS
Study Design and Population
The Leading Innovation for Transgender Women's Health and Empowerment (LITE) Study was a multisite prospective cohort study that examined HIV incidence and other health outcomes among transfeminine adults in the eastern and southern United States. The cohort was informed by formative research [20, 21] and methods have been previously described [22]. The study included both site-based recruitment in 6 cities and a completely digital, remote mode [23]. Activities were similar between modes, except STI testing was not conducted for the digital mode. Here, we present baseline data from the site-based mode.
Participants were recruited via convenience sampling methods, including peer referral, social media, dating apps, and referrals from clinics and community-based organizations. Eligibility criteria included being 18 years or older, endorsing a transfeminine identity [24, 25], and residing in a site-based city (Atlanta, Baltimore, Boston, Miami, New York City [NYC], and Washington, DC). Enrollment to the baseline survey was status neutral, although final enrollment was restricted to people without HIV at baseline, given the primary aim to measure HIV incidence [26]. At baseline, all participants completed a facility-based study visit, which included a sociobehavioral survey (self-administered or interviewer administered, if literacy was low), laboratory-confirmed HIV and bacterial STI testing, and specimen collection (serum, plasma, and urine) [22]. Plasma samples were stored at −80°C.
Between March 2018 and August 2020, 1030 TGW completed a baseline assessment. Twelve participants missing all bacterial STI test results were excluded, yielding an analytic sample of 1018 participants.
Laboratory Testing
Bacterial STI testing was conducted locally at facility-based visits. Self-collected urine, anorectal, and vaginal (for those with vaginoplasty) swabs were tested for presence of Chlamydia trachomatis and Neisseria gonorrhoeae infection using nucleic acid amplification testing with the Gen-Probe Aptima Combo 2 assay (Hologic).
For participants in Baltimore, initial antibody testing was performed for Treponema pallidum, followed by confirmatory rapid plasma reagin (RPR) testing with quantitative titers. All other sites performed initial RPR testing, followed by confirmatory treponemal antibody testing. A diagnosis of syphilis was defined as a newly reactive RPR with a positive treponemal test or a 4-fold increase in RPR titers after past diagnosis and treatment based on review of available clinical records.
Participants self-administrated the OraQuick In-Home HIV Test (OraSure Technologies) during the facility visit with support from trained study staff. All participants with a positive HIV test underwent confirmatory testing and were referred to a local HIV care facility [22, 27].
Outcomes
The primary outcome was the prevalence of at least 1 bacterial STI (C. trachomatis, N. gonorrhoeae, or syphilis) at any anatomic site. Among the 1018 participants, 9 were missing syphilis results. Three of these participants had a positive C. trachomatis infection and were coded as having a bacterial STI, while the remaining 6 participants were negative for C. trachomatis and N. gonorrhoeae infection and were coded as not having a bacterial STI. We also examined the overall and anatomic-site specific prevalence of each individual bacterial STI.
Covariates
Data on covariates were ascertained on the baseline survey via self-report. Sociodemographic variables included age, race and ethnicity (non-Hispanic white, non-Hispanic black, Hispanic/Latinx, and non-Hispanic other/mixed race), geographic region (Northeast [Boston/NYC], Upper South Atlantic [Baltimore/Washington DC], and Lower South Atlantic [Atlanta/Miami]), gender identity (female/women, transwoman, and genderqueer/nonbinary/other), marital status (single/casually dating, committed relationship/married, other), and educational attainment (no high school completion, high school completion, and completed some college or more). Sparse response categories for gender identity (eg, transfeminine/trans femme, woman of trans experience, person of trans experience, 2-spirit, and “prefer not to answer”) were combined to form the “genderqueer/nonbinary/other” category.
Data on sexual behaviors in the past 12 months included sexual activity (anal or vaginal sex) (yes/no), partner concurrency (having more than 1 sexual partner at the same time) (yes/no), any condomless anal or vaginal sex (yes/no), and gender of sexual partner(s). The question regarding gender of sexual partner(s) included the answers: “cisgender (nontransgender) man,” “cisgender (nontransgender) woman,” “transgender woman/male-to-female,” “transgender man/female-to-male,” “genderqueer or gender nonconforming (female at birth),” and “genderqueer or gender nonconforming (male at birth).” Participants who reported only cisgender male sexual partners were categorized as having partners that were “cisgender men only.” Participants who reported the gender of their sexual partner(s) as cisgender woman, transgender woman, transgender male, and/or genderqueer were categorized as having partners that were “non-cisgender men only.” Participants who reported sexual partners in both categories were classified as having “multiple partners of different genders.” Data on the number of sexual partners in the past 3 months were dichotomized a priori (0–1 vs >1). Preexposure prophylaxis (PrEP) use in the past 30 days (yes/no) was assessed among participants without HIV.
We also evaluated situated vulnerabilities relating to substance use, sociostructural factors, and health care access. The Alcohol Use Disorder Identification Test-Consumption (AUDIT-C) score of ≥4 was used to classify high-risk alcohol use [28]. Lifetime psychological (4 items), physical (6 items), and sexual violence (4 items) was assessed using an adaptation of the Conflicts Tactics Scale [29]; we created a binary measure for each type of violence (never/ever). Other sociostructural factors included lifetime history of sex work (never/ever), incarceration (never/ever), and homelessness (never, former [≥3 months ago], and current [<3 months ago]). We also evaluated health insurance status (insured/uninsured), concern about safety in transit to health care (yes/no), and receipt of gender-affirming health services, including gender identity therapy or counseling (never/former/current), exogenous hormone use (never/former/current), or genital surgery (ie, vaginoplasty/orchiectomy) (yes/no). Among TGW with HIV, we assessed engagement in HIV care within the past 12 months (yes/no).
Statistical Analysis
We estimated the prevalence of any bacterial STI and each individual and anatomic site-specific bacterial STI overall and by HIV status. To quantify the association between HIV status and STI outcomes, we estimated crude prevalence ratios (PR) using Poisson regression with robust variance estimators. We also estimated adjusted prevalence ratios (aPRs) using multivariable models that adjusted for age, race and ethnicity, and geographic region as these demographic covariates were expected a priori to be associated with prevalent HIV and STIs. Next, we examined sociodemographic, sexual behavior, and situated vulnerability-related correlates of any bacterial STI stratified by HIV status. Univariable and multivariable regression models were constructed separately for each covariate of interest; the multivariable model for each covariate of interest was consistently adjusted for age, race and ethnicity, and geographic region. An available-case approach was used to handle missing covariate data in regression models (<6% for all variables except for engagement in HIV care [13%]).
Ethics Approval
All participants provided written informed consent and study procedures were approved by the Johns Hopkins University School of Medicine Institutional Review Board.
RESULTS
Sample Characteristics
Overall (n = 1018), the median age was 31 years, 29% (n = 296) were non-Hispanic/Latinx black, 27% (n = 275) were Hispanic, and HIV prevalence was 27% (n = 276) (Table 1). There were 79 (8%) participants who self-reported a history of vaginoplasty. The distribution of sociodemographic characteristics, sexual behaviors, and situated vulnerability-related characteristics varied by HIV status (Table 1).
Table 1.
Characteristics of the Study Population, Transgender Women in the Eastern and Southern United States, by HIV Status
| Characteristic | Overall (n = 1018) | Without HIV (n = 742) | With HIV (n = 276) | |||
|---|---|---|---|---|---|---|
| Median age, y (IQR) | 31 (25–42) | 29 (24–38) | 40 (31–51) | |||
| Race and ethnicity | ||||||
| White, non-Hispanic/Latinx | 286 | (28) | 274 | (37) | 12 | (4) |
| Black, non-Hispanic/Latinx | 296 | (29) | 145 | (20) | 151 | (55) |
| Hispanic, any race | 275 | (27) | 201 | (27) | 74 | (27) |
| Other/mixed race, non-Hispanic/Latinx | 161 | (16) | 122 | (16) | 39 | (14) |
| Geographic region | ||||||
| Northeast | ||||||
| Boston | 184 | (18) | 170 | (23) | 14 | (5) |
| New York City | 253 | (25) | 216 | (29) | 37 | (13) |
| Upper South Atlantic | ||||||
| Baltimore | 131 | (13) | 78 | (11) | 53 | (19) |
| Washington, DC | 182 | (18) | 122 | (16) | 60 | (22) |
| Lower South Atlantic | ||||||
| Atlanta | 115 | (11) | 66 | (9) | 49 | (18) |
| Miami | 153 | (15) | 90 | (12) | 63 | (23) |
| Gender identity | ||||||
| Female or woman | 347 | (34) | 253 | (34) | 94 | (34) |
| Transwoman | 488 | (48) | 346 | (47) | 142 | (51) |
| Genderqueer/nonbinary/othera | 183 | (18) | 143 | (19) | 40 | (14) |
| Marital status | ||||||
| Single or casually dating | 692 | (68) | 490 | (66) | 202 | (73) |
| Committed or married | 267 | (26) | 204 | (27) | 63 | (23) |
| Other | 44 | (4) | 42 | (6) | 2 | (1) |
| Unknown | 15 | (1) | 6 | (1) | 9 | (3) |
| Educational attainment | ||||||
| No high school completion | 169 | (17) | 92 | (12) | 77 | (28) |
| High school completion | 267 | (26) | 167 | (23) | 100 | (36) |
| Completed some college or moreb | 573 | (56) | 476 | (64) | 97 | (35) |
| Unknown | 9 | (1) | 7 | (1) | 2 | (1) |
| Sexual activity, 12 mo | ||||||
| No | 230 | (23) | 165 | (22) | 65 | (24) |
| Yes | 776 | (76) | 575 | (77) | 201 | (73) |
| Unknown | 12 | (1) | 2 | (0.3) | 10 | (4) |
| Gender(s) of sexual partner(s), 12 moc | ||||||
| Cisgender men only | 472 | (61) | 304 | (53) | 168 | (84) |
| Non-cisgender men only | 97 | (13) | 92 | (16) | 5 | (2) |
| Multiple partners of different gendersd | 204 | (26) | 177 | (31) | 27 | (13) |
| Unknown | 3 | (0.4) | 2 | (0.3) | 1 | (0.5) |
| Sexual partner concurrency, 12 moc | ||||||
| No | 431 | (56) | 308 | (54) | 123 | (61) |
| Yes | 333 | (43) | 259 | (45) | 74 | (37) |
| Unknown | 12 | (2) | 8 | (1) | 4 | (2) |
| Condomless sex, 12 moc | ||||||
| No | 212 | (27) | 126 | (22) | 86 | (43) |
| Yes | 549 | (71) | 440 | (77) | 109 | (54) |
| Unknown | 15 | (2) | 9 | (2) | 6 | (3) |
| Multiple sex partners, 3 mo | ||||||
| No | 542 | (53) | 391 | (53) | 151 | (55) |
| Yes | 464 | (46) | 349 | (47) | 115 | (42) |
| Unknown | 12 | (1) | 2 | (0.3) | 10 | (4) |
| PrEP use | ||||||
| Never | … | … | 526 | (71) | … | … |
| Former, ≥30 d ago | … | … | 84 | (11) | … | … |
| Current, <30 d ago | … | … | 119 | (16) | … | … |
| Unknown | … | … | 13 | (2) | … | … |
| AUDIT-C | ||||||
| Score < 4 | 681 | (67) | 485 | (65) | 196 | (71) |
| Score ≥ 4 | 298 | (29) | 232 | (31) | 66 | (24) |
| Unknown | 39 | (4) | 25 | (3) | 14 | (5) |
| Psychological violence | ||||||
| Never | 223 | (22) | 131 | (18) | 92 | (33) |
| Ever | 774 | (76) | 602 | (81) | 172 | (62) |
| Unknown | 21 | (2) | 9 | (1) | 12 | (4) |
| Physical violence | ||||||
| Never | 378 | (37) | 268 | (36) | 110 | (40) |
| Ever | 614 | (60) | 463 | (62) | 151 | (55) |
| Unknown | 26 | (3) | 11 | (1) | 15 | (5) |
| Sexual violence | ||||||
| Never | 559 | (55) | 408 | (55) | 151 | (55) |
| Ever | 431 | (42) | 321 | (43) | 110 | (40) |
| Unknown | 28 | (3) | 13 | (2) | 15 | (5) |
| Sex work | ||||||
| Never | 484 | (48) | 408 | (55) | 76 | (28) |
| Ever | 513 | (50) | 324 | (44) | 189 | (68) |
| Unknown | 21 | (2) | 10 | (1) | 11 | (4) |
| Incarceration history | ||||||
| Never | 705 | (69) | 575 | (77) | 130 | (47) |
| Ever | 273 | (27) | 143 | (19) | 130 | (47) |
| Unknown | 40 | (4) | 24 | (3) | 16 | (6) |
| Homelessness | ||||||
| Never | 499 | (49) | 397 | (54) | 102 | (37) |
| Ever, ≥3 mo ago | 365 | (36) | 247 | (33) | 118 | (43) |
| Ever, <3 mo ago | 131 | (13) | 83 | (11) | 48 | (17) |
| Unknown | 23 | (2) | 15 | (2) | 8 | (3) |
| Health insurance | ||||||
| Insured | 915 | (90) | 660 | (89) | 255 | (92) |
| Uninsured | 86 | (8) | 75 | (10) | 11 | (4) |
| Unknown | 17 | (2) | 7 | (1) | 10 | (4) |
| Safety concern in transit to health care | ||||||
| No | 565 | (56) | 433 | (58) | 132 | (48) |
| Yes | 444 | (44) | 304 | (41) | 140 | (51) |
| Unknown | 9 | (1) | 5 | (1) | 4 | (1) |
| Gender identity therapy or counseling | ||||||
| Never | 282 | (28) | 191 | (26) | 91 | (33) |
| Former, ≥3 mo ago | 299 | (29) | 226 | (30) | 73 | (26) |
| Current, <3 mo ago | 418 | (41) | 318 | (43) | 100 | (36) |
| Unknown | 19 | (2) | 7 | (1) | 12 | (4) |
| Hormone treatment use | ||||||
| Never | 144 | (14) | 82 | (11) | 62 | (22) |
| Former, ≥3 mo ago | 74 | (7) | 44 | (6) | 30 | (11) |
| Current, <3 mo ago | 783 | (77) | 609 | (82) | 174 | (63) |
| Unknown | 17 | (2) | 7 | (1) | 10 | (4) |
| Genital surgerye | ||||||
| Never | 844 | (83) | 612 | (82) | 232 | (84) |
| Ever | 141 | (14) | 111 | (15) | 30 | (11) |
| Unknown | 33 | (3) | 19 | (3) | 14 | (5) |
| Engagement in HIV care, 12 mo | ||||||
| No | … | … | … | … | 34 | (12) |
| Yes | … | … | … | … | 207 | (75) |
| Unknown | … | … | … | … | 35 | (13) |
Data are No. (%) except where indicated as an interquartile range. Percentages may not total to 100% due to rounding.
Abbreviations: AUDIT-C, Alcohol Use Disorder Identification Test-Consumption; HIV, human immunodeficiency virus; IQR, interquartile range; PrEP, preexposure prophylaxis.
aOther includes transfeminine/trans femme, woman of trans experience, person of trans experience, 2-spirit, other identity, and prefer not to answer.
bIncludes associate's degree and technical college.
cAmong those who are sexually active in the past 12 months (overall, n = 776; without HIV, n = 575; with HIV, n = 201).
dGenders include man, woman, transgender woman, transgender man, genderqueer (female at birth), and genderqueer (male at birth).
eVaginoplasty or orchiectomy.
Bacterial STI Prevalence Overall and by HIV Status
The overall prevalence of any bacterial STI was 16% (C. trachomatis [5%], N. gonorrhoeae [2%], and syphilis [11%]; Table 2). Of the 53 participants with C. trachomatis infection, most were detected at the rectal site (n = 49) versus the urogenital site (n = 6). Two participants had C. trachomatis infections detected at both rectal and urogenital sites. Of the 50 participants with a history of vaginoplasty that were tested for C. trachomatis, C. trachomatis infection was detected at the neovaginal site and urogenital site for 1 participant. Of the 24 participants with N. gonorrhoeae infection, all had N. gonorrhoeae detected at the rectal site with 2 participants also having N. gonorrhoeae detected at the urogenital site.
Table 2.
Prevalence of Bacterial STIs Overall By HIV Status
| Overall | Without HIV | With HIV | PR (95% CI)a | Adjusted PR (95% CI)a,b | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tested, No. | Positive, No. (%) | Tested, No. | Positive, No. (%) | Tested, No. | Positive, No. (%) | ||||||
| ≥1 Bacterial STI | 1018 | 167 | (16) | 742 | 79 | (11) | 276 | 88 | (32) | 2.99 (2.28–3.93) | 1.91 (1.39–2.62) |
| Chlamydia | 1018 | 53 | (5) | 742 | 32 | (4) | 276 | 21 | (8) | 1.76 (1.04–3.01) | 1.77 (.95–3.28) |
| Rectal | 1013 | 49 | (5) | 737 | 31 | (4) | 276 | 18 | (7) | … | … |
| Urogenital | 1014 | 6 | (0.6) | 739 | 2 | (0.3) | 275 | 4 | (1) | … | … |
| Vaginal | 50 | 1 | (2) | 41 | 0 | (0) | 9 | 1 | (11) | … | … |
| Gonorrhea | 1018 | 24 | (2) | 742 | 13 | (2) | 276 | 11 | (4) | 2.27 (1.03–5.02) | 2.53 (.86–7.38) |
| Rectal | 1012 | 24 | (2) | 737 | 13 | (2) | 275 | 11 | (4) | … | … |
| Urogenital | 1012 | 2 | (0.2) | 738 | 0 | (0) | 274 | 2 | (0.7) | … | … |
| Vaginal | 48 | 0 | (0) | 40 | 0 | (0) | 8 | 0 | (0) | … | … |
| Syphilis | 1009 | 112 | (11) | 740 | 40 | (5) | 269 | 72 | (27) | 4.95 (3.45–7.10) | 2.62 (1.72–4.00) |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; PR, prevalence ratio; STI, sexually transmitted infection.
aPrevalence ratios of each STI outcome were estimated by HIV status using Poisson regression models with robust variance estimation. The reference group was people without HIV.
bFor each STI outcome variable, a separate multivariable regression model was used that adjusted for age, race and ethnicity, and geographic region.
Compared to TGW without HIV, TGW with HIV had a higher prevalence of any bacterial STI (PR = 2.99; 95% confidence interval [CI] = 2.28–3.93), C. trachomatis infection (PR = 1.76; 95% CI = 1.04–3.01), N. gonorrhoeae infection (PR = 2.27; 95% CI = 1.03–5.02), and syphilis (PR = 4.95; 95% CI = 3.45–7.10) (Table 2). After adjustment for age, race and ethnicity, and geographic region, HIV infection (vs no HIV infection) remained significantly associated with any bacterial STI (aPR = 1.91; 95% CI = 1.39–2.62) and syphilis (aPR = 2.62; 95% CI = 1.72–4.00).
The prevalence of any bacterial STI coinfection was 0.8% among TGW without HIV and 5% among TGW with HIV (PR = 6.42; 95% CI = 2.49–16.54 and aPR = 6.67; 95% CI = 2.00–22.22) (Supplementary Figure 1).
Correlates of Bacterial STI Prevalence by HIV Status
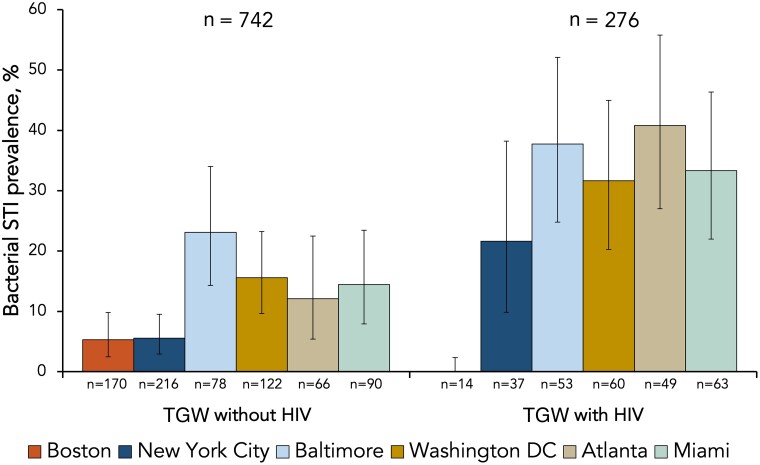
Among TGW without HIV, prevalence of any bacterial STI did not vary with age but was significantly higher in non-Hispanic black and Hispanic TGW compared to non-Hispanic white TGW (PR = 8.03; 95% CI = 3.82–16.90 and PR = 4.77; 95% CI = 2.22–10.25, respectively) (Table 3). Bacterial STI prevalence varied by city among TGW without HIV (Figure 1 and Supplementary Table 1). Participants residing in the Upper (Baltimore/Washington, DC) and Lower (Atlanta/Miami) South Atlantic regions were more likely to have a bacterial STI compared to those residing in the Northeast (Boston/NYC) (PR = 3.40; 95% CI = 2.05–5.65 and PR = 2.47; 95% CI = 1.39–4.40, respectively) (Table 3). Among TGW without HIV, participants who identified as gender nonbinary, genderqueer, or another gender identity were 79% more likely to have a bacterial STI compared to participants identifying as transwomen (PR = 1.79; 95% CI = 1.10–2.92). Among TGW without HIV, race and ethnicity, geographic region, and gender identity remained significantly associated with bacterial STI prevalence in demographic-adjusted models.
Table 3.
Sociodemographic Correlates of Bacterial STI Prevalence by HIV Status
| Sociodemographics | Outcome: ≥1 Bacterial STI | |||||||
|---|---|---|---|---|---|---|---|---|
| TGW Without HIV | TGW With HIV | |||||||
| No. | Prevalence, No. (%) | Crude PR (95% CI)a | Adjusted PR (95% CI)a,b | No. | Prevalence, No. (%) | Crude PR (95% CI)a | Adjusted PR (95% CI)a,b | |
| Age, y, continuous | 742 | … | 0.96 (.87–1.06) | 0.95 (.85–1.06) | 276 | … | 0.92 (.85–.99) | 0.93 (.86–.997) |
| Race and ethnicity | ||||||||
| White, non-Hispanic/Latinx | 274 | 8 (3) | Ref | Ref | 12 | 5 (42) | 1.12 (.56–2.27) | 1.31 (.69–2.51) |
| Black, non-Hispanic/Latinx | 145 | 34 (23) | 8.03 (3.82–16.90) | 6.37 (3.05–13.30) | 151 | 56 (37) | Ref | Ref |
| Hispanic, any race | 201 | 28 (14) | 4.77 (2.22–10.25) | 4.62 (2.11–10.09) | 74 | 17 (23) | 0.62 (.39–.99) | 0.69 (.43–1.11) |
| Other/mixed race, non-Hispanic/Latinx | 122 | 9 (7) | 2.53 (.998–6.40) | 2.50 (.99–6.31) | 39 | 10 (26) | 0.69 (.39–1.23) | 0.69 (.39–1.25) |
| Geographic region | ||||||||
| Northeast | 386 | 21 (5) | Ref | Ref | 51 | 8 (16) | Ref | Ref |
| Upper South Atlantic | 200 | 37 (19) | 3.40 (2.05–5.65) | 2.75 (1.65–4.58) | 113 | 39 (35) | 2.20 (1.11–4.37) | 1.98 (.97–4.01) |
| Lower South Atlantic | 156 | 21 (13) | 2.47 (1.39–4.40) | 1.97 (1.11–3.49) | 112 | 41 (37) | 2.33 (1.18–4.62) | 2.21 (1.12–4.38) |
| Gender identity | ||||||||
| Female or woman | 253 | 27 (11) | 1.19 (.73–1.94) | 1.05 (.65–1.69) | 94 | 26 (28) | 0.80 (.54–1.19) | 0.83 (.56–1.24) |
| Transwoman | 346 | 31 (9) | Ref | Ref | 142 | 49 (35) | Ref | Ref |
| Genderqueer/non-binary/otherc | 143 | 21 (15) | 1.64 (.98–2.75) | 1.79 (1.10–2.92) | 40 | 13 (33) | 0.94 (.57–1.56) | 0.91 (.55–1.52) |
| Marital status | ||||||||
| Single or casually dating | 490 | 62 (13) | Ref | Ref | 202 | 64 (32) | Ref | Ref |
| Committed or married | 204 | 16 (8) | 0.62 (.37–1.05) | 0.74 (.44–1.25) | 63 | 19 (30) | 0.95 (.62–1.46) | 1.03 (.67–1.57) |
| Other | 42 | 1 (2) | 0.19 (.03–1.32) | 0.26 (.04–1.70) | 2 | 1 (50) | … | … |
| Educational attainment | ||||||||
| No high school completion | 92 | 17 (18) | Ref | Ref | 77 | 17 (22) | Ref | Ref |
| High school completion | 167 | 23 (14) | 0.75 (.42–1.32) | 0.82 (.48–1.41) | 100 | 40 (40) | 1.81 (1.12–2.94) | 1.56 (.96–2.55) |
| Completed some college or mored | 476 | 39 (8) | 0.44 (.26–.75) | 0.75 (.44–1.27) | 97 | 30 (31) | 1.40 (.84–2.35) | 1.40 (.83–2.37) |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; PR, prevalence ratio; Ref, reference; STI, sexually transmitted infection; TGW, transgender women.
aPRs were estimated using Poisson regression models with robust variance estimation.
bFor each STI outcome variable, a separate multivariable regression model was used that adjusted for age, race and ethnicity, and geographic region.
cOther includes transfeminine/trans femme, woman of trans experience, person of trans experience, 2-spirit, other identity, and prefer not to answer.
dIncludes associate's degree and technical college.
Figure 1.
Bacterial STI prevalence by site and HIV status among transgender women in the eastern and southern United States. 95% confidence intervals are presented. Abbreviations: HIV, human immunodeficiency virus; STI, sexually transmitted infection; TGW, transgender women.
Among TGW with HIV, older age was inversely associated with a bacterial STI in univariable (PR = 0.92; 95% CI = .85–.99) and the demographic-adjusted model (aPR = 0.93; 95% CI = .86–.997). Compared to non-Hispanic black TGW with HIV, Hispanic TGW with HIV were less likely to have a bacterial STI (PR = 0.62; 95% CI = .39–.99) although this was slightly attenuated in the demographic-adjusted model (aPR = 0.69; 95% CI = .43–1.11). TGW with HIV in the Upper and Lower South Atlantic regions were also more likely to have a bacterial STI compared to those in the Northeast (PR = 2.20; 95% CI = 1.11–4.37 and PR = 2.33; 95% CI = 1.18–4.62, respectively); residing in the Lower South Atlantic region remained significantly associated in demographic-adjusted models (aPR = 2.21; 95% CI = 1.12–4.38).
Among TGW without HIV, reporting multiple sexual partners was positively associated with bacterial STI prevalence (aPR = 1.91; 95% CI = 1.21–3.02), independent of demographic factors (Table 4). Bacterial STI prevalence was significantly lower among participants who reported only non-cisgender male partner(s) compared to those who reported exclusively cisgender male partner(s) (aPR = 0.12; 95% CI = .02–.88). Current PrEP use was positively associated with a prevalent bacterial STI in the univariable analysis (PR = 1.71; 95% CI = 1.05–2.80), but this association was attenuated in the demographic-adjusted model. No sexual behaviors were significantly associated with bacterial STI prevalence among TGW with HIV.
Table 4.
Sexual Behavioral Correlates of Bacterial STI Prevalence by HIV Status
| Sexual Behaviors | Outcome: ≥1 Bacterial STI | |||||||
|---|---|---|---|---|---|---|---|---|
| TGW Without HIV | TGW With HIV | |||||||
| No. | Prevalence, No. (%) | Crude PR (95% CI)a | Adjusted PR (95% CI)a,b | No. | Prevalence, No. (%) | Crude PR (95% CI)a | Adjusted PR (95% CI)a,b | |
| Sexual activity, 12 mo | ||||||||
| No | 165 | 9 (5) | Ref | Ref | 65 | 22 (34) | Ref | Ref |
| Yes | 575 | 70 (12) | 2.23 (1.14–4.37) | 1.64 (.85–3.14) | 201 | 61 (30) | 0.90 (.60–1.34) | 0.84 (.56–1.25) |
| Gender(s) of sexual partner(s), 12 moc | ||||||||
| Cisgender men only | 304 | 54 (18) | Ref | Ref | 168 | 53 (32) | Ref | Ref |
| Non-cisgender men only | 92 | 1 (1) | 0.06 (.01–.44) | 0.12 (.02–.88) | 5 | 1 (20) | 0.63 (.11–3.73) | 0.61 (.08–4.78) |
| Multiple partners of different gendersd | 177 | 15 (8) | 0.48 (.28–.82) | 0.76 (.43–1.32) | 27 | 7 (26) | 0.82 (.42–1.62) | 0.68 (.36–1.28) |
| Sexual partner concurrency, 12 moc | ||||||||
| No | 308 | 41 (13) | Ref | Ref | 123 | 36 (29) | Ref | Ref |
| Yes | 259 | 29 (11) | 0.84 (.54–1.31) | 1.12 (.72–1.77) | 74 | 22 (30) | 1.02 (.65–1.59) | 0.85 (.53–1.35) |
| Condomless sex, 12 moc | ||||||||
| No | 126 | 16 (13) | Ref | Ref | 86 | 25 (29) | Ref | Ref |
| Yes | 440 | 52 (12) | 0.93 (.55–1.57) | 0.86 (.53–1.40) | 109 | 35 (32) | 1.10 (.72–1.70) | 1.02 (.67–1.57) |
| Multiple sex partners, 3 mo | ||||||||
| No | 391 | 24 (6) | Ref | Ref | 151 | 51 (34) | Ref | Ref |
| Yes | 349 | 55 (16) | 2.57 (1.62–4.06) | 1.91 (1.21–3.02) | 115 | 32 (28) | 0.82 (.57–1.19) | 0.74 (.51–1.05) |
| PrEP use | ||||||||
| Never | 526 | 49 (9) | Ref | Ref | … | … | … | … |
| Former, ≥30 d ago | 84 | 11 (13) | 1.41 (.76–2.59) | 1.07 (.58–1.99) | … | … | … | … |
| Current, <30 d ago | 119 | 19 (16) | 1.71 (1.05–2.80) | 1.25 (.76–2.05) | … | … | … | … |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; PR, prevalence ratio; PrEP, preexposure prophylaxis; Ref, reference; STI, sexually transmitted infection; TGW, transgender women.
aPRs were estimated using Poisson regression models with robust variance estimation.
bFor each STI outcome variable, a separate multivariable regression model was used that adjusted for age, race and ethnicity, and geographic region.
cAmong those who are sexually active in the past 12 months.
dGenders include man, woman, transgender woman, transgender man, genderqueer (female at birth), and genderqueer (male at birth).
Situated vulnerability-related correlates of bacterial STI prevalence differed by HIV status (Table 5). For example, hazardous alcohol use was positively associated with bacterial STI prevalence among TGW without HIV (aPR = 1.64; 95% CI = 1.08–2.47), but inversely associated among TGW with HIV (aPR = 0.57; 95% CI = .35–.93). While a history of physical and sexual violence was not associated with bacterial STI among TGW without HIV, they were negatively associated among TGW with HIV (aPR = 0.70; 95% CI = .50–.996 and aPR = 0.59; 95% CI = .40–.89, respectively). Uniquely among TGW without HIV, recent homelessness and concern about safety in transit to health care were positively associated with bacterial STI (aPR = 1.63; 95% CI = 1.01–2.64 and aPR = 1.68; 95% CI = 1.11–2.54, respectively), and receipt of gender-affirming health services was negatively associated with bacterial STI (eg, receipt of genital surgery; aPR = 0.34; 95% CI = .13–.89). Several other sociostructural factors (eg, history of sex work and incarceration) were positively associated with bacterial STI among TGW wthout HIV, but these associations were attenuated in demographic-adjusted models.
Table 5.
Situated Vulnerability-Related Correlates of Bacterial STI Prevalence by HIV Status
| Outcome: ≥1 Bacterial STI | ||||||||
|---|---|---|---|---|---|---|---|---|
| TGW Without HIV | TGW With HIV | |||||||
| Situated Vulnerabilities | No. | Prevalence, No. (%) |
Crude PR (95% CI)a |
Adjusted PR (95% CI)a,b |
No. | Prevalence, No. (%) |
Crude PR (95% CI)a |
Adjusted PR (95% CI)a,b |
| AUDIT-C | ||||||||
| Score < 4 | 485 | 44 (9) | Ref | Ref | 196 | 69 (35) | Ref | Ref |
| Score ≥ 4 | 232 | 32 (14) | 1.52 (.99–2.33) | 1.64 (1.08–2.47) | 66 | 14 (21) | 0.60 (.36–.997) | 0.57 (.35–.93) |
| Psychological violence | ||||||||
| Never | 131 | 25 (19) | Ref | Ref | 92 | 36 (39) | Ref | Ref |
| Ever | 602 | 54 (9) | 0.47 (.30–.73) | 0.71 (.46–1.10) | 172 | 49 (28) | 0.73 (.51–1.03) | 0.74 (.53–1.05) |
| Physical violence | ||||||||
| Never | 268 | 33 (12) | Ref | Ref | 110 | 44 (40) | Ref | Ref |
| Ever | 463 | 45 (10) | 0.79 (.52–1.21) | 1.01 (.67–1.54) | 151 | 40 (26) | 0.66 (.47–.94) | 0.70 (.50–.996) |
| Sexual violence | ||||||||
| Never | 408 | 46 (11) | Ref | Ref | 151 | 60 (40) | Ref | Ref |
| Ever | 321 | 33 (10) | 0.91 (.60–1.39) | 1.03 (.69–1.55) | 110 | 24 (22) | 0.55 (.37–.82) | 0.59 (.40–.89) |
| Sex work | ||||||||
| Never | 408 | 30 (7) | Ref | Ref | 76 | 23 (30) | Ref | Ref |
| Ever | 324 | 49 (15) | 2.06 (1.34–3.16) | 1.55 (.995–2.41) | 189 | 62 (33) | 1.08 (.73–1.61) | 1.06 (.71–1.58) |
| Incarceration history | ||||||||
| Never | 575 | 52 (9) | Ref | Ref | 130 | 43 (33) | Ref | Ref |
| Ever | 143 | 23 (16) | 1.78 (1.13–2.81) | 1.24 (.76–2.02) | 130 | 39 (30) | .91 (.63–1.30) | 0.88 (.61–1.26) |
| Homelessness | ||||||||
| Never | 397 | 35 (9) | Ref | Ref | 102 | 32 (31) | Ref | Ref |
| Ever, ≥3 mo ago | 247 | 27 (11) | 1.24 (.77–2.00) | 1.07 (.67–1.71) | 118 | 35 (30) | 0.95 (.63–1.41) | 0.95 (.64–1.42) |
| Ever, <3 mo ago | 83 | 16 (19) | 2.19 (1.27–3.76) | 1.63 (1.01–2.64) | 48 | 16 (33) | 1.06 (.65–1.74) | 0.95 (.58–1.55) |
| Health insurance | ||||||||
| Insured | 660 | 65 (10) | Ref | Ref | 255 | 80 (31) | Ref | Ref |
| Uninsured | 75 | 14 (19) | 1.90 (1.12–3.21) | 1.57 (.93–2.65) | 11 | 5 (45) | 1.45 (.74–2.84) | 1.28 (.70–2.36) |
| Safety concern in transit to health care | ||||||||
| No | 433 | 35 (8) | Ref | Ref | 132 | 43 (33) | Ref | Ref |
| Yes | 304 | 44 (14) | 1.79 (1.18–2.72) | 1.68 (1.11–2.54) | 140 | 44 (31) | 0.96 (.68–1.37) | 0.85 (.60–1.19) |
| Gender identity therapy or counseling | ||||||||
| Never | 191 | 38 (20) | Ref | Ref | 91 | 32 (35) | Ref | Ref |
| Former, ≥3 mo ago | 226 | 15 (7) | 0.33 (.19–.59) | 0.45 (.26–.79) | 73 | 21 (29) | 0.82 (.52–1.29) | 0.91 (.58–1.41) |
| Current, <3 mo ago | 318 | 25 (8) | 0.40 (.25–.63) | 0.52 (.33–.83) | 100 | 29 (29) | 0.82 (.54–1.25) | 0.84 (.56–1.25) |
| Hormone treatment use | ||||||||
| Never | 82 | 15 (18) | Ref | Ref | 62 | 17 (27) | Ref | Ref |
| Former, ≥3 mo ago | 44 | 9 (20) | 1.12 (.53–2.35) | 1.28 (.63–2.63) | 30 | 14 (47) | 1.70 (.97–2.97) | 1.70 (.99–2.91) |
| Current, <3 mo ago, | 609 | 54 (9) | 0.48 (.29–.82) | 0.67 (.40–1.11) | 174 | 52 (30) | 1.09 (.68–1.74) | 1.14 (.73–1.79) |
| Genital surgeryc | ||||||||
| Never | 612 | 72 (12) | Ref | Ref | 232 | 73 (31) | Ref | Ref |
| Ever | 111 | 4 (4) | 0.31 (.11–.82) | 0.34 (.13–.89) | 30 | 9 (30) | 0.95 (.53–1.70) | 1.19 (.66–2.14) |
| Engagement in HIV care, 12 mo | ||||||||
| No | … | … | … | … | 34 | 9 (26) | 0.79 (.44–1.44) | .72 (.42–1.25) |
| Yes | … | … | … | … | 207 | 69 (33) | Ref | Ref |
Abbreviations: AUDIT-C, Alcohol Use Disorder Identification Test-Consumption; CI, confidence interval; HIV, human immunodeficiency virus; PR, prevalence ratio; Ref, reference; STI, sexually transmitted infection; TGW, transgender women.
aPRs were estimated using Poisson regression models with robust variance estimation.
bFor each STI outcome variable, a separate multivariable regression model was used that adjusted for age, race and ethnicity, and geographic region.
cVaginoplasty or orchiectomy.
DISCUSSION
Among this community-based sample of adult TGW from 6 eastern and southern US cities, 16% of participants had at least 1 bacterial STI. TGW with HIV had a higher bacterial STI prevalence than TGW without HIV, although prevalence in both groups was considerably higher than the general US population [30, 31]. We also observed geographical and racial disparities among TGW without HIV. Many factors associated with bacterial STI among TGW without HIV (eg, multiple sexual partners, recent homelessness, and receipt of gender-affirming health services) were not found among TGW with HIV. The differential distribution of STIs among these 2 populations underscores the necessity of developing interventions tailored to the unique needs and health service utilization of each population.
Bacterial STI prevalence was 3-fold higher among TGW with HIV compared to TGW without HIV. The higher bacterial STI prevalence among TGW with HIV is consistent with several clinic-based studies of TGW with HIV [32–34]; however, additional research is needed to understand HIV-related disparities in bacterial STI prevalence. Beyond demographic differences, TGW with HIV were more likely than TGW without HIV to report many situated vulnerabilities (eg, history of sex work, incarceration, and homelessness). A differential distribution of other unmeasured sociostructural, network, and individual-level risk factors by HIV status may also explain the disproportionate STI burden among TGW with HIV (eg, intersectional stigma, sexual networks with high STI prevalence, and lifetime sexual behaviors).
By leveraging a multisite, diverse cohort of TGW, we detected geographic heterogeneity in bacterial STI prevalence. Among TGW with and without HIV, STI prevalence was highest in the Upper and Lower South Atlantic cities. STI burden in other populations has also been shown to be disproportionately higher in southern cities as compared to other regions in the United States [1]. Geographic differences in STI prevalence have important implications for resource allocation. While the geographic differences in STI prevalence observed in this study among TGW without HIV may also reflect the pronounced racial and ethnic disparities in STI prevalence among TGW without HIV, these associations were also independent of each other. Notably, black participants without HIV were over 6 times more likely to have a bacterial STI compared to white participants without HIV. Among TGW with HIV, we suspect the low proportion of white participants (<5%) reduced power to detect racial and ethnic disparities. Compared to white TGW, TGW of color experience intersectional stigma (eg, transphobia and racism) [35], which place them at heightened risk for HIV and STI acquisition. There have been recent efforts to develop and implement culturally tailored interventions to improve engagement in HIV care among TGW of color [36]. Similar approaches are needed to enhance access to and engagement in STI services.
Among TGW without HIV, we observed a positive crude association between current PrEP use and bacterial STI prevalence, which is consistent with guidelines recommending frequent bacterial STI testing among people on PrEP [37]. Having multiple sexual partners in the past 3 months was also associated with bacterial STI prevalence among TGW without HIV. We additionally found that TGW without HIV who reported only non-cisgender male sexual partners within the past 12 months were less likely to have a bacterial STI compared to TGW who reported only cisgender male sex partners. For TGW who have sex with cisgender men, it is important to consider the increased biological risks of STI acquisition via condomless anal sex [38, 39]. Research exploring the role of network-level drivers of bacterial STI incidence among TGW is needed. Specifically, there is a need to contextualize the role of these sexual behaviors in relation to one's sexual network, such as understanding how different core, periphery, and adjacent groups in sexual networks of TGW facilitate STI transmission.
Participants without HIV who reported concern about their safety in transit to health care were more likely to have a bacterial STI than those who did not, highlighting transportation as a potential barrier to prompt STI diagnosis and treatment among TGW. Indeed, black TGW in Baltimore and Washington, DC have reported fears for safety getting to and from health care settings [40]. TGW using public transportation to attend health care visits may face harassment, discrimination, or violence, hindering engagement in necessary health care services [40, 41]. Partnerships between health and transportation systems (eg, ride hailing companies) to provide nonemergency medical transportation services has been proposed as a strategy to mitigate transportation challenges and promote health equity [42]; this type of strategy may also facilitate improvements in STI testing and treatment among TGW.
Receipt of several gender-affirming health services, including counseling and genital surgery, were associated with a lower bacterial STI prevalence among TGW without HIV. Medical gender affirmation therapies have been shown to improve psychological functioning and quality of life for transgender adults [43], but it is unknown whether these interventions mitigate STI risk or reflect increased health service utilization. Among transgender youth, receipt of medical gender affirmation has been related to increased likelihood of condomless sex and a lifetime history of STI testing [13, 44]. Further investigation is needed to elucidate the role of medical gender affirmation in sexual health-related outcomes.
We identified limited factors associated with bacterial STI prevalence among participants with HIV. Older age was inversely associated with bacterial STI prevalence, consistent with prior studies [32]. The lack of associations of recent sexual behaviors and bacterial STI prevalence among TGW with HIV may be due to misreporting of recent sexual behaviors, detection of prevalent STI that reflect lifetime sexual behaviors, or due to a larger role of sexual network-level factors (eg, a high network-level prevalence of bacterial STI) in conferring bacterial STI risk [8]. Some findings among TGW with HIV were also in an unexpected direction (eg, hazardous alcohol use, physical and sexual violence) [45, 46]. This may be due to potential unmeasured confounding (eg, prior STI testing and treatment). The inverse association with lifetime physical and sexual violence may reflect survival bias or behavioral modifications, such as changes in partner selection following experiences of violence. Nonetheless, the high prevalence of bacterial STI observed among TGW with HIV underscores the need to strengthen comprehensive sexual health services for TGW with HIV, including integration of HIV and STI services, to improve suboptimal STI testing and treatment rates among TGW [32, 47–49].
A key study strength was that we examined multiple laboratory-confirmed STIs at multiple anatomic sites. Our data indicate vaginoplasty remains fairly uncommon among TGW in the United States. At the neovaginal site, we only detected C. trachomatis infection in 1 participant (and no N. gonorrhoeae infections). Additional research is needed to confirm the low neovaginal bacterial STI prevalence among TGW with a history of vaginoplasty. It is also notable that coinfection with different bacterial STIs was higher among TGW with HIV than TGW without HIV, consistent with the overall higher burden of bacterial STIs among TGW with HIV. Bacterial STI coinfection is known to vary by population characteristics [50].
This study has limitations. First, causal inferences cannot be drawn from reported associations. Second, self-reported covariates may be subject to reporting biases (eg, social desirability bias). Third, this study used convenience sampling. While participants represented a sample diverse in age, race, ethnicity, and gender identity, nonprobability-based sampling can lead to selection bias and may limit generalizability. Finally, small numbers within some variable categories may have resulted in sparse data bias.
Although participants with and without HIV had a high prevalence of bacterial STIs, the variation in bacterial STI prevalence and correlates seen between TGW with and without HIV highlights the differential burden and needs of these 2 populations. Elucidating the ways in which situated vulnerabilities are associated with STI risk may help inform more tailored intervention strategies for each population. Research is needed to identify STI determinants unique to TGW with and without HIV and guide future prevention and treatment efforts that are responsive to the diverse needs of TGW.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Contributor Information
Erin E Brown, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, Baltimore, Maryland, USA.
Eshan U Patel, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Tonia C Poteat, Department of Social Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Kenneth Mayer, Department of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, Massachusetts, USA.
Andrew J Wawrzyniak, Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine, Miami, Florida, USA.
Asa E Radix, Callen-Lorde Community Health Center, New York, New York, USA.
Erin E Cooney, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Oliver Laeyendecker, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, Baltimore, Maryland, USA.
Sari L Reisner, Brigham and Women's Hospital, Boston, Massachusetts, USA.
Andrea L Wirtz, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Notes
Author contributions. A. L. W., S. L. R., and T. C. P. developed the study concept. E. E. C., T. C. P., A. E. R., A. J. W., and K. M. provided extensive input to the original grant submission or the study protocol. E. E. B. and E. U. P. led the statistical analyses with support from O. L. and A. L. W. E. E. B. and A. L. W. have accessed and verified the data. E. E. B., E. U. P., A. L. W., and O. L. wrote the first draft of the manuscript and were responsible for the decision to submit the manuscript for publication. All authors reviewed the manuscript, provided scientific input to the manuscript, and agreed with the decision to submit the manuscript. All authors had full access to all the data in the study.
Acknowledgments. We express our gratitude to the transgender women who participated in this study. The LITE study would not have been possible without their experiences and participation. We also appreciate the contributions of the LITE Community Advisory Board. We also thank all the research staff who spent their time and effort to actualize this study and connect with study participants in meaningful ways. The following are members of the collaborative author, American Cohort to Study HIV Acquisition Among Transgender Women (also known as the LITE cohort): Sari Reisner (multiple Principle Investigator [PI]; Brigham and Women's Hospital); Andrea Wirtz (multiple PI; Johns Hopkins University [JHU]); Keri Althoff (JHU); Chris Beyrer (JHU); James Case (JHU); Erin Cooney (JHU); Meg Stevenson (JHU); Dee Adams (JHU); Oliver Laeyendecker (National Institute of Allergy and Infectious Diseases); Charlotte Gaydos (JHU); Tonia Poteat (University of North Carolina); Kenneth Mayer (Fenway Health); Asa Radix (Callen-Lorde Community Health Center); Christopher Cannon (Whitman-Walker Health); Jason Schneider (Emory University and Grady Hospital); J. Sonya Haw (Emory University and Grady Hospital); Allan Rodriguez (University of Miami); Andrew J. Wawrzyniak (University of Miami); and the LITE Community Advisory Board, including the following individuals: Sherri Meeks, Sydney Shackelford, Nala Toussaint, SaVanna Wanzer, as well as those who have remained anonymous.
Disclaimer . The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported jointly by the National Institute of Allergy and Infectious Diseases (NIAID), the National Institute of Mental Health, and the National Institute of Child Health and Human Development, of the National Institutes of Health (grant number UG3/UH3AI133669 to A. L. W. and S. L. R.); NIAID (grant number T32AI102623 to E. U. P.); the National Institute on Drug Abuse (grant number F31DA054849 to E. U. P.), the National Institute on Minority Health and Health Disparities (grant number R01MD013498 to T. C. P.); the National Institute of Mental Health (grant numbers R25MH087217 to A. E. R. and F31MH124582 to E. E. C.); and the HIV/AIDS, Hepatitis, STD, and TB Administration, Washington, DC, Department of Health. O. L. and E. E. B. were supported by the Division of Intramural Research, NIAID. The LITE study also appreciates support from the Centers for AIDS Research at partner institutions, including Johns Hopkins University (grant number P30AI094189), Emory University (grant number P30AI050409), Harvard University (grant number P30AI060354), DC CFAR (grant number P30AI117970), and the University of Miami (grant number P30AI073961).
References
- 1. Centers for Disease Control and Prevention . Sexually transmitted disease surveillance 2019. https://www.cdc.gov/std/statistics/2019/std-surveillance-2019.pdf. Accessed 31 December 2022.
- 2. World Health Organization . Sexually transmitted infections (STIs). http://who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis). Accessed 31 December 2022.
- 3. Chesson HW, Mayaud P, Aral SO. Sexually transmitted infections: impact and cost-effectiveness of prevention. In: Holmes KK, Bertozzi S, Bloom BR, Jha P, eds. Major infectious diseases. 3rd ed. Washington, DC: The International Bank for Reconstruction and Development/The World Bank; 2017:14–24. [PubMed] [Google Scholar]
- 4. Becasen JS, Denard CL, Mullins MM, Higa DH, Sipe TA. Estimating the prevalence of HIV and sexual behaviors among the US transgender population: a systematic review and meta-analysis, 2006–2017. Am J Public Health 2019; 109:e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Gerwen OT, Jani A, Long DM, Austin EL, Musgrove K, Muzny CA. Prevalence of sexually transmitted infections and human immunodeficiency virus in transgender persons: a systematic review. Transgend Health 2020; 5:90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nuttbrock L, Hwahng S, Bockting W, et al. Lifetime risk factors for HIV/sexually transmitted infections among male-to-female transgender persons. J Acquir Immune Defic Syndr 2009; 52:417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shannon CL, Keizur EM, Fehrenbacher A, et al. Sexually transmitted infection positivity among adolescents with or at high-risk for human immunodeficiency virus infection in Los Angeles and New Orleans. Sex Transm Dis 2019; 46:737–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Poteat T, Scheim A, Xavier J, Reisner S, Baral S. Global epidemiology of HIV infection and related syndemics affecting transgender people. J Acquir Immune Defic Syndr 2016; 72(Suppl 3):S210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herbst JH, Jacobs ED, Finlayson TJ, McKleroy VS, Neumann MS, Crepaz N. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: a systematic review. AIDS Behav 2008; 12:1–17. [DOI] [PubMed] [Google Scholar]
- 10. Ragonnet-Cronin M, Hu YW, Morris SR, Sheng Z, Poortinga K, Wertheim JO. HIV transmission networks among transgender women in Los Angeles County, CA, USA: a phylogenetic analysis of surveillance data. Lancet HIV 2019; 6:e164–e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Long JE, Tordoff DM, Reisner SL, et al. HIV transmission patterns among transgender women, their cisgender male partners, and cisgender MSM in Lima, Peru: a molecular epidemiologic and phylodynamic analysis. Lancet Reg Health Am 2022; 6:100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reisner SL, Poteat T, Keatley J, et al. Global health burden and needs of transgender populations: a review. Lancet 2016; 388:412–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reisner SL, Jadwin-Cakmak L, Sava L, Liu S, Harper GW. Situated vulnerabilities, sexual risk, and sexually transmitted infections’ diagnoses in a sample of transgender youth in the United States. AIDS Patient Care STDS 2019; 33:120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poteat T, Reisner SL, Radix A. HIV epidemics among transgender women. Curr Opin HIV AIDS 2014; 9:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. MacCarthy S, Poteat T, Xia Z, et al. Current research gaps: a global systematic review of HIV and sexually transmissible infections among transgender populations. Sex Health 2017; 14:456–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poteat T, White RH, Footer KHA, et al. Characterising HIV and STIs among transgender female sex workers: a longitudinal analysis. Sex Transm Infect 2021; 97:226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bowleg L. The problem with the phrase women and minorities: intersectionality-an important theoretical framework for public health. Am J Public Health 2012; 102:1267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Logie CH, James L, Tharao W, Loutfy MR. HIV, gender, race, sexual orientation, and sex work: a qualitative study of intersectional stigma experienced by HIV-positive women in Ontario, Canada. PLoS Med 2011; 8:e1001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pitasi MA, Kerani RP, Kohn R, et al. Chlamydia, gonorrhea, and human immunodeficiency virus infection among transgender women and transgender men attending clinics that provide sexually transmitted disease services in six US cities: results from the sexually transmitted disease surveillance network. Sex Transm Dis 2019; 46:112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wirtz AL, Cooney EE, Chaudhry A, Reisner SL; American Cohort to Study HIVAATW . Computer-mediated communication to facilitate synchronous online focus group discussions: feasibility study for qualitative HIV research among transgender women across the United States. J Med Internet Res 2019; 21:e12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reisner SL, Chaudhry A, Cooney E, Garrison-Desany H, Juarez-Chavez E, Wirtz AL. ‘It all dials back to safety’: a qualitative study of social and economic vulnerabilities among transgender women participating in HIV research in the USA. BMJ Open 2020; 10:e029852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wirtz AL, Poteat T, Radix A, et al. American cohort to study HIV acquisition among transgender women in high-risk areas (the LITE study): protocol for a multisite prospective cohort study in the Eastern and Southern United States. JMIR Res Protoc 2019; 8:e14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wirtz AL, Cooney EE, Stevenson M, et al. Digital epidemiologic research on multilevel risks for HIV acquisition and other health outcomes among transgender women in Eastern and Southern United States: protocol for an online cohort. JMIR Res Protoc 2021; 10:e29152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sausa LA, Sevelius J, Keatley J, Iñiguez JR, Reyes M. Policy recommendations for inclusive data collection of trans people in HIV prevention, care and services: Center of Excellence for Transgender HIV Prevention. San Francisco: University of California, 2009. [Google Scholar]
- 25. Tate CC, Ledbetter JN, Youssef CP. A two-question method for assessing gender categories in the social and medical sciences. J Sex Res 2013; 50:767–76. [DOI] [PubMed] [Google Scholar]
- 26. Wirtz AL, Humes E, Althoff KN, et al. HIV incidence and mortality in transgender women in the eastern and southern USA: a multisite cohort study. Lancet HIV 2023; 10:e308–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Branson BM, Owen, S.M., Wesolowski, L.G., et al. Centers for Disease Control and Prevention . Laboratory testing for the diagnosis of HIV infection: updated recommendations. https://stacks.cdc.gov/view/cdc/23447. Accessed 27 February 2023.
- 28. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction 1993; 88:791–804. [DOI] [PubMed] [Google Scholar]
- 29. Straus MA, Hamby SL, Boney-McCoy S, Sugarman DB. The revised conflict tactics scales (CTS2): development and preliminary psychometric data. J Fam Issues 1996; 17:283–316. [Google Scholar]
- 30. Kreisel KM, Spicknall IH, Gargano JW, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2018. Sex Transm Dis 2021; 48:208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kreisel KM, Weston EJ, St Cyr SB, Spicknall IH. Estimates of the prevalence and incidence of chlamydia and gonorrhea among US men and women, 2018. Sex Transm Dis 2021; 48:222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van Gerwen OT, Tamhane A, Westfall AO, et al. Prevalence of and factors associated with genital and extragenital chlamydia and gonorrhea among transgender women in HIV care in the United States, 2005 to 2016. Sex Transm Dis 2021; 48:410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shover CL, DeVost MA, Beymer MR, Gorbach PM, Flynn RP, Bolan RK. Using sexual orientation and gender identity to monitor disparities in HIV, sexually transmitted infections, and viral hepatitis. Am J Public Health 2018; 108:S277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Secco AA, Akselrod H, Czeresnia J, et al. Sexually transmitted infections in persons living with HIV infection and estimated HIV transmission risk: trends over time from the DC cohort. Sex Transm Infect 2020; 96:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sevelius JM. Gender affirmation: a framework for conceptualizing risk behavior among transgender women of color. Sex Roles 2013; 68:675–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rebchook GM, Chakravarty D, Xavier JM, et al. An evaluation of nine culturally tailored interventions designed to enhance engagement in HIV care among transgender women of colour in the United States. J Int AIDS Soc 2022; 25(Suppl 5):e25991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Centers for Disease Control and Prevention. US Public Health Service . Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update: a clinical practice guideline. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf. Accessed 12 October 2023.
- 38. Nemoto T, Bodeker B, Iwamoto M, Sakata M. Practices of receptive and insertive anal sex among transgender women in relation to partner types, sociocultural factors, and background variables. AIDS Care 2014; 26:434–40. [DOI] [PubMed] [Google Scholar]
- 39. Coan DL, Schrager W, Packer T. The role of male sexual partners in HIV infection among male-to-female transgendered individuals. Int J Transgend 2005; 8:21–30. [Google Scholar]
- 40. Sherman ADF, Balthazar MS, Daniel G, et al. Barriers to accessing and engaging in healthcare as potential modifiers in the association between polyvictimization and mental health among black transgender women. PLoS One 2022; 17:e0269776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lubitow A, Abelson MJ, Carpenter E. Transforming mobility justice: gendered harassment and violence on transit. J Transp Geogr 2020; 82:102601. [Google Scholar]
- 42. Chen KL, Brozen M, Rollman JE, et al. How is the COVID-19 pandemic shaping transportation access to health care? Transp Res Interdiscip Perspect 2021; 10:100338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hughto JM W, Reisner SL. A systematic review of the effects of hormone therapy on psychological functioning and quality of life in transgender individuals. Transgend Health 2016; 1:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Andrzejewski J, Dunville R, Johns MM, Michaels S, Reisner SL. Medical gender affirmation and HIV and sexually transmitted disease prevention in transgender youth: results from the survey of today’s adolescent relationships and transitions, 2018. LGBT Health 2021; 8:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Santos GM, Rapues J, Wilson EC, et al. Alcohol and substance use among transgender women in San Francisco: prevalence and association with human immunodeficiency virus infection. Drug Alcohol Rev 2014; 33:287–95. [DOI] [PubMed] [Google Scholar]
- 46. Wirtz AL, Poteat TC, Malik M, Glass N. Gender-based violence against transgender people in the United States: a call for research and programming. Trauma Violence Abuse 2020; 21:227–41. [DOI] [PubMed] [Google Scholar]
- 47. Trujillo D, Arayasirikul S, Xie H, et al. Disparities in sexually transmitted infection testing and the need to strengthen comprehensive sexual health services for trans women. Transgend Health 2022; 7:230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kennedy CE, Haberlen SA, Narasimhan M. Integration of sexually transmitted infection (STI) services into HIV care and treatment services for women living with HIV: a systematic review. BMJ Open 2017; 7:e015310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Town K, Tie Y, Dasgupta S, et al. Sexually transmitted infection testing among transgender women living with human immunodeficiency virus in the United States: medical monitoring project, 2015–2019. Clin Infect Dis 2021; 73:899–902. [DOI] [PubMed] [Google Scholar]
- 50. Forward KR. Risk of coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in Nova Scotia. Can J Infect Dis Med Microbiol 2010; 21:e84–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.