Abstract
The landscape of current tumor treatment has been revolutionized by the advent of immunotherapy based on PD-1/PD-L1 inhibitors. Leveraging its capacity to mobilize systemic antitumor immunity, which is primarily mediated by T cells, there is growing exploration and expansion of its potential value in various stages of clinical tumor treatment. Neoadjuvant immunotherapy induces a robust immune response against tumors prior to surgery, effectively facilitating tumor volume reduction, early eradication or suppression of tumor cell activity, and control of potential metastatic spread, to improve curative surgical resection rates, and prevent tumor recurrence. This review delineates the theoretical basis of neoadjuvant immunotherapy from preclinical research evidence, discusses specific challenges in clinical application, and provides a comprehensive overview of clinical research progress in neoadjuvant immunotherapy for gastrointestinal tumors. These findings suggest that neoadjuvant immunotherapy has the potential to ameliorate immunosuppressive states and enhance cytotoxic T cell function while preserving lymphatic drainage in the preoperative period. However, further investigations are needed on specific treatment regimens, suitable patient populations, and measurable endpoints. Despite numerous studies demonstrating the promising efficacy and manageable adverse events of neoadjuvant immunotherapy in gastrointestinal tumors, the availability of high-quality randomized controlled trials is limited, which highlights the necessity for further research.
Keywords: gastrointestinal tumor, neoadjuvant immunotherapy, PD-1/L1 inhibitors, tumor microenvironment
Introduction
Highlights
This study extensively summarizes the current preclinical evidence that has been confirmed within the field of neoadjuvant immunotherapy.
Latest advancements in clinical trials exploring neoadjuvant immunotherapy in gastrointestinal tumors is extensively investigated, showing significant advantages and promising potential in gastrointestinal tumor.
High-quality RCTs and drugs targeting other immune checkpoints in the context of neoadjuvant therapy are still lacking, necessitating continued exploration in the future.
Neoadjuvant therapy is a comprehensive tumor treatment modality involving the administration of radiation, chemotherapy, targeted agents, or their combination prior to curative surgery that improves patient survival and is recommended for the treatment of various cancers, such as breast, esophageal, colorectal, and prostate cancer1,2. However, conventional neoadjuvant therapy primarily relies on cytotoxic drugs or physical interventions to treat tumors that unavoidably disrupt the immune system and create an immunosuppressive microenvironment, which may facilitate tumor cell dissemination and growth under selective pressure3,4.
In contrast to conventional radiotherapy and chemotherapy, immune checkpoint inhibitors (ICIs) therapy targeting the PD-1/L1 pathway has the potential to activate the host immune response and harness cytotoxic T cells for tumor recognition and eradication while minimizing damage to normal cells5,6. The combination of immunotherapy with traditional treatments can further synergistically enhances efficacy and improves patient survival. Based on these characteristics, immunotherapy has emerged as a cornerstone of systemic tumor treatment, and is progressively extending its applications from advanced tumors to various stages of tumor therapy, including neoadjuvant treatment7–10.
In an ideal scenario, neoadjuvant immune therapy induces a robust immune response against the tumor prior to surgery to effectively facilitate tumor volume reduction, early eradication or suppression of tumor cell activity and control of potential metastatic spread. This approach leads to pathological remission, delays tumor recurrence, and improves patient survival2,11. Given the promising application prospects of neoadjuvant immunotherapy, the present study comprehensively analyzed the preclinical research basis of neoadjuvant immunotherapy based on PD-1/L1 inhibitors and investigated the existing clinical challenges associated with neoadjuvant immunotherapy. Furthermore, this study also provides a comprehensive summary of recent clinical trial outcomes and ongoing research in the field of gastrointestinal tumors, explicating indices of interest for surgeons such as resection rate, objective response rate (ORR), pathological response rate, etc. The main objective is to contribute valuable insights and assistance to advance progress in this field.
The theoretical basis of neoadjuvant immunotherapy based on preclinical evidence
Ameliorating immune suppression and enhancing the cytotoxic T Cells
Surgical radical resection is one of the most effective strategies for achieving long-term survival in patients with solid tumors. However, patients with successful curative surgery still face the risk of tumor recurrence at the regional or distant level, which is attributed to numerous factors, such as undetected residual tumors, micro-metastasis, and the persistent presence of a carcinogenic environment. Therefore, it is imperative to use viable approaches during the perioperative period to enhance thorough resection and prevent relapse. Current research revealed that surgery induced an immunosuppressive environment that directly hindered T-cell proliferation and led to the apoptosis of initial T cells12. Meanwhile, the immunosuppressive microenvironment surrounding the excised lesion facilitates the growth of tumor cells and predisposes patients to tumor recurrence13,14. Studies have shown that PD-1/L1 blockade in the neoadjuvant setting activated and expanded pre-existing cytotoxic T cells within the tumor microenvironment. Simultaneously, dendritic cells within the primary tumor microenvironment acquire tumor-specific antigens and migrate to lymph nodes for tolerance presentation, which caused the functional conversion of tumor-specific T cells to a state of dysfunction. However, PD-1/L1 blockade enhanced the activity of tumor-specific T cells and reversed immune tolerance in select T cells, which ameliorated the overall immunosuppressive status of the organism11. In addition, tumor-related inflammation can lead to immune dysregulation within organisms, which impairs the function of T cells. This tumor-associated inflammation-induced immune suppression is closely associated with tumor burden and stage15. Therefore, neoadjuvant immunotherapy has the potential to better mobilize the body’s immune defense mechanisms in the early stages of cancer.
Certain gene mutations within tumor cells can give rise to tumor-specific neoantigens that are recognized by the immune system. Tumor cells with greater numbers of somatic mutations have a greater abundance of neoantigens, which leads to improved efficacy of ICIs therapy. Compared to potential residual tumor cells after surgery, neoadjuvant immunotherapy achieves sufficient antigen presentation in the context of a larger tumor burden, which fully activates T cells and mobilizes the immune system. Preclinical studies using animal models demonstrated that neoadjuvant immunotherapy prior to surgery increased the expansion of tumor-specific CD8+ T lymphocytes. Survival rates were significantly greater in mice receiving neoadjuvant ICIs therapy compared to postoperative adjuvant immunotherapy, which was also observed in preclinical investigations8. Preoperative ICI treatment led to the development of immune memory in a murine model of oral cancer, which conferred resistance against transplanted tumor tissues following surgery. Conversely, postoperative immunotherapy failed to elicit a similar response. Neoadjuvant immunotherapy significantly enhanced the proportion of immune memory T cells in the spleens of mice16. Studies tracking and monitoring of T cells before and after PD-1 blockade therapy found a potential advantage in the generation of tumor-specific T cells via the recognition of primary tumor antigens17. Therefore, neoadjuvant immunotherapy appears beneficial for full activation of T cell-mediated immune cytotoxicity during periods of high tumor antigen burden. Most tumor-killing immune cells undergo apoptosis, but some antigen-specific T cells persist as circulating memory T cells (TCMs) and tissue-resident memory T cells (TRMs), which provide long-term protection against tumor recurrence18,19.
Intact lymph node structure facilitates antitumor immunity
The concept of the tumor immune cycle suggests that lymph nodes also play a critical role as antigen-presenting cells in the delivery of tumor antigens to T cells. Specialized antigen-presenting cells exist within tumors that cross-present within the tumor microenvironment and migrate to draining lymph nodes to present tumor antigens. Studies using in situ tumor models for the analysis of tumor-specific T cell subtypes demonstrated that the initiation of T cell priming and PD-1 expression initially occur in tumor-draining lymph nodes before the appearance of PD-L1-expressing T cells within the tumor itself20. In preclinical studies using prostate cancer and colon cancer models, PD-L1 expression occurred on exosomes derived from tumors and not just on tumor cells themselves. This finding suggested that PD-L1-mediated immune evasion in tumors also occurred in draining lymph nodes rather than solely at the T cell-tumor interaction21. Therefore, maintaining the integrity of lymph nodes prior to immunotherapy is beneficial for maximizing therapeutic efficacy. Neoadjuvant studies in melanoma patients demonstrated a direct correlation between the presence of draining lymph nodes and the efficacy of ICI therapy22. The administration of PD-1/PD-L1 blockade agents enhanced the cancer-immunity cycle process within tumor-draining lymph nodes23.
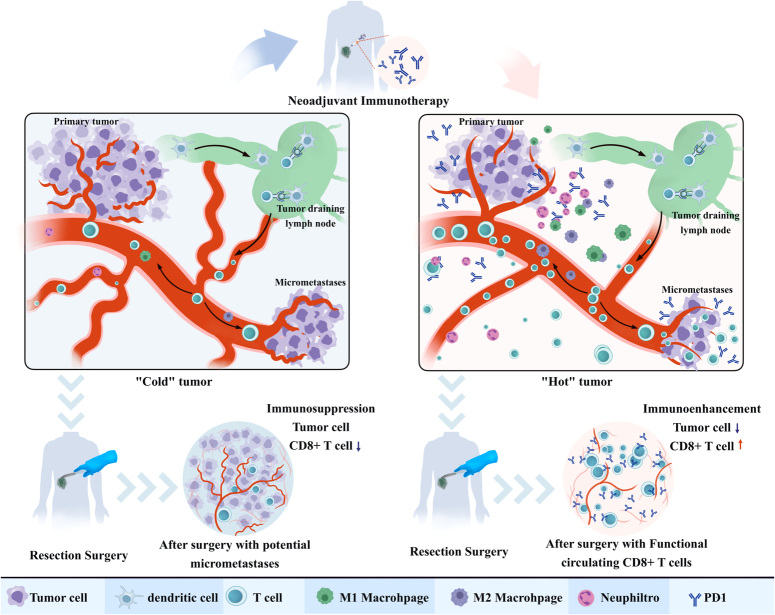
Studies have analyzed the dynamic changes in CD8+ T cells in patient lymph nodes, blood, and the tumor microenvironment and found that precursors of exhausted T cells in nonmetastatic lymph nodes differentiated into transitional intermediate exhausted T cells after receiving immunotherapy. A substantial number of Tex cells migrate into the peripheral blood, proliferate, and infiltrate the tumor, where they exert immune cytotoxicity24. Therefore, intact tumor-draining lymph nodes play a crucial role as interfaces to facilitate tumor immune priming. Neoadjuvant immunotherapy initiates tumor immunity when the structure of the tumor-draining lymph nodes remains intact, which benefits the lymphatic circulation of tumor-specific T cells and ultimately promotes treatment efficacy (Fig. 1)24,25.
Figure 1.
The potential immune-enhancing mechanisms in neoadjuvant immunotherapy. In the presence of primary tumors, the antitumor immune response is suppressed. Limited antigen presentation from the primary tumor and potential micrometastatic lesions results in a deficiency of tumor-specific CTLs. Following neoadjuvant immunotherapy, dendritic cells efficiently acquire a substantial quantity of tumor-specific antigens and effectively present them to T cells. This process leads to the expansion of CTLs and the alleviation of tumor-mediated inhibition of CD8+ T cells. Therefore, these activated CD8+ T cells exert their cytotoxic effects on the primary tumor site and micro-metastatic lesions, which results in tumor regression and an ameliorated immunosuppressive state. Notably, after curative surgery, patients who received neoadjuvant immunotherapy demonstrated a greater abundance and greater cytotoxicity of CD8+ T cells compared to patients who did not receive this treatment. These patients exhibit improved chances of eradicating micro-metastases. CTLs, cytotoxic T lymphocytes.
Clinical challenges faced by neoadjuvant immunotherapy
Currently representative neoadjuvant immunotherapy trials and clinical insights
Preclinical research on neoadjuvant immunotherapy has gradually advanced with some promising results. The highly anticipated SWOG 1801 study compared the efficacy of neoadjuvant therapy with pembrolizumab monotherapy as an adjuvant treatment for resectable stage III/IV melanoma. The results from a 14.7-month follow-up revealed that the neoadjuvant group (154 patients) had significantly prolonged disease-free survival compared to the adjuvant group (159 patients, P=0.004). The 2-year disease-free survival rate was 72% in the neoadjuvant group and 49% in the adjuvant group. Both groups exhibited comparable adverse events (AEs)10. The AEGEAN study is a global multicenter phase III clinical trial that evaluated the efficacy and safety of neoadjuvant immunotherapy with durvalumab followed by surgery and adjuvant immunotherapy in patients with resectable stage IIA-IIIB (N2) nonsmall cell lung cancer (NSCLC) lacking EGFR and ALK mutations26. The primary endpoint revealed that neoadjuvant treatment with durvalumab in combination with chemotherapy significantly improved the pathological complete response rate in surgically resectable early-stage (IIA-IIIB) NSCLC patients compared to neoadjuvant chemotherapy alone. The incidence of common AEs was similar between the two groups and consistent with the known AE profile.
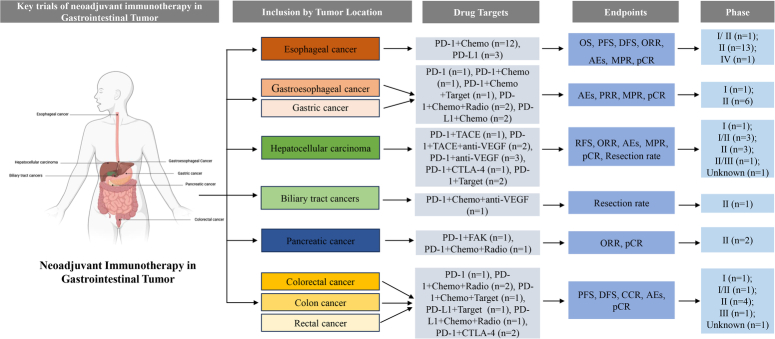
Compared to hematological malignancies, gastrointestinal tumors have a complex tumor microenvironment and are more prone to the development of resistant tumors during treatment. As previously mentioned, neoadjuvant immunotherapy has the potential to elicit a robust antitumor immune response in the setting of a substantial tumor burden, which reduces tumor activity. Therefore, neoadjuvant immunotherapy for gastrointestinal tumors is gaining significant momentum, and several Phase III clinical trials are underway. The Figure 2 provides an overview of the current landscape of neoadjuvant immune therapy in the field of gastrointestinal tumors, while detailed clinical trial information is provided in Supplement Table 1 (Supplemental Digital Content 1, http://links.lww.com/JS9/C206) for ease of reference.
Figure 2.
The current landscape of neoadjuvant immunotherapy in the field of gastrointestinal tumors.
Patient population, treatment regimens, and observation endpoints for neoadjuvant immunotherapy
Potentially eligible population for neoadjuvant immunotherapy
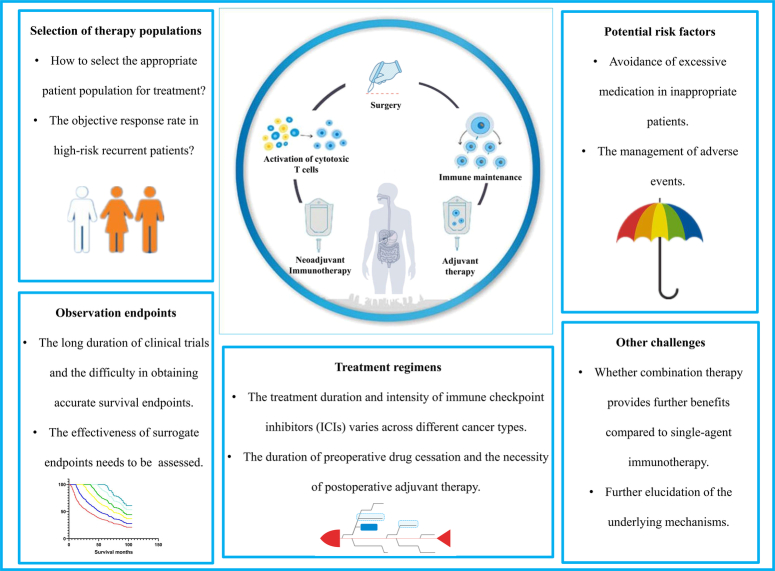
Despite the remarkable treatment responses achieved by ICIs in advanced-stage tumors, a considerable proportion of patients experience limited benefits, especially when ICIs are administered as monotherapy. Neoadjuvant immunotherapy, as a novel therapeutic option, requires careful consideration of this issue, because of the potential risks of delaying or impeding surgery due to the indiscriminate use of ICIs as preoperative antitumor agents. The effective identification of patients who will benefit from neoadjuvant immunotherapy and the avoidance of inappropriate treatment for individuals who may not derive significant benefits pose significant challenges in current immunotherapy practices. Addressing these challenges is crucial for ensuring the overall success of immune-based therapies and the specific clinical implementation of neoadjuvant immunotherapy (Fig. 3).
Figure 3.
Clinical challenges for neoadjuvant immunotherapy. The overall treatment process of neoadjuvant immunotherapy is represented by the enclosed circle in the diagram. The outer rectangular box highlights potential clinical challenges, including patient selection, identification of potential risk factors, determination of therapy regimens, observation endpoints and other factors.
Current exploration of biomarkers offers a crucial approach to address this issue. Studies have indicated that utilizing baseline genomic, transcriptomic, and microbiome profiles can effectively stratify patients according to treatment response, thereby identifying potential beneficiaries. The melanoma OpACIN Ib and OpACIN-neo studies27 performed biomarker analysis and revealed that high tumor mutation burden (TMB) and high interferon-gamma-related gene expression signature (IFN-γ score) were associated with durable responses and a decreased risk of recurrence. Patients with a high IFN-γ score/TMB had a 100% pathological response rate (pRR), and patients with a high IFN-γ score/low TMB or low IFN-γ score/high TMB had pRRs of 91 and 88%, respectively. However, patients with a low IFN-γ score or low TMB had a pRR of only 39%. Studies on dual immune checkpoint blockade in the neoadjuvant setting analyzed tumor-infiltrating immune cell levels in resected tissues during the treatment process and found that CD3+ CD8+ lymphocytes, tissue-resident T cells and effector T cells were abundant in postneoadjuvant therapy tumor samples. These findings suggested that nivolumab and ipilimumab combination therapy, as neoadjuvant treatments, effectively enhanced intratumoral immune cells to exert immune cytotoxicity. Patients who responded better to this regimen had higher PD-L1 expression. Notably, responses to this treatment approach were observed even in patients without PD-L1 expression. These findings indicate that higher levels of PD-L1 expression are associated with better neoadjuvant immune therapy outcomes, but patients without PD-L1 expression can still receive ICIs as neoadjuvant treatment28. Neoadjuvant therapy in the context of breast cancer demonstrated a greater rate of pCR in lymph node-negative patients compared to patients with lymph node involvement. Notably, there was no significant difference in PD-L1 expression between patients who achieved pCR.
Specific treatment modalities and regimens
Malignant tumors exhibit significant biological heterogeneity, which results in a lack of standardized targeted therapeutic approaches. ICIs mobilize the immune system to combat tumors and are considered a broad-spectrum cancer treatment modality, but the specific drug class, dosage, and sequence vary across tumor types. Therefore, neoadjuvant therapy regimens are diverse. In various cancers, such as melanoma and lung cancer, combination therapy, as opposed to monotherapy, facilitated antigen exposure, which enhanced T cell-mediated immune cytotoxicity and achieved higher rates of pathological response.
Although clinical trials demonstrated that some patients achieved pCR after neoadjuvant immunotherapy, post-treatment strategies remain crucial, and definitive surgical resection is necessary. Current research identified four patterns of residual tumor after neoadjuvant therapy: large shallow residual disease, large deep residual disease, small shallow residual disease, and small deep residual disease. Previous studies on solid tumors following neoadjuvant chemoradiotherapy revealed that tumor cells remained in the mucosal layer (60.9%), submucosal layer (69.6%), muscular layer (52.2%), serosal layer (30.4%), and lymph nodes (30.4%) after treatment. Therefore, a clinical complete response (cCR) does not equate to pCR, and a certain risk of tumor recurrence remained even when neoadjuvant therapy achieved pCR. Therefore, neoadjuvant therapy represents a continuum of treatment rather than a curative method.
Observation endpoints
Neoadjuvant therapy provides an opportunity for early tumor assessment, but selecting appropriate endpoints for perioperative treatment evaluation is challenging. Given the prolonged overall survival (OS) observed in patients after surgical resection in neoadjuvant studies, the use of OS as an endpoint necessitates long study durations and a substantial number of samples. The OS of patients is significantly influenced by later-line treatment settings. Disease-free survival (DFS), which is the time without tumor recurrence after surgery, is a primary endpoint for neoadjuvant therapy. Once a tumor recrudesces, patients experience related symptoms and face a dismal prognosis. Therefore, DFS is used as a main research endpoint, which allows for shorter study durations and reduced sample sizes. One of the prominent advantages of neoadjuvant immunotherapy is its ability to downstage tumors prior to surgery, which leads to a major pathological response (MPR) and even pCR, to ultimately improve OS benefits. Therefore, most phase II prospective studies use pCR as the primary endpoint for assessing the potential value of neoadjuvant immunotherapy in predicting tumor regression. Indeed, it is important to note that although neoadjuvant immunotherapy studies can choose surrogate endpoints as primary endpoints, OS remains the gold standard and is crucial for evaluating patient benefit.
Neoadjuvant therapy followed by surgical intervention provides the unique advantage of abundant tissue samples for comprehensive multidimensional biomarker analysis. Evaluating the pathological response of postoperative specimens, metabolic changes in the tumor microenvironment, immune cell infiltration classification, and the identification of specific gene expression patterns will greatly contribute to advancing research on specific biomarkers. These analyses will enable precise patient classification and improve treatment efficacy.
Potential adverse factors
For earlier stages of cancer, surgery can achieve curative resection. Although neoadjuvant immunotherapy has the potential to provide long-term survival benefits for patients by leveraging the antitumor immune response, further rigorous clinical trials with higher levels of evidence are needed to explore the incremental benefits of incorporating these treatment modalities. There is no consensus on whether neoadjuvant therapy is necessary for early-stage tumors amenable to curative resection. It is imperative to accurately select patient populations who would truly benefit from these treatments to avoid potential overtreatment. For example, the Neo-AEGIS study investigated the efficacy of perioperative chemotherapy compared to neoadjuvant chemoradiotherapy in patients with adenocarcinoma of the esophagus and gastroesophageal junction cancer. The results demonstrated no significant difference in the 3-year OS rate between patients who underwent neoadjuvant chemoradiotherapy and patients who received perioperative chemotherapy (56 vs. 57%)9.
Conversely, for tumors harboring high-risk factors for recurrence and considered potentially resectable, neoadjuvant immunotherapy offers the advantages of improving the resection rate and reducing the likelihood of relapse. However, it is crucial to consider the unconventional AEs, including hyper-progression and pseudo-progression, after receiving immunotherapy. Patients with locally advanced esophageal cancer who are eligible for surgical resection may experience intolerable adverse reactions when receiving neoadjuvant treatment due to the cumulative toxicity of radiation therapy and chemotherapy. These adverse reactions may adversely affect the surgical procedure and postoperative recovery. Therefore, further assessment is warranted to evaluate the potential increase in AEs resulting from the addition of neoadjuvant immunotherapy in these patients.
Clinical advances in neoadjuvant immunotherapy in gastrointestinal cancer
Esophageal cancer
Esophageal cancer manifests as the outward growth of carcinoma from the esophageal mucosa. From the perspective of gene alterations, esophageal cancer has a high tumor mutation burden and ranks among the top gastrointestinal tumors, which suggests that immune therapies based on PD-1/L1 have efficacy in the treatment of esophageal cancer29.
For resectable esophageal cancer patients, an integrated treatment approach centered around surgical intervention has been acknowledged for its therapeutic efficacy. Approximately 70% of locally advanced patients require neoadjuvant or adjuvant therapy during the perioperative period. Combination immunotherapy based on PD-1/L1 checkpoint inhibitors demonstrated synergistic antitumor effects. Multiple clinical studies investigated neoadjuvant therapy for esophageal cancer and revealed overall pCR rates ranging from 20 to 42.5%. A multicenter phase II clinical study demonstrated that in patients with stage II–IVA esophageal squamous cell carcinoma located in the thoracic segment, after receiving two cycles of neoadjuvant therapy with camrelizumab combined with albumin-bound paclitaxel and cisplatin, 51 patients underwent surgery, and 50 of these patients (98.0%) achieved R0 resection. In 20 patients (39.2%), a pCR with ypT0N0 was observed, which indicated no residual tumor in the primary site or lymph nodes. Five patients (9.8%) achieved complete resolution of the primary tumor but had residual disease in the lymph nodes (ypT0N+)30. To further explore the effectiveness and safety of neoadjuvant immunotherapy combined with chemotherapy in the perioperative treatment of esophageal cancer, the NIC-ESCC2019 study recruited 56 patients31. The patients underwent two cycles of pembrolizumab combined with chemotherapy as preoperative neoadjuvant treatment, with each cycle lasting 21 days. Radical surgery for esophageal cancer was performed 6 weeks after the completion of neoadjuvant treatment. The results showed that among the patients who underwent surgical treatment (n=51), the R0 resection rate reached 100%, and the complete pathological response (CPR) rate was 35.3%. Sixteen patients (31.4%) achieved CPR of the primary tumor lesions and lymph nodes. The ORR of the study was 66.7% (95% CI: 40.0–70.4). It is worth noting that in terms of safety, the adverse reactions of pembrolizumab combined with chemotherapy were manageable, with significantly fewer toxic side effects compared to traditional radiotherapy and chemotherapy. Severe adverse reactions, such as esophageal perforation, were relatively rare.
In addition to the favorable therapeutic effects of immunotherapy combined with chemotherapy as a neoadjuvant treatment for esophageal cancer, clinical studies reported significant findings of immunotherapy combined with targeted therapy and continued immunotherapy combined with chemotherapy. A Phase Ib study investigated the safety and efficacy of pembrolizumab in combination with chemotherapy and apatinib (pembrolizumab plus albumin-bound paclitaxel plus nedaplatin plus apatinib), as neoadjuvant therapy for locally advanced esophageal squamous cell carcinoma32. The study included a total of 30 enrolled patients, 5 of whom underwent two cycles of neoadjuvant treatment, and 1 patient missed the second cycle due to a grade 3 increase in alanine transaminase (ALT) levels. Notably, the remaining 24 patients completed the full course of four cycles of neoadjuvant treatment. Eleven patients (36.7%) experienced grade 3 treatment-related adverse events (TRAEs). The most common grade 3 TRAE was neutropenia (23.3%). Among the 29 patients who underwent esophageal tumor resection after neoadjuvant treatment, 5 patients experienced surgical delays due to adverse reactions. Of the 29 patients who underwent esophageal resection, 15 patients (51.7%) achieved MPR, and 7 patients (24.1%) achieved pCR. Among the 24 patients who completed the full course of four cycles of neoadjuvant treatment, the pCR and MPR rates were 29.2 and 58.3%, respectively. One study of neoadjuvant chemotherapy followed by sequential immunotherapy included 30 patients with T3, T4, or lymph node-positive esophageal squamous cell carcinoma33. The results demonstrated that the pCR rates in the experimental (receiving toripalimab 2 days after chemotherapy) and control (simultaneously receiving chemotherapy and toripalimab) groups were 36.4% (n=4) and 7.7% (n=1), respectively. The experimental group showed a trend toward a higher pCR rate (P=0.079).
Neoadjuvant immunotherapy combined with chemotherapy holds promise as a reliable treatment option for locally advanced resectable esophageal cancer. However, whether these results translate into survival benefits requires further validation over time, and numerous clinical studies are currently underway to investigate this aspect and are frequently updates at leading conferences such as American Society of Clinical Oncology (ASCO) and European Society for Medical Oncology (ESMO) annual meetings (Table 1).
Table 1.
Key trials of neoadjuvant immunotherapy in esophagogastric cancers.
| Study/trial identifier | Tumor type | Phase | Participants | Combination regimen type | Resection rate | Clinical endpoints | Main Results | Status | Reference |
|---|---|---|---|---|---|---|---|---|---|
| ChiCTR2000040330 | ESCC | Phase 4 | 150 | PD-1+Chemotherapy VS Chemotherapy | N/A | 5-year OS rate | pCR:25/90 (27.8%) VS 6/60 (10%) | Recruiting | 2023 ASCO1 |
| NCT05323890 | ESCC | Phase 2 | 18 | PD-1+Chemotherapy+Radiotherapy | R0:100% | MPR, pCR | pCR:50.0%, MPR:72.2% | Recruiting | 2023 ASCO2 |
| NCT05807542 | ESCC | Phase 2 | 15 | PD-1+Chemotherapy | 100% | pCR, MPR<10% | pCR:20% (3/15), MPR:13% (2/15) | Completed | 2023 ASCO3 |
| NCT05476380 | ESCC | Phase 2 | 38 | PD-1+Chemotherapy | R0:89.5% | pCR | MPR:67.6% (23/34), pCR:17.6% (6/34) | Recruiting | 2023 ASCO4 |
| NCT04460066 | ESCC | Phase 2 | 64 | PD-L1+Chemotherapy VS Chemotherapy+Placebo | R0:100% VS 98.6% | MPR | MPR:68.97% VS 62.07%, pCR:41.4% VS 27.6% | Active, not recruiting | Li Y5 |
| ChiCTR2000033252 | ESCC | Phase 2 | 37 | PD-1+Chemotherapy | R0:100% (31/31) | PFS | ORR:46.7%, pCR:20% | Recruiting | 2023 ESMO6 |
| ChiCTR2100050057 | ESCC | Phase 2 | 70 | PD-1+Chemotherapy | 84.29% | pCR | pCR:41.7%, MPR:66.7% | Recruiting | 2023 ESMO7 |
| NCT04215471 | ESCC | Phase 2 | 30 | PD-L1 | 83.33% | ORR | 2-year OS:92% | Recruiting | Yin J8 |
| ChiCTR2000037488 | ESCC | Phase 2 | 45 | PD-1+Chemotherapy | 80% | MPR | MPR:72.0%; pCR:50.0% | Completed | Yan X9 |
| ChiCTR1900027160 | ESCC | Phase 2 | 60 | PD-1+Chemotherapy | R0:98.21% | MPR | MPR:49.09%; pCR:29.09% | Completed | Zhang G10 |
| ChiCTR1900026240 | ESCC | Phase 2 | 60 | PD-1+Chemotherapy | R0:98.0%(50/51) | pCR | pCR:39.2% | Completed | Liu J11 |
| NCT03946969 | ESCC | Phase 2 | 30 | PD-1+Chemotherapy | 100% | Safety and feasibility | Grade 3-4 TRAEs:36.7% | Active, not recruiting | Chen X12 |
| ChiCTR2000029807 | ESCC | Phase 2 | 47 | PD-1+Chemotherapy | R0:89.4% | MPR | MPR:64.3% | Completed | Yang G13 |
| ChiCTR1900026593 | ESCC | Phase 2 | 47 | PD-1+Chemotherapy | R0:97.8% | MPR | MPR:44.4% | Completed | Zhang Z14 |
| NCT03448835 | GC and GEC | Phase 2 | 20 | PD-L1+Chemotherapy | 100% | AEs | pCR:45%, MPR:70% | Recruiting | 2022 ESMO15 |
| ChiCTR2000030610 | GC or GJA | Phase 2 | 63 | Chemotherapy VS PD-1+Chemotherapy | R0:91% VS 100% | pCR | pCR:4% VS 13%. | Recruiting | 2022 ESMO16 |
| NCT04061928 | GJA | Phase 2 | 24 | PD-1+Chemotherapy+Radiotherapy | R0:66.7% | MPR | CR:33.3%, MPR:72.2% | Recruiting | 2022 ESMO17 |
| NCT03064490 | Esophageal and gastric cancers | Phase 2 | 38 | PD-1+Chemotherapy+Radiotherapy | R0:97% | pCR | pCR:35.7% (10/28) | Active, not recruiting | 2023 ASCO18 |
| NCT03878472 | GC | Phase 2 | 25 | PD-1 | 76.0% | PRR | MPR:26.3% | Recruiting | Li S19 |
| JapicCTI-183895 | GC | Phase 1 | 31 | PD-1 | 97% | AEs | AEs:23% | Completed | Hasegawa H20 |
| NCT03288350 | Gastroesophageal adenocarcinoma | Phase 2 | 50 | PD-L1+Chemotherapy | R0:96% | pCR | pCR:14% | Recruiting | 2023 ASCO21 |
| NCT03221426 | G/GEJ | Phase3 | 203 | PD-1+Chemotherapy VS Placebo+Chemotherapy | R0:79 VS 80% | EFS | pCR:17% VS 6.8 | Active, not recruiting | 2024 ASCO GI22 |
| NCT04661150 | G/GEJ | Phase2 | 42 | PD-L1+Chemotherapy+Target VS Target+Chemotherapy | N/A | pCR | pCR:38.1% VS 14.3% | Active, not recruiting | 2024 ASCO GI23 |
| NCT03914443 | ESCC | Phase1 | 12 | PD-1+Chemotherapy | R0:91.7% | Rate of participants with dose limiting toxicities (DLTs) | pCR:41.7% | Active, not recruiting | 2024 ASCO GI24 |
| ChiCTR2200064848 | ESCC | Phase2 | 25 | PD-1+Target | 76% | pCR | pCR:18.8% | Recruiting | 2024 ASCO GI25 |
| NCT03064490 | Esophago-gastric diseases | Phase2 | 35 | PD-1+Chemotherapy+Radiotherapy | 85.70% | pCR | pCR:35.5%, MPR:50% | Completed | 2024 ASCO GI26 |
| NCT05189730 | ESCC | Phase2 | 32 | PD-1+Chemotherapy+Radiotherapy | 75% | pCR | MPR:16 (66.7%), ORR:21(87.5%) | Unknown | 2023 ESMO27 |
| NCT04389177 | ESCC | Phase2 | 45 | PD-1+Chemotherapy | N/A | MPR | MPR:73.3%, pCR:42.2%,ORR:95.6%,DCR:100%. | Active, not recruiting | 2023 ESMO28 |
| CTR2100051599 | ESCC | Phase2 | 26 | PD-1+Chemotherapy+Radiotherapy | R0:100% | pCR | CR:9 (42.8%), MPR:14 (66.6%) | Recruiting | 2023 ESMO29 |
| NCT04819971 | GC/EGJC | Phase2 | 18 | PD-1+Chemotherapy+Target | 61.10% | pCR | pCR:6(54.5%), MPR:7 (63.6%) | Recruiting | 2023 ESMO30 |
| NCT04159974 | esophageal adenocarcinoma | Phase2 | 56 | PD-1+Chemotherapy+Radiotherapy | 93% | Evaluation of safety and efficacy | anastomotic leakage:6% (3/52) | Recruiting | 2023 ESMO31 |
| NCT05007145 | Thoracic esophageal squamous cell cancer | Phase2 | 85 | PD-1+Chemotherapy VS Chemotherapy+Radiotherapy | 78.1% VS 63.6% | pCR | pCR:40.6% (13/32) VS 35.7% (10/28),MPR:62.5% (20/32) VS 71.4% (20/28) | Unknown | 2023 ESMO32 |
| NCT03221426 | G/GEJ | Phase3 | 804 | PD-1+Chemotherapy VS Chemotherapy+Placebo | N/A | EFS | pCR:12.9%VS2.0% | Active, not recruiting | Shitara K33 |
| NCT04208347 | G/GEJ | Phase3 | 360 | PD-1+Chemotherapy+Target VS Chemotherapy+Target | R0:98.7% (SOXRC) vs 94.2% (SOX). | pCR | pCR:18.3% (95% CI 13.0-24.8) VS 5.0% (95% CI 2.3-9.3) | Active, not recruiting | 2023 ESMO34 |
| NCT03421288 | ESCC | Phase2 | 295 | PD-L1+Chemotherapy VS Chemotherapy | R0:96% VS 95% | EFS | Surgical morbidity 45% VS 42%,60-day mortality 3% VS 2% | Recruiting | Lorenzen S35 |
AEs, adverse events; CR, complete response; DFS, disease-free survival; ESCC, esophageal squamous cell carcinoma; GC, gastric cancer; GEC, gastroesophageal cancer; GJA, gastroesophageal junction adenocarcinoma; MPR, major pathological response; N/A, not applicable; OR, objective response; OS, overall survival; pCR, pathological complete response; PFS, progression free survival; PRR, pathological remission rate; TRAEs, treatment-related adverse events.
Gastric cancer
Curative resection is the primary means of achieving a cure for early-stage gastric cancer. However, for locally advanced gastric cancer, current guidelines and consensuses suggest the need for neoadjuvant therapy prior to surgery. The FLOT4-AIO, RESOLVE, and RESONANCE studies demonstrated promising prospects for the application of neoadjuvant chemotherapy in gastric cancer. Gastric cancer is classified into different molecular subtypes, including Epstein–Barr virus (EBV)-infected, microsatellite instability (MSI), genomically stable, and chromosomal instability types. EBV-positive patients exhibit increased immune cell infiltration, which suggests a favorable response to immunotherapy. MSI-high patients have been approved for treatment with PD-1 antibodies, and chromosomal instability (CIN) patients may benefit from specific targeted therapies combined with immunotherapy. Therefore, gastric cancer also presents a solid foundation for immunotherapy. Despite achieving an effective rate of 40–50% in advanced gastric cancer, the addition of immunotherapy to conventional neoadjuvant therapy has the potential to further enhance tumor treatment responses, improve the proportion of resection, and prolong patient survival.
The combination of chemotherapy and immunotherapy with trastuzumab in combination with the SOX regimen for neoadjuvant treatment in advanced GC/GEJ patients achieved a pCR rate of 25% and an MPR rate of 53.1%, which suggests that immunotherapy combined with chemotherapy holds promising prospects for neoadjuvant and conversion therapy in GC/GEJ patients34. The phase II clinical trial PERSIST investigated the efficacy of a new neoadjuvant treatment regimen that combines SOX chemotherapy with sintilimab compared to SOX alone in patients with locally advanced gastric cancer. The results demonstrated that the combination of sintilimab and SOX achieved a pCR rate of 26.9% and an MPR rate of 69.2%, and the SOX alone group had a pCR rate of 4.8% and an MPR rate of 28.6%. Three patients in the combination group experienced grades 3–4 surgical complications35. A multicenter prospective study explored the efficacy of neoadjuvant SOX chemotherapy combined with apatinib and PD-1 inhibitors in the treatment of locally advanced gastric cancer. The 1-year and 2-year DFS rates were 96.6 and 77.7%, respectively, and the 1-year and 2-year OS rates were 96.6 and 90.1%, respectively. Ipilimumab plus durvalumab was evaluated as a neoadjuvant treatment for resectable GAC/GEJAC patients with MSI, mismatch repair deficiency (dMMR), or EBV negativity. Of the 15 assessable patients, 1 patient experienced disease progression, and 14 patients underwent surgical resection. The study reported a pCR rate of 60% (9/15) and an MPR (residual viable cells <10%) rate of 80%36. The phase II NEONIPIGA study evaluated the efficacy of neoadjuvant nivolumab combined with ipilimumab and adjuvant monotherapy with nivolumab in patients with resectable MSI/dMMR tumors (13). Among the 32 patients enrolled, 27 (84%) completed all six cycles of neoadjuvant treatment. Eight patients (32%) experienced grade 3/4 AEs during neoadjuvant therapy. Twenty-nine patients underwent surgery, with a median delay of 35 days from the last injection to surgery. Preliminary findings suggest the feasibility and manageable safety profile of adding CTLA-4 to PD-1 inhibitor-based therapy37.
Although there are ongoing clinical studies investigating various therapeutic combinations (Table 1), there is still a lack of high-quality multicenter phase III trials. The optimal timing and duration of immunotherapy for the treatment of advanced gastric cancer, and other related questions, require further investigation using randomized prospective studies38.
Hepatobiliary cancers
Curative liver resection has long been the backbone treatment for hepatocellular carcinoma (HCC), and it yields optimal clinical benefit. However, the high postoperative recurrence rate remains a major obstacle to long-term survival39,40. The ability of neoadjuvant therapy to minimize the risk of recurrence, eliminate potential microscopic metastases, and convert unresectable disease into resectable disease in patients with nonhepatic neoplasms has been validated41. The low efficacy, poor tolerability, and frequent drug resistance of prior alternative medications have hindered the development of neoadjuvant treatment strategies for HCC. In the era of molecular targeted therapy and ICIs, the number of staged therapy options for HCC has increased over the last decade, as have the number of well-known advances in systemic therapy and postoperative adjuvant therapy that have been considered in guidelines42,43. However, there are no widely accepted protocols to guide the application of immunotherapy, particularly ICIs-based regimens, in the field of neoadjuvant therapy. Fortunately, emerging studies are underway on the efficacy and safety of ICIs-based neoadjuvant immunotherapy, with inspiring initial results44.
A single-arm phase Ib study by Ho et al.45 investigating neoadjuvant cabozantinib and nivolumab is the first report of the conversion of locally advanced HCC into resectable disease. In this study of 15 patients, 12 (80%) underwent successful marginal-negative resection, and 5 of the 12 (42%) resected patients achieved major pathological responses (>90% necrosis). Marron et al.46 evaluated the potential efficacy of neoadjuvant cemiplimab (an anti-PD-1) monotherapy in 21 patients with initially resectable HCC with stage Ib, II, or IIIb disease. Among the 20 patients who underwent resection, 7 patients (35%) had 50% or greater tumor necrosis, 4 patients (20%) had significant tumor necrosis (>70% necrosis), and no grade 4 or 5 AEs were observed. Based on published and ongoing trials, the paradigm of combining ICIs with antitumor drugs or local therapies to increase benefits is an emerging current trend (Table 2). A recently released study of 56 individuals treated with lenvatinib plus anti-PD-1 antibodies for preoperative conversion achieved a conversion success rate of 55.4% (31/56) and an R0 resection rate of 85.7% (18/21) among surgical patients47. The patients in the conversion success group had significantly longer OS and PFS. The most recent meta-analysis demonstrated that neoadjuvant ICIs, whether used as single or dual ICIs or in combination with antitumor drugs, were associated with a statistically significant pCR and a tolerable safety profile48.
Table 2.
Key trials of neoadjuvant immunotherapy in hepatobiliary and pancreatic cancers.
| Study/trial identifier | Tumor type | Phase | Participants | Combination regimen type | Resection rate | Clinical endpoints | Main Results | Status | Reference |
|---|---|---|---|---|---|---|---|---|---|
| ChiCTR2100050410 | uHCC | Unknown | 55 | TACE+PD-1+Target | 47.3% | ORR | ORR:72.0%, DCR:84.0%. | Completed | 2023 ASCO36 |
| NCT04521153 | HCC | Phase 2/3 | 119 | PD-1+Target+TACE VS TACE | 89.7% VS N/A | MPR | MPR:46.2% (24/52) | Recruiting | 2023 ASCO37 |
| NCT03682276 | HCC | Phase 1B | 25 | CTLA-4+PD-1 | 84% | AEs | Grade 3 AEs:24% (n=6) | Recruiting | 2023 ASCO38 |
| NCT04701060 | HCC | Phase 2 | 31 | PD-1+Target | 93.5% | ORR | MPR:38.5%, pCR:7.7% | Recruiting | 2023 ASCO39 |
| NCT04843943 | Resectable intermediate HCC | Phase 2 | 30 | PD-1+Target | 43.3% | AEs | ORR:23.3%, DCR:90% | Recruiting | 2022 ESMO40 |
| NCT04930315 | HCC | Phase 2 | 24 | Target+PD-1 VS PD-1 | R0:100% VS 100% | 1-year tumor recurrence-free rate | ORR:57.1%, DCR:92.9% | Recruiting | 2022 ESMO41 |
| NCT04997850 | uHCC | Phase 1/2 | 142 | TACE+Target+PD-1 VS TACE | 50.7% VS 15.5% | Conversion resection rate | ORR (mRECIST):78.9% VS 16.9% | Enrolling by invitation | 2022 ESMO42 |
| NCT03299946 | HCC | Phase 1 | 15 | PD-1+Targeted therapy | 80% | AEs | MPR: 42% | Completed | Ho WJ43 |
| NCT04297202 | HCC | Phase 2 | 18 | PD-1+Targeted | 94.4% | MPR | MPR:17.6% (3/17) | Completed | Xia Y44 |
| NCT05036798 | Biliary tract cancers | Phase 2 | 25 | PD-1+Chemotherapy+Target | R0:52% | R0 resection rate | ORR:56%, DCR:92%. | Active, not recruiting | 2022 ESMO45 |
| NCT03727880 | PDAC | Phase 2 | 36 | PD-1+Target VS PD-1 | N/A | pCR | Patients in Arm A demonstrated a 5.44-fold average increase in CD8+ T-cell percentage compared to a 2.01-fold average in Arm B | Recruiting | 2023 ASCO46 |
| ChiCTR2000032955 | Resectable pancreatic cancer | Phase 2 | 25 | PD-1+Chemotherapy+Radiotherapy | R0:90% | ORR | ORR:60%, DCR:100%. | Completed | 2022 ESMO47 |
| ChiCTR2100048249 | HCC | Phase 2 | 29 | PD-1+Target+TACE | R0:100% | RFS | ORR:89.7% (26/29, CR 4, PR 22), DCR:96.7% | Recruiting | 2024 ASCO GI48 |
| NCT04615143 | HCC | Phase 2 | 12 | PD-1+Target | 85.7% | DFS | CR:25% (3/12) | Recruiting | 2024 ASCO GI49 |
| NCT05185531 | HCC | Phase 1B | 11 | PD-1+Chemotherapy | 63.6% | Delay to surgery | DCR:100% (RECIST: 1 PR, 6 SD; mRECIST: 1 CR, 2 PR, 3 SD, 1 CR+SD) | Active, not recruiting | 2023 ESMO50 |
| NCT04308174 | Biliary tract cancer (BTC) | Phase 2 | 31 | PD-L1+Chemotherapy VS Chemotherapy | 68% (n=21) VS 36% (n=5) | R0 resection rate | R0 resection:48% (n=15) VS 36% (n=5) | Active, not recruiting | 2023 ESMO51 |
AEs, adverse events; DCR, disease control rate; MPR, major pathological response; N/A, not applicable; ORR, overall response rate; pCR, pathological complete response; PDAC, pancreatic ductal adenocarcinoma; uHCC, unresectable hepatocellular carcinoma.
BTCs are frequently diagnosed in the advanced-stage, and only a small fraction of patients are candidates for surgery. The presence of positive surgical margins (R1) and lymph node metastasis negatively impact the 5-year OS of these patients49. The introduction of innovative molecular-targeted medicines has contributed to the evolution of treatment paradigms, and the results have been extensively reviewed and evaluated elsewhere. Recent studies demonstrated the safety and efficacy of ICIs-based immunotherapy in advanced BTCs50–52. However, unlike other gastrointestinal tumors, the application and benefits of neoadjuvant immunotherapy in resectable BTCs have not been well studied. At the ASCO-2023 annual meeting, Gbolahan and colleagues reported a novel combined neoadjuvant therapy protocol of durvalumab and tremelimumab with platinum-based chemotherapy (gemcitabine and cisplatin)53. In addition to efficacy and safety evaluations, they intend to investigate the relationship between treatment response and clinical and tumor microenvironment-related factors.
In neoadjuvant immunotherapy for hepatobiliary cancers, ICIs are expected to enhance the effectiveness of subsequent surgery by reducing the tumor burden and enabling more radical resection, but several challenges remain. Key areas of subsequent research include the identification of reliable predictive biomarkers and optimal patient selection criteria, the balancing of immune-mediated adverse events (imAEs) and perioperative safety, and standardization of indications and surgical criteria. The Chinese expert consensus on neoadjuvant therapy for hepatocellular carcinoma (2023 edition) recommends that the eligible population for neoadjuvant therapy is the group of resectable patients with high-risk recurrence factors (Recommended: 2A). Neoadjuvant immunotherapy improves patient prognosis and increases the CPR rate, and eligible patients are recommended to participate in clinical trials investigating neoadjuvant immunotherapy (Recommended: 2B)54.
Pancreatic cancer
Pancreatic cancer is a malignant tumor of the digestive system that is characterized by an immunosuppressive tumor microenvironment and an extremely poor prognosis55,56. The clinical treatments for most patients with advanced pancreatic cancer are limited, and systemic therapies are the mainstay of disease control57. Preoperative and postoperative neoadjuvant therapy reduce recurrence and improve the prognosis of patients with advanced cancer58. However, evidence in favor of neoadjuvant therapy for the management of advanced pancreatic cancer is controversial. A recent study by Yang et al.59 showed that neoadjuvant therapy effectively improved the prognosis of resectable pancreatic cancer patients. In contrast, an oral abstract session at 2023 ASCO by Labori et al. suggested that neoadjuvant FOLFIRNOX did not improve the OS of patients with resectable pancreatic cancer.
Emerging areas of PD‐1/PD-L1 inhibitors provide hope for cancer immunotherapy60. Immunologically ‘hot’ tumors have a greater response to immunotherapies. However, pancreatic cancer is a ‘cold’ tumor with a low tumor mutation burden, which makes immunotherapy ineffective61. Therefore, activators of the immune system may be needed to jump-start the immune response. Anti-CD40 Abs may transform ‘cold’ tumors into ‘hot’ tumors and improve cancer treatment. The combined application of nivolumab and the anti-CD40 Ab APX005M holds great promise for pancreatic cancer treatment (NCT03214250)62. Neoadjuvant immunotherapy has become a new option for improving the prognosis of advanced cancer patients. Currently, several prospective clinical trials of neoadjuvant immunotherapy for pancreatic cancer have been performed (NCT03979066, NCT02451982, NCT03767582, and NCT05604560). Du et al. at the 2023 ASCO showed that PD-1 inhibitors plus chemotherapy plus SBRT improved local control, which further prolonged the survival of patients with borderline resectable and locally advanced pancreatic cancer. Drug efficacy may be seriously hindered by the tumor microenvironment. Immunotherapy approaches have not yet affected pancreatic cancer prognosis due to the immunosuppressive nature of the tumor microenvironment. Li et al. performed multiomic analyses and reported that neoadjuvant immunotherapy reshaped the immune microenvironment of pancreatic cancer patients and may improve the efficacy of immunotherapy63.
Colorectal cancer
With the recent emergence and application of preoperative and postoperative therapies, such as chemotherapy, radiation therapy, targeted therapy, and immunotherapy, the trend of surgical treatment for colorectal cancer (CRC) has become increasingly precise and personalized64. Although ICIs have revolutionized advanced gastrointestinal cancer treatment, the effects of ICIs are mostly limited to patients with deficient mismatch repair (dMMR) CRC, which is characterized by high infiltration of CD8+ T cells. Numerous neoadjuvant trials are underway to estimate the benefit of immunotherapy-based regimens in the neoadjuvant setting for CRC patients.
ICI monotherapy led to significantly longer survival than chemotherapy for dMMR/MSI-H metastatic CRC patients65. However, ICI monotherapy has not shown meaningful effects in pMMR/MSS CRC patients66. Chen et al.67 demonstrated that anti-PD-1 monotherapy was effective and tolerable for advanced rectal cancer patients with dMMR/MSI-H tumors. A meeting abstract at the 2023 ASCO by Vora et al.68 also indicated that neoadjuvant immunotherapy showed promise for MSI-H gastrointestinal cancers. However, Fouchardiere et al.69 observed a limited pCR to short-course neoadjuvant immunotherapy. Real-world data from Chakrabarti et al.70 indicated the efficacy and safety of neoadjuvant immunotherapy in dMMR/MSI-H colon cancer patients. Studies have also suggested that neoadjuvant immunotherapy achieved better clinical outcomes in dMMR/MSI-H patients, which indicated that molecular subtyping was an effective strategy to identify patients who respond efficiently to neoadjuvant immunotherapy71. Therefore, the molecular subtypes of CRC patients must be studied, and additional potential prognostic markers must be assessed. dMMR/MSI-H CRC patients are more responsive to immunotherapy than pMMR/MSS CRC patients, which is likely due to their high tumor mutational burden, but the frequency of dMMR/MSI-H tumors in CRC is ~10–15%72. pMMR/MSS CRC is a ‘cold’ tumor with low tumor mutation burden, which makes immunotherapy ineffective. Other investigators explored various treatment options for the neoadjuvant immunotherapy of pMMR/MSS CRC, including the application of neoadjuvant immunotherapy in early-stage CRC, neoadjuvant immunotherapy combined with chemotherapy or a combination of two immunotherapies73,74 (Table 3). Intratumoral injection of an influenza vaccine immunologically transformed ‘cold’ tumors into ‘hot’ tumors and served as an immunotherapy for cancer75. Gögenur et al.76 demonstrated that the neoadjuvant intratumoral influenza vaccine was safe and feasible and induced CD8+ T cell infiltration. Jiang et al.77 recently indicated that neoadjuvant immunotherapy combined with targeted therapy and concurrent chemoradiotherapy plus surgery showed promise for the treatment of MSS rectal cancer. Another issue worth considering is that pseudoprogression and pseudoresidue are unique and prevalent response patterns in dMMR/MSI-H rectal cancer patients after neoadjuvant immunotherapy. Xie et al.78 revealed that pseudoprogression and pseudoresidue were present in three CRC patients (23.1%) and 10 CRC patients (76.9%), respectively.
Table 3.
Key trials of neoadjuvant immunotherapy in colorectal cancers.
| Study/trial identifier | Tumor type | Phase | Participants | Combination regimen type | Resection rate | Clinical endpoints | Main Results | Status | Reference |
|---|---|---|---|---|---|---|---|---|---|
| NCT03503630 | Rectal adenocarcinoma | Phase 2 | 44 | PD-L1+Chemotherapy+Radiotherapy | 90% | pCR | pCR:41.6% (15/36) | Active, not recruiting | 2023 ASCO1 |
| NCT04165772 | RC | Phase 2 | 12 | PD-1+Chemotherapy+Radiotherapy | N/A | CCR | CCR:100% | Recruiting | Cercek A2 |
| NCT03926338 | CRC | Phase 2 | 34 | PD-1 VS Chemotherapy+Radiotherapy | R0:100% | pCR | pCR:88% VS 65% | Recruiting | Hu H3 |
| NCT02754856 | CRC | Phase 1 | 23 | PD-1+CTLA-4 | 74% | AEs | RFS:9.7 (95% CI: 8.1–17.8) months | Completed | Kanikarla Marie P4 |
| NCT03026140 | Colon cancer | Phase 2 | 19 | PD-1+LAG-3 | 100% | irAEs | irAEs:74% | Recruiting | 2023 ESMO5 |
| NCT05571293 | CRC | Phase 3 | 12 | PD-1+CTLA-4 | 100% | EFS | Response≥90:4/12 | Active, not recruiting | 2024 ASCO GI6 |
| NCT04518280 | RC | Phase2 | 104 | PD-1+Chemotherapy+Radiotherapy VS Chemotherapy+Radiotherapy | 56.70% | CR | CR:57.4% VS 54.0% | Recruiting | 2023 ESMO7 |
| NCT04083365 | RC | Phase2 | 55 | PD-L1+Chemotherapy+Radiotherapy | 98.1% | pCR | pCR:17/46 (37%) | Recruiting | Grassi E8 |
| NCT05307198 | RC | Phase2 | 20 | PD-1+Chemotherapy | 60.0% | pCR | pCR:4/12 (33.3%) pCR | Recruiting | 2023 ESMO9 |
| EudraCT 2018-004835-56 | RC | Phase2 | 61 | PD-1+Chemotherapy+Radiotherapy | 92% | pCR | pCR:22 /56(39%) | Recruiting | 2023 ESMO10 |
AEs, adverse events; CCR, complete clinical response; CRC, colorectal cancer; DCR, disease control rate; DFS, disease-free survival; irAEs, immune-related adverse events; MPR, major pathological response; N/A, not applicable; ORR, overall response rate; pCR, pathological complete response; PFS, progression free survival; RC: rectal cancer; RFS, relapse-free survival.
Liver metastasis is common in CRC patients, and ~70% of patients die from liver metastases79. Intrahepatic recurrence is also common after resection for CRC metastases to the liver80. The primary advantage of neoadjuvant therapy is the potential to down-stage metastatic disease to facilitate hepatic resection in patients with colorectal liver metastasis. The application of neoadjuvant therapy for colorectal liver metastasis patients remains controversial, especially because of the potential risk of inducing liver injury prior to hepatectomy. Although Zhou et al.81 demonstrated the safety of neoadjuvant anti-CTLA-4 therapy and an anti-PD-L1 antibody in patients with metastatic CRC, the role of immunotherapy in the perioperative setting has been relatively poorly studied. A recent study demonstrated that dostarlimab-gxly bound to PD-1 receptors with high affinity and effectively blocked interactions with PD-L1 and PD-L282. Based on several lines of evidence, the 2023 National Comprehensive Cancer Network recommended dostarlimab-gxly may be used as neoadjuvant therapy in patients with operable colorectal liver metastases, with a category 2A level of evidence83.
Future perspectives
Preclinical studies and clinical trials have established that neoadjuvant immunotherapy is a novel and effective modality for tumor treatment. From a patient-oriented standpoint, neoadjuvant immunotherapy facilitates a degree of pathological remission before surgery, which reduces tumor cell activity and the risk of recurrence to culminate in enhanced patient survival. From an oncological perspective, neoadjuvant trials provide a unique opportunity to conduct very detailed spatial analysis of the tumor microenvironment after immunotherapy by providing large tumor specimens, which is not achievable with the small biopsy specimens typically obtained in late-stage disease trials. Despite its potential, neoadjuvant immunotherapy faces certain limitations. These limitations include the need for long-term follow-up to determine the effectiveness of treatment in early-stage patients to avoid overtreatment and the fact that neoadjuvant immunotherapy may lead to potential severe immune-related side effects, which deprive patients of the opportunity for curative surgery. As summarized in Table 4, an increasing number of phase 3 studies with large sample sizes are being conducted, which are expected to elucidate these issues. Gastrointestinal tumors, with their intricate stromal elements and complex tumor immune microenvironments, are areas where neoadjuvant combination immunotherapy has been extensively studied, and it exhibited considerable advantages and promising potential. Despite these advances, there remains a paucity of high-quality randomized controlled trials necessitating ongoing investigation.
Table 4.
Ongoing phase 3 clinical trials of combined immunotherapy in gastrointestinal cancers.
| Study/trial identifier | Tumor type | Phase | Participants | Combination regimen type | Clinical endpoints | ICIs Type | Status |
|---|---|---|---|---|---|---|---|
| NCT04848753 | ESCC | 3 | 663 | PD-1+Chemotherapy VS Chemotherapy | EFS | PD-1 | Active, not recruiting |
| NCT04807673 | ESCC | 3 | 342 | PD-1+Chemotherapy VS Chemotherapy+Radiotherapy | EFS | PD-1 | Recruiting |
| NCT04973306 | ESCC | 2/3 | 176 | PD-1+Chemotherapy+Radiotherapy VS Chemotherapy+Radiotherapy | pCR | PD-1 | Recruiting |
| NCT05213312 | ESCC | 2/3 | 90 | PD-1+Chemotherapy VS Chemotherapy | pCR | PD-1 | Recruiting |
| NCT05244798 | ESCC | 3 | 420 | PD-1+Chemotherapy VS PD-1+Chemotherapy+Radiotherapy VS Chemotherapy+Radiotherapy | pCR | PD-1 | Not yet recruiting |
| NCT05357846 | ESCC | 3 | 422 | PD-1+Chemotherapy+Radiotherapy VS Chemotherapy+Radiotherapy | OS | PD-1 | Recruiting |
| NCT05610332 | GC | 3 | 216 | PD-1+Chemotherapy VS Chemotherapy | ORR | PD-1 | Not yet recruiting |
| NCT04139135 | GC | 3 | 642 | PD-1+Chemotherapy vs Placebo +Chemotherapy | EFS | PD-1 | Recruiting |
| NCT05593458 | GC | 3 | 190 | PD-1+Chemotherapy | MPR | PD-1 | Recruiting |
| NCT05699655 | GC | 2/3 | 130 | PD-1+Chemotherapy VS Chemotherapy | pCR | PD-1 | Recruiting |
| NCT05270824 | Gastric Adenocarcinoma | 3 | 120 | PD-1+Chemotherapy+Target VS Chemotherapy | The number of CD8+ TILs in tumor tissue and adjacent tissue before and after treatment | PD-1 | Not yet recruiting |
| NCT04882241 | Gastric or GEJ Adenocarcinoma | 3 | 120 | PD-1+Chemotherapy VS Chemotherapy | EFS | PD-1 | Active, not recruiting |
| NCT04592913 | GC and GJC | 3 | 958 | PD-L1+Chemotherapy VS Chemotherapy | EFS | PD-L1 | Active, not recruiting |
| NCT05250843 | HCC | 2/3 | 90 | HAIC/TACE+PD-1+Target | RFS | PD-1 | Not yet recruiting |
| NCT04928807 | Rectal Cancer | 3 | 230 | PD-1+Chemotherapy+Radiotherapy | pCR | PD-1 | Recruiting |
| NCT06017583 | Rectal Cancer | 3 | 48 | PD-1+Chemotherapy+Radiotherapy VS Chemotherapy+Radiotherapy | CR | PD-1 | Recruiting |
| NCT05215379 | Rectal Cancer | 2/3 | 180 | PD-1+Chemotherapy+Radiotherapy | cCR | PD-1 | Recruiting |
cCR, clinical complete response; CR, complete response; EFS, event free survival; ESCC, esophageal squamous cell carcinoma; GC, gastric cancer; GEJ, gastroesophageal junction; GJC, gastroesophageal junction carcinoma; HCC, hepatocellular carcinoma; MPR, major pathological response; ORR, objective response rate; OS, overall survival; pCR, pathological complete response; RFS, recurrence-free survival; TIL, tumor-infiltrating lymphocyte.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Sources of funding
This work was supported by the National Natural Science Foundation of China (Grant No. 82073200 & 81874178 & 82203000), Major basic research of Shandong Provincial Natural Science Foundation (Grant No. ZR2021ZD26), Founds for Independent Cultivation of Innovative Team from Universities in Jinan (Grant No. 2020GXRC023), the Taishan Scholars Program of Shandong Province (tstp20221158, tsqnz20221164), and Shandong Provincial Natural Science Foundation (ZR202111120102).
Author contribution
D.-X.W. and T.L.: designed the study; D.-X.W., H.L., J.-C.T., and D.-L.Z.: searched the literature and wrote the manuscript; L.-J.Y., Z.-N.D., and H.L.: helped to collect literature and participated in discussions; Y.-C.Y. and Z.-R.D.: examined and verified the study. All authors read and approved the final manuscript.
Conflicts of interest disclosure
The authors declare that they have no competing interests.
Research registration unique identifying number (UIN)
Name of the registry: not applicable.
Unique identifying number or registration ID: not applicable.
Hyperlink to your specific registration (must be publicly accessible and will be checked): not applicable.
Guarantor
Dong-Xu Wang and Tao Li.
Data availability statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Acknowledgements
All of the figures were initially designed using BioRender (https://biorender.com/) and Photoshop (Adobe). Images for some figures include icons from BioRender.
Footnotes
Dong-Xu Wang, Hui Liu, Jin-Cheng Tian, and Dao-Lin Zhang are equal contributors.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.lww.com/international-journal-of-surgery.
Published online 21 March 2024
Contributor Information
Dong-Xu Wang, Email: drwangdongxu@163.com.
Hui Liu, Email: liuhuidoct@163.com.
Jin-Cheng Tian, Email: drtianjc@163.com.
Dao-Lin Zhang, Email: 2039384468@qq.com.
Lun-Jie Yan, Email: 1780794572@qq.com.
Zi-Niu Ding, Email: 419688449@qq.com.
Han Li, Email: 1301312243@qq.com.
Yu-Chuan Yan, Email: 17865195183@163.com.
Zhao-Ru Dong, Email: dongzhaoru0911@163.com.
Tao Li, Email: litao7706@163.com.
References
- 1.Aquina CT, Ejaz A, Tsung A, et al. National trends in the use of neoadjuvant therapy before cancer surgery in the US from 2004 to 2016. JAMA Network Open 2021;4:e211031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Versluis JM, Long GV, Blank CU. Learning from clinical trials of neoadjuvant checkpoint blockade. Nat Med 2020;26:475–484. [DOI] [PubMed] [Google Scholar]
- 3.Rapoport BL, Anderson R. Realizing the clinical potential of immunogenic cell death in cancer chemotherapy and radiotherapy. Int J Mol Sci 2019;20:959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mota Reyes C, Teller S, Muckenhuber A, et al. Neoadjuvant therapy remodels the pancreatic cancer microenvironment via depletion of protumorigenic immune cells. Clin Cancer Res 2020;26:220–231. [DOI] [PubMed] [Google Scholar]
- 5.Li W, Wu F, Zhao S, et al. Correlation between PD-1/PD-L1 expression and polarization in tumor-associated macrophages: a key player in tumor immunotherapy. Cytokine Growth Factor Rev 2022:67;49–57. [DOI] [PubMed] [Google Scholar]
- 6.Guan WL, He Y, Xu RH. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol 2023;16:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma W, Xue R, Zhu Z, et al. Increasing cure rates of solid tumors by immune checkpoint inhibitors. Exp Hematol Oncol 2023;12:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Blake SJ, Yong MC, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov 2016;6:1382–1399. [DOI] [PubMed] [Google Scholar]
- 9.Reynolds JV, Preston SR, O’Neill B, et al. Neo-AEGIS (Neoadjuvant Trial in Adenocarcinoma of the Esophagus and Esophago-Gastric Junction International Study): final primary outcome analysis. J Clin Oncol 2023;41(suppl_4):295, 295.36179271 [Google Scholar]
- 10.Patel SP, Othus M, Chen Y, et al. Neoadjuvant-adjuvant or adjuvant-only pembrolizumab in advanced melanoma. N Engl J Med 2023;388:813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 2020;367:eaax0182. doi: 10.1126/science.aax0182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S, Sun Q, Ren X. Novel strategies for cancer immunotherapy: counter-immunoediting therapy. J Hematol Oncol 2023;16:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu P, Zhang P, Sun Z, et al. Surgical trauma induces postoperative T-cell dysfunction in lung cancer patients through the programmed death-1 pathway. Cancer Immunol Immunother 2015;64:1383–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim R. Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J Transl Med 2018;16:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bi Q, Wu J-Y, Qiu X-M, et al. Tumor-associated inflammation: the tumor-promoting immunity in the early stages of tumorigenesis. J Immunol Res 2022;2022:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman J, Moore EC, Zolkind P, et al. Neoadjuvant PD-1 immune checkpoint blockade reverses functional immunodominance among tumor antigen-specific T cells. Clin Cancer Res 2020;26:679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yost KE, Satpathy AT, Wells DK, et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat Med 2019;25:1251–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Notarbartolo S, Abrignani S. Human T lymphocytes at tumor sites. Semin Immunopathol 2022;44:883–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luoma AM, Suo S, Wang Y, et al. Tissue-resident memory and circulating T cells are early responders to pre-surgical cancer immunotherapy. Cell 2022;185:2918–2935.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callahan MK, Wolchok JD. Recruit or reboot? How does Anti-PD-1 therapy change tumor-infiltrating lymphocytes? Cancer Cell 2019;36:215–217. [DOI] [PubMed] [Google Scholar]
- 21.Poggio M, Hu T, Pai C-C, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell 2019;177:414–427. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Q, Wu X, Wang Z, et al. The primordial differentiation of tumor-specific memory CD8(+) T cells as bona fide responders to PD-1/PD-L1 blockade in draining lymph nodes. Cell 2022;185:4049–4066.e25. [DOI] [PubMed] [Google Scholar]
- 23.Dammeijer F, van Gulijk M, Mulder EE, et al. The PD-1/PD-L1-checkpoint restrains T cell immunity in tumor-draining lymph nodes. Cancer Cell 2020;38:685–700.e8. [DOI] [PubMed] [Google Scholar]
- 24.Rahim MK, Okholm TLH, Jones KB, et al. Dynamic CD8(+) T cell responses to cancer immunotherapy in human regional lymph nodes are disrupted in metastatic lymph nodes. Cell 2023;186:1127–1143.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown CC, Wolchok JD. PD-L1 blockade therapy: location, location, location. Cancer Cell 2020;38:615–617. [DOI] [PubMed] [Google Scholar]
- 26.Haager B, Heymach J, Harpole D, et al. AEGEAN: a phase 3 trial of neoadjuvant durvalumab+ chemotherapy followed by adjuvant durvalumab in patients with resectable NSCLC. Zentralblatt für Chirurgie 2023;148:S87. [Google Scholar]
- 27.Versluis J, Menzies A, Sikorska K, et al. Survival update of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma in the OpACIN and OpACIN-neo trials. Ann Oncol 2023;34:420–430. [DOI] [PubMed] [Google Scholar]
- 28.Cascone T, William WN, Weissferdt A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med 2021;27:504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L, Bai L, Lin H, et al. Multiomics analysis of tumor mutational burden across cancer types. Comput Struct Biotechnol J 2021;19:5637–5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Yang Y, Liu Z, et al. Multicenter, single-arm, phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J Immunother Cancer 2022;10;e004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J, Li J, Lin W, et al. Neoadjuvant camrelizumab plus chemotherapy for resectable, locally advanced esophageal squamous cell carcinoma (NIC-ESCC2019): a multicenter, phase 2 study. Int J Cancer 2022;151:128–137. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z. Neoadjuvant camrelizumab combined with chemotherapy and apatinib for locally advanced thoracic esophageal squamous cell carcinoma (ESCC): a single-arm, open-label, phase Ib study. J Clin Oncol 2021;39(suppl_15):4047, 4047. [Google Scholar]
- 33.Xu X, Sun Z, Zhang Y, et al. Neoadjuvant chemoradiotherapy combined with perioperative toripalimab in locally advanced esophageal cancer. J Clin Oncol 2022;40(suppl_16):e16065, e16065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin Y, Lin Y, Yang M, et al. Neoadjuvant tislelizumab and tegafur/gimeracil/octeracil (S-1) plus oxaliplatin in patients with locally advanced gastric or gastroesophageal junction cancer: Early results of a phase 2, single-arm trial. Original Research. Front Oncol 2022;12:959295. doi: 10.3389/fonc.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding X, Wang X, Li B, et al. PERSIST: a multicenter, randomized phase II trial of perioperative oxaliplatin and S-1 (SOX) with or without sintilimab in resectable locally advanced gastric/gastroesophageal junction cancer (GC/GEJC). J Clin Oncol 2023;41(suppl_4):364.35878085 [Google Scholar]
- 36.Andre T, Tougeron D, Piessen G, et al. Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in patients (pts) with localized microsatellite instability-high (MSI)/mismatch repair deficient (dMMR) oeso-gastric adenocarcinoma (OGA): the GERCOR NEONIPIGA phase II study. J Clin Oncol 2022;40(suppl_4):244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pietrantonio F, Raimondi A, Lonardi S, et al. INFINITY: a multicentre, single-arm, multi-cohort, phase II trial of tremelimumab and durvalumab as neoadjuvant treatment of patients with microsatellite instability-high (MSI) resectable gastric or gastroesophageal junction adenocarcinoma (GAC/GEJAC). J Clin Oncol 2023;41(suppl_4):358. [Google Scholar]
- 38.Wu D, Huang H, Zhang M, et al. The global landscape of neoadjuvant and adjuvant anti-PD-1/PD-L1 clinical trials. J Hematol Oncol 2022;15:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xia Y, Tang W, Qian X, et al. Efficacy and safety of camrelizumab plus apatinib during the perioperative period in resectable hepatocellular carcinoma: a single-arm, open label, phase II clinical trial. J Immunother Cancer 2022;10:e004656. doi: 10.1136/jitc-2022-e004656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6. [DOI] [PubMed] [Google Scholar]
- 41.Marron TU, Schwartz M, Corbett V, et al. Neoadjuvant immunotherapy for hepatocellular carcinoma. J Hepatocell Carcinoma 2022;9:571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tian JC, Liu H, Yan LJ, et al. Adverse events of immune checkpoint inhibitors in hepatocellular carcinoma: a systemic review and meta-analysis. Clin Exp Med 2022;23:2115–2129. [DOI] [PubMed] [Google Scholar]
- 43.Yang C, Zhang H, Zhang L, et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2023;20:203–222. [DOI] [PubMed] [Google Scholar]
- 44.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L . EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- 45.Ho WJ, Zhu Q, Durham J, et al. Neoadjuvant cabozantinib and nivolumab converts locally advanced HCC into resectable disease with enhanced antitumor immunity. Nat Cancer 2021;2:891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marron TU, Fiel MI, Hamon P, et al. Neoadjuvant cemiplimab for resectable hepatocellular carcinoma: a single-arm, open-label, phase 2 trial. Lancet Gastroenterol Hepatol 2022;7:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang W, Tong S, Hu B, et al. Lenvatinib plus anti-PD-1 antibodies as conversion therapy for patients with unresectable intermediate-advanced hepatocellular carcinoma: a single-arm, phase II trial. J Immunother Cancer 2023;11:e007366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao M, Chen S, Li C, et al. Neoadjuvant immune checkpoint inhibitors for resectable hepatocellular carcinoma: a systematic review and meta-analysis. Cancers (Basel) 2023;15:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roth GS, Neuzillet C, Sarabi M, et al. Cholangiocarcinoma: what are the options in all comers and how has the advent of molecular profiling opened the way to personalised medicine. Eur J Cancer 2023;179:1–14. [DOI] [PubMed] [Google Scholar]
- 50.Harding JJ, Khalil DN, Fabris L, et al. Rational development of combination therapies for biliary tract cancers. J Hepatol 2023;78:217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kelley RK, Ueno M, Yoo C, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023;401:1853–1865. [DOI] [PubMed] [Google Scholar]
- 52.You R, Jiang H, Xu Q, et al. Preintervention MCP-1 serum levels as an early predictive marker of tumor response in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Transl Cancer Res 2021;10:966–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gbolahan OB, Akce M, Outlaw DA, et al. An open-label window of opportunity trial to evaluate the activity of durvalumab and tremelimumab with platinum-based chemotherapy (gemcitabine and cisplatin) in intrahepatic cholangiocarcinoma. J Clin Oncol 2023;41(suppl_16):TPS4189–TPS4189. [Google Scholar]
- 54.Alliance of Chinese Expert Consensus on Neoadjuvant Therapy for Hepatocellular Carcinoma, Committee of Digestive Surgery of Chinese Research Hospital Association, Committee of Liver Cancer, Chinese Anti-Cancer Association. 肝癌新辅助治疗中国专家共识(2023版) [Chinese expert consensus on neoadjuvant therapy for hepatocellular carcinoma (2023 ed)]. Zhonghua Wai Ke Za Zhi. 2023;61:1035–1045. [DOI] [PubMed] [Google Scholar]
- 55.Zheng B, Qu J, Ohuchida K, et al. LAMA4 upregulation is associated with high liver metastasis potential and poor survival outcome of pancreatic cancer. Theranostics 2020;10:10274–10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Narayanan JSS, Ray P, Hayashi T, et al. Irreversible electroporation combined with checkpoint blockade and TLR7 stimulation induces antitumor immunity in a murine pancreatic cancer model. Cancer Immunol Res 2019;7:1714–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang D, Lin J, Yang X, et al. Combination regimens with PD-1/PD-L1 immune checkpoint inhibitors for gastrointestinal malignancies. J Hematol Oncol 2019;12:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei X, Jiang Y, Zhang X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J Clin Oncol 2019;37:2141–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang B, Chen K, Liu W, et al. The benefits of neoadjuvant therapy for patients with resectable pancreatic cancer: an updated systematic review and meta-analysis. Clin Exp Med 2023;23:3159–3169. [DOI] [PubMed] [Google Scholar]
- 60.Liu H, Yang CC, Ma YL, et al. Identification of the most effective subgroup of advanced hepatocellular carcinoma from immune checkpoint blocker treatment: a meta-analysis. Immunotherapy 2023;15:669–678. [DOI] [PubMed] [Google Scholar]
- 61.Li K, Tandurella JA, Gai J, et al. Multi-omic analyses of changes in the tumor microenvironment of pancreatic adenocarcinoma following neoadjuvant treatment with anti-PD-1 therapy. Cancer Cell 2022;40:1374–1391.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Hara MH, O’Reilly EM, Varadhachary G, et al. CD40 agonistic monoclonal antibody APX005M (sotigalimab) and chemotherapy, with or without nivolumab, for the treatment of metastatic pancreatic adenocarcinoma: an open-label, multicentre, phase 1b study. Lancet Oncol 2021;22:118–131. [DOI] [PubMed] [Google Scholar]
- 63.Ruffolo LI, Jackson KM, Kuhlers PC, et al. GM-CSF drives myelopoiesis, recruitment and polarisation of tumour-associated macrophages in cholangiocarcinoma and systemic blockade facilitates antitumour immunity. Gut 2022;71:1386–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bridgewater JA, Pugh SA, Maishman T, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 2020;21:398–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.André T, Shiu KK, Kim TW, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med 2020;383:2207–2218. [DOI] [PubMed] [Google Scholar]
- 66.Lee JL, Desai C, Park SH, et al. Avelumab first-line maintenance plus best supportive care (BSC) vs. BSC alone for advanced urothelial carcinoma: JAVELIN Bladder 100 Asian subgroup analysis. Urol Oncol 2023;41:256.e17–256.e25. [DOI] [PubMed] [Google Scholar]
- 67.Chen G, Jin Y, Guan WL, et al. Neoadjuvant PD-1 blockade with sintilimab in mismatch-repair deficient, locally advanced rectal cancer: an open-label, single-centre phase 2 study. Lancet Gastroenterol Hepatol 2023;8:422–431. [DOI] [PubMed] [Google Scholar]
- 68.Vora KB, Ouyang B, Pappas L, et al. Assessment of complete pathologic response after neoadjuvant immunotherapy for MSI-H gastrointestinal cancers. J Clin Oncol 2023;41(suppl_4):43.35737919 [Google Scholar]
- 69.De La Fouchardiere C, Zaanan A, Cohen R, et al. Immunotherapy for localized dMMR/MSI tumors: first interim analysis of the IMHOTEP trial. J Clin Oncol 2023;41(16_suppl):2591. [Google Scholar]
- 70.Chakrabarti S, Parish M, Peterson C, et al. The efficacy and safety of neoadjuvant immunotherapy in patients with deficient mismatch repair/microsatellite instability–high (dMMR/MSI-H) localized and oligometastatic colon cancer: data from the real world. J Clin Oncol 2023;41(suppl_4):105. [Google Scholar]
- 71.Li J, Wu C, Hu H, et al. Remodeling of the immune and stromal cell compartment by PD-1 blockade in mismatch repair-deficient colorectal cancer. Cancer Cell 2023;41:1152–1169.e7. [DOI] [PubMed] [Google Scholar]
- 72.Zhang J, Cai J, Deng Y, et al. Complete response in patients with locally advanced rectal cancer after neoadjuvant treatment with nivolumab. Oncoimmunology 2019;8:e1663108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kanikarla Marie P, Haymaker C, Parra ER, et al. Pilot clinical trial of perioperative durvalumab and tremelimumab in the treatment of resectable colorectal cancer liver metastases. Clin Cancer Res 2021;27:3039–3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Overman MJ, Yothers G, Jacobs SA, et al. NRG-GI004/SWOG-S1610: Colorectal Cancer Metastatic dMMR Immuno-Therapy (COMMIT) study—A randomized phase III study of atezolizumab (atezo) monotherapy versus mFOLFOX6/bevacizumab/atezo in the first-line treatment of patients (pts) with deficient DNA mismatch repair (dMMR) or microsatellite instability high (MSI-H) metastatic colorectal cancer (mCRC). J Clin Oncol 2023;41(suppl_4):TPS258–TPS258. [Google Scholar]
- 75.Newman JH, Chesson CB, Herzog NL, et al. Intratumoral injection of the seasonal flu shot converts immunologically cold tumors to hot and serves as an immunotherapy for cancer. Proc Natl Acad Sci U S A 2020;117:1119–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gögenur M, Balsevicius L, Bulut M, et al. Neoadjuvant intratumoral influenza vaccine treatment in patients with proficient mismatch repair colorectal cancer leads to increased tumor infiltration of CD8+ T cells and upregulation of PD-L1: a phase 1/2 clinical trial. J Immunother Cancer 2023;11:e006774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiang T, Wang D, Cheng J, et al. Efficacy and safety of camrelizumab and apatinib combined with neoadjuvant concurrent chemoradiation for MSS locally advanced rectal cancer. J Clin Oncol 2023;41(suppl_16):e15647. [Google Scholar]
- 78.Xie Y, Lin J, Zhang N, et al. Prevalent pseudoprogression and pseudoresidue in patients with rectal cancer treated with neoadjuvant immune checkpoint inhibitors. J Natl Compr Canc Netw 2023;21:133–142.e3. [DOI] [PubMed] [Google Scholar]
- 79.Harvey JB, Phan LH, Villarreal OE, et al. CD73’s Potential as an Immunotherapy Target in Gastrointestinal Cancers. Front Immunol 2020;11:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bu P, Chen KY, Xiang K, et al. Aldolase B-mediated fructose metabolism drives metabolic reprogramming of colon cancer liver metastasis. Cell Metab 2018;27:1249–1262.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou L, Yang XQ, Zhao GY, et al. Meta-analysis of neoadjuvant immunotherapy for non-metastatic colorectal cancer. Front Immunol 2023;14:1044353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oaknin A, Tinker AV, Gilbert L, et al. Clinical activity and safety of the anti-programmed death 1 monoclonal antibody dostarlimab for patients with recurrent or advanced mismatch repair-deficient endometrial cancer: a nonrandomized phase 1 clinical trial. JAMA Oncol 2020;6:1766–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Colon Cancer. Version 4. 2023. https://www.nccn.org/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.