Abstract
A prospective cohort study investigated the effectiveness of platelet-rich plasma (PRP) infusion for refractory thin endometrium in 38 infertile patients. Patients showed significant improvement in endometrial thickness post-PRP injection, leading to successful implantation and pregnancy. The study revealed a negative correlation between antimullerian hormone (AMH) levels and the need for PRP interventions, suggesting higher ovarian reserve may reduce the necessity for repeated treatments. This implies AMH levels could serve as a prognostic indicator for treatment outcomes, aiding clinicians in optimizing protocols and reducing patient burden. Further research is needed to confirm these findings in larger and more diverse populations, along with exploring long-term reproductive success rates post-PRP treatment.
Keywords: antimullerian hormone, endometrial thickness, platelet-rich plasma, thin endometrium
1. Introduction
The endometrium includes the basal layer and the functional layer, which is the site of embryo implantation and it experiences periodic changes during the menstrual, proliferation, and secretion periods.[1] The endometrium is shedded monthly in the absence of pregnancy without leaving a scar while keeping its function.[2] The optimal endometrial thickness suitable for pregnancy is ≥ 6 mm.[3] A thin endometrium is an endometrium that reached a thickness of <6 mm and is usually diagnosed through vaginal ultrasonography.[4] Refractory thin endometrium lowers the probability of conception whether through normal coitus or by using assisted reproductive technique (ART).[5] Refractory thin endometrium has a wide etiology including infections, iatrogenic, and idiopathic.[6]
Refractory thin endometrium non-responsive to standard treatments usually results in cycle cancellation and is still a challenge in ART. Various strategies have been suggested and developed for the treatment of refractory thin endometrium, including extended use of exogenous estrogen,[7] vitamin E[8] vaginal sildenafil citrate,[9] electroacupuncture,[10] and application of granulocyte colony stimulation factor.[11] Intrauterine infusion of platelet-rich plasma (PRP) is a new approach suggested for treating refractory thin endometrium.[12]
Antimullerian hormone (AMH) was found to be correlated positively with endometrial thickness in the pre-ovulatory phase, and a great predictor of endometrial thickness and pregnancy outcomes.[13]
PRP is a plasma obtained from autologous whole blood and is enriched with platelets. After centrifugation, the plasma layer is re-centrifuged again to obtain PRP ready for injection. It is used as a non-operative therapeutic option in broad areas of medicine such as sports medicine and orthopedics. The PRP is prepared by obtaining an amount of autologous venous blood at 21°C and 24°C and 3 to 5 mL of PRP can be obtained from 30 mL of venous blood[14]
Different classifications of PRP preparation depend on the method of preparation, the contents of plasma, and the proposed application. Platelets include ɑ-granules, which contain high concentrations of various growth factors such as vascular endothelial growth factor, epidermal growth factor, platelet-derived growth factor, transforming growth factor, and other cytokines that stimulate proliferation and growth, and other substances, which are released when the platelets are activated. The high concentration of growth factors in PRP can potentially speed up the healing process and initiate other biological effects.[14]
PRP has recently been used in several disorders, including gynecological disorders such as cervical ectopy, vulvar dystrophy, reconstructive surgery for vulvar cancer, skin lesions and wound healing, urogenital disorders (genital fistulae, genital prolapse and urinary incontinence), reproductive medicine (premature ovarian failure, ovarian torsion, refractory thin endometrium, repeated implantation failure and premature rupture of membranes), and aesthetic gynecology (breast reconstruction and female sexual dysfunction).[14]
2. Materials and methods
2.1. Participants
This study was a prospective cohort clinical study. We included 38 Patients aged 22 to 47 who suffer from infertility and are diagnosed with refractory thin endometrium with:
maximal endometrial thickness of < 6 mm, low AMH levels (AMH < 1.5 ng/mL) or normal/high AMH after excluding other possible causes of irregular periods, idiopathic infertility after trying conventional treatment methods, patients with secondary infertility after treating the underlined cause.
Exclusion criteria were effective hormonal therapy, effective in vitro fertilization (IVF), or intracytoplasmic sperm injection (ICSI).
3. Measurements and outcomes
3.1. Intervention
The intervention included several PRP injections in the cervix in the locations 6 and 12 o’clock and the quadriceps femoris and the contralateral triceps brachii simultaneously, and PRP infusion in the uterine cavity, PRP administration was continued until the targeted endometrial thickness of 6 to 8 mL is reached.
3.2. PRP Obtaining and preparing
Autologous PRP was obtained by drawing 20 cm of venous blood from the patient arm in anticoagulant-containing tubes, the blood was then centrifuged at 1500 rpm (round per minute) for 15 minutes, the blood then separated into 3 layers: an upper layer which contains the platelets and white blood cells, an intermediate thin layer (the buffy coat) which contains white blood cells and a bottom layer that contains red blood cells. The lower layer is discarded, then the plasma is centrifuged again at 2000 rpm for 10 minutes to help with the formation of soft pellets (erythrocytes and platelets) at the bottom of the tube.
After the initial preparation, approximately 3ml of PRP is treated with a special activation solution that consists of physiological fluid enriched with vitamins, minerals, and nutrients, the remaining plasma (6–8 mL) is also treated with the same activation solution. Administration is done within 15 minutes of PRP activation.
3.3. PRP administration
The procedure is conducted by injecting approximately 1.5 mL of activated PRP in the cervix (0.75 mL in the location at 6 O’clock, and another 0.75 mL in the location at 12 O’clock).
This procedure is done within the first 10 days of the menstrual cycle and is repeated periodically on the same day of the cycle until the targeted endometrial thickness of 6 to 8 mL is reached. The endometrial thickness is measured 10 days after the injection using transvaginal ultrasonography.
3.4. Laboratory and imaging diagnostics
Transvaginal ultrasonography was performed for all patients to measure the endometrial thickness, and AMH levels were measured for all patients as demonstrated.
3.5. Statistical methodology
Statistical analysis was performed using spss 26.0, counts and percentages explained the variables. Mean and standard deviation explored the data. The Shapiro–Wilk test demonstrated the shape of the data. The Wilcoxon signed rank and Kruskall Willis tests were conducted to reveal the significance mediated by the intervention and across age groups. A P value of <.05 was considered statistically significant. Spearman rank-order correlations were used to determine the correlation between non-parametric data. An ordinal logistic regression analysis was conducted to estimate the change in log odds on the number of interventions variable.
3.6. Ethical approval
Ethical approval was obtained from - and provided to all participants.
4. Results
4.1. Patients’ characteristics
Thirty-eight women were recruited in this study aged between 22 and 48 with a mean and standard deviation of 36.25 and 6.92 respectively. The age was divided into 3 age groups as follows: group 1 (22–30) enrolled 13 women, Group 2 (31–39) enrolled 14 women and Group 3 (40–48) included 11 women.
4.2. Endometrial thickness before and after PRP injection
The Shapiro–Wilk test showed a significant departure from normality W (38) = 0.81, P < .001 for thickness before PRP injection and W (38) = 0.86, P < .001 for thickness after PRP injection.
Endometrial thickness before PRP injection was <3 mm in 12 patients (31.6%), <4 mm in 15 patients (39.5%), and <5 mm in 11 patients (28.9%). The mean and sd for thickness before PRP injection was 3.97 and 0.79.
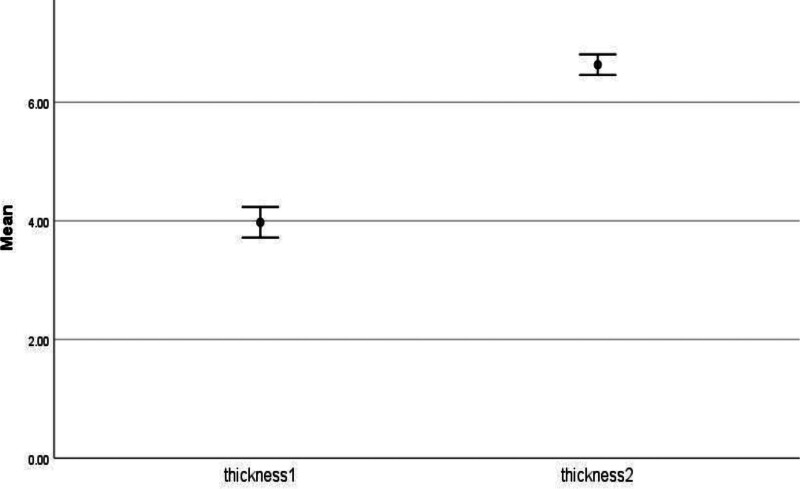
Endometrial thickness after PRP injection was 6 mm in 11 patients (28.9%), 6.5 mm in 12 patients (31.6%), 7 mm in 9 patients (23.7%) and 7.5 mm in 6 patients (15.8%). The mean and sd for thickness after PRP injection was 6.63 and.53 (Fig. 1).
Figure 1.
Thickness before and after PRP injection. PRP = platelet-rich plasma.
The mean and SD for AMH were.58 and.495, Ranging between 0.02 and 1.60.
4.3. Number of PRP rounds
Nine patients (23.7 %) underwent 1 round of PRP, 23 patients (60.5%) received 2 rounds, 4 patients (10.5%) and 2 patients (5.3%) had 3 and 4 rounds, respectively. The mean number of PRP rounds was 1.97 and the standard deviation was 0.75. The Wilcoxon signed rank test revealed that endometrial thickness was significantly higher after intervention (Md = 4, N = 38) compared to (Md = 6.5, N = 38), Z = -5.39, P value < .001, with a larger effect size r = 0.87.
Spearman rank-order correlation was run to examine the relationship between the level of AMH and the number of interventions. There were negative and significant correlations rs = −0.39, n = 38, P = .02.
An ordinal logistic regression analysis to investigate if there is a relationship between AMH and the number of interventions was conducted. The predictor variable, AMH, in the ordinal logistic regression analysis, was found to contribute to the model. B = −1.74, SE = 0.73, WALD = 5.78, P = .02. AMH was a significant negative predictor of the number of interventions. For every 1 unit increase in AMH, there is a predicted decrease of 1.74 in the log odds of being on a higher level of number of interventions. The odds ratio indicates that the odds of being on a higher level on the number of interventions decrease by a factor of 0.18 and 95% CI (0.04, 0.72) for every 1 unit increase on AMH.
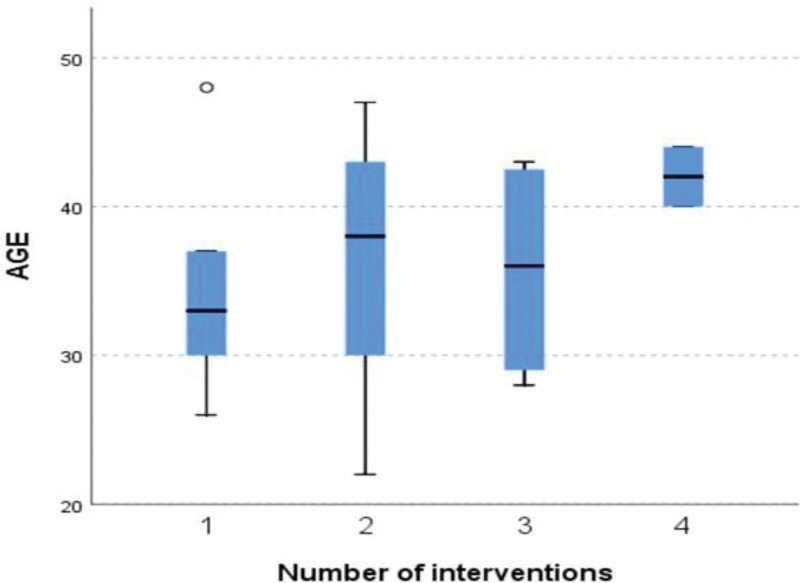
The Kruskall Willis test revealed no statistically significance difference across the number of interventions in thickness after PRP injection χ2 (3, N = 38) = 3.28, P value = .35, in thickness before PRP injection χ2 (3, N = 38) = 4.49, P value = .21 and in age χ2 (3, N = 38) = 3.72, P value = .29. The distribution of the thickness before and after PRP injection, age, and AMH is the same across categories of interventions (Fig. 2).
Figure 2.
The age distribution across the number of intervention groups.
The Kruskall Willis test revealed no statistically significance difference across age groups in thickness before PRP injection χ2 (2, N = 38) = 4.58, P value = .10 and in thickness after PRP injection χ2 (2, N = 38) = 0.05, P value = .97. The distribution of the thickness before and after PRP injection is the same across categories of age
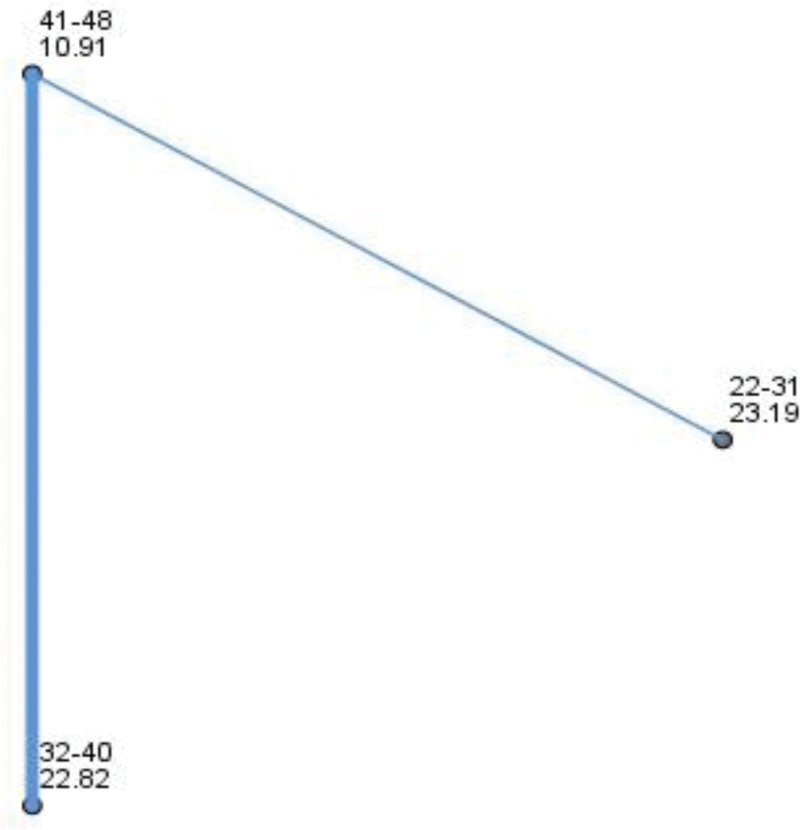
The Kruskall Willis test revealed a statistically significance difference across age groups in AMH χ2 (2, N = 38) = 9.29, P value = .01, with a large effect size of the eta-squared value of 0.21. The distribution of the AMH is different across categories of age (Fig. 3).
Figure 3.
Pairwise comparisons between age groups in AMH. Each node shows the sample average rank of age groups. AMH = antimullerian hormone.
3. Discussion
PRP administration is a relatively new procedure that has recently been utilized in many aspects of medicine, most notably in orthopedic and musculoskeletal medicine. It has been hypothesized that PRP produces its effects through stimulating proliferation and cell regeneration using various growth factors and cytokines present in platelets. PRP is autologous blood plasma that has a 4 folds platelet concentration of the blood, it is obtained through centrifugation of peripheral venous blood, therefore it entails no risk for hypersensitivity reactions and low risk for complications, however, there has been no regulated formula nor a consensus on the standard preparation of PRP.[15]
Activation of PRP refers to the process of stimulating the platelets to release the desired growth factors (GFs) and cytokines through various activation solutions that are usually ready-to-use commercial sets.[15] However, for our study, we used our activation solution, consisting of physiological fluid enriched with vitamins, minerals, and nutrients. Thereby bypassing trading sanctions applied on the country, all while being more cost-effective than the available commercial sets.
The Implementation of PRP in refractory thin endometrium is a relatively new therapeutic procedure as the first trial regarding this procedure was done in 2015 by Cheng et al on a group of 5 women undergoing IVF who suffer from refractory thin endometrium, their application protocol included re-infusing PRP 72h after the initial infusion, all 5 women had successful pregnancies.[12]
A diverse representation allowed comprehensive analysis across different demographic segments in this cohort study with marked thickness improvement after PRP injection yielding successful implantation and pregnancy results. A negative correlation was observed between AMH levels and the number of PRP interventions suggesting higher ovarian reserve with fewer interventions required to achieve optimal endometrial thickness thus adequate counseling could be informed. In addition to the large effect size of the PRP injection in improving endometrial thickness. These findings suggest that higher AMH levels may predict a reduced need for repeated PRP interventions, offering a valuable prognostic indicator for clinicians in optimizing treatment protocols and minimizing patient burden.
Interestingly there were no variations, in thickness or patient age among the various treatment groups. Nevertheless, a marked contrast in AMH levels was evident across age brackets suggesting varying reserve and treatment response based on age. This emphasizes the significance of factoring in both age and ovarian reserve when formulating treatment plans for individuals, with endometrium.
The results, from the Kruskal Wallis test revealed that there were no variations in thickness after the intervention regardless of the number of treatments given. This suggests that the positive impacts of PRP therapy can be attained regardless of how many rounds re-administered. This discovery is in line with the observation that there were no differences in thickness among different age groups following PRP injection indicating that age does not play a role, in determining the effectiveness of PRP treatment.
Zedehmoderres et al conducted a similar trial in 2016 on a group of 10 patients undergoing frozen-thawed embryo transfer, resulting in similar findings as all 10 patients were pregnant by the end of the trial.[16]
In recent years, the number of studies regarding this procedure has increased year after year with various study designs,[16–22] and this paper adds to the limited research done in this area.
Another study done hysteroscopically by Agarwal et al resulted in similar findings by implementing PRP through a hysteroscope on 32 patients undergoing IVF, an endometrium thicker than 7 mm was noted in 75% of them resulting in clinical and/or chemical pregnancy.[20]
Eftekhar et al performed a randomized controlled trial on 83 patients with refractory thin endometrium using the same method of administering PRP in the context of IVF, they found a significant increase in endometrial thickness in the PRP group compared to the control.[21]
Maliki-hajiagha and his colleagues performed a systematic review and meta-analysis in 2020 regarding PRP application in refractory thin endometrium, 7 studies, and 625 patients were included, and they found a significantly higher chance of pregnancy in women who received PRP compared to controls (P < .001), they also concluded the need for more research to be done regarding this new therapy.[22]
In contrast to these methods, in our study, we re-administered the PRP before the 10th day of every menstrual cycle for about (1–4) cycles, all while utilizing more convenient and low-cost methods such as natural fertilization, IUI, and ICSI thereby cutting the cost of IVF used in similar trials, and being more cost-effective through limiting PRP re-administration to once per menstrual cycle.
Randomized controlled trials are needed to reach a comprehensive methodology that ensures the best cost-effectiveness, in a concern for our paper, we recommend conducting an RCT comparing the results of the conventional method of administering PRP coined by Cheng et al[12] compared to our way of administration of once every menstrual cycle.
Dawood et al published a significant paper reviewing most of the research conducted regarding this therapeutic approach in refractory thin endometrium patients, they found it to be an effective and easy-to-implement therapeutic procedure while having a low risk of complications.[14]
The low cost of this procedure, combined with it being easy to perform and readily available to be prepared locally, and its low risk of complications all while being effective make this innovative method of treatment a good resort for patients with refractory thin endometrium.
4. Conclusion
This study has demonstrated that PRP therapy is a promising treatment for refractory thin endometrium, with the potential to improve endometrial receptivity and enhance fertility outcomes. The correlation between AMH levels and the number of PRP interventions needed provides a basis for further research into personalized treatment protocols. Future studies should aim to validate these findings in larger, more diverse populations and explore the long-term reproductive success rates following PRP treatment.
Acknowledgments
We wish to show our appreciation to Stemosis for Scientific Research, a Syria-based scientific research youth Official team managed by Dr Nafiza Martini, for the scientific environment they provided.
Author contributions
Visualization: Haitham Abbassi.
Writing – original draft: Rasha Abbassi, Sultaneh Haddad, Farah Haneyah, Mohammed Subhi Murad, Abdulmoez Mohammed Issa, Ahmad Alkheder, Adel Azar, Majd dakhalalah bani hani.
Writing – review & editing: Wael Nakawa.
Abbreviations:
- AMH
- antimullerian hormone
- ART
- assisted reproductive technique
- PRP
- platelet-rich plasma
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Abbassi R, Haddad S, Haneyah F, Nakawa W, Murad MS, Issa AM, Alkheder A, Azar A, dakhalalah bani hani M, Abbassi H. Treating refractory thin endometrium through a novel way of activation and administration of Platelet-rich plasma in sexually active women: An interventional prospective cohort clinical study. Medicine 2024;103:24(e38554).
Contributor Information
Rasha Abbassi, Email: haitham.abbassi@damascusuniversity.edu.sy.
Farah Haneyah, Email: farahhanieh9@gmail.com.
Wael Nakawa, Email: Wael.nakawa@gmail.com.
Mohammed Subhi Murad, Email: mohammedsubhi9murad@gmail.com.
Abdulmoez Mohammed Issa, Email: abdulmoez.issa@gmail.com.
Ahmad Alkheder, Email: alkhederahmed@gmail.com.
Adel Azar, Email: adelazar99@gmail.com.
Majd dakhalalah bani hani, Email: Majdjihad@outlook.com.
Haitham Abbassi, Email: haitham.abbassi@damascusuniversity.edu.sy.
References
- [1].Bai X, Zheng L, Li D, et al. Research progress of endometrial receptivity in patients with polycystic ovary syndrome: a systematic review. Reprod Biol Endocrinol. 2021;19:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Critchley HO, Maybin JA, Armstrong GM, et al. Physiology of the endometrium and regulation of menstruation. Physiol Rev. 2020;17:1–5. [DOI] [PubMed] [Google Scholar]
- [3].Shapiro H, Cowell C, Casper RF. The use of vaginal ultrasound for monitoring endometrial preparation in a donor oocyte program**Supported by a grant from the Medical Research Council of Canada, Ottawa, Ontario, Canada (to R.F.C.). Fertil Steril. 1993;59:1055–8. [DOI] [PubMed] [Google Scholar]
- [4].Mahajan N, Sharma S. The endometrium in assisted reproductive technology: How thin is thin? J Hum Reprod Sci. 2016;9:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mouhayar Y, Franasiak JM, Sharara FI. Obstetrical complications of thin endometrium in assisted reproductive technologies: a systematic review. J Assist Reprod Genet. 2019;36:607–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Coles MJ, Palmer N, Casper R. The Refractory Endometrium is Still Refractory. J Obstet Gynaecol Canada. 2017;39:1188–91. [DOI] [PubMed] [Google Scholar]
- [7].Chen MJ, Yang JH, Peng FH, et al. Extended estrogen administration for women with thin endometrium in frozen-thawed in-vitro fertilization programs. J Assist Reprod Genet. 2006;23:337–42. [DOI] [PubMed] [Google Scholar]
- [8].Takasaki A, Tamura H, Miwa I, et al. Endometrial growth and uterine blood flow: a pilot study for improving endometrial thickness in the patients with a thin endometrium. Fertil Steril. 2010;93:1851–8. [DOI] [PubMed] [Google Scholar]
- [9].Sher G, Fisch JD. Effect of vaginal sildenafil on the outcome of in vitro fertilization (IVF) after multiple IVF failures attributed to poor endometrial development. Fertil Steril. 2002;78:1073–6. [DOI] [PubMed] [Google Scholar]
- [10].Ho M, Huang LC, Chang YY, et al. Electroacupuncture reduces uterine artery blood flow impedance in infertile women. Taiwanese J Obstet Gynecol. 2009;48:148–51. [DOI] [PubMed] [Google Scholar]
- [11].Gleicher N, Vidali A, Barad DH. Successful treatment of unresponsive thin endometrium. Fertil Steril. 2011;95:2123.e13–7. [DOI] [PubMed] [Google Scholar]
- [12].Chang Y, Li J, Chen Y, et al. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int J Clin Exp Med. 2015;8:1286–90. [PMC free article] [PubMed] [Google Scholar]
- [13].Vagios S, Hammer K, Sacha C, et al. The Association of AMH with endometrial thickness in gonadotropin stimulation/intrauterine insemination cycles. Fertil Steril. 2020;114:1–15. [Google Scholar]
- [14].Dawood AS, Salem HA. Current clinical applications of platelet-rich plasma in various gynecological disorders: an appraisal of theory and practice. Clin Exp Reprod Med. 2018;45:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Everts P, Onishi K, Jayaram P, et al. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci . 2020;21:7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zadehmodarres S, Salehpour S, Saharkhiz N, et al. Treatment of thin endometrium with autologous platelet-rich plasma: a pilot study. JBRA Assist Reprod. 2017;21:54–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Russell SJ, Kwok YSS, Nguyen, TTN, et al. Autologous platelet-rich plasma improves the endometrial thickness and live birth rate in patients with recurrent implantation failure and thin endometrium. J Assist Reprod Genet. 2022;39:1305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Frantz N, Ferreira M, Kulmann MI, et al. Platelet-rich plasma as an effective alternative approach for improving endometrial receptivity - a clinical retrospective study. JBRA Assist Reprod. 2020;24:442–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nazari L, Salehpour S, Hoseini S, et al. Effects of autologous platelet-rich plasma on endometrial expansion in patients undergoing frozen-thawed embryo transfer: a double-blind RCT. Int J Reprod Biomed. 2019;17:443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Agarwal M, Mettler L, Jain S, et al. Management of a thin endometrium by hysteroscopic instillation of platelet-rich plasma into the endomyometrial junction: a pilot study. J Clin Med. 2020;9:2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Eftekhar M, Neghab N, Naghshineh E, et al. Can autologous platelet rich plasma expand endometrial thickness and improve pregnancy rate during frozen-thawed embryo transfer cycle? A randomized clinical trial. Taiwanese J Obstet Gynecol. 2018;57:810–3. [DOI] [PubMed] [Google Scholar]
- [22].Maleki-Hajiagha A, Razavi M, Rouholamin S, et al. Intrauterine infusion of autologous platelet-rich plasma in women undergoing assisted reproduction: a systematic review and meta-analysis. J Reprod Immunol. 2020;137: 103078. [DOI] [PubMed] [Google Scholar]