Abstract
Surgeons must be confident that the instruments they use do not pose risk of infection to patients due to bioburden or contamination. Despite this importance, surgeons are not necessarily aware of the steps required to ensure that an instrument has been properly sterilized, processed, and prepared for the next operation. At the end of an operation, instruments must be transported to the sterile processing unit. There, instruments are decontaminated before being sterilized by heat, chemical, or radiation-based methods. Following this, they are stored before being brought back into use. This review highlights the intricacies of the processing of surgical instruments at the conclusion of an operation so that they are ready for the next one.
Mini-abstract
Purpose: This review seeks to elucidate the process and importance of surgical instrument sterilization.
Methods: This was a narrative review conducted at a single academic hospital.
Results: Sterilization can be accomplished by high-temperature and low-temperature methods. Steam sterilization is the most common method in developed countries. Sterility assurance levels represent the probability of finding a single microbe on an instrument after it has been sterilized and levels of 10−6 are typical.
Conclusion: Surgical instrument sterilization is nuanced and critical for operational capabilities.
INTRODUCTION
From needle drivers to scalpels, a multitude of instruments are used by surgeons every day. Despite the importance of these instruments with respect to daily operational capabilities, many surgeons are not aware of the effort and science required to return a used instrument back to a functional status for the next surgery. For many surgeons, tenotomy scissors used in an operation are simply sterilized, and hopefully sharp, for their next case. This article seeks to shed insight into the lifecycle of surgical instruments and describe the nuances that allow an instrument to return to the operating theater. While there are variations depending on regional access and resources, this review will focus on the process as it occurs in the United States and nations with similar access to resources.
The framework for modern instrument sterilization starts with William Henry, the English physician and chemist who famously discovered the gas law that bears his namesake. In 1832, Henry described that pressurized heat was capable of decontaminating clothes bearing typhus and measles.1,2 Louis Pasteur, the French chemist and microbiologist, and his colleague, Charles Chamberland, built upon this work in 1876 when they noted that moist heat was more effective than dry heat with respect to its germicidal potential.2,3 Pasteur’s germ theory inspired the Scottish surgeon, Joseph Lister, to use phenol as an antiseptic. In 1865, this had the effective result of decreasing postamputation mortality from ~50% to 15%.2 At the end of the 1800s, German scientist Wilhelm Röntgen invented X-rays, and 3 years after this discovery X-rays were found to be hazardous to pathogens.4 Science continued to progress and in 1933 Gross and Dixon submitted the United States patent for sterilization with ethylene oxide. These moments highlight the path that ultimately led to modern surgical instrument sterilization.
MODERN INSTRUMENT PROCESSING
Modern decontamination defines cleaning, disinfection, and sterilization as technical terms. Cleaning removes foreign material, allowing for better surface contact and reducing the risk of chemical inactivation. Disinfection involves the destruction or removal of microorganisms. However, with disinfection bacterial spores may still survive. Sterilization is the highest level of decontamination. This process renders an instrument free from both viable bacteria, viruses, and spores.5 The World Health Organization (WHO) recommends different levels of decontamination for different medical devices, with sterilization being recommended for surgical instruments that violate skin or mucous membranes.6
Responsibility for sterilizing surgical instruments varies globally, but in the United States and similar countries it usually falls under a distinct sterile services unit.6 The WHO recommends each technician in the sterile services unit processes 1500–2000 sets of instruments per year.6 The sterile processing unit must be designed with care to decrease the risk of contamination. The general workflow is such that instruments progressively move from more contaminated areas to less contaminated areas before being stored. Surfaces in the sterile processing unit should be smooth and made of nonporous material, like stainless steel, to facilitate cleaning.6,7 The flooring should avoid corners with gentle slopes instead as this decreases the likelihood of debris accumulation. Adequate ventilation is another important facet to ensure proper sterilization.6,7
Preparation
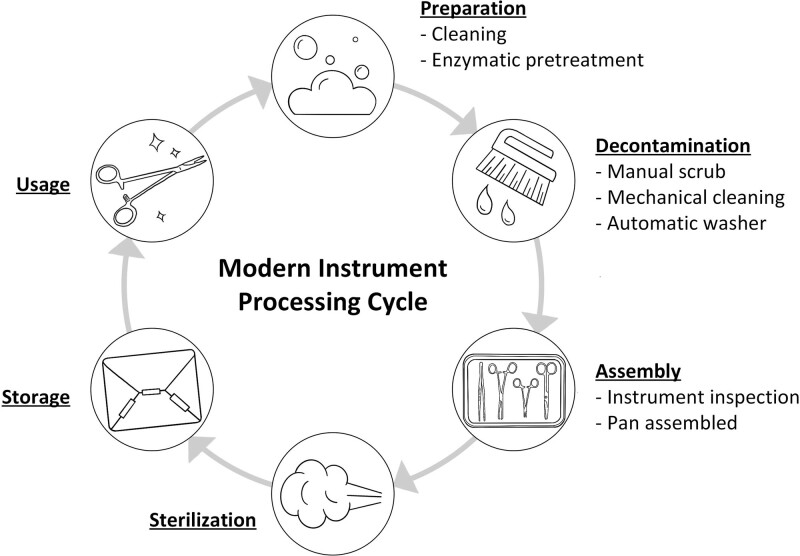
At the end of an operation, the first step in the lifecycle of a surgical instrument is preparing instruments for transfer to the sterile services unit. The general lifecycle of instruments can be seen in Figure 1. Instruments must be grossly cleaned by the scrub technician to remove blood, tissue, and other material. Prompt cleaning to decrease contact time with blood and using sterile water rather than saline decreases the risk of instrument corrosion.8 Another important step that can be overlooked is the use of an enzymatic transport gel or foam. These products vary but provide a neutral pH and moisture. This pretreatment facilitates cleaning in the sterile processing unit and significantly decreases the burden of cleaning for the sterilization technicians. A sterile processing technician at our institution was interviewed and he stated that he wished to raise surgeon awareness of this critical step more than any other because if it is omitted it will increase the difficulty of instrument decontamination and significantly prolong instrument turnover time. Supplemental Video 1 demonstrates the use of pretreatment and its effectiveness in removing blood from used instruments. A photo of enzymatic pretreatment is shown in Figure 2. One other point in the transport phase to emphasize is that fragile instruments, like microsurgical instruments, must be placed in special trays that prevent excessive movement and wear. This increases the utility and longevity of the instruments.
FIGURE 1.
The cycle of surgical instrument use and sterilization.
FIGURE 2.
A photo of enzymatic pretreatment being applied to instruments.
Decontamination
Once the instruments are successfully transported to the sterile processing unit, the first step is to decontaminate them. This starts with workers manually scrubbing instruments with nylon brushes. This can be seen in Supplemental Video 1. After this initial step, the instruments are subjected to a form of mechanical cleaning. A common variation of this is an ultrasonic cleaner, which is exemplified in Figure 3. Within such a machine, instruments are plunged into a bath of detergent. Vibrations are created with high-frequency sound to take advantage of cavitation, a process by which bubbles are formed and progressively enlarge until they implode.8 This implosion creates a vacuum that helps remove debris from difficult areas of instruments to clean. Ultrasonic cleaning usually takes 5 to 20 minutes.8 Supplemental Video 1 depicts instruments being lowered into an ultrasonic cleaner. Following this, the instruments are then moved to an automatic washer, which sprays instruments with cleaning solutions. This is seen in Figure 4. The instruments are often placed into the washer from the decontamination zone of the sterile processing unit and removed from the washer on the opposite side and taken into the assembly area. It takes approximately 30 minutes to wash instruments in this automated fashion.
FIGURE 3.
An example of an ultrasonic cleaner.
FIGURE 4.
Photo of an automatic washer.
Assembly
Following decontamination, instruments are then brought to the assembly area. Here, technicians assemble set pans of instruments. The technicians will inspect instruments to ensure functionality, lack of corrosion, and absence of gross contamination. They will then replace missing instruments to ensure that pans are complete. At our institution, this takes roughly 30 minutes per pan. At this point, pans are placed into a container in which they will ultimately be sterilized in. Common container materials include aluminum, double linen wraps, polypropylene, or a sterilization pouch. Pans will be wrapped according to protocoled sterilization methods and storage concerns. For example, a polypropylene wrap can be used for steam, ethylene oxide, or hydrogen peroxide sterilization. With respect to storage, pans that are unusual sizes, like certain craniotomy hardware, cannot be stored in aluminum trays, as they will either not fit or will not be secured in the tray. This can cause excessive movement during transport and damage to the contents. In addition, aluminum trays can occupy more storage space, which is important given the volume of instruments that is circulated. Supplemental Video 1 demonstrates a pan being placed in an aluminum tray and a pan being wrapped in double linen.
Sterilization
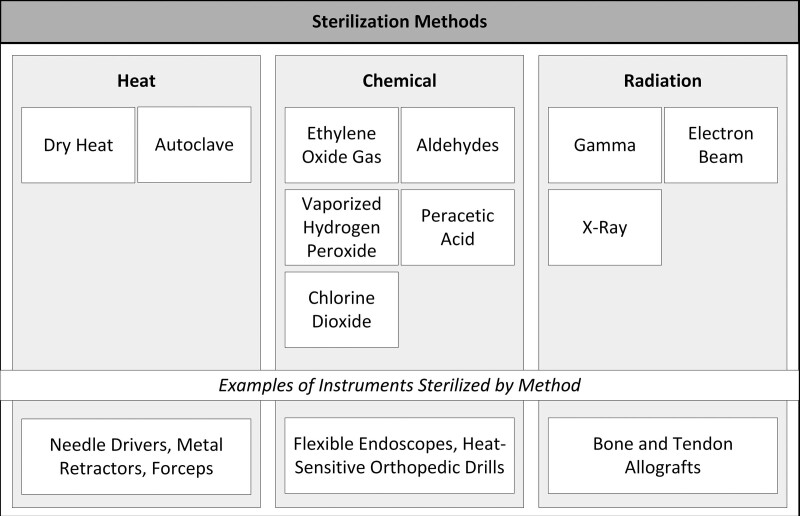
The sterility of instruments is determined by the sterility assurance level, which is the probability of finding a single microbe on an instrument after it has been sterilized. Sterility assurance levels of 10−6 are typical for surgical sterilization.9 There are several ways to successfully sterilize instruments. These can be categorized as high-temperature or low-temperature methods. Figure 5 depicts the common categories of sterilization. Within high-temperature sterilization, dry or moist heat can be used.4,10 Dry heat destroys microorganisms via oxidation and has the advantages of requiring a less complex apparatus than moist heat, having no requirement for pressure, and being a lighter-in-weight system.4,11 Moist sterilization utilizes steam and is known as autoclave sterilization. Autoclaves are more effective than dry heat and represent the most common form of sterilization in developed nations. Autoclaves use pressurized systems which allows for greater heat penetration and neutralization of pathogens via irreversible coagulation and denaturation of critical proteins.9–11 Advantages of autoclaves compared to dry heat include shorter run times, greater heat penetration, and lower temperature requirements. Autoclaves require around 1 hour for sterilization, but settings can vary. Ideally, the sterilized pans cool for 5 hours in the sterile processing unit. This cooling time allows for moisture to completely migrate out of the packaging. Both methods are nontoxic, but do require electricity for operation.9,11 Different types of instruments are sterilized using different methods according to manufacturer instructions. Many instruments like needle drivers, forceps, and metal retractors can be sterilized using an autoclave. Many modern fiberoptic cables can be sterilized using an autoclave and will be damaged if soaked in chemicals.12,13 Similarly, many rigid endoscopes can be autoclaved.6
FIGURE 5.
Common methods of surgical instrument sterilization.
Interestingly, an indicator containing spores of the thermophile, Geobacillus stearothermophilus is used to confirm sufficient autoclave sterilization.9–11 G. stearothermophilus is a spore-producing aerobe that inhabits soil, hot springs, desert climates, and arctic waters.14 These spores are very resistant to moist heat due to their core dehydration and mineralization.15 Indicators may consist of suspensions or test strips and change color to demonstrate a successful or failed sterilization cycle.14,16 The frequency of unsuccessful autoclave runs is not well documented in the literature and is affected by autoclave settings and operational practices.17,18 However, 1 review from 2019 found that the rate of autoclave sterilization failure ranges from 1.5% to 43%.19 Figure 6 displays a standard autoclave.
FIGURE 6.
Photograph of an autoclave.
Heat sterilization can be used for many materials, but for heat-sensitive materials, low-temperature sterilization should be used. Low-temperature sterilization consists of either chemical or radiation-based methods. A common method of chemical sterilization is ethylene oxide, which has been used since the 1950s. Ethylene oxide is compatible with many materials that are not resistant to moisture or heat.9 Therefore, it is a frequently used method of sterilization when autoclaves may be damaging to certain instruments. Ethylene oxide destroys pathogens via alkylation. Unlike heat sterilization, ethylene oxide is toxic, and technicians must be cautious with its use.9 Vaporized hydrogen peroxide, a weak acid, is another chemical method of sterilization that is noncarcinogenic and attacks pathogens by oxidation.20 Other methods of chemical sterilization include chlorine dioxide, peracetic acid, and aldehydes.4 Flexible endoscopes are examples of instruments that are heat sensitive and must be sterilized with liquid or gas chemicals.5,21
Radiation for sterilization comes in the form of gamma rays, electron beams, or X-rays.9 Gamma rays have the highest energy and X-rays have the greatest penetration. Benefits of radiation sterilization include a lack of residue, short duration, and broad applicability to various materials. Gamma radiation is ideal for the sterilization of biologic tissues, including allografts.22
Once instruments are successfully sterilized, they are then placed in storage. The WHO recommends that, depending on the number of surgical procedures per day, the storage area be roughly double the assembly and decontamination areas combined.6 There is evidence that instruments can be stored for at least 96 weeks.23 When instruments are required for surgical use, they are obtained from the storage area and brought to the operating room for use. At our institution, the quickest turnaround time possible is 2 hours, which should be borne in mind when considering operational capabilities.
It is estimated that the lifespan of an instrument is 300–900 cycles of sterilization.24 In the majority of surgical settings, the number of sterilization cycles for each instrument is not tracked. As a result, it is difficult for surgeons to identify faulty instruments until they are attempted to be used in the operating room and about 5.9% of trays were found in 1 study to have broken instruments.24 New technologies, like applying radiofrequency tags to instruments, are being developed to enhance institutional capabilities with respect to tracking instrument usage and sterilization.25 Reducing unnecessary surgical instrument sterilization would increase the longevity of surgical instruments and decrease the costs associated with sterilization. The budget for supplies can consume over 50% of the perioperative budget at academic institutions.26 This is compared to ~35% of perioperative budgets being directed towards nonphysician salaries and benefits.26 Moreover, the cost of sterilizing and packaging a reusable instrument is estimated to be between $0.59 and $11.52.24 The magnitude of excessive sterilization further contributes to the financial impact of sterilization. The usage rate of sterilized instruments in a tray that is brought into an operating room typically ranges from 13.0% to 21.9% and the number of instruments in a tray is inversely proportional to the usage rate.24 Simultaneously, the Virginia Mason Medical Center found that a 70% reduction in excessive sterilization would correspond to an estimated savings up to $2.8 million per year at their institution.27 Given that surgery centers can sterilize more than 2 million instruments per year, streamlining and optimizing surgical trays to reduce excessive sterilization would be beneficial with regard to enhancing the lifespan of surgical instruments and offering significant cost savings.24,26,27 A concern for operative staff and surgeons may be that reducing the size of instrument trays could lead to an increase in case duration due to circulating nurses having to leave the OR to acquire a needed instrument. However, optimizing tray sizes with the removal of rarely used instruments has been shown to have no effect on surgical times and actually decreases the setup times of cases.27
CONCLUSION
In conclusion, the lifecycle of surgical instruments, starting with use in the operating room and ending in the designated storage area, is a nuanced one. This paper describes in detail how surgical instruments are processed at the end of an operation and made ready for the next operation. There are a multitude of steps and interesting science that serve to decrease bioburden, allowing surgeons to operate without significant risk of disease transmission to patients due to contaminated instruments. Lastly, the financial impact of sterilization is important for institutions to be aware of, as many instruments are sterilized unnecessarily which leads to unnecessary costs.
Acknowledgments
We would like to acknowledge the sterile processing technicians at our institution who kindly took the time to demonstrate the techniques they use during instrument sterilization, and for sharing their perspectives on the process.
Footnotes
Published online 22 February 2024
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
Disclosure: The authors declare that they have nothing to disclose.
All authors participated in the writing of the paper.
REFERENCES
- 1.Wisniak J. William Henry: his achievements and his law. J Chem Educ. 2001;6:62–68. [Google Scholar]
- 2.Hugo WB. A brief history of heat, chemical and radiation preservation and disinfection. J Appl Bacteriol. 1991;71:9–18. [PubMed] [Google Scholar]
- 3.Cavaillon JM, Legout S. Louis Pasteur: between myth and reality. Biomolecules. 2022;12:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharti B, Li H, Ren Z, et al. Recent advances in sterilization and disinfection technology: a review. Chemosphere. 2022;308:136404. [DOI] [PubMed] [Google Scholar]
- 5.Rowinski A, Von Schreeb J. Decontamination of surgical instruments for safe wound care surgeries in disasters: what are the options? a scoping review. Prehosp Disaster Med. 2021;36:645–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Decontamination and Reprocessing of Medical Devices for Health-Care Facilities. Available at: http://www.who.int. [Google Scholar]
- 7.of Veterans Affairs D, of Construction O, Management F, Standards Service F. PG-18-9 Space Planning Criteria PG-18-5 Equipment Guide List PG-18-12 Design Guide 284: Logistics Service 285. Sterile Processing Service; 2015. [Google Scholar]
- 8.Chobin N. Surgical instrument decontamination: a multistep process. AORN J. 2019;110:253–262. [DOI] [PubMed] [Google Scholar]
- 9.Sastri VR. Material requirements for plastics used in medical devices. In: Plastics in Medical Devices. Elsevier; 2022:65–112. [Google Scholar]
- 10.Sebben J. Sterilization and care of surgical instruments and supplies. J Am Acad Dermatol. 1984;11:381–392. [DOI] [PubMed] [Google Scholar]
- 11.Taiwo Mubarak M, Ozsahin I, Uzun Ozsahin D. Evaluation of sterilization methods for medical devices.
- 12.American Medicals. Medical Fiber Optic Cable Care. Available at: https://www.americanmedicals.com/medical-fiber-optic-cable-care.aspx#:~:text=Sterilization%3A,Sterilize%20the%20cable%20separately. Accessed October 16, 2023. [Google Scholar]
- 13.Elmed Inc. Fiber-optic Cables & Fiber-optic Retractors: Cleaning & Sterilization Instructions. Available at: https://www.elmed.com/wp-content/uploads/2021/11/FIBER-OPTIC-CABLES-FIBER-OPTIC-RETRACTORS-IFU-11.3.2021-1.pdf. Accessed October 16, 2023. [Google Scholar]
- 14.Kotzekidou P. Bacillus: Geobacillus stearothermophilus (Formerly Bacillus stearothermophilus). In: Encyclopedia of Food Microbiology. 2nd ed. Elsevier Inc.; 2014:129–134. [Google Scholar]
- 15.Guizelini BP, Vandenberghe LPS, Sella SRBR, et al. Study of the influence of sporulation conditions on heat resistance of Geobacillus stearothermophilus used in the development of biological indicators for steam sterilization. Arch Microbiol. 2012;194:991–999. [DOI] [PubMed] [Google Scholar]
- 16.3MTM AttestTM 1262/1262P Biological Indicator Product Description; 2016. [Google Scholar]
- 17.Garibaldi BT, Reimers M, Ernst N, et al. Validation of autoclave protocols for successful decontamination of category a medical waste generated from care of patients with serious communicable diseases. J Clin Microbiol. 2017;55:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skaug N, Lingaas E, Nielsen O, et al. Biological monitoring of sterilizers and sterilization failures in Norwegian dental offices in 1985 and 1996. Acta Odontol Scand. 1999;57:175–180. [DOI] [PubMed] [Google Scholar]
- 19.Panta G, Richardson AK, Shaw IC. Effectiveness of autoclaving in sterilizing reusable medical devices in healthcare facilities. J Infect Dev Ctries. 2019;13:858–864. [DOI] [PubMed] [Google Scholar]
- 20.McEvoy B, Rowan NJ. Terminal sterilization of medical devices using vaporized hydrogen peroxide: a review of current methods and emerging opportunities. J Appl Microbiol. 2019;127:1403–1420. [DOI] [PubMed] [Google Scholar]
- 21.Vesley D, Norlien KG, Nelson B, Beverly Ott C, Andrew Streifel CJ, Minneapolis M. Significant factors in the disinfection and sterilization of flexible endoscopes. [DOI] [PubMed]
- 22.Singh R, Singh D, Singh A. Radiation sterilization of tissue allografts: a review. World J Radiol. 2016;8:355–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhumisirikul W, Bhumisirikul P, Pongchairerks P. Long-term storage of small surgical instruments in autoclaved packages. Asian J Surg. 2003;26:202–204. [DOI] [PubMed] [Google Scholar]
- 24.Stockert EW, Langerman A. Assessing the magnitude and costs of intraoperative inefficiencies attributable to surgical instrument trays. J Am Coll Surg. 2014;219:646–655. [DOI] [PubMed] [Google Scholar]
- 25.Hill I, Olivere L, Helmkamp J, et al. Measuring intraoperative surgical instrument use with radio-frequency identification. JAMIA Open. 2022;5:ooac003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park KW, Dickerson C. Can efficient supply management in the operating room save millions?. Curr Opin Anaesthesiol. 2009;22:242–248. [DOI] [PubMed] [Google Scholar]
- 27.Farrokhi FR, Gunther M, Williams B, et al. Application of lean methodology for improved quality and efficiency in operating room instrument availability. J Healthc Qual. 2015;37:277–286. [DOI] [PubMed] [Google Scholar]