Abstract
Human aging is marked by the emergence of a tapestry of clonal expansions in dividing tissues, particularly evident in blood as clonal hematopoiesis (CH). CH, linked to cancer risk and aging-related phenotypes, often stems from somatic mutations in a set of established genes. However, the majority of clones lack known drivers. Here we infer gene-level positive selection in whole blood exomes from 200,618 individuals in UK Biobank. We identify 17 additional genes, ZBTB33, ZNF318, ZNF234, SPRED2, SH2B3, SRCAP, SIK3, SRSF1, CHEK2, CCDC115, CCL22, BAX, YLPM1, MYD88, MTA2, MAGEC3 and IGLL5, under positive selection at a population level, and validate this selection pattern in 10,837 whole genomes from single-cell-derived hematopoietic colonies. Clones with mutations in these genes grow in frequency and size with age, comparable to classical CH drivers. They correlate with heightened risk of infection, death and hematological malignancy, highlighting the significance of these additional genes in the aging process.
Subject terms: Population genetics, DNA sequencing
Fitness-based analysis of 200,618 UK Biobank exomes and single-cell-derived hematopoietic clones identifies 17 genes under positive selection, including novel drivers of clonal hematopoiesis.
Main
Human cells accumulate somatic mutations, leading to an evolving tapestry of clones throughout our tissues as we age1–10. The inferred mechanism in replicating tissues is that a stem cell gains a mutation providing a fitness benefit, leading to a clonal expansion due to selection rather than drift11–13. Clones with increased fitness from specific mutations can certainly drive cancer14–16, but expanded clones can influence other diseases both directly, as in chronic liver disease, and through indirect mechanisms, such as in blood17–21.
In the past decade, genomic sequencing of blood samples has revealed that clonal hematopoiesis (CH) is common in elderly individuals with apparently normal hematopoiesis, with large-scale retrospective studies identifying associations of CH with hematological malignancies, cardiovascular disease and all-cause mortality22. Initial estimates identified CH in >10% of those over the age of 70 when screening for mutations in a known set of genes23. However, the prevalence of CH is highly dependent on the sensitivity of sequencing assays, with very small CH clones reported in most individuals over the age of 50 when using highly sensitive sequencing24. Bulk approaches generally detect one to two small clones in individuals. Using single-cell sequencing approaches, dozens of parallel clonal expansions can, in fact, be found in blood in all individuals by the seventh to eighth decade of life, with most expansions lacking known driver mutations25,26. Similarly, CH identified on the basis of the presence of passenger mutations within clones that lack known driver mutations has been shown to account for the majority of CH in blood10,27,28. As a result, there are ongoing efforts to comprehensively map the drivers of CH29 to better understand clonal selection and aging phenotypes of blood.
The UK Biobank (UKBB) provides a large cohort with which to assess gene fitness effects and their associated health effects30. In this Article, we exploited the idea that a gene can be identified as under positive selection if one finds an enrichment of nonsynonymous mutations compared to neutral synonymous mutations within that gene’s coding sequence11 and comprehensively examine 200,618 UKBB exomes of blood-derived buffy coat for the presence of positive selection leading to clonal expansions. We validate our findings in 10,837 genomes from single-cell-derived hematopoietic cells and examine the associated clinical phenotypes and outcomes, as reported here.
Results
Global positive selection in 200,618 whole blood exomes
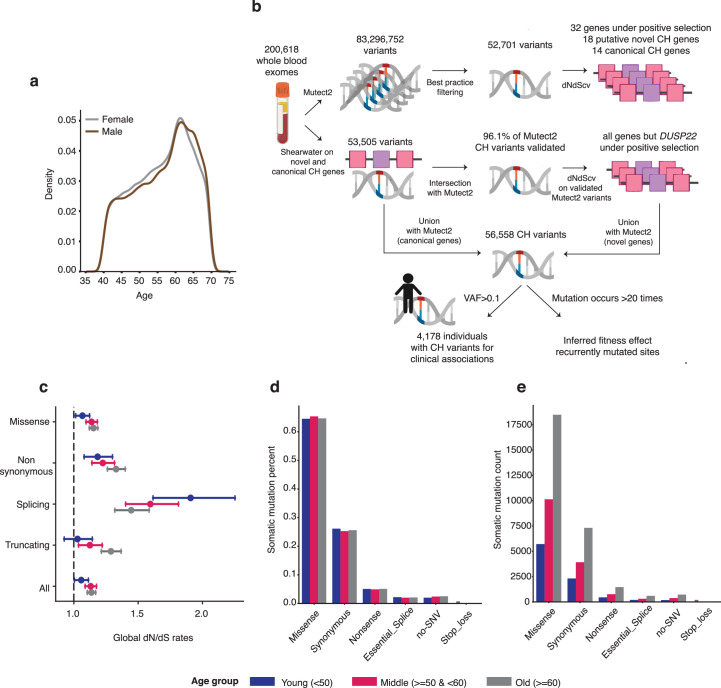
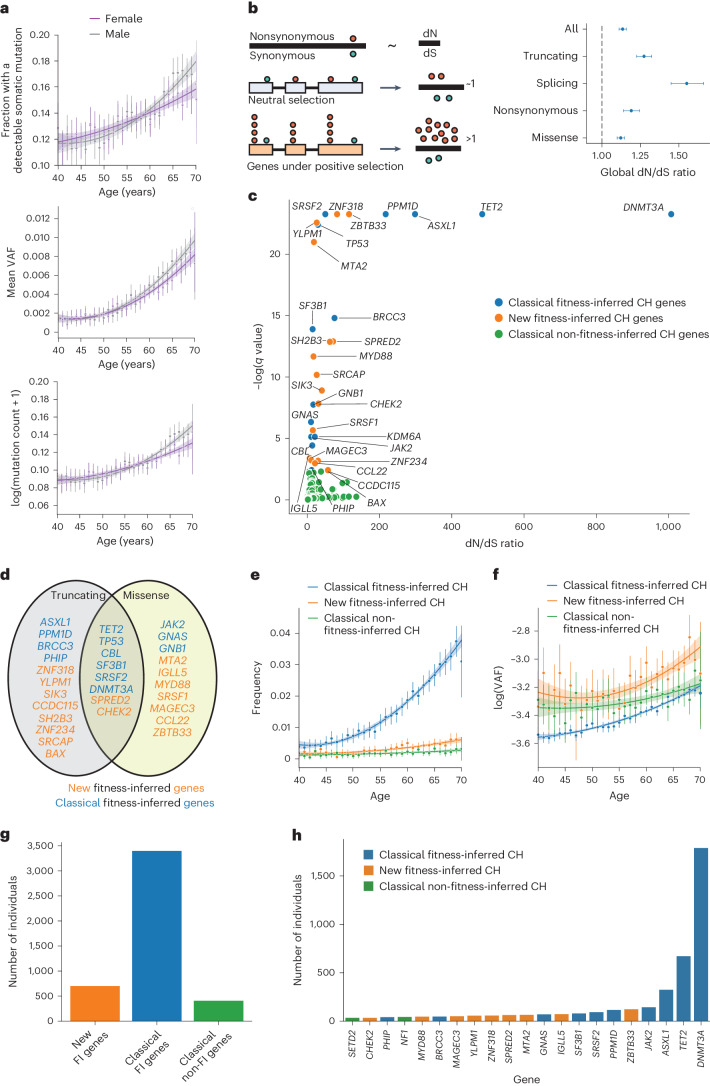
We analyzed whole blood exome sequencing from 200,618 UKBB individuals aged 40–70 years (Extended Data Fig. 1a) to identify somatic mutations in blood. Following variant calling using Mutect2 (ref. 31) and stringent filtering to remove artifacts and germline variants (Methods), we initially identified 52,701 putative coding somatic variants across 38,211 individuals (Supplementary Table 1 and Extended Data Fig. 1b). As expected, the fraction of individuals with somatic mutations, their variant allele frequency (VAF) and number of somatic mutations per individual increased with age (Fig. 1a). Using the normalized ratio of nonsynonymous to synonymous somatic mutations (dN/dS, R package dNdScv) across all variants, as well as on a per gene basis, we were able to distinguish genes and specific mutations under purifying, neutral and positive selection11 (Methods, Fig. 1b and Supplementary Table 2). Globally, we found that, across all types of nonsynonymous coding mutation, the dN/dS ratio was 1.13 (95% confidence interval (CI) 1.11–1.16; Fig. 1b), suggesting that one in every eight (CI 7–10) nonsynonymous mutations detected in this dataset was under selection. Specifically, 1 in every 8–11 missense mutations, 1 in every 4–5 truncating mutations and 1 in ~3 splicing mutations (predominantly affecting DNMT3A; Supplementary Table 2) showed evidence of positive selection in blood. Positive dN/dS was found in both young and older individuals (Extended Data Fig. 1c–e), suggesting that the rate of entry of somatic mutations under selection may not be significantly different over this age range. These data validate our recent findings of pervasive positive selection on somatic mutations from >3,000 single-cell-derived whole genomes from a small number of healthy individuals25 and extend these observations to blood of the UK population, as represented by samples collected by the UKBB.
Extended Data Fig. 1. Variant calling, global selection and somatic mutations in UK Biobank.
a. Age distribution from UKBB. b. Pipeline for identifying exome wide selection on somatic mutations and new genes under positive selection in UKBB. c. Global dN/dS estimates per age group. Error bars represent the 95% CI of the dN/dS parameter for that mutation type and age group. N = 52,701 mutations. d. Percent of mutation type per age group. e. Number of mutations for each mutation type per age group.
Fig. 1. Pervasive selection in whole blood exomes in UKBB.
a, Exome-wide somatic mutation frequency, VAF and mutation counts in individuals increase with age. The error bars represent 2× standard error of the mean. The smoothed line represents a second-degree polynomial fit of the actual data, and the shading represents the CI. N = 200,618. b, Left: dN/dS is the normalized ratio of nonsynonymous to synonymous mutations. dN represents the rate of nonsynonymous mutations per nonsynonymous site, and dS represents the rate of synonymous mutations per synonymous site. A dN/dS of ~1 is expected under neutrality. Genes with a dN/dS ratio >1, taking into account a trinucleotide-specific mutation rate, indicates the gene is under positive selection (‘fitness inferred’, FI). HSCs with a mutation under positive selection will clonally expand to result in CH. Right: global positive selection in blood detectable at missense, essential splice site and truncating mutations (comprising nonsense substitutions and frameshift insertions/deletions). Nonsynonymous mutations comprise missense and nonsense single base substitutions. The error bars represent the 95% CI of the dN/dS parameter for that mutation type. N = 52,701 mutations. c, Classical fitness-inferred (FI) CH genes (in blue), Classical non-fitness-inferred (non-FI) genes (in green), and new fitness-inferred (FI) CH genes (in orange) representing both novel genes and several recently reported. The graph shows the dN/dS ratio for nonsense and/or missense variants >1, q value <0.1, plotting the maximum dN/dS value. d. New fitness-inferred CH genes (in orange) alongside classical fitness-inferred CH genes (in blue), and the types of nonsynonymous mutation they are under positive selection for. e,f, The frequency of individuals (e) and mutation log(VAF) (f) for new and classical FI CH genes and classical non-FI CH genes versus age. The error bars represent the 2× standard error of the mean. The smoothed line represents a second-degree polynomial fit of the actual data, and the shading represents the CI. N = 200,618 individuals. g, The number of individuals in UKBB with detectable somatic mutations in driver genes associated with CH. h, The number of individuals carrying CH conferring variants per gene.
Novel fitness-inferred CH drivers are common and increase with age
Of the set of 74 genes typically used to identify CH from recently published large population studies17,32 (Supplementary Table 3), 14 were under positive selection as measured by dN/dS in UKBB (Supplementary Table 2). This includes the most frequently mutated and recognizable drivers of CH such as mutated TET2, DNMT3A, ASXL1, PPM1D, JAK2, TP53, SRSF2 and SF3B1 (Fig. 1c) and also less frequently mutated BRCC3, PHIP, CBL, KDM6A, GNB2 and GNAS. Genes within this canonical set for CH (Supplementary Table 3) found to be under positive selection we call ‘classical fitness-inferred drivers’. U2AF1 may be missing from this set due to recognized issues with the hg38 reference assembly genome33. We identify a high dN/dS ratio for missense variation in DNMT3A and TET2, but not for ASXL1 and PPM1D (Supplementary Table 2), in agreement with the known mutation landscape in these drivers34. We initially identified 18 genes with large significant dN/dS ratios not in the canonical set of 74 CH genes (Supplementary Table 2). To ensure the additional genes identified were not a reflection of our mutation calling strategy, we validated our approach by independently calling somatic mutations in the additional and classical CH genes using Shearwater35 and retesting for selection using dN/dS (Extended Data Fig. 1b). A total of 96.1% of variants identified by Mutect2 were independently called by Shearwater (Supplementary Tables 4 and 5), with all additional and classical CH genes also under strong positive selection with the sole exception of DUSP22. We called the remaining set of 17 genes ‘new fitness-inferred drivers’ of CH, to distinguish them from the classical set of CH genes, as shown in Fig. 1c,d (Supplementary Table 6). These genes—BAX, CCL22, CCDC115, CHEK2, IGLL5, SH2B3, SIK3, SPRED2, SRCAP, SRFS1, MAGEC3, MTA2, MYD88, YLPM1, ZBTB33, ZNF234 and ZNF318—included novel genes, recently reported candidate drivers of CH in independent datasets, and some previously reported in association with malignancy (Supplementary Table 6). Mutations in these genes had dN/dS ratios between 5 and 660 (indicating that there were 5–660 times more nonsynonymous mutations than expected by chance for these genes) (Fig. 1c and Supplementary Table 2). Many previously reported genes associated with CH were not found to be under significant positive selection in UKBB (Supplementary Table 2), such as RUNX1, PTEN and CUX1, which may reflect their mutation infrequency, their lower prevalence in healthy individuals compared to those with hematological malignancy, or the sensitivity of sequencing in UKBB. Such genes within the canonical set of CH (as defined in refs. 17,32; Supplementary Table 3) that were not under positive selection in UKBB we call ‘classical non-fitness-inferred drivers’.
Overall, 23% of UKBB (47,026 individuals) had a detectable mutation in a classical or new CH gene. A total of 51,264 somatic variants were found in classical fitness-inferred (n = 45,770) and classical non-fitness-inferred (n = 5,494) CH genes, and an additional 5,294 variants were found in new fitness-inferred CH genes. Non-‘DTA’ (DNMT3A, TET2 and ASXL1 mutated) CH was boosted by >50%. As expected, both the VAF and population frequency for classical drivers of CH increased with age in the UKBB (Fig. 1e,f). Crucially, new fitness-inferred CH gene mutation frequency also increased with age (Fig. 1e, β = 0.00015, P value <0.001) and showed a strikingly similar VAF distribution to classical fitness-inferred CH genes (β = 0.00852, P value <0.001; Fig. 1f).
When restricting to only larger CH clones (VAF > 0.1), 1.8% of the UKBB exome cohort (n = 3,565) had at least one classical CH mutation, similar to other studies of this age range and sequencing sensitivity10,32. Of these, classical fitness-inferred drivers were present in 3,228 individuals and classical non-fitness-inferred drivers affected 371 individuals, with 34 individuals carrying mutations in both CH categories. A total of 681 variants (VAF > 0.1) in new fitness-inferred drivers were identified in 660 individuals (Fig. 1g), the majority of whom (93%, n = 613) did not otherwise harbor mutations in classical CH genes, thus representing an 18% increase to the cohort of individuals with large clone (VAF > 0.1) CH in UKBB.
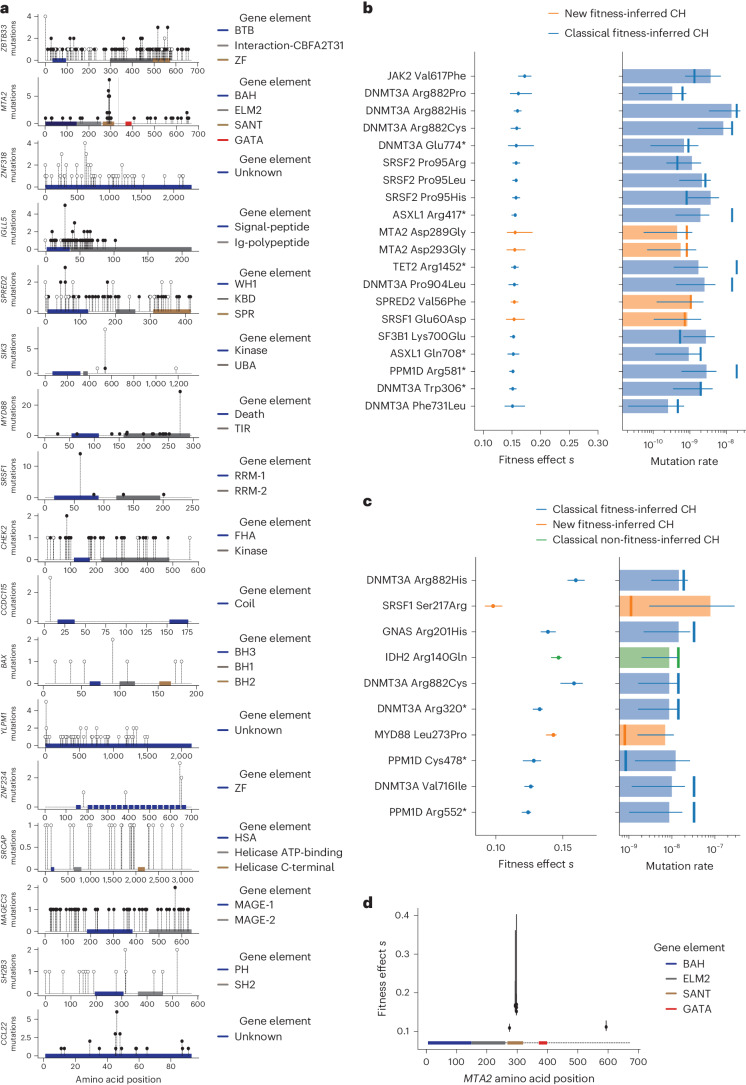
On a per gene basis, new fitness-inferred drivers were among the most common associated with CH status in individuals in the UKBB (Fig. 1h), with many genes showing distinct mutation landscapes (Fig. 2a). Of these, BAX, CHEK2, SH2B3 and MYD88 mutations were also recently identified as candidate markers of CH in blood from patients with other tumors29, with CHEK2 mutations associated with chemotherapy exposure29,36, and BAX mutations reported following BCL2 inhibitor therapy in chronic lymphocytic leukemia (CLL)37.
Fig. 2. New CH gene-specific mutation landscapes and fitness effects.
a, Location and type of mutation across the gene body for new drivers of CH. Filled black circles, missense mutations; filled white circles, truncating mutations (frameshift indel or nonsense mutations). b, Twenty-five recurrently mutated sites with the highest estimated fitness effects include several mutations in new CH driver genes. The error bars for the inferred fitness effect and mutation rate parameters are the CI. The number of observations of VAF for each mutation used to infer parameters is presented in Supplementary Table 4. c, Ten recurrently mutated sites with the highest estimated mutation rate. Of note, MYD88 Leu273Pro, which lies outside the SANT domain, has one of the highest mutation rates within the dataset but did not have a significantly increased fitness effect. The error bars for the inferred fitness effect and mutation rate parameters are the CI. The number of observations of VAF for each mutation used to infer parameters is presented in Supplementary Table 4. d, Fitness estimates for MTA2 plotted across the gene body, which primarily localize to the SANT domain. The error bars for the inferred fitness effect and mutation rate parameters are the CI. The number of observations of VAF for each mutation used to infer parameters is presented in Supplementary Table 4. Amino acid positions are colored by the gene elements shown in the key. Orange shading refers to new fitness-inferred genes of CH; classical fitness-inferred genes of CH are shown in blue, and classical non-fitness-inferred CH genes are shown in green.
Several of the genes identified have been reported in myeloid or lymphoid malignancies. Mutations in MYD88 are well recognized in Waldenstroms macroglobulinemia and diffuse large B cell lymphoma but also CLL and IgM monoclonal gammopathy of uncertain significance38,39. CCL2 mutations have been reported in chronic lymphoproliferative disorder of natural killer cells40, and IGLL5 mutations in CLL41 and diffuse large B cell lymphoma42. It is therefore likely that these mutations derive from the lymphoid compartment in blood. MTA2 and MAGEC3 mutations have been reported rarely in acute myeloid leukemia10,43 and T cell acute lymphoblastic leukemia44 respectively, where their significance is uncertain, and SH2B3 mutations have been reported in myeloproliferative neoplasms45.
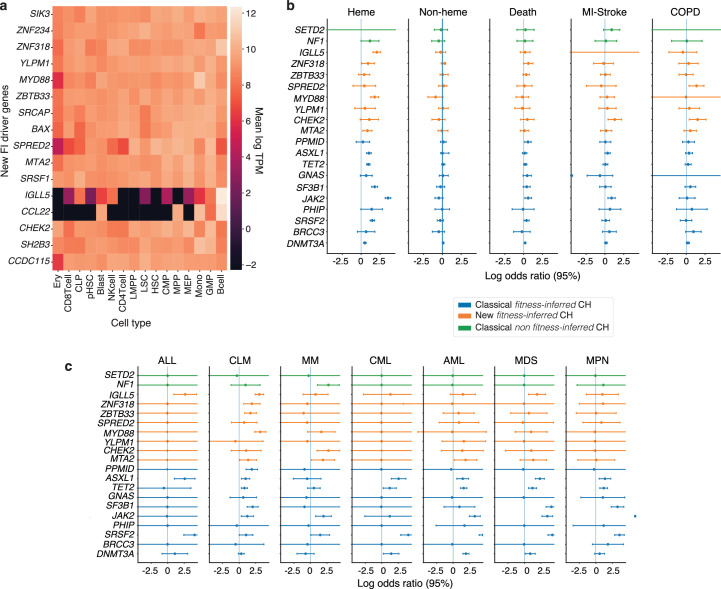
Four genes (ZNF318, YLPM1, SRCAP and ZBTB33) were also recently identified as new drivers of CH in whole blood exomes from the ExAC database46, with mutational landscapes similar to those in UKBB. Truncating mutations in SIK3, SPRED2 and ZNF234, as well as missense mutations in SRSF1, have not been previously described in blood but were under positive selection in UKBB. Most new CH genes are expressed throughout the hematopoietic compartment, with IGLL5 and CCL22 gene expression restricted to B cells47 (Extended Data Fig. 2a).
Extended Data Fig. 2. Gene expression and poor health outcome associations of recurrently mutated clonal haematopoiesis genes.
Gene expression of new ‘fitness-inferred’ (FI)-driver genes in haematopoietic compartment. Expression data was taken from Corces et al.47. Common lymphoid progenitor, CLP; megakaryocyte-erythroid progenitor cell, MEP; common myeloid progenitor, CMP; granulocyte-macrophage progenitor cell, GMP; lymphoid-primed multipotent progenitor cell, LMPP; preleukemic HSC, pHSC; leukemia stem cells, LSC; erythroid cell, Ery; leukemic blast cell, blasts; multipotent progenitor cell, MPP; Mono, monocyte; NK cell, natural killer cell; HSC, haematopoietic stem cell. b, c. Logistic regression log odds ratios for a wide range of poor health outcomes for commonly mutated (genes with >35 mutations) driver genes of CH. Error bars represent the 95% CI. N = 200,618 individuals. Note the log odds ratio for JAK2 mutations and MPN is shown as ‘>’ as it is >5 (5.96, 95% CI 5.56–6.36). Acute lymphoblastic leukaemia, ALL; Common lymphoid malignancies, CLM; multiple myeloma and related, MM; chronic myeloid leukaemia, CML; acute myeloid leukaemia, AML; myelodysplastic diseases, MDS; myeloproliferative neoplasms, MPN; acute upper respiratory infection, AURI; acute lower respiratory infection, ALRI; intestinal infections, II. Orange shading refers to new FI genes of CH; classical FI genes of CH are shown in blue, and classical non FI CH genes are shown in green.
To appreciate how these additional fitness-inferred genes drive CH in the context of more commonly studied CH mutations, and which regions of these genes may be relevant for conferring fitness (Fig. 2b), we inferred mutation-specific fitness effects as described recently12 for variants identified >20 times in UKBB (regardless of clone size; Extended Data Fig. 1b). We find that several of these new fitness-inferred driver genes confer some of the strongest fitness effects (Fig. 2b). For example, mutations such as MTA2 p.Asp289Gly and p.Asp293Gly, SPRED2 p.Val56Phe and SRSF1 p.Glu60Asp confer comparable fitness effects to the hotspot variant DNMT3AR882H and other drivers of CH12, providing a clonal advantage that corresponds to an excess hematopoietic stem cell (HSC) division rate of 15–20% per year (Fig. 2b). Mutations in the SANT domain of MTA2, required for recruitment of HDAC1 and nucleosome remodeling, confer particularly strong fitness (Fig. 2d). Interestingly, the most commonly mutated new fitness-inferred driver gene site, MYD88 p.Leu273Pro (also commonly referred to as Leu265Pro when protein annotation uses transcript ENST00000396334), was not among sites conferring strong selection (Fig. 2b), and recurrent mutations at this site might be observed because of an intrinsically high mutation rate (Fig. 2c) rather than strong selection.
Additional CH drivers under selection in blood colonies
To further validate these additional fitness-inferred drivers and identify the cell types affected, we looked for corresponding mutations in several large datasets we have recently published comprising whole-genome sequencing (WGS) of single-cell-derived hematopoietic colonies from individuals with healthy hematopoiesis and blood cancers13,25,26,48–50. Clonal WGS data do not suffer from the same artifacts as bulk exome sequencing data, thereby also providing robust orthogonal validation. The combined data comprised WGS from a total of 10,837 individual HSC, myeloid and lymphoid single-cell-derived colonies, from 50 individuals with either healthy hematopoiesis or blood cancer. This includes 10,202 colonies derived from myeloid progenitors and stem cells from healthy aging individuals25,26,48, cord blood25, human fetal hematopoiesis25,49, individuals who have undergone allogeneic stem cell transplantation50, individuals with myeloproliferative neoplasms13, additional myeloid malignancies such as therapy-related acute myeloid leukemia, chronic myeloid leukemia and essential thrombocythemia), and 635 B/T cell-derived lymphoid colonies48. In total, 150 nonsynonymous variants were identified in 16 of the 17 new fitness-inferred CH drivers across these individuals (Supplementary Tables 7 and 9).
Within the myeloid datasets (both healthy hematopoiesis and hematological disease), we found 127 variants within 15/17 new fitness-inferred driver genes (Supplementary Table 7), with the majority (n = 102) of mutations detected in colonies from healthy individuals (Supplementary Table 10). Ten genes had evidence of positive selection by dN/dS (CHEK2, SRCAP, ZNF318, ZBTB33, MAGEC3, SPRED2, SIK3, YLPM1, BAX and SH2B3, q value <0.1, restricted hypothesis testing) with the mutation landscapes resembling those found in the UKBB data (Supplementary Table 8). Mutations that are either rarely acquired, or only selected for in a subset of individuals, may not have been validated by dN/dS due to the far lower number of individuals in the clonal WGS dataset (17 healthy individuals, 10 allogeneic stem cell transplant recipients and 23 individuals with blood cancers).
Within the lymphoid clonal WGS data, among other variants in new fitness-inferred CH genes, there were 15 nonsynonymous mutations in IGLL5—a gene found at the immunoglobulin lambda locus—found in three healthy individuals (Supplementary Table 9), and this was the only gene found here to be under selection by dNdScv. This suggests that somatic mutations within the lymphoid compartment are driving their occurrence in bulk whole blood exome sequencing in UKBB. Intriguingly, 14/15 mutations in IGLL5 occurred in memory B cell-derived colonies, even though, overall, memory B cells accounted for <12% of the lymphoid dataset. Overall ~15% of memory B cell colonies had IGLL5 mutations. Both synonymous and nonsynonymous mutations clustered around the N-terminus in B cell-derived colonies from healthy individuals and in whole blood exome sequencing in UKBB (Fig. 2a), a finding also echoed in IGLL5 mutations in lymphoid neoplasms found in the COSMIC database34. This implies that the gene may be subject to highly localized mutational processes—for example, during B cell somatic hypermutation41—that could invalidate assumptions underlying the dN/dS methodology. This raises the possibility that mutations in IGLL5 may be a passenger event during memory B cell expansion rather than driving clonal expansion themselves. No mutations in other suspected lymphoid genes, for example, MYD88, were found in this dataset, though it is notable that not all lymphoid compartments were represented within the single-cell-derived lymphoid colonies.
Overall, across >10,000 single-cell-derived clonal hematopoietic cell colonies derived from 50 individuals, we identified 150 somatic mutations in 16 of the 17 new fitness-inferred drivers, with a strikingly similar pattern of nonsynonymous mutations to bulk whole-exome sequencing data from UKBB. The majority of variants (n = 125 of 150) were from myeloid and lymphoid colonies from individuals with healthy hematopoiesis (Supplementary Table 10), with no history of malignancy or chemotherapy exposure, demonstrating that positive selection acts on mutations in new fitness-inferred CH genes during healthy hematopoiesis. Importantly, 10 of the 17 new fitness-inferred CH genes were also under positive selection, as estimated by dN/dS in single-cell-derived colonies from a small set of individuals, validating UKBB findings. Two of the remaining seven genes (SRSF1 and MTA2) did show independent evidence of positive selection based on the distribution of the VAFs of recurrently mutated sites (Fig. 2b). Excluding IGLL5, the significance of which remains to be determined, and MYD88, a previously reported driver of lymphoid malignancies, there are three remaining genes (CCDC115, ZNF234 and CCL22) identified as under positive selection in UKBB that we could not independently validate. While two ZNF234 variants were found in hematopoietic colonies, including one nonsense mutation, and a missense mutation in CCL22 was present in a naive B cell colony, these numbers were too low to display gene-wide positive selection. Incidentally, these three genes were also the least frequently mutated in UKBB, and they should be considered provisional CH genes pending future studies of larger datasets where their potentially increased variant numbers may allow further elucidation.
Increased risk of hematological neoplasm, death and infection
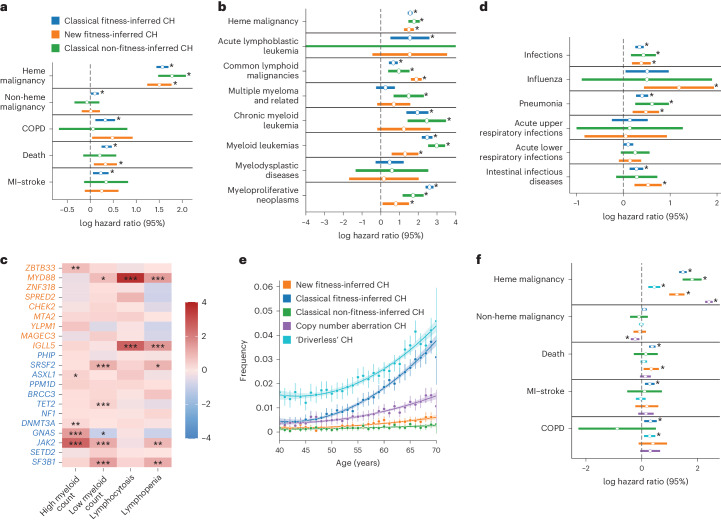
The UKBB provides continuously updated electronic health records that we used to assess for the impact of clonal expansions associated with new fitness-inferred CH genes on subsequent health events (Supplementary Table 11). Many of the new fitness-inferred CH drivers have been proposed as candidate drivers of CH previously, or are known to be mutated in specific hematological malignancies (Supplementary Table 6), but their clinical associations have not been characterized at a population scale. We considered individuals with CH driver mutations at an allele frequency of >0.1 (Supplementary Table 5) as the clinical effect of CH is known to be attenuated in smaller clones17,51,52. Individuals were removed from association analyses if they had chronic obstructive pulmonary disease (COPD), myocardial infarction or stroke, nonhematological malignancy and hematological malignancy events before blood draw for whole-exome sequencing, as well as if there was a mismatch between X chromosome zygosity and reported sex53. We performed a Cox-proportional hazard regression to assess for the association of drivers of CH (both new and classical, VAF >0.1) with hematological malignancies. We found that new fitness-inferred driver genes confer a significant hazard for hematological malignancy (Fig. 3a, log(hazard ratio (HR)) 1.5, P value <0.001) similar to recognized drivers of CH. This hazard is strongest for common lymphoid malignancies (Fig. 3b, CLM, log(HR) 1.9, P value <0.001), driven primarily by mutations in MYD88 (log(odds ratio (OR)) 2.5, P value <0.001) associating with chronic lymphoid malignancy, and IGLL5 (log(OR) 2.8, P value <0.001) associating with acute lymphoblastic leukemia and chronic lymphoid malignancy, with weaker associations with other malignancies (Extended Data Fig. 2b,c). Both genes have been reported as drivers of lymphoid malignancies38,39,41,42. The presence of mutations in many new fitness-inferred CH genes were mirrored by changes in peripheral blood counts, with ZBTB33 mutations associated with increased myeloid counts and MYD88/IGLL5 mutations associated with abnormal lymphocyte counts (Fig. 3c).
Fig. 3. Clinical outcome associations with new drivers of CH.
a,b,d, Cox-proportional HRs for a range of poor health outcomes against CH categories with CIs of log(HR) plotted. N = 200,618 individuals. Different categories of poor health outcomes are shown in a, hematological malignancies are shown in b, and infections are shown in d. *P < 0.05. Heme, hematological; COPD, chronic obstructive pulmonary disease; MI, myocardial infarct. c, Complete blood count associations of commonly mutated fitness-inferred driver genes. The stars are based on the FDR-adjusted P values from logistic regression (*P value >0.01 to <0.05, **P value >0.001 to <0.01, ***P value <0.001). e, The incidence of ‘driverless’ CH and autosomal mosaic copy number variants with age alongside other CH categories. The error bars represent the 2× standard error of the mean incidence. The smoothed line represents a second-degree polynomial fit of the actual data, and the shading represents the CI. N = 200,618 individuals. f, log(HR) with the 95% CI plotted for different categories of CH across poor health outcomes. N = 200,618 individuals. The orange shading refers to new fitness-inferred genes of CH, classical fitness-inferred genes of CH are shown in dark blue, classical non-fitness-inferred CH genes are shown in green, CH clones with copy number aberrations on autosomes are shown in purple, and CH clones without detectable candidate driver mutations are shown in light blue.
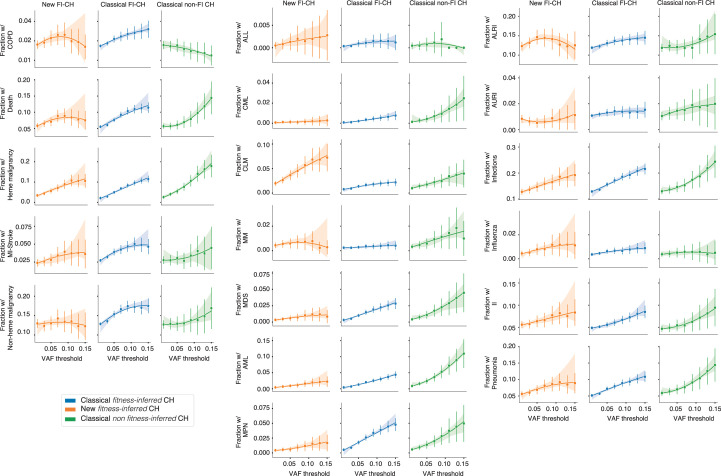
CH has been associated with increased risk for myocardial infarction, ischemic events, COPD and all-cause mortality17,18,54,55. Similar to classical fitness-inferred drivers, individuals with new fitness-inferred driver mutations also have a significantly increased hazard for death (Fig. 3a, P = 0.01, log(HR) 0.36). Crucially, incidence of poor health outcomes was dependent on clone size, which is a phenomenon also observed in classical CH55 (Extended Data Fig. 3). Recently, mosaic copy number variants in blood were associated with an increased risk for a wide range of infections20. CH driven by nonsynonymous/short indel mutations have not yet been shown to confer an increased risk for these outcomes. We show here that all categorizations of CH associate with increased hazards for infections, intestinal infectious diseases and pneumonia (Fig. 3d).
Extended Data Fig. 3. Clone size effect on poor health outcomes.
Clone size effect on poor-health outcomes (malignancy, stroke, death, different haematological neoplasms and infections) incidence by CH driver mutation category. Poor health outcomes incidence increases as clone variant allele frequency increases to 0.1 after which the relationship becomes unstable which has been noted previously for DNMT3A and TET2 mutant clones and cardiovascular risk. Error bars represent the 2*standard error of the mean incidence. The smoothed line represents a second degree polynomial fit of the actual data and the shading represents the 95% CI. N = 200,618 individuals. Orange shading refers to new FI genes of CH; classical FI genes of CH are shown in blue, and classical non FI CH genes are shown in green.
The majority of clonal expansions remain unexplained
‘Driverless’ CH is the occurrence of clonal expansions in blood without a known driver10,25 and is estimated to drive the majority of clonal expansions in the elderly. We identified individuals with a high mutational burden without any known CH driver (as identified by this study; Methods) or copy number variants (as described in ref. 56). We confirm that ‘driverless’ CH in UKBB is very common and increases with age as previously seen (Fig. 3e), conferring a significantly increased risk for COPD and hematological malignancies but not all-cause mortality (Fig. 3f).
An ongoing debate is whether ‘driverless’ CH is caused by somatic driver mutations that are yet to be identified, nongenetic factors (for example, epigenetic changes) or neutral drift. Our dN/dS analysis of mutations >0.11 VAF—indicating that 1/8 of nonsynonymous mutations are under positive selection—suggests that unrecognized driver mutations still remain a large contributor. With our expanded definition of CH, we estimate that we now identify ~50% (CI 43–59%) of the total number of drivers of CH in blood (Supplementary Note 1). This is an increase on previous estimates of positive selection in blood27 and suggests that we are now ‘halfway there’ to identifying driver mutations that are able to produce large clones in blood. Since dNdScv appears to linearly recover increased numbers of drivers of CH within this cohort (Supplementary Note 2), there would be merit in a future substantially larger study combining genome sequencing of several different population cohorts to identify further genes under positive selection in blood.
Discussion
Somatic mutation acquisition in human cells occurs stochastically, reflecting tissue-specific rates and mutational processes, with only a minority of these mutations confering a fitness advantage that drives clonal expansion. However, recent studies have suggested that clonal selection on somatic mutations in blood extends beyond that attributable to known driver mutations in a set of <100 genes10,25,27. In whole blood exomes from ~200,000 individuals from UKBB, over 10% of nonsynonymous mutations that we identified may be under positive selection. Of note, the high dN/dS ratios in UKBB whole blood exomes compared to other studies25 could be due to ascertainment bias. First, bulk exome sequencing data will only capture mutations that occurred before the expansion of the most recent common ancestor of a detectable clone, and not any subsequent mutations. Secondly, in the absence of single-molecule sequencing, driver mutations that occurred earlier in life are more likely to be captured than later driver mutation acquisitions due to the greater duration for clonal expansion. Both of these factors could have the impact of inflating the number of driver mutations compared to background mutations. Nevertheless, at a gene-specific level, we identify 17 further candidates in this clonal apparatus. While some of these genes have been recently reported as candidate drivers46, or identified in the context of concurrent malignancies29 and therapy36,37,56, here we show that positive selection on these genes is detectable in unselected populations, within individuals with healthy myeloid and/or lymphoid hematopoiesis, and that those who carry a nonsynonymous mutation in these genes (at VAF >0.1) having significantly increased hazards for a wide range of adverse health outcomes.
In this study, we used one of the many methods available to identify regions within exome sequences that are under positive selection to identify additional driver mutations in whole blood exomes (restricted to those associated with large clones >0.1) together with validation of positive selection in single-cell-derived hematopoietic myeloid and lymphoid colonies. Inclusion of mutations in these fitness-inferred CH genes increases the prevalence of CH (restricted to large clones >0.1) by 18% in the UKBB cohort. Other methods that infer selection coefficients from variant VAFs12 or mosaic copy number variants20,56 may lead to more CH driving loci associated with poor health outcomes. Studies of different hematopoietic populations, such as HSCs or specific lymphoid populations not represented in whole blood, may also identify additional genetic sites under positive selection. Nevertheless, it is clear that many drivers of clonal expansion in blood are still unknown, in stark contrast to other tissues such as the esophagus, where our compendium of fitness-inferring somatic mutations appears much more complete27. Future efforts enabling characterization of smaller clonal expansions, using both more sensitive and less error-prone sequencing technologies, as well as combined population datasets, will enable a more comprehensive examination of the evolutionary substrate under positive selection in blood.
The pervasive impact of age-associated oligoclonality and gene-specific clonal expansions on health outcomes is increasingly clear. Future research is needed to determine the extent to which aging phenotypes are encoded by the landscape of clonal expansions, in both blood and other self-renewing tissues, to determine the degree to which modulating the burden of somatically mutated clones can improve health outcomes.
Methods
Ethics statement
Informed consent was obtained for all individuals providing samples for hematopoietic colonies under NHS Research Ethics Committee approval 18/EE/0199 and 07/MRE/44. Participants for in-house whole genomes were recruited under Cambridge Blood and Stem Cell Biobank ethics, 18/EE/0199 and 07/MRE/44, following written informed consent, and for published genomes as reported previously13,25,26,48–50. UKBB analysis was conducted using the UKBB resource under application 18448.
Somatic variant calling in UKBB and dNdScv analysis
Putative somatic variants were initially identified using Mutect2 (ref. 31) (broadinstitute/gatk:4.1.3.0) from exome sequencing CRAM files (UKBB resources 23143 and 23144). We used 1000 Genomes, ExAC and gnomAD to remove sequencing artifacts and common germline single-nucleotide polymorphisms (SNPs)57–59. Variants were called within target capture regions (UKBB resource 3801) and 100 bp either side and annotated using SNPeff60 (v4_3) and dbSNP build GRCh38.86. A total of 83,396,753 putative variants underwent quality control filtering to remove (1) variants with a median base quality difference of >5 between the alternate and reference allele, (2) mutation sites showing extreme strand bias (P < 0.01, chi-square test) due to overrepresentation of sequencing artifacts61, (3) variants with features commonly associated with false positives, such as alleles only supported by the end of the read, or reads with excessive edit distance, using FINGs v1.7.1 (ref. 62) (using thresholds as follows: minbasequality 30, zeroproportion 0.05, minmapquality 50, minmapqualitydifference 5, enddistance 10, enddistancemad 3, editdistance 4, maxoaftumor 0.04, maxsecondtumor 0.05, foxog 0.9, snvcluster50 2, snvcluster100 4, repeats 12), (4) indels with 10 or fewer reads supporting the alternate allele17, and (5) variants in the gene MUC, olfactory genes and genes with recurrent synonymous mutations due to high rates of false positivity63,64. Variants with VAF <0.11 and <3 reads supporting the alternate allele were also excluded to reduce false positive mutations. This threshold was chosen because our desired number of false positive mutations was <1 for the entire exome cohort. The total number of sites tested for our project is equal to the size of the targeted exome region in UKBB (38,997,831 base pairs65) times the number of individuals (200,618). Given a sequencing error in 1/1,000 base pairs, we wanted to find the VAF threshold for the median locus depth, 45 reads, which would lead to <1 false positive somatic mutation from sequencing error. Using the formula Number of false positives = Number of bases tested xsequencing error(median loci depth × VAF), led to the choice of a VAF threshold of 0.11. Additional filters were applied to remove (1) variants which appeared germline (binomial test, P = 0.5, n represents depth of locus, k represents read count of alternate allele, P < 1 × 10−4), (2) variants with a −log10 population minor allele frequency in gnomAD of <3.35 as this threshold allowed removal of germline variants while retaining known drivers of CH common in gnomAD, such as JAK2V617F, (3) variants that occurred more often than DNMT3AR882H as well as mutations with more than 50% of their occurrences having a VAF >0.15 (ref. 46), (4) variants in homopolymers regions of length 4 (ref. 66), (5) high depth (>150×) variants, and (6) variants within 50 bp of one another. A total of 52,701 variants remained following the above filtering (Extended Data Fig. 1b). U2AF1 mutations may have been missing from this list of variants due to recognized issues with mutation calling relating to the hg38 reference assembly genome33. We used dNdScv11 (https://github.com/im3sanger/dndscv) to perform neutrality tests by calculating dN/dS ratios on the 52,701 filtered Mutect2 variants on both a global and a per gene level for different classes of mutations (missense, nonsense, indel and essential splice site). A dN/dS ratio >1 for any type of variation (q value <0.1) was considered evidence of positive selection. To validate both variants and genes under selection, and to gain sensitivity for detecting low VAF clones in putative genes of interest, we next ran the somatic variant caller Shearwater35 (v3_11) on all 200,618 UKBB exomes for all classical (Supplementary Table 3) and new fitness-inferred CH genes (Supplementary Table 6). We included uniquely mapped reads with mapping quality >30. CosmicCodingMut.vcf from COSMIC v94 (ref. 34) was used to create a prior of Shearwater. We considered variants to validate our Mutect2 call with a Bayes factor <0.5. Finally, we reran dNdScv only on the intersection of variants (96.1%) that were called by both Mutect2 and Shearwater. All new and classical fitness-inferred driver genes except DUSP22 were still under significant positive selection using these high-quality validated variant calls. DUSP22 was removed from subsequent analysis.
Determining CH status in UKBB and clinical correlates
We began with the union of unfiltered variant calls from Shearwater and Mutect2 to identify which variants would confer CH status (Extended Data Fig. 1b). Shearwater variants were included only if they were recurrently found (n > 5) in the Mutect2 call set. We then removed variants with (1) VAF >0.5 unless they were on chromosome X in males, (2) excess strand bias as described above, (3) a −log10 population minor allele frequency <3.35 according to gnomAD, and (4) if >50% of a variant’s occurrences had a VAF >0.15. We required variants to have an allele frequency of >0.1 as the clinical effect of CH is known to be decreased in smaller clones17,51,52. This provided the final variant list for conferring CH status. We divided CH identifying genes into three categories: (1) classical fitness-inferred drivers, that is, previously described genes in CH17,32 under positive selection in UKBB; (2) classical non-fitness-inferred drivers, that is, mutations in genes historically associated with CH but not found to be under positive selection in the UKBB; (3) new fitness-inferred drivers, that is, mutations in both novel genes and several recently reported genes that have not routinely been used to identify CH status and under strong positive selection in UKBB. Genes were under differential selection for missense versus nonsense mutation. For example, MTA2 was under positive selection only for missense variation according to dNdScv, so only missense variants in MTA2 were considered to confer new fitness-inferred CH status. For nonsense variation, any truncating variant was used to confer new fitness-inferred CH status. We used linear regression to identify relationships between age and frequency of each category of CH as well as log(VAF). The log (VAF) distribution was assumed to be normal, but this was not formally tested. We identified poor health outcome events and their corresponding dates from UKBB data (Supplementary Table 11). Individuals were removed from association analyses if they had COPD, Myocardial infarct-stroke, nonhematological malignancy and hematological malignancy events before blood draw, and if there was a mismatch between X chromosome zygosity and reported sex53. We used Cox-proportional hazard regression with new fitness-inferred CH status, classical fitness-inferred CH status, classical non-fitness-inferred status, body fat percentage, pack-years of smoking, age at recruitment, age at recruitment2, self-reported sex, hypertension, systolic, diastolic, low-density lipoprotein, high-density lipoprotein, cholesterol and type 2 diabetes, as covariates (Supplementary Table 12). Per gene effects for genes mutated in >30 individuals in UKBB were inferred using logistic regression using Python statsmodels (0.12.2) for each health outcome as described above (Supplementary Table 13).
Inferring fitness coefficients for recurrently mutated sites
We performed maximum likelihood estimates for the fitness parameter of the 150 most commonly mutated sites in the UKBB, following the method described by Watson et al.12. We included all variants at these sites called by Shearwater or Mutect2 with VAF <0.5. Fitness effects were estimated as selection coefficients (s), which represents the increased birth rate of an HSC relative to wild-type HSCs as a result of the driver mutation. We estimated Nτ, where N represents the total number of HSCs and τ represents the time in years between symmetric divisions, and σ, the standard deviation of the age of the cohort using the most common mutation in our cohort, DNMT3AR882H. Our estimated parameters for Nτ (140,000) and s (0.159) were similar to previous estimates2,12. We removed variants from consideration where the CI for s was >0.2.
Determining mosaic CNV and ‘driverless’ CH status and clinical correlates
Individuals with mosaic CNV were identified from Loh et al.67. To identify large ‘driverless’ CH, we identified individuals with an excessive number of somatic mutations10 (≥95th percentile, n = 3 somatic mutations). We removed individuals with known driver mutations (VAF >0.02) or autosomal mosaic CNV events. This identified 5,873 individuals with ‘driverless’ CH. To identify associated clinical outcomes, we used the same set of outcomes and covariates as described above for our Cox-proportional hazard regression for individuals with >0.1 cell fraction of mosaic CNV and all identified ‘driverless’ CH.
Gene expression and gene set enrichment
We visualized normalized expression log(transcripts per million sequencing reads) data of sequenced bulk RNA from sorted normal, preleukemic and leukemic cells of the hematopoietic lineage from Corces et al.47 (GSE74912) plotting mean log(transcripts per million sequencing reads) per cell type for each gene on a heatmap. Gene set enrichment analysis was performed using the q-value ranked list of genes from dNdScv to identify significantly enriched (false discovery rate (FDR) cutoff of 0.1) MSigDB hallmark gene sets68,69.
dN/dS analysis of mutations from hematopoietic colonies
WGS of single-cell-derived colonies was derived from published13,25,26,48–50 and unpublished datasets. Mutations from published single-cell colony datasets13,25,26,48–50 and unpublished datasets were divided by whether they contained predominantly hematopoietic stem cell/multipotent progenitor (HSC/MPP) and myeloid progenitor colonies, or lymphoid colonies. The myeloid and HSC/MPP datasets included those where colonies were grown on methylcellulose agar or were flow-sorted HSC/MPPs. Given that colonies from the same individual may share somatic mutations that occurred in a common ancestor, we only considered unique mutations from each individual. Combined mutation sets across patients were analyzed using the dndscv function from the R package ‘dndscv’ (https://github.com/im3sanger/dndscv) using default settings except for the following arguments: max_muts_per_gene_per_sample = Inf, use_indel_sites=T, max_coding_muts_per_sample = Inf. This part of the analysis was not aimed at new driver discovery but rather validation of the new genes discovered from UKBB data. Therefore, analysis was restricted to the 17 new genes: SPRED2, MTA2, YLPM1, ZBTB33, ZNF318, ZNF234, SRSF1, IGLL5, MYD88, SIK3, CHEK2, MAGEC3, CCDC115, BAX, SRCAP, SH2B3 and CCL22, which were set within the ‘gene_list’ argument of the dndscv function. Genes were considered to have evidence of positive selection if the reported dN/dS q value was <0.1 for missense mutations, truncating variants, all substitutions or indels.
Peripheral blood count associations with CH
To quantify the impact of new genes associated with CH on peripheral blood counts, we used the complete blood count parameters obtained by the UKBB. These parameters allowed us to categorize individuals into five categories: high myeloid cell parameters, low myeloid cell parameters, lymphocytosis, lymphopenia and normal blood count parameters, as previously described70. We then used logistic regression using the Python package stansmodels (0.12.2) to identify the relationship between each peripheral blood count abnormality and the new genes associated with CH using the individuals with normal blood count parameters as the control group. Pack-years smoking, age at recruitment and sex were used as covariates.
Statistics and reproducibility statement
No statistical methods were used to predetermine sample sizes. No data were excluded from the analysis. The experiments were not randomized and the investigators were not blinded to allocation during experiments and outcome assessment. Data collection and analysis were not performed blind to the conditions of the experiments.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41588-024-01755-1.
Supplementary information
Supplementary Notes 1 and 2 and Fig. 1 (to go with Supplementary Note 2).
Supplementary Tables 1–13, together with README at start.
Source data
These are the source data files for plots in Fig. 1. Those not included here are already present in the supplementary tables.
These are the source data files for plots in Fig. 3. Those not included here already present in the supplementary tables.
These are the source data files for plots in Extended Data Fig. 2. Those not included here are already present in the supplementary tables.
These are the source data files for plots in Extended Data Fig. 3.
Acknowledgements
We thank N. Rubinstein, E. Sorokin, M. Cule, C. Connelly, E. Melamud and F. Harding for their feedback and guidance. UKBB analysis was funded by Calico Life Sciences LLC and conducted under UKBB application 18448. J.N. is a Cancer Research UK (CRUK) Advanced Clinician Scientist Fellow. K.N. is supported by CRUK and the Wellcome Trust. Work in the Nangalia lab is supported by CRUK, Wellcome core funding at Wellcome Sanger Institute, Alborada Trust and Rosetrees Trust.
Extended data
Author contributions
N.B. performed UKBB analysis with Z.C. under supervision of R.L.C. and J.N. R.L.C. conceived the study. R.L.C. and J.N. directed the research. M.S.C. performed analysis of hematopoietic colonies with E.M. and P.J.C. N.B., K.N., M.S.C., R.L.C. and J.N. prepared the manuscript. All authors reviewed the manuscript. R.L.C. and J.N. jointly supervised this work.
Peer review
Peer review information
Nature Genetics thanks Alexander Bick, Ross Levine and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
Individual level data will be uploaded to UKBB in keeping with UKBB’s data sharing agreement. Nonindividualized mutation data have been provided in the supplementary tables. Hematopoietic colony-level data are available as follows: (1) healthy hematopoietic colonies (WGS accession numbers EGAD00001007684 and EGAD00001007851), (2) myeloproliferative neoplasm colonies (WGS accession number EGAD00001007714), (3) fetal hematopoiesis colonies (WGS accession number EGAD00001006162), (4) lymphoid colonies (WGS accession number EGAD00001008107), (5) chronic myeloid leukemia colonies (WGS accession number EGAD00001015353), (6) allogeneic stem cell transplant (WGS accession number EGAD00001010872) and (7) single individual with therapy-related acute myeloid leukemia (WGS accession number EGAD00001015339). Source code for figures has been provided as Supplementary Information. Source data are provided with this paper.
Code availability
Code for analyses is available online at 10.5281/zenodo.10891332 ref. 71 and can also be found at https://github.com/nangalialab/UKBB_ClonalHaem_Novel_Drivers as well as at https://github.com/mspencerchapman/Pervasive_positive_selection_in_blood.
Competing interests
N.B. is an ex-employee of Calico Life Sciences LLC; R.L.C. and Z.C. are employees of Calico Life Sciences LLC. P.J.C. is a cofounder and shareholder of FL86 Inc. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Nicholas Bernstein, Michael Spencer Chapman.
Contributor Information
Robert L. Cohen, Email: rlc@calicolabs.com
Jyoti Nangalia, Email: jn5@sanger.ac.uk.
Extended data
is available for this paper at 10.1038/s41588-024-01755-1.
Supplementary information
The online version contains supplementary material available at 10.1038/s41588-024-01755-1.
References
- 1.Colom B, et al. Spatial competition shapes the dynamic mutational landscape of normal esophageal epithelium. Nat. Genet. 2020;52:604–614. doi: 10.1038/s41588-020-0624-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee-Six H, et al. Population dynamics of normal human blood inferred from somatic mutations. Nature. 2018;561:473–478. doi: 10.1038/s41586-018-0497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olafsson S, et al. Somatic evolution in non-neoplastic IBD-affected colon. Cell. 2020;182:672–684.e11. doi: 10.1016/j.cell.2020.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yizhak K, et al. RNA sequence analysis reveals macroscopic somatic clonal expansion across normal tissues. Science. 2019;364:eaaw0726. doi: 10.1126/science.aaw0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martincorena I, et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science. 2015;348:880–886. doi: 10.1126/science.aaa6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonason AS, et al. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc. Natl Acad. Sci. USA. 1996;93:14025–14029. doi: 10.1073/pnas.93.24.14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blokzijl F, et al. Tissue-specific mutation accumulation in human adult stem cells during life. Nature. 2016;538:260–264. doi: 10.1038/nature19768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martincorena I, et al. Somatic mutant clones colonize the human esophagus with age. Science. 2018;362:911–917. doi: 10.1126/science.aau3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yokoyama A, et al. Age-related remodelling of oesophageal epithelia by mutated cancer drivers. Nature. 2019;565:312–317. doi: 10.1038/s41586-018-0811-x. [DOI] [PubMed] [Google Scholar]
- 10.Zink F, et al. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood. 2017;130:742–752. doi: 10.1182/blood-2017-02-769869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martincorena I, et al. Universal patterns of selection in cancer and somatic tissues. Cell. 2017;171:1029–1041.e21. doi: 10.1016/j.cell.2017.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watson CJ, et al. The evolutionary dynamics and fitness landscape of clonal hematopoiesis. Science. 2020;367:1449–1454. doi: 10.1126/science.aay9333. [DOI] [PubMed] [Google Scholar]
- 13.Williams N, et al. Life histories of myeloproliferative neoplasms inferred from phylogenies. Nature. 2022;602:162–168. doi: 10.1038/s41586-021-04312-6. [DOI] [PubMed] [Google Scholar]
- 14.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 15.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481:306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- 17.Jaiswal S, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaiswal S, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuster JJ, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zekavat SM, et al. Hematopoietic mosaic chromosomal alterations increase the risk for diverse types of infection. Nat. Med. 2021;27:1012–1024. doi: 10.1038/s41591-021-01371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng SWK, et al. Convergent somatic mutations in metabolism genes in chronic liver disease. Nature. 2021;598:473–478. doi: 10.1038/s41586-021-03974-6. [DOI] [PubMed] [Google Scholar]
- 22.Jaiswal S, Ebert BL. Clonal hematopoiesis in human aging and disease. Science. 2019;366:eaan4673. doi: 10.1126/science.aan4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kar SP, et al. Genome-wide analyses of 200,453 individuals yield new insights into the causes and consequences of clonal hematopoiesis. Nat. Genet. 2022;54:1155–1166. doi: 10.1038/s41588-022-01121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young AL, Challen GA, Birmann BM, Druley TE. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat. Commun. 2016;7:12484. doi: 10.1038/ncomms12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell E, et al. Clonal dynamics of haematopoiesis across the human lifespan. Nature. 2022;606:343–350. doi: 10.1038/s41586-022-04786-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fabre MA, et al. The longitudinal dynamics and natural history of clonal haematopoiesis. Nature. 2022;606:335–342. doi: 10.1038/s41586-022-04785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poon GYP, Watson CJ, Fisher DS, Blundell JR. Synonymous mutations reveal genome-wide levels of positive selection in healthy tissues. Nat. Genet. 2021;53:1597–1605. doi: 10.1038/s41588-021-00957-1. [DOI] [PubMed] [Google Scholar]
- 28.Stacey SN, et al. Genetics and epidemiology of mutational barcode-defined clonal hematopoiesis. Nat. Genet. 2023 doi: 10.1038/s41588-023-01555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pich O, Reyes-Salazar I, Gonzalez-Perez A, Lopez-Bigas N. Discovering the drivers of clonal hematopoiesis. Nat. Commun. 2022;13:4267. doi: 10.1038/s41467-022-31878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bycroft C, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benjamin, D. et al. Calling somatic SNVs and indels with Mutect2. Preprint at bioRxiv10.1101/861054 (2019).
- 32.Bick, A. G. et al. Inherited causes of clonal haematopoiesis in 97,691 whole genomes. Nature586, 763–768 (2020). [DOI] [PMC free article] [PubMed]
- 33.Miller CA, et al. Failure to detect mutations in U2AF1 due to changes in the GRCh38 reference sequence. J. Mol. Diagn. 2022;24:219–223. doi: 10.1016/j.jmoldx.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tate JG, et al. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res. 2019;47:D941–D947. doi: 10.1093/nar/gky1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerstung M, Papaemmanuil E, Campbell PJ. Subclonal variant calling with multiple samples and prior knowledge. Bioinformatics. 2014;30:1198–1204. doi: 10.1093/bioinformatics/btt750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolton KL, et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat. Genet. 2020;52:1219–1226. doi: 10.1038/s41588-020-00710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blombery P, et al. Clonal hematopoiesis, myeloid disorders and BAX-mutated myelopoiesis in patients receiving venetoclax for CLL. Blood. 2022;139:1198–1207. doi: 10.1182/blood.2021012775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu X, et al. MYD88 L265P mutation in lymphoid malignancies. Cancer Res. 2018;78:2457–2462. doi: 10.1158/0008-5472.CAN-18-0215. [DOI] [PubMed] [Google Scholar]
- 39.Shuai W, et al. Clinicopathological characterization of chronic lymphocytic leukemia with MYD88 mutations: L265P and non-L265P mutations are associated with different features. Blood Cancer J. 2020;10:86. doi: 10.1038/s41408-020-00351-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baer C, et al. CCL22 mutations drive natural killer cell lymphoproliferative disease by deregulating microenvironmental crosstalk. Nat. Genet. 2022;54:637–648. doi: 10.1038/s41588-022-01059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kasar S, et al. Whole-genome sequencing reveals activation-induced cytidine deaminase signatures during indolent chronic lymphocytic leukaemia evolution. Nat. Commun. 2015;6:8866. doi: 10.1038/ncomms9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan Z, et al. Comprehensive characterization of driver genes in diffuse large B cell lymphoma. Oncol. Lett. 2020;20:382–390. doi: 10.3892/ol.2020.11552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corces-Zimmerman MR, Hong W-J, Weissman IL, Medeiros BC, Majeti R. Preleukemic mutations in human acute myeloid leukemia affect epigenetic regulators and persist in remission. Proc. Natl Acad. Sci. USA. 2014;111:2548–2553. doi: 10.1073/pnas.1324297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keersmaecker KD, et al. Exome sequencing identifies mutation in CNOT3 and ribosomal genes RPL5 and RPL10 in T-cell acute lymphoblastic leukemia. Nat. Genet. 2013;45:186–190. doi: 10.1038/ng.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nangalia J, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N. Engl. J. Med. 2013;369:2391–2405. doi: 10.1056/NEJMoa1312542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beauchamp EM, et al. ZBTB33 is mutated in clonal hematopoiesis and myelodysplastic syndromes and impacts RNA splicing. Blood Cancer Discov. 2021;2:500–517. doi: 10.1158/2643-3230.BCD-20-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corces MR, et al. Lineage-specific and single-cell chromatin accessibility charts human hematopoiesis and leukemia evolution. Nat. Genet. 2016;48:1193–1203. doi: 10.1038/ng.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Machado HE, et al. Diverse mutational landscapes in human lymphocytes. Nature. 2022;608:724–732. doi: 10.1038/s41586-022-05072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spencer Chapman M, et al. Lineage tracing of human development through somatic mutations. Nature. 2021;595:85–90. doi: 10.1038/s41586-021-03548-6. [DOI] [PubMed] [Google Scholar]
- 50.Spencer Chapman M, et al. Clonal dynamics after haematopoietic stem cell transplantation using genome-wide somatic mutations. Blood. 2022;140:1572–1573. [Google Scholar]
- 51.Abelson S, et al. Prediction of acute myeloid leukaemia risk in healthy individuals. Nature. 2018;559:400–404. doi: 10.1038/s41586-018-0317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Desai P, et al. Somatic mutations precede acute myeloid leukemia years before diagnosis. Nat. Med. 2018;24:1015–1023. doi: 10.1038/s41591-018-0081-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marees AT, et al. A tutorial on conducting genome-wide association studies: quality control and statistical analysis. Int J. Methods Psychiatr. Res. 2018;27:e1608. doi: 10.1002/mpr.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buscarlet M, et al. DNMT3A and TET2 dominate clonal hematopoiesis and demonstrate benign phenotypes and different genetic predispositions. Blood. 2017;130:753–762. doi: 10.1182/blood-2017-04-777029. [DOI] [PubMed] [Google Scholar]
- 55.Bick AG, et al. Genetic Interleukin 6 signaling deficiency attenuates cardiovascular risk in clonal hematopoiesis. Circulation. 2020;141:124–131. doi: 10.1161/CIRCULATIONAHA.119.044362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slavin TP, et al. Association between clonal hematopoiesis and late nonrelapse mortality after autologous hematopoietic cell transplantation. Biol. Blood Marrow Transpl. 2019;25:2517–2521. doi: 10.1016/j.bbmt.2019.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siva N. 1000 Genomes project. Nat. Biotechnol. 2008;26:256. doi: 10.1038/nbt0308-256b. [DOI] [PubMed] [Google Scholar]
- 58.Lek M, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karczewski KJ, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cingolani P, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff. Fly. 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo Y, et al. The effect of strand bias in Illumina short-read sequencing data. BMC Genomics. 2012;13:666. doi: 10.1186/1471-2164-13-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wardell CP, Ashby C, Bauer MA. FiNGS: high quality somatic mutations using filters for next generation sequencing. BMC Bioinform. 2021;22:77. doi: 10.1186/s12859-021-03995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lawrence MS, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Przytycki PF, Singh M. Differential analysis between somatic mutation and germline variation profiles reveals cancer-related genes. Genome Med. 2017;9:79. doi: 10.1186/s13073-017-0465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Szustakowski JD, et al. Advancing human genetics research and drug discovery through exome sequencing of the UK Biobank. Nat. Genet. 2021;53:942–948. doi: 10.1038/s41588-021-00885-0. [DOI] [PubMed] [Google Scholar]
- 66.Koboldt DC. Best practices for variant calling in clinical sequencing. Genome Med. 2020;12:91. doi: 10.1186/s13073-020-00791-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Loh P-R, et al. Insights into clonal haematopoiesis from 8,342 mosaic chromosomal alterations. Nature. 2018;559:350–355. doi: 10.1038/s41586-018-0321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liberzon A, et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1:417–425. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Subramanian A, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Niroula A, et al. Distinction of lymphoid and myeloid clonal hematopoiesis. Nat. Med. 2021;27:1921–1927. doi: 10.1038/s41591-021-01521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.nangalialab/UKBB_ClonalHaem_Novel_Drivers: initial release. Zenodo10.5281/zenodo.10891332 (2024).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Notes 1 and 2 and Fig. 1 (to go with Supplementary Note 2).
Supplementary Tables 1–13, together with README at start.
These are the source data files for plots in Fig. 1. Those not included here are already present in the supplementary tables.
These are the source data files for plots in Fig. 3. Those not included here already present in the supplementary tables.
These are the source data files for plots in Extended Data Fig. 2. Those not included here are already present in the supplementary tables.
These are the source data files for plots in Extended Data Fig. 3.
Data Availability Statement
Individual level data will be uploaded to UKBB in keeping with UKBB’s data sharing agreement. Nonindividualized mutation data have been provided in the supplementary tables. Hematopoietic colony-level data are available as follows: (1) healthy hematopoietic colonies (WGS accession numbers EGAD00001007684 and EGAD00001007851), (2) myeloproliferative neoplasm colonies (WGS accession number EGAD00001007714), (3) fetal hematopoiesis colonies (WGS accession number EGAD00001006162), (4) lymphoid colonies (WGS accession number EGAD00001008107), (5) chronic myeloid leukemia colonies (WGS accession number EGAD00001015353), (6) allogeneic stem cell transplant (WGS accession number EGAD00001010872) and (7) single individual with therapy-related acute myeloid leukemia (WGS accession number EGAD00001015339). Source code for figures has been provided as Supplementary Information. Source data are provided with this paper.
Code for analyses is available online at 10.5281/zenodo.10891332 ref. 71 and can also be found at https://github.com/nangalialab/UKBB_ClonalHaem_Novel_Drivers as well as at https://github.com/mspencerchapman/Pervasive_positive_selection_in_blood.