Abstract
Salmonella enterica is comprised of genetically distinct ‘serovars’ that together provide an intriguing model for exploring the genetic basis of pathogen evolution. Although the genomes of numerous Salmonella isolates with broad variations in host range and human disease manifestations have been sequenced, the functional links between genetic and phenotypic differences among these serovars remain poorly understood. Here, we conduct high-throughput functional genomics on both generalist (Typhimurium) and human-restricted (Typhi and Paratyphi A) Salmonella at unprecedented scale in the study of this enteric pathogen. Using a comprehensive systems biology approach, we identify gene networks with serovar-specific fitness effects across 25 host-associated stresses encountered at key stages of human infection. By experimentally perturbing these networks, we characterize previously undescribed pseudogenes in human-adapted Salmonella. Overall, this work highlights specific vulnerabilities encoded within human-restricted Salmonella that are linked to the degradation of their genomes, shedding light into the evolution of this enteric pathogen.
Subject terms: Microbial genetics, Microbial genetics
Random barcoded transposon sequencing screens of generalist and typhoidal Salmonella determine the fitness effects of genes in a range of stress conditions and during macrophage infection, characterizing unknown genes and identifying typhoidal-specific vulnerabilities.
Main
Salmonella enterica—an enteric human pathogen causing 100 million infections and 200,000 deaths annually1–3—is comprised of distinct strains, or ‘serovars’, with varied host ranges and disease presentations. Nontyphoidal serovars like S. enterica serovar Typhimurium (S. Typhimurium), are generalists with broad host range causing self-limiting gastroenteritis in humans1,4, while typhoidal Salmonella, including S. Typhi and S. Paratyphi A, are human-restricted and induce enteric fever4–6—a severe systemic infection (Fig. 1a). Despite decades of study, the molecular mechanisms governing host range and disease differences among these serovars remain largely unclear.
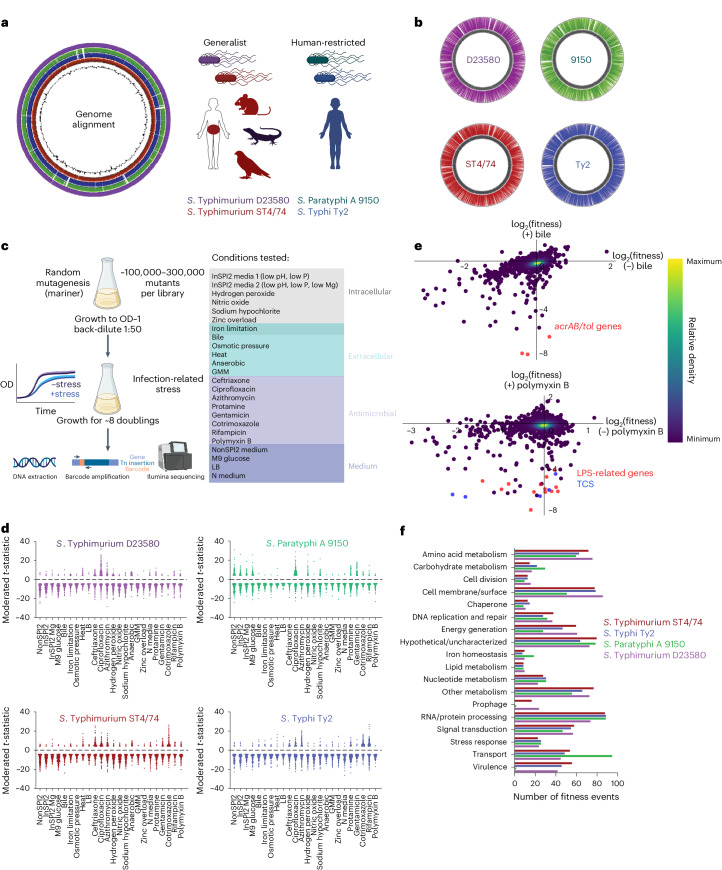
Fig. 1. Construction and validation of Rb-Tn-seq libraries in four serovars.
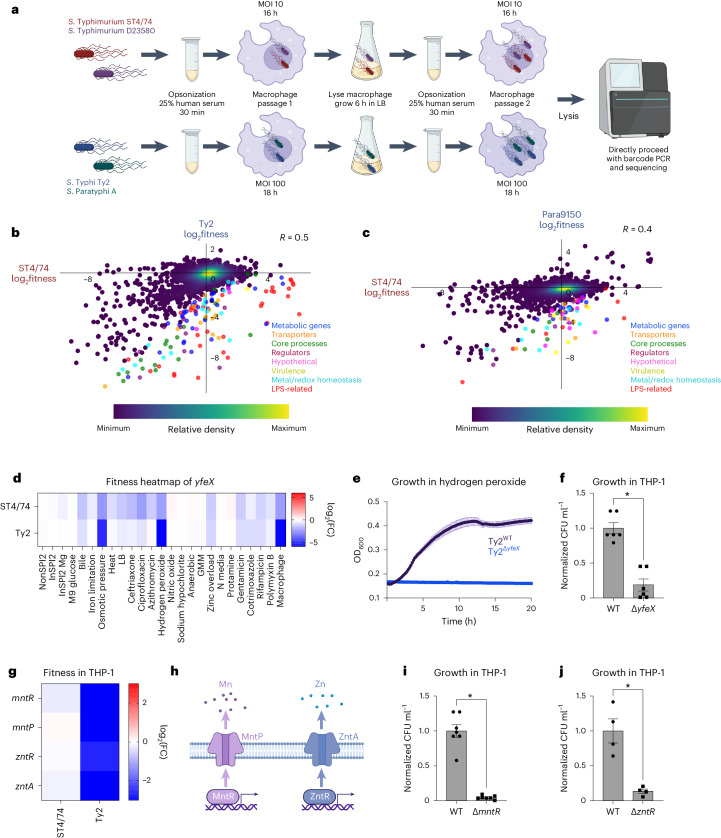
a, Left, genome alignments of S. Typhimurium ST4/74, S. Typhi Ty2, S. Paratyphi A 9150 and S. Typhimurium D23580. GC skew is indicated by the internal black trace. Right, schematic displaying the host range of generalist and human-restricted Salmonella. b, Location of all barcoded transposon insertions in each genome, as indicated by the colored lines on the outside of the gray circle (representing the chromosome). c, Schematic of Rb-Tn-seq workflow. Left, general growth scheme for all Rb-Tn-seq experiments. Right, 24 plate-based in vitro conditions tested, sorted by the type of stressor. Iron limitation can be encountered both intracellularly and extracellularly and is depicted in the overlap region. d, Plots showing all significant fitness changes (|t| > 4) for each isolate, across all 24 conditions. Red, S. Typhimurium ST4/74; blue, S. Typhi Ty2; green, S. Paratyphi A 9150; purple, S. Typhimurium D23580. Data are combined from two biologically independent Rb-Tn-seq replicates. e, Top, correlation of gene fitness changes between (−) bile (LB only) and (+) bile (LB + 4% ox bile) in S. Typhi Ty2, with the acrAB/tol genes highlighted in red and all other genes shown in purple. Bottom, correlation of gene fitness changes between (−) polymyxin B and (+) polymyxin B in S. Typhimurium D23580, with LPS modification genes in red (including arn operon) and pmrABD genes highlighted in blue; all other genes shown in purple. For both panels in e, data are derived from n = 2 biologically independent Rb-Tn-seq replicates and shown as a density plot, where colors range from dark purple (low) to yellow (high), representing the kernel density estimation from low to high density. f, Functional classification of genes with significant fitness changes for each serovar, based on available GO terms and manual classifications. In a, b and f, S. Typhimurium ST4/74 is in red, S. Typhi Ty2 is in blue, S. Paratyphi A 9150 is in green, and S. Typhimurium D23580 is in purple. TCS, two-component system. Created with Biorender.com.
Genomic analyses highlight notable genetic variation and serovar-specific genes in typhoidal Salmonella4,7–12, particularly within Salmonella pathogenicity islands (SPIs). For example, SPI-7 in S. Typhi encodes the Vi capsule, which inhibits complement binding13, dampens immune responses14 and prevents uptake by neutrophils15. SPI-11 of S. Typhi encodes the typhoid toxin16—a typhoid-specific virulence factor inducing DNA damage in host cells17,18. Importantly, typhoidal serovars harbor hundreds of pseudogenes, some of which are involved in intestinal colonization when functional1,19–21, indicating genomic decay in human-restricted Salmonella during its evolution to an extraintestinal pathogen7.
Despite abundant bioinformatic data, our understanding of how genotypic differences among Salmonella isolates correlate with phenotypic variations remains limited. Bioinformatic approaches have identified hundreds of typhoid-specific genes, but many lack known physiological functions1,4. Moreover, although human-restricted Salmonella encode hundreds of pseudogenes7,11,12, the functional consequences of pseudogene accumulation have not been studied systematically. Bioinformatics also cannot easily determine whether genes conserved across Salmonella serovars have distinct functions in different isolates. Thus, more detailed functional studies are needed to better characterize the impact of genetic variation across Salmonella.
Transposon sequencing (Tn-seq) is a powerful method linking genotype to phenotype, extensively contributing to Salmonella biology by uncovering genes crucial for survival under various infection-related stresses22–33. However, most Salmonella Tn-seq studies have focused on generalist serovars like S. Typhimurium, while human-adapted strains remain severely understudied. Moreover, Tn-seq is costly and low-throughput, limiting the number of conditions assayed in each study. Recent advances in random barcoded Tn-seq (Rb-Tn-seq) overcome this limitation by enabling high-throughput assessment of microbial fitness34–36, but Rb-Tn-seq has not been applied systematically to interrogate Salmonella virulence and evolution.
Here, we employ Rb-Tn-seq to explore genotypic and phenotypic differences among generalist and human-restricted Salmonella serovars. We capture thousands of significant fitness events across 25 host-associated stresses encountered at key stages of Salmonella infection within humans. We use a systems biology approach to identify serovar-specific changes in fitness within gene networks, including those involved in lipopolysaccharide (LPS) modification, amino acid metabolism and metal homeostasis. We perturb these networks experimentally to identify specific pseudogenes, including several previously undescribed pseudogenes, contributing to typhoidal-specific fitness effects. Overall, our results provide a comprehensive functional perspective on how genetic differences between generalist and host-restricted Salmonella have influenced the evolution of this enteric pathogen.
Results
Set-up of Rb-Tn-seq experiments
We constructed Rb-Tn-seq libraries to study Salmonella stress response37 in four phylogenetically distinct and genetically tractable serovars. These included two generalist isolates (S. Typhimurium ST4/74 and D23850) and two human-restricted isolates (S. Typhi Ty2 and S. Paratyphi A 9150). On average, each library contained 166,905 unique genome-wide transposon insertion sites which integrated every 27.7 bp. The median insertions per gene ranged from 12 to 44 across these serovars, with central Tn insertions in ~90–92% of coding genes. Barcoded transposons showed even distribution across chromosomes and plasmids, ensuring high genome coverage with minimal strand or coverage bias (Fig. 1b, Supplementary Fig. 1a–e and Supplementary Table 4). The insertion index of each gene was calculated to identify 427 to 476 putative essential genes for each serovar (Supplementary Data 1 and Supplementary Fig. 2a–d), several of which were serovar-specific, including igaA in Typhimurium ST4/74 and D23580, rpoE in S. Typhi Ty2 and various iron homeostasis genes in Paratyphi A (Supplementary Fig. 2e and Supplementary Note 1)38.
We conducted fitness assays on each library, evaluating their response to (1) extracellular stresses encountered in the intestinal tract and/or systemic tissues, (2) intracellular stresses within host cells, including macrophages and (3) exposure to a diverse suite of antibiotics (Fig. 1c and Supplementary Table 5). Stressor concentrations were optimized to achieve ~30–50% growth reduction38 (Fig. 1c and Supplementary Fig. 3a–d). Each experiment included biological duplicates, which were tightly correlated and passed published quality-control metrics36 (Supplementary Fig. 3e–h and Supplementary Data 2).
We used a moderated t-like statistic with |t| > 4 to identify significant fitness events36, leading to the identification of hundreds of genes with significant fitness effects for each serovar across our conditions (Fig. 1d and Supplementary Data 3 and 4). We then used agglomerative clustering to generate heatmaps with all genes with |t| > 4 in at least 1 condition, retaining 678 to 781 genes for each serovar (Supplementary Figs. 4–7). These clustered heatmaps revealed patterns linking similar conditions, including grouping intracellular stresses (for example, InSPI2, InSPI2 Mg, H2O2, NO, bleach), extracellular stresses (for example bile, heat stress, anaerobiosis, gut microbiota media (GMM)) and another grouping linking various antibiotics (for example, ciprofloxacin, azithromycin, rifampicin).
We identified many functionally related gene clusters involved in stress response (Supplementary Data 5). For instance, acrAB/tolC Tn insertions displayed significant fitness defects during bile stress for typhoidal Salmonella (Fig. 1e)39,40. LPS modification arn operon and pmrAB mutations exhibited reduced fitness under polymyxin B in Typhimurium D23580 (Fig. 1e and Supplementary Fig. 8a)41–44. Mutations in DNA repair genes (for example recDGNQX) led to decreased D23580 fitness with ciprofloxacin—an antibiotic inducing DNA damage (Supplementary Fig. 8b)45,46. Tn insertions in iron homeostasis genes (for example, entDEF, exbD, tonB) caused decreased fitness under iron restriction in Paratyphi A 9150 (Supplementary Fig. 8c). Tn insertions in molybdenum (Mo) metabolism genes (for example moeA, moaA, mog, mobA) showed fitness defects in GMM in several serovars (Supplementary Fig. 8d)47,48. LPS-synthesizing gene mutations (for example, rfaL, rfbBD, wzyO4, waaK) increased sensitivity to both intracellular and extracellular stresses, underscoring the broad role of LPS during bacterial stress response49,50 (Supplementary Fig. 8e). Intriguingly, mutations in barA and sirA exhibited increased fitness under multiple stresses (Supplementary Fig. 8f), possibly explaining their frequent occurrence in chronically infected Salmonella patients51.
Several SPI-encoded genes displayed significant phenotypes (Supplementary Data 6)4. For instance, mutations in hilD—an SPI-1 encoded transcription factor—increased fitness during bile stress in S. Typhi Ty2 and heat shock in S. Typhimurium D23580 (Supplementary Data 6), aligning with a study proposing a role for HilD role in enhancing membrane permeability52. SPI-3-encoded magnesium importers mgtB and mgtC mutations decreased S. Paratyphi A 9150 survival in macrophage-mimicking media InSPI2 Mg (Supplementary Data 6). Mutations in SPI-7-encoded Vi capsule genes in Typhi increased fitness under protamine stress—a positively charged antimicrobial peptide (Supplementary Data 6). Despite Typhi and Paratyphi A encoding unique genes not found in Typhimurium genomes, only a small proportion of these unique genes exhibited significant fitness effects (3.9% in Typhi, 3.2% in Paratyphi A) (Supplementary Data 7). In contrast, a higher proportion of shared orthologs had significant phenotypes in Typhi (18.5%) and Paratyphi A (16.5%) (Supplementary Table 6). To highlight specific processes involved in Salmonella stress response, we sorted significant fitness events by annotated gene ontology (GO) terms and BioCyc-derived functional annotations, grouped into various functional classes (Fig. 1f). Dozens of fitness events involved uncharacterized genes, suggesting that our Rb-Tn-seq dataset holds rich uncharacterized biology (Fig. 1f).
Systems biology approach to analyze fitness profiles
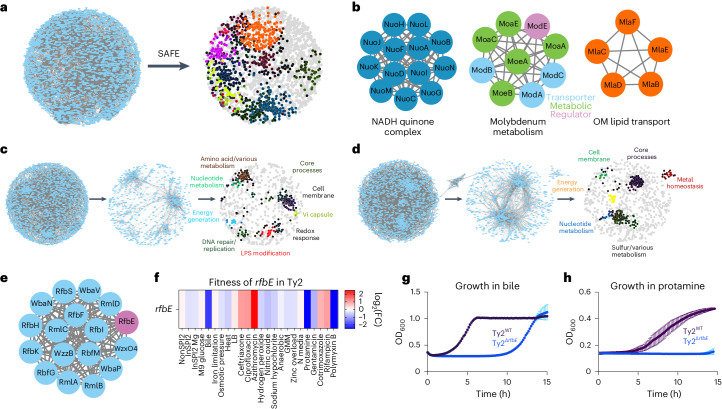
To systematically analyze the thousands of fitness effects captured through Rb-Tn-seq, we employed cofitness network analysis and spatial analysis of functional enrichment (SAFE) to overlay functional data onto network maps38, which has been performed previously in Saccharomyces cerevisiae53 and Streptococcus pneumoniae38. Briefly, we constructed correlation matrices reflecting the log2 fitness changes for each gene across all conditions. These matrices were transformed into cofitness interaction networks, where nodes represented genes and edges indicated correlation values, using a Pearson’s correlation of R > 0.75 to identify closely related fitness profiles (Fig. 2a and Supplementary Data 8). Stability testing38 indicated high significance across our networks (Supplementary Data 8).
Fig. 2. Cofitness network analysis and SAFE identify serovar-specific gene fitness changes.
a, Left, cofitness network analysis of all genes in S. Typhimurium ST4/74 with r > 0.75; all blue nodes are genes and all gray lines connect pairs of cofit (r > 0.75) genes. Right, SAFE highlights regions of the network that are enriched in certain functional terms. Each colored area represents a different functionally enriched area on the network. b, Examples of subclusters of genes that are identified through SAFE. c,d, Left, cofitness network analysis of all genes in S. Typhi Ty2 (c) and S. Paratyphi A 9150 (d) with r > 0.75; all blue nodes are genes and all gray lines are between pairs of cofit genes. Middle, filtered network that only includes genes with fitness changes that are (1) significant in S. Typhi/S. Paratyphi A but not in S. Typhimurium and (2) > 2-FC in S. Typhi/S. Paratyphi A compared with S. Typhimurium. Right, SAFE highlights regions of the network that are enriched in certain functional terms on these filtered maps. e, An LPS modification cluster including rfbE (purple) is identified through SAFE on the S. Typhi filtered map. f, Heatmap showing the fitness values of Tn insertions in rfbE across 24 plate-based stresses for S. Typhi Ty2. Color gradient is derived from the log2(FC) from each condition in the Rb-Tn-seq experiments. g, Growth curves of Ty2WT (black) and Ty2ΔrfbE (blue) when exposed to 4% bile, with reads taken at OD600 once every 10 min. h, Growth curves of Ty2WT (black) and Ty2ΔrfbE (blue) when exposed to 2.3 µg ml−1 protamine, with reads taken at OD600 once every 10 min. For growth curve experiments (g,h), each point and error bar indicates the mean ± s.e.m. of OD600, derived from n = 4 (g) and n = 3 (h) biologically independent experiments.
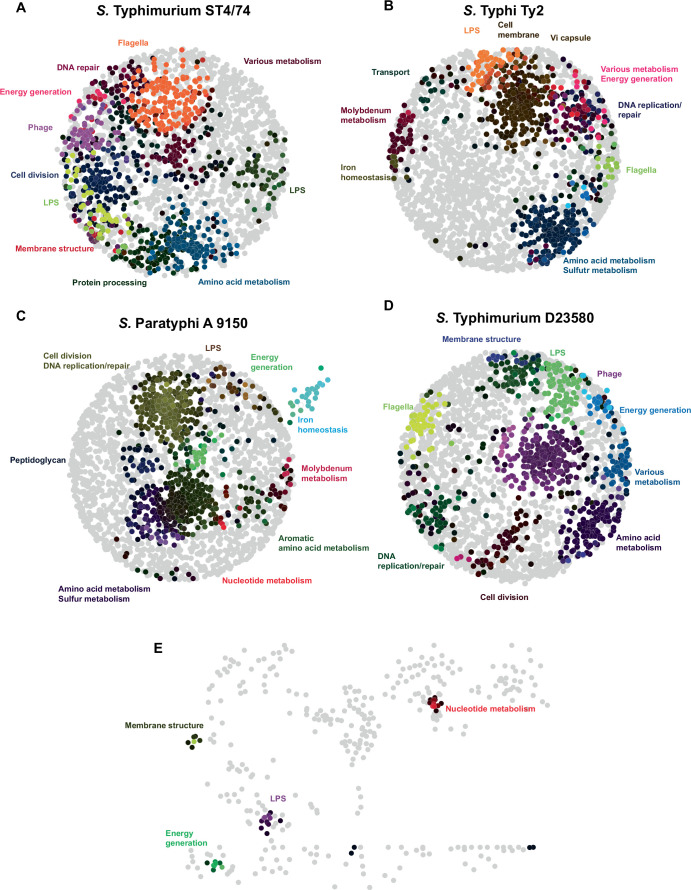
SAFE was then applied to annotate each node based on BioCyc classifications and GO terms (Supplementary Data 9)54, through which we identified local network neighborhoods enriched for specific functional classes38,55 (Fig. 2a and Extended Data Fig. 1a–d). To validate this analysis, we searched SAFE outputs for gene networks expected to cluster together based on related functionality, finding clusters for the NADH quinone oxidoreductase complex, molybdenum metabolism genes and genes associated with lipid trafficking to the outer membrane (Fig. 2b). Intriguingly, ~15–20% submodules contain at least one hypothetical gene, including RS16480/STM_3341 in ST4/74, which is correlated strongly with cpxR, and RS03310/t0654 in Typhi Ty2, which is correlated strongly with 24 amino acid metabolism genes (Supplementary Data 10 and Supplementary Note 2)56.
Extended Data Fig. 1. Co-fitness and SAFE network analysis across four Salmonella isolates.
a–d) Co-fitness network analysis using r > |0.75| and SAFE for S. Typhimurium ST4/74 (A), S. Typhi Ty2 (B), S. Paratyphi A 9150 (C) and S. Typhimurium D23580 (D), with regions enriched in certain functional attributes highlighted in different colors, as indicated on the maps. E) Filtered network for S. Typhimurium D23580, which includes genes with fitness changes that are 1) significant (|t| > 4) in S. Typhimurium D23580 but not in S. Typhimurium ST4/74, and 2) >|2-fold| higher fitness change in S. Typhimurium D23580 compared to S. Typhimurium ST4/74. Colors indicate areas on these filtered maps that are enriched in certain functional terms through SAFE.
We used the cofitness network pipeline to pinpoint typhoid-specific fitness changes. To this end, we applied an additional filtering step where, for each condition, we only retained genes that (1) had a significant fitness change (|t| > 4) in Typhi Ty2 or Paratyphi A 9150 but not in Typhimurium ST4/74 and (2) had a fold change (FC) that was at least twofold greater in Typhi or Paratyphi compared with Typhimurium (Fig. 2c,d and Supplementary Data 11–12). These filters removed ~75% and 65% of the nodes and connections within each cofitness network for Typhi and Paratyphi, respectively, but still retained thousands of serovar-specific correlations. We then applied SAFE to these filtered networks and identified gene clusters with serovar-specific fitness changes (Fig. 2c,d), including those involved in LPS modification, amino acid metabolism and metal homeostasis. In contrast, only ~3% of the network was retained in D23580 when doing this same analysis, indicating similar fitness profiles between these Typhimurium isolates (Extended Data Fig. 1e and Supplementary Data 13).
We then examined several gene networks with serovar-specific changes in typhoidal Salmonella, focusing on three categories for further mechanistic investigation. The first includes clusters with genes unique to typhoidal Salmonella, including rfbE (Fig. 2). The second comprises gene clusters where all genes are shared between nontyphoidal and typhoidal Salmonella but exhibit different phenotypes in distinct isolates (for example, metIQN in Fig. 3). The third involves gene clusters containing at least one uncharacterized gene in Salmonella (for example, ybdZ in Fig. 4).
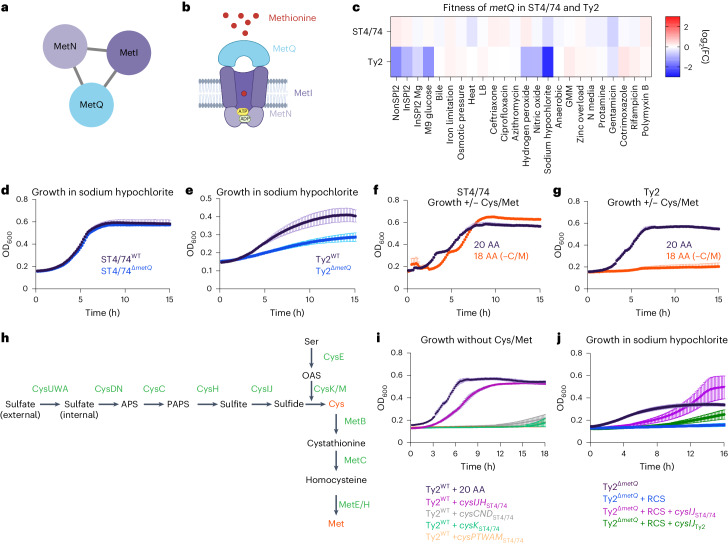
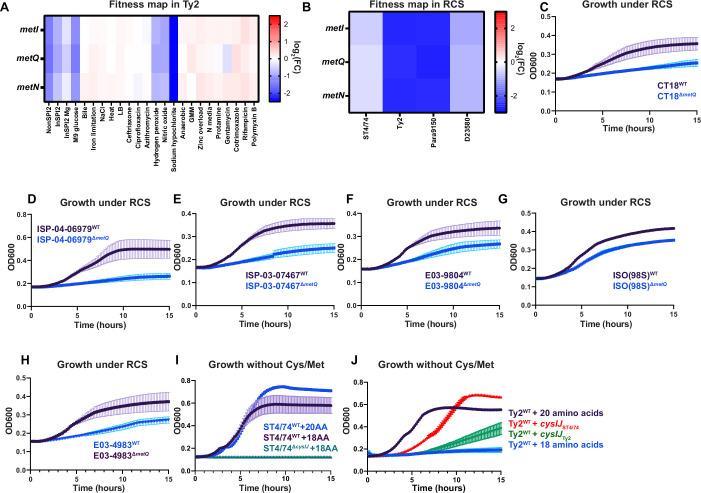
Fig. 3. metIQN has typhoid-specific fitness defects during RCS.
a, metIQN cluster, derived from the S. Typhi Ty2 filtered network. b, Schematic of MetIQN. c, Heatmap showing fitness of metQ Tn insertions across 24 conditions for S. Typhimurium ST4/74 and S. Typhi Ty2. Color gradient is derived from the log2(FC) from Rb-Tn-seq. d, Growth of ST4/74WT (black) and ST4/74ΔmetQ (blue) exposed to 12.5 µg ml−1 sodium hypochlorite, derived from n = 3 biologically independent experiments. e, Growth of Ty2WT (black) and Ty2ΔmetQ (blue) exposed to 12.5 µg ml−1 sodium hypochlorite, derived from n = 3 biologically independent experiments. f,g, Growth of ST4/74WT (f) and Ty2WT(g) when grown in defined minimal medium with a mix of all 20 amino acids added (20 AA; purple), or with 18 amino acids added, without Cys/Met (18AA (−C/M); orange), derived from n = 3 biologically independent experiments. h, Schematic of endogenous synthesis pathway for Cys/Met. Intermediate substrates are shown in black and enzymes are shown in green. Cys/Met are shown in red. i, Growth of WT and complemented strains in which different operons in the endogenous Cys/Met pathway from S. Typhimurium ST4/74 are expressed in S. Typhi Ty2. As a control, Ty2WT was grown in minimal medium supplemented with 20 AA and is shown in black (n = 3 biologically independent experiments). All complementation growth curves were run in minimal medium supplemented with 18AA, but no Cys/Met (−C/M), and are derived from n = 4 biologically independent experiments; magenta, CysIJHST4/74; gray, CysCNDST4/74; green, CysKST4/74; orange, CysAWUMST4/74. j, Growth curves of WT, mutant and complementation strains when exposed to a lethal dose of 25 µg ml−1 sodium hypochlorite. Black, Ty2ΔmetQ growth in the absence of RCS; blue, Ty2ΔmetQ growth with RCS; purple, growth of Ty2ΔmetQ + cysIJST4/74 in the presence of RCS; green, growth of Ty2ΔmetQ + cysIJTy2. All curves are derived from n = 5 biologically independent experiments. For all growth curves (d–g,i,j), each point and error bar indicates the mean ± s.e.m. of OD600 with reads taken at OD600 once every 10 min. APS, adenosine 5′-phosphosulfate; OAS, O-acetyl-Ser; PAPS, 3′-phosphoadenosine 5′-phosphosulfate. Created with Biorender.com.
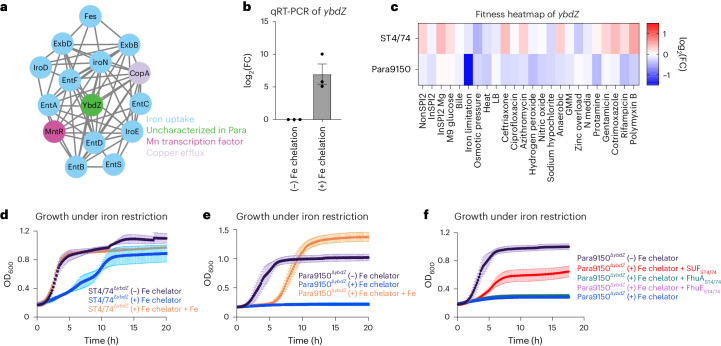
Fig. 4. A ybdZ-containing gene cluster with paratyphoid-specific fitness effect.
a, Cluster of genes involved in metal homeostasis, identified through SAFE on the S. Paratyphi A 9150 filtered network. b, Transcript levels of ybdZ measured by qRT-PCR and normalized to a control gene (rpoD). Bars indicate mean ± s.e.m., with individual measurements shown (black dots). Each bar is derived from n = 3 biologically independent experiments. c, Heatmap showing the fitness values of Tn insertions in ybdZ across 24 stress conditions for S. Typhimurium ST4/74 and S. Paratyphi A 9150. Color gradient is derived from the log2(FC) from each condition in the Rb-Tn-seq experiments. d, Growth curves of ST4/74WT (black) and ST4/74ΔybdZ (blue) when exposed to 100 µM 2,2′-dipyridyl, +/− addition of exogenous 1 mM FeCl3 (orange), with reads taken at OD600 once every 10 min, derived from n = 3 biologically independent experiments. e, Growth curves of Para9150WT (black) and Para9150ΔybdZ (blue) when exposed to 100 µM 2,2′-dipyridyl, +/− addition of exogenous 1 mM FeCl3 (orange), with reads taken at OD600 once every 10 min, derived from n = 3 biologically independent experiments. f, Growth curves of Para9150ΔybdZ and complementation strains in which functional versions of different iron-related pseudogenes from S. Typhimurium ST4/74 are expressed in Para9150ΔybdZ under iron restriction, with reads taken at OD600 once every 10 min; SUFST4/74 is shown in red, FhuAST4/74 is shown in green, and FhuEST4/74 is shown in purple. For controls, the growth of Para9150ΔybdZ without iron restriction (−100 µM 2,2′-dipyridyl) is shown in black, while the growth of Para9150ΔybdZ under iron restriction (+100 µM 2,2′-dipyridyl) is shown in blue. Each point and error bar indicates the mean ± s.e.m. of OD600, where n = 3 biologically independent experiments for all curves except SUFST4/74 in red, which was derived from n = 7 biologically independent experiments.
We were intrigued by an LPS modification gene cluster containing rfbE (Fig. 2e), a typhoid-specific gene that modifies the O-antigen terminal sugar to tyvelose56,57. While tyvelose is used for serotyping, its biological function in typhoidal Salmonella remains unclear. Tn insertions in rfbE sensitize typhoidal Salmonella to bile acids, protamine and polymyxin B (Fig. 2f and Supplementary Fig. 9). To validate these results, we deleted rfbE in S. Typhi Ty2 and found that Ty2ΔrbfE exhibited decreased survival in response to bile acids (Fig. 2g) and protamine (Fig. 2h) compared with Ty2WT, suggesting that this unique tyvelose moiety protects Typhi from membrane-insulting stresses and antibiotics.
A typhoid-specific phenotype for metQ during reactive chlorine stress
metIQN encodes the main methionine transporter in Salmonella and was identified as a gene cluster with typhoid-specific fitness effects by SAFE58 (Fig. 3a,b). Tn insertions in metIQN rendered both S. Typhi Ty2 and S. Paratyphi A highly susceptible (~sevenfold) to reactive chlorine stress (RCS) but did not sensitize S. Typhimurium ST4/74 (Fig. 3c and Extended Data Fig. 2a,b). To confirm these Rb-Tn-seq results, we deleted metQ in both S. Typhi Ty2 and S. Typhimurium ST4/74 and found that Ty2ΔmetQ had a more severe growth defect under RCS than ST4/74ΔmetQ (Fig. 3d,e). Deleting metQ in six S. Typhi clinical isolates, including CT18 and multidrug resistance strains belonging to the H58 lineage (ISP-04-06979, ISP-03-07467, E03-9804, ISO(98S) and E03-4983)59, also increased RCS sensitivity (Extended Data Fig. 2c–h).
Extended Data Fig. 2. Tn insertions in metIQN lead to a typhoid-specific fitness effect during RCS.
a) Heatmap showing the fitness values of Tn insertions in metIQN across 24 stress conditions for S. Typhi Ty2. Color gradient is derived from the log2(fitness) from each condition in the Rb-Tn-seq experiments. b) Heatmap showing the fitness values of Tn insertions in metIQN during sodium hypochlorite stress (RCS) for all isolates. Color gradient is derived from the log2(fitness) from each condition in the Rb-Tn-seq experiments. c–h): Representative growth curves of CT18 (C), ISP-04-06979 (D), ISP-03-07467 (E), E03-9804 (F), ISO(98 S) (G), E03-4983 (H), with WT (black) and ΔmetQ mutants (blue) in the presence of 12.5 µg/mL sodium hypochlorite, with reads taken at OD600 once every 10 minutes, each derived from n = 3 biologically independent experiments. i) Growth curves of WT and mutant strains of Typhimurium ST4/74, with reads taken at OD600 once every 10 minutes, derived from n = 3 biologically independent experiments. ST4/74WT growth in minimal media supplemented with all 20 amino acids is shown in blue, and ST4/74WT growth in minimal media supplemented with 18 amino acids lacking Cys/Met is shown in black. ST4/74ΔcysIJ growth in minimal media supplemented with 18 amino acids lacking Cys/Met is shown in green. j) Growth curves of WT and complementation strains in which the cysIJ locus from both S. Typhimurium ST4/74 (red) and S. Typhi Ty2 (green) is expressed in the WT S. Typhi Ty2 background, with reads taken at OD600 once every 10 minutes, derived from n = 4 biologically independent experiments. Each complementation growth experiment was run in minimal media supplemented with 18 amino acids, but no Cys/Met. For controls, Ty2WT grown in minimal media supplemented with all 20 amino acids is shown in black, while Ty2WT grown in minimal media supplemented with 18 amino acids without Cys/Met is shown in blue. For these curves, reads were taken at OD600 once every 10 minutes, and each point and error bar indicates the mean ± SEM of OD600.
RCS kills bacteria by oxidizing cysteine (Cys) and methionine (Met)60. Salmonella replenishes these sulfated amino acids by either importing them via dedicated transporters like MetIQN or through de novo synthesis58. Given the increased susceptibility of Ty2ΔmetQ to RCS, we reasoned that endogenous Cys/Met in Typhi may be impaired. To test this hypothesis, we cultured both S. Typhimurium ST4/74 and S. Typhi Ty2 in defined minimal medium. Both strains grew when all 20 amino acids (AA) were added (Fig. 3f,g). However, in medium lacking Cys/Met (18AA −Cys −Met), ST4/74 continued to grow, while Ty2 failed to grow (Fig. 3f,g), suggesting that S. Typhi cannot synthesize Cys/Met endogenously.
To pinpoint the compromised part of the Cys/Met synthesis pathway in S. Typhi (Fig. 3h), we expressed plasmid-borne functional versions of each operon in this pathway (cysIJH, cysCND, cysK, cysPTWAM) from S. Typhimurium in S. Typhi Ty2. Only cysIJH from ST4/74 restored Ty2 growth in the 18AA medium lacking Cys/Met (Fig. 3i). Furthermore, deleting cysIJ in ST4/74 ablated growth of this isolate in medium without Cys/Met (Extended Data Fig. 2i), emphasizing the importance of CysI/J in both nontyphoidal and typhoidal Salmonella under Cys/Met limitation. Importantly, cysIJST4/74 expression rescued Ty2ΔmetQ growth under a lethal dose of RCS (Fig. 3j). In contrast, expressing cysIJ from Ty2 only partially rescued S. Typhi growth in Cys/Met-deficient media (Extended Data Fig. 2j), and weakly in Ty2ΔmetQ under lethal RCS exposure (Fig. 3j), further suggesting that CysIJTy2 function is impaired. Collectively, these findings indicate the increased sensitivity of Ty2ΔmetQ to RCS is driven by defects in endogenous Cys/Met synthesis.
A serovar-specific phenotype for ybdZ under iron restriction
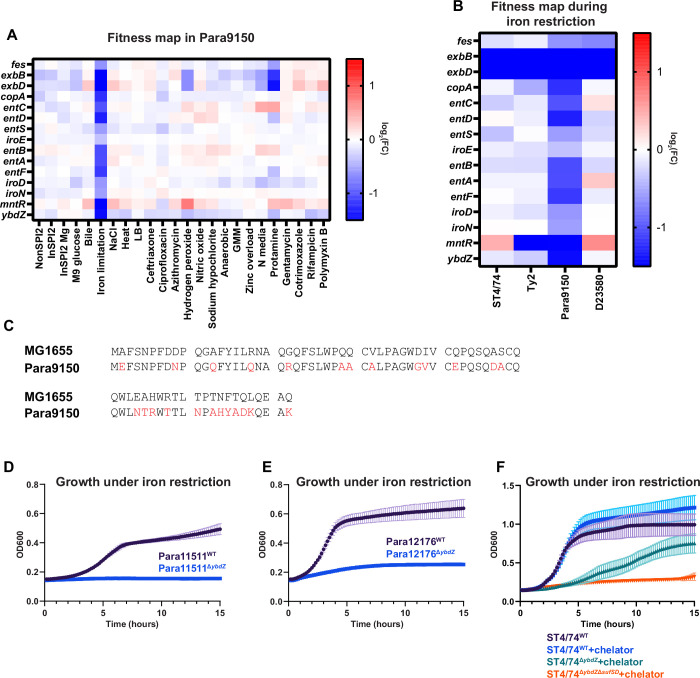
We identified a paratyphoid-specific gene network displaying fitness defects, primarily consisting of iron-related genes and featuring an unannotated gene RS10805, sharing ~60% identity with ybdZ in Escherichia coli (Fig. 4a and Extended Data Fig. 3a–c), which enhances enterobactin production61. Quantitative real-time (qRT)-PCR showed an increase in ybdZ expression of ~200-fold during iron restriction in S. Paratyphi A (Fig. 4b), consistent with a role during iron limitation in Salmonella. Tn insertions in ybdZ caused a more pronounced fitness defect under iron limitation in S. Paratyphi A than in S. Typhimurium (Fig. 4c). To confirm this Rb-Tn-seq result, we deleted ybdZ in both S. Paratyphi A 9150 and S. Typhimurium ST4/74 and observed that Para9150ΔybdZ had a stronger growth defect under iron limitation than ST4/74ΔybdZ (Fig. 4d,e). Deleting ybdZ in other Paratyphi A strains (Para11511 and Para12176) also led to heightened sensitivity to iron restriction, and exogenous iron rescued these phenotypes (Extended Data Fig. 3d,e).
Extended Data Fig. 3. Tn insertions in ybdZ lead to a paratyphoid-specific fitness defect during iron limitation.
a) Heatmap of genes that comprise the sub-network of LPS-synthesis genes in Paratyphi A 9150 shown in Fig. 4a. Color gradient is derived from the log2(fitness) from each condition in the Rb-Tn-seq experiments. All fitness values on these heatmaps were generated from the average of two biologically independent biological replicates. b) Heatmap showing the fitness values of Tn insertions in iron homeostasis genes during iron restriction for all isolates. Color gradient is derived from the log2(fitness) from each condition in the Rb-Tn-seq experiments. c) Alignment of ybdZ from E. coli MG1655 and S. Paratyphi A 9150. Residues that are different in S. Paratyphi A are highlighted in red. d-e) Growth curves of S. Paratyphi A 11511 (D) and S. Paratyphi A 12176 (E) with WT (black) and ΔybdZ mutants (blue) in the presence of 100 µM 2,2′-dipyridyl, with reads taken at OD600 once every 10 minutes, derived from n = 3 biologically independent experiments. (f) Growth curves of WT and mutant strains in Typhimurium ST4/74. ST4/74WT growth in LB without iron restriction is shown in black (n = 3 biologically independent experiments), and ST4/74WT growth under iron restriction (100 µM 2,2′-dipyridyl) is shown in blue (n = 4 biologically independent experiments). ST4/74ΔybdZ growth in LB supplemented with 100 µM 2,2′-dipyridyl is shown in green (n = 6 biologically independent experiments). ST4/74ΔybdZsufSD growth in LB supplemented with 100 µM 2,2′-dipyridyl is shown in orange (n = 4 biologically independent experiments). For these curves, reads were taken at OD600 once every 10 minutes, and each point and error bar indicates the mean ± SEM of OD600.
To understand why Para9150ΔybdZ exhibits a more severe growth defect under iron limitation than ST4/74ΔybdZ, we investigated the impact of pseudogenes in typhoidal Salmonella involved in iron acquisition, including fhuA, fhuE, sufD and sufS7. SufS/D are components of the SUF complex, one of two multiprotein complexes that synthesizes iron-sulfur clusters. In E. coli, SUF contributes to survival during iron restriction62–64. Strikingly, expressing functional versions of these genes from S. Typhimurium in Para9150ΔybdZ revealed that only SUFST4/74 expression restored growth during iron restriction (Fig. 4f), suggesting that functional SUF is sufficient to rescue this growth defect. To determine whether SUF is also necessary for survival under iron limitation in a ΔybdZ mutant background, we constructed a double deletion mutant of ybdZ and sufSD (ΔybdZΔsufSD) in Typhimurium ST4/74 and found that it was highly sensitive to iron restriction (Extended Data Fig. 3f), confirming that SUF contributes to survival under iron restriction in both nontyphoidal and typhoidal Salmonella. Notably, sufS and/or sufD are pseudogenes in all deposited Paratyphi A sequences on BioCyc, indicating the increased sensitivity of Paratyphi A to iron restriction is likely a general feature of Paratyphi A.
Serovar-specific fitness during macrophage infection
We aimed to identify serovar-specific phenotypes in a host-associated setting. Salmonella replication within mammalian macrophages is a key feature of this pathogen65–67. Despite previous Tn-seq studies examining Salmonella fitness in macrophages27–29, a systematic comparison of intracellular fitness profiles between generalist and human-restricted Salmonella within human macrophages is lacking. To address this gap, we performed Rb-Tn-seq on human THP-1 macrophages infected with our four barcoded libraries, using a multiplicity of infection and infection duration favoring Salmonella replication while minimizing host cell death (Fig. 5a and Supplementary Fig. 10a–g). Two macrophage passages were conducted to enrich subtle phenotypes (Fig. 5a). Consistent with published work, the absence of Vi capsule increased S. Typhi uptake into macrophages68 (Supplementary Fig. 10h); thus, we performed the Typhi Rb-Tn-seq in a ΔVi capsule background to increase intracellular bacteria.
Fig. 5. Serovar-specific fitness changes during macrophage infection.
a, Schematic of macrophage infections, showing opsonization, cell infection, lysis and passaging. b,c, Pearson’s correlation (R) of gene fitness changes between the S. Typhimurium ST4/74 and S. Typhi Ty2 (b) or S. Paratyphi A 9150 (c) macrophages Rb-Tn-seq experiments, with serovar-specific changes in fitness colored according to functional class. Blue, metabolic genes; orange, transporters; green, core process genes (for example, replication, transcription, translation); burgundy, regulators; pink, hypothetical genes; gold, virulence genes; light blue, metal/redox homeostasis genes; red, LPS-related genes; dark purple, all other genes. Data are shown as a density scatterplot, where colors range from dark purple (low) to yellow (high), representing the kernel density estimation from low to high density. d, Heatmap showing the fitness values of Tn insertions in yfeX across 24 stress conditions for S. Typhimurium ST4/74 and S. Typhi Ty2. Color gradient is derived from the log2(FC) from each condition in the Rb-Tn-seq experiments. e, Growth curves of Ty2WT (black) and Ty2ΔyfeX (blue) when exposed to 250 µM hydrogen peroxide, with reads taken at OD600 once every 10 min. Each point and error bar indicates the mean ± s.e.m. of OD600, derived from n = 3 biologically independent experiments. f, Normalized intracellular Ty2WT and Ty2ΔyfeX bacterial counts recovered after 5 h of infection of LPS-activated THP-1 cells (P = 4.03 × 10−5). g, Heatmap showing the fitness values of Tn insertions in metal homeostasis genes during THP-1 infection by S. Typhimurium ST4/74 and S. Typhi Ty2. Color gradient is derived from the log2(FC) from each condition in the Rb-Tn-seq experiments. h, Schematic of Mn and Zn homeostasis systems in Salmonella. i, Normalized intracellular Ty2WT and Ty2ΔmntR bacterial counts recovered after 5 h of infection in LPS-activated THP-1 cells (P = 2.05 × 10−7). j, Normalized intracellular Ty2WT and Ty2ΔzntR bacterial counts after 5 h of infection in LPS-activated THP-1 cells (P = 0.00259). For all macrophage CFU plots (f, i and j), bars indicate the mean ± s.e.m. of the normalized bacterial count recovered from macrophages, derived from n = 6 biologically independent experiments (f), n = 7 biologically independent experiments (i) and n = 4 biologically independent experiments (j). Significance was calculated using a two-tailed t-test; *P < 0.01. Created with Biorender.com.
We used agglomerative clustering to generate a heatmap from these macrophage experiments, retaining all genes with significant fitness effects (P < 0.05) in at least one serovar (Supplementary Fig. 11). This heatmap revealed that Typhi and Paratyphi A cluster together while Typhimurium ST4/74 and D23580 cluster together (Supplementary Fig. 11), suggesting that the genes involved in macrophage infection exhibit greater similarity within typhoidal strains and nontyphoidal isolates, respectively. This heatmap captured several SPI-related gene clusters (Supplementary Table 7). Tn insertions in SPI-2 genes displayed decreased fitness in S. Typhimurium ST4/74, D23580 and S. Typhi Ty2 (Supplementary Table 7 and Supplementary Fig. 12a), consistent with previous findings highlighting the critical role of SPI-2 in intracellular S. Typhimurium28,29,65,68 and S. Typhi proliferation69. Intriguingly, no significant fitness defects in SPI-2 genes were observed in S. Paratyphi A 9150. This might suggest that Paratyphi A does not rely solely on SPI-2 for intracellular survival69, or could reflect the low intracellular biosynthetic capacity of S. Paratyphi A70. Mutations in the Typhimurium-specific effectors sseK1 and sseK3 led to decreased fitness in ST4/74 and D23580 within macrophages (Supplementary Data 14). Mutations in the SPI-1 encoded sitABCD Fe/Mn import system decreased Typhi and Paratyphi fitness, suggesting high sensitivity of typhoidal Salmonella to intracellular perturbations in Fe/Mn pools (Supplementary Data 14). Similarly, mutations in the SPI-3 encoded Mg2+ importers mgtB and mgtC decreased S. Paratyphi A 9150 fitness during macrophage infection (Supplementary Data 14).
Beyond SPI-genes, Tn insertions in several two-component signaling genes (for example, phoPQ, envZ/ompR, arcAB) and redox-related genes (for example, trxA, trxB, sodA, oxyR) decreased fitness during macrophage infection (Supplementary Data 14). In contrast, increased fitness was observed with Tn insertions in fepE in S. Paratyphi A 9150 (Supplementary Data 14). The regulation of very long O-antigen chains by FepE in Paratyphi71,72 indicates a potential effect of this modified LPS structure during macrophage uptake, akin to the role of Vi capsule in reducing Typhi phagocytosis68. Tn insertions in many LPS genes increased fitness in S. Typhimurium ST4/74 and D23580, consistent with previous studies highlighting enhanced invasiveness of O-antigen deficient S. Typhimurium (Supplementary Fig. 12b and Supplementary Table 7)29. Conversely, mutations in these O-antigen genes decreased Typhi fitness, emphasizing the importance of the O-antigen layer in shielding typhoidal isolates from membrane-disrupting stresses. For example, Ty2ΔrfbE, lacking its O-antigen tyvelose moiety (Fig. 2f), exhibited reduced fitness within macrophages (Supplementary Fig. 13a). Intriguingly, Tn insertions in chemotaxis genes (for example, cheARWY, tar) increased fitness in all four isolates (Supplementary Fig. 12c and Supplementary Table 7).
To identify serovar-specific fitness changes during macrophage infection, we correlated the fitness profiles between S. Typhimurium ST4/74 and the other isolates; the highest correlation was observed between the two Typhimurium isolates (R = 0.6; Supplementary Fig. 14), followed by Typhi (R = 0.5; Fig. 5b) and Paratyphi A (R = 0.4; Fig. 5c). We identified dozens of genes with typhoidal-specific changes in fitness, defined as genes that had FC greater than fourfold higher in S. Typhi Ty2 (Supplementary Table 8) or S. Paratyphi A 9150 (Supplementary Table 9) compared with S. Typhimurium ST4/74. For example, Tn insertions in yfeX, encoding a putative iron-dependent peroxidase, strongly reduced fitness within THP-1 cells for S. Typhi Ty2 and S. Paratyphi A 9150 (~120× and ~30× lower, respectively) and moderately for S. Typhimurium ST4/74 (~4×) (Fig. 5d). Accordingly, deleting this putative peroxidase in S. Typhi (Ty2ΔyfeX) increased sensitivity to 250 µM H2O2 (Fig. 5e), and impaired survival in LPS-stimulated THP-1 macrophages compared with Ty2WT (Fig. 5f and Supplementary Fig. 13b).
Tn insertions in genes involved in manganese (Mn) and zinc (Zn) homeostasis induced stronger fitness defects in S. Typhi during macrophage infection than in the other serovars (Fig. 5g–h). To confirm these results, we deleted mntR and zntR in S. Typhi Ty2 and observed that these mutants survived worse within LPS-activated THP-1 macrophages compared with the wild-type (Fig. 5i–j and Supplementary Fig. 13c,d), indicating heightened sensitivity to changes in intracellular Mn2+ and Zn2+ levels in human-restricted Salmonella.
A typhoid-specific phenotype for mntR under stress
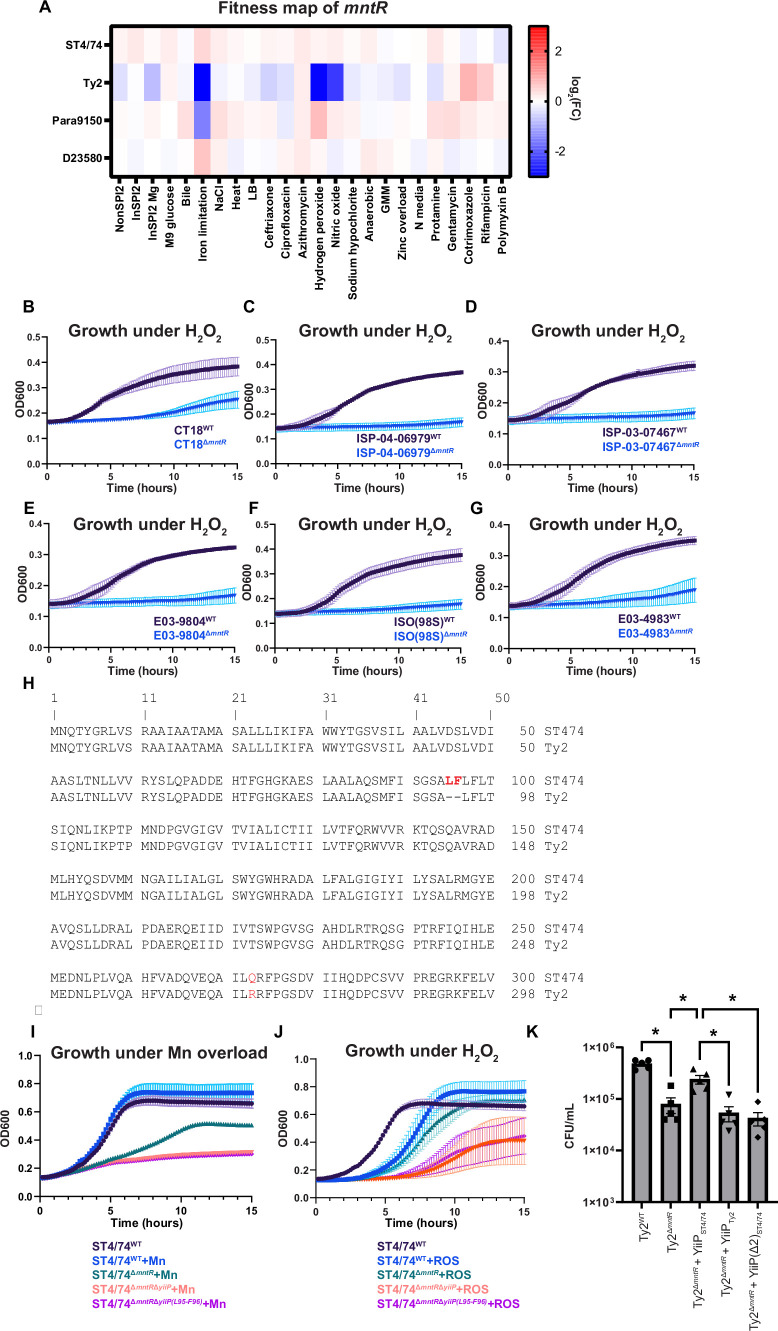
We sought to understand why S. Typhi Ty2ΔmntR has decreased intramacrophage survival. Tn insertions in mntR rendered S. Typhi sensitive to iron limitation, H2O2 and nitric oxide (NO) (Fig. 6a)—all of which all encountered within macrophages73–75. In contrast, Tn insertions in mntR had no impact on the fitness of other serovars (Fig. 6a and Extended Data Fig. 4a). Deleting mntR in both S. Typhimurium ST4/74 and S. Typhi Ty2 validated these results; ST4/74ΔmntR did not have a growth defect with H2O2 (Fig. 6b), whereas Ty2ΔmntR exhibited marked growth defects under iron restriction (Fig. 6c) and H2O2 (Fig. 6d), confirming its serovar-specific phenotype. Deleting mntR in six additional S. Typhi strains sensitized each strain to H2O2, indicating conservation of this phenotype across clinical Typhi isolates (Extended Data Fig. 4b–g).
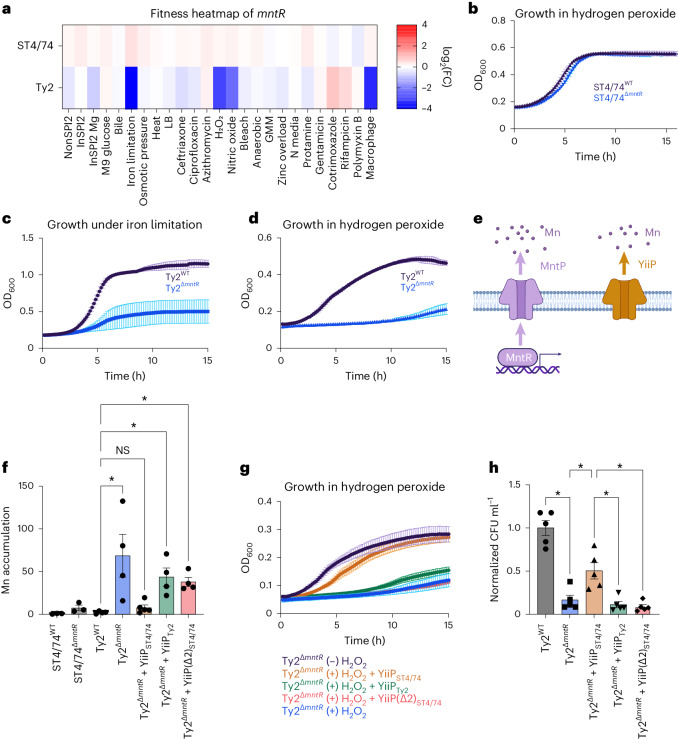
Fig. 6. YiiP pseudogenization sensitizes S. Typhi to stress.
a, Fitness heatmap of mntR Tn insertions for S. Typhimurium ST4/74 and S. Typhi Ty2. Color gradient is derived from the log2(FC) for each condition from Rb-Tn-seq. b, Growth of ST4/74WT (black) and ST4/74ΔmntR (blue) when exposed to 250 µM H2O2, derived from n = 4 biologically independent experiments. c, Growth of Ty2WT (black) and Ty2ΔmntR (blue) when exposed to 100 µM 2,2′-dipyridyl, derived from n = 4 biologically independent experiments. d, Growth of Ty2WT (black) and Ty2ΔmntR (blue) when exposed to 250 µM H2O2, derived from n = 3 biologically independent experiments. e, Schematic of Mn efflux systems in Salmonella. The MntR/MntP system is shown in purple, while YiiP is in orange. f, ICP-OES of Mn accumulation in WT and mutant strains of S. Typhimurium ST4/74 and S. Typhi Ty2 under Mn challenge, shown as the ratio of accumulated Mn in the (+) versus (−) Mn challenged samples, normalized to viable cell count. Each point and error bar indicates the mean ± s.e.m. of normalized Mn and is derived from n = 3 (ST4/74 strains), n = 4 (Ty2WT, Ty2ΔmntR, Ty2ΔmntR+YiiPTy2, Ty2ΔmntR+YiiP(Δ2)ST4/74) and n = 5 (Ty2ΔmntR+YiiPST4/74) biologically independent experiments. g, Growth curves of Ty2ΔmntR and complementation strains of Ty2ΔmntR under 250 µM H2O2. For controls, Ty2ΔmntR grown without H2O2 is in black, while Ty2ΔmntR growth with H2O2 is in blue, both derived from n = 3 biologically independent replicates. For complementation curves, YiiP(Δ2)ST4/74 is in red (n = 5 biologically independent replicates), while YiiPST4/74 is in orange and YiiPTy2 is in green, both derived from n = 7 biologically independent replicates. h, Normalized intracellular bacterial counts recovered from LPS-activated macrophages, with Ty2WT in gray and Ty2ΔmntR in blue. For complementation strains, YiiPST4/74 is in orange, YiiPTy2 is in green and YiiP(Δ2)ST4/74 is in red. Bars indicate the mean ± s.e.m. of the normalized bacterial count recovered from macrophages with individual values shown, derived from n = 5 biologically independent experiments. For all growth curves (b–d, g), each point and error bar indicates the mean ± s.e.m. of OD600, with reads taken at OD600 once every 10 min. For f and h, significance was calculated using a one-way ANOVA; *P < 0.05, with multiple comparisons corrected by the Benjamini, Krieger and Yekutieli method (Supplementary Table 10).
Extended Data Fig. 4. Tn insertions in mntR lead to a typhoid-specific fitness defect during macrophage-associated stress.
a) Heatmap of mntR across 24 stresses. Color gradient is derived from the log2(fitness) from each condition in the Rb-Tn-seq experiments. All fitness values on these heatmaps were generated from the average of two biologically independent biological replicates. b–g): Growth of CT18 (B), ISP-04-06979 (C), ISP-03-07467 (D), E03-9804 (E), ISO(98 S) (F), E03.4983 (G), with WT (black) and ΔmntR mutants (blue) with 250 µM hydrogen peroxide, derived from n = 3 biologically independent experiments. h) Alignment of yiiP from S. Typhimurium ST4/74 and S. Typhi Ty2. Residues that are different in S. Typhi Ty2 are highlighted in red. i) Growth of WT and mutant strains in Typhimurium ST4/74 under Mn challenge. ST4/74WT growth in LB without Mn challenge is shown in black and ST4/74WT growth under Mn challenge (200 µM Mn) is shown in blue (both derived from n = 3 biologically independent experiments). ST4/74ΔmntR growth in LB supplemented with 200 µM Mn is shown in green, and ST4/74ΔmntRyiiP growth in LB supplemented with 200 µM Mn is shown in orange (both derived from n = 5 biologically independent experiments). ST4/74ΔmntRyiiP(L95-F96) growth in LB supplemented with 200 µM Mn is shown in purple (n = 6 biologically independent experiments). j) Growth curves of WT and mutant strains in Typhimurium ST4/74 under hydrogen peroxide challenge (ROS). ST4/74WT growth in InSPI2 media without hydrogen peroxide is shown in black, and ST4/74WT growth under hydrogen peroxide stress (600 µM) is shown in blue, both derived from n = 3 biologically independent experiments. ST4/74ΔmntR growth under 600 µM hydrogen peroxide is shown in green (n = 4 biologically independent experiments). ST4/74ΔmntRyiiP growth with 600 µM hydrogen peroxide is shown in orange and ST4/74ΔmntRyiiP(L95-F96) growth under 600 µM hydrogen peroxide is shown in purple, both derived from n = 5 biologically independent experiments. k) Intracellular bacterial counts for Ty2WT and Ty2ΔmntR complementation strains recovered after 5 hours in LPS-activated THP-1 macrophages, derived from n = 5 independent biological experiments. Significance was calculated using a one-way ANOVA, with * indicating p < 0.05 and multiple comparisons corrected by the Benjamini, Krieger, and Yekutieli method; a list of exact P values is found in Supplemental Table 10. For all growth curves (B-G, I-J), reads were taken at OD600 once every 10 minutes, and each point and error bar indicates the mean ± SEM of OD600.
We next explored why Ty2ΔmntR is sensitive to infection-related stress. MntR—a Mn-responsive transcription factor—activates MntP, a Mn efflux pump, to restore homeostasis76,77. Nontyphoidal and typhoidal Salmonella also encode YiiP—a constitutively active Mn pump78,79 (Fig. 6e). While MntP sequences are identical between S. Typhimurium and S. Typhi, Ty2 YiiP has a two amino acid deletion (ΔL95–F96) found in all Typhi isolates on BioCyc (Extended Data Fig. 4h), suggesting that YiiPTy2 may not be fully functional. Accordingly, we challenged WT and ΔmntR mutants of S. Typhimurium ST4/74 and S. Typhi Ty2 with 200 µM manganese and quantified intracellular Mn accumulation. Neither WT ST4/74 nor WT Ty2 accumulated Mn, indicating functional Mn efflux (Fig. 6f). The ST4/74ΔmntR modestly accumulated Mn (around fourfold), suggesting that YiiP removes most intracellular Mn even without MntR in this isolate (Fig. 6f). In contrast, Ty2ΔmntR accumulated high Mn (~70-fold), indicating severely impaired Mn efflux without MntR, likely due to a nonfunctional YiiP efflux pump (Fig. 6f).
To investigate whether mutated YiiP underlies the fitness defects of Ty2ΔmntR, we expressed functional YiiP from S. Typhimurium in Ty2ΔmntR; this complemented strain no longer accumulated Mn during Mn challenge (Fig. 6f) or exhibited strong sensitivity to H2O2 (Fig. 6g). In contrast, expressing YiiP from Ty2 in the Ty2ΔmntR strain still resulted in high Mn accumulation during Mn challenge (Fig. 6f) and sustained sensitivity to H2O2 (Fig. 6g), further indicating that YiiP in S. Typhi is likely nonfunctional. Similarly, introducing the mutated YiiPST4/74 pump (YiiP(Δ2)ST4/74), with an in-frame deletion of L95–F96, into Ty2ΔmntR failed to correct growth defects under Mn or H2O2 stress (Fig. 6f–g). Moreover, deleting either full-length yiiP or just the L95–F96 sequence of this pump in ST4/74ΔmntR sensitized ST4/74 to both Mn and H2O2 stress (Extended Data Fig. 4i–j), indicating that YiiP is both necessary and sufficient for Salmonella survival to macrophage-associated stresses. Importantly, expressing YiiPST4/74 rescued Ty2ΔmntR survival in LPS-activated THP-1 macrophages to a significantly higher extent than expressing yiiPTy2 or mutated yiiP(Δ2)ST4/74 (Fig. 6h and Extended Data Fig. 4k). Collectively, our results indicate that yiiP pseudogenization in human-adapted Salmonella leads to a vulnerability during macrophage infection.
Discussion
Decades of genomics research have revealed extensive genetic diversity in Salmonella, yet a functional understanding of its genetic evolution remains unclear. Here, we performed hundreds of high-throughput fitness assays with representative nontyphoidal and typhoidal Salmonella serovars, capturing thousands of fitness events across 25 infection-relevant conditions. We characterized serovar-specific fitness profiles and gene networks using a comprehensive systems biology approach and experimentally perturbed gene networks to pinpoint specific pseudogenes in typhoidal Salmonella contributing to serovar-specific fitness effects. Overall, these findings advance our functional understanding of how genetic differences between generalist and host-restricted Salmonella correlate with serovar behavior.
Our functional genomics approach has experimentally identified typhoid-specific phenotypes not detected in previous bioinformatic-based genomic comparisons7,9–11. Unlike traditional pseudogene identification relying on early stop codons and frameshift mutations7, our approach identified putative pseudogenes with missense mutations or small, internal inframe deletions that likely disrupt gene function. Examples include cysIJ and yiiP, which contribute to the fitness defects of Ty2ΔmetQ and Ty2ΔmntR under RCS and macrophage-associated stresses, respectively. Our systematic identification of pseudogenes hints at a potentially higher number of nonfunctional genes in host-adapted Salmonella than currently estimated.
Our results further indicate that pseudogenes arise in redundant pathways. For instance, Salmonella acquires Met/Cys through high-affinity transporters or de novo synthesis, processes redundant in S. Typhimurium58. However, S. Typhi cannot synthesis Cys/Met endogenously, heightening its sensitivity to RCS. Similarly, Salmonella encodes ISC and SUF complexes for iron-sulfur cluster synthesis80,81, but SUF pseudogenization in Paratyphi increases sensitivity to iron restriction. Additionally, Salmonella encodes two Mn efflux pumps, MntP and YiiP, that mediate Mn homeostasis78. However, YiiP is likely nonfunctional in Typhi, sensitizing Typhi to macrophage-associated stresses. Overall, typhoidal Salmonella tolerates pseudogenization in redundant pathways, but accumulation of these mutations can sensitize these isolates to infection-related stress.
In summary, our Rb-Tn-seq screens create a genome-wide atlas for both generalist and human-restricted Salmonella, providing a critical public resource for further mechanistic understanding of how diverse serovars withstand human infection stresses. Although our study focuses on four representative isolates, we acknowledge the vast genetic landscape of this pathogen. Expanding these screens to encompass more genetically diverse Salmonella will lead to additional insights into how isolates respond to infection-related stress or sublethal doses of antibiotics. Furthermore, uncovering additional pseudogenes in typhoidal Salmonella will highlight vulnerabilities within the genomes of these human-restricted pathogens. Ultimately, these efforts will pinpoint genes and pathways that could serve as targets for rational drug design against Salmonella-related illnesses.
Methods
Ethics declaration
The authors have complied with all ethics guidelines and have no competing interests to declare. All study protocols were approved by Stanford University.
Experimental model and subject details
Salmonella Typhimurium ST4/74 and D23580, Salmonella Typhi Ty2 and Salmonella Paratyphi A 9150 were utilized for Rb-Tn-seq experiments. Both S. Paratyphi A 9150 and S. Typhi Ty2 are laboratory-adapted isolates, and S. Typhi Ty2 is an RpoS(−) strain that is attenuated in virulence82. For all cloning experiments, the conjugative E. coli strain Jke201 was used to move plasmids into Salmonella83, and E. coli strain DH5α was used for storage and sequencing of plasmids. For additional experiments in clinical isolates, the published H58 isolates ISP-04-06979, ISP-03-07467, E03-9804, ISO (98 S) and E03.4983 were used59, which display chloramphenicol, ampicillin and tetracycline resistance. For all experiments and cloning, Salmonella and E. coli strains were grown overnight in Luria-Bertani (LB) medium at 37 °C under shaking conditions before subculturing into specific stress conditions (see below). When applicable, antibiotics were added at the following concentrations for all strains: 50 μg ml−1 kanamycin, 20 μg ml−1 gentamicin and 100 μg ml−1 carbenicillin. Strains, plasmids and primers used are listed in Supplementary Tables 1–3. For macrophage experiments, THP-1 human macrophages (ATCC TIB202) were grown routinely in RPMI medium supplemented with 10% FBS and 2 mM Glutamax and incubated at 37 °C and 5% CO2. Cell cultures were passaged every ~3–5 days and passages between 4 and 9 were used for Rb-Tn-seq. Cells were genotyped at ATCC with STR profiling. Mycoplasma testing was done routinely by PCR every 3–6 months.
General cloning procedures
Chromosomal-based modifications in Salmonella were done with allelic exchange, as previously described83. Briefly, the pFOK and pFOG vectors were utilized, which confer kanamycin and gentamicin sensitivity, respectively. These vectors also encode sucrose and anhydrotetracycline (AHT) sensitivity for counterselection. For all deletions, fragments ~500–1,000 bp upstream and downstream of the target gene were amplified from Salmonella genomic DNA (gDNA) using a KAPA Hifi PCR kit (Roche). Each fragment contained ~20–30-bp overlap regions. In turn, all vectors were assembled by HiFi Gibson Assembly (NEB) and transformed into DH5α E. coli for storage. Plasmids with the correct sequences were miniprepped and transformed into JKe201 E. coli on LB agar plates supplemented with 300 µM 2,6-diaminopimelic acid (DAP) and appropriate antibiotics.
For conjugation into Salmonella, JKe201 E. coli and relevant Salmonella strains were grown overnight for 16 h. The next morning, 500 µl of E. coli and Salmonella were mixed, centrifuged and resuspended in 50 μl LB + DAP. This mixture was then pipetted onto the center of a LB + 300 µM DAP plate and grown at 30 °C for mating to proceed. Mating times were 16 h for S. Typhimurium ST4/74 and D23580, 3 h for S. Typhi Ty2 and 6 h for S. Paratyphi A 9150. The mixtures were then scraped and resuspended in 1 ml PBS. Then, 100 μl of these mixtures were plated on LB supplemented with either Kan (for pFOK) or gent (for pFOG). Single colonies were streaked onto plain LB plates supplemented with 15% sucrose and 0.5 µg ml−1 AHT for counterselection. Colonies were screened for chromosomal modifications using colony PCR and sanger sequencing; clones with the correct constructs were collected and stored at −80 °C.
The low-copy pWSK29 plasmid was used to generate complementation constructs84. Briefly, inserts of interest were PCR amplified from Salmonella gDNA using the KAPA Hifi PCR kit (Roche), and designed such that they contained ~20–30 bp of overlap with the multicloning site (MCS) of the pWSK29 plasmid. A ~500-bp upstream region was included for each gene to capture the native promoter. For plasmid assembly, the pWSK29 was digested using the EcoRV-HF restriction enzyme (NEB), according to manufacturer protocols. Digested pWSK29 and PCR-amplified sequences were then assembled using the HiFi Gibson Assembly kit (NEB) and transformed into DH5α E. coli for plasmid storage and whole plasmid sequencing, as described above. Sequence verified plasmids were then electroporated into electrocompetent Salmonella strains. A 1 μl sample of assembled vector was then electroporated into 100 μl of competent Salmonella. Cells were allowed to recover for 1 h at 37 °C and then plated on LB agar plates supplemented with carbenicillin for overnight growth.
Set-up of growth experiments
All growth experiments and Rb-Tn-seq screens were done in 24-well plates (Falcon), in which each well contained 1 ml of cells. Briefly, cultures were first grown overnight in LB at 37 °C with shaking for 16 h. The next morning, the overnight cultures were backdiluted 1:100 into fresh LB and grown for ~3 h at 37 °C with shaking until an OD of ~1 (input sample). Then, 1 ml of these ‘time-zero’ input samples were centrifuged at 8,000g for 3 min and frozen at −80 °C until further processing. Cultures were then backdiluted 1:50 into the 24-well plate format (20 μl into 1 ml of medium per well), and growth was monitored on a BioTek Synergy plate reader overnight with reads at OD600 every 15 min, or until around six to eight doublings had been achieved (output sample). For these output samples, 1-ml cultures from the 24-well plates were collected by centrifugation at 8,000g for 3 min and then frozen at −80 °C until further processing. Log-transformed optical density plots of all growth curves in the main figures are shown in Supplementary Fig. 15 to show growth rates.
The NonSPI2 medium was composed of 80 mM MOPS (pH 7.4), 4 mM tricine, 376 μM K2SO4, 50 mM NaCl, 25 mM K2HPO4, 0.4% glucose, 15 mM NH4Cl, 1 mM MgSO4, 10 μM CaCl2, 10 nM Na2MoO4, 10 nM Na2SeO3, 4 nM H3BO3, 300 nM CoCl2, 100 nM CuSO4, 800 nM MnCl2, 1 nM ZnSO4 and 100 μM FeCl3 (ref. 37). InSPI2 medium was the same as NonSPI2, except it contained 80 mM MES instead of 80 mM MOPS, was at pH 5.8 instead of 7.4 and contained 0.4 mM K2HPO4 instead of 25 mM K2HPO4. InSPI2 Mg was the same as InSPI2 except it contained 10 μM MgSO4 instead of 1 mM MgSO4. All redox stress experiments (for example, hydrogen peroxide, nitric oxide, sodium hypochlorite) were run in InSPI2 medium to mimic intracellular conditions. Protamine, which is insoluble in LB, was added to N medium85, which contains 100 mM Tris-HCl (pH 7.4), 1 mM MgCl2, 0.2% casamino acids and 0.2% glycerol. GMM86 comprised 0.2% tryptone, 0.1% yeast extract, 2.2 mM glucose, 3.2 mM cysteine, 2.9 mM cellobiose, 2.8 mM maltose, 2.2 mM fructose, 0.5% meat extract, 100 mM KH2PO4, 0.008 mM MgSO4, 4.8 mM NaHCO3, 1.37 mM NaCl, 0.8% CaCl2, 5.8 mM vitamin K, 1.4 mM FeSO4, 0.1% histidine hematin, 0.05% tween 80, 1% ATCC vitamin mix, 1% ATCC trace mineral mix, 30 mM acetic acid, 1 mM isovaleric acid, 8 mM propionic acid and 4 mM butyric acid. All other stressors were run in LB. All stressor concentrations used for each serovar are listed in Supplementary Table 5.
Preparation of barcoded Tn libraries
Overnight cultures (25 ml) of each Salmonella strain were grown in LB at 37 °C overnight with shaking for 14 h. Following the 14-h growth for Typhimurium strains, these cultures were incubated at 47 °C for an additional 2 h to increase conjugation efficiency, as has been done in other bacteria87,88. In parallel, 1 ml aliquots of previously constructed conjugative E. coli strains harboring barcoded transposon plasmids36 were thawed and grown in 50 ml of LB + 300 µM DAP + 50 μg ml−1 kanamycin for ~3 h, until the OD reached ~1. The donor E. coli and recipient Salmonella strains were then mixed at a ratio of two donor:one recipient in a total volume of 2 ml and centrifuged at 8,000g for 3 min. The pellet was resuspended in 100 μl of LB and placed on a 0.45 μm nitrocellulose filter on a LB + 300 µM DAP agar plate for conjugation. S. Typhimurium conjugations were allowed to proceed overnight, while S. Typhi Ty2 and S. Paratyphi A conjugations proceeded for 3 and 5 h, respectively. The filters were then resuspended in LB and vortexed for 30 s to dislodge bacteria. Dilutions of this solution were plated onto 245 mm (Fluotics) square Petri plates with LB agar + 50 μg ml−1 kanamycin and incubated overnight. Colonies were then scraped into 25 ml LB, grown for 1 h at 37 °C, and frozen as 1 ml glycerol stocks at −80 °C. A total of ~100–300 K colonies were collected for each library.
Construction and sequencing of Tn-seq libraries
Tn libraries were prepared according to previously published protocols36. Briefly, gDNA from each library was extracted using the QIAamp DNA Mini Kit (Qiagen). gDNA was diluted to ~15 ng μl−1 in 130 μl of nuclease-free water (Ambion) and sheared to an average size of 300 bp using the Covaris S2 ultrasonicator. AMPure XP beads (Beckman Coulter) were then used to size-select for 300-bp-sized fragments. Fragments were then subjected to end-repair and A-tailing using the KAPA Hyper Prep kit (Roche). Splinkerette adapters were ligated to these fragments to allow for the selection of barcode-containing fragments, and contaminants were removed using SPRI beads (Beckman Coulter). These fragments were then amplified for 15 cycles (98 °C for 15 s, 60 °C for 30 s, 72 °C for 30 s) and final extension at 72 °C for 60 s using the KAPA HiFi HotStart ReadyMix kit (Roche). In these reactions, one primer binds to the splinkerette and the other one binds to the transposon, thereby allowing for the selective amplification of sequences containing both a barcode and a transposon36. These amplified fragments were cleaned up with SPRI beads (Beckman Coulter) and then subjected to another round of PCR using the KAPA HiFi HotStart ReadyMix kit (Roche) for eight cycles (98 °C for 15 s, 60 °C for 30 s, 72 °C for 30 s and final extension at 72 °C for 60 s), using a universal P5 Illumina primer and a different indexed P7 Ilumina primer for each reaction36. SPRI-cleaned samples were sequenced on an Illumina HiSeq4000 instrument using paired-end sequencing (PE150) at Novogene. Typically, up to eight different Tn libraries were sequenced on one lane. A published perl script (MapTnSeq.pl) was used to analyze Tn-seq reads and identify the associated barcode for each Tn insertion36. Essential genes were mapped as previously described89,90. Briefly, we calculated the insertion index of each gene by dividing the number of unique Tn insertions for each gene by its length. We then plotted a histogram of this data, which showed a clear bimodal distribution (Supplementary Fig. 2). We fit these two peaks to gamma distributions using the scipy.stats function in python 3.8. Log-likelihood scores were then calculated for each gene; a gene that was 16× more likely to belong to the left-peak than the right-peak was considered essential.
Preparation and sequencing of Rb-Tn-seq samples
Rb-Tn-seq fitness experiments were done in biological duplicate. gDNA from frozen pellets was extracted using the QIAamp DNA Mini Kit (Qiagen). The Q5 HiFi polymerase (NEB) was used to amplify the barcode regions associated with each Tn insertion; each barcode is flanked by universal primer binding sites. One primer (P5) is universal and binds to the upstream common priming site for Rb-Tn-seq, while the other primer (P7) has a unique index sequence that is different for each sample and binds to the downstream common priming site. The PCR was amplified for 25 cycles (98 °C for 15 s, 55 °C for 30 s, 72 °C for 30 s, final extension of 72 °C for 5 min). PCR samples were run on 1.2% agarose gels to verify the presence of bands (~200 bp) for each sample. Equal volumes of all samples (10 μl) were pooled and cleaned up using a PCR purification kit (Qiagen). Samples were then sequenced on an Illumina HiSeq4000 instrument using paired-end sequencing (PE150) at Novogene. Typically, up to 48 different Rb-Tn-seq experiments were sequenced on one lane. Mutant fitness was calculated using several published perl and R scripts (for example, Multicodes.pl and FEBA.R)36. The fitness of each strain is approximately equal to the normalized log2 ratio of counts for each Tn-associated barcoded between the initial ‘time-zero’ sample and the final sample collected after six to eight doublings in the presence of a stressor. The fitness of each gene is the weighted average of all strains within the central portion (10–90%) of that gene36. A previously described moderated t-like statistic (|t| > 4) was used to identify statistically significant genes; this statistic considers the consistency of fitness effects for all Tn insertions within a given gene36. To verify that this moderated t-statistic fits well to the standard normal distribution, we performed control comparisons between replicate time-zero samples for each of the four serovars used in this paper, as previously described36. Using a quartile–quartile (QQ) plot, we observed that the moderated t-statistic between these replicate time-zero samples is indeed distributed normally for each isolate (Supplementary Fig. 16).
For gene fitness, only insertions with the 10–50% and 50–90% of the gene region are used. In addition, genes without at least 15 time-zero reference reads are filtered out. The quality of each experiment was evaluated using a series of published metrics36, including gMed (the median reads per gene in the sample), cor12 (measurement of the consistency of fitness data for each gene, taken by comparing the fitness of a gene using Tn insertions within the first half of the gene versus the second half of the gene using a Spearman rank correlation), mad12 (measurement of the mean absolute difference between fitness values from Tn insertions in the first versus second half of gene) and opcor (measurement of the consistency of fitness data for all genes within an operon).
Cofitness analysis and SAFE
Cofitness analysis and SAFE were performed according to published pipelines38. Briefly, a gene × condition matrix was first assembled for each Salmonella serovar, consisting of all genes with captured log2 fitness effects in the Rb-Tn-seq experiments, and all 24 plate-based conditions tested. These matrices were then used to build a cofitness network, which calculates the Pearson’s correlation coefficient for all gene pairs in the network38. We used a correlation cutoff of R > 0.75 for two reasons. First, this value was used in previous publications performing cofitness analysis and SAFE38. Second, we empirically tried several different cut-offs, ranging from 0.6 to 0.9, and conducted a manual analysis of the generated SAFE clusters, aiming to identify an optimal cutoff point that preserves genes expected to cluster together due to their related functionality while minimizing the clustering of genes with no functional association. Using R > 0.75 led to the retention of nodes and edges in the network between pairs of genes with similar fitness profiles. Statistical significance of each correlation value was determined using t = r, where r is the correlation and n is the sample size of 24 conditions. P values were calculated from the t-statistics in Python, and false discovery rate (FDR) was calculated using the Benjamini–Yekutieli method. We then performed an additional stability test according to published methods38. Briefly, we built a correlation matrix using partial data by repeatedly hiding random conditions and performed our correlation analysis with 20 out of the 24 possible conditions 100 times. This resulted in 100 binary matrices that we then summed up, with each possible correlation receiving a score between 0 and 100; we considered scores >75 to be ‘stable,’ in line with published work38.
These networks were then visualized in Cytoscape using the edge-weighted spring layout, with the absolute correlation value used as the edge weight. Next, SAFE was used to identify functionally enriched clusters of genes on these networks55. SAFE attributes were assigned using available GO term-based functional annotations from the BioCyc database for each Salmonella serovar (Supplementary Data 9). The distance threshold was set to 2% of the map-weighed distance, and the Jaccard similarity index was set to 0.5.
RNA extraction and qRT-PCR
RNA was purified using previously published methods91. Briefly, 200 μl of cells were pelleted at 12,000g for 2 min and resuspended in 300 μl of Cell Lysis solution (Epicentre) with 2 μl of 50 μg μl−1 proteinase K. This lysis solution was incubated for 30 min at 65 °C, chilled on ice for 5 min, and then mixed with 175 μl of MPC Protein Precipitation Reagent (Epicentre) to remove all proteins. Samples were then spun for 15 min at 15,000g at 4 °C to remove insoluble precipitated proteins. The supernatant was collected and then mixed with 500 μl of isopropanol to precipitate nucleic acids. This mixture was centrifuged for another 15 min at 15,000g at 4 °C, and the resulting pellet was washed with 70% ethanol twice to remove contaminants. The pellet was then air-dried for 5 min and resuspended in 50 μl of nuclease-free water (Ambion). Then, 1.5 μl of Turbo DNAse (Invitrogen) was then added for 30 min at 37 °C to remove contaminating DNA from the preparation. DNAse was removed using DNAse inactivation reagent (Invitrogen), and the resulting supernatant containing purified RNA was collected and placed at −80 °C for long-term storage.
cDNA for qRT-PCR experiments was prepared using the ProtoScript II reaction mix (NEB), as described previously92. Briefly, 500 ng of purified RNA was mixed with 2 μl of random hexamers, 2 μl of Protoscript enzyme and 10 μl of ProtoScript II reaction mix. Samples were then incubated at 25 °C for 5 min and then 42 °C for 1 h The Sybr Fast qPCR kit (Roche) was used to perform all qRT-PCR experiments. Briefly, 10 ng of cDNA was mixed with 300 nM of each primer, nuclease-free water, and 2× Sybr master mix (Roche), in a final volume of 10 μl. All qRT-PCR experiments were run in 384-well plate format on a LightCycler 480 system (Roche). A program of 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s and 72 °C for 30 s was used. FC was calculated using the ddCT method, using rpoD as the reference gene.
Rb-Tn-seq experiments in THP-1 macrophages
THP-1 infections were carried out according to published methods69. Briefly, for Rb-Tn-seq experiments, THP-1 cells were seeded at a density of 5 × 107 cells on a large 150 × 15 mm Petri dish (Falcon). A final phorbol myristate acetate concentration of 5 ng ml−1 was used for differentiation. Cells were allowed to rest for 48 h in fresh medium before Salmonella infection experiments. For infections, S. Typhimurium ST4/74 and D23580 were used at a multiplicity of infection (MOI) of 10, while S. Typhi Ty2 and S. Paratyphi A 9150 were used at an MOI of 100. Frozen 1 ml aliquots of each library were thawed in 50 ml of LB + kanamycin and were allowed to grow until OD ~0.5. Based on the desired MOI, appropriate volumes of these libraries were then centrifuged and resuspended in 200 μl of 25% human serum (MP Biomedicals) and incubated at 22 °C for 30 min. Opsonized solutions of bacteria were passed three times through a 25-gauge needle to separate clumps of cells and then allowed to infect differentiated THP-1 macrophages (passage 1). Following incubation at 37 °C for 1 h, cells were washed once with PBS and then incubated with THP-1 medium supplemented with 100 μg ml−1 gentamicin for 1 h to kill extracellular bacteria. The plates were then washed again with PBS and incubated with THP-1 medium supplemented with 10 μg ml−1 gentamicin for 16 h (S. Typhimurium strains) or 18 h (S. Typhi and S. Paratyphi A). The THP-1 macrophages were then lysed for 1 h at 37 °C with 2% saponin, and the resulting lysate was added to 5 ml of LB + kanamycin for passaging and allowed to grow at 37 °C for 6 h to increase the number of bacteria. These passaged libraries were then subjected to the same treatment as above, starting with opsonization of these libraries in 25% human serum, and allowed to infect a fresh plate of differentiated THP-1 macrophages (passage 2), using the same parameters as above.
At the end of the second passage, macrophages were again lysed with 2% saponin to release intracellular bacteria. Bacterial counts were measured routinely by plating serial dilutions of this lysate on LB + kanamycin plates; typical recovery from Rb-Tn-seq experiments ranged from ~3 × 107 to 1 × 108 colony forming units (CFU) per milliliter. Macrophage lysates were centrifuged to concentrate intracellular Salmonella, and these samples were processed directly using the MasterPure DNA/RNA extraction kit (Epicentre), which has been used previously to extract nucleic acids from small numbers of bacteria91. Briefly, the cell pellets were resuspended in 300 μl of Cell Lysis solution (Epicentre) with 2 μl of 50 μg μl−1 proteinase K and incubated for 1 h at 65 °C. These samples were then chilled on ice for 5 min and mixed with 175 μl of MPC Protein Precipitation Reagent (Epicentre) to remove all proteins. Samples were then spun for 15 min at 12,000g at 4 °C, and the supernatant was recovered and mixed with 500 μl isopropanol to precipitate DNA. These samples were then spun down at 12,000g for 15 min at 4 °C to pellet the DNA, which was then washed with 70% ethanol twice and resuspended in nuclease-free water at a final volume at 30 μl. Rb-Tn-seq using the Q5 polymerase was then used to amplify all barcodes captured from these samples, as described above. Changes in gene fitness and significance within macrophages were calculated using the DEseq2 method, as has previously been done for Salmonella macrophage Tn-seq experiments29.
Salmonella mutant strain validation in THP-1 cells was performed in a similar manner as described above. Briefly, THP-1 cells were seeded on six-well plates, at a density of 1 × 106 THP-1 cells per well. The same MOIs as above were used for these infection assays. To capture stronger phenotypes in these experiments, THP-1 cells were stimulated with 100 ng ml−1 of LPS for 24 h, as previously described93. Salmonella strains were allowed to infect these activated THP-1 cells for 5 h using the same gentamicin protection assay described above. Macrophages were then lysed with 2% saponin, and serial dilutions of the lysate were plated on LB agar plates to calculate CFUs from WT and mutant Salmonella strains.
Sytox green and replication assays using the pFCcGI plasmid were performed on the Incucyte imaging platform (Satorius). For these assays, 96-well black plates with clear bottoms (Falcon) were used for seeding THP-1 cells, which were seeded at a density of 5 × 104 cells per well using the same general seeding protocol as above. For cell death assays, Sytox Green was added at a final concentration of 20 nM to each well during the low gentamicin incubation step; cell death was then monitored by the green signal on the Incucyte with measurements once every hour. A final spike-in of 10% Triton-X 100 was used to kill all cells for a full lysis control. For pFCcGI-based experiments, bacteria were grown to OD ~0.5 in the presence of 0.2% arabinose to induce GFP production. Infections were then allowed to proceed in the same way as described above, with imaging for both GFP and mCherry signals on the Incucyte once an hour.
Inductively coupled plasma-optical emission spectroscopy
Overnight cultures were diluted 1:100 into fresh LB and grown until an OD ~0.5. Cells were then challenged with either 0 or 200 μM MnCl2 for 2 h. After Mn challenge, cells were spun down at 8,000g for 5 min and washed once in 10 mM HEPES + 2 mM EDTA (pH 7.5), followed by two washes in 10 mM HEPES (pH 7.5). Cell pellets were then dried for 3 h in a speed vac and resuspended in 600 μl of 30% v/v nitric acid (Sigma). These solutions were incubated at 95 °C for 1 h to release intracellular Mn, with vortexing every 15 min; 500 μl of these solutions were then diluted into 4.5 ml of 3% v/v nitric acid. All samples were filtered sterilized through a 0.22 μM filter before running on the inductively coupled plasma-optical emission spectroscopy (ICP-OES) instrument. Each ICP-OES run also included blank controls (3% v/v nitric acid) and serially diluted commercially available metal standards (Accustandard). All samples were run on a ICAP 6300 Duo View Spectrometer (Thermo Scientific), at the Stanford Environmental Measurements Facility. Intracellular Mn concentrations were normalized to viable bacterial counts, as previously described78.
Statistics and reproducibility
Two independent biological replicates were used for all Rb-Tn-seq experiments and exhibited a high degree of correlation (Supplementary Data 2). All other assays (for example, growth curves, ICP-OES, and so on) were done with at least three biologically independent experiments, with exact n values indicated in each figure legend. Sample sizes were not predetermined, but all experiments were highly reproducible and our sample sizes were similar to previous related publications36,38. The experiments were not randomized. Data collection and analysis was not blinded. Pairwise statistical comparisons were done with two-sided t-tests, while multiple comparisons were done with one-way analysis of variance (ANOVA) with FDR correction, as described in each figure legend. No data were excluded from this study.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41588-024-01779-7.
Supplementary information
Supplementary Figs. 1–16 and Notes 1 and 2.
Table 1, All strains used in this study. Table 2, All plasmids used in this study. Table 3, All primers used in this study. Table 4, Summary of barcoded libraries used in this study, including the number of genes with central insertions and bias metrics. Table 5, Summary of all conditions tested, with concentrations of each stressor indicated for each serovar. Table 6, List of orthologs for all shared genes among S. Typhimurium ST4/74, S. Typhi Ty2, S. Paratyphi A 9150, and S. Typhimurium D23580. Table 7, List of genes in all clusters from the heatmaps in Supplementary Fig. 11, along with their log2 fitness changes during macrophage infection. Table 8, List of all Typhi-specific changes in fitness during macrophage infection, defined as genes that have a FC > 4× higher in S. Typhi Ty2 compared with S. Typhimurium ST4/74. Table 9, List of all Paratyphi-specific changes in fitness, defined as genes that have a FC > 4× higher in S. Paratyphi A 9150 compared with S. Typhimurium ST4/74. Table 10, List of all P values for one-way ANOVA tests from Fig. 6f,h and Extended Data Fig. 4k, with multiple comparisons corrected by controlling the FDR according to the Benjamini, Krieger and Yekutieli method.
Supplemental Data 1, List of all essential genes for each serovar. S. Typhimurium ST4/74 genes are on sheet 1, S. Typhi Ty2 genes are on sheet 2, S. Paratyphi A 9150 genes are on sheet 3, and S. Typhimurium D23580 genes are on sheet 4.
Supplemental Data 2, List of all quality-control metrics for each Rb-Tn-seq experiment. S. Typhimurium ST4/74 experiments are on sheet 2, S. Typhi Ty2 experiments are on sheet 3, S. Paratyphi A 9150 experiments are on sheet 4, and S. Typhimurium D23580 experiments are on sheet 5. N-used refers to the total number of reads in central portions of genes (10–90% of the gene sequence). gMed is the median reads per gene in the sample. Cor12 is a measurement of how consistent the fitness data is for each gene. This metric is calculated by comparing the fitness of a gene using only Tn insertions in the first half of each gene region with the fitness from Tn insertions in the second half of each gene region. The cor12 value is the Spearman rank correlation of these two sets of values. Mad12 measures the median absolute difference between the fitness according to Tn insertions in the first half versus the second half of each gene. Opcor measures the consistency of fitness data for each operon, as genes within the same operon should have similar fitness values. In this metric, a Spearman rank correlation on the fitness value for each upstream and downstream gene within an operon is calculated. Sheet 1 contains R-values (correlation) between all biological replicate samples, for each condition and each serovar.
Supplemental Data 3, All FC for each serovar for all conditions, for the 24 plate-based assays. S. Typhimurium ST4/74 genes are on sheet 1, S. Typhi Ty2 genes are on sheet 2, S. Paratyphi A 9150 genes are on sheet 3, and S. Typhimurium D23580 genes are on sheet 4.
Supplemental Data 4, All moderated t-like statistics for each serovar for all conditions, for the 24 plate-based assays. S. Typhimurium ST4/74 genes are on sheet 1, S. Typhi Ty2 genes are on sheet 2, S. Paratyphi A 9150 genes are on sheet 3, and S. Typhimurium D23580 genes are on sheet 4.
Supplemental Data 5, List of genes in all clusters from the heatmaps in Supplementary Figs. 4–7, along with their moderated t-statistics in all conditions. Only genes with t > 4 are included. S. Typhimurium ST4/74 genes are on sheet 1, S. Typhi Ty2 genes are on sheet 2, S. Paratyphi A 9150 genes are on sheet 3, and S. Typhimurium D23580 genes are on sheet 4.
Supplemental Data 6, Moderated t-like statistics for all SPI-encoded genes, for the 24 plate-based assays. S. Typhimurium ST4/74 genes are on sheet 1, S. Typhi Ty2 genes are on sheet 2, S. Paratyphi A 9150 genes are on sheet 3, and S. Typhimurium D23580 genes are on sheet 4. Any genes with t > 4 are in bold.
Supplemental Data 7, List of moderated t-statistics for all genes uniquely encoded by S. Typhi Ty2 (sheet 1) and S. Paratyphi A 9150 (sheet 2).
Supplemental Data 8, List of all correlations with R > 0.75 among pairs of genes in the cofitness network maps shown in Extended Data Fig. 1a–d. Each correlation also includes a stability score, in which the correlation matrices were generated by hiding subsets of the conditions 100×; the stability score ranges from 0 to 100, which scores > 75 being considered stable; t-statistics were calculated from each correlation using a sample size of 24 conditions, which were then converted to P values using a cumulative distribution function of the Student’s t-distribution, and FDR calculations were performed using the Benjamini–Yekutieli method. Correlations for S. Typhimurium ST4/74 are on sheet 1, correlations for S. Typhi Ty2 are on sheet 2, correlations for S. Paratyphi A 9150 are on sheet 3, and correlations for S. Typhimurium D23580 are on sheet 4.
Supplemental Data 9, All BioCyc/GO term annotations for making cofitness network and SAFE maps. S. Typhimurium ST4/74 genes are on sheet 1, S. Typhi Ty2 genes are on sheet 2, S. Paratyphi A 9150 genes are on sheet 3, and S. Typhimurium D23580 genes are on sheet 4.
Supplemental Data 10, Filtered list of all correlations with R > 0.75 that contain at least one hypothetical gene. Correlations for S. Typhimurium ST4/74 are on sheet 1, correlations for S. Typhi Ty2 are on sheet 2, correlations for S. Paratyphi A 9150 are on sheet 3, and correlations for S. Typhimurium D23580 are on sheet 4.
Supplemental Data 11, List of all Typhi-specific changes in fitness, defined as genes that (1) have significant fitness changes (t > 4) in S. Typhi Ty2 but not in S. Typhimurium ST4/74 and (2) have a FC > 2-fold higher in S. Typhi Ty2 compared with S. Typhimurium ST4/74 in at least one condition. All significance values are on sheet 1 and all FC are on sheet 2.
Supplemental Data 12, List of all Paratyphi A-specific changes in fitness, defined as genes that (1) have significant fitness changes (t > 4) in S. Paratyphi A 9150 but not in S. Typhimurium ST4/74 and (2) have a FC > 2-fold higher in S. Paratyphi A 9150 compared with S. Typhimurium ST4/74 in at least one condition. All significance values are on sheet 1, and all FC are on sheet 2.
Supplemental Data 13, List of all D23580-specific changes in fitness, defined as genes that (1) have significant fitness changes (t > 4) in S. Typhimurium D23580 but not in S. Typhimurium ST4/74 and (2) have a FC > 2-fold higher in S. Typhimurium D23580 compared with S. Typhimurium ST4/74 in at least one condition. All significance values are on sheet 1, and all FC are on sheet 2.
Supplemental Data 14, All FC and statistics for each of the four serovars from the macrophage infection Rb-Tn-seq experiment. S. Typhimurium ST4/74 genes are on sheet 1, S. Typhi Ty2 genes are on sheet 2, S. Paratyphi A 9150 genes are on sheet 3, and S. Typhimurium D23580 genes are on sheet 4. FC and P values were calculated using DEseq2 analysis.
Acknowledgements
We thank the members of the Monack laboratory for valuable discussions, especially D. Arve-Butler, B. Di Luccia and T. Pham. We thank A. Bhatt for insights into our paper. We thank A. Deutschbauer (University of California, Berkeley), D. Bumann (University of Basel) and D. Pickard (University of Cambridge) for generously providing strains and protocols. We thank T. Van Opijnen for helpful discussions about Tn-seq computational analysis. We thank Y. Le Guen at the Stanford Quantitative Sciences unit for help with coding. This work was supported by the grants R01-AI116059 and R01-AI095396 from the National Institute of Allergy and Infectious Diseases (to D.M.M.), the Paul Allen Stanford Discovery Center on Systems Modeling of Infection (to D.M.M.), the Maternal and Child Health Research Institute (to B.X.W.) and the A.P. Giannini Postdoctoral Fellowship (to B.X.W.). L.L. is funded by an EMBO fellowship (ALTF 95-2023). This work was supported in part by a Wellcome Trust Investigator Award (grant no. 222528/Z/21/Z) to J.C.D.H. For the purpose of open access, the authors have applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission.
Extended data
Author contributions
B.X.W. and M.T. performed the experiments. B.X.W., L.L. and D.L. performed computational analysis. D.L. provided scripts used in this work. J.C.D.H. contributed to writing the paper. J.C.D.H. and K.H. created the interactive SalComFit browser associated with this work. B.X.W. and D.M.M. wrote the paper and conceived the ideas in this work.
Peer review
Peer review information
Nature Genetics thanks Thierry Wirth and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
All strains from this study are available by request to the corresponding author. All raw sequencing data and processed fitness data in this study can be found on NCBI GEO at accession numbers GSE261860, GSE261867, GSE261873, GSE262768, GSE262769, GSE262848, GSE261757, GSE261749 and GSE261214. All raw FC and statistics from the dataset are in Supplementary Data 3, 4 and 14. All raw correlations for network analysis are in Supplementary Data 8. All source data for SAFE annotations are in Supplementary Data 9. All fitness changes from this work can be searched on an interactive website (https://bioinf.gen.tcd.ie/cgi-bin/salcomfit.pl).
Code availability
Code used in this study is derived from previous publications36,38, which we have deposited to Zenodo at 10.5281/zenodo.10963611 (ref. 94).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/20/2024
In the version of the article initially published, "Created with Biorender.com" was missing from the legends of Figs. 1, 3 and 5 and has now been added in the HTML and PDF versions of the article.
Extended data
Extended data is available for this paper at 10.1038/s41588-024-01779-7.
Supplementary information
The online version contains supplementary material available at 10.1038/s41588-024-01779-7.
References
- 1.Wang BX, Butler DS, Hamblin M, Monack DM. One species, different diseases: the unique molecular mechanisms that underlie the pathogenesis of typhoidal Salmonella infections. Curr. Opin. Microbiol. 2023;72:102262. doi: 10.1016/j.mib.2022.102262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull. World Health Organ. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 3.Majowicz SE, et al. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010;50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 4.Sabbagh SC, Forest CG, Lepage C, Leclerc JM, Daigle F. So similar, yet so different: uncovering distinctive features in the genomes of Salmonella enterica serovars Typhimurium and Typhi. FEMS Microbiol. Lett. 2010;305:1–13. doi: 10.1111/j.1574-6968.2010.01904.x. [DOI] [PubMed] [Google Scholar]
- 5.Gal-Mor O, Boyle EC, Grassl GA. Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front Microbiol. 2014;5:391. doi: 10.3389/fmicb.2014.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson R, Mylona E, Frankel G. Typhoidal Salmonella: distinctive virulence factors and pathogenesis. Cell Microbiol. 2018;20:e12939. doi: 10.1111/cmi.12939. [DOI] [PubMed] [Google Scholar]
- 7.McClelland M, et al. Comparison of genome degradation in Paratyphi A and Typhi, human-restricted serovars of Salmonella enterica that cause typhoid. Nat. Genet. 2004;36:1268–1274. doi: 10.1038/ng1470. [DOI] [PubMed] [Google Scholar]
- 8.Baddam R, Kumar N, Shaik S, Lankapalli AK, Ahmed N. Genome dynamics and evolution of Salmonella Typhi strains from the typhoid-endemic zones. Sci. Rep. 2014;4:7457. doi: 10.1038/srep07457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou Q-H, Li R-Q, Liu G-R, Liu S-L. Comparative genomic analysis between typhoidal and non-typhoidal Salmonella serovars reveals typhoid-specific protein families. Infect. Genet. Evol. 2014;26:295–302. doi: 10.1016/j.meegid.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Thomson NR, et al. Comparative genome analysis of Salmonella Enteritidis PT4 and Salmonella Gallinarum 287/91 provides insights into evolutionary and host adaptation pathways. Genome Res. 2008;18:1624–1637. doi: 10.1101/gr.077404.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng W, et al. Comparative genomics of Salmonella enterica serovar Typhi strains Ty2 and CT18. J. Bacteriol. 2003;185:2330–2337. doi: 10.1128/JB.185.7.2330-2337.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holt KE, et al. High-throughput sequencing provides insights into genome variation and evolution in Salmonella Typhi. Nat. Genet. 2008;40:987–993. doi: 10.1038/ng.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson RP, et al. The Vi capsular polysaccharide prevents complement receptor 3-mediated clearance of Salmonella enterica serotype Typhi. Infect. Immun. 2011;79:830–837. doi: 10.1128/IAI.00961-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson RP, et al. The Vi-capsule prevents Toll-like receptor 4 recognition of Salmonella. Cell Microbiol. 2008;10:876–890. doi: 10.1111/j.1462-5822.2007.01090.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang LF, et al. The Vi capsular polysaccharide of Salmonella Typhi promotes macrophage phagocytosis by binding the human C-type lectin DC-SIGN. mBio. 2022;13:e0273322. doi: 10.1128/mbio.02733-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song J, Gao X, Galán JE. Structure and function of the Salmonella Typhi chimaeric A2B5 typhoid toxin. Nature. 2013;499:350–354. doi: 10.1038/nature12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibler AEM, et al. Typhoid toxin exhausts the RPA response to DNA replication stress driving senescence and Salmonella infection. Nat. Commun. 2019;10:4040. doi: 10.1038/s41467-019-12064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Bel Belluz L, et al. The typhoid toxin promotes host survival and the establishment of a persistent asymptomatic infection. PLoS Pathog. 2016;12:e1005528. doi: 10.1371/journal.ppat.1005528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bronner DN, et al. Genetic ablation of butyrate utilization attenuates gastrointestinal Salmonella disease. Cell Host Microbe. 2018;23:266–273.e4. doi: 10.1016/j.chom.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, et al. MUC1 is a receptor for the Salmonella SiiE adhesin that enables apical invasion into enterocytes. PLoS Pathog. 2019;15:e1007566. doi: 10.1371/journal.ppat.1007566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorsey CW, Laarakker MC, Humphries AD, Weening EH, Bäumler AJ. Salmonella enterica serotype Typhimurium MisL is an intestinal colonization factor that binds fibronectin. Mol. Microbiol. 2005;57:196–211. doi: 10.1111/j.1365-2958.2005.04666.x. [DOI] [PubMed] [Google Scholar]
- 22.Khatiwara A, et al. Genome scanning for conditionally essential genes in Salmonella enterica serotype Typhimurium. Appl. Environ. Microbiol. 2012;78:3098–3107. doi: 10.1128/AEM.06865-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langridge GC, et al. Simultaneous assay of every Salmonella Typhi gene using one million transposon mutants. Genome Res. 2009;19:2308–2316. doi: 10.1101/gr.097097.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandal RK, Jiang T, Kwon YM. Genetic determinants in Salmonella enterica serotype Typhimurium required for overcoming in vitro stressors in the mimicking host environment. Microbiol. Spectr. 2021;9:e0015521. doi: 10.1128/Spectrum.00155-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karash S, Kwon YM. Iron-dependent essential genes in Salmonella Typhimurium. BMC Genomics. 2018;19:610. doi: 10.1186/s12864-018-4986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu D, et al. Genome-wide identification of genes involved in acid stress resistance of Salmonella Derby. Genes (Basel) 2021;12:476. doi: 10.3390/genes12040476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabbagh, S. C., Lepage, C., McClelland, M. & Daigle, F. Selection of Salmonella enterica serovar Typhi genes involved during interaction with human macrophages by screening of a transposon mutant library. PLoS One7, e36643 (2012). [DOI] [PMC free article] [PubMed]
- 28.Chan K, Kim CC, Falkow S. Microarray-based detection of Salmonella enterica serovar Typhimurium transposon mutants that cannot survive in macrophages and mice. Infect. Immun. 2005;73:5438–5449. doi: 10.1128/IAI.73.9.5438-5449.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canals R, et al. The fitness landscape of the African Salmonella Typhimurium ST313 strain D23580 reveals unique properties of the pBT1 plasmid. PLoS Pathog. 2019;15:e1007948. doi: 10.1371/journal.ppat.1007948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fong WY, et al. Genome-wide fitness analysis identifies genes required for in vitro growth and macrophage infection by African and global epidemic pathovariants of Salmonella enterica Enteritidis. Micro. Genom. 2023;9:mgen001017. doi: 10.1099/mgen.0.001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grant AJ, et al. Genes required for the fitness of Salmonella enterica serovar Typhimurium during infection of immunodeficient gp91−/− phox mice. Infect. Immun. 2016;84:989–997. doi: 10.1128/IAI.01423-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karlinsey JE, et al. Genome-wide analysis of Salmonella enterica serovar Typhi in humanized mice reveals key virulence features. Cell Host Microbe. 2019;26:426–434.e6. doi: 10.1016/j.chom.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawley TD, et al. Genome-wide screen for Salmonella genes required for long-term systemic infection of the mouse. PLoS Pathog. 2006;2:e11. doi: 10.1371/journal.ppat.0020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price MN, et al. Mutant phenotypes for thousands of bacterial genes of unknown function. Nature. 2018;557:503–509. doi: 10.1038/s41586-018-0124-0. [DOI] [PubMed] [Google Scholar]
- 35.Cain AK, et al. A decade of advances in transposon-insertion sequencing. Nat. Rev. Genet. 2020;21:526–540. doi: 10.1038/s41576-020-0244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wetmore KM, et al. Rapid quantification of mutant fitness in diverse bacteria by sequencing randomly bar-coded transposons. mBio. 2015;6:e00306–e00315. doi: 10.1128/mBio.00306-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kröger C, et al. An infection-relevant transcriptomic compendium for Salmonella enterica serovar Typhimurium. Cell Host Microbe. 2013;14:683–695. doi: 10.1016/j.chom.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Leshchiner D, et al. A genome-wide atlas of antibiotic susceptibility targets and pathways to tolerance. Nat. Commun. 2022;13:3165. doi: 10.1038/s41467-022-30967-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thanassi DG, Cheng LW, Nikaido H. Active efflux of bile salts by Escherichia coli. J. Bacteriol. 1997;179:2512–2518. doi: 10.1128/jb.179.8.2512-2518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baucheron S, et al. Bile-mediated activation of the acrAB and tolC multidrug efflux genes occurs mainly through transcriptional derepression of ramA in Salmonella enterica serovar Typhimurium. J. Antimicrob. Chemother. 2014;69:2400–2406. doi: 10.1093/jac/dku140. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Z, et al. Lipid A modifications in polymyxin-resistant Salmonella Typhimurium: PMRA-dependent 4-amino-4-deoxy-L-arabinose, and phosphoethanolamine incorporation. J. Biol. Chem. 2001;276:43111–43121. doi: 10.1074/jbc.M106960200. [DOI] [PubMed] [Google Scholar]
- 42.Olaitan AO, Morand S, Rolain J-M. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front. Microbiol. 2014;5:643. doi: 10.3389/fmicb.2014.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gunn JS. The Salmonella PmrAB regulon: lipopolysaccharide modifications, antimicrobial peptide resistance and more. Trends Microbiol. 2008;16:284–290. doi: 10.1016/j.tim.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Shi Y, Cromie MJ, Hsu F-F, Turk J, Groisman EA. PhoP-regulated Salmonella resistance to the antimicrobial peptides magainin 2 and polymyxin B. Mol. Microbiol. 2004;53:229–241. doi: 10.1111/j.1365-2958.2004.04107.x. [DOI] [PubMed] [Google Scholar]
- 45.Imlay, J. A., Chin, S. M. & Linn, S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science240, 640–642 (1988). [DOI] [PubMed]
- 46.Ojkic N, et al. A roadblock-and-kill mechanism of action model for the DNA-targeting antibiotic ciprofloxacin. Antimicrob. Agents Chemother. 2020;64:e02487–19. doi: 10.1128/AAC.02487-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Denkel LA, Rhen M, Bange F-C. Biotin sulfoxide reductase contributes to oxidative stress tolerance and virulence in Salmonella enterica serovar Typhimurium. Microbiology (Reading) 2013;159:1447–1458. doi: 10.1099/mic.0.067256-0. [DOI] [PubMed] [Google Scholar]
- 48.Zhong Q, Kobe B, Kappler U. Molybdenum enzymes and how they support virulence in pathogenic bacteria. Front Microbiol. 2020;11:615860. doi: 10.3389/fmicb.2020.615860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, et al. LPS remodeling is an evolved survival strategy for bacteria. Proc. Natl Acad. Sci. USA. 2012;109:8716–8721. doi: 10.1073/pnas.1202908109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bertani, B. & Ruiz, N. Function and biogenesis of lipopolysaccharides. EcoSal Plus8, 10.1128/ecosalplus.ESP-0001-2018 (2018). [DOI] [PMC free article] [PubMed]
- 51.Grote A, et al. Persistent Salmonella infections in humans are associated with mutations in the BarA/SirA regulatory pathway. Cell Host Microbe. 2024;32:79–92.e7. doi: 10.1016/j.chom.2023.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sobota M, et al. The expression of virulence genes increases membrane permeability and sensitivity to envelope stress in Salmonella Typhimurium. PLoS Biol. 2022;20:e3001608. doi: 10.1371/journal.pbio.3001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baryshnikova A. Systematic functional annotation and visualization of biological networks. Cell Syst. 2016;2:412–421. doi: 10.1016/j.cels.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 54.Karp PD, et al. The BioCyc collection of microbial genomes and metabolic pathways. Brief. Bioinform. 2019;20:1085–1093. doi: 10.1093/bib/bbx085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baryshnikova, A. Spatial analysis of functional enrichment (SAFE) in large biological networks. Methods Mol. Biol.1819, 249–268 (2018). [DOI] [PubMed]
- 56.Zuñiga J, et al. Salmonella enterica serovar Typhi O:1,9,12 polysaccharide-protein conjugate as a diagnostic tool for typhoid fever. J. Clin. Microbiol. 2005;43:4545–4550. doi: 10.1128/JCM.43.9.4545-4550.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koropatkin NM, Liu H, Holden HM. High resolution X-ray structure of tyvelose epimerase from Salmonella Typhi. J. Biol. Chem. 2003;278:20874–20881. doi: 10.1074/jbc.M301948200. [DOI] [PubMed] [Google Scholar]
- 58.Husna AU, et al. Methionine biosynthesis and transport are functionally redundant for the growth and virulence of Salmonella Typhimurium. J. Biol. Chem. 2018;293:9506–9519. doi: 10.1074/jbc.RA118.002592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holt KE, et al. Emergence of a globally dominant IncHI1 plasmid type associated with multiple drug resistant typhoid. PLoS Negl. Trop. Dis. 2011;5:e1245. doi: 10.1371/journal.pntd.0001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang G, Nauseef WM. Salt, chloride, bleach, and innate host defense. J. Leukoc. Biol. 2015;98:163–172. doi: 10.1189/jlb.4RU0315-109R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schomer, R. A., Park, H., Barkei, J. J. & Thomas, M. G. Alanine scanning of YbdZ, an MbtH-like protein, reveals essential residues for functional interactions with its nonribosomal peptide synthetase partner EntF. Biochemistry57, 4125–4134 (2018). [DOI] [PMC free article] [PubMed]
- 62.Outten FW, Djaman O, Storz G. A suf operon requirement for Fe-S cluster assembly during iron starvation in Escherichia coli. Mol. Microbiol. 2004;52:861–872. doi: 10.1111/j.1365-2958.2004.04025.x. [DOI] [PubMed] [Google Scholar]
- 63.Roche B, et al. Iron/sulfur proteins biogenesis in prokaryotes: formation, regulation and diversity. Biochim. Biophys. Acta. 2013;1827:455–469. doi: 10.1016/j.bbabio.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 64.Wollers, S. et al. Iron-sulfur (Fe-S) cluster assembly: the SufBCD complex is a new type of Fe-S scaffold with a flavin redox cofactor. J. Biol. Chem.285, 23331–23341 (2010). [DOI] [PMC free article] [PubMed]
- 65.Figueira R, Watson KG, Holden DW, Helaine S. Identification of Salmonella pathogenicity island-2 type III secretion system effectors involved in intramacrophage replication of S. enterica serovar typhimurium: implications for rational vaccine design. mBio. 2013;4:e00065. doi: 10.1128/mBio.00065-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saliba AE, et al. Single-cell RNA-seq ties macrophage polarization to growth rate of intracellular Salmonella. Nat. Microbiol. 2016;2:16206. doi: 10.1038/nmicrobiol.2016.206. [DOI] [PubMed] [Google Scholar]
- 67.Pham THM, et al. Salmonella-driven polarization of granuloma macrophages antagonizes TNF-mediated pathogen restriction during persistent infection. Cell Host Microbe. 2020;27:54–67.e5. doi: 10.1016/j.chom.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Monack DM, Detweiler CS, Falkow S. Salmonella pathogenicity island 2-dependent macrophage death is mediated in part by the host cysteine protease caspase-1. Cell Microbiol. 2001;3:825–837. doi: 10.1046/j.1462-5822.2001.00162.x. [DOI] [PubMed] [Google Scholar]
- 69.Hamblin M, Schade R, Narasimhan R, Monack DM. Salmonella enterica serovar Typhi uses two type 3 secretion systems to replicate in human macrophages and colonize humanized mice. mBio. 2023;14:e0113723. doi: 10.1128/mbio.01137-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reuter T, Scharte F, Franzkoch R, Liss V, Hensel M. Single cell analyses reveal distinct adaptation of typhoidal and non-typhoidal Salmonella enterica serovars to intracellular lifestyle. PLoS Pathog. 2021;17:e1009319. doi: 10.1371/journal.ppat.1009319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crawford RW, et al. Loss of very-long O-antigen chains optimizes capsule-mediated immune evasion by Salmonella enterica serovar Typhi. mBio. 2013;4:e00232–13. doi: 10.1128/mBio.00232-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mylona E, et al. Very long O-antigen chains of Salmonella Paratyphi A inhibit inflammasome activation and pyroptotic cell death. Cell Microbiol. 2021;23:e13306. doi: 10.1111/cmi.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hébrard M, Viala JPM, Méresse S, Barras F, Aussel L. Redundant hydrogen peroxide scavengers contribute to Salmonella virulence and oxidative stress resistance. J. Bacteriol. 2009;191:4605–4614. doi: 10.1128/JB.00144-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ronneau S, Michaux C, Helaine S. Decline in nitrosative stress drives antibiotic persister regrowth during infection. Cell Host Microbe. 2023;31:993–1006.e6. doi: 10.1016/j.chom.2023.05.002. [DOI] [PubMed] [Google Scholar]
- 75.Ganz T. Iron in innate immunity: starve the invaders. Curr. Opin. Immunol. 2009;21:63–67. doi: 10.1016/j.coi.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Waters LS, Sandoval M, Storz G. The Escherichia coli MntR miniregulon includes genes encoding a small protein and an efflux pump required for manganese homeostasis. J. Bacteriol. 2011;193:5887–5897. doi: 10.1128/JB.05872-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Que Q, Helmann JD. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol. Microbiol. 2000;35:1454–1468. doi: 10.1046/j.1365-2958.2000.01811.x. [DOI] [PubMed] [Google Scholar]
- 78.Ouyang A, Gasner KM, Neville SL, McDevitt CA, Frawley ER. MntP and YiiP contribute to manganese efflux in Salmonella enterica serovar Typhimurium under conditions of manganese overload and nitrosative stress. Microbiol Spectr. 2022;10:e0131621. doi: 10.1128/spectrum.01316-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ha N, Lee E-J. Manganese transporter proteins in Salmonella enterica serovar Typhimurium. J. Microbiol. 2023;61:289–296. doi: 10.1007/s12275-023-00027-7. [DOI] [PubMed] [Google Scholar]
- 80.Vergnes A, et al. The iron-sulfur cluster sensor IscR is a negative regulator of Spi1 type III secretion system in Salmonella enterica. Cell Microbiol. 2017;19:e12680. doi: 10.1111/cmi.12680. [DOI] [PubMed] [Google Scholar]
- 81.Mettert EL, Kiley PJ. Coordinate regulation of the Suf and Isc Fe-S cluster biogenesis pathways by IscR is essential for viability of Escherichia coli. J. Bacteriol. 2014;196:4315–4323. doi: 10.1128/JB.01975-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robbe-Saule V, Norel F. The rpoS mutant allele of Salmonella typhi Ty2 is identical to that of the live typhoid vaccine Ty21a. FEMS Microbiol. Lett. 1999;170:141–143. doi: 10.1111/j.1574-6968.1999.tb13366.x. [DOI] [PubMed] [Google Scholar]
- 83.Cianfanelli FR, Cunrath O, Bumann D. Efficient dual-negative selection for bacterial genome editing. BMC Microbiol. 2020;20:129. doi: 10.1186/s12866-020-01819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murray GL, Attridge SR, Morona R. Altering the length of the lipopolysaccharide O antigen has an impact on the interaction of Salmonella enterica serovar Typhimurium with macrophages and complement. J. Bacteriol. 2006;188:2735–2739. doi: 10.1128/JB.188.7.2735-2739.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Honeycutt JD, et al. Genetic variation in the MacAB-TolC efflux pump influences pathogenesis of invasive Salmonella isolates from Africa. PLoS Pathog. 2020;16:e1008763. doi: 10.1371/journal.ppat.1008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goodman AL, et al. Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice. Proc. Natl Acad. Sci. USA. 2011;108:6252–6257. doi: 10.1073/pnas.1102938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kirk JA, Fagan RP. Heat shock increases conjugation efficiency in Clostridium difficile. Anaerobe. 2016;42:1–5. doi: 10.1016/j.anaerobe.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zeng X, Ardeshna D, Lin J. Heat shock-enhanced conjugation efficiency in standard Campylobacter jejuni strains. Appl. Environ. Microbiol. 2015;81:4546–4552. doi: 10.1128/AEM.00346-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gray J, et al. Transposon mutagenesis screen in Klebsiella pneumoniae identifies genetic determinants required for growth in human urine and serum. eLife. 2023;12:RP88971. [Google Scholar]
- 90.Barquist L, et al. A comparison of dense transposon insertion libraries in the Salmonella serovars Typhi and Typhimurium. Nucleic Acids Res. 2013;41:4549–4564. doi: 10.1093/nar/gkt148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang BX, et al. Mucin glycans signal through the sensor kinase RetS to inhibit virulence-associated traits in Pseudomonas aeruginosa. Curr. Biol. 2021;31:90–102.e7. doi: 10.1016/j.cub.2020.09.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang BX, et al. Host‐derived O‐glycans inhibit toxigenic conversion by a virulence‐encoding phage in Vibrio cholerae. EMBO J. 2023;42:e111562. doi: 10.15252/embj.2022111562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brewer SM, et al. A Salmonella Typhi RNA thermosensor regulates virulence factors and innate immune evasion in response to host temperature. PLoS Pathog. 2021;17:e1009345. doi: 10.1371/journal.ppat.1009345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang, B., Monack, D. & Dmitry, L. Scripts used in “high-throughput fitness experiments reveal specific vulnerabilities of human-adapted salmonella during stress and infection”. Zenodo10.5281/zenodo.10963611 (2024). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figs. 1–16 and Notes 1 and 2.
Table 1, All strains used in this study. Table 2, All plasmids used in this study. Table 3, All primers used in this study. Table 4, Summary of barcoded libraries used in this study, including the number of genes with central insertions and bias metrics. Table 5, Summary of all conditions tested, with concentrations of each stressor indicated for each serovar. Table 6, List of orthologs for all shared genes among S. Typhimurium ST4/74, S. Typhi Ty2, S. Paratyphi A 9150, and S. Typhimurium D23580. Table 7, List of genes in all clusters from the heatmaps in Supplementary Fig. 11, along with their log2 fitness changes during macrophage infection. Table 8, List of all Typhi-specific changes in fitness during macrophage infection, defined as genes that have a FC > 4× higher in S. Typhi Ty2 compared with S. Typhimurium ST4/74. Table 9, List of all Paratyphi-specific changes in fitness, defined as genes that have a FC > 4× higher in S. Paratyphi A 9150 compared with S. Typhimurium ST4/74. Table 10, List of all P values for one-way ANOVA tests from Fig. 6f,h and Extended Data Fig. 4k, with multiple comparisons corrected by controlling the FDR according to the Benjamini, Krieger and Yekutieli method.
Supplemental Data 1, List of all essential genes for each serovar. S. Typhimurium ST4/74 genes are on sheet 1, S. Typhi Ty2 genes are on sheet 2, S. Paratyphi A 9150 genes are on sheet 3, and S. Typhimurium D23580 genes are on sheet 4.
Supplemental Data 2, List of all quality-control metrics for each Rb-Tn-seq experiment. S. Typhimurium ST4/74 experiments are on sheet 2, S. Typhi Ty2 experiments are on sheet 3, S. Paratyphi A 9150 experiments are on sheet 4, and S. Typhimurium D23580 experiments are on sheet 5. N-used refers to the total number of reads in central portions of genes (10–90% of the gene sequence). gMed is the median reads per gene in the sample. Cor12 is a measurement of how consistent the fitness data is for each gene. This metric is calculated by comparing the fitness of a gene using only Tn insertions in the first half of each gene region with the fitness from Tn insertions in the second half of each gene region. The cor12 value is the Spearman rank correlation of these two sets of values. Mad12 measures the median absolute difference between the fitness according to Tn insertions in the first half versus the second half of each gene. Opcor measures the consistency of fitness data for each operon, as genes within the same operon should have similar fitness values. In this metric, a Spearman rank correlation on the fitness value for each upstream and downstream gene within an operon is calculated. Sheet 1 contains R-values (correlation) between all biological replicate samples, for each condition and each serovar.
Supplemental Data 3, All FC for each serovar for all conditions, for the 24 plate-based assays. S. Typhimurium ST4/74 genes are on sheet 1, S. Typhi Ty2 genes are on sheet 2, S. Paratyphi A 9150 genes are on sheet 3, and S. Typhimurium D23580 genes are on sheet 4.
Supplemental Data 4, All moderated t-like statistics for each serovar for all conditions, for the 24 plate-based assays. S. Typhimurium ST4/74 genes are on sheet 1, S. Typhi Ty2 genes are on sheet 2, S. Paratyphi A 9150 genes are on sheet 3, and S. Typhimurium D23580 genes are on sheet 4.
Supplemental Data 5, List of genes in all clusters from the heatmaps in Supplementary Figs. 4–7, along with their moderated t-statistics in all conditions. Only genes with t > 4 are included. S. Typhimurium ST4/74 genes are on sheet 1, S. Typhi Ty2 genes are on sheet 2, S. Paratyphi A 9150 genes are on sheet 3, and S. Typhimurium D23580 genes are on sheet 4.
Supplemental Data 6, Moderated t-like statistics for all SPI-encoded genes, for the 24 plate-based assays. S. Typhimurium ST4/74 genes are on sheet 1, S. Typhi Ty2 genes are on sheet 2, S. Paratyphi A 9150 genes are on sheet 3, and S. Typhimurium D23580 genes are on sheet 4. Any genes with t > 4 are in bold.
Supplemental Data 7, List of moderated t-statistics for all genes uniquely encoded by S. Typhi Ty2 (sheet 1) and S. Paratyphi A 9150 (sheet 2).
Supplemental Data 8, List of all correlations with R > 0.75 among pairs of genes in the cofitness network maps shown in Extended Data Fig. 1a–d. Each correlation also includes a stability score, in which the correlation matrices were generated by hiding subsets of the conditions 100×; the stability score ranges from 0 to 100, which scores > 75 being considered stable; t-statistics were calculated from each correlation using a sample size of 24 conditions, which were then converted to P values using a cumulative distribution function of the Student’s t-distribution, and FDR calculations were performed using the Benjamini–Yekutieli method. Correlations for S. Typhimurium ST4/74 are on sheet 1, correlations for S. Typhi Ty2 are on sheet 2, correlations for S. Paratyphi A 9150 are on sheet 3, and correlations for S. Typhimurium D23580 are on sheet 4.
Supplemental Data 9, All BioCyc/GO term annotations for making cofitness network and SAFE maps. S. Typhimurium ST4/74 genes are on sheet 1, S. Typhi Ty2 genes are on sheet 2, S. Paratyphi A 9150 genes are on sheet 3, and S. Typhimurium D23580 genes are on sheet 4.
Supplemental Data 10, Filtered list of all correlations with R > 0.75 that contain at least one hypothetical gene. Correlations for S. Typhimurium ST4/74 are on sheet 1, correlations for S. Typhi Ty2 are on sheet 2, correlations for S. Paratyphi A 9150 are on sheet 3, and correlations for S. Typhimurium D23580 are on sheet 4.
Supplemental Data 11, List of all Typhi-specific changes in fitness, defined as genes that (1) have significant fitness changes (t > 4) in S. Typhi Ty2 but not in S. Typhimurium ST4/74 and (2) have a FC > 2-fold higher in S. Typhi Ty2 compared with S. Typhimurium ST4/74 in at least one condition. All significance values are on sheet 1 and all FC are on sheet 2.
Supplemental Data 12, List of all Paratyphi A-specific changes in fitness, defined as genes that (1) have significant fitness changes (t > 4) in S. Paratyphi A 9150 but not in S. Typhimurium ST4/74 and (2) have a FC > 2-fold higher in S. Paratyphi A 9150 compared with S. Typhimurium ST4/74 in at least one condition. All significance values are on sheet 1, and all FC are on sheet 2.
Supplemental Data 13, List of all D23580-specific changes in fitness, defined as genes that (1) have significant fitness changes (t > 4) in S. Typhimurium D23580 but not in S. Typhimurium ST4/74 and (2) have a FC > 2-fold higher in S. Typhimurium D23580 compared with S. Typhimurium ST4/74 in at least one condition. All significance values are on sheet 1, and all FC are on sheet 2.
Supplemental Data 14, All FC and statistics for each of the four serovars from the macrophage infection Rb-Tn-seq experiment. S. Typhimurium ST4/74 genes are on sheet 1, S. Typhi Ty2 genes are on sheet 2, S. Paratyphi A 9150 genes are on sheet 3, and S. Typhimurium D23580 genes are on sheet 4. FC and P values were calculated using DEseq2 analysis.
Data Availability Statement
All strains from this study are available by request to the corresponding author. All raw sequencing data and processed fitness data in this study can be found on NCBI GEO at accession numbers GSE261860, GSE261867, GSE261873, GSE262768, GSE262769, GSE262848, GSE261757, GSE261749 and GSE261214. All raw FC and statistics from the dataset are in Supplementary Data 3, 4 and 14. All raw correlations for network analysis are in Supplementary Data 8. All source data for SAFE annotations are in Supplementary Data 9. All fitness changes from this work can be searched on an interactive website (https://bioinf.gen.tcd.ie/cgi-bin/salcomfit.pl).
Code used in this study is derived from previous publications36,38, which we have deposited to Zenodo at 10.5281/zenodo.10963611 (ref. 94).