Abstract
Background
Latina women experience disproportionately higher rates of HPV infection, persistence, and progression to cervical dysplasia and cancer compared to other racial–ethnic groups. This systematic review explores the relationship between the cervicovaginal microbiome and human papillomavirus infection, cervical dysplasia, and cervical cancer in Latinas.
Methods
The review abides by the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines. PubMed, EMBASE, and Scopus databases were searched from January 2000 through November 11, 2022. The review included observational studies reporting on the cervicovaginal microbiota in premenopausal Latina women with human papillomavirus infection, cervical dysplasia, and cervical cancer.
Results
Twenty-five articles were eligible for final inclusion (N = 131,183). Forty-two unique bacteria were reported in the cervicovaginal microbiome of Latinas. Seven bacteria: Lactobacillus crispatus, Lactobacillus iners, Chlamydia trachomatis, Prevotella spp., Prevotella amnii, Fusobacterium spp. and Sneathia spp. were enriched across multiple stages of cervical carcinogenesis in Latinas. Therefore, the total number of reported bacteria includes four bacteria associated with the healthy state, 16 bacteria enriched in human papillomavirus outcomes, 24 unique bacteria associated with abnormal cytology/dysplasia, and five bacteria associated with cervical cancer. Furthermore, three studies reported significantly higher alpha and beta diversity in Latinas with cervical dysplasia and cancer compared to controls. Lactobacillus depletion and an increased abundance of L. iners in Latinas compared to non-Latinas, regardless of human papillomavirus status or lesions, were observed.
Conclusions
The identification of 42 unique bacteria and their enrichment in cervical carcinogenesis can guide future cervicovaginal microbiome research to better inform cervical cancer prevention strategies in Latinas.
Supplementary Information
The online version contains supplementary material available at 10.1007/s44197-024-00201-z.
Keywords: Microbiome, Cervical cancer, Health disparities, Human papillomavirus, Latin America and the Caribbean, Latinas
Introduction
Cervical cancer is the fourth most common cancer affecting women worldwide [1]. Incident rates vary among high- and low-income countries due to public health efforts targeting this preventable disease. As a result of population-based cancer screening and human papillomavirus (HPV) vaccination programs, cervical cancer incidence and mortality rates have largely declined in high-income countries [1]. However, in Latin America and the Caribbean (LAC), HPV prevalence and cervical cancer mortality are among the highest in the world [2]. By 2025, 126,000 Latinas are predicted to be diagnosed with cervical cancer in LAC—a 75% rise in the frequency of the disease from 2002 [2].
Latina women are disproportionately affected by risk factors for HPV infection, persistence, and progression to cervical dysplasia and cancer [3–9]. Latinas experience the highest rates of HPV infection, with an incidence of over 40%, and are 40% more likely to be diagnosed with cervical cancer compared to other racial–ethnic groups [6–8]. In addition, Latinas have the highest cervical cancer incidence of any racial–ethnic group and are 24% more likely to die from cervical cancer compared to non-Hispanic White women [7]. This disparity could be exacerbated by systemic barriers that prevent Latinas from receiving adequate healthcare services, including HPV vaccination, cervical cancer screenings, and health education [2, 8, 9].
The premalignant precursor of cervical cancer, cervical intraepithelial neoplasia (CIN), is caused by persistent infection with high-risk HPV genotypes [10]. Although over 90% of HPV infections are cleared, reinfection of HPV can occur, and persistent HPV infection is linked to carcinogenesis [11]. The optimal environment that promotes HPV clearance is not completely understood; however, evidence shows that cervicovaginal microbiota play a dual role in HPV clearance or persistence and cervical cancer development and progression [10, 12, 13].
A cervicovaginal microbiome dominated by Lactobacillus species is often a proxy for vaginal health. Lactobacillus species facilitate homeostasis by creating a lactic acid-enriched microenvironment; this competitive niche adaptation indirectly protects the host from invading pathogens [10, 14–16]. Bacterial vaginosis (BV) is characterized by a depletion in Lactobacillus species and an increase in microaerophilic and anaerobic microbes, such as Fannyhessea/Atopobium, Gardnerella, Prevotella, and Sneathia species [15–18]. Notably, BV is associated with an increased risk of sexually transmitted infections (STIs), including HPV [10, 14–21].
Two recent systematic reviews in non-Hispanic White women have (1) established a causal link between vaginal dysbiosis and cervical carcinogenesis [12] and (2) assessed community state types in women with HPV infection, cervical dysplasia, and cervical cancer [22]. However, an individual analysis of specific cervicovaginal bacteria in Latina women who are particularly high risk for adverse gynecologic sequelae has not been conducted. The cervicovaginal microbiota of Latinas must be further investigated, given the significantly high rates of vaginal dysbiosis, HPV infection, and cervical cancer in this population [2, 3, 6–8, 21, 23, 24].
Objectives
The purpose of the present review was to identify bacteria reported in the cervicovaginal microbiome of Latinas relating to HPV infection, cervical dysplasia, and cervical cancer as well as better understand the role of the microbiome in these disease states worldwide. These data could provide novel insights and approaches to address health disparities in HPV infection, persistence, and cervical cancer morbidity and mortality in this historically understudied, underrepresented, and underreported population of women.
Methods
This systematic review followed the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analyses) guidelines and was registered in PROSPERO (CRD42022367244) on 11/21/2022 [25]. No similar systematic review or protocol was registered.
Eligibility Criteria, Information Sources, Search Strategy
Studies in which participants were pre-menopausal women identifying as Hispanic or Latina according to the U.S Census Bureau definition: “a person of Cuban, Mexican, Puerto Rican, South or Central American, or other Spanish culture or origin regardless of race” were included in the systematic review [26]. Furthermore, studies were only included if participants were diagnosed with either HPV, dysplasia, and/or cervical cancer or were healthy controls for studying these disease states. In addition, studies were required to be observational and describe analyses related to cervicovaginal microbiota. Interventional studies, article reviews, or commentaries were excluded. Studies evaluating pregnant people or non-human subjects were also excluded.
The following electronic databases were searched from January 2000 through November 11, 2022: PubMed, EMBASE (Elsevier), and Scopus. A search of ClinicalTrials.gov was not applicable because the studies for this review were not interventional. The search strategy employed a combination of terms related to “vagina/cervix” and “microbiota.” This review used the Latinx/Hispanic US Population Search Hedge developed by the Medical Library Association Latinx Caucus for this literature search [27]. The search was restricted to articles published after January 2000 on “female(s)” with no language restrictions. A more detailed report of the search strategy can be found in Supplementary Table 1. All records were exported into the bibliographic software EndNote X9 to remove duplicates. The unique records were then transferred to a web-based systematic review software, DistillerSR [28].
Study Selection
Titles and abstracts of retrieved articles were screened independently by two authors (V.M., N.R.J.). Any discrepancies were included for full-text screening to determine eligibility. The same two authors (V.M., N.R.J.) independently screened full-text articles for eligibility, with discrepancies resolved by a third author (M.M.H–K).
Data Extraction
Two authors (V.M., N.R.J.) independently performed data extraction. A standardized data collection form in DistillerSR was created to collect: (1) participant characteristics (i.e., age, menopausal status, and gender identity), (2) geographic characteristics, (3) study characteristics (i.e., recruitment site, sample collection method, number of participants) (4) clinical characteristics (i.e., HPV genotypes, indication of abnormal Papanicolaou (Pap) smears, histological stage/grade, and comorbidities), and (5) microbiome methodology (i.e., identification methods for the microbiome and HPV, community state types, Lactobacillus dominance/depletion, alpha and beta diversity, and bacteria associated with outcome groups). Discrepancies in data collection were resolved after discussion and reviewing the full-text article. The compiled data from the full-text extraction are provided in Supplementary Table 2.
Assessment of Risk of Bias
The Risk of Bias in Non-randomized Studies of Exposure (ROBINS-E) tool was utilized to evaluate the risk of bias for observational studies [29]. The ROBINS-E tool evaluates each study according to seven domains of bias: (1) due to confounding, (2) arising from measurement of the exposure, (3) in selection of participants into the study (or into the analysis), (4) due to post-exposure interventions, (5) due to missing data, (6) arising from measurement of the outcome, and (7) in selection of the reported result. The risk of bias for each domain and overall bias for each study was consolidated into a summary table; see Table 1.
Table 1.
ROBINS-E Risk of Bias for Included Articles
| ID# | First Author | Domain 1: Risk of bias due to confoundinga | Domain 2: Risk of bias arising from measurement of the exposure | Domain 3: Risk of bias in selection of participants into the study | Domain 4: Risk of bias due to post-exposure interventions | Domain 5: Risk of bias due to missing data | Domain 6: Risk of bias arising from measurement of the outcome | Domain 7: Risk of bias in selection of the reported result | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Nieves-Ramirez et al. | Low | Low | Some concerns | Low | High | Some concerns | Some concerns | High |
| 2 | Mosmann et al. | Some concerns | Low | Some concerns | Some concerns | Low | Low | Some concerns | Some concerns |
| 3 | Carrillo-Ng et al. | High | Low | Some concerns | Some concerns | Some concerns | Low | Low | High |
| 4 | Hernandez-Rosas et al. | High | Low | Some concerns | Some concerns | Some concerns | Low | Low | High |
| 5 | Conde-Ferráez et al. | Very High | ⎽ | ⎽ | ⎽ | ⎽ | ⎽ | ⎽ | Very High |
| 6 | Vargas-Robles et al. | High | Low | Some concerns | Some concerns | Some concerns | Low | Low | High |
| 7 | Torres-Poveda et al. | High | Low | Some concerns | Some concerns | Low | Low | Low | High |
| 8 | Bristow et al. | Very High | ⎽ | ⎽ | ⎽ | ⎽ | ⎽ | ⎽ | Very High |
| 9 | Romero-Morelos et al. | High | Low | Some concerns | Some concerns | Some concerns | Low | Low | High |
| 10 | Melo et al. | Very High | ⎽ | ⎽ | ⎽ | ⎽ | ⎽ | ⎽ | Very High |
| 11 | Mongelos et al. | Some concerns | Low | Some concerns | Some concerns | Low | Low | Low | Some concerns |
| 12 | Lippman et al. | High | Low | Some concerns | Some concerns | Some concerns | Some concerns | Low | High |
| 13 | Soto et al. | Very High | ⎽ | ⎽ | ⎽ | ⎽ | ⎽ | ⎽ | Very High |
| 14 | DeLuca et al. | Very High | ⎽ | ⎽ | ⎽ | ⎽ | ⎽ | ⎽ | Very High |
| 15 | Tonon et al. | High | Low | Some concerns | Some concerns | High | Low | Low | High |
| 16 | Somesh-Vikramdeo et al. | Very High | ⎽ | ⎽ | ⎽ | ⎽ | ⎽ | ⎽ | Very High |
| 17 | Manzanares-Leal et al. | High | Low | Some concerns | Some concerns | Some concerns | Low | Low | High |
| 18 | Sanchez-Garcia et al. | High | Low | Some concerns | Some concerns | Low | Low | Low | High |
| 19 | de Oliveira Ignacio et al. | Very High | ⎽ | ⎽ | ⎽ | ⎽ | ⎽ | ⎽ | Very High |
| 20 | Godoy-Vitorino et al. | High | Low | Some concerns | Some concerns | Some concerns | Low | Low | High |
| 21 | Łaniewski et al. | Some concerns | Low | Some concerns | Some concerns | Some concerns | Low | Low | Some concerns |
| 22 | Gomes de Oliveira et al. | High | Low | Some concerns | Some concerns | High | Low | Low | High |
| 23 | Audirac-Chalifour et al. | Low | Low | Some concerns | Some concerns | Low | Low | Low | Some concerns |
| 24 | Clarke et al. | High | Low | Some concerns | Some concerns | Some concerns | Low | Low | High |
| 25 | Escarcega-Tame et al. | High | Low | Some concerns | Some concerns | High | Low | Low | High |
ROBINS-E risk of bias associated with each of the seven domains and overall risk of bias for each included study are reported. The level of bias can be categorized as low risk, some concerns, high risk, or very high risk. The dash symbol denotes “no rating.” The ROBINS-E assessment concludes as very high risk after reporting the highest risk of bias rating in Domain 1. aFive confounding variables must be controlled to rate Domain 1 as low risk
Data Synthesis
A data collection form was developed and completed for each article in DistillerSR. Data was synthesized in a tabulated form by DistillerSR and downloaded as an Excel spreadsheet (Supplementary Table 2).
Results
Study Selection
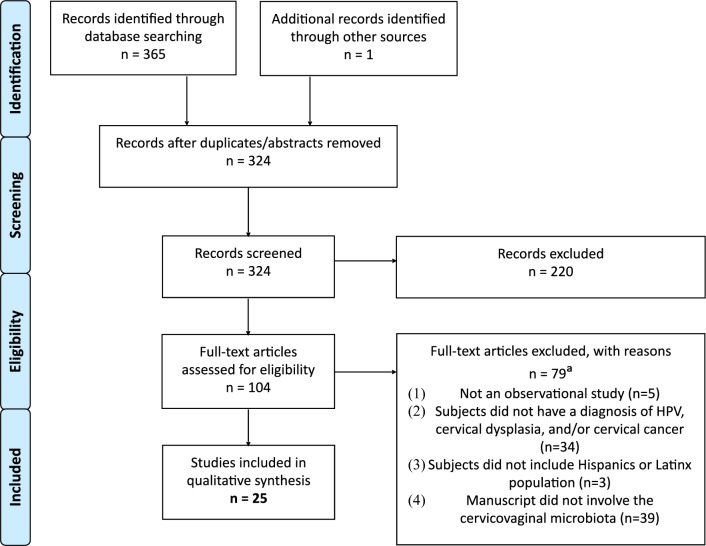
The initial search yielded a total of 365 articles from three databases: PubMed (178), EMBASE (168), and Scopus (19). One article was included due to a similar and relevant title during the full-text upload of documents. Twenty-five conference abstracts and 17 duplicates were removed, resulting in 324 unique articles. A title–abstract screening was performed on 324 articles, leading to the exclusion of 220 articles. One hundred four full-text articles were assessed for eligibility. Seventy-nine studies were excluded for not meeting the inclusion criteria. Nearly, half of the excluded articles (39/79) were ineligible because they lacked information on the cervicovaginal microbiome. This search method yielded a final sample of 25 full-text articles; see Fig. 1 for the PRISMA methodological flowchart.
Fig. 1.
PRISMA Methodological Flowchart. The flowchart depicts the identification, selection, and final inclusion of articles. Records of excluded articles and reasons for exclusion are also included. aThe total number of full-text articles excluded was n = 79. However, articles could be excluded for more than one reason. Two articles were excluded for multiple reasons, therefore the total sum for each reason is 81
Study Characteristics
The key characteristics of the 25 included observational studies are featured in Table 2. Some longitudinal studies were included but most studies were cross-sectional. The types of cross-sectional studies featured include descriptive, population-based, and prevalence studies. No qualitative studies were included. The studies were published between 2004 and 2022. The geographical regions of the studies encompass North [ID# 1, 4, 5, 7–9, 16–18, 20, 21, 23, 25], Central [ID# 24], and South America [ID# 2, 3, 6, 10–12, 14, 15, 19, 22, 24], including the Caribbean [ID# 13]. Studies from ten different countries were included. The total number of participants in the review was 131,183. With the exception of three articles [ID# 9, 13, 21], most studies reported the age of participants with the minimum reported age of 12 years old [ID# 6] and the maximum reported age of 100 years old [ID# 24]. The two most common methods of sample collection were by vaginal swab (n = 10, 40%) [ID# 1, 6, 8, 12, 17–21, 24] and cytobrush (n = 10, 40%) [ID# 2–5, 9–11, 14, 15, 19], followed by biopsy (n = 2, 8%) [ID# 7, 16], cervicovaginal lavage (n = 1, 4%) [ID# 19], endo/ectocervical scrapings (n = 1, 4%) [ID# 13], and endocervical swab (n = 1, 4%) [ID# 25]. Two studies did not report sample collection methods (n = 2, 8%) [ID# 22, 23]. In addition, two studies used multiple methods of sample collection, which accounts for the overall percentage above 100% [ID# 19, 21]. Of the 25 included articles, 22 studies reported on HPV outcomes (88%) [ID# 1, 3–7, 9–16, 18–25]. The most common HPV genotypes reported were HPV16, HPV31, and HPV6 (Supplementary Table 2). In addition, 13 studies [ID# 1, 2, 8, 9, 13–16, 20–23, 25] reported on abnormal cytology/dysplasia outcomes (46%), and four studies [ID# 17, 21, 23, 25] reported on cervical cancer outcomes (16%). Ten articles [ID# 1, 9, 13, 15, 16, 20–23, 25] reported on more than one outcome; three studies reported on all three outcomes [ID# 21, 23, 25].
Table 2.
Study Characteristics and Key Findings of the Included Articles
| ID# | First Author (Publication Year) | NParticipants | NOutcomeGrp | NCtrlGrp | Age range | Location | HPV outcome | Abnormal cytology outcome | Cervical cancer outcome | Sample collections | Bacteria identified | Other findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Nieves-Ramirez et al. (2021) | 228 | 121 | 107 | 21 + | Mexico City, Mexico | ✓ | ✓ | ⎽ | Vaginal swab | ⎽ | Alpha diversity: individuals with SILs had significantlty higher species richness and evenness compared to non-SILs; beta diversity: SILs explains 1.4% of the variation in vaginal bacterial community structure |
| 2 | Mosmann et al. (2021) | 100 | 50 | 50 | 18–67 | Córdoba, Argentina | ⎽ | ✓ | ⎽ | Cytobrush | Chlamydia trachomatis | ⎽ |
| 3 | Carrillo-Ng et al. (2021) | 833 | 200 | 633 | 27–43 | Cajamarca, Peru | ✓ | ⎽ | ⎽ | Cytobrush | Eubacterium, Actinobacteria, Fusobacterium, Bacteroides, Bifidobacterium, Enterococcus | ⎽ |
| 4 | Hernandez-Rosas et al. (2021) | 1000 | 272 | 728 | 18–65 | Sonora, Mexico | ✓ | ⎽ | ⎽ | Cytobrush | Actinomyces | ⎽ |
| 5 | Conde-Ferráez et al. (2017) | 233 | 63 | 170 | 15–49 | Merida, Yucatan, Mexico | ✓ | ⎽ | ⎽ | Cytobrush | Chlamydia trachomatis | ⎽ |
| 6 | Vargas-Robles et al. (2020) | 111 | 74 | 21 | 12–53 | Venezuela | ✓ | ⎽ | ⎽ | Vaginal swab | Anaerococcus tetradius, Coriobacteriaceae, Prevotella spp., Prevotella amnii | Lactobacillus dominance: L. iners was the most abundant taxa; alpha diversity: no associations were detected for any of the HPV types; beta diversity: microbiota diversity did not differ significantly according to HPV status |
| 7 | Torres-Poveda et al. (2019) | 115,651 | 15,040 | 100,611 | 25–64 | Mexico | ✓ | ⎽ | ⎽ | Biopsy | ⎽ | ⎽ |
| 8 | Bristow et al. (2019) | 300 | 35 | 265 | 18 + | Tijuana, Mexico | ⎽ | ✓ | ⎽ | Vaginal swab | Chlamydia trachomatis | ⎽ |
| 9 | Romero-Morelos et al. (2018) | 177 | 73 | 104 | ⎽ | Guerrero, Mexico | ✓ | ✓ | ⎽ | Cytobrush | ⎽ | ⎽ |
| 10 | Melo et al. (2016) | 151 | 57 | 94 | 18–24 | Temuco, Chile | ✓ | ⎽ | ⎽ | Cytobrush | Chlamydia trachomatis | ⎽ |
| 11 | Mongelos et al. (2015) | 181 | 42 | 139 | 23–41 | Paraguay | ✓ | ⎽ | ⎽ | Cytobrush | Chlamydia trachomatis, Mycoplasma hominis | ⎽ |
| 12 | Lippman et al. (2010) | 386 | 135 | 251 | 18–40 | Sao Paulo, Brazil | ✓ | ⎽ | ⎽ | Vaginal swab | ⎽ | ⎽ |
| 13 | Soto et al. (2007) | 60 | 30 | 30 | ⎽ | Havana, Cuba | ✓ | ✓ | ⎽ | Ecto/endocervical scrapings | Mycoplasma spp. | ⎽ |
| 14 | DeLuca et al. (2006) | 189 | 89 | 100 | 15–58 | Resistencia, Argentina | ✓ | ✓ | ⎽ | Cytobrush | Chlamydia trachomatis | ⎽ |
| 15 | Tonon et al. (2004) | 207 | 133 | 74 | 12–64 | Misiones, Argentina | ✓ | ✓ | ⎽ | Cytobrush | Gardnerella vaginalis | ⎽ |
| 16 | Somesh-Vikramdeo et al. (2022) | 11 | 7 | 4 | 20–62 | Mobile, Alabama | ✓ | ✓ | ⎽ | Biopsy | Rubellimicrobium, Pedobacter, Brevibacterium, Paracoccus, Fannyhessea, Brevundimonous, Comamonas, Novosphingobium | Lactobacillus depletion: decreased abundance of Lactobacillus in Hispanic compared to Non-Hispanic White suggests an increased risk of colonization of the pathogenic microbes; alpha diversity: no difference in species richness and evenness according to CIN or HPV status |
| 17 | Manzanares-Leal et al. (2022) | 120 | 60 | 60 | 21–71 | Mexico | ⎽ | ⎽ | ✓ | Vaginal swab | Corynebacterium amycolatum, Staphylococcus epidermidis | ⎽ |
| 18 | Sanchez-Garcia et al. (2019) | 201 | 78 | 123 | 18–50 | Tabasco, Mexico | ✓ | ⎽ | ⎽ | Vaginal swab | Chlamydia trachomatis | ⎽ |
| 19 | de Oliveira Ignacio et al. (2018) | 150 | 68 | 82 | 19–50 + | Botucatu, São Paulo, Brazil | ✓ | ⎽ | ⎽ | Vaginal swab, Cytobrush | ⎽ | ⎽ |
| 20 | Godoy-Vitorino et al. (2018) | 62 | 52 | 10 | 21–50 | San Juan, Puerto Rico | ✓ | ✓ | ⎽ | Vaginal swab | Lactobacillus kitasatonis, Tissierella praeacuta, Lactobacillus acidophilus, Gardnerella vaginalis, Prevotella timonensis, Lactobacillus fornicalis, Prevotella amnii, Parvimonas micra, Lactobacillus crispatus | Lactobacillus dominance: L. iners was the most common high-abundance bacterial taxon, present in more than 83% of the samples, regardless of HPV risk and lesions; alpha—Shannon bacterial diversity was significantly higher in introitus and cervix of CIN 3 compared to CIN 1 patients (P = 0.033 and P = 0.031) |
| 21 | Łaniewski et al. (2018) | 100 | 49 | 51 | ⎽ | Phoenix, Arizona | ✓ | ✓ | ✓ | Vaginal swab, Cervicovaginal lavage | Lactobacillus iners, Sneathia spp., Fannyhessea spp., Parvimonas spp., Gardnerella vaginalis, Prevotella spp., Megasphaera spp., Lactobacillus crispatus, Shuttleworthia spp. | Lactobacillus depletion: Hispanic ethnicity was associated with depletion of lactobacilli |
| 22 | Gomes de Oliveira et al. (2017) | 1346 | 1192 | 154 | 17–81 | Fortaleza, Brazil | ✓ | ✓ | ⎽ | Not specified | ⎽ | ⎽ |
| 23 | Audirac-Chalifour et al. (2016) | 32 | 12 | 20 | 22–61 | Morelos, Mexico & Mexico City, Mexico | ✓ | ✓ | ✓ | Not specified | Sneathia spp., Megasphaera elsdenii, Shuttleworthia satelles, Lactobacillus iners, Lacobacillus crispatus, Fusobacterium necrophorum | Beta diversity: the CC samples showed the highest variation among groups (HPV, cervical dysplasia, and CC); phylogenetic diversity—significant difference in SIL vs. CC where microbiota diversity in CC cases is higher than in the non-cervical lesions group |
| 24 | Clarke et al. (2012) | 9165 | 3065 | 6100 | 18–100 | Guanacaste, Costa Rica | ✓ | ⎽ | ⎽ | Vaginal swab | ⎽ | ⎽ |
| 25 | Escarcega-Tame et al. (2020) | 189 | 107 | 82 | 18–50 | Mexico City, Mexico | ✓ | ✓ | ✓ | Endocervical brush | Chlamydia trachomatis | ⎽ |
The data extraction table reports study authors and publication year, number of participants, number of participants in the outcome and control group, the age range of participants, the location of the study, the types of outcomes studied: HPV, abnormal cytology or dysplasia, and cervical cancer, sample collection method, bacteria identified in outcome or control groups, and other microbiome metrics, such as Lactobacillus dominance, depletion, and diversity
Quality Assessment
The quality of the included studies was assessed by performing the ROBINS-E risk of bias for each article (Table 1). Out of the seven domains assessed, the domain with the highest risk of bias was considered the study’s overall risk of bias [29]. The first domain, bias due to confounding, had the highest reported biases. For this domain, the authors developed a list of major risk factors influencing cervicovaginal microbiota composition and referred to confounding variables included in a similar systematic review during this process [12]. As a result, five major confounding factors influencing cervicovaginal microbiota composition were included: age [30, 31], parity [32], hormonal contraceptive use [33, 34], smoking [35], and STIs, including human immunodeficiency virus (HIV) [36, 37], herpes simplex virus (HSV) [38, 39], chlamydia [40, 41], gonorrhea [42, 43], and trichomoniasis [44, 45]. Seven articles did not control or adjust for the listed confounding variables contributing to their overall “very high” risk of bias score (Table 1) [ID# 5, 8, 10, 13, 14, 16, 19]. Thirteen articles controlled for a limited number of confounding variables contributing to their overall “high risk” of bias rating [ID# 3, 4, 6, 7, 9, 12, 15, 17, 18, 20, 22, 24, 25]. Three articles controlled for most confounding variables and were categorized as having “some concerns” of bias [ID# 2, 11, 21]. Two studies controlled for all five confounding variables and had a low risk of bias for Domain 1 [ID# 1, 23]. Due to further bias ratings, the two studies were categorized as having “some concerns” [ID# 23] and a “high” overall risk of bias [ID# 1]. Domains 2–7 were consistently rated as “some concerns” or “low” bias, with the exception of Domain 5 which resulted in four “high” bias ratings due to missing data [ID# 1, 15, 22, 25].
Synthesis of Results
Eighteen studies reported on bacteria associated with outcome groups (Table 2) [ID# 2–6, 8, 10, 11, 13–18, 20, 21, 23, 25]. Five studies [ID# 1, 6, 16, 20, 23] reported on alpha diversity using the Chao index [ID# 1], Simpson’s index [ID# 6], Shannon index [ID# 6, 16, 20], observed features [ID# 16], and phylogenetic diversity whole tree (Table 2 and Supplementary Table 1) [ID# 23]. In two studies, a significantly higher species richness and diversity were observed in Latinas with high-grade cervical intraepithelial neoplasia (CIN3) compared to low-grade cervical intraepithelial neoplasia (CIN1) [ID# 20] and Latinas with squamous intraepithelial lesions (SILs) compared to individuals without dysplasia [ID# 1]. One study reported on phylogenetic diversity and found that in Latinas, the microbiota diversity was higher in cervical cancer cases compared to the non-cervical lesions group [ID# 23]. Three studies reported on beta diversity using the Bray–Curtis distance metric [ID# 1], the Unweighted UniFrac distance metric [ID# 6, 23], and the Mann–Whitney U test (Table 2 and Supplementary Table 1) [ID# 23]. Regarding beta diversity, one study reported that 1.4% of the microbiome composition is contributed by SILs [ID# 1]. A second study reporting on beta diversity revealed that cervical cancer samples demonstrated the most variation in microbiota composition compared to HPV and cervical dysplasia groups [ID# 23]. Four studies reported on Lactobacillus [ID# 6, 16, 20, 21]. Both studies reporting on Lactobacillus dominance found that Lactobacillus iners was the most abundant bacteria amongst Latinas (n = 37, 45%; n = 48, 83%) [ID# 6, 20]. Moreover, two included studies revealed significant depletion of Lactobacillus species amongst Latinas compared to non-Latinas regardless of HPV status or lesions (n = 47, 42–86% [ID# 21]) [ID# 16, 21]. Statistical analyses performed in the aforementioned articles are listed in Table 2.
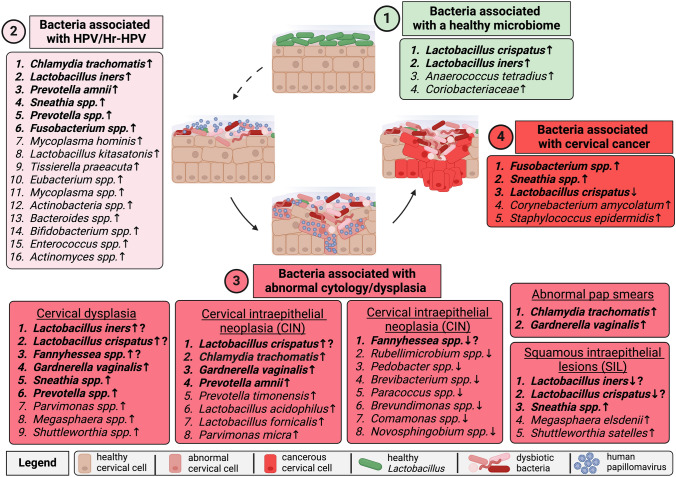
The reported bacteria were categorized into each outcome group to display cervical cancer progression in Latinas; see Fig. 2. Panel 1 depicts a healthy microbiome in Latinas, which was associated with the enrichment of four bacteria: Lactobacillus crispatus [ID# 23], L. iners [ID# 23], Anaeroccoccus [ID# 6], and Coriobacteriaceae [ID# 6]. Panel 2 highlights 16 bacteria from nine articles indicating enrichment in Latinas that were HPV-positive or high-risk HPV (Hr-HPV) positive [ID# 3, 4, 6, 10, 11, 13, 14, 20, 21]. Chlamydia trachomatis was the most consistently reported bacteria in Latinas with HPV or Hr-HPV infection (n = 4, 1.7%, n = 17, 11.2%; n = 12, 21%; n = 100, 52.9%, n = 51, 28%) [ID# 5, 10, 11, 14, 25]. Panel 3 incorporates various conditions related to abnormal cytology or dysplasia, such as abnormal Pap smears, squamous intraepithelial lesions (SILs), cervical dysplasia, and cervical intraepithelial neoplasia (CIN), and highlights 32 total associations among 24 unique bacteria across nine articles [ID# 2, 8, 15, 16, 18, 20, 21, 23, 25]. C. trachomatis (n = 30, 60%; n = 17, 48.6%; n = 6, 3%) [ID# 2, 8, 18] and Gardnerella vaginalis (n = 42, 20%) [ID# 15] were enriched in Latinas with abnormal Pap smears. Sneathia spp., Megasphaera elsdenii, and Shuttleworthia satelles were enriched in Latinas with SILs, while L. iners and L. crispatus were depleted [ID# 23]. Nine bacteria were enriched in Latinas with cervical dysplasia [ID# 21]. In Latinas with CIN, eight bacteria were enriched [ID# 20]. One study reported on the depletion of eight bacteria in the vaginal microbiome of Latinas with CIN [ID# 16]. Overall, the following bacteria were consistently enriched in Latinas with abnormal cytology/dysplasia: Sneathia spp. (n = 3, 10.3% [ID# 23]) [ID# 21, 23], C. trachomatis (n = 30, 60% n = 17, 48.6%; n = 6, 3%, n = 11, 14.3%) [ID# 2, 8, 18, 25], and G. vaginalis (n = 42, 20% [ID# 15]) [ID# 15, 20, 21]. Lastly, Panel 4 shows that Latinas with cervical cancer had enrichment of four cervicovaginal bacteria [ID# 17, 21, 23] and depletion of L. crispatus [ID# 23].
Fig. 2.
Cervicovaginal Bacteria Associated With Cervical Carcinogenesis in Latinas. Differences and similarities in the cervicovaginal microbiome composition from (1) healthy state to (2) HPV infection, (3) abnormal cytology/dysplasia, and (4) cervical cancer are depicted. Panel 1 is a light green color indicating an association with vaginal health. The following disease conditions proceed in a pink-to-red gradient according to severity: Panel 2 is light pink for HPV infection, Panel 3 is dark pink for abnormal cytology/dysplasia, and Panel 4 is bright red for cervical cancer. Enrichment or depletion of bacterial taxa associated with each stage is indicated with an up or down arrow, respectively. A question mark denotes differences in reports with regard to bacterial enrichment or depletion within the same panel. Bolded bacteria were reported in more than one study
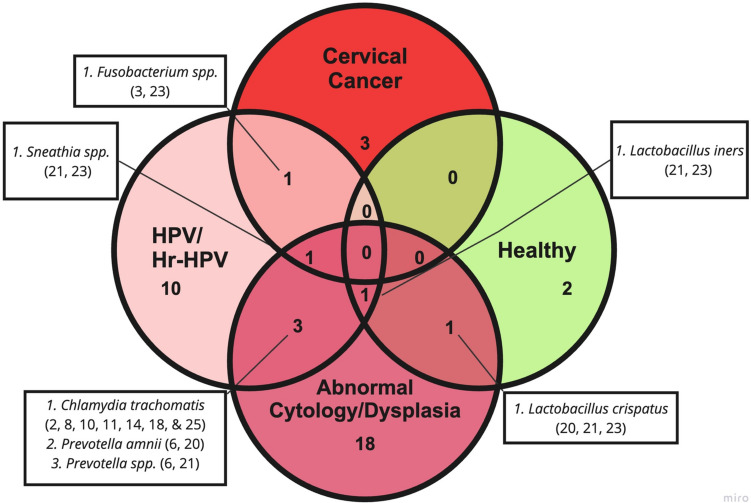
Approximately 42 unique bacteria in Latinas were associated with a healthy microbiome, HPV infection, abnormal cytology/dysplasia, and/or cervical cancer outcome groups. Some bacteria were enriched across multiple stages of cervical carcinogenesis in Latinas, which is summarized in a Venn diagram; see Fig. 3. L. crispatus was associated with health and abnormal cytology/dysplasia [ID# 20, 21, 23], whereas L. iners was associated with health, HPV/Hr-HPV infection, and abnormal cytology/dysplasia [ID# 21, 23]. Three bacteria, C. trachomatis (n = 30, 60%; n = 17, 48.6%; n = 17, 11.2%; n = 12, 21%; n = 100, 52.9%; n = 6, 3%; n = 51, 28%) [ID# 2, 8, 10, 11, 14, 18, 25], Prevotella amnii [ID# 6, 20], and Prevotella spp. [ID# 6, 21] were enriched in the HPV/Hr-HPV and abnormal cytology/dysplasia groups. Over a quarter of included studies reported C. trachomatis enrichment in HPV infection and abnormal cytology/dysplasia (n = 30, 60%; n = 17, 48.6%; n = 17, 11.2%; n = 12, 21%; n = 100, 52.9%; n = 6, 3%; n = 51, 28%) [ID# 2, 8, 10, 11, 14, 18, 25]. Fusobacterium and Sneathia spp. [ID# 21, 23] were enriched in Latinas with cervical cancer. Sneathia spp. were enriched in Latinas across all stages of cervical carcinogenesis [ID# 21, 23]. Specific statistical analyses in the aforementioned studies can be found in Supplementary Table 2.
Fig. 3.
Cervicovaginal Bacteria Enriched Across Stages of Cervical Carcinogenesis in Latinas. The quadruple Venn diagram highlights seven bacteria enriched across outcome groups, including healthy controls. Sneathia spp. and L. iners were associated with multiple outcome groups across cervical carcinogenesis. The numbers listed after each bacterial species correspond to the article reporting on the enrichment of the bacteria (reference Table 2)
Discussion
Principal Findings
This systematic review summarizes the currently available literature reporting on cervicovaginal bacteria associated with HPV infection, cervical dysplasia, and cervical cancer in North, Central, and South American Latinas. Of 324 studies, 25 articles met our inclusion criteria, revealing 42 unique cervicovaginal bacteria associated with HPV infection, cervical dysplasia, and/or cervical cancer in Latinas. Other significant findings related to alpha and beta diversity and Lactobacillus dominance or depletion in Latinas were also reported.
Comparison with Existing Literature
In our review, two bacteria, L. crispatus and L. iners, were identified in two articles reporting on the healthy cervicovaginal microbiome of Latinas. The literature suggests that L. crispatus is associated with HPV-resistance and clearance which was confirmed in our review on Latinas [46]. L. iners, on the other hand, is not associated with HPV clearance and was consistently reported in women with bacterial vaginosis in a longitudinal study [46, 47]. In addition, our review revealed that in some studies, L. crispatus was reportedly enriched in health and abnormal cytology/dysplasia, while L. iners was enriched in health, HPV/Hr-HPV infection, and abnormal cytology/dysplasia in Latinas. These findings are supported by a systematic review of 29 articles which reports a generally high relative abundance of L. iners and a lower relative abundance of L. crispatus in non-Latina individuals with precancerous lesions and cervical cancer [48]. Although, the review did not define specific values for “high” and “low” abundances of Lactobacillus species. Multiple studies have linked L. iners enrichment to HPV infection [22, 49, 50] and even cervical cancer [22, 51]. In contrast to women with L. iners-dominant microbiomes, those with L. crispatus-dominant microbiomes have a more stable microenvironment and are more likely to clear HPV infection [13, 22, 52].
Sixteen bacteria were enriched in the cervicovaginal microbiome of Latinas with HPV/Hr-HPV infection across nine studies in our review. Across studies related to HPV/Hr-HPV infection, C. trachomatis and P. amnii were significantly enriched while four bacteria were consistently enriched, although not significant: Sneathia spp., L. iners, Prevotella spp., and Fusobacterium spp.. Among the listed bacteria, C. trachomatis, P. amnii, and Prevotella spp. were enriched across eight studies in our review reporting on Latinas with HPV/Hr-HPV infection and abnormal cytology/dysplasia. C. trachomatis infection has been previously associated with HPV and Hr-HPV infection and persistence [53]. In addition, HPV/C. trachomatis co-infection has been identified as a significant risk factor for cervical cytological abnormalities [54–57]. Notably, a study based in Morocco revealed that women coinfected with HPV/C. trachomatis were three times more at risk of developing cervical abnormalities [57]. Previous studies in non-Latina cohorts reveal an increased abundance of Prevotella spp. has been associated with HPV infection, persistent Hr-HPV infection, and cervical lesions compared to control groups [58–60]. Moreover, Prevotella spp. have been associated with persistent CIN lesions and slower regression of disease [52]. Despite limited reports on P. amnii, it was the most abundant bacteria in women with low-grade SILs in a Chinese study cohort [60].
Abnormal cytology or dysplasia, such as abnormal Pap smears, SILs, cervical dysplasia, and CIN in Latinas, yielded associations with 24 unique bacteria. Across multiple studies in this category, C. trachomatis was significantly enriched, and seven additional bacteria were consistently enriched although not significant: Fannyhessea spp., G. vaginalis, L. crispatus, L. iners, Prevotella spp., P. amnii, and Sneathia spp.. In our review, some conditions included depleted bacteria, while most reported enriched bacteria. For example, L. iners and L. crispatus were depleted in women with SILs, and Fannyhessea spp. were depleted in women with CIN. An enrichment of G. vaginalis was observed across three conditions: abnormal Pap smears, cervical dysplasia, and CIN. An abundance of G. vaginalis has been frequently reported in the vaginal microbiome of women with HPV and Hr-HPV infection and is also associated with HPV persistence [61, 62]. A study revealed that coinfections with HPV and G. vaginalis increased risk for SILs and cervical cancer [61]. This may be due to the ability of G. vaginalis and other BV-associated bacteria to secrete sialidase, which is an enzyme that has been linked to an increased risk of cervical lesions [63]. In our review, L. iners, L. crispatus, and Fannyhessea spp. had conflicting relative abundance reports in women with cervical dysplasia. Existing literature suggests that Fannyhessea spp. are frequently detected in women with high-grade CIN [64, 65] and cervical cancer [64–66] in Chinese, South Korean, and Slovak cohorts.
Our literature review revealed that cervical cancer includes the enrichment of four bacteria and the depletion of one in Latinas. The depleted bacterium in Latinas with cervical cancer was L. crispatus, which is generally associated with a healthy cervicovaginal microbiome [46]. Fusobacterium and Sneathia spp. were enriched across three studies in our review on cervical cancer in Latinas. Fusobacterium spp. were enriched in Latinas with HPV/Hr-HPV infection and cervical cancer. Existing evidence demonstrates that Fusobacterium spp. abundance is strongly associated with HPV infection and high-grade dysplasia [22, 52, 58]. Moreover, after examining 112 cervical cancer tumor tissues, Huang et al. (2020) suggest Fusobacterium spp. may be a potential diagnostic and prognostic biomarker for cervical cancer [67]. Lastly, Sneathia spp. were enriched across HPV/Hr-HPV infection, abnormal cytology/dysplasia, and cervical cancer in Latinas in two included articles. One study considered Sneathia spp. a microbiological marker of Hr-HPV infection, given that Sneathia spp. were detected three times more frequently in women with Hr-HPV infection [52]. Moreover, previous studies on non-Latinas reported a significantly greater abundance of Sneathia spp. in women with cervical intraepithelial lesions, high-grade SILs, and cervical cancer compared to healthy controls [68, 69]. Furthermore, our lab demonstrated that Sneathia spp. exhibit potential oncogenic mechanisms based on the altered immunometabolic microenvironment in a 3D model of the human cervix [70, 71]. Although we were unable to run a meta-analysis, Sneathia spp. are microbes of interest and require further investigation in Latina cohorts and longitudinal studies considering they are enriched in all stages of cervical carcinogenesis.
Other significant findings related to alpha and beta diversity in Latinas were also reported. Two studies in our review reported higher species richness and diversity in Latinas with high-grade cervical intraepithelial neoplasia (CIN3) compared to low-grade cervical intraepithelial neoplasia (CIN1) and Latinas with SILs compared to non-SILs. Existing literature reports higher alpha diversity among women with abnormal cervical pathology, with a trend to increase the more severe the cervical lesion [72]. In addition, a previous study reported higher alpha diversity among women with cervical cancer, which was confirmed in our review on Latinas [73]. Our review also revealed a significantly higher beta diversity in women with SILs compared to non-SILs and women with cervical cancer compared to those with HPV infection and cervical dysplasia. Significant differences in beta diversity among non-Latina women with HPV infection, SILs, and cervical cancer compared to controls were also reported in a systematic review [74]. Overall, our review revealed a trend toward increased alpha and beta diversity and cervical carcinogenesis progression in Latinas.
Regarding Lactobacillus dominance, two studies in our review reported L. iners, a transitional bacterium, as the most abundant among Latinas [47]. Lastly, two studies in our review revealed significant depletion of Lactobacillus species among Latinas compared to non-Latinas. Previously, Ravel et al. (2011) demonstrated a racial–ethnic difference in microbiome composition as Black and Latina women harbor increased levels of diverse anaerobes and lower levels of health-associated, Lactobacillus species compared to White and Asian women who possess Lactobacillus-dominant vaginal microbiomes [75, 76]. In our review, we observed similar overall low levels of Lactobacillus and increased bacterial diversity across Latinas globally.
Our review featured studies from ten countries across North, Central, and South America and the Caribbean. Another review of women from Latin America and the Caribbean (LAC) revealed that early age at first sexual intercourse, number of sexual partners, and sexual behavior of the partner are associated with increased risk of genital HPV acquisition [77]. Additional co-factors for cervical cancer in LAC include high parity, long-term use of oral contraceptives, high prevalence of smoking, and co-infection with the human immunodeficiency virus (HIV) [77]. In addition to these risk factors, women in LAC face systematic barriers to healthcare such as uneven resource distribution, variable infrastructure, and healthcare service availability [77, 78]. Rural, low-resourced, and underserved populations in LAC are especially impacted and are less likely to have access to HPV vaccination, cervical cancer prevention, and screening [52]. Studies along the U.S.–Mexican border also demonstrate the need for increased health education and awareness regarding the HPV vaccine and HPV/cervical disease diagnoses to prevent cervical cancer with screening, early diagnosis, and treatment [9, 79].
Strengths and Limitations
To our knowledge, this is the first systematic review to report on the cervicovaginal bacteria in studies related to HPV infection, cervical dysplasia, and cancer in Latinas. The strengths of this review include strict criteria for inclusion, yielding only articles relevant to our research question. While our inclusion criteria were strict, our search generated a broad range of articles focused on our population of interest. By including the extensive Latinx search hedge, we were able to account for the racial–ethnic, geographic, and linguistic range the individual “Hispanic” search term lacks [27].
The limitations are those inherent to a systematic review, which includes relying on the information and quality of data available. Consequently, the varying study designs and general lack of homogeneity of reported analyses (e.g., few and dissimilar statistical tests, differing variables, etc.) failed to meet the basic criteria for unbiased meta-analytic methods. In an effort to update the review and reassess the potential for a meta-analysis, the search could be re-conducted after a few years following publication of additional studies. With more data available, significant associations of specific bacteria can be determined in Latinas with HPV, cervical dysplasia, and cervical cancer. Second, some studies adjusted for confounding factors and excluded individuals with STIs (i.e., C. trachomatis), while other studies included all patients, even those with STIs. This leads to a discrepancy in the inclusion or exclusion of individuals with C. trachomatis in the 25 included studies. As such, the C. trachomatis findings must be interpreted with caution. Hence, controlling for confounding variables involves balancing the need to include all individuals with the demand of strict bias criteria. Lastly, in order to establish a causal connection between the enriched bacteria and cervical carcinogenesis, longitudinal microbiome studies must be performed [80]. Longitudinal microbiome studies and larger cohort studies, including Latinas will help determine the role of bacteria as drivers (influential disease-causing agents), passengers (less influential agents favoring the environment), or a consequence of disease in this population of women [80].
Conclusions and Implications
The systematic review identified 42 unique bacteria across 25 studies related to HPV infection, cervical dysplasia, and cervical cancer in Latinas. L. crispatus was enriched in healthy Latinas and those with abnormal cytology/dysplasia, while L. iners was enriched in multiple states ranging from healthy to abnormal cytology/dysplasia groupings. C. trachomatis, P. amnii, and Prevotella spp. were enriched in Latinas with HPV/Hr-HPV infection and abnormal cytology/dysplasia. Notably, Fusobacterium spp. were enriched in Latinas with HPV/Hr-HPV infection and cervical cancer. Importantly, Sneathia spp. were enriched across all stages in cervical cancer progression—HPV/Hr-HPV infection, abnormal cytology/dysplasia, and cervical cancer. In addition to more research on Latina populations, barriers related to social determinants of health and structural racism must be considered to improve outcomes against a preventable disease like cervical cancer amongst Latinas. Advanced public health efforts, including community-based participatory research projects with Latinas, can reduce health disparities in HPV infection and cervical cancer [81, 82]. In conclusion, future epidemiological studies must intentionally include Latina women in order to ultimately create primary or secondary preventative strategies for this susceptible population.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to express our gratitude to Dr. Paweł Łaniewski and Phoebe Crossley for critically reviewing the manuscript. We would also like to acknowledge the Medical Library Association Latinx Caucus for developing the Latinx and Hispanic U.S. Population Search Hedge to facilitate our search strategy.
Abbreviations
- HPV
Human papillomavirus
- Hr-HPV
High-risk human papillomavirus
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- LAC
Latin America and the Caribbean
- CIN
Cervical intraepithelial neoplasia
- CIN1
Cervical intraepithelial neoplasia, low grade
- CIN3
Cervical intraepithelial neoplasia, high grade
- BV
Bacterial vaginosis
- STI
Sexually transmitted infection
- ROBINS-E
Risk Of Bias In Non-randomized Studies of Exposure
- L.iners
Lactobacillus iners
- C.trachomatis
Chlamydia trachomatis
- L.crispatus
Lactobacillus crispatus
- G.vaginalis
Gardnerella vaginalis
- Spp.
Species
- P.amnii
Prevotella amnii
- SILs
Squamous intraepithelial lesions
- Pap smear
Papanicolaou smear
- HIV
Human immunodeficiency virus
- U.S
United States
- NParticipants
Number of total participants
- NOutcomeGrp
Number of participants in the outcome group
- NCtrlGrp
Number of participants in the control group
- CC
Cervical cancer
Author Contributions
V.M., N.R.J., N.S.B., M.F., and M.M.H–K. contributed to the conception, planning, and study design. Material preparation, data collection, and analysis were performed by V.M. and N.R.J.. N.S.B. assisted with software access and training V.M., N.R.J and M.M.H-K. on DistillerSR. The first draft of the manuscript was written by V.M., and N.R.J., N.S.B., M.F., and M.M.H–K. critically reviewed and provided edits on the manuscript. M.M.H–K provided supervision and funding for the work. All authors read and approved the final manuscript.
Funding
This work was supported by the Flinn Foundation (Grant# 2244) to M.M.H–K.. N.R.J. was supported by the Sidney Hopkins, Mayola B. Vail, and Patricia Ann Hanson Postdoctoral Fellowship award from the Community Foundation for Southern Arizona. M.F. was supported by the National Heart, Lung, and Blood Institute under K99HL15761101. N.R.J. is supported by the Guiding U54 Investigator Development to Sustainability (GUIDeS) shared resource through the Partnership for Native American Cancer Prevention; the partnership is funded the National Cancer Institute grant U54CA143924. The funding sources were not involved in the study design, data collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Availability of Data and Materials
All manuscript data are included as electronic supplementary material.
Declarations
Conflict of Interest
M.M.H.–K. is a paid consultant for Vaginal Biome Sciences and serves on the scientific advisory board for Freya Biosciences. None of this work or related to was shared with or was licensed to this company or any other commercial entity. V.M., N.R.J., N.S.B., and M.F. declare no financial interests. The authors have no relevant non-financial interests to disclose.
Ethical Approval and Consent to Participate
Human participants were not involved in the systematic review. Ethics committee approval and consent to participate was not required.
Consent for Publication
Human participants were not involved in the systematic review. Consent to the submission was not required.
References
- 1.Buskwofie A, David-West G, Clare CA. A review of cervical cancer: incidence and disparities. J Natl Med Assoc. 2020;112(2):229–232. doi: 10.1016/j.jnma.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Almonte M, Bruni L, Clifford G, Curado MP, Piñeros M. Burden and trends of type-specific human papillomavirus infections and related diseases in the Latin America and Caribbean Region. Vaccine. 2008;26:L1–L15. doi: 10.1016/j.vaccine.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 3.Kombe Kombe AJ, Li B, Zahid A, et al. Epidemiology and burden of human papillomavirus and related diseases, molecular pathogenesis, and vaccine evaluation. Front Public Health. 2020;8:552028. doi: 10.3389/fpubh.2020.552028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pflieger JC, Cook EC, Niccolai LM, Connell CM. Racial/ethnic differences in patterns of sexual risk behavior and rates of sexually transmitted infections among female young adults. Am J Public Health. 2013;103(5):903–909. doi: 10.2105/AJPH.2012.301005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith SJ. Risky sexual behavior among young adult latinas: are acculturation and religiosity protective? J Sex Res. 2015;52(1):43–54. doi: 10.1080/00224499.2013.821443. [DOI] [PubMed] [Google Scholar]
- 6.Hariri S, Unger ER, Sternberg M, et al. Prevalence of genital human papillomavirus among females in the United States, the national health and nutrition examination survey, 2003–2006. J Infect Dis. 2011;204(4):566–573. doi: 10.1093/infdis/jir341. [DOI] [PubMed] [Google Scholar]
- 7.Reiter PL, Pennell ML, Martinez GA, Perkins RB, Katz ML. HPV vaccine coverage across Hispanic/Latinx subgroups in the United States. Cancer Causes Control. 2020;31(10):905–914. doi: 10.1007/s10552-020-01331-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ortiz AP, Soto-Salgado M, Calo WA, et al. Elimination of cervical cancer in U.S. Hispanic populations: Puerto Rico as a case study. Prevent Med. 2021;144:106336. doi: 10.1016/j.ypmed.2020.106336. [DOI] [PubMed] [Google Scholar]
- 9.Morales-Campos DY, Snipes SA, Villarreal EK, Crocker LC, Guerrero A, Fernandez ME. Cervical cancer, human papillomavirus (HPV), and HPV vaccination: exploring gendered perspectives, knowledge, attitudes, and cultural taboos among Mexican American adults. Ethn Health. 2021;26(2):206–224. doi: 10.1080/13557858.2018.1494821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitra A, MacIntyre DA, Marchesi JR, Lee YS, Bennett PR, Kyrgiou M. The vaginal microbiota, human papillomavirus infection, and cervical intraepithelial neoplasia: what do we know and where are we going next? Microbiome. 2016;4(1):58. doi: 10.1186/s40168-016-0203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plummer M, Schiffman M, Castle PE, Maucort-Boulch D, Wheeler CM, ALTS Group A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis. 2007;195(11):1582–1589. doi: 10.1086/516784. [DOI] [PubMed] [Google Scholar]
- 12.Brusselaers N, Shrestha S, van de Wijgert J, Verstraelen H. Vaginal dysbiosis and the risk of human papillomavirus and cervical cancer: systematic review and meta-analysis. Am J Obstet Gynecol. 2019;221(1):9–18.e8. doi: 10.1016/j.ajog.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Shi W, Zhu H, Yuan L, et al. Vaginal microbiota and HPV clearance: a longitudinal study. Front Oncol. 2022;12:955150. doi: 10.3389/fonc.2022.955150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tachedjian G, Aldunate M, Bradshaw CS, Cone RA. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res Microbiol. 2017;168(9):782–792. doi: 10.1016/j.resmic.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Petrova MI, Lievens E, Malik S, Imholz N, Lebeer S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front Physiol. 2015;6:81. doi: 10.3389/fphys.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Lu Y, Chen T, Li R. The female vaginal microbiome in health and bacterial vaginosis. Front Cell Infect Microbiol. 2021 doi: 10.3389/fcimb.2021.631972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muzny CA, Taylor CM, Swords WE, et al. An updated conceptual model on the pathogenesis of bacterial vaginosis. J Infect Dis. 2019;220(9):1399–1405. doi: 10.1093/infdis/jiz342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lev-Sagie A, De Seta F, Verstraelen H, Ventolini G, Lonnee-Hoffmann R, Vieira-Baptista P. The vaginal microbiome: II. Vaginal dysbiotic conditions. J Low Genit Tract Dis. 2022;26(1):79. doi: 10.1097/LGT.0000000000000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Seta F, Lonnee-Hoffmann R, Campisciano G, et al. The vaginal microbiome: III. The vaginal microbiome in various urogenital disorders. J Low Genit Tract Dis. 2022;26(1):85. doi: 10.1097/LGT.0000000000000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brotman RM. Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. J Clin Invest. 2011;121(12):4610–4617. doi: 10.1172/JCI57172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peipert JF, Lapane KL, Allsworth JE, Redding CA, Blume JD, Stein MD. Bacterial vaginosis, race, and sexually transmitted infections: does race modify the association? Sex Transm Dis. 2008;35(4):363–367. doi: 10.1097/OLQ.0b013e31815e4179. [DOI] [PubMed] [Google Scholar]
- 22.Norenhag J, Du J, Olovsson M, Verstraelen H, Engstrand L, Brusselaers N. The vaginal microbiota, human papillomavirus and cervical dysplasia: a systematic review and network meta-analysis. BJOG. 2020;127(2):171–180. doi: 10.1111/1471-0528.15854. [DOI] [PubMed] [Google Scholar]
- 23.Peebles K, Velloza J, Balkus JE, McClelland RS, Barnabas RV. High global burden and costs of bacterial vaginosis: a systematic review and meta-analysis. Sex Transm Dis. 2019;46(5):304–311. doi: 10.1097/OLQ.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 24.Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007;34(11):864–869. doi: 10.1097/OLQ.0b013e318074e565. [DOI] [PubMed] [Google Scholar]
- 25.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.United States Census Bureau. About the Hispanic Population and its Origin. Accessed Oct 2022. https://www.census.gov/topics/population/hispanic-origin/about.html
- 27.Medical Library Association Latinx Caucus. Latinx/Hispanic US Population Search Hedges. Accessed Oct 2022. http://hdl.handle.net/20.500.12613/6949
- 28.DistillerSR. Version 2.35. DistillerSR Inc.; 2023. Accessed Nov 2022-June 2023. https://www.distillersr.com/.
- 29.ROBINS-E Development Group, Higgins J, Morgan R, Rooney A, et al. Risk Of Bias In Non-randomized Studies - of Exposure (ROBINS-E). Accessed Feb 2023. https://www.riskofbias.info/welcome/robins-e-tool.
- 30.Auriemma RS, Scairati R, del Vecchio G, et al. The vaginal microbiome: a long urogenital colonization throughout woman life. Front Cell Infect Microbiol. 2021;11:686167. doi: 10.3389/fcimb.2021.686167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muhleisen AL, Herbst-Kralovetz MM. Menopause and the vaginal microbiome. Maturitas. 2016;91:42–50. doi: 10.1016/j.maturitas.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Romero R, Theis KR, Gomez-Lopez N, et al. The vaginal microbiota of pregnant women varies with gestational age, maternal age, and parity. Microbiol Spectr. 2023;11(4):e03429–e3522. doi: 10.1128/spectrum.03429-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bastianelli C, Farris M, Bianchi P, Benagiano G. The effect of different contraceptive methods on the vaginal microbiome. Expert Rev Clin Pharmacol. 2021;14(7):821–836. doi: 10.1080/17512433.2021.1917373. [DOI] [PubMed] [Google Scholar]
- 34.Vodstrcil LA, Hocking JS, Law M, et al. Hormonal contraception is associated with a reduced risk of bacterial vaginosis: a systematic review and meta-analysis. PLoS ONE. 2013;8(9):e73055. doi: 10.1371/journal.pone.0073055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brotman RM, He X, Gajer P, et al. Association between cigarette smoking and the vaginal microbiota: a pilot study. BMC Infect Dis. 2014;14(1):471. doi: 10.1186/1471-2334-14-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klein C, Gonzalez D, Samwel K, et al. Relationship between the cervical microbiome, HIV Status, and precancerous lesions. MBio. 2019 doi: 10.1128/mbio.02785-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gosmann C, Anahtar MN, Handley SA, et al. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased Hiv acquisition in young south African women. Immunity. 2017;46(1):29–37. doi: 10.1016/j.immuni.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masese L, Baeten JM, Richardson BA, et al. Incident herpes simplex virus type 2 infection increases the risk of subsequent episodes of bacterial vaginosis. J Infect Dis. 2014;209(7):1023–1027. doi: 10.1093/infdis/jit634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esber A, Vicetti Miguel RD, Cherpes TL, Klebanoff MA, Gallo MF, Turner AN. Risk of bacterial vaginosis among women with herpes simplex virus type 2 infection: a systematic review and meta-analysis. J Infect Dis. 2015;212(1):8–17. doi: 10.1093/infdis/jiv017. [DOI] [PubMed] [Google Scholar]
- 40.Filardo S, Di Pietro M, Porpora MG, et al. Diversity of cervical microbiota in asymptomatic Chlamydia trachomatis genital infection: a pilot study. Front Cell Infect Microbiol. 2017;7:321. doi: 10.3389/fcimb.2017.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raimondi S, Candeliere F, Amaretti A, et al. Vaginal and anal microbiome during Chlamydia trachomatis infections. Pathogens. 2021;10(10):1347. doi: 10.3390/pathogens10101347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallo MF, Macaluso M, Warner L, et al. Bacterial vaginosis, gonorrhea, and chlamydial infection among women attending a sexually transmitted disease clinic: a longitudinal analysis of possible causal links. Ann Epidemiol. 2012;22(3):213–220. doi: 10.1016/j.annepidem.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Lovett A, Seña AC, Macintyre AN, Sempowski GD, Duncan JA, Waltmann A. Cervicovaginal microbiota predicts neisseria gonorrhoeae clinical presentation. Front Microbiol. 2021;12:790531. doi: 10.3389/fmicb.2021.790531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rathod SD, Krupp K, Klausner JD, Arun A, Reingold AL, Madhivanan P. Bacterial vaginosis and risk for trichomonas vaginalis infection: a longitudinal analysis. Sex Transm Dis. 2011;38(9):882–886. doi: 10.1097/OLQ.0b013e31821f91a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brotman RM, Bradford LL, Conrad M, et al. Association between trichomonas vaginalis and vaginal bacterial community composition among reproductive-age women. Sex Transm Dis. 2012;39(10):807. doi: 10.1097/OLQ.0b013e3182631c79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai W, Du H, Li S, Wu R. Cervicovaginal microbiome factors in clearance of human papillomavirus infection. Front Oncol. 2021;11:722639. doi: 10.3389/fonc.2021.722639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ravel J, Brotman RM, Gajer P, et al. Daily temporal dynamics of vaginal microbiota before, during and after episodes of bacterial vaginosis. Microbiome. 2013;1:29. doi: 10.1186/2049-2618-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang X, Da M, Zhang W, Qi Q, Zhang C, Han S. Role of Lactobacillus in cervical cancer. CMAR. 2018;10:1219–1229. doi: 10.2147/CMAR.S165228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nicolò S, Tanturli M, Mattiuz G, et al. Vaginal lactobacilli and vaginal dysbiosis-associated bacteria differently affect cervical epithelial and immune homeostasis and anti-viral defenses. Int J Mol Sci. 2021;22(12):6487. doi: 10.3390/ijms22126487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moscicki AB, Shi B, Huang H, Barnard E, Li H. Cervical-vaginal microbiome and associated cytokine profiles in a prospective study of HPV 16 acquisition, persistence, and clearance. Front Cell Infect Microbiol. 2020;10:569022. doi: 10.3389/fcimb.2020.569022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei B, Chen Y, Lu T, Cao W, Tang Z, Yang H. Correlation between vaginal microbiota and different progression stages of cervical cancer. Genet Mol Biol. 2022;45(2):e20200450. doi: 10.1590/1678-4685-GMB-2020-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitra A, MacIntyre DA, Ntritsos G, et al. The vaginal microbiota associates with the regression of untreated cervical intraepithelial neoplasia 2 lesions. Nat Commun. 2020;11(1):1999. doi: 10.1038/s41467-020-15856-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naldini G, Grisci C, Chiavarini M, Fabiani R. Association between human papillomavirus and chlamydia trachomatis infection risk in women: a systematic review and meta-analysis. Int J Public Health. 2019;64(6):943–955. doi: 10.1007/s00038-019-01261-w. [DOI] [PubMed] [Google Scholar]
- 54.Madaan N, Pandhi D, Sharma V, et al. Association of abnormal cervical cytology with coinfection of human papillomavirus and Chlamydia trachomatis. Indian J Sex Transm Dis AIDS. 2019;40(1):57–63. doi: 10.4103/ijstd.IJSTD_9_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martinelli M, Musumeci R, Rizzo A, et al. Prevalence of chlamydia trachomatis infection, serovar distribution and co-infections with seven high-risk HPV types among italian women with a recent history of abnormal cervical cytology. Int J Environ Res Public Health. 2019;16(18):3354. doi: 10.3390/ijerph16183354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mosmann JP, Zayas S, Kiguen AX, Venezuela RF, Rosato O, Cuffini CG. Human papillomavirus and Chlamydia trachomatis in oral and genital mucosa of women with normal and abnormal cervical cytology. BMC Infect Dis. 2021;21(1):422. doi: 10.1186/s12879-021-06118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferrera L, Rogua H, El Mansouri N, et al. The association of Chlamydia trachomatis and human papillomavirus co-infection with abnormal cervical cytology among women in south of Morocco. Microb Pathog. 2023;175:105971. doi: 10.1016/j.micpath.2023.105971. [DOI] [PubMed] [Google Scholar]
- 58.Lee JE, Lee S, Lee H, et al. Association of the vaginal microbiota with human papillomavirus infection in a Korean twin cohort. PLoS ONE. 2013;8(5):e63514. doi: 10.1371/journal.pone.0063514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dong B, Huang Y, Cai H, et al. Prevotella as the hub of the cervicovaginal microbiota affects the occurrence of persistent human papillomavirus infection and cervical lesions in women of childbearing age via host NF-κB/C-myc. J Med Virol. 2022;94(11):5519–5534. doi: 10.1002/jmv.28001. [DOI] [PubMed] [Google Scholar]
- 60.Chen Y, Qiu X, Wang W, et al. Human papillomavirus infection and cervical intraepithelial neoplasia progression are associated with increased vaginal microbiome diversity in a Chinese cohort. BMC Infect Dis. 2020;20(1):629. doi: 10.1186/s12879-020-05324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suehiro TT, Malaguti N, Damke E, et al. Association of human papillomavirus and bacterial vaginosis with increased risk of high-grade squamous intraepithelial cervical lesions. Int J Gynecol Cancer. 2019 doi: 10.1136/ijgc-2018-000076. [DOI] [PubMed] [Google Scholar]
- 62.Di Paola M, Sani C, Clemente AM, et al. Characterization of cervico-vaginal microbiota in women developing persistent high-risk human papillomavirus infection. Sci Rep. 2017;7(1):10200. doi: 10.1038/s41598-017-09842-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Govinden G, Parker JL, Naylor KL, Frey AM, Anumba DOC, Stafford GP. Inhibition of sialidase activity and cellular invasion by the bacterial vaginosis pathogen Gardnerella vaginalis. Arch Microbiol. 2018;200(7):1129–1133. doi: 10.1007/s00203-018-1520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wei W, Xie LZ, Xia Q, et al. The role of vaginal microecology in the cervical cancer. J Obstet Gynaecol Res. 2022;48(9):2237–2254. doi: 10.1111/jog.15359. [DOI] [PubMed] [Google Scholar]
- 65.So KA, Yang EJ, Kim NR, et al. Changes of vaginal microbiota during cervical carcinogenesis in women with human papillomavirus infection. PLoS ONE. 2020;15(9):e0238705. doi: 10.1371/journal.pone.0238705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rokos T, Holubekova V, Kolkova Z, et al. Is the physiological composition of the vaginal microbiome altered in high-risk HPV infection of the uterine cervix? Viruses. 2022;14(10):2130. doi: 10.3390/v14102130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang ST, Chen J, Lian LY, et al. Intratumoral levels and prognostic significance of Fusobacterium nucleatum in cervical carcinoma. Aging (Albany NY) 2020;12(22):23337–23350. doi: 10.18632/aging.104188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mitra A, MacIntyre DA, Lee YS, et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci Rep. 2015;5:16865. doi: 10.1038/srep16865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xie Y, Feng Y, Li W, et al. Revealing the disturbed vaginal micobiota caused by cervical cancer using high-throughput sequencing technology. Front Cell Infect Microbiol. 2020;10:538336. doi: 10.3389/fcimb.2020.538336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Łaniewski P, Herbst-Kralovetz MM. Bacterial vaginosis and health-associated bacteria modulate the immunometabolic landscape in 3D model of human cervix. NPJ Biofilms Microbiomes. 2021;7:88. doi: 10.1038/s41522-021-00259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maarsingh JD, Łaniewski P, Herbst-Kralovetz MM. Immunometabolic and potential tumor-promoting changes in 3D cervical cell models infected with bacterial vaginosis-associated bacteria. Commun Biol. 2022;5:725. doi: 10.1038/s42003-022-03681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu S, Ding X, Kong Y, et al. The feature of cervical microbiota associated with the progression of cervical cancer among reproductive females. Gynecol Oncol. 2021;163(2):348–357. doi: 10.1016/j.ygyno.2021.08.016. [DOI] [PubMed] [Google Scholar]
- 73.Tsementzi D, Pena-Gonzalez A, Bai J, et al. Comparison of vaginal microbiota in gynecologic cancer patients pre- and post-radiation therapy and healthy women. Cancer Med. 2020;9(11):3714–3724. doi: 10.1002/cam4.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu M, Li H, Yu H, et al. Disturbances of vaginal microbiome composition in human papillomavirus infection and cervical carcinogenesis: a qualitative systematic review. Front Oncol. 2022 doi: 10.3389/fonc.2022.941741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci. 2011;108(supplement_1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.MacIntyre DA, Chandiramani M, Lee YS, et al. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci Rep. 2015;5(1):8988. doi: 10.1038/srep08988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Almonte M, Albero G, Molano M, Carcamo C, García PJ, Pérez G. Risk factors for human papillomavirus exposure and co-factors for cervical cancer in Latin America and the Caribbean. Vaccine. 2008;26:L16–L36. doi: 10.1016/j.vaccine.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 78.Lopez MS, Baker ES, Maza M, et al. Cervical cancer prevention and treatment in Latin America. J Surg Oncol. 2017;115(5):615–618. doi: 10.1002/jso.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fernandez ME, McCurdy SA, Arvey SR, et al. HPV knowledge, attitudes, and cultural beliefs among Hispanic men and women living on the Texas-Mexico border. Ethn Health. 2009;14(6):607–624. doi: 10.1080/13557850903248621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Łaniewski P, Ilhan ZE, Herbst-Kralovetz MM. The microbiome and gynaecological cancer development, prevention and therapy. Nat Rev Urol. 2020;17(4):232. doi: 10.1038/s41585-020-0286-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barnack-Tavlaris JL, Garcini L, Sanchez O, Hernandez I, Navarro AM. Focus group discussions in community-based participatory research to inform the development of a human papillomavirus (HPV) Educational Intervention for Latinas in San Diego. J Cancer Educ. 2013 doi: 10.1007/s13187-013-0516-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baezconde-Garbanati L, Martinez B, Ochoa C, et al. Optimizing engagement of the latino community in cancer research. In: Ramirez AG, Trapido EJ, et al., editors. Advancing the science of cancer in latinos: building collaboration for action. Springer International Publishing; 2023. pp. 101–113. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All manuscript data are included as electronic supplementary material.